Importance of Melatonin in Assisted Reproductive Technology and Ovarian Aging
Abstract
1. Introduction
2. Antioxidative Effects of Melatonin
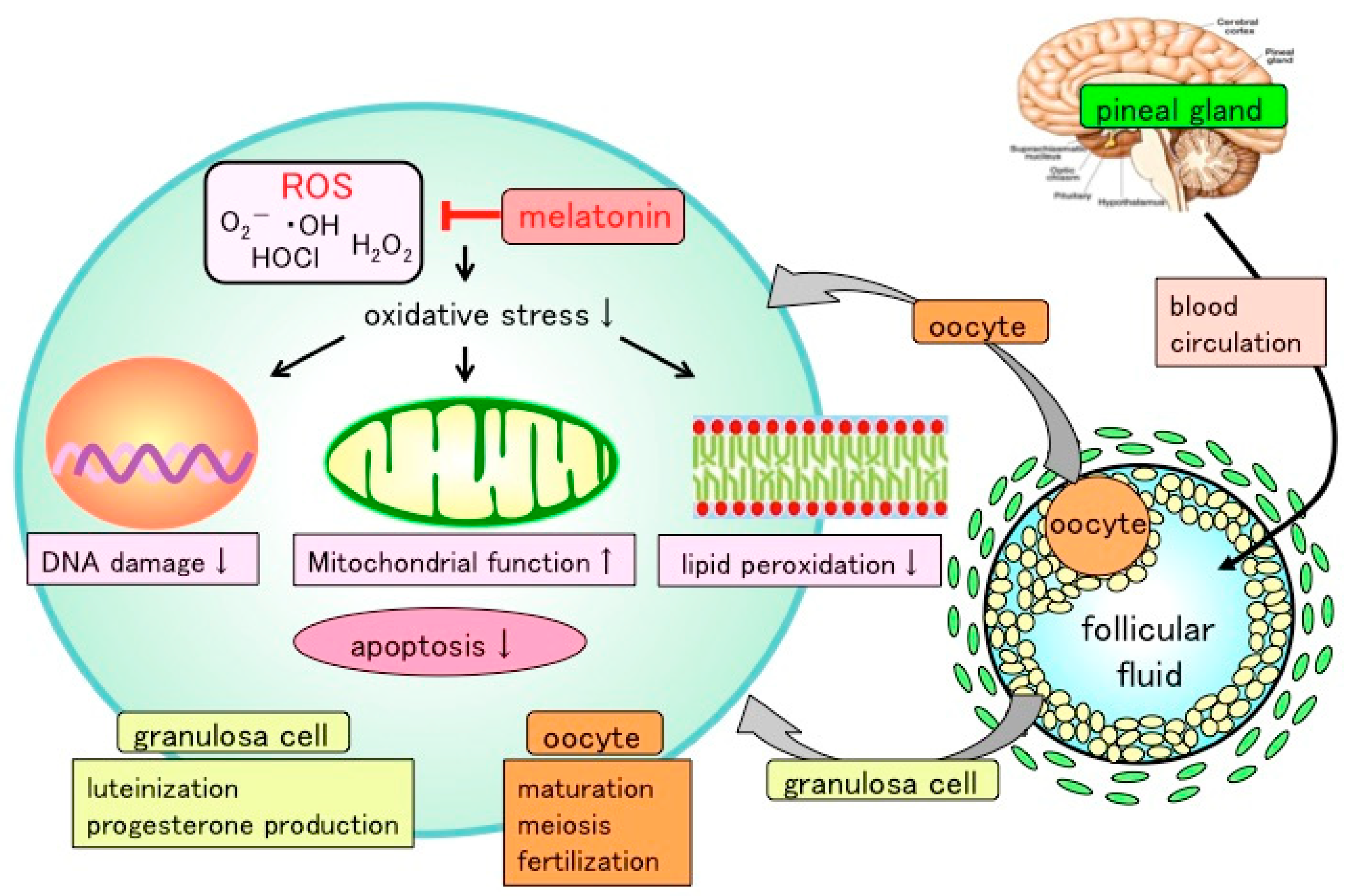
3. Reactive Oxygen and Reproductive Function
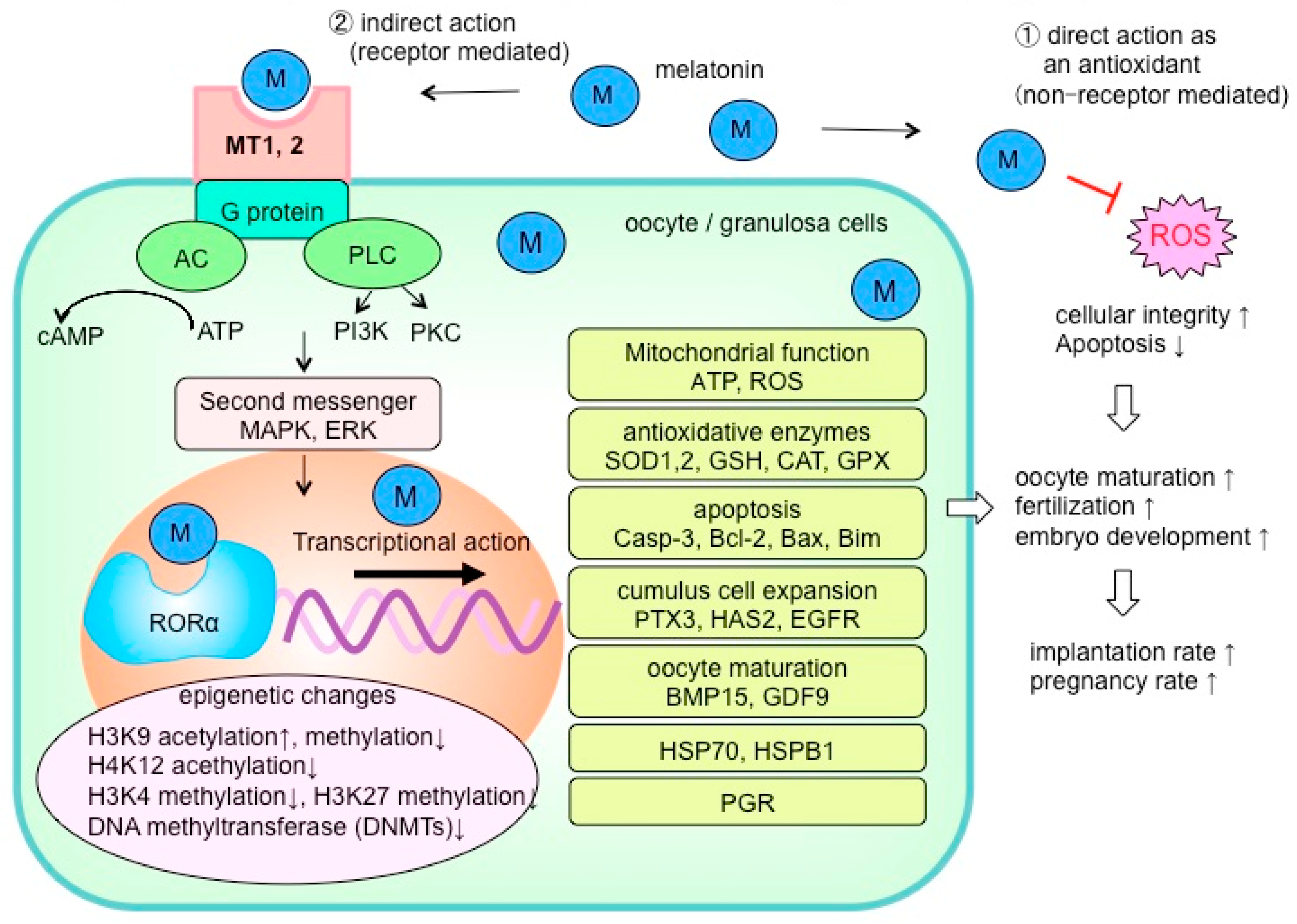
4. Melatonin in the Ovaries
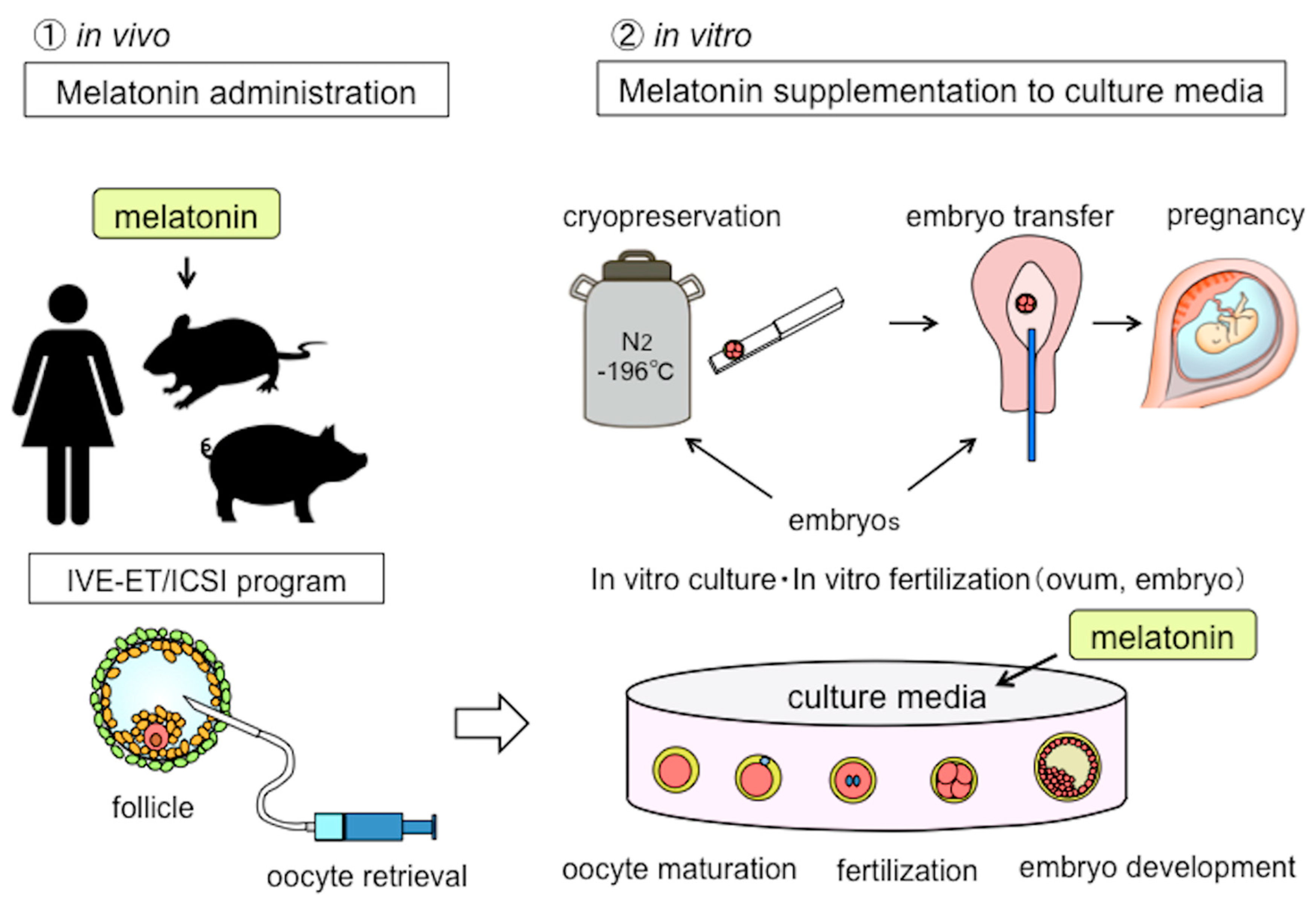
5. The Clinical Application of Melatonin in the Field of Reproductive Medicine
5.1. Melatonin in Assisted Reproductive Technology (ART)
5.2. Oocyte Maturation, Embryo Development, and Melatonin
6. Reduced Fertility Associated with Ovarian Aging
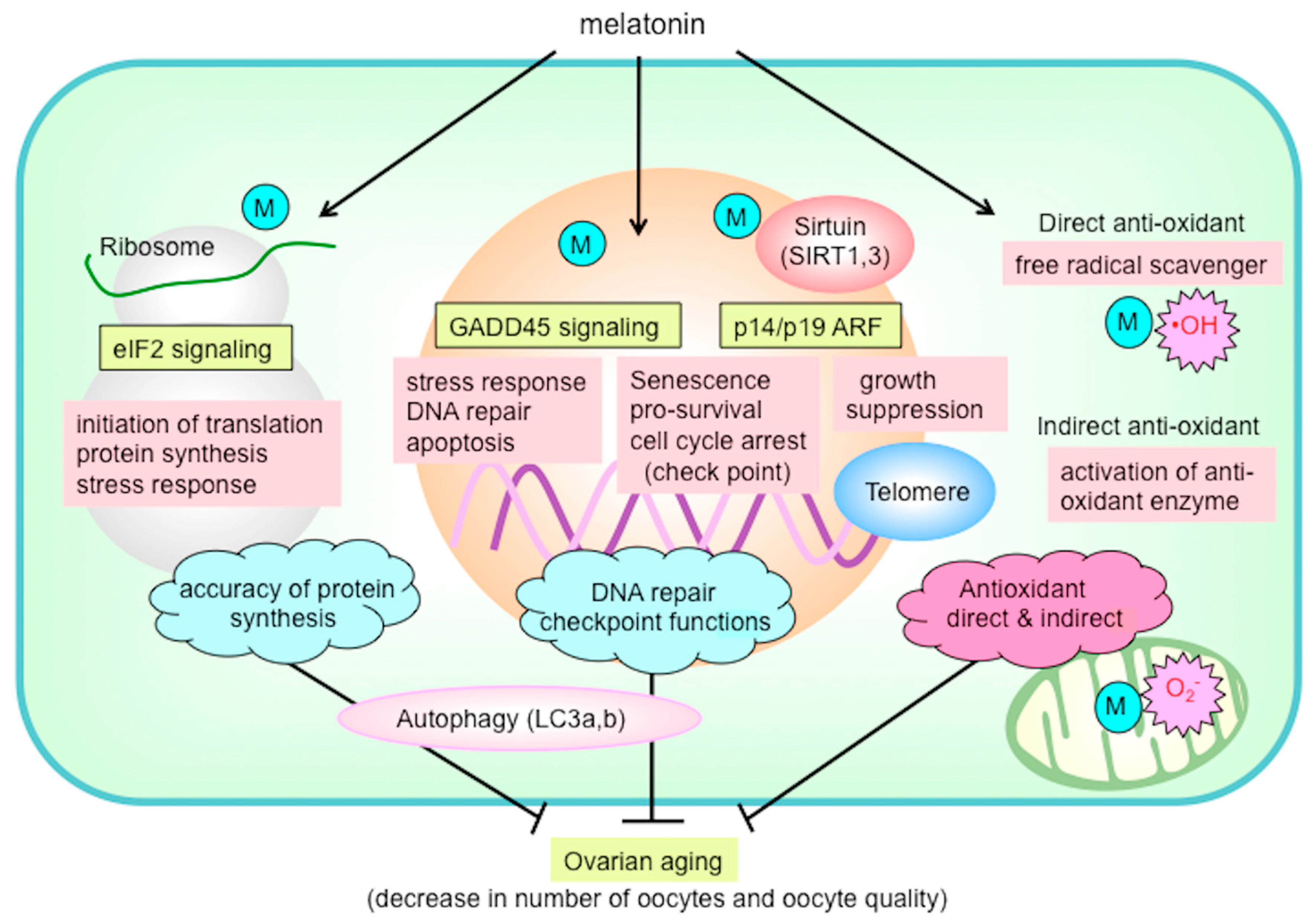
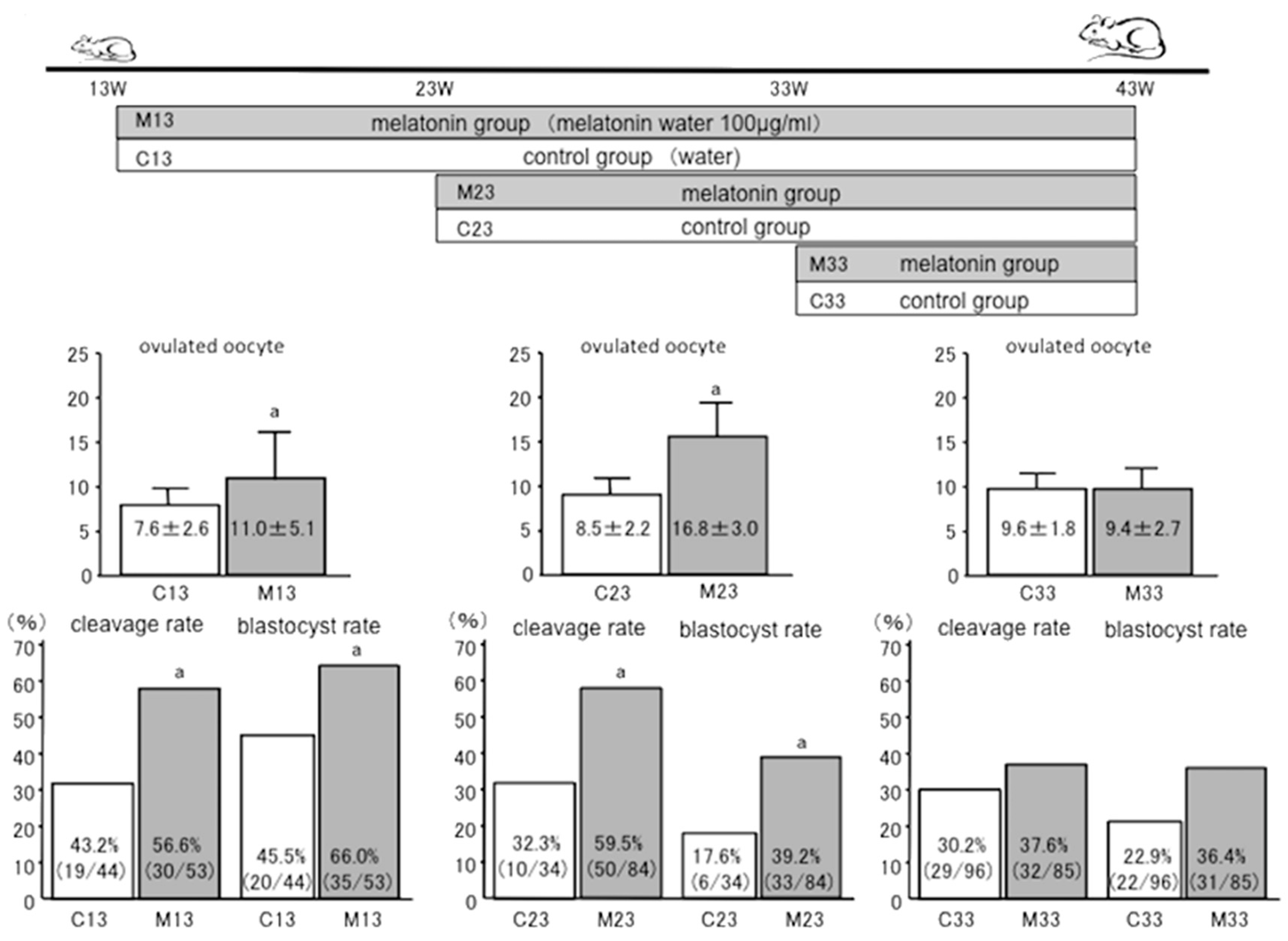
7. Anti-Aging Effects of Melatonin
Author Contributions
Funding
Ethics Approval and Consent to Participate
Conflicts of Interest
References
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin synthesis and function: Evolutionary history in animals and plants. Front. Endocrinol. 2019, 10, 249. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Galano, A. Melatonin: Exceeding expectations. Physiology (Bethesda) 2014, 29, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Acuna-Castroviejo, D.; Escames, G.; Venegas, C.; Diaz-Casado, M.E.; Lima-Cabello, E.; Lopez, L.C.; Rosales-Corral, S.; Tan, D.X.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef] [PubMed]
- Majidinia, M.; Reiter, R.J.; Shakouri, S.K.; Yousefi, B. The role of melatonin, a multitasking molecule, in retarding the processes of ageing. Ageing Res. Rev. 2018, 47, 198–213. [Google Scholar] [CrossRef]
- Otsuka, F. Modulation of bone morphogenetic protein activity by melatonin in ovarian steroidogenesis. Reprod. Med. Biol. 2018, 17, 228–233. [Google Scholar] [CrossRef]
- Tamura, H.; Nakamura, Y.; Narimatsu, A.; Yamagata, Y.; Takasaki, A.; Reiter, R.J.; Sugino, N. Melatonin treatment in peri- and postmenopausal women elevates serum high-density lipoprotein cholesterol levels without influencing total cholesterol levels. J. Pineal Res. 2008, 45, 101–105. [Google Scholar] [CrossRef]
- Tamura, H.; Nakamura, Y.; Takiguchi, S.; Kashida, S.; Yamagata, Y.; Sugino, N.; Kato, H. Melatonin directly suppresses steroid production by preovulatory follicles in the cyclic hamster. J. Pineal Res. 1998, 25, 135–141. [Google Scholar] [CrossRef]
- Vriend, J.; Reiter, R.J. Melatonin, bone regulation and the ubiquitin-proteasome connection: A review. Life Sci. 2016, 145, 152–160. [Google Scholar] [CrossRef]
- Zhao, C.N.; Wang, P.; Mao, Y.M.; Dan, Y.L.; Wu, Q.; Li, X.M.; Wang, D.G.; Davis, C.; Hu, W.; Pan, H.F. Potential role of melatonin in autoimmune diseases. Cytokine Growth Factor Rev. 2019, 48, 1–10. [Google Scholar] [CrossRef]
- Favero, G.; Moretti, E.; Bonomini, F.; Reiter, R.J.; Rodella, L.F.; Rezzani, R. Promising antineoplastic actions of melatonin. Front. Pharmacol. 2018, 9, 1086. [Google Scholar] [CrossRef]
