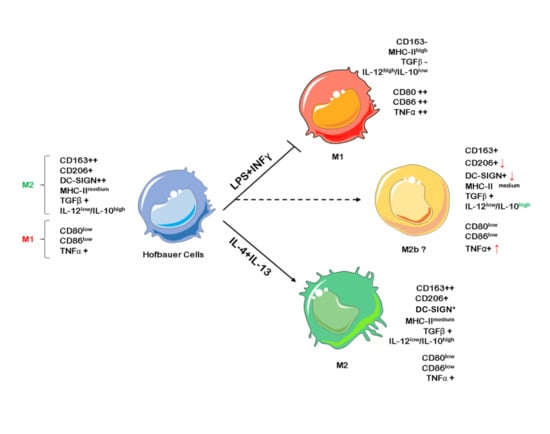
Placental Hofbauer Cell Polarization Resists Inflammatory Cues In Vitro
Abstract
1. Introduction
2. Results
2.1. Hofbauer Cells Do not Polarize Towards an M1 Phenotype upon LPS + INF-γ Treatment
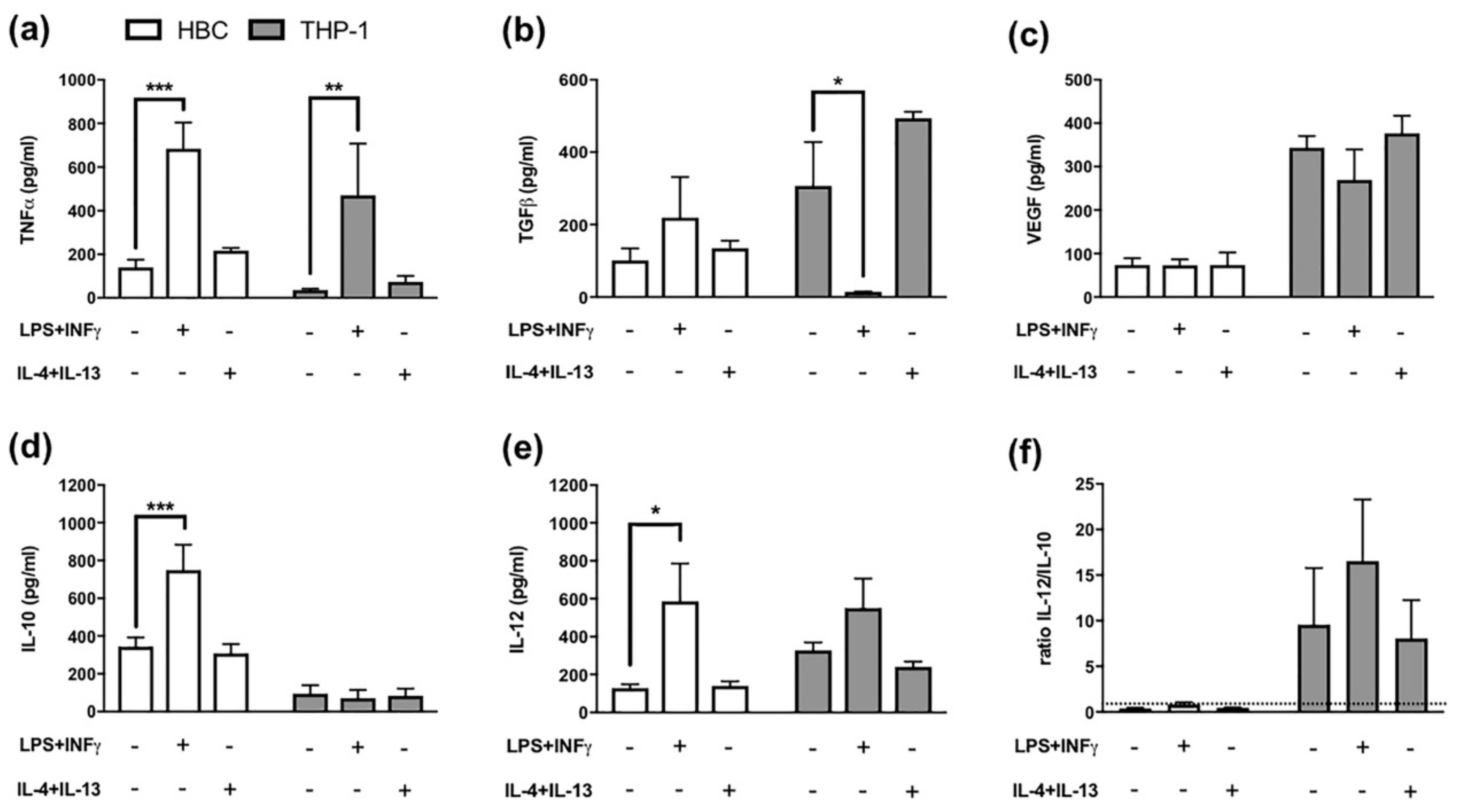
2.2. LPS + INF-γ Stimulation Increases TNF-α and IL-12 but also IL-10 Release from HBCs
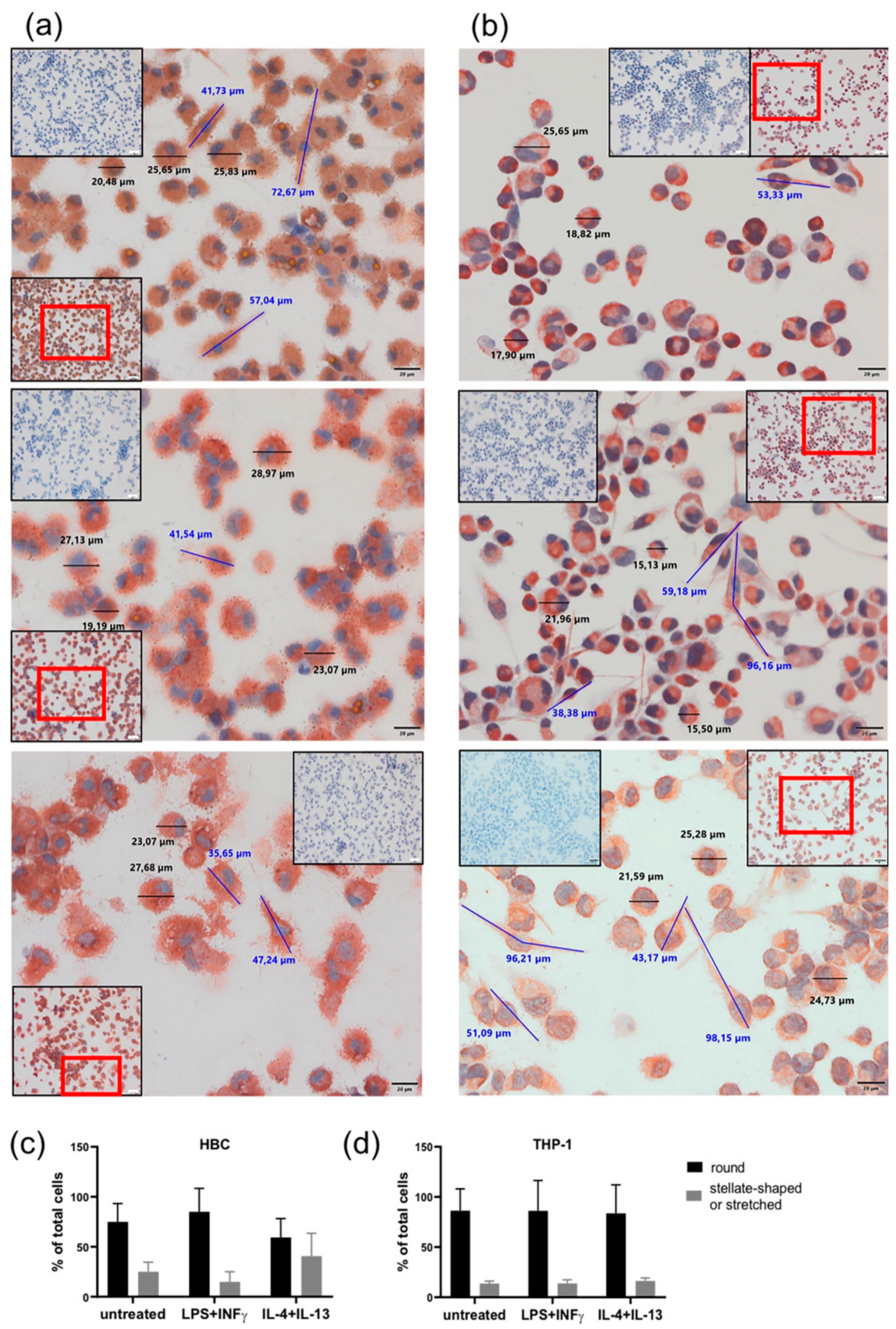
2.3. LPS + INF-γ Stimulation Alters Cell Morphology of HBCs but not THP-1 Cells
3. Discussion
4. Materials and Methods
4.1. Isolation of Hofbauer Cells
4.2. Cultivation of THP-1 Cells and Macrophage Differentiation
4.3. Phenotypical Stimulation of THP-1 Macrophages and Hofbauer Cells
4.4. Flow Cytometry
4.5. Enzyme Linked Immunosorbent Assays
4.6. Immune Cytochemistry (ICC)
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HBCs | Hofbauer cells |
| LPS | Lipopolysaccharid |
| TNF-α | Tumor necrosis factor alpha |
| TGF-β | Transforming growth factor beta |
| INF-ɣ | Interferon gamma |
| IL- | Interleukin |
| TAM | Tumor associated macrophage |
| CAM | Chorioamnionitis |
| VUE | Villitis of Unknown Etiology |
| T1DM | Type 1 Diabetes Mellitus |
| GDM | Gestational Diabetes Mellitus |
References
- Martinez, F.; Sica, A.; Mantovani, A.; Locati, M. Macrophage activation and polarization. Front. Biosci. 2008, 1, 453–461. [Google Scholar] [CrossRef]
- Italiani, P.; Boraschi, D. From monocytes to M1/M2 macrophages: Phenotypical vs. functional differentiation. Front. Immunol. 2014, 5, 1–22. [Google Scholar]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Rőszer, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediat. Inflamm. 2015, 2015, 1–16. [Google Scholar] [CrossRef]
- Vinchi, F.; Muckenthaler, M.U.; Da Silva, M.C.; Balla, G.; Balla, J.; Jeney, V. Atherogenesis and iron: From epidemiology to cellular level. Front. Pharmacol. 2014, 5, 94. [Google Scholar] [CrossRef]
- Martinoli, C.; Castellucci, M.; Zaccheo, D.; Kaufmann, P. Scanning electron microscopy of stromal cells of human placental villi throughout pregnancy. Cell Tissue Res. 1984, 235, 647–655. [Google Scholar] [CrossRef]
