Small Vessel Disease-Related Dementia: An Invalid Neurovascular Coupling?
Abstract
1. Introduction
2. Vascular Dementia and Small Vessel Disease-Related Dementia
3. Anatomical and Structural Weaknesses in Small Vessel Disease
3.1. Arteriolosclerosis as a Functional Model for SVD
3.2. Hypoperfusion and Neuroinflammation
3.3. Cholinergic Role in Small Vessel Disease
4. Chronic Hypoxia and Brain Response
5. Endothelium and SVD
6. Astrocytes and SVD
7. Oxidative Stress in Angiogenesis and Vascular Disease
8. SVD: Inflammation as a Promoter or a Marker
9. Conclusions
- Small vessel disease and related dementia are two unambiguous nosographic entities that deserve attention for their relevance and impact in clinical practice.
- The arterioles are the targets of small vessel disease, with degeneration of the smooth muscle layer and replacement by hyaline fibrosis, leading to a subtotal luminal occlusion.
- To be added, the more intriguing emerging aspects are those concerning the altered endothelium activation, astrocytes modifications, microglia activation, pericytes, and BBB disruption.
- SVD is related to altered neurovascular coupling.
- The SVD is an ongoing process, which begins with altered microvessels and pial arteries and ends in a subcortical dementia; CBF regional selective decrease seems to be one of the critical factors for the progression from small vessel disease to small vessel disease-related dementia.
- Altered response to inflammation, oxidative stress are crucial aspects of possible irreversible post-transcriptional modifications: these processes are auto-expanding features of irreversible changes of a primarily aging-related benign process.
- Neuromodulators, i.e., Acethlycholine, GABA, and endothelium-acting molecules, such as NO, VEGF, ICAM, prostanoids interact, and are deeply involved in the perpetuation of the ongoing pathological cascade of events.
- The modality of changing from typical aging small vessel disease towards dementia.
- The impact of aging and the age-gene interactions in arteriolosclerosis, endothelium dysfunction, pericytes alterations, and astrocytes modifications, which are caused, promoted, or potentiated by hypoperfusion and metabolic disruption.
- The altered neurovascular coupling is a secondary or a causative (primary) defect in SVD.
- The potential clinical rescue from SVD, knowing different times of its development, probably by acting precociously, simultaneously via various strategies (anti-inflammatory, anti-oxidant, anti-thrombotic, and perhaps via nutraceutical promotions [368], acting as co-enzymatic promoters.
Author Contributions
Funding
Conflicts of Interest
References
- Pantoni, L.; Gorelick, P. Cerebral Small Vessel Disease, 1st ed.; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Xu, W.H. Large artery: An important target for cerebral small vessel diseases. Ann. Transl. Med. 2014, 2, 78. [Google Scholar] [CrossRef]
- Poels, M.M.; Zaccai, K.; Verwoert, G.C.; Vernooij, M.W.; Hofman, A.; vander Lugt, A.; Witteman, J.C.M.; Breteler, M.M.B.; Mattace-Raso, F.U.S.; Ikram, M.A. Arterial stiffness and cerebral small vessel disease: The Rotterdam Scan Study. Stroke 2012, 43, 2637–2642. [Google Scholar] [CrossRef]
- Pantoni, L. Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010, 9, 689–701. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Smith, E.F.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–832. [Google Scholar] [CrossRef]
- Haffner, C.; Malik, R.; Dichgans, M. Genetic factors in cerebral small vessel disease and their impact on stroke and dementia. J. Cereb. Blood Flow Metab. 2015. [Google Scholar] [CrossRef] [PubMed]
- Moody, D.M.; Brown, W.R.; Challa, V.R.; Anderson, R.L. Periventricular venous collagenosis: Association with leukoaraiosis. Radiology 1995, 194, 469–476. [Google Scholar] [CrossRef]
- Smith, E.E.; Vijayappa, M.; Lima, F.; Delgado, P.; Wendell, L.; Rosand, J.; Greenberg, S.M. Impaired visual evoked flow velocity response in cerebral amyloid angiopathy. Neurology 2008, 71, 1424–1430. [Google Scholar] [CrossRef]
- Park, L.; Koizumi, K.; ElJamal, S.; Zhou, P.; Previti, M.L.; VanNostrand, W.E.; Carlson, G.; Iadecola, C. Age-dependent neurovascular dysfunction and damage in a mouse model of cerebral amyloid angiopathy. Stroke 2014, 45, 1815–1821. [Google Scholar] [CrossRef]
- Karakis, I.; Pase, M.P.; Beiser, A.; Booth, S.L.; Jacques, P.F.; Rogers, G.; DeCarli, C.; Vasan, R.S.; Wang, T.J.; Himali, J.J.; et al. Association of serum vitamin D with the risk of incident dementia and subclinical indices of brain aging: The Framingham Heart Study. J. Alzheimers Dis. 2016, 51, 451–461. [Google Scholar] [CrossRef]
- Román, G.C.; Tatemichi, T.K.; Erkinjuntti, T.; Cummings, J.L.; Masdeu, J.C.; Garcia, J.H.; Amaducci, L.; Orgogozo, J.M.; Brun, A.; Hofman, A.; et al. Vascular dementia: Diagnostic criteria for Research studies. Reports of the NINDS-AIREN International Workshop. Neurology 1993, 43, 250–260. [Google Scholar] [CrossRef]
- World Health Organization for Vascular Dementia. The ICD-10 Classification of Mental and Behavioral Disorders; Diagnostic Criteria for Research; World Health Organization: Geneva, Switzerland, 1993; pp. 48–50. [Google Scholar]
- Chui, H. Vascular dementia, a new beginning: Shifting focus from clinical phenotype to ischemic brain injury. Neurol. Clin. 2000, 18, 951–978. [Google Scholar] [CrossRef]
- Román, G.C.; Goldstein, M. A population-based study of dementia in 85-year-olds. N. Engl. J. Med. 1993, 328, 153–158. [Google Scholar]
- Shi, Y.; Wardlaw, J.M. Update on cerebral small vessel disease. A dynamic whole-brain disease. Stroke Vasc. Neurol. 2016, 1, 83–92. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Major or Mild Vascular Neurocognitive disorders. In Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Washington DC, USA, 2013; pp. 612–615. [Google Scholar]
- Sinha, P.; Bharath, S.; Chandra, S.R. DSM-5 in vascular dementia. Comparison with other diagnostic criteria in a retrospective study. EC Neurology 2015, 2, 135–143. [Google Scholar]
- Black, S.; Gao, F.; Bilbao, J. Understanding white matter disease: Imaging-pathological correlations in vascular cognitive impairment. Stroke 2009, 40 (Suppl. 3), S48–S52. [Google Scholar] [CrossRef] [PubMed]
- De Laat, K.F.; Van Norden, A.G.W.; Gons, R.A.R.; van Oudheusden, L.J.; van Uden, I.W.; Bloem, B.R.; Zwiers, M.P.; de Leeuw, F.E. Gait in elderly with cerebral small vessel disease. Stroke 2010, 41, 1652–1658. [Google Scholar] [CrossRef]
- Jellinger, K.A. Pathology and pathogenesis of vascular cognitive impairment—A critical update. Front. Aging Neurosci. 2013, 5, 17. [Google Scholar] [CrossRef]
- Patel, B.; Markus, H.S. Magnetic resonance imaging in cerebral small vessel disease and its use as a surrogate disease marker. Int. J. Stroke 2011, 6, 47–59. [Google Scholar] [CrossRef]
- Erkinjunnti, T.; Inzitari, D.; Pantoni, L.; Wallin, A.; Scheltens, P.; Rockwood, K.; Roman, G.C.; Chui, H.; Desmond, D.W. Research criteria for subcortical vascular dementia in clinical trials. J. Neural Transm. Suppl. 2000, 59, 23–30. [Google Scholar]
- Roman, G.C.; Erkinjunnti, T.; Wallin, A.; Pantoni, L.; Chui, H.C. Subcortical ischemic vascular dementia. Lancet Neurol. 2002, 1, 426–436. [Google Scholar] [CrossRef]
- Jani, B.I.; Rajkumar, C. Ageing and vascular ageing. Postgrad Med. J. 2006, 82, 357–362. [Google Scholar] [CrossRef]
- De la Torre, J.C. Vascular basis of Alzheimer’s pathogenesis. Ann. N. Y. Acad. Sci. 2002, 977, 196–215. [Google Scholar] [CrossRef]
- Mathias, C.J.; Kimber, J.R. Postural hypotension: Causes, clinical features, investigation, and management. Ann. Rev. Med. 1999, 50, 317–336. [Google Scholar] [CrossRef]
- Roriz-Filho, J.S.; Bernardes Silva Filho, S.R.; Rosset, I.; Roriz-Cruz, M. Postural blood pressure dysregulation and dementia: Evidence for a vicious circle and implications for neurocardiovascular rehabilitation. In Cardiac Rehabilitation; Halliday, J.T., Ed.; Novascience Publisher Inc.: New York, NY, USA, 2009; pp. 1–37. ISBN 987-1-60741-918-1. [Google Scholar]
- Salloway, S. Subcortical Vascular Dementia: Binswanger’s and CADASIL; 8AC.006-2; American Academy of Neurology (AAN): Honolulu, HI, USA, 2003; pp. 1–29. [Google Scholar]
- Pantoni, L.; Garcia, J.H.; Gutierrez, J.A. Cerebral white matter is highly vulnerable to ischemia. Stroke 1996, 27, 1641–1647. [Google Scholar] [CrossRef]
- Schmidt, R.; Schmidt, H.; Haybaeck, J.; Loitfelder, M.; Weis, S.; Cavalieri, M.; Seiler, S.; Enzinger, C.; Ropele, S.; Erkinjuntti, T.; et al. Heterogeneity in age-related white matter changes. Acta Neuropathol. 2011, 122, 171–185. [Google Scholar] [CrossRef]
- Hommet, C.; Mondon, K.; Constans, T.; Beaufils, E.; Desmidt, T.; Camus, V.; Cottier, J.P. Review of cerebral microangiopathy and Alzheimer’s disease: Relation between white matter hyperintensities and microbleeds. Dement. Geriatr. Cogn. Disord. 2011, 32, 367–378. [Google Scholar] [CrossRef]
- Munoz, D.G.; Hastak, S.M.; Harper, B.; Lee, D.; Hachinski, V.C. Pathologic correlates of increased signals of the centrum ovale on magnetic resonance imaging. Arch. Neurol. 1993, 50, 492–497. [Google Scholar] [CrossRef]
- Mirski, M.A. Pharmacology of Blood Pressure Management during Cerebral Ischemia; 5PC-004; American Academy of Neurology (AAN): Miami, FL, USA, 2005; pp. 456–469. [Google Scholar]
- Wallin, A.; Blennow, K.; Gottfries, C.G. Neurochemical abnormalities in vascular dementia. Dementia 1989, 1, 120–130. [Google Scholar]
