Elucidating the H+ Coupled Zn2+ Transport Mechanism of ZIP4; Implications in Acrodermatitis Enteropathica
Abstract
1. Introduction
2. Results
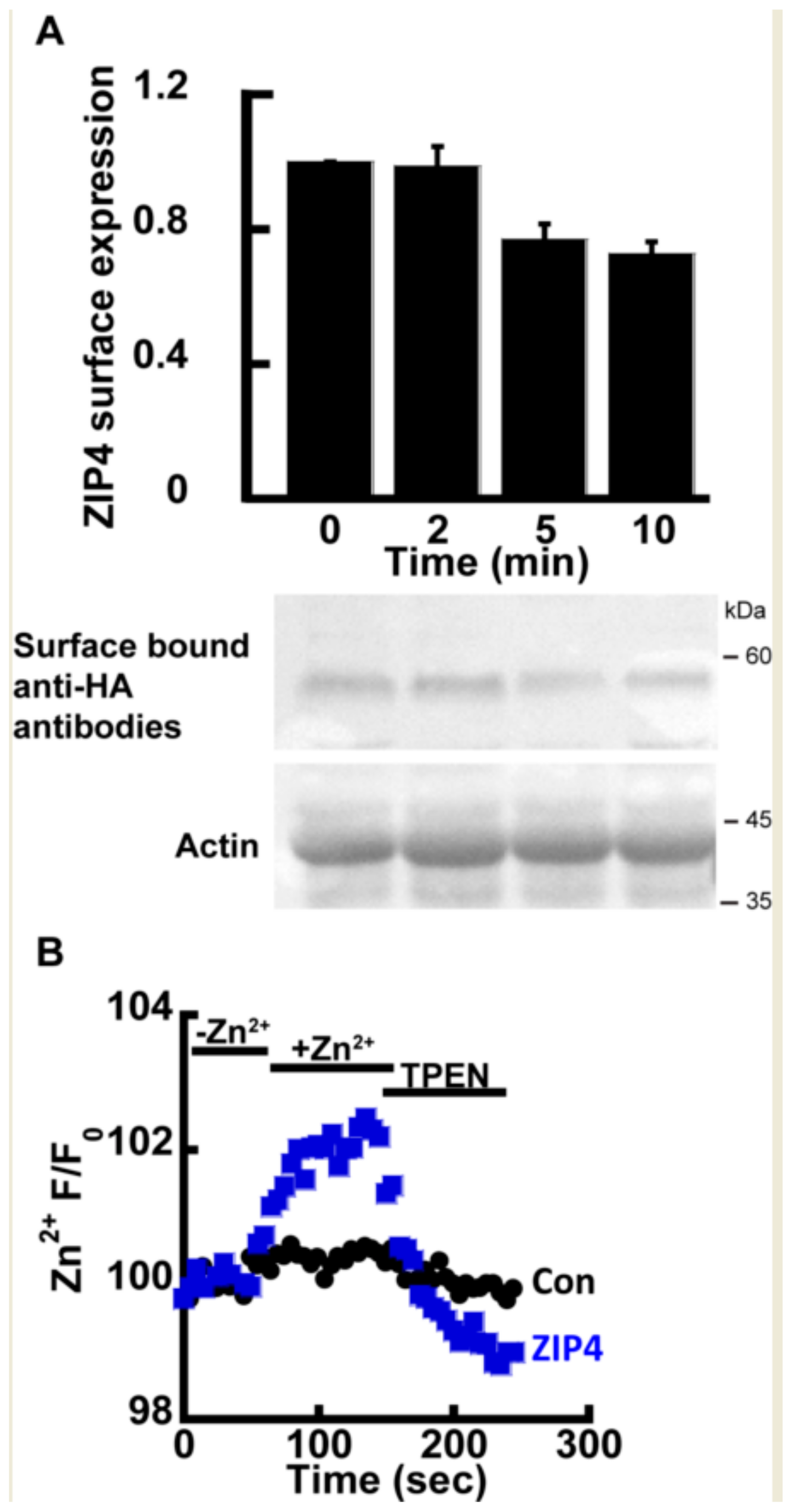
2.1. Zn2+ Transport by ZIP4
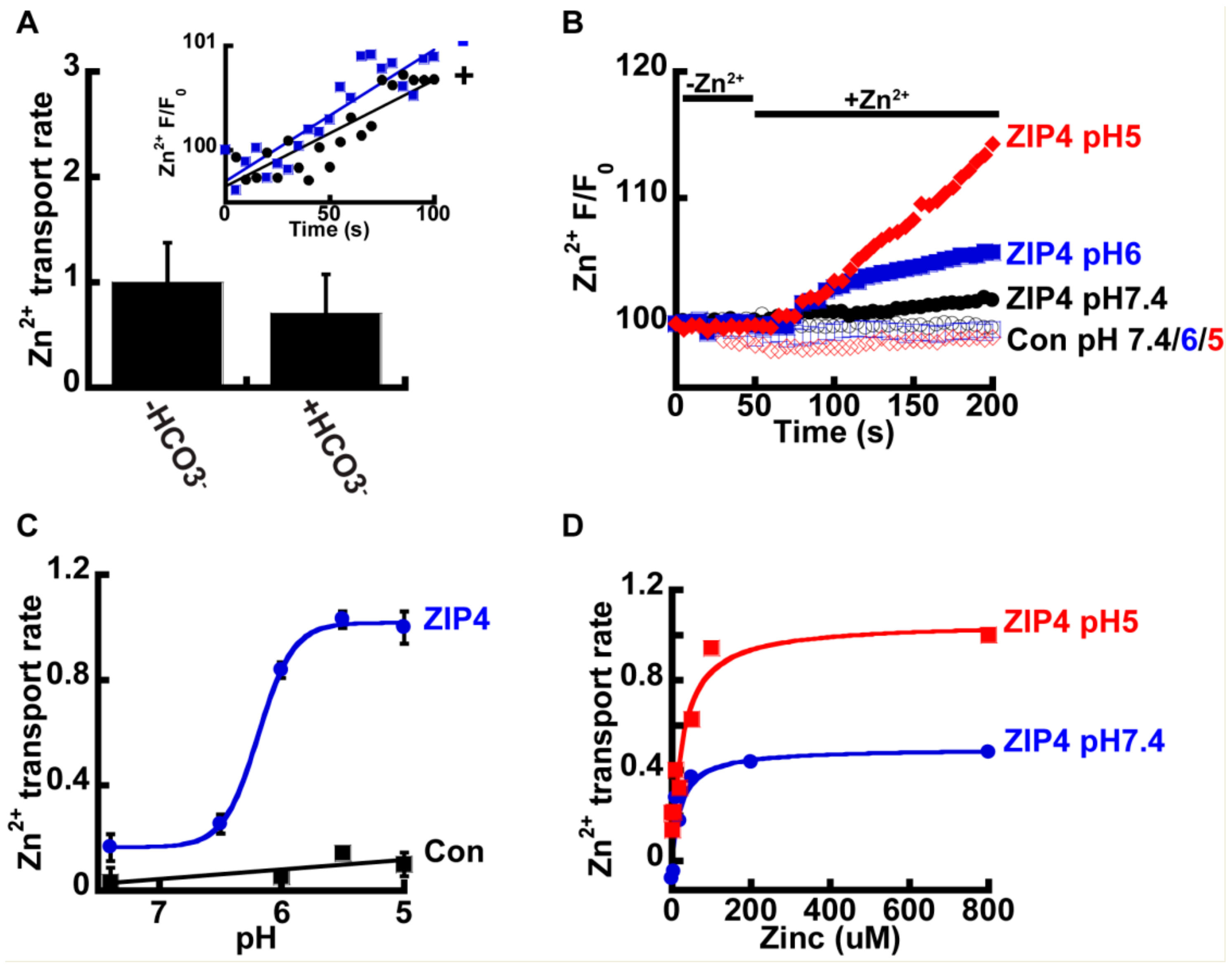
2.2. Zn2+ Uptake by ZIP4 is pH Dependent
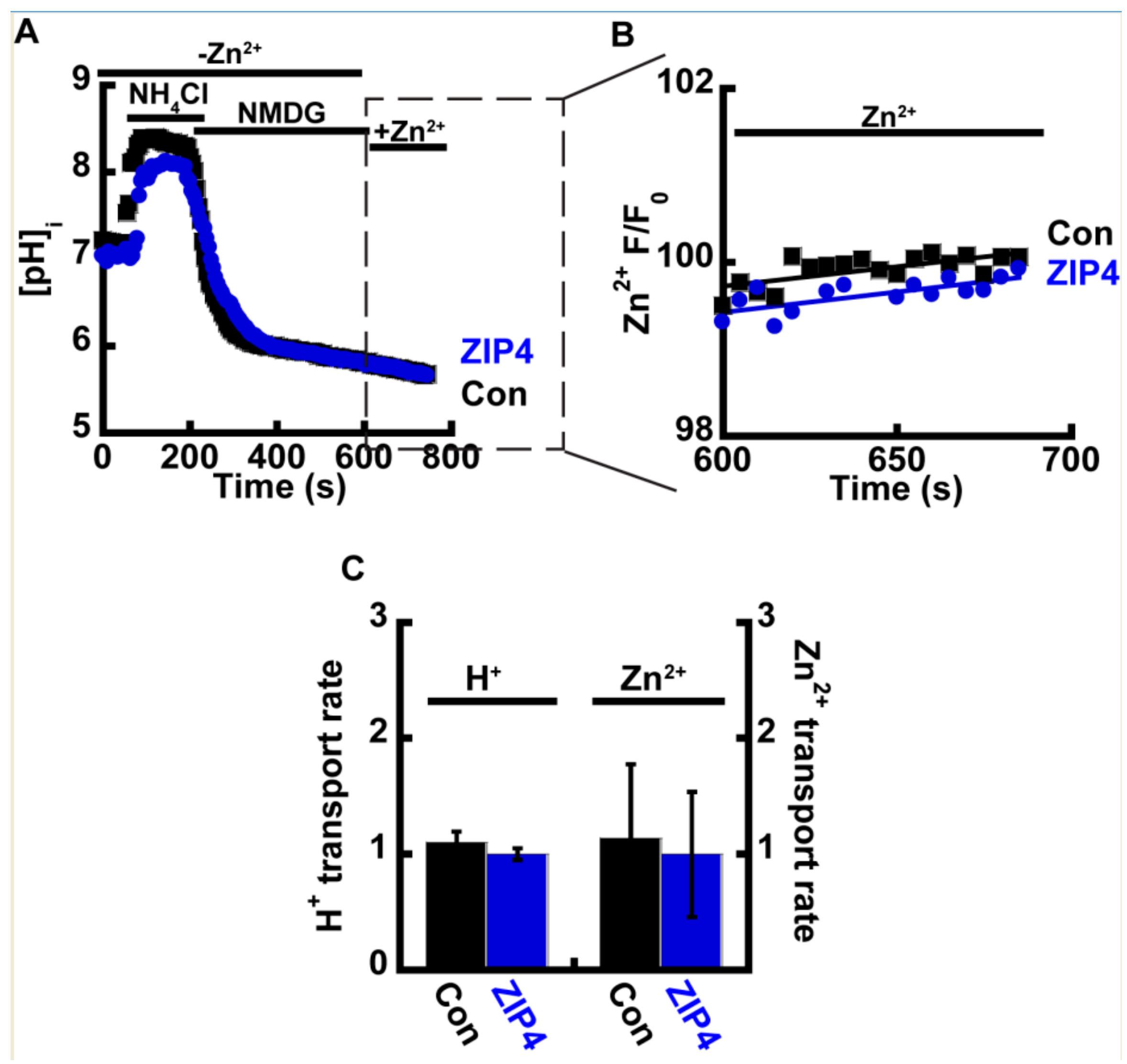
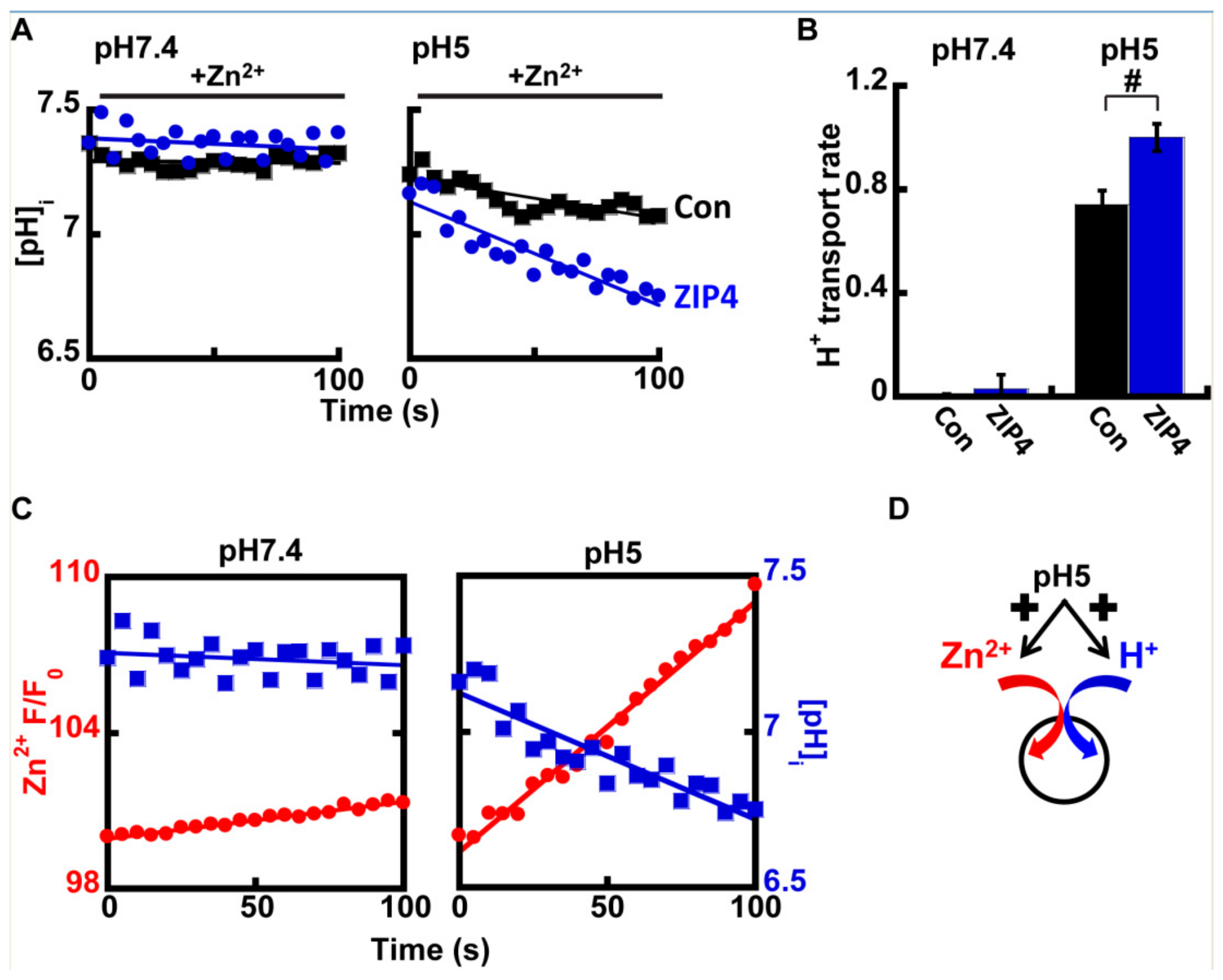
2.3. Zn2+ and H+ Transport by ZIP4 Are Coupled
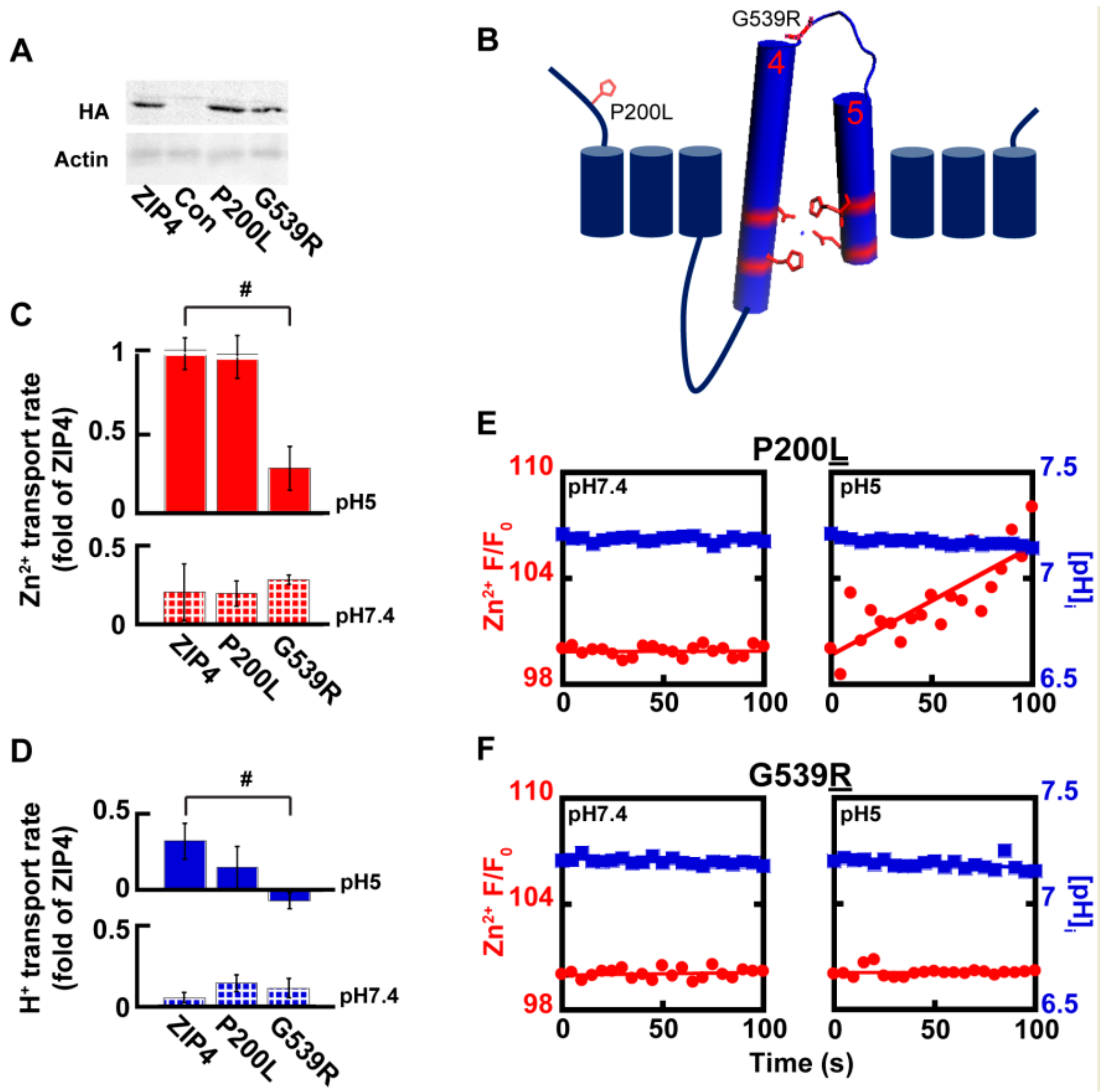
2.4. Acrodermatitis Enteropathica Associated Variants Disrupt Zn2+ Transport by ZIP4
3. Discussion
3.1. ZIP4 Mediates H+/Zn+ Co-Transport
3.2. Genetic Variants Associated with Zn2+ Deficiency in AE Patients Are Linked to Either Catalytic or Non-Catalytic Domains of ZIP4
4. Materials and Methods
4.1. Cell Cultures
4.2. Plasmid Transfection
4.3. Immunoblot Analysis
4.4. Live Cell Fluorescent Imaging
4.5. Statistics
Author Contributions
Funding
Conflicts of Interest
References
- Vallee, B.L.; Falchuk, K.H. The biochemical basis of zinc physiology. Physiol. Rev. 1993, 73, 79–118. [Google Scholar] [CrossRef]
- Prasad, A.S. Zinc deficiency. BMJ 2003, 326, 409–410. [Google Scholar] [CrossRef]
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Counting the Zinc-Proteins Encoded in the Human Genome. J. Proteome Res. 2006, 5, 196–201. [Google Scholar] [CrossRef]
- Eide, D.J. Zinc transporters and the cellular trafficking of zinc. Biochim. Biophys. Acta 2006, 1763, 711–722. [Google Scholar] [CrossRef]
- Chimienti, F.; Devergnas, S.; Favier, A.; Seve, M. Identification and cloning of a beta-cell-specific zinc transporter, ZnT-8, localized into insulin secretory granules. Diabetes 2004, 53, 2330–2337. [Google Scholar] [CrossRef]
- Chimienti, F.; Devergnas, S.; Pattou, F.; Schuit, F.; Garcia-Cuenca, R.; Vandewalle, B.; Kerr-Conte, J.; Lommel, L.V.; Grunwald, D.; Favier, A.; et al. In vivo expression and functional characterization of the zinc transporter ZnT8 in glucose-induced insulin secretion. J. Cell Sci. 2006, 119, 4199–4206. [Google Scholar] [CrossRef]