- Castellucci, M.; Zaccheo, D.; Pescetto, G. A three-dimensional study of the normal human placental villous core. I. The Hofbauer cells. Cell Tissue Res. 1980, 210, 235–247. [Google Scholar] [CrossRef]
- Kim, J.; Romero, R.; Kim, M.; Kim, M.; Friel, L.; Espinoza, J.; Kim, C. Involvement of Hofbauer cells and maternal T cells in villitis of unknown etiology. Histopathology 2008, 52, 457–464. [Google Scholar] [CrossRef]
- Bockle, B.C.; Solder, E.; Kind, S.; Romani, N.; Sepp, N.T. DC-SIGN+ CD163+ Macrophages Expressing Hyaluronan Receptor LYVE-1 Are Located within Chorion Villi of the Placenta. Placenta 2008, 29, 187–192. [Google Scholar] [CrossRef]
- Joerink, M.; Rindsjö, E.; van Riel, B.; Alm, J.; Papadogiannakis, N. Placental macrophage (Hofbauer cell) polarization is independent of maternal allergen-sensitization and presence of chorioamnionitis. Placenta 2011, 32, 380–385. [Google Scholar] [CrossRef]
- Svensson, J.; Jenmalm, M.C.; Matussek, A.; Geffers, R.; Berg, G.; Ernerudh, J. Macrophages at the fetal-maternal interface express markers of alternative activation and are induced by M-CSF and IL-10. J. Immunol. 2011, 187, 3671–3682. [Google Scholar] [CrossRef]
- Kim, S.Y.; Romero, R.; Tarca, A.L.; Bhatti, G.; Kim, C.J.; Lee, J.; Sonia, S. Methylome of Fetal and Maternal Monocytes and Macrophages at the Feto-Maternal Interface. Am. J. Reprod. Immunol. 2012, 68, 8–27. [Google Scholar] [CrossRef]
- Svensson-Arvelund, J.; Mehta, R.B.; Lindau, R.; Mirrasekhian, E.; Rodriguez-Martinez, H.; Berg, G.; Lash, G.E.; Jenmalm, M.C.; Ernerudh, J. The Human Fetal Placenta Promotes Tolerance against the Semiallogeneic Fetus by Inducing Regulatory T Cells and Homeostatic M2 Macrophages. J. Immunol. 2015, 194, 1534–1544. [Google Scholar] [CrossRef]
- Anteby, E.Y.; Natanson-Yaron, S.; Greenfield, C.; Goldman-Wohl, D.; Haimov-Kochman, R.; Holzer, H.; Yagel, S. Human placental Hofbauer cells express sprouty proteins: A possible modulating mechanism of villous branching. Placenta 2005, 26, 476–483. [Google Scholar] [CrossRef]