- Caruso, P.; Signori, R.; Moretti, R. Small vessel disease to subcortical dementia: A dynamic model, which interfaces aging, cholinergic dysregulation and the neurovascular unit. Vasc. Health Risk Manag. 2019, 15, 259–281. [Google Scholar] [CrossRef]
- Bohnen, N.I.; Muller, M.L.T.M.; Kuwabara, H.; Ocnstantien, G.M.; Studentski, S.A. Age-associated leukoaraiosis and cortical cholinergic deafferentation. Neurology 2009, 72, 1411–1416. [Google Scholar] [CrossRef]
- Román, G.C. Brain hypoperfusion: A critical factor in vascular dementia. Neurol. Res. 2004, 26, 454–458. [Google Scholar] [CrossRef]
- Zhan, S.S.; Beyreuther, K.; Schmitt, H.P. Synaptophysin immunoreactivity of the cortical neuropil in vascular dementia of Binswanger type compared with the dementia of Alzheimer type and non-demented controls. Dementia 1994, 5, 79–87. [Google Scholar] [CrossRef]
- Ahtiluoto, S.; Polvikoski, T.; Peltonen, M.; Solomon, A.; Tuomilehto, J.; Winblad, B.; Sulkava, R.; Kivipelto, M. Diabetes, Alzheimer disease, and vascular dementia: A population-based neuropathologic study. Neurology 2010, 75, 1195–1202. [Google Scholar] [CrossRef]
- Englund, E.A.; Person, B. Correlations between histopathologic white matter changes and proton MR relaxation times in dementia. Alzheimer Dis. Assoc. Disord. 1987, 1, 156–170. [Google Scholar] [CrossRef]
- Román, G.C. Senile dementia of the Binswanger type: A vascular form of dementia in the elderly. JAMA 1987, 258, 1782–1788. [Google Scholar] [CrossRef]
- Vinters, H.V.; Ellis, W.G.; Zarow, C.; Zaias, B.W.; Jagust, W.J.; Mack, W.J.; Chui, H.C. Neuropathological substrate of ischemic vascular dementia. J. Neuropathol. Exp. Neurol. 2000, 59, 931–945. [Google Scholar] [CrossRef]
- Garcia, J.H.; Lassen, N.A.; Weiller, C.; Sperling, B.; Nakagawara, J. Ischemic stroke and incomplete infarction. Stroke 1996, 27, 761–765. [Google Scholar] [CrossRef]
- Dalkara, T.; Alarcon-Martinez, L. Cerebral micro-vascular signaling in health and disease. Brain Res. 2015, 1623, 3–17. [Google Scholar] [CrossRef]
- Giannakopoulos, P.; Gold, G.; Kowaru, E.; von Gunten, A.; Imhof, A.; Bouras, C. Assessing the cognitive impact of Alzheimer disease pathology and vascular burden in the aging brain: The Geneva experience. Acta Neuropathol. 2007, 113, 1–12. [Google Scholar] [CrossRef]
- Launer, L.J.; Hughes, T.M.; White, L.R. Microinfarcts, brain atrophy, and cognitive function: The Honolulu Asia Aging Study Autopsy Study. Ann. Neurol. 2011, 70, 774–780. [Google Scholar] [CrossRef]
- Van der Veen, P.H.; Muller, M.; Vinken, K.L.; Hendrikse, J.; Mali, W.P.; van der Graaf, Y.; Geerlings, M.I.; SMART Study Group. Longitudinal relationship between cerebral small vessel disease and cerebral blood flow. The second manifestations of arterial disease-magnetic resonance study. Stroke 2015, 46, 1233–1238. [Google Scholar] [CrossRef]
- Gouw, A.A.; van Der Flier, W.M.; Fazekas, F.; van Straaten, E.C.; Pantoni, L.; Poggesi, A.; Inzitari, D.; Erkinjuntti, T.; Wahlund, L.O.; Waldemar, G.; et al. Progression of white matter hyperintensities and incidence of new lacunes over a 3-year period: The leukoaraiosis and disability study. Stroke 2008, 39, 1414–1420. [Google Scholar] [CrossRef]
- Schmidt, R.; Seiler, S.; Loitfelder, M. Longitudinal change of small vessel disease related brain abnormalities. J. Cereb. Blood Flow Metab. 2016, 36, 26–39. [Google Scholar] [CrossRef]
- Munoz-Maniega, S.; Chappell, F.M.; Valdes-Henrandez, M.L.; Armitage, P.A.; Makin, S.D.; Heye, A.K.; Thrippleton, M.J.; Sakka, E.; Shuler, K.; Dennis, M.S.; et al. Integrity of normal appearing white matter: Influence of age, visible lesion burden and hypertension in patients with small-vessel disease. J. Cereb. Blood Flow Metab. 2016, 37, 644–656. [Google Scholar] [CrossRef]
- Smallwood, A.; Oulhaj, A.; Joachim, C.; Christie, S.; Sloan, C.; Smith, A.D.; Esiri, M. Cerebral subcortical small vessel disease and its relation to cognition in elderly subjects: A pathological study in the Oxford Project to Investigate Memory and Ageing (OPTIMA) cohort. Neuropathol. Appl. Neurobiol. 2012, 38, 337–343. [Google Scholar] [CrossRef]
- Kramer, J.H.; Reed, B.R.; Mungas, D.; Weiner, M.W.; Chui, H. Executive dysfunction in subcortical ischaemic vascular disease. J. Neurol. Neurosurg. Psychiatr. 2002, 72, 217–220. [Google Scholar] [CrossRef]
- Burton, E.; Ballard, C.; Stephens, S.; Kenny, R.A.; Kalaria, R.; Barber, R.; O’Brien, J. Hyperintensities and fronto-subcortical atrophy on MRI are substrates of mild cognitive deficits after stroke. Dement. Geriatr. Cogn. Disord. 2003, 16, 113–118. [Google Scholar] [CrossRef]
- Tullberg, M.; Fletcher, E.; DeCarli, C.; Mungas, D.; Reed, B.R.; Harvey, D.J.; Weiner, M.W.; Chui, H.C.; Jagust, W.J. White matter lesions impair frontal lobe function regardless of their location. Neurology 2004, 63, 246–253. [Google Scholar] [CrossRef]
- Gold, G.; Kovari, E.; Herrmann, F.R.; Canuto, A.; Hof, P.R.; Michel, J.P.; Bouras, C.; Giannakopoulos, P. Cognitive consequences of thalamic, basal ganglia, and deep white matter lacunes in brain aging and dementia. Stroke 2005, 36, 1184–1188. [Google Scholar] [CrossRef]
- Cheng, B.; Golsari, A.; Fiehler, J.; Rosenkranz, M.; Gerloff, C.; Thomalla, G. Dynamics of regional distribution of ischemic lesions in middle cerebral artery trunk occlusion relates to collateral circulation. J. Cereb. Blood Flow Metab. 2010, 31, 36–40. [Google Scholar] [CrossRef]
- Dijkhuizen, R.M.; Knollema, S.; van der Worp, H.B.; Ter Horst, G.J.; De Wildt, D.J.; Berkelbach van der Sprenkel, J.W.; Tulleken, K.A.; Nicolay, K. Dynamics of cerebral tissue injury and perfusion after temporary hypoxia-ischemia in the rat: Evidence for region-specific sensitivity and delayed damage. Stroke 1998, 29, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.H.; Liu, K.F.; Ye, Z.R.; Gutierrez, J.A. Incomplete infarct and delayed neuronal death after transient middle cerebral artery occlusion in rats. Stroke 1997, 28, 2303–2309. [Google Scholar] [CrossRef] [PubMed]
- Konaka, K.; Miyashita, K.; Naritomi, H. Changes in diffusion-weighted magnetic resonance imaging findings in the acute and subacute phases of anoxic encephalopathy. J. Stroke Cerebrovasc. Dis. 2007, 16, 82–83. [Google Scholar] [CrossRef] [PubMed]
- Ravens, J.R. Vascular changes in the human senile brain. Adv. Neurol. 1978, 20, 487–501. [Google Scholar]
- Klassen, A.C.; Sung, J.H.; Stadlan, E.M. Histological changes in cerebral arteries with increasing age. J. Neuropathol. Exp. Neurol. 1968, 27, 607–623. [Google Scholar] [CrossRef]
- Cummings, J.L. Frontal-subcortical circuits and human behavior. Arch. Neurol. 1993, 50, 873–880. [Google Scholar] [CrossRef]
- Mega, M.S.; Cummings, J.L. Frontal-subcortical circuits and neuropsychiatric disorders. J. Neuropsychiatry Clin. Neurosci. 1994, 6, 358–370. [Google Scholar]
- Yao, H.; Sadoshima, S.; Kuwabara, Y.; Ichiya, Y.; Fujishima, M. Cerebral blood flow and oxygen metabolism in patients with vascular dementia of the Binswanger type. Stroke 1990, 21, 1694–1699. [Google Scholar] [CrossRef]
- Furuta, A.; Ishii, N.; Nishihara, Y.; Horie, A. Medullary arteries in aging and dementia. Stroke 1991, 22, 442–446. [Google Scholar] [CrossRef]
- Tak, S.; Yoon, S.J.; Jang, J.; Yoo, K.; Jeong, Y.; Ye, J.C. Quantitative analysis of hemodynamic and metabolic changes in subcortical vascular dementia using simulataneous near-infrared spectroscopy and FMRI measurements. Neuroimage 2011, 55, 176–184. [Google Scholar] [CrossRef]
- Schroeter, M.; Cutini, S.; Wahl, M.; Scheid, R.; Yves von Cramon, D. Neurovascular coupling is impaired in cerebral microangiopathy an event related stroop study. Neuroimage 2007, 34, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Bar, K.; Boettger, M.; Seidler, N.; Mentzelh, H.J.; Terborg, C.; Sauer, H. Influence of galantamine on vasomotor reactivity in AD and vascular dementia due to microangiopathy. Stroke 2007, 38, 3186–3192. [Google Scholar] [CrossRef] [PubMed]
- De Reuck, J.; Decoo, D.; Marchau, M.; Santens, P.; Lemahieu, I.; Strijckmans, K. Positron emission tomography in vascular dementia. J. Neurol. Sci. 1998, 154, 55–61. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Murase, K.; Oku, N.; Kitigawa, K.; Imaizumi, M.; Takasawa, M.; Nishikawa, T.; Matsumoto, M.; Hatazawa, J.; Hori, M. Statistical image analysis of cerebral blood flow in vascular dementia with small-vessel disease. J. Nucl. Med. 2003, 44, 505–511. [Google Scholar]
- Yang, D.; Kim, B.; Park, J.; Kim, S.; Kim, E.; Sohn, H. Analysis of cerebral blood flow of subcortical vascular dementia with single photon emission computed tomography: Adaptation of statistical parametric mapping. J. Neurol. Sci. 2002, 203, 199–205. [Google Scholar] [CrossRef]
- Ramirez-Gomez, C.; Zheng, C.; Reed, B.; Kramer, J.; Mungas, D.; Zarow, C.; Vinters, H.; Ringman, J.M.; Chui, H. Neuropsychological profiles differentiate Alzheimer Disease from Subcortical Ischemic vascular dementia in an autopsy-defined cohort. Dement. Aging Cogn. Disord. 2017, 44, 1–11. [Google Scholar] [CrossRef]
- Cummings, J.L. Vascular subcortical dementias: Clinical aspects. Dementia 1994, 5, 177–180. [Google Scholar] [CrossRef]
- Desmond, D.W.; Erkinjuntti, T.; Sano, M.; Cummings, J.L.; Bowler, J.V.; Pasquier, F.; Moroney, J.T.; Ferris, S.H.; Stern, Y.; Sachdev, P.S.; et al. The cognitive syndrome of vascular dementia: Implications for clinical trials. Alzh. Dis. Assoc. Dis. 1999, 13 (Suppl. 3), S21–S29. [Google Scholar] [CrossRef]
- Sachdev, P.S.; Brodaty, H.; Valenzuela, M.J.; Lorents, L.; Looi, J.C.L.; Wen, W.; Zagami, A.S. The neuropsychological profile of vascular cognitive impairment in stroke and TIA patients. Neurology 2004, 62, 912–62919. [Google Scholar] [CrossRef]