- Reiter, R.J.; Rosales-Corral, S.A.; Tan, D.X.; Acuna-Castroviejo, D.; Qin, L.; Yang, S.F.; Xu, K. Melatonin, a full service anti-cancer agent: Inhibition of initiation, progression and metastasis. Int. J. Mol. Sci. 2017, 18, 834. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Barcelo, E.J.; Rueda, N.; Mediavilla, M.D.; Martinez-Cue, C.; Reiter, R.J. Clinical uses of melatonin in neurological diseases and mental and behavioural disorders. Curr. Med. Chem. 2017, 24, 3851–3878. [Google Scholar] [CrossRef] [PubMed]
- Cipolla-Neto, J.; Amaral, F.G.; Afeche, S.C.; Tan, D.X.; Reiter, R.J. Melatonin, energy metabolism, and obesity: A review. J. Pineal Res. 2014, 56, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Favero, G.; Franceschetti, L.; Bonomini, F.; Rodella, L.F.; Rezzani, R. Melatonin as an anti-inflammatory agent modulating inflammasome activation. Int. J. Endocrinol. 2017, 1835195. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, J.; Reiter, R.J.; Ma, X. Melatonin mediates mucosal immune cells, microbial metabolism, and rhythm crosstalk: A therapeutic target to reduce intestinal inflammation. Med. Res. Rev. 2019. [Google Scholar] [CrossRef]
- Moradkhani, F.; Moloudizargari, M.; Fallah, M.; Asghari, N.; Heidari Khoei, H.; Asghari, M.H. Immunoregulatory role of melatonin in cancer. J. Cell. Physiol. 2020, 235, 745–757. [Google Scholar] [CrossRef]
- Alghamdi, B.S. The neuroprotective role of melatonin in neurological disorders. J. Neurosci. Res. 2018, 96, 1136–1149. [Google Scholar] [CrossRef]
- Cardinali, D.P. Melatonin: Clinical perspectives in neurodegeneration. Front. Endocrinol. 2019, 10, 480. [Google Scholar] [CrossRef]
- Karamitri, A.; Jockers, R. Melatonin in type 2 diabetes mellitus and obesity. Nat. Rev. Endocrinol. 2019, 15, 105–125. [Google Scholar] [CrossRef]
- Wu, H.; Liu, J.; Yin, Y.; Zhang, D.; Xia, P.; Zhu, G. Therapeutic opportunities in colorectal cancer: Focus on melatonin antioncogenic action. BioMed Res. Int. 2019, 2019, 9740568. [Google Scholar] [CrossRef]
- Alston, M.; Cain, S.W.; Rajaratnam, S.M.W. Advances of melatonin-based therapies in the treatment of disturbed sleep and mood. Handb. Exp. Pharmacol. 2019, 253, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Galano, A.; Zhou, X.J.; Xu, B. Mitochondria: Central organelles for melatonin’s antioxidant and anti-aging actions. Molecules 2018, 23, 509. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Reiter, R.J.; Poeggeler, B.; Tan, D.X. The significance of the metabolism of the neurohormone melatonin: Antioxidative protection and formation of bioactive substances. Neurosci. Biobehav. Rev. 1993, 17, 347–357. [Google Scholar] [CrossRef]
- Poeggeler, B.; Reiter, R.J.; Tan, D.X.; Chen, L.D.; Manchester, L.C. Melatonin, hydroxyl radical-mediated oxidative damage, and aging: A hypothesis. J. Pineal Res. 1993, 14, 151–168. [Google Scholar] [CrossRef]
- Reiter, R.J. Functional pleiotropy of the neurohormone melatonin: Antioxidant protection and neuroendocrine regulation. Front. Neuroendocrinol. 1995, 16, 383–415. [Google Scholar] [CrossRef]