- Suzuki, T.; Ishihara, K.; Migaki, H.; Ishihara, K.; Nagao, M.; Yamaguchi-Iwai, Y.; Kambe, T. Two different zinc transport complexes of cation diffusion facilitator proteins localized in the secretory pathway operate to activate alkaline phosphatases in vertebrate cells. J. Biol. Chem. 2005, 280, 30956–30962. [Google Scholar] [CrossRef]
- Suzuki, T.; Ishihara, K.; Migaki, H.; Matsuura, W.; Kohda, A.; Okumura, K.; Nagao, M.; Yamaguchi-Iwai, Y.; Kambe, T. Zinc transporters, ZnT5 and ZnT7, are required for the activation of alkaline phosphatases, zinc-requiring enzymes that are glycosylphosphatidylinositol-anchored to the cytoplasmic membrane. J. Biol. Chem. 2005, 280, 637–643. [Google Scholar] [CrossRef]
- Ohana, E.; Hoch, E.; Keasar, C.; Kambe, T.; Yifrach, O.; Hershfinkel, M.; Sekler, I. Identification of the Zn2+ binding site and mode of operation of a mammalian Zn2+ transporter. J. Biol. Chem. 2009, 284, 17677–17686. [Google Scholar] [CrossRef]
- Kawachi, M.; Kobae, Y.; Mimura, T.; Maeshima, M. Deletion of a Histidine-rich Loop of AtMTP1, a Vacuolar Zn2+/H+ Antiporter of Arabidopsis thaliana, Stimulates the Transport Activity. J. Biol. Chem. 2008, 283, 8374–8383. [Google Scholar] [CrossRef]
- Chao, Y.; Fu, D. Kinetic study of the antiport mechanism of an Escherichia coli zinc transporter, ZitB. J. Biol. Chem. 2004, 279, 12043–12050. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Fu, D. Structure of the zinc transporter YiiP. Science 2007, 317, 1746–1748. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Walker, J.M.; Wang, F.; Park, J.G.; Palmer, A.E.; Giunta, C.; Rohrbach, M.; Steinmann, B.; Eide, D.J. Promotion of vesicular zinc efflux by ZIP13 and its implications for spondylocheiro dysplastic Ehlers-Danlos syndrome. Proc. Natl. Acad. Sci. USA 2012, 109, E3530–E3538. [Google Scholar] [CrossRef] [PubMed]