- Traykov, L.; Baudic, S.; Thibaudet, M.C.; Rigaud, A.S.; Samgghe, A.; Boller, F. Neuropsychological deficit in early subcortical vascular dementia: Comparison to Alzheimer’s disease. Dement. Geriatr. Cogn. Dis. 2002, 14, 26–32. [Google Scholar] [CrossRef]
- Moretti, R.; Signori, R. Neural correlates for apathy: Frontal-prefrontal and parietal cortical-subcortical circuits. Front. Aging Neurosci. 2016, 9, 8–289. [Google Scholar] [CrossRef] [PubMed]
- Ishii, N.; Nashihara, Y.; Imamura, T. Why do frontal lobe symptoms predominate in vascular dementia with lacunes? Neurology 1986, 36, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.T.; Inman, C.B.; Weller, R.O. Interrelationships of the pia mater and the perivascular (Wirchov-Robin) spaces in the human cerebrum. J. Anat. 1990, 170, 111–123. [Google Scholar]
- Iadecola, C. The neurovascular Unit coming of age: A journey through neurovascular coupling in health and disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef]
- Hendrikx, D.; Smits, A.; Lavanga, M.; De Wel, O.; Thewissen, L.; Jansen, K.; Caicedo, A.; Van Huffel, S.; Naulaers, G. Measurement of Neurovascular Coupling in Neonates. Front. Physiol. 2019, 10, 65. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Abbott, N.J.; Pizzo, M.E.; Preston, J.E.; Janigro, D.; Thorne, R.G. The role of brain barriers in fluid movement in the CNS: Is there a ‘glymphatic’ system? Acta Neuropathol. 2018, 135, 387–407. [Google Scholar] [CrossRef]
- Huijts, M.; Duits, A.; Staals, J.; Kroon, A.A.; de Leeuw, P.W.; van Oostenbrugge, R.J. Basal ganglia enlarged perivascular spaces are linked to cognitive function in patients with cerebral small vessel disease. Curr. Neurovasc. Res. 2014, 11, 136–141. [Google Scholar] [CrossRef]
- Jiménez-Balado, J.; Riba-Llena, I.; Garde, E.; Valor, M.; Gutiérrez, B.; Pujadas, F.; Delgado, P. Prevalence of hippocampal enlarged perivascular spaces in a sample of patients with hypertension and their relation with vascular risk factors and cognitive function. J. Neurol. Neurosurg. Psychiatry 2018, 89, 651–656. [Google Scholar] [CrossRef]
- Potter, G.M.; Marlborough, F.J.; Wardlaw, J.M. Wide variation in definition, detection and description of lacunar lesions on imaging. Stroke 2011, 42, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Potter, G.M.; Doubal, F.N.; Jackson, C.A.; Chappell, F.M.; Sudlow, C.L.; Dennis, M.S.; Wardlaw, J.M. Counting cavitating lacunes underestimates the burden of lacunar infarction. Stroke 2010, 41, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Ciacciarelli, A.; Sette, G.; Giubilei, F.; Orzi, F. Chronic cerebral hypoperfusion: An undefined, relevant entity. J. Clin. Neurosci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.M.; Doubal, F.; Armitage, P.; Chappell, F.; Carpenter, T.; Muñoz Maniega, S.; Farrall, A.; Sudlow, C.; Dennis, M.; Dhillon, B. Lacunar stroke is associated with diffuse blood brain barrier dysfunction. Ann. Neurol. 2009, 65, 194–202. [Google Scholar] [CrossRef]
- De Silva, T.M.; Faraci, F.M. Microvascular dysfunction and cognitive impairment. Cell Mol. Neurobiol. 2016, 36, 241–258. [Google Scholar] [CrossRef]
- Joutel, A.; Faraci, F.M. Cerebral small vessel disease; insights and opportunities from mouse models of collagen IV related small vessel disease and cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Stroke 2014, 45, 1215–1221. [Google Scholar] [CrossRef]
- Lammie, A.G. Small vessel disease. In Cerebrovascular Diseases; Kalimo, H., Ed.; Wiley Press: New York, NY, USA, 2005; pp. 85–91. [Google Scholar]
- Rosenberg, G.A. Neurological diseases in relation to the blood-brain barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Pantoni, L. Pathophysiology of age-related cerebral white matter changes. Cerebrovasc. Dis 2002, 13, 7–10. [Google Scholar] [CrossRef]
- Englund, E. White matter pathology of vascular dementia. In Vascular Dementia; Chui, E., Dunitz, M., Eds.; Martin Dunitz: London, UK, 2004; pp. 117–130. [Google Scholar]
- Kumar, V.; Cotran, R.S.; Robbins, S.L. Basic Pathology, 8th ed.; Saunders: Philadelphia, PA, USA, 2007. [Google Scholar]
- Lodder, J.; Bamford, J.M.; Sandercock, P.A.; Jones, L.N.; Warlow, C.P. Are hypertension or cardiac embolism likely causes of lacunar infarction? Stroke 1990, 21, 375–381. [Google Scholar] [CrossRef]
- Gamble, C. The pathogenesis of hyaline arteriosclerosis. Am. J. Pathol. 1986, 122, 410–420. [Google Scholar]
- Pavelka, M.; Roth, J. Hyaline Arteriolosclerosis. In Functional Ultrastructure; Springer: Vienna, Austria, 2010; pp. 256–257. [Google Scholar]
- Najjar, S.S.; Scuteri, A.; Lakatta, E.G. Arterial aging: Is it an immutable cardiovascular risk factor? Hypertension 2005, 46, 454–462. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, M.F.; Safar, M.E. Relationship between aortic stiffening and microvascular disease in brain and kidney: Cause and logic of therapy. Hypertension 2005, 46, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Cervós-Navarro, J.; Matakas, F.; Roggendorf, W.; Christmann, U. The morphology of spastic intracerebral arterioles. Neuropathol. Appl. Neurobiol. 1978, 4, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Irving, E.A.; Nicoll, J.; Graham, D.I.; Dewar, D. Increased tau immunoreactivity in oligodendrocytes following human stroke and head injury. Neurosci. Lett. 1996, 213, 189–192. [Google Scholar] [CrossRef]
- Irving, E.A.; Yatsushiro, K.; McCullough, J.; Dewar, R. Rapid alteration of tau in oligodendrocytes after focal ischemic injury in the rat: Involvement of free radical. J. Cererb. Blood Flow Metab. 1997, 17, 612–622. [Google Scholar] [CrossRef]
- Furukawa, S.; Sameshima, H.; Yang, L.; Hariskuma, M.; Ikenoue, T. Regional differences of microglial accumulation within 72 hours of hypoxia-ischemia and the effect of acetylcholine receptor agonist on brain damage and microglial activation in newborn rats. Brain Res. 2014, 1562, 52–58. [Google Scholar] [CrossRef]
- Petito, C.K. Trasnformation of postisichemic perineuronal glial cells. J. Cereb. Blood Flow Metabol. 1986, 6, 616–624. [Google Scholar] [CrossRef]
- Petito, C.K.; Olarte, J.P.; Roberts, B.; Nowak, T.S.; Pulsinelli, W.A. Selective glial vulnerability following transient global ischemia in rat brain. J. Neuropathol. Exp. Neurol. 1998, 57, 231–238. [Google Scholar] [CrossRef]
- Gehrmann, J.; Bonnekoh, P.; Miyazawa, T. Immunoistochemical study of an early microglial activation in ischemia. J. Cereb. Blood Flow Metab. 1992, 12, 257–269. [Google Scholar] [CrossRef]
- Rupalla, K.; Allegrin, P.R.; Saver, D. Time course of microglia activation and apoptosis in various brain regions after permanent focal cerebral ischemia in mice. Acta Neuropathol. 1998, 96, 172–178. [Google Scholar] [CrossRef]
- Masuda, T.; Croom, D.; Hida, H.; Kirov, S.A. Capillary blood flow around microglial somata determines dynamics of microglial processes in ischemic conditions. Glia 2011, 59, 1744–1753. [Google Scholar] [CrossRef] [PubMed]
- Ju, F.; Ran, Y.; Zhu, L.; Cheng, X.; Gao, H.; Xi, X.; Yang, Z.; Zhang, S. Increased BBB Permeability Enhances Activation of Microglia and Exacerbates Loss of Dendritic Spines after Transient Global Cerebral Ischemia. Front. Cell Neurosci. 2018, 12, 236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S. Microglial activation after ischaemic stroke. Stroke Vasc. Neurol. 2019, 4, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Yenari, M.A.; Xu, L.; Tang, X.N.; Qiao, Y.; Giffard, R.G. Microglia potentiate damage to blood-brain barrier constituents: Improvement by minocycline in vivo and in vitro. Stroke 2006, 37, 1087–1093. [Google Scholar] [CrossRef]
- Szalay, G.; Martinecz, B.; Lénárt, N.; Környei, Z.; Orsolits, B.; Judák, L.; Császár, E.; Fekete, R.; West, B.L.; Katona, G.; et al. Microglia protect against brain injury and their selective elimination dysregulates neuronal network activity after stroke. Nat. Commun. 2016, 7, 11499. [Google Scholar] [CrossRef]
- Czeh, M.; Gressens, P.; Kaindl, A.M. The Yin and Yang of microglia. Dev. Neurosci. 2011, 33, 199–209. [Google Scholar] [CrossRef]
- Yuan, T.H.; Yu, H.M. Notch signaling: Key role in intrauterin infection/inflammation, embryonic development and white matter damage. J. Neurosci. Res. 2010, 88, 461–468. [Google Scholar] [CrossRef]
- Scremin, O.U.; Sonneschein, R.R.; Rubinstein, E.H. Cholinergic cerebral vasodilatation in the rabbit: Absence of concomitant metabolic activation. J. Cereb. Blood Flow Metab. 1982, 2, 241–247. [Google Scholar] [CrossRef]
- Morrison, H.W.; Filosa, J.A. A quantitative spatiotemporal analysis of microglia morphology during ischemic stroke and reperfusion. J. Neuroinflamm. 2013, 10, 4. [Google Scholar] [CrossRef]
- Perego, C.; Fumagalli, S.; De Simoni, M.G. Temporal pattern of expression and colocalization of microglia/macrophage phenotype markers following brain ischemic injury in mice. J. Neuroinflamm. 2011, 8, 174. [Google Scholar] [CrossRef]
- Wakita, H.; Tomimoto, H.; Akiguchi, I.; Kimura, J. Glial activation and white matter changes in the rat brain induced by chronic cerebral hypoperfusion: An immunoistochemical study. Acta Neuropathol. 1994, 87, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Farkas, E.; Donka, G.; de Vous, R.A.I.; Mihaly, A.; Bari, F.; Luiten, P.G.M. Experimental cerebral hypoeprfusion induces white matter injury and microglial activation in the rat brain. Acta Neuropathol. 2004, 108, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.G.; Bower, L.; Zhang, R.L.; Chen, S.; Windham, J.P.; Chopp, M. Three dimensional measurement of cerebral microvascular plasma perfusion, glial fibrillary acid protein and microtubule associated P-2 immunoreactivity after embolic stroke in rats: A double fluorescent labeled laser scanning confocal microscopic study. Brain Res. 1999, 844, 55–66. [Google Scholar] [CrossRef]