- Acuna-Castroviejo, D.N.-N.M.; Reiter, R.J.; Escames, G. Melatonin actions in the heart; more than a hormone. Melatonin Res. 2018, 1, 21–26. [Google Scholar] [CrossRef]
- Leon, J.; Acuna-Castroviejo, D.; Escames, G.; Tan, D.X.; Reiter, R.J. Melatonin mitigates mitochondrial malfunction. J. Pineal Res. 2005, 38, 1–9. [Google Scholar] [CrossRef]
- Paradies, G.; Petrosillo, G.; Paradies, V.; Reiter, R.J.; Ruggiero, F.M. Melatonin, cardiolipin and mitochondrial bioenergetics in health and disease. J. Pineal Res. 2010, 48, 297–310. [Google Scholar] [CrossRef]
- Reiter, R.J.; Paredes, S.D.; Manchester, L.C.; Tan, D.X. Reducing oxidative/nitrosative stress: A newly-discovered genre for melatonin. Crit. Rev. Biochem. Mol. Biol. 2009, 44, 175–200. [Google Scholar] [CrossRef]
- Venegas, C.; Garcia, J.A.; Escames, G.; Ortiz, F.; Lopez, A.; Doerrier, C.; Garcia-Corzo, L.; Lopez, L.C.; Reiter, R.J.; Acuna-Castroviejo, D. Extrapineal melatonin: Analysis of its subcellular distribution and daily fluctuations. J. Pineal Res. 2012, 52, 217–227. [Google Scholar] [CrossRef]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolin, I.; Herrera, F.; Martin, V.; Reiter, R.J. Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 2004, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.R.R. Mitochondria: The birth place, battle ground and the site of melatonin metabolism in cells. Melatonin Res. 2019, 2, 44–46. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Manchester, L.C.; Lopez-Burillo, S.; Sainz, R.M.; Mayo, J.C. Melatonin: Detoxification of oxygen and nitrogen-based toxic reactants. Adv. Exp. Med. Biol. 2003, 527, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Ghosal, I.; Das, D. and Chakraborty, S.B. Melatonin ameliorates H2O2-induced oxidative stress through modulation of Erk/Akt/NFkB pathway. Biol. Res. 2018, 51, 17. [Google Scholar] [CrossRef]
- Kala, M.; Shaikh, M.V.; Nivsarkar, M. Equilibrium between anti-oxidants and reactive oxygen species: A requisite for oocyte development and maturation. Reprod. Med. Biol. 2017, 16, 28–35. [Google Scholar] [CrossRef]
- He, C.; Wang, J.; Zhang, Z.; Yang, M.; Li, Y.; Tian, X.; Ma, T.; Tao, J.; Zhu, K.; Song, Y.; et al. Mitochondria synthesize melatonin to ameliorate its function and improve mice oocyte’s quality under in vitro conditions. Int. J. Mol. Sci. 2016, 17, 939. [Google Scholar] [CrossRef]
- Tamura, H.; Takasaki, A.; Taketani, T.; Tanabe, M.; Kizuka, F.; Lee, L.; Tamura, I.; Maekawa, R.; Aasada, H.; Yamagata, Y.; et al. The role of melatonin as an antioxidant in the follicle. J. Ovarian. Res. 2012, 5, 5. [Google Scholar] [CrossRef]
- Tamura, H.; Takasaki, A.; Miwa, I.; Taniguchi, K.; Maekawa, R.; Asada, H.; Taketani, T.; Matsuoka, A.; Yamagata, Y.; Shimamura, K.; et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J. Pineal Res. 2008, 44, 280–287. [Google Scholar] [CrossRef]
- Cruz, M.H.; Leal, C.L.; Cruz, J.F.; Tan, D.X.; Reiter, R.J. Essential actions of melatonin in protecting the ovary from oxidative damage. Theriogenology 2014, 82, 925–932. [Google Scholar] [CrossRef]