- Giunta, C.; Elçioglu, N.H.; Albrecht, B.; Eich, G.; Chambaz, C.; Janecke, A.R.; Yeowell, H.; Weis, M.; Eyre, D.R.; Kraenzlin, M.; et al. Spondylocheiro dysplastic form of the Ehlers-Danlos syndrome—An autosomal-recessive entity caused by mutations in the zinc transporter gene SLC39A13. Am. J. Hum. Genet. 2008, 82, 1290–1305. [Google Scholar] [CrossRef] [PubMed]
- Fukada, T.; Civic, N.; Furuichi, T.; Shimoda, S.; Mishima, K.; Higashiyama, H.; Idaira, Y.; Asada, Y.; Kitamura, H.; Yamasaki, S.; et al. The zinc transporter SLC39A13/ZIP13 is required for connective tissue development; its involvement in BMP/TGF-beta signaling pathways. PLoS ONE 2008, 3, e3642. [Google Scholar] [CrossRef]
- Dalton, T.P.; He, L.; Wang, B.; Miller, M.L.; Jin, L.; Stringer, K.F.; Chang, X.; Baxter, C.S.; Nebert, D.W. Identification of mouse SLC39A8 as the transporter responsible for cadmium-induced toxicity in the testis. Proc. Natl. Acad. Sci. USA 2005, 102, 3401–3406. [Google Scholar] [CrossRef]
- Gitan, R.S.; Shababi, M.; Kramer, M.; Eide, D.J. A cytosolic domain of the yeast Zrt1 zinc transporter is required for its post-translational inactivation in response to zinc and cadmium. J. Biol. Chem. 2003, 278, 39558–39564. [Google Scholar] [CrossRef]
- Taylor, K.M.; Nicholson, R.I. The LZT proteins; the LIV-1 subfamily of zinc transporters. Biochim. Biophys. Acta 2003, 1611, 16–30. [Google Scholar] [CrossRef]
- Antala, S.; Ovchinnikov, S.; Kamisetty, H.; Baker, D.; Dempski, R.E. Computation and Functional Studies Provide a Model for the Structure of the Zinc Transporter hZIP4. J. Biol. Chem. 2015, 290, 17796–17805. [Google Scholar] [CrossRef]
- Rogers, E.E.; Eide, D.J.; Guerinot, M.L. Altered selectivity in an Arabidopsis metal transporter. Proc. Natl. Acad. Sci. USA 2000, 97, 12356–12360. [Google Scholar] [CrossRef]