- Tomimoto, H.; Akiguchi, I.; Wakita, H.; Svenaga, T.; Nakamura, S.; Kimura, J. Regressive changes of astroglia in white matter lesions in cerebrovascular disease and AD patients. Acta Neuropathol. 1997, 94, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C. The pathobiology of vascular dementia. Neuron 2013, 80, 844–866. [Google Scholar] [CrossRef] [PubMed]
- Filous, A.S.; Silver, J. Targeting astrocytes in CNS injury and disease: A translational research approach. Prog. Neurobiol. 2016, 144, 173–187. [Google Scholar] [CrossRef]
- Chen, A.; Akinyemi, R.O.; Hase, Y.; Firbank, M.J.; Ndung’u, M.N.; Foster, V.; Craggs, L.J.; Washida, K.; Okamoto, Y.; Thomas, A.J.; et al. Frontal white matter hyperintensities, clasmatodendrosis and gliovascular abnormalities in ageing and post-stroke dementia. Brain 2016, 139, 242–258. [Google Scholar] [CrossRef]
- Forsberg, K.M.E.; Zhang, Y.; Reiners, J.; Ander, M.; Niedermayer, A.; Fang, L.; Neugebauer, H.; Kassubek, J.; Katona, I.; Weis, J.; et al. Endothelial damage, vascular bagging and remodeling of the microvascular bed in human microangiopathy with deep white matter lesions. Acta Neuropathol. Commun. 2018, 6, 128. [Google Scholar] [CrossRef]
- Hicks, P.; Rolsten, C.; Brizzee, D.; Samorajski, T. Age-related changes in rat brain capillaries. Neurobiol. Aging 1983, 4, 69–75. [Google Scholar] [CrossRef]
- Peters, A.; Sethares, C. Age-related changes in the morphology of cerebral capillaries do not correlate with cognitive decline. J. Comp. Neurol. 2012, 520, 1339–1347. [Google Scholar] [CrossRef]
- Akiguchi, I.; Tomimoto, H.; Suenaga, T.; Wakita, H.; Budka, H. Blood-brain barrier dysfunction in Binswanger’s disease; an immunohistochemical study. Acta Neuropathol. 1998, 95, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Bridges, L.R.; Andoh, J.; Lawrence, A.J.; Khoong, C.H.; Poon, W.W.; Esiri, M.M.; Markus, H.S.; Hainsworth, A.H. Blood-brain barrier dysfunction and cerebral small vessel disease (arteriolosclerosis) in brains of older people. J. Neuropathol. Exp. Neurol. 2014, 73, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- McAleese, K.E.; Alafuzoff, I.; Charidimou, A.; De Reuck, J.; Grinberg, L.T.; Hainsworth, A.H.; Hortobagyi, T.; Ince, P.; Jellinger, K.; Gao, J.; et al. Post-mortem assessment in vascular dementia: Advances and aspirations. BMC Med. 2016, 14, 129. [Google Scholar] [CrossRef] [PubMed]
- Young, V.G.; Halliday, G.M.; Kril, J.J. Neuropathologic correlates of white matter hyperintensities. Neurology 2008, 71, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Attwell, D.; Mishra, A.; Hall, C.N.; O’Farrell, F.M.; Dalkara, T. What is a pericyte? J. Cereb. Blood Flow Metab. 2016, 36, 451–455. [Google Scholar] [CrossRef]
- Brown, W.R. A review of string vessels or collapsed, empty basement membrane tubes. J. Alzheimers Dis. 2010, 21, 725–739. [Google Scholar] [CrossRef]
- Irvine, A.R.; Wood, I.S. Radiation retinopathy as an experimental model for ischemic proliferative retinopathy and rubeosis iridis. Am. J. Ophthalmol. 1987, 103, 790–797. [Google Scholar] [CrossRef]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Wang, H.; Abumrad, N.; Eaton, J.W.; Tracey, K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000, 405, 458–462. [Google Scholar] [CrossRef]
- Wang, H.; Yu, M.; Ochani, M.; Amella, C.A.; Tanovic, M.; Susarla, S.; Li, J.H.; Wang, H.; Yang, H.; Ulloa, L.; et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 2003, 421, 384–838. [Google Scholar] [CrossRef]
- Conejero-Goldberg, C.; Davies, P.; Ulloa, L. Alpha7 nicotinic acetylcholine receptor: A link between inflammation and neurodegeneration. Neurosci. Biobehav. Rev. 2008, 32, 693–706. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Tracey, K.J. Controlling inflammation: The cholinergic anti-inflammatory pathway. Biochem. Soc. Trans. 2006, 34 Pt 6, 1037–1040. [Google Scholar] [CrossRef]
- Kalaria, R.N.; Maestre, G.E.; Arizaga, R.; Friedland, R.P.; Galasko, D.; Hall, K.; Luchsinger, J.A.; Ogunniyi, A.; Perry, E.K.; Potocnik, F.; et al. Alzheimer’s disease and vascular dementia in developing countries: Prevalence, management, and risk factors. Lancet Neurol. 2008, 7, 812–826. [Google Scholar] [CrossRef]
- Kim, H.J.; Moon, W.J.; Han, S.H. Differential cholinergic pathway involvement in Alzheimer’s disease and subcortical ischemic vascular dementia. J. Alzheimers Dis. 2013, 35, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kang, H.S.; Kim, H.J.; Moon, Y.; Ryu, H.J.; Kim, M.Y.; Han, S.H. The effect of ischemic cholinergic damage on cognition in patients with subcortical vascular cognitive impairment. J. Geriatr. Psychiatry Neurol. 2012, 25, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhu, Z.; Teipel, S.J.; Yang, J.; Xing, Y.; Tang, Y.; Jia, J. White Matter Damage in the Cholinergic System Contributes to Cognitive Impairment in Subcortical Vascular Cognitive Impairment, No Dementia. Front. Aging Neurosci. 2017, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Tohgi, H.; Abe, T.; Kimura, M.; Saheki, M.; Takahashi, S. Cerebrospinal fluid acetylcholine and choline in vascular dementia of Binswanger and multiple small infarct types as compared with Alzheimer-type dementia. J. Neural Transm. 1996, 103, 1211–1220. [Google Scholar] [CrossRef]
- Wallin, A.; Sjogren, M.; Blennow, K.; Davidsson, P. Decreased cerebrospinal fluid acetylcholinesterase in patients with subcortical ischemic vascular dementia. Dement. Geriatr. Cogn. Disord. 2003, 16, 200–207. [Google Scholar] [CrossRef]
- Yamada, M.; Lamping, K.G.; Duttaroy, A.; Zhang, W.; Cui, Y.; Bymaster, F.P.; McKinzie, D.L.; Felder, C.C.; Deng, C.X.; Faraci, F.M.; et al. Cholinergic dilatation of cerebral blood vessels is abolished in M5 muscarinic acetylcoline receptor knockout mice. Prc. Natl. Acd. Sci. USA 2001, 98, 14096–14101. [Google Scholar] [CrossRef]
- Togashi, H.; Matsumoto, M.; Yoshioka, M.; Hirokami, M.; Minami, M.; Saito, H. Neurochemical profiles in cerebrospinal fluid of stroke-prone spontaneously hypertensive rats. Behav. Lett. 1994, 166, 117–120. [Google Scholar]
- Togashi, H.; Kimura, S.; Matsumoto, M.; Yoshioka, M.; Minami, M.; Saito, H. Cholinergic changes in the hippocampus of stroke-prone spontaneously hypertensive rats. Stroke 1996, 27, 520–526. [Google Scholar] [CrossRef]
- Scremin, O.U.; Li, M.G.; Scremin, A.M.E.; Jenden, D.J. Cholinesterase inhibition improves blood flow in the ischemic cerebral cortex. Brain Res. Bull. 1997, 42, 59–70. [Google Scholar] [CrossRef]
- Court, J.; Perry, E.; Kalaria, R. Neurotransmitter control of the cerebral vasculature and abnormalities in vascular dementia. In Vascular Cognitive Impairment; Erkinjuntti, T., Gauthier, S., Eds.; Martin Dunitz: London, UK, 2002; pp. 167–185. [Google Scholar]
- Szilagy, A.K.; Nemeth, A.; Martini, E.; Lendvai, B.; Venter, V. Serum and CSF cholinesterase activity in various kind of dementia. Eur. Arch. Psychiatry Neurol. Sci. 1987, 236, 309–311. [Google Scholar] [CrossRef] [PubMed]
- Mertens, J.C.; Leenaerts, D.; Brouns, R.; Engelborghs, S.; Ieven, M.; De Deyn, P.P.; Lambeir, A.M.; Hendriks, D. Procarboxypeptidase U (proCPU, TAFI, proCPB2) in cerebrospinal fluid during ischemic stroke is associated with stroke progression, outcome and blood-brain barrier dysfunction. J. Thromb. Haemost. 2018, 16, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Chou, W.H.; Fang, C.P.; Liu, T.H.; Tsou, H.H.; Wang, Y.; Liu, Y.L. Serum Level and Activity of Butylcholinesterase: A Biomarker for Post-Stroke Dementia. J. Clin. Med. 2019, 8, E1778. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Saito, H.; Minami, M.; Togashi, H.; Nakamura, N.; Nemoto, M.; Parvez, H.S. Pathogenesis of vascular dementia in stroke-prone spontaneously hypertensive rats. Toxicology 2000, 153, 167–187. [Google Scholar] [CrossRef]
- Furukawa, S.; Sameshima, H.; Yang, L.; Ikenove, T. Activation of acetylcholine receptros and microglia in hypoxic-ischemic brain damage in newborn rats. Brain Dev. 2013, 35, 607–613. [Google Scholar] [CrossRef]
- Hejmadi, M.V.; Dajas-BAilador, F.; Barns, S.M.; Jones, B.; Wonnacott, S. Neuroprotection by nicotine against hypoxia-induced apopotosis in cortical cultures involves activation of multiple nicotinic acethylcholine receptor subtypes. Mol. Cell. Neurosci. 2003, 24, 779–786. [Google Scholar] [CrossRef]
- Albuquerque, E.X.; Pereira, E.F.; Alkondon, M.; Rogers, S.W. Mammalian nicotinic acetylcholine receptors: From structure to function. Physiol. Rev. 2009, 89, 73–120. [Google Scholar] [CrossRef]
- Alkondon, M.; Pereira, E.F.; Eisenberg, H.M.; Albuquerque, E.X. Choline and selective antagonists identify two subtypes of nicotinic acetylcholine receptors that modulate GABA release from CA1 interneurons in rat hippocampal slices. J. Neurosci. 1999, 19, 2693–2705. [Google Scholar] [CrossRef]
- Alkondon, M.; Albuquerque, E.X. The nicotinic acetylcholine receptor subtypes and their function in the hippocampus and cerebral cortex. Prog. Brain Res. 2004, 145, 109–120. [Google Scholar]