- Seval, Y.; Korgun, E.T.; Demir, R. Hofbauer Cells in Early Human Placenta: Possible Implications in Vasculogenesis and Angiogenesis. Placenta 2007, 28, 841–845. [Google Scholar] [CrossRef]
- Rosenberg, A.Z.; Yu, W.; Hill, D.A.; Reyes, C.A.; Schwartz, D.A. Placental Pathology of Zika Virus: Viral Infection of the Placenta Induces Villous Stromal Macrophage (Hofbauer Cell) Proliferation and Hyperplasia. Arch. Pathol. Lab. Med. 2017, 141, 43–48. [Google Scholar] [CrossRef]
- Johnson, E.L.; Chakraborty, R. Placental Hofbauer cells limit HIV-1 replication and potentially offset mother to child transmission (MTCT) by induction of immunoregulatory cytokines. Retrovirology 2012, 9, 101. [Google Scholar] [CrossRef]
- Tamblyn, J.A.; Lissauer, D.M.; Powell, R.; Cox, P.; Kilby, M.D. The immunological basis of villitis of unknown etiology—review. Placenta 2013, 34, 846–855. [Google Scholar] [CrossRef]
- Amara, A.B.; Gorvel, L.; Baulan, K.; Buffat, C.; Vérollet, C.; Textoris, J.; Ghigo, E.; Bretelle, F.; Maridonneau-Parini, I.; Mege, J.L. Placental macrophages are impaired in chorioamnionitis, an infectious pathology of the placenta. J. Immunol. 2013, 191, 5501–5514. [Google Scholar] [CrossRef]
- Sisino, G.; Bouckenooghe, T.; Aurientis, S.; Fontaine, P.; Storme, L.; Vambergue, A. Diabetes during pregnancy influences Hofbauer cells, a subtype of placental macrophages, to acquire a pro-inflammatory phenotype. Biochimica et Biophysica Acta-Molecular Basis Disease 2013, 1832, 1959–1968. [Google Scholar] [CrossRef]
- Schliefsteiner, C.; Peinhaupt, M.; Kopp, S.; Lögl, J.; Lang-Olip, I.; Hiden, U.; Heinemann, A.; Desoye, G.; Wadsack, C. Human Placental Hofbauer Cells Maintain an Anti-inflammatory M2 Phenotype despite the Presence of Gestational Diabetes Mellitus. Front. Immunol. 2017, 8, 1–17. [Google Scholar] [CrossRef]
- Tang, Z.; Buhimschi, I.A.; Buhimschi, C.S.; Tadesse, S.; Norwitz, E.; Niven-Fairchild, T.; Huang, S.T.J.; Guller, S. Decreased Levels of Folate Receptor-β and Reduced Numbers of Fetal Macrophages (Hofbauer Cells) in Placentas from Pregnancies with Severe Preeclampsia (PE). Am. J. Reprod. Immunol. 2014, 70, 104–115. [Google Scholar] [CrossRef]