- Wurtman, R.J.; Axelrod, J.; Potter, L.T. The uptake of H3-melatonin in endocrine and nervous tissues and the effects of constant light exposure. J. Pharmacol. Exp. Ther. 1964, 143, 314–318. [Google Scholar] [PubMed]
- Nakamura, Y.; Tamura, H.; Takayama, H.; Kato, H. Increased endogenous level of melatonin in preovulatory human follicles does not directly influence progesterone production. Fertil. Steril. 2003, 80, 1012–1016. [Google Scholar] [CrossRef]
- Tanabe, M.; Tamura, H.; Taketani, T.; Okada, M.; Lee, L.; Tamura, I.; Maekawa, R.; Asada, H.; Yamagata, Y.; Sugino, N. Melatonin protects the integrity of granulosa cells by reducing oxidative stress in nuclei, mitochondria, and plasma membranes in mice. J. Reprod. Dev. 2015, 61, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.J.; Schatten, H.; Zhang, C.L.; Sun, Q.Y. Oocyte ageing and epigenetics. Reproduction 2015, 149, R103–R114. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J. Targeting oocyte maturation to improve fertility in older women. Cell Tissue Res. 2015, 363, 57–68. [Google Scholar] [CrossRef]
- Batioglu, A.S.; Sahin, U.; Gurlek, B.; Ozturk, N.; Unsal, E. The efficacy of melatonin administration on oocyte quality. Gynecol. Endocrinol. 2012, 28, 91–93. [Google Scholar] [CrossRef]
- Eryilmaz, O.G.; Devran, A.; Sarikaya, E.; Aksakal, F.N.; Mollamahmutoglu, L.; Cicek, N. Melatonin improves the oocyte and the embryo in IVF patients with sleep disturbances, but does not improve the sleeping problems. J. Assist. Reprod Genet. 2011, 28, 815–820. [Google Scholar] [CrossRef]
- Jahromi, B.N.; Sadeghi, S.; Alipour, S.; Parsanezhad, M.E.; Alamdarloo, S.M. Effect of melatonin on the outcome of assisted reproductive technique cycles in women with diminished ovarian reserve: A double-blinded randomized clinical trial. Iran. J. Med. Sci. 2017, 42, 73–78. [Google Scholar]
- Nishihara, T.; Hashimoto, S.; Ito, K.; Nakaoka, Y.; Matsumoto, K.; Hosoi, Y.; Morimoto, Y. Oral melatonin supplementation improves oocyte and embryo quality in women undergoing in vitro fertilization-embryo transfer. Gynecol. Endocrinol. 2014, 30, 359–362. [Google Scholar] [CrossRef]
- Espino, J.; Macedo, M.; Lozano, G.; Ortiz, A.; Rodriguez, C.; Rodriguez, A.B.; Bejarano, I. Impact of melatonin supplementation in women with unexplained infertility undergoing fertility treatment. Antioxidants 2019, 8, 338. [Google Scholar] [CrossRef]
- Keshavarzi, S.; Salehi, M.; Farifteh-Nobijari, F.; Hosseini, T.; Hosseini, S.; Ghazifard, A.; Ghaffari Novin, M.; Fallah-Omrani, V.; Nourozian, M.; Hosseini, A. Melatonin modifies histone acetylation during in vitro maturation of mouse oocytes. Cell. J. 2018, 20, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Lee, J.E.; Kang, J.W.; Oqani, R.K.; Cho, E.S.; Kim, S.B.; Jin, D., II. Melatonin supplementation during prolonged in vitro maturation improves the quality and development of poor-quality porcine oocytes via anti-oxidative and anti-apoptotic effects. Mol. Reprod Dev. 2018, 85, 665–681. [Google Scholar] [CrossRef] [PubMed]