- Roman, G.C.; Kalaria, R.N. Vascular determinants of cholinergic deficits in AD and vascular dementia. Neurobiol. Aging 2006, 27, 1769–1785. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, E.; Afkhami Aghda, F.; Rezvani, M.E.; Shahrokhi Raeini, A.; Hafizibarjin, Z.; Zare Mehrjerdi, F. Effect of endogenous sulfur dioxide on spatial learning and memory and hippocampal damages in the experimental model of chronic cerebral hypoperfusion. J. Basic Clin. Physiol. Pharmacol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, J.; Purve, M.J. The cholinergic pathway to cerebral blood vessels. I. Morphological studies. Pflugers Arch. 1979, 379, 157–163. [Google Scholar] [CrossRef]
- Tong, X.K.; Hamel, E. Regional cholinergic denervation of cortical microvessels and nitric oxid synthase-containing neurons in, A.D. Neuroscience 1999, 92, 163–175. [Google Scholar] [CrossRef]
- Cauli, B.; Tong, X.K.; Rancillac, A.; Serluca, N.; Lambolez, B.; Rossier, J.; Hamel, E. Cortical GABA interneurons in neurovascular coupling: Relays for the subcortical vasoactive pathways. J. Neurosci. 2004, 24, 8940–8949. [Google Scholar] [CrossRef]
- Vanhoutte, P.M. Endothelium and control of vascular function. State of the Art lecture. Hypertension 1989, 13, 658–667. [Google Scholar] [CrossRef]
- Kocharyan, A.; Fernandes, P.; Tong, X.P.; Vaucher, E.; Hamel, E. Specific subtypes of cortical GABA interneurons contribute to the neurovascular coupling response to basal forebrain stimulation. J. Cereb. Blood Flow Metab. 2008, 28, 221–231. [Google Scholar] [CrossRef]
- Lacombe, P.; Sercombe, R.; Vaucher, E.; Seylaz, J. reduced cortical vasodilatory response to stimulation of the nucleus basalis of Meynert in the aged rats and evidence for a control of the cerebral circulation. Ann. N. Y. Acad. Sci. 1997, 826, 410–415. [Google Scholar] [CrossRef]
- Elhusseiny, A.; Hamel, E. muscarinic but not nicotinic acetylcholine receptros mediated nitric oxide dependent dilatation in brain cortical arterioles: A possible role for the M5 receptor subtype. J. Cereb. Blood Flow Metab. 2000, 20, 298–305. [Google Scholar] [CrossRef]
- Katusic, Z.S.; Austin, S.A. Endothelial nitric oxide: Protector of a healthy mind. Eur. Heart J. 2014, 35, 888–894. [Google Scholar] [CrossRef]
- Hamner, J.W.; Tan, C.O.; Lee, K.; Cohen, M.A.; Taylor, J.A. Sympathetic control of the cerebral vasculature in humans. Stroke 2010, 41, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.H.; Ju, G.S.; Chung, Y.Y.; Shin, H.K.; Kim, D.J.; Choi, M.S.; Kim, S.T.; Son, K.M. Differential Expression of Vascular Endothelial Growth Factor in the Cortex and Hippocampus upon Cerebral Hypoperfusion. In Vivo 2020, 34, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Yang, G.; Ebner, T.J.; Chen, G. Local and propagated vascular responses evoked by focal synaptic activity in cerebellar cortex. J. Neurophysiol. 1997, 78, 651–659. [Google Scholar] [CrossRef]
- Tomimoto, H.; Ohtani, R.; Shibata, M.; Nakamura, N.; Ihara, M. Loss of cholinergic pathways in vascular dementia of the Binswanger type. Dement. Geriatr. Cogn. Disord. 2005, 19, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, H.Y.; Tang, X.C. Cholinergic deficiency involved in vascular dementia: Possible mechanism and strategy of treatment. Acta Pharamcol. Sin. 2009, 30, 879–888. [Google Scholar] [CrossRef]
- Mann, D.M.; Yates, P.D.; Marcyniuk, B. The Nucleus Basalis of Meynert in multi-infarct dementia. Acta Neuropathol. 1986, 71, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Zarow, C.; Mack, W.J.; Zheng, L.; Vinters, H.V.; Ellis, W.G.; Lyness, S.A.; Chui, H.C. Preservation of neurons of the nucleus basalis in subcortical ischemic vascular disease. Arch Neurol. 2012, 69, 879–886. [Google Scholar] [CrossRef][Green Version]
- Swartz, R.H.; Sahlas, D.J.; Black, S.E. Strategic involvement of cholinergic pathways and executive dysfunction: Does location of white matter signal hyperintensities matter? J. Stroke Cerebrovasc. Dis. 2003, 12, 29–36. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Smith, C.; Dichgans, M. Mechanism of sporadic cerebral small vessel disease: Insight from neuroimaging. Lancet Neurol. 2013, 12, 483–497. [Google Scholar] [CrossRef]
- De Silva, T.M.; Miller, A.A. Cerebral small vessel disease: Targeting oxidative stress as a novel therapeutic strategy. Front. Pharmacol. 2016, 7, 61. [Google Scholar] [CrossRef]
- Chan, S.L.; Umesalma, S.; Baumbach, G.L. Epidermal growth factor receptor is critical for angiotensin II mediated hypertrophy in cerebral arterioles. Hypertension 2015, 65, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Umesalma, S.; Houwen, F.K.; Baumbach, G.L.; Chan, S.L. Roles of ceveolin-1 in angiotensin-ii induced hypertrophy and inward remodeling of cerebral pial arterioles. Hypertension 2016, 67, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Attwell, D.; Buchan, A.M.; Charpak, S.; Lauritzen, M.; Macvicar, B.A.; Newman, E.A. Glial and neuronal control of brain blood flow. Nature 2010, 468, 232–243. [Google Scholar] [CrossRef]
- Cipolla, M.J. The cerebral circulation. In Integrated Systems Physiology: From Molecule to Function; Granger, D.N.G., Granger, J., Eds.; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2009; pp. 1–59. [Google Scholar]
- Jackman, K.; Iadecola, C. Neurovascular regulation in the ischemic brain. Antioxid. Redox Signal. 2014, 10, 149–160. [Google Scholar] [CrossRef]
- Li, W.Z.; Wu, W.Y.; Huang, H.; Wu, Y.Y.; Yin, Y.Y. Protective effect of bilobalide on learning and memory impairment in rats with vascular dementia. Mol. Med. Rep. 2013, 8, 935–941. [Google Scholar] [CrossRef]
- Ma, X.; Sun, Z.; Liu, Y.; Jia, Y.; Zhang, B.; Zhang, J. Resveratrol improves cognition and reduces oxidative stress in rats with vascular dementia. Neural Regen. Res. 2013, 8, 2050–2059. [Google Scholar]
- Gill, R.; Tsung, A.; Billiar, T. Linking oxidative stress to inflammation: Toll-like receptors. Free Radic. Biol. Med. 2010, 48, 1121–1132. [Google Scholar] [CrossRef]
- Tomimoto, H.; Ihara, M.; Wakita, H.; Ohtani, R.; Lin, X.J.; Akiguchi, I.; Kinoshita, M.; Shibasaki, H. Chronic cerebral hypoperfusion induces white matter lesions and loss of oligodendroglia with DNA fragmentation in the rat. Acta Neuropathol. 2003, 106, 527–534. [Google Scholar] [CrossRef]
- Brian, J.E.; Faraci, F.M.; Heistad, D.D. Recent insights into the regulation of cerebral circulation. Clin. Exp. Pharmacol. Physiol. 1996, 23, 449–457. [Google Scholar] [CrossRef]
- Sillau, H.A.; Mccullough, R.E.; Dyckes, R.; White, M.M.; Moore, L.G. Chronic hypoxia increases MCA contractile response to U-46619 by reducing NO production and activity. J. Appl. Physiol. 2001, 92, 1859–1864. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, J.; Xiong, L.; Deng, M.; Wang, J.; Xin, J.; Liu, H. Cognitive improvement induced by environment enrichment in chronic cerebral hypoperfusion rats: A result of upregulated endogenous neuroprotection? J. Mol. Neurosci. 2015, 56, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Farkas, E.; Luiten, P.G.; Bari, F. Permanent, bilateral common carotid artery occlusion in the rat: A model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res. Rev. 2007, 54, 162–180. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, J. Cerebral hypoperfusion and cognitive impairment: The pathogenic role of vascular oxidative stress. Int. J. Neurosci. 2012, 122, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Choy, M.; Ganesan, V.; Thomas, D.L.; Thornton, J.S.; Proctor, E.; King, M.D.; van der Weerd, L.; Gadian, D.G.; Lythgoe, M.F. The chronic vascular and hemodynamic response after permanent bilateral common carotid occlusion in newborn and adult rats. J. Cereb. Blood Flow. Metab. 2006, 26, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Manalo, D.J.; Rowan, A.; Lavoie, T.; Natarajan, L.; Kelly, B.D.; Ye, S.Q.; Garcia, J.G.; Semenza, G.L. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood 2005, 105, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Correia, S.C.; Moreira, P.I. Hypoxia-inducible factor 1: A new hope to counteract neurodegeneration? J. Neurochem. 2010, 112, 1–12. [Google Scholar] [CrossRef]
- Benarroch, E.E. Hypoxia-induced mediators, and neurologic disease. Neurology 2009, 73, 560–565. [Google Scholar] [CrossRef]
- Yang, Y.; Ju, J.; Deng, M.; Wang, J.; Liu, H.; Xiong, L.; Zhang, J. Hypoxia Inducible Factor 1 Promotes Endogenous Adaptive Response in Rat Model of Chronic Cerebral Hypoperfusion. Int. J. Mol. Sci. 2017, 18, 3. [Google Scholar] [CrossRef]
- Bauer, A.T.; Burgers, H.F.; Rabie, T.; Marti, H.H. Matrix metalloproteinase-9 mediates hypoxia-induced vascular leakage in the brain via tight junction rearrangement. J. Cereb. Blood Flow Metab. 2010, 30, 837–848. [Google Scholar] [CrossRef]
- Rolett, E.L.; Azzawi, A.; Liu, K.J.; Yongbi, M.N.; Swartz, H.M.; Dunn, J.F. Critical oxygen tension in rat brain: A combined 31P-NMR and EPR oximetry study. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R9–R16. [Google Scholar] [CrossRef]
- Erecinska, M.; Silver, I.A. Tissue oxygen tension, and brain sensitivity to hypoxia. Respir. Physiol. 2001, 128, 263–276. [Google Scholar] [CrossRef]
- Flamme, I.; Frohlich, T.; von Reutern, M.; Kappel, A.; Damert, A.; Risau, W. HRF, a putative basic helix-loop-helix-PAS-domain transcription factor is closely related to hypoxia-inducible factor-1 and developmentally expressed in blood vessels. Mech. Dev. 1997, 63, 51–60. [Google Scholar] [CrossRef]
- Jalal, F.Y.; Yang, Y.; Thompson, J.F.; Roitbak, T.; Rosenberg, G.A. Hypoxia-induced neuroinflammatory white-matter injury reduced by minocycline in SHR/SP. J. Cereb. Blood Flow Metab. 2015, 35, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Craggs, L.J.; Hagel, C.; Kuhlenbaeumer, G.; Borjesson-Hanson, A.; Andersen, O.; Viitanen, M.; Kalimo, H.; McLean, C.A.; Slade, J.Y.; Hall, R.A.; et al. Quantitative vascular pathology and phenotyping familial and sporadic cerebral small vessel diseases. Brain Pathol. 2013, 23, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Hainsworth, A.H.; Oommen, A.T.; Bridges, L.R. Endothelial Cells and Human Cerebral Small Vessel Disease. Brain Pathol. 2015, 25, 44–50. [Google Scholar] [CrossRef]