- Hoch, E.; Lin, W.; Chai, J.; Hershfinkel, M.; Fu, D.; Sekler, I. Histidine pairing at the metal transport site of mammalian ZnT transporters controls Zn2+ over Cd2+ selectivity. Proc. Natl. Acad. Sci. USA 2012, 109, 7202–7207. [Google Scholar] [CrossRef] [PubMed]
- Dufner-Beattie, J.; Wang, F.; Kuo, Y.-M.; Gitschier, J.; Eide, D.; Andrews, G.K. The acrodermatitis enteropathica gene ZIP4 encodes a tissue-specific, zinc-regulated zinc transporter in mice. J. Biol. Chem. 2003, 278, 33474–33481. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-E.; Wang, F.; Dufner-Beattie, J.; Andrews, G.K.; Eide, D.J.; Petris, M.J. Zn2+-stimulated endocytosis of the mZIP4 zinc transporter regulates its location at the plasma membrane. J. Biol. Chem. 2004, 279, 4523–4530. [Google Scholar] [CrossRef] [PubMed]
- Weaver, B.P.; Dufner-Beattie, J.; Kambe, T.; Andrews, G.K. Novel zinc-responsive post-transcriptional mechanisms reciprocally regulate expression of the mouse Slc39a4 and Slc39a5 zinc transporters (Zip4 and Zip5). Biol. Chem. 2007, 388, 1301–1312. [Google Scholar] [CrossRef]
- Dufner-Beattie, J.; Kuo, Y.-M.; Gitschier, J.; Andrews, G.K. The adaptive response to dietary zinc in mice involves the differential cellular localization and zinc regulation of the zinc transporters ZIP4 and ZIP5. J. Biol. Chem. 2004, 279, 49082–49090. [Google Scholar] [CrossRef]
- Küry, S.; Dréno, B.; Bézieau, S.; Giraudet, S.; Kharfi, M.; Kamoun, R.; Moisan, J.-P. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat. Genet. 2002, 31, 239–240. [Google Scholar] [CrossRef]
- Wang, K.; Zhou, B.; Kuo, Y.-M.; Zemansky, J.; Gitschier, J. A novel member of a zinc transporter family is defective in acrodermatitis enteropathica. Am. J. Hum. Genet. 2002, 71, 66–73. [Google Scholar] [CrossRef]
- Nakano, A.; Nakano, H.; Nomura, K.; Toyomaki, Y.; Hanada, K. Novel SLC39A4 mutations in acrodermatitis enteropathica. J. Invest. Dermatol. 2003, 120, 963–966. [Google Scholar] [CrossRef]