- Buxadé, M.; Huerga Encabo, H.; Riera-Borrull, M.; Quintana-Gallardo, L.; López-Cotarelo, P.; Tellechea, M.; Martínez-Martínez, S.; Redondo, J.M.; Martín-Caballero, J.; Flores, J.M.; et al. Macrophage-specific MHCII expression is regulated by a remote Ciita enhancer controlled by NFAT5. J. Exp. Med. 2018, 215, 2901–2918. [Google Scholar] [CrossRef]
- Shrivastava, R.; Shukla, N. Attributes of alternatively activated (M2) macrophages. Life Sci. 2019, 224, 222–231. [Google Scholar] [CrossRef]
- Jetten, N.; Verbruggen, S.; Gijbels, M.J.; Post, M.J.; De Winther, M.P.J.; Donners, M.M.P.C. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis 2014, 17, 109–118. [Google Scholar] [CrossRef]
- Taylor, P.R.; Martinez-Pomares, L.; Stacey, M.; Lin, H.-H.; Brown, G.D.; Gordon, S. Macrophage receptors and immune recognition. Annu. Rev. Immunol. 2005, 23, 901–944. [Google Scholar] [CrossRef]
- Porcheray, F.; Viaud, S.; Rimaniol, A.C.; Leone, C.; Samah, B.; Dereuddre-Bosquet, N.; Dormont, D.; Gras, G. Macrophage activation switching: An asset for the resolution of inflammation. Clin. Exp. Immunol. 2005, 142, 481–489. [Google Scholar] [CrossRef]
- Vogel, D.Y.; Glim, J.E.; Stavenuiter, A.W.; Breur, M.; Heijnen, P.; Amor, S.; Dijkstra, C.D.; Beelen, R.H. Human macrophage polarization in vitro: Maturation and activation methods compared. Immunobiology 2014, 219, 695–703. [Google Scholar] [CrossRef]
- Eligini, S.; Crisci, M.; Bono, E.; Songia, P.; Tremoli, E.; Colombo, G.I.; Colli, S. Human monocyte-derived macrophages spontaneously differentiated in vitro show distinct phenotypes. J. Cell. Physiol. 2013, 228, 1464–1472. [Google Scholar] [CrossRef]
- Tedesco, S.; Bolego, C.; Toniolo, A.; Nassi, A.; Fadini, G.P.; Locati, M.; Cignarella, A. Phenotypic activation and pharmacological outcomes of spontaneously differentiated human monocyte-derived macrophages. Immunobiology 2015, 220, 545–554. [Google Scholar] [CrossRef]