- Marques, T.C.; da Silva Santos, E.C.; Diesel, T.O.; Leme, L.O.; Martins, C.F.; Dode, M.; Alves, B.G.; Costa, F.; de Oliveira, E.B.; Gambarini, M.L. Melatonin reduces apoptotic cells, SOD2 and HSPB1 and improves the in vitro production and quality of bovine blastocysts. Reprod. Domest. Anim. 2018, 53, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Qin, Y.; Hu, X.; Ren, L.; Zhang, C.; Wang, X.; Wang, W.; Zhang, Z.; Hao, J.; Guo, M.; et al. Melatonin protects in vitro matured porcine oocytes from toxicity of Aflatoxin B1. J. Pineal Res. 2019, 66, e12543. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Park, S.Y.; Kim, J.W.; Yang, S.G.; Kim, M.J.; Jegal, H.G.; Kim, I.S.; Choo, Y.K.; Koo, D.B. Melatonin improves oocyte maturation and mitochondrial functions by reducing bisphenol A-derived superoxide in porcine oocytes in vitro. Int. J. Mol. Sci. 2018, 19, 3422. [Google Scholar] [CrossRef]
- An, Q.; Peng, W.; Cheng, Y.; Lu, Z.; Zhou, C.; Zhang, Y.; Su, J. Melatonin supplementation during in vitro maturation of oocyte enhances subsequent development of bovine cloned embryos. J. Cell. Physiol. 2019, 234, 17370–17381. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jin, J.X.; Taweechaipaisankul, A.; Kim, G.A.; Ahn, C.; Lee, B.C. Melatonin influences the sonic hedgehog signaling pathway in porcine cumulus oocyte complexes. J. Pineal Res. 2017, 63, e12424. [Google Scholar] [CrossRef]
- Pang, Y.; Zhao, S.; Sun, Y.; Jiang, X.; Hao, H.; Du, W.; Zhu, H. Protective effects of melatonin on the in vitro developmental competence of bovine oocytes. Anim. Sci. J. 2018, 89, 648–660. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, J.; Li, Y.; Guo, X.; Li, J.; Zhong, R.; Zhang, X. Melatonin-induced demethylation of antioxidant genes increases antioxidant capacity through RORalpha in cumulus cells of prepubertal lambs. Free Radic. Biol. Med. 2019, 131, 173–183. [Google Scholar] [CrossRef]
- Fang, Y.; Deng, S.; Zhang, J.; Liu, H.; Li, Y.; Zhang, X.; Liu, Y. Melatonin-mediated development of ovine cumulus cells, perhaps by regulation of DNA methylation. Molecules 2018, 23, 494. [Google Scholar] [CrossRef]
- Liu, L.; Labani, N.; Cecon, E.; Jockers, R. Melatonin target proteins: Too many or not enough? Front. Endocrinol. 2019, 10, 791. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jin, J.X.; Taweechaipaisankul, A.; Kim, G.A.; Lee, B.C. Stimulatory effects of melatonin on porcine in vitro maturation are mediated by MT2 receptor. Int. J. Mol. Sci. 2018, 19, 1581. [Google Scholar] [CrossRef] [PubMed]