- Wang, F.; Kim, B.-E.; Dufner-Beattie, J.; Petris, M.J.; Andrews, G.; Eide, D.J. Acrodermatitis enteropathica mutations affect transport activity, localization and zinc-responsive trafficking of the mouse ZIP4 zinc transporter. Hum. Mol. Genet. 2004, 13, 563–571. [Google Scholar] [CrossRef]
- Prasad, A.S. The role of zinc in gastrointestinal and liver disease. Clin. Gastroenterol. 1983, 12, 713–741. [Google Scholar]
- Zhang, T.; Liu, J.; Fellner, M.; Zhang, C.; Sui, D.; Hu, J. Crystal structures of a ZIP zinc transporter reveal a binuclear metal center in the transport pathway. Sci. Adv. 2017, 3, e1700344. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, Y.; Liu, Z.; Bharadwaj, U.; Wang, H.; Wang, X.; Zhang, S.; Liuzzi, J.P.; Chang, S.-M.; Cousins, R.J.; et al. Aberrant expression of zinc transporter ZIP4 (SLC39A4) significantly contributes to human pancreatic cancer pathogenesis and progression. Proc. Natl. Acad. Sci. USA 2007, 104, 18636–18641. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, Y.; Bharadwaj, U.; Zhai, Q.J.; Ahern, C.H.; Fisher, W.E.; Brunicardi, F.C.; Logsdon, C.D.; Chen, C.; Yao, Q. Down-regulation of ZIP4 by RNA interference inhibits pancreatic cancer growth and increases the survival of nude mice with pancreatic cancer xenografts. Clin. Cancer Res. 2009, 15, 5993–6001. [Google Scholar] [CrossRef] [PubMed]
- Gaither, L.A.; Eide, D.J. Functional expression of the human hZIP2 zinc transporter. J. Biol. Chem. 2000, 275, 5560–5564. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Girijashanker, K.; Dalton, T.P.; Reed, J.; Li, H.; Soleimani, M.; Nebert, D.W. ZIP8, member of the solute-carrier-39 (SLC39) metal-transporter family: Characterization of transporter properties. Mol. Pharmacol. 2006, 70, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Girijashanker, K.; He, L.; Soleimani, M.; Reed, J.M.; Li, H.; Liu, Z.; Wang, B.; Dalton, T.P.; Nebert, D.W. Slc39a14 gene encodes ZIP14, a metal/bicarbonate symporter: Similarities to the ZIP8 transporter. Mol. Pharmacol. 2008, 73, 1413–1423. [Google Scholar] [CrossRef]