- Frischer, J.M.; Pipp, I.; Stavrou, I.; Trattnig, S.; Hainfellner, J.A.; Knosp, E. Cerebral cavernous malformations: Congruency of histopathological features with the current clinical definition. J. Neurol. Neurosurg. Psychiatry 2008, 79, 783–788. [Google Scholar] [CrossRef]
- Giwa, M.O.; Williams, J.; Elderfield, K.; Jiwa, N.S.; Bridges, L.R.; Kalaria, R.N.; Markus, H.S.; Esiri, M.M.; Hainsworth, A.H. Neuropathologic evidence of endothelial changes in cerebral small vessel disease. Neurology 2012, 78, 167–174. [Google Scholar] [CrossRef]
- Zlokovic, B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011, 12, 723–728. [Google Scholar] [CrossRef]
- Cai, W.; Zhang, K.; Li, P.; Zhu, L.; Xu, J.; Yang, B.; Hu, X.; Lu, Z.; Chen, J. Dysfunction of the neurovascular unit in ischemic stroke and neurodegenerative diseases: An aging effect. Ageing Res. Rev. 2017, 34, 77–87. [Google Scholar] [CrossRef]
- Prisby, R.D.; Ramsey, M.W.; Behnke, B.J.; Dominguez, J.M.; Donato, A.J.; Allen, M.R.; Delp, M.D. Aging reduces skeletal blood flow endothelium dependent vasodilation, and NO bioavailability in Rats. J. Bone Miner. Res. 2007, 22, 1280–1288. [Google Scholar] [CrossRef]
- Nicholson, W.T.; Vaa, B.; Hesse, C.; Eisenach, J.H.; Joyner, M.J. Aging is associated with reduced prostacyclin-mediated dilation in the human forearm. Hypertenison 2009, 53, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Long, D.A.; Newaz, M.A.; Prabahakar, S.S.; Price, K.L.; Truong, L.; Feng, L.; Mu Oyekan, A.O.; Johnson, R.J. Loss of nitric oxide and endothelial-derived hyperpolarizing factor-mediated responses in ageing. Kidney Int. 2005, 68, 2154–2163. [Google Scholar] [CrossRef] [PubMed]
- Van der Loo, B.; Labugger, R.; Skepper, J.N.B.; Achschmid, M.; Kilo, J.; Powell, J.M.; Palacios-Callendere, M.; Erusalimsky, J.D.; Quaschning, T.; Malinski, T. Enhanced peroxynitrite formation is associated with vascular ageing. J. Exp. Med. 2000, 18, 1731–1744. [Google Scholar] [CrossRef]
- Puca, A.A.; Carrizzo, A.; Ferrario, A.; Villa, F.; Vecchione, C. Endothelial nitric oxide synthase, vascular integrity and human exceptional longevity. Immun. Ageing 2012, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Flentje, A.; Kalsi, R.; Monahan, T.S. Small GTPases and Their Role in Vascular Disease. Int. J. Mol. Sci. 2019, 20, 917. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, S.; Ridley, A.J.; Lutz, S. The Function of Rho-Associated Kinases ROCK1 and ROCK2 in the Pathogenesis of Cardiovascular Disease. Front. Pharmacol. 2015, 6, 276. [Google Scholar] [CrossRef]
- Pestonjamasp, K.; Amieva, M.R.; Strassel, C.P.; Nauseef, W.M.; Furthmayr, H.; Luna, E.J. Moesin, ezrin, and p205 are actin-binding proteins associated with neutrophil plasma membranes. Mol. Biol. Cell. 1995, 6, 247–259. [Google Scholar] [CrossRef]
- Cicek, F.A.; Kandilci, H.B.; Turan, B. Role of ROCK upregulation in endothelial and smooth muscle vascular functions in diabetic rat aorta. Cardiovasc. Diabetol. 2013, 12, 51. [Google Scholar] [CrossRef]
- Noma, K.; Oyama, N.; Liao, J.K. Physiological role of ROCKs in the cardiovascular system. Am. J. Physiol. Cell Physiol. 2006, 290, C661–C668. [Google Scholar] [CrossRef]
- Hassan, A.; Gormley, K.; O’Sullivan, M.; Knight, J.; Sham, P.; Vallance, P.; Bamford, J.; Markus, H. Endothelial Nitric Oxide Gene Haplotypes and Risk of Cerebral Small-Vessel Disease. Stroke 2004, 35, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Van Nieuw Amerongen, G.P.; Koolwijk, P.; Versteilen, A.; van Hinsbergh, V.W. Involvement of RhoA/Rho kinase signaling in VEGF-induced endothelial cell migration and angiogenesis in vitro. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Uwatoku, T.; Oi, K.; Abe, K.; Hattori, T.; Morishige, K.; Eto, Y.; Fukumoto, Y.; Nakamura, K.I.; Shibata, Y.; et al. Long-term inhibition of Rho-kinase suppresses neointimal formation after stent implantation in porcine coronary arteries: Involvement of multiple mechanisms. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Szulcek, R.; Beckers, C.M.; Hodzic, J.; de Wit, J.; Chen, Z.; Grob, T.; Musters, R.J.; Minshall, R.D.; van Hinsbergh, V.W.; van Nieuw Amerongen, G.P. Localized RhoA GTPase activity regulates dynamics of endothelial monolayer integrity. Cardiovasc. Res. 2013, 99, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Van Nieuw Amerongen, G.P.; Beckers, C.M.; Achekar, I.D.; Zeeman, S.; Musters, R.J.; van Hinsbergh, V.W. Involvement of Rho kinase in endothelial barrier maintenance. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2332–2339. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, H.; Chen, B.; Li, Q.; Huang, X.; Wang, L.; Guo, X.; Huang, Q. RhoA/ROCK-dependent moesin phosphorylation regulates AGE-induced endothelial cellular response. Cardiovasc. Diabetol. 2012, 11, 7. [Google Scholar] [CrossRef]
- Sun, H.; Breslin, J.W.; Zhu, J.; Yuan, S.Y.; Wu, M.H. Rho and ROCK signaling in VEGF-induced microvascular endothelial hyperpermeability. Microcirculation 2006, 13, 237–247. [Google Scholar] [CrossRef]
- Gradinaru, D.; Borsa, C.; Ionescu, C.; Prada, G.I. Oxidized LDL and NO synthesis-biomarkers of endothelial dysfunction and ageing. Mech. Ageing Dev. 2015, 151, 101–113. [Google Scholar] [CrossRef]
- Deplanque, D.; Lavallee, P.C.; Labreuche, J.; Gongora-Rivera, F.; Jaramillo, A.; Brenner, D.; Abboud, H.; Klein, I.F.; Touboul, P.J.; Vicaut, E.; et al. Cerebral and extracerebral vasoreactivity in symptomatic lacunar stroke patients: A case-control study. Int. J. Stroke 2013, 8, 413–421. [Google Scholar] [CrossRef]
- Gunarathne, A.; Patel, J.V.; Kausar, S.; Gammon, B.; Hughes, E.A.; Lip, G.Y. Glycemic status underlies increased arterial stiffness and impaired endothelial function in migrant South Asian stroke survivors compared to European Caucasians: Pathophysiological insights from the West Birmingham Stroke Project. Stroke 2009, 40, 2298–2306. [Google Scholar] [CrossRef]
- Markus, H.S.; Lythgoe, D.J.; Ostegaard, L.; O’Sullivan, M.; Williams, S.C. Reduced cerebral blood flow in white matter in ischaemic leukoaraiosis demonstrated using quantitative exogenous contrast based perfusion MRI. J. Neurol. Neurosurg. Psychiatry 2000, 69, 48–53. [Google Scholar] [CrossRef]
- O’Sullivan, M.; Lythgoe, D.J.; Pereira, A.C.; Summers, P.E.; Jarosz, J.M.; Williams, S.C.; Markus, H.S. Patterns of cerebral blood flow reduction in patients with ischemic leukoaraiosis. Neurology 2002, 59, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Markus, H.S.; Allan, C.L.; Ebmeier, K.P. Cerebral hemodynamics in cerebral small vessel disease. In Cerebral Small Vessel Disease; Pantoni, L., Gorelick, P.B., Eds.; Cambridge University Press: Cambridge, UK, 2014; pp. 180–191. [Google Scholar]
- Gallin, J.I.; Snyderman, R. Inflammation: Basic Principles and Clinical Correlates, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1999. [Google Scholar]
- de Leeuw, F.E.; de Kleine, M.; Frijns, C.J.; Fijnheer, R.; van Gijn, J.; Kappelle, L.J. Endothelial cell activation is associated with cerebral white matter lesions in patients with cerebrovascular disease. Ann. N. Y. Acad. Sci. 2002, 977, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Rouhl, R.P.; van Oostenbrugge, R.J.; Theunissen, R.O.; Knottnerus, I.L.; Staals, J.; Henskens, L.H. Autoantibodies against oxidized low-density lipoprotein in cerebral small vessel disease. Stroke 2010, 41, 2687–2689. [Google Scholar] [CrossRef] [PubMed]
- Wada, M.; Takahashi, Y.; Iseki, C.; Kawanami, T.; Daimon, M.; Kato, T. Plasma fibrinogen, global cognitive function, and cerebral small vessel disease: Results of a cross-sectional study in community-dwelling Japanese elderly. Int. Med. 2011, 50, 999–1007. [Google Scholar] [CrossRef][Green Version]
- Markus, H.S.; Hunt, B.; Palmer, K.; Enzinger, C.; Schmidt, H.; Schmidt, R. Markers of endothelial and hemostatic activation and progression of cerebral white matter hyperintensities: Longitudinal results of the Austrian Stroke Prevention Study. Stroke 2005, 36, 1410–1414. [Google Scholar] [CrossRef]
- Knottnerus, I.L.; Govers-Riemslag, J.W.; Hamulyak, K.; Rouhl, R.P.; Staals, J.; Spronk, H.M. Endothelial activation in lacunar stroke subtypes. Stroke 2010, 41, 1617–1622. [Google Scholar] [CrossRef][Green Version]
- Knottnerus, I.L.; Cate, H.; Lodder, J.; Kessels, F.; van Oostenbrugge, R.J. Endothelial dysfunction in lacunar stroke: A systematic review. Cerebrovasc. Dis. 2009, 27, 519–526. [Google Scholar] [CrossRef]
- Stevenson, S.F.; Doubal, F.N.; Shuler, K.; Wardlaw, J.M. A systematic review of dynamic cerebral and peripheral endothelial function in lacunar stroke versus controls. Stroke 2010, 41, e434–e442. [Google Scholar] [CrossRef]
- Fernando, M.S.; Simpson, J.E.; Matthews, F.; Brayne, C.; Lewis, C.E.; Barber, R.; Kalaria, R.N.; Forster, G.; Esteves, F.; Wharton, S.B.; et al. White matter lesions in an unselected cohort of the elderly: Molecular pathology suggests origin from chronic hypoperfusion injury. Stroke 2006, 37, 1391–1398. [Google Scholar] [CrossRef]
- Esiri, M.M.; Wilcock, G.K.; Morris, J.H. Neuropathological ssessment of the lesions of significance in vascular dementia. J. Neurol. Neurosurg. Psychiatry 1997, 63, 749–753. [Google Scholar] [CrossRef]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013, 9. [Google Scholar] [CrossRef]