- Saeedabadi, S.; Abazari-Kia, A.H.; Rajabi, H.; Parivar, K.; Salehi, M. Melatonin improves the developmental competence of goat oocytes. Int. J. Fertil. Steril. 2018, 12, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Soto-Heras, S.; Catala, M.G.; Roura, M.; Menendez-Blanco, I.; Piras, A.R.; Izquierdo, D.; Paramio, M.T. Effects of melatonin on oocyte developmental competence and the role of melatonin receptor 1 in juvenile goats. Reprod. Domest. Anim. 2019, 54, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Wang, F.; Zhang, L.; He, C.; Ji, P.; Wang, J.; Zhang, Z.; Lv, D.; Abulizi, W.; Wang, X.; et al. Beneficial effects of melatonin on the in vitro maturation of sheep oocytes and its relation to melatonin receptors. Int. J. Mol. Sci. 2017, 18, 824. [Google Scholar] [CrossRef]
- Pang, Y.W.; Jiang, X.L.; Wang, Y.C.; Wang, Y.Y.; Hao, H.S.; Zhao, S.J.; Du, W.H.; Zhao, X.M.; Wang, L.; Zhu, H.B. Melatonin protects against paraquat-induced damage during in vitro maturation of bovine oocytes. J. Pineal Res. 2019, 66, e12532. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, S. Melatonin Promotes Ubiquitination of Phosphorylated pro-apoptotic protein Bcl-2-interacting mediator of cell death-extra long (BimEL) in porcine granulosa cells. Int. J. Mol. Sci. 2018, 19, 3431. [Google Scholar] [CrossRef]
- Yang, M.; Tao, J.; Chai, M.; Wu, H.; Wang, J.; Li, G.; He, C.; Xie, L.; Ji, P.; Dai, Y.; et al. Melatonin improves the quality of inferior bovine oocytes and promoted their subsequent IVF embryo development: Mechanisms and Results. Molecules 2017, 22, 2059. [Google Scholar] [CrossRef]
- Tian, X.; Wang, F.; Zhang, L.; Ji, P.; Wang, J.; Lv, D.; Li, G.; Chai, M.; Lian, Z.; Liu, G. Melatonin promotes the in vitro development of microinjected pronuclear mouse embryos via its anti-oxidative and anti-apoptotic effects. Int. J. Mol. Sci. 2017, 18, 988. [Google Scholar] [CrossRef]
- Ezzati, M.; Roshangar, L.; Soleimani Rad, J.; Karimian, N. Evaluating the effect of melatonin on HAS2, and PGR expression, as well as cumulus expansion, and fertility potential in mice. Cell. J. 2018, 20, 108–112. [Google Scholar] [CrossRef]
- Nie, J.; Xiao, P.; Wang, X.; Yang, X.; Xu, H.; Lu, K.; Lu, S.; Liang, X. Melatonin prevents deterioration in quality by preserving epigenetic modifications of porcine oocytes after prolonged culture. Aging (Albany N.Y.) 2018, 10, 3897–3909. [Google Scholar] [CrossRef] [PubMed]
- Younis, J.S. Ovarian aging and implications for fertility female health. Minerva Endocrinol. 2012, 37, 41–57. [Google Scholar] [PubMed]
- Pellestor, F.; Andreo, B.; Arnal, F.; Humeau, C.; Demaille, J. Maternal aging and chromosomal abnormalities: New data drawn from in vitro unfertilized human oocytes. Hum. Genet. 2003, 112, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xi, Q.; Wang, D.; Li, J.; Wang, M.; Li, D.; Zhu, L.; Jin, L. Mitochondrial dysfunction and endoplasmic reticulum stress involved in oocyte aging: An analysis using single-cell RNA-sequencing of mouse oocytes. J. Ovarian. Res. 2019, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Luderer, U. Oxidative damage increases and antioxidant gene expression decreases with aging in the mouse ovary. Biol. Reprod. 