- Qiu, A.; Hogstrand, C. Functional expression of a low-affinity zinc uptake transporter (FrZIP2) from pufferfish (Takifugu rubripes) in MDCK cells. Biochem. J. 2005, 390, 777–786. [Google Scholar] [CrossRef]
- Antala, S.; Dempski, R.E. The human ZIP4 transporter has two distinct binding affinities and mediates transport of multiple transition metals. Biochemistry 2012, 51, 963–973. [Google Scholar] [CrossRef]
- Lin, W.; Chai, J.; Love, J.; Fu, D. Selective electrodiffusion of zinc ions in a Zrt-, Irt-like protein, ZIPB. J. Biol. Chem. 2010, 285, 39013–39020. [Google Scholar] [CrossRef]
- Kiedrowski, L. Cytosolic acidification and intracellular zinc release in hippocampal neurons. J. Neurochem. 2012, 121, 438–450. [Google Scholar] [CrossRef]
- Sensi, S.L.; Ton-That, D.; Sullivan, P.G.; Jonas, E.A.; Gee, K.R.; Kaczmarek, L.K.; Weiss, J.H. Modulation of mitochondrial function by endogenous Zn2+ pools. Proc. Natl. Acad. Sci. USA 2003, 100, 6157–6162. [Google Scholar] [CrossRef] [PubMed]
- Azriel-Tamir, H.; Sharir, H.; Schwartz, B.; Hershfinkel, M. Extracellular Zinc Triggers ERK-dependent Activation of Na+/H+ Exchange in Colonocytes Mediated by the Zinc-sensing Receptor. J. Biol. Chem. 2004, 279, 51804–51816. [Google Scholar] [CrossRef] [PubMed]
- Outten, C.E.; O’Halloran, T.V. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 2001, 292, 2488–2492. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Dufner-Beattie, J.; Kim, B.-E.; Petris, M.J.; Andrews, G.; Eide, D.J. Zinc-stimulated endocytosis controls activity of the mouse ZIP1 and ZIP3 zinc uptake transporters. J. Biol. Chem. 2004, 279, 24631–24639. [Google Scholar] [CrossRef]