- Selkov, S.A.; Selutin, A.V.; Pavlova, O.M.; Khromov-Borisov, N.N.; Pavlov, O.V. Comparative phenotypic characterization of human cord blood monocytes and placental macrophages at term. Placenta 2013, 34, 836–839. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Ganesh, K.; Khanna, S.; Sen, C.K.; Roy, S. Engulfment of Apoptotic Cells by Macrophages: A Role of MicroRNA-21 in the Resolution of Wound Inflammation. J. Immunol. 2014, 192, 1120–1129. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.F.; Hong, Y.X.; Feng, G.J.; Zhang, G.F.; Rogers, H.; Lewis, M.A.; Williams, D.W.; Xia, Z.F.; Song, B.; Wei, X.Q. Lipopolysaccharide-Induced M2 to M1 Macrophage Transformation for IL-12p70 Production Is Blocked by Candida albicans Mediated Up-Regulation of EBI3 Expression. PLoS ONE 2013, 8, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Heusinkveld, M.; van Steenwijk, P.J.D.V.; Goedemans, R.; Ramwadhdoebe, T.H.; Gorter, A.; Welters, M.J.; van Hall, T.; van der Burg, S.H. M2 Macrophages Induced by Prostaglandin E2 and IL-6 from Cervical Carcinoma Are Switched to Activated M1 Macrophages by CD4+ Th1 Cells. J. Immunol. 2011, 187, 1157–1165. [Google Scholar] [CrossRef]
- Brown, M.B.; von Chamier, M.; Allam, A.B.; Reyes, L. M1/M2 Macrophage Polarity in Normal and Complicated Pregnancy. Front. Immunol. 2014, 5, 1–10. [Google Scholar]
- Zhang, Y.H.; He, M.; Wang, Y.; Liao, A.H. Modulators of the balance between M1 and M2 macrophages during pregnancy. Front. Immunol. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Young, O.M.; Tang, Z.; Niven-Fairchild, T.; Tadesse, S.; Krikun, G.; Norwitz, E.R.; Mor, G.; Abrahams, V.M.; Guller, S. Toll-like Receptor-Mediated Responses by Placental Hofbauer Cells (HBCs): A Potential Pro-Inflammatory Role for Fetal M2 Macrophages*. Am. J. Clin. Obst. 2015, 73, 22–35. [Google Scholar] [CrossRef]
- Wang, L.X.; Zhang, S.X.; Wu, H.J.; Rong, X.L.; Guo, J. M2b macrophage polarization and its roles in diseases. J. Leukoc. Biol. 2018, 106, 1–14. [Google Scholar] [CrossRef]