- Drake, C.T.; Iadecola, C. The role of the neuronal signaling in controlling cerebral blood flow. Brain Lang 2007, 102, 141–152. [Google Scholar] [CrossRef]
- Iadecola, C.; Duering, M.; Hachinski, V.; Joutel, A.; Pendlebury, S.T.; Schneider, J.A.; Dichgans, M. Vascular Cognitive Impairment and Dementia: JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 2019, 2, 3326–3344. [Google Scholar] [CrossRef]
- Tallini, Y.N.; Brekke, J.F.; Shui, B.; Doran, R.; Hwang, S.M.; NAkai, J.; Salama, G.; Segal, S.S.; Kotlikoff, M.I. Propagated endothelial Ca++ waves and arteriolar dilatation in vivo: Measurements in Cx40 BAC GCaMP2 transgenic mice. Circ. Res. 2007, 7, 1300–1309. [Google Scholar] [CrossRef]
- Segal, S.S. Integration and modulation of intracellular signaling underlying blood flow control. J. Vasc. Res. 2015, 52, 136–157. [Google Scholar] [CrossRef] [PubMed]
- Hen, B.P.; Kozberg, M.G.; Bouchard, M.B.; Shaik, M.A.; Hillman, E.M.C. A critical role for the vascular endothelium in functional neurovascular coupling in the brain. J. Am. Heart Assoc. 2014, 3, e000787. [Google Scholar] [CrossRef]
- Longden, T.A.; Hill-Eubanks, D.C.; Nelosn, M.T. ion channel networks in the control of cerebral blood flow. J. Cereb. Blood Flow Metab. 2016, 36, 492–512. [Google Scholar] [CrossRef] [PubMed]
- Bagher, P.; Segal, S.S. Regulation of blood flow in the microcirculation: Role of the conducted vasodilation. Acta Physiol. 2011, 202, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Uhurovoa, H.; Kilic, K.; Tian, P.; Thunemann, M.; Desjardins, M.; Desjardins, M.; Saisan, P.A.; Sakadžić, S.; Ness, T.V.; Mateo, C.; et al. Cell-type specificity of neurovascular coupling in cerebral cortex. eLife 2016, 5, 155. [Google Scholar] [CrossRef]
- Longden, T.A.; Dabertrand, F.; Koide, M.; Gonzales, A.L.; Tykochi, N.T.; Brayden, J.E.; Hill-Eubanks, D.; Nelosn, M.T. Capillary K+ sensing initiates retrograde hyperpolarization to increase local cerebral blood flow. Nat. Neurosci. 2017, 275, 717–726. [Google Scholar] [CrossRef]
- Chung, W.S.; Barres, B.A. The role of glial cells in synapse elimination. Curr. Opin. Neurobiol. 2012, 22, 438–445. [Google Scholar] [CrossRef]
- Wolburg, H.; Noell, S.; Wolburg-Buchholz, K.; Mack, A.; Fallier-Becker, P. Agrin, Aquaporin-4, and Astrocyte Polarity as an Important Feature of the Blood-Brain Barrier. Neuroscientist 2009, 15, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Esposito, E.; Wang, X.; Terasaki, Y.; Liu, Y.; Xing, C.; Ji, X.; Lo, E.H. Transfer of mitochondria from astrocytes to neurons after stroke. Nature 2016, 535, 551–555. [Google Scholar] [CrossRef]
- Brix, B.; Mesters, J.R.; Pellerin, L.; Johren, O. Endothelial cell-derived nitric oxide enhances aerobic glycolysis in astrocytes via HIF-1alpha-mediated target gene activation. J. Neurosci. 2012, 32, 9727–9735. [Google Scholar] [CrossRef]
- Allaman, I.; Belanger, M.; Magistretti, P.J. Astrocyte-neuron metabolic relationships: For better and for worse. Trends Neurosci. 2011, 34, 76–87. [Google Scholar] [CrossRef]
- Sidoryk-Wegrzynowicz, M.; Wegrzynowicz, M.; Lee, E.; Bowman, A.B.; Aschner, M. Role of astrocytes in brain function and disease. Toxicol. Pathol. 2011, 39, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 2009, 32, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Phatnani, H.; Maniatis, T. Astrocytes in Neurodegenerative Disease. Cold Spring Harb. Perspect. Biol. 2015, 7, a020628. [Google Scholar] [CrossRef]
- Nagele, R.G.; D’Andrea, M.R.; Lee, H.; Venkataraman, V.; Wang, H.Y. Astrocytes accumulate Ab42 and give rise to astrocytic amyloid plaques in Alzheimer’s disease brains. Brain Res. 2003, 971, 197–209. [Google Scholar] [CrossRef]
- Olabarria, M.; Noristani, H.N.; Verkhratsky, A.; Rodriguez, J.J. Concomitant astroglial atrophy and astrogliosis in a triple transgenic animal model of Alzheimer’s disease. Glia 2010, 58, 831–838. [Google Scholar] [CrossRef]
- Simpson, J.E.; Ince, P.G.; Lace, G.; Forster, G.; Shaw, P.J.; Matthews, F.; Savva, G.; Brayne, C.; Wharton, S.B.; Function, M.R.C.C.; et al. Astrocyte phenotype concerning Alzheimer-type pathology in the ageing brain. Neurobiol. Aging 2010, 31, 578–590. [Google Scholar] [CrossRef]
- Wyss-Coray, T.; Loike, J.D.; Brionne, T.C.; Lu, E.; Anankov, R.; Yan, F.; Silverstein, S.C.; Husemann, J. Adult mouse astrocytes degrade amyloid-b in vitro and in situ. Nat. Med. 2003, 9, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Koistinaho, M.; Lin, S.; Wu, X.; Esterman, M.; Koger, D.; Hanson, J.; Higgs, R.; Liu, F.; Malkani, S.; Bales, K.R.; et al. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-b peptides. Nat. Med. 2004, 10, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Kuchibhotla, K.V.; Lattarulo, C.R.; Hyman, B.T.; Bacskai, B.J. Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science 2009, 323, 1211–1215. [Google Scholar] [CrossRef] [PubMed]
- Nagy, J.I.; Li, W.; Hertzberg, E.L.; Marotta, C.A. Elevated connexin43 immunoreactivity at sites of amyloid plaques in Alzheimer’s disease. Brain Res. 1996, 717, 173–178. [Google Scholar] [CrossRef]
- Mei, X.; Ezan, P.; Giaume, C.; Koulakoff, A. Astroglial connexin immunoreactivity is specifically altered at b-amyloid plaques in b-amyloid precursor protein/presenilin1 mice. Neuroscience 2010, 171, 92–105. [Google Scholar] [CrossRef]
- Orellana, J.A.; Froger, N.; Ezan, P.; Jiang, J.X.; Bennett, M.V.; Naus, C.C.; Giaume, C.; Saez, J.C. ATP and glutamate released via astroglial connexin 43 hemichannels mediate neuronal death through activation of pannexin 1 hemichannels. J. Neurochem. 2011, 118, 826–840. [Google Scholar] [CrossRef]
- Orellana, J.A.; Shoji, K.F.; Abudara, V.; Ezan, P.; Amigou, E.; Saez, P.J.; Jiang, J.X.; Naus, C.C.; Saez, J.C.; Giaume, C. Amyloid b-induced death in neurons involves glial and neuronal hemichannels. J. Neurosci. 2011, 31, 4962–4977. [Google Scholar] [CrossRef]
- Zareha, M.; Manshaeia, M.M.; Adibib, M.; Montazeria, M.A. Neurons and astrocytes interaction in neuronal network: A game-theoretic approach. J. Theor. Biol. 2019, 470, 76–89. [Google Scholar] [CrossRef]
- Molofsky, A.V.; Krencik, R.; Ullian, E.M.; Tsai, H.H.; Deneen, B.; Richardson, W.D.; Barres, B.A.; Rowitch, D.H. Astrocytes and disease: A neurodevelopmental perspective. Genes Dev. 2012, 26, 891–907. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.H.; Li, H.; Fuentealba, L.C.; Molofsky, A.V.; Taveira-Marques, R.; Zhuang, H.; Tenney, A.; Murnen, A.T.; Fancy, S.P.; Merkle, F.; et al. Regional astrocyte allocation regulates synaptogenesis and repair. CNS Sci. 2012, 337, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Price, B.R.; Norris, C.M.; Sompol, P.; Wilcock, D.M. An emerging role of astrocytes in vascular contributions to cognitive impairment and dementia. J. Neurochem. 2018, 144, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Akiguchi, I.; Tomimoto, H.; Suenaga, T.; Wakita, H.; Budka, H. Alterations in glia and axons in the brains of Binswanger’s disease patients. Stroke 1997, 28, 1423–1429. [Google Scholar] [CrossRef]
- Price, D.L.; Ludwig, J.W.; Mi, H.; Schwarz, T.L.; Ellisman, M.H. Distribution of rSlo Ca2+-activated K+ channels in rat astrocyte perivascular endfeet. Brain Res. 2002, 956, 183–193. [Google Scholar] [CrossRef]
- Wilcock, D.M.; Vitek, M.P.; Colton, C.A. Vascular amyloid alters astrocytic water and potassium channels in mouse models and humans with Alzheimer’s disease. Neuroscience 2009, 159, 1055–1069. [Google Scholar] [CrossRef]
- Noell, S.; Wolburg-Buchholz, K.; Mack, A.F.; Beedle, A.M.; Satz, J.S.; Campbell, K.P.; Wolburg, H.; Fallier-Becker, P. Evidence for a role of dystroglycan regulating the membrane architecture of astroglial endfeet. Eur. J. Neurosci. 2011, 33, 2179–2186. [Google Scholar] [CrossRef]
- Gondo, A.; Shinotsuka, T.; Morita, A.; Abe, Y.; Yasuihannels, M.; Nuriya, M. Sustained down-regulation of beta-dystroglycan and associated dysfunctions of astrocytic endfeet in epileptic cerebral cortex. J. Biol. Chem. 2014, 289, 30279–30288. [Google Scholar] [CrossRef]
- Michaluk, P.; Kolodziej, L.; Mioduszewska, B.; Wilczynski, G.M.; Dzwonek, J.; Jaworski, J.; Gorecki, D.C.; Ottersen, O.P.; Kaczmarek, L. Beta-dystroglycan as a target for MMP-9, in response to enhanced neuronal activity. J. Biol. Chem. 2007, 282, 16036–16041. [Google Scholar] [CrossRef]
- Yin, K.J.; Cirrito, J.R.; Yan, P.; Hu, X.; Xiao, Q.; Pan, X.; Bateman, R.; Song, H.; Hsu, F.F.; Turk, J.; et al. Matrix metalloproteinases expressed by astrocytes mediate extracellular amyloid-beta peptide catabolism. J. Neurosci. 2006, 26, 10939–10948. [Google Scholar] [CrossRef]
- Weekman, E.M.; Wilcock, D.M. Matrix metalloproteinase in blood-brain barrier breakdown in dementia. J. Alzheimers Dis. 2016, 49, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Sudduth, T.L.; Powell, D.K.; Smith, C.D.; Greenstein, A.; Wilcock, D.M. Induction of hyperhomocysteinemia models vascular dementia by induction of cerebral microhemorrhages and neuroinflammation. J. Cereb. Blood Flow Metab. 2013, 33, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Sudduth, T.L.; Weekman, E.M.; Brothers, H.M.; Braun, K.; Wilcock, D.M. Beta-amyloid deposition is shifted to the vasculature and memory impairment is exacerbated when hyperhomocysteinemia is induced in APP/PS1 transgenic mice. Alzheimers Res. Ther. 2014, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Sudduth, T.L.; Weekman, E.M.; Price, B.R.; Gooch, J.L.; Woolums, A.; Norris, C.M.; Wilcock, D.M. Time-course of glial changes in the hyperhomocysteinemia model of vascular cognitive impairment and dementia (VCID). Neuroscience 2017, 341, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, B.; Nie, K.; Jia, Y.; Yu, J. Effects of acupuncture on declined cerebral blood flow, impaired mitochondrial respiratory function and oxidative stress in multi-infarct dementia rats. Neurochem. Int. 2014, 65, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.L.; Fu, S.T.; Jiang, Y.Y.; Cao, Y.B.; Guo, M.L.; Wang, Y.; Xu, Z. Protective effects of Nicotiflorin on reducing memory dysfunction, energy metabolism failure, and oxidative stress in multi-infarct dementia model rats. Pharmacol. Biochem. Behav. 2007, 86, 741–748. [Google Scholar] [CrossRef]