2011, 84, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Kawamoto, M.; Sato, S.; Tamura, I.; Maekawa, R.; Taketani, T.; Aasada, H.; Takaki, E.; Nakai, A.; Reiter, R.J.; et al. Long-term melatonin treatment delays ovarian aging. J. Pineal Res. 2017, 62, e12381. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Peng, W.; Yin, S.; Zhao, J.; Fu, B.; Zhang, J.; Mao, T.; Wu, H.; Zhang, Y. Melatonin improves age-induced fertility decline and attenuates ovarian mitochondrial oxidative stress in mice. Sci. Rep. 2016, 6, 35165. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Wang, J.; Lv, D.; Zhu, T.; Wang, F.; Tian, X.; Yao, Y.; Ji, P.; Liu, G. Melatonin regulates the activities of ovary and delays the fertility decline in female animals via MT1/AMPK pathway. J. Pineal Res. 2019, 66, e12550. [Google Scholar] [CrossRef]
| Patients | Number | Technique | Melatonin Treatment | Result | Mechanisms | Year | Author/Reference |
|---|---|---|---|---|---|---|---|
| infertile women | 115 (56M/59C) | IVF-ET | 3mg/day orally | improved fertilization rate | reduced 8-OHdG in FF increased M in FF | 2008 | Tamura [6] |
| infertile women | 60 (30M/30C) | IVF-ET | 3mg/day orally | increased mature oocyte increased good quality embryos | 2011 | Eryilmaz [47] | |
| infertile women | 85 (40M/45C) | IVF-ET | 3mg/day orally | increased mature oocyte increased good quality embryos | 2012 | Batioglu [46] | |
| infertile women | 97 (97M/97C) | IVF, ICSI | 3mg/day orally | improved fertilization rate increased good quality embryos | 2014 | Nishihara [49] | |
| infertile women diminished ovarian reserve | 66 (32M/24C) | IVF, ICSI | 3mg/day orally | increased mature oocyte increased good quality embryos | 2017 | Jahromi [48] | |
| infertile women | 30 (10C/10M, 10M) | IVF, ICSI | 3 or 6mg/day orally | increased no of oocyte retrieved increased good quality embryos | increased M, TAC in FF decreased 8-OHdG in FF | 2019 | Espino [50] |
| Animal | Design | Melatonin Treatment | Result | Year | Author/Reference |
|---|---|---|---|---|---|
| mouse | vitro COCs | 10−6 M | Cumulus expansion, M-Ⅱ ↑ ROS, Acetyla on level of H4k12 ↓ | 2017 | Keshavarzi Somayeh [51] |
| mouse | vitro, IVM, implantation | 10−7 M | blastocyst rate, hatching blastocyst rate and blastocyst cell number ↑ pregnancy rate and birth rate↑, (ROS) production and cellular apoptosis ↓ | 2017 | Tian Xiuzhi [69] |
| sheep | vitro, IVM | 10−7 M | rates of nuclear maturation, cumulus cells expansion, cleavage, and blastocyst ↑ MT1 and MT2 were expressed in oocytes, cumulus cells, and granulosa cells BMP15, PTX3, HAS2, EGFR ↑, cAMP level ↓, cGMP ↑ | 2017 | Tian Xiuzhi [65] |
| bovine | vitro, IVM | 10−9 M | ROS↓, GSH↑,mitochondrial normal distribution increase ATP level upregulated ATPase 6, BMP-15, GDF-9, SOD-1, Gpx-4, and Bcl-2 downregulated apoptotic gene expression of caspase-3. | 2017 | Yang Minghui [68] |
| porcine | vitro IVM COCs | 10−7, 10−6, 10−5 M | oocyte quality, embryo development ↑ ROS generation, apoptosis, and DNA damage ↓, GSX, OCT4, H2AX | 2018 | Lin Tao [52] |