- Thwaites, D.T.; Anderson, C.M.H. H+-coupled nutrient, micronutrient and drug transporters in the mammalian small intestine. Exp. Physiol. 2007, 92, 603–619. [Google Scholar]
- Hummel, C.S.; Lu, C.; Loo, D.D.F.; Hirayama, B.A.; Voss, A.A.; Wright, E.M. Glucose transport by human renal Na+/D-glucose cotransporters SGLT1 and SGLT2. Am. J. Physiol. Cell Physiol. 2011, 300, C14–C21. [Google Scholar] [CrossRef]
- Wright, E.M. Renal Na(+)-glucose cotransporters. Am. J. Physiol. Renal Physiol. 2001, 280, F10–F18. [Google Scholar] [CrossRef]
- Wright, E.M.; Hirsch, J.R.; Loo, D.D.; Zampighi, G.A. Regulation of Na+/glucose cotransporters. J. Exp. Biol. 1997, 200, 287–293. [Google Scholar]
- Choi, D.W.; Koh, J.Y. Zinc and brain injury. Annu. Rev. Neurosci. 1998, 21, 347–375. [Google Scholar] [CrossRef]
- Weiss, J.H.; Sensi, S.L.; Koh, J.Y. Zn(2+): A novel ionic mediator of neural injury in brain disease. Trends Pharmacol. Sci. 2000, 21, 395–401. [Google Scholar] [CrossRef]
- Kambe, T. Molecular architecture and function of ZnT transporters. Curr. Top. Membr. 2012, 69, 199–220. [Google Scholar] [PubMed]
- Qin, Y.; Thomas, D.; Fontaine, C.P.; Colvin, R.A. Silencing of ZnT1 reduces Zn2+ efflux in cultured cortical neurons. Neurosci. Lett. 2009, 450, 206–210. [Google Scholar] [CrossRef]
- Kambe, T.; Andrews, G.K. Novel proteolytic processing of the ectodomain of the zinc transporter ZIP4 (SLC39A4) during zinc deficiency is inhibited by acrodermatitis enteropathica mutations. Mol. Cell. Biol. 2009, 29, 129–139. [Google Scholar] [CrossRef]
- Markwell, M.A.; Haas, S.M.; Bieber, L.L.; Tolbert, N.E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 1978, 87, 206–210. [Google Scholar] [CrossRef]