- Daigneault, M.; Preston, J.A.; Marriott, H.M.; Whyte, M.K.; Dockrell, D.H. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS ONE 2010, 5, e8668. [Google Scholar] [CrossRef]
- Lund, M.E.; To, J.; O’Brien, B.A.; Donnelly, S. The choice of phorbol 12-myristate 13-acetate differentiation protocol influences the response of THP-1 macrophages to a pro-inflammatory stimulus. J. Immunol. Methods 2016, 430, 64–70. [Google Scholar] [CrossRef]
- Tang, M.X.; Hu, X.H.; Liu, Z.Z.; Kwak-Kim, J.; Liao, A.H. What are the roles of macrophages and monocytes in human pregnancy? J. Reprod. Immunol. 2015, 112, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Xu, X.H.; Jin, L. Macrophage Polarization in Physiological and Pathological Pregnancy. Front. Immunol. 2019, 10, 792. [Google Scholar] [CrossRef] [PubMed]
- Loegl, J.; Hiden, U.; Nussbaumer, E.; Schliefsteiner, C.; Cvitic, S.; Lang, I.; Wadsack, C.; Huppertz, B.; Desoye, G. Hofbauer cells of M2a, M2b and M2c polarization may regulate feto-placental angiogenesis. Reproduction 2016, 152, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Okabe, Y.; Medzhitov, R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell 2014, 157, 832–844. [Google Scholar] [CrossRef] [PubMed]
- Soumelis, V.; Pattarini, L.; Michea, P.; Cappuccio, A. Systems approaches to unravel innate immune cell diversity, environmental plasticity and functional specialization. Curr. Opin. Immunol. 2015, 32, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.W.; Cho, E.H.; Choi, S.Y.; Lee, Y.K.; Park, J.H.; Kim, M.K.; Park, J.Y.; Choi, H.J.; Lee, J.I.; Ko, H.M.; et al. DC-SIGN expression in Hofbauer cells may play an important role in immune tolerance in fetal chorionic villi during the development of preeclampsia. J. Reprod. Immunol. 2017, 124, 30–37. [Google Scholar] [CrossRef]
- Bulmer, J.N.; Williams, P.J.; Lash, G.E. Immune cells in the placental bed. Int. J. Dev. Biol. 2010, 54, 281–294. [Google Scholar] [CrossRef]
- Goldstein, J.; Braverman, M.; Salafia, C.; Buckley, P. The phenotype of human placental macrophages and its variation with gestational age. Am. J. Pathol. 1988, 133, 648–659. [Google Scholar]