| bovine | vitro, IVM | 10−9 M | G1 blastocyst ↑, cell number ↑, apoptotic cell ↓ glutathione content, mitochondrial membrane potential ↑ antioxidant gene (SOD2) heat shock protein (HSPB1) ↑ | 2018 | Marques TC [53] |
| porcine | vitro prolonged culture | 10−3 M | blastocyst rate↑ methylation at H3K4me2 and H3K27me2 ↓ imprinted gene NNAT ↓ | 2018 | Nie Junyu [71] |
| bovine | vitro, IVM | 10−9 M | blastocyst, total cell number ↑, apoptotic cell ↓ ROS ↓, GSH ↑ caspase-3 ↓, BCL-2, XIAP, CAT, HSP70 ↑ | 2018 | Pang Yunwei [66] |
| Goat | vitro, IVM | 10−9 M, 10−12 M | M-II stage, blastocyst ↑, GSH ↑, MTNR1A in cumulus cell and oocytes DNA methyltransferases (DNMTs) global DNA methylation ↓ | 2018 | Saeedabadi Saghar [63] |
| mouse | vitro, IVM | 10 μM | fertilization rate ↑ hyaluronan synthase-2 (HAS2) and Progesterone receptor (PGR) ↑ | 2018 | Ezzati Maryam [70] |
| porcine | vitro COCs | 10−9 M | blastocyst, cell number, cumulus expansion ↑, apoptosis ↓ MT2 was expressed in both oocytes and cumulus cells M effects were abolished when either luzindole or 4P-PDOT (MT antagonist) | 2018 | Lee Sanghoon [62] |
| porcine | vitro COCs | 10−9 M | pro-apoptotic protein BimEL, ERK-mediated phosphorylation M only promoted the ubiquitination of phosphorylated BimEL M action was independent of its receptor and its antioxidant properties | 2018 | Wang Yingzheng [67] |
| bovine | vitro cloned embryo | 10−9 M | cloned embryo development ↑ oxidative stress, apoptosis, mitochondria, chromosome alignment epigenetic modifications, H3K9 acetylation ↑, H3K9 methylation ↓ | 2019 | An Quanli [56] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamura, H.; Jozaki, M.; Tanabe, M.; Shirafuta, Y.; Mihara, Y.; Shinagawa, M.; Tamura, I.; Maekawa, R.; Sato, S.; Taketani, T.; et al. Importance of Melatonin in Assisted Reproductive Technology and Ovarian Aging. Int. J. Mol. Sci. 2020, 21, 1135. https://doi.org/10.3390/ijms21031135
Tamura H, Jozaki M, Tanabe M, Shirafuta Y, Mihara Y, Shinagawa M, Tamura I, Maekawa R, Sato S, Taketani T, et al. Importance of Melatonin in Assisted Reproductive Technology and Ovarian Aging. International Journal of Molecular Sciences. 2020; 21(3):1135. https://doi.org/10.3390/ijms21031135
Chicago/Turabian StyleTamura, Hiroshi, Mai Jozaki, Manabu Tanabe, Yuichiro Shirafuta, Yumiko Mihara, Masahiro Shinagawa, Isao Tamura, Ryo Maekawa, Shun Sato, Toshiaki Taketani, and et al. 2020. "Importance of Melatonin in Assisted Reproductive Technology and Ovarian Aging" International Journal of Molecular Sciences 21, no. 3: 1135. https://doi.org/10.3390/ijms21031135
APA StyleTamura, H., Jozaki, M., Tanabe, M., Shirafuta, Y., Mihara, Y., Shinagawa, M., Tamura, I., Maekawa, R., Sato, S., Taketani, T., Takasaki, A., Reiter, R. J., & Sugino, N. (2020). Importance of Melatonin in Assisted Reproductive Technology and Ovarian Aging. International Journal of Molecular Sciences, 21(3), 1135. https://doi.org/10.3390/ijms21031135