- Ventura-Bixenshpaner, H.; Asraf, H.; Chakraborty, M.; Elkabets, M.; Sekler, I.; Taylor, K.M.; Hershfinkel, M. Enhanced ZnR/GPR39 Activity in Breast Cancer, an Alternative Trigger of Signaling Leading to Cell Growth. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Machen, T.E.; Leigh, M.J.; Taylor, C.; Kimura, T.; Asano, S.; Moore, H.-P.H. pH of TGN and recycling endosomes of H+/K+-ATPase-transfected HEK-293 cells: Implications for pH regulation in the secretory pathway. Am. J. Physiol. Cell Physiol. 2003, 285, C205–C214. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Component | Amount |
|---|---|
| dsDNA template (100 ng) | 0.333 µL |
| Primer F (0.3 µM) | 0.75 µL |
| Primer R (0.3 µM) | 0.75 µL |
| dNTPs | 1.5 µL |
| Enzyme | 0.5 µL |
| ddH20 (a total of 25 µL) | 21.167 µL |
| Total volume | 25 µL |
| Cycles | Step | Temperature | Time (min:s) |
|---|---|---|---|
| 1 | Initial denaturation | 95 °C | 5:00 |
| 20 | Denaturation | 98 °C | 0:20 |
| Annealing | 78 °C | 0:20 | |
| Elongation | 72 °C | 0:30/1 kb template | |
| 1 | Final elongation | 78 °C | 5:00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoch, E.; Levy, M.; Hershfinkel, M.; Sekler, I. Elucidating the H+ Coupled Zn2+ Transport Mechanism of ZIP4; Implications in Acrodermatitis Enteropathica. Int. J. Mol. Sci. 2020, 21, 734. https://doi.org/10.3390/ijms21030734
Hoch E, Levy M, Hershfinkel M, Sekler I. Elucidating the H+ Coupled Zn2+ Transport Mechanism of ZIP4; Implications in Acrodermatitis Enteropathica. International Journal of Molecular Sciences. 2020; 21(3):734. https://doi.org/10.3390/ijms21030734
Chicago/Turabian StyleHoch, Eitan, Moshe Levy, Michal Hershfinkel, and Israel Sekler. 2020. "Elucidating the H+ Coupled Zn2+ Transport Mechanism of ZIP4; Implications in Acrodermatitis Enteropathica" International Journal of Molecular Sciences 21, no. 3: 734. https://doi.org/10.3390/ijms21030734
APA StyleHoch, E., Levy, M., Hershfinkel, M., & Sekler, I. (2020). Elucidating the H+ Coupled Zn2+ Transport Mechanism of ZIP4; Implications in Acrodermatitis Enteropathica. International Journal of Molecular Sciences, 21(3), 734. https://doi.org/10.3390/ijms21030734