- Zaccheo, D.; Pistoia, V.; Castellucci, M.; Martinoli, C. Isolation and characterization of Hofbauer cells from human placental villi. Arch. Gynecol. Obstet. 1989, 246, 189–200. [Google Scholar] [CrossRef]
- Mor, G.; Cardenas, I.; Abrahams, V.; Guller, S. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 2011, 1221, 80–87. [Google Scholar] [CrossRef]
- Houser, B.L.; Tilburgs, T.; Hill, J.; Nicotra, M.L.; Strominger, J.L. Two Unique Human Decidual Macrophage Populations. J. Immunol. 2011, 186, 2633–2642. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, S.; Takayama, T.; Yamada, T.; Usui, R.; Izumi, A.; Watanabe, T.; Ohkuchi, A.; Shibahara, H.; Sato, I.; Suzuki, M. Hofbauer Cell Activation and its increased Glucose-6-Phosphate Dehydrogenase Activity in Second Trimester-spontaneous Abortion: An Ultrastructural Dual Staining Enzyme-cytochemical Study. Am. J. of Reprod. Immunol. 2003, 49, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Niven-Fairchild, T.; Tadesse, S.; Norwitz, E.R.; Buhimschi, C.S.; Buhimschi, I.; Guller, S. Glucocorticoids enhance CD163 expression in placental Hofbauer cells. Endocrinology 2013, 154, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.; Oomomian, Y.; Stephen, G.; Shynlova, O.; Tower, C.L.; Garrod, A.; Lye, S.J.; Jones, R.L. Macrophages Infiltrate the Human and Rat Decidua During Term and Preterm Labor: Evidence That Decidual Inflammation Precedes Labor1. Biol. Reprod. 2012, 86, 39–41. [Google Scholar] [CrossRef]
- Vinnars, M.-T.N.; Rindsjö, E.; Ghazi, S.; Sundberg, A.; Papadogiannakis, N. The Number of CD68+ (Hofbauer) Cells is Decreased in Placentas with Chorioamnionitis and with Advancing Gestational Age. Pediatric Dev. Pathol. 2010, 13, 300–304. [Google Scholar] [CrossRef]
- Tang, Z.; Tadesse, S.; Norwitz, E.; Mor, G.; Abrahams, V.M.; Guller, S. Isolation of Hofbauer Cells from Human Term Placentas with High Yield and Purity. Am. J. Reprod. Immunol. 2011, 66, 336–348. [Google Scholar] [CrossRef]
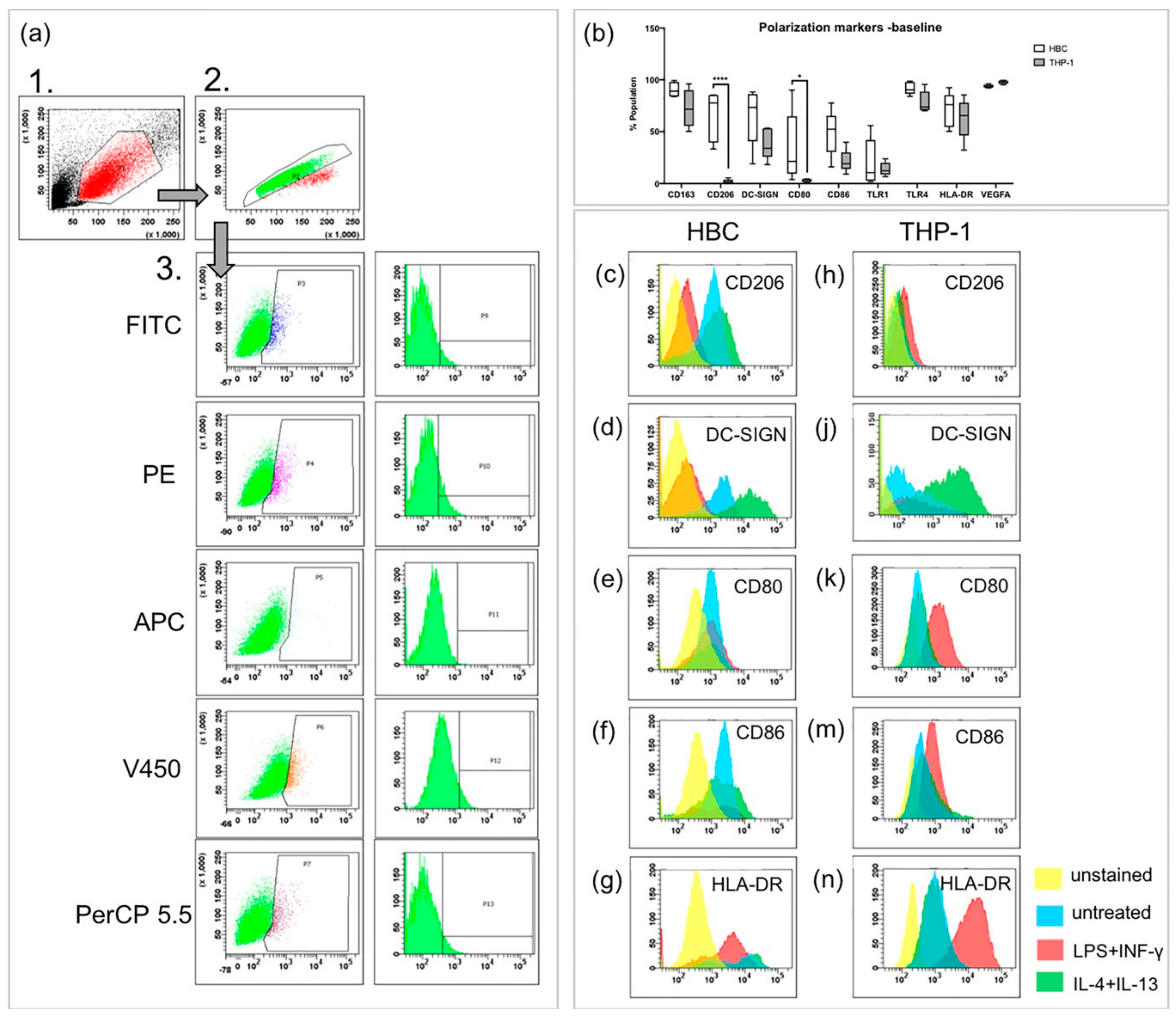
| HBC% pos. Population | THP-1% pos. Population | |||||||
|---|---|---|---|---|---|---|---|---|
| Untreated | LPS + INF-γ | IL-4 + IL-13 | p-Value | Untreated | LPS + INF-γ | IL-4 + IL-13 | p-Value | |
| CD163 | 82.9 ± 13.7 | 67.7 ± 31.5 | 79.0 ± 11.5 | 70.3 ± 24.7 | 91.5 ± 4.9 | 78.0 ± 18.4 | ||
| CD206 | 76.7 ± 18.4 | 27.6 ± 6.6** | 81.0 ± 4.7 | ** 0.01 | 2.7 ± 1.5 | 6.2 ± 0.7* | 2.7 ± 1.7 | *0.02 |
| DC-SIGN | 77.7 ± 11.4 | 26.9 ± 16.4* | 93.1 ± 2.7 | * 0.03 | 41.4 ± 10.5 | 34.7 ± 15.6 | 57.0 ± 32.2* | *0.01 |
| CD80 | 40.4 ± 20.4 | 32.8 ± 14.0 | 42.0 ± 20.2 | 4.9 ± 3.5 | 46.9 ± 18.5* | 3.2 ± 1.9 | *0.03 | |
| CD86 | 67.6 ± 18.1 | 50.1 ± 14.0 | 72.8 ± 11.3 | 24.8 ± 8.1 | 61.2 ± 19.7* | 23.1 ± 9.8 | *0.02 | |
| TLR1 | 31.4 ± 24.4 | 26.6 ± 11.3 | 34.7 ± 19.3 | 14.1 ± 5.7 | 16.1 ± 5.3 | 7.7 ± 3.6* | *0.04 | |
| TLR4 | 96.0 ± 3.6 | 84.6 ± 13.1 | 91.7 ± 4.3 | 74.3 ± 14.1 | 93.2 ± 12.0 | 66.3 ± 16.5 | ||
| HLA-DR | 74.8 ± 16.1 | 62.8 ± 12.8 | 77.5 ± 10.4 | 64.9 ± 13.7 | 95.8 ± 7.2** | 43.8 ± 8.9* | **0.005; *0.025 | |
| VEGF | 94.4 ± 6.5 | 88.8 ± 7.9 | 92.1 ± 7.8 | 96.8 ± 1.8 | 99.3 ± 0.5 | 96.8 ± 2.5 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schliefsteiner, C.; Ibesich, S.; Wadsack, C. Placental Hofbauer Cell Polarization Resists Inflammatory Cues In Vitro. Int. J. Mol. Sci. 2020, 21, 736. https://doi.org/10.3390/ijms21030736
Schliefsteiner C, Ibesich S, Wadsack C. Placental Hofbauer Cell Polarization Resists Inflammatory Cues In Vitro. International Journal of Molecular Sciences. 2020; 21(3):736. https://doi.org/10.3390/ijms21030736
Chicago/Turabian StyleSchliefsteiner, Carolin, Sandra Ibesich, and Christian Wadsack. 2020. "Placental Hofbauer Cell Polarization Resists Inflammatory Cues In Vitro" International Journal of Molecular Sciences 21, no. 3: 736. https://doi.org/10.3390/ijms21030736
APA StyleSchliefsteiner, C., Ibesich, S., & Wadsack, C. (2020). Placental Hofbauer Cell Polarization Resists Inflammatory Cues In Vitro. International Journal of Molecular Sciences, 21(3), 736. https://doi.org/10.3390/ijms21030736