- Halliwell, B. Role of free radicals in the neurodegenerative diseases: Therapeutic implications for antioxidant treatment. Drugs Aging 2001, 18, 685–716. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Raz, N. The aging brain: Structural changes and their implications for cognitive aging. In New Frontiers in Cognitive Aging; Dixon, R., Bäckman, L., Nilsson, L., Eds.; Oxford University Press: Telangana, India, 2004; pp. 115–134. [Google Scholar]
- Touyz, R.M.; Briones, A.M. Reactive oxygen species and vascular biology: Implications in human hypertension. Hypertens. Res. 2011, 34, 5–14. [Google Scholar] [CrossRef]
- Ushio-Fukai, M.; Alexander, R.W. Reactive oxygen species as mediators of angiogenesis signaling: Role of NAD(P)H oxidase. Mol. Cell. Biochem. 2004, 264, 85–97. [Google Scholar] [CrossRef]
- Takac, I.; Schr¨oder, K.; Brandes, R.P. The Nox family of NADPH oxidases: Friend or foe of the vascular system? Curr. Hypertens. Rep. 2012, 14, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.A.; Drummond, G.R.; DeSilva, T.M.; Mast, A.E.; Hickey, H.; Williams, J.P.; Broughton, B.R.; Sobey, C.G. NADPH oxidase activity is higher in cerebral versus systemic arteries of four animal species: Role of Nox2. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H220–H225. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.A.; Drummond, G.R.; Schmidt, H.H.; Sobey, C.G. NADP Hoxidase activity and function are profoundly greater in cerebral versus systemic arteries. Circ. Res. 2005, 97, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Paravicini, T.M.; Miller, A.A.; Drummond, G.R.; Sobey, C.G. Flow-induced cerebral vasodilatation in vivo involves activation of phosphatidylinositol-3kinase, NADPH-oxidase, and nitric oxide synthase. J. Cereb. Blood Flow Metab. 2006, 26, 836–845. [Google Scholar] [CrossRef]
- De Silva, T.M.; Brait, V.H.; Drummond, G.R.; Sobey, C.G.; Miller, A.A. Nox2 oxidase activity accounts for the oxidative stress and vasomotor dysfunction in mouse cerebral arteries following ischemic stroke. PLoS ONE 2011, 6, e28393. [Google Scholar] [CrossRef] [PubMed]
- Mayhan, W.G.; Arrick, D.M.; Sharpe, G.M.; Sun, H. Age-related alterations in reactivity of cerebral arterioles: Role of oxidative stress. Microcirculation 2008, 15, 225–236. [Google Scholar] [CrossRef]
- Dong, Y.F.; Kataoka, K.; Toyama, K.; Sueta, D.; Koibuchi, N.; Yamamoto, E.; Yata, K.; Tomimoto, H.; Ogawa, H.; Kim-Mitsuyama, S. Attenuation of brain damage and cognitive impairment by direct renin inhibition in mice with chronic cerebral hypoperfusion. Hypertension 2011, 58, 635–642. [Google Scholar] [CrossRef]
- Xie, H.; Ray, P.E.; Short, B.L. NF-kappa B activation plays a role in superoxide-mediated cerebral endothelial dysfunction after hypoxia/reoxygenation. Stroke 2005, 36, 1047–1052. [Google Scholar] [CrossRef]
- Didion, S.P.; Lynch, C.M.; Baumbach, G.L.; Faraci, F.M. Impaired Endothelium-Dependent Responses and Enhanced Influence of Rho-Kinase in Cerebral Arterioles in Type II Diabetes. Stroke 2005, 36, 342–347. [Google Scholar] [CrossRef]
- Aghajanian, A.; Wittchen, E.S.; Campbell, S.L.; Burridge, K. Direct activation of RhoA by reactive oxygen species requires a redox-sensitive motif. PLoS ONE 2009, 4, e8045. [Google Scholar] [CrossRef]
- Laufs, U.; Liao, J.K. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J. Biol. Chem. 1998, 273, 24266–24271. [Google Scholar] [CrossRef] [PubMed]
- Faraco, G.; Moraga, A.; Moore, J.; Anrather, J.; Pickel, V.M.; Iadecola, C. Circulating endothelin-1 alters critical mechanisms regulating cerebral microcirculation. Hypertension 2013, 62, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Bochkov, V.N.; Philippova, M.; Oskolkova, O.; Kadl, A.; Furnkranz, A.; Karabeg, E.; Afonyushkin, T.; Gruber, F.; Breuss, J.; Minchenko, A.; et al. Oxidized phospholipids stimulate angiogenesis via autocrine mechanisms, implicating a novel role for lipid oxidation in the evolution of atherosclerotic lesions. Circ. Res. 2006, 99, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Podrez, E.A.; Poliakov, E.; Shen, Z.; Zhang, R.; Deng, Y.; Sun, M.; Finton, P.J.; Shan, L.; Febbraio, M.; Hajjar, D.P.; et al. A novel family of atherogenic oxidized phospholipids promotes macrophage foam cell formation via the scavenger receptor CD36 and is enriched in atherosclerotic lesions. J. Biol. Chem. 2002, 277, 38517–38523. [Google Scholar] [CrossRef] [PubMed]
- Davidson, Y.; Gibbons, L.; Purandare, N.; Byrne, J.; Hardicre, J.; Wren, J.; Payton, A.; Pendleton, N.; Horan, M.; Burns, A.; et al. Apolipoprotein E epsilon4 allele frequency in vascular dementia. Dement. Geriatr. Cogn. Disord. 2006, 22, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Tai, L.M.; Thomas, R.; Marottoli, F.M.; Koster, K.P.; Kanekiyo, T.; Morris, A.W.; Bu, G. The role of APOE in cerebrovascular dysfunction. Acta Neuropathol. 2016, 131, 709–723. [Google Scholar] [CrossRef]
- Bath, P.M.; Wardlaw, J.M. Pharmacological treatment and prevention of cerebral small vessel disease: A review of potential interventions. Int. J. Stroke 2015, 10, 469–478. [Google Scholar] [CrossRef]
- Poggesi, A.; Pasi, M.; Pescini, F.; Pantoni, L.; Inzitari, D. Circulating biologic markers of endothelial dysfunction in cerebral small vessel disease: A review. J. Cereb. Blood Flow Metab. 2016, 36, 72–94. [Google Scholar] [CrossRef]
- Bullen, M.L.; Miller, A.A.; Andrews, K.L.; Irvine, J.C.; Ritchie, R.H.; Sobey, C.G.; Kemp-Harper, B.K. Nitroxyl (HNO) as a vasoprotective signaling molecule. Antioxid. Redox Signal. 2011, 14, 1675–1686. [Google Scholar] [CrossRef]
- Miller, A.A.; Maxwell, K.F.; Chrissobolis, S.; Bullen, M.L.; Ku, J.M.; De Silva, T.; Selemidis, S.; Hooker, E.U.; Drummond, G.R.; Sobey, C.G.; et al. Nitroxyl (HNO) suppresses vascular Nox2 oxidase activity. Free Radic. Biol. Med. 2013, 60, 264–271. [Google Scholar] [CrossRef]
- Drummond, G.R.; Selemidis, S.; Griendling, K.K.; Sobey, C.G. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat. Rev. Drug Discov. 2011, 10, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Gopalan, Y.; Shuaib, I.L.; Magosso, E.; Ansari, M.A.; Abu Bakar, M.R.; Wong, J.W.; Khan, N.A.; Liong, W.C.; Sundram, K.; Ng, B.H.; et al. Clinical investigation of the protective effects of palm vitamin E tocotrienols on brain white matter. Stroke 2014, 45, 1422–1428. [Google Scholar] [CrossRef]
- Ueno, Y.; Koike, M.; Shimada, Y.; Shimura, H.; Hira, K.; Tanaka, R.; Uchiyama, Y.; Hattori, N.; Urabe, T. L-carnitine enhances axonal plasticity and improves white-matter lesions after chronic hypoperfusion in the rat brain. J. Cereb. Blood Flow Metabol. 2015, 35, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Study Collaborative, G. MRC/BHF heart protection study of antioxidant vitamin supplementation in 20,536 high-risk individuals: A randomized placebo-controlled trial. Lancet 2002, 360, 23–33. [Google Scholar] [CrossRef]
- Moretti, R.; Dal Ben, M.M.; Gazzin, S.; Tiribelli, C. Homocysteine in Neurology: From Endothelium to Neurodegeneration. Curr. Nutr. Food Sci. 2017, 13, 163–175. [Google Scholar] [CrossRef]
- Modrick, M.L.; Didion, S.P.; Sigmund, C.D.; Faraci, F.M. Role of oxidative stress and AT1 receptors in cerebral vascular dysfunction with aging. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1914–H1919. [Google Scholar] [CrossRef] [PubMed]
- Han, B.H.; Zhou, M.L.; Johnson, A.W.; Singh, I.; Liao, F.; Vellimana, A.K.; Nelson, J.W.; Milner, E.; Cirrito, J.R.; Basak, J.; et al. Contribution of reactive oxygen species to cerebral amyloid angiopathy, vasomotor dysfunction, and microhemorrhage in aged Tg2576 mice. Proc. Natl. Acad. Sci. USA 2015, 112, E881–E890. [Google Scholar] [CrossRef]
- Allen, C.L.; Bayraktutan, U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int. J. Stroke 2009, 4, 461–470. [Google Scholar] [CrossRef]
- Altenhofer, S.; Radermacher, K.A.; Kleikers, P.W.; Wingler, K.; Schmidt, H.H. Evolution of NADPH oxidase inhibitors: Selectivity and mechanisms for target engagement. Antioxid. Redox Signal. 2015, 23, 406–427. [Google Scholar] [CrossRef]
- Bedard, K.; Whitehouse, S.; Jaquet, V. Challenges progress, and promises for developing future NADPH oxidase therapeutics. Antioxid. Redox Signal. 2015, 23, 355–357. [Google Scholar] [CrossRef]
- Pretnar-Oblak, J.; Sebestjen, M.; Sabovic, M. Statin treatment improves cerebral more than systemic endothelial dysfunction in patients with arterial hypertension. Am. J. Hypertens. 2008, 21, 674–678. [Google Scholar] [CrossRef]
- Tong, X.K.; Nicolakakis, N.; Fernandes, P.; Ongali, B.; Brouillette, J.; Quirion, R.; Hamel, E. Simvastatin improves cerebrovascular function and counters soluble amyloid-beta, inflammation, and oxidative stress in aged APP mice. Neurobiol. Dis. 2009, 35, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Amarenco, P.; Benavente, O.; Goldstein, L.B.; Callahan, A.; Sillesen, H.; Hennerici, M.G.; Gilbert, S.; Rudolph, A.E.; Simunovic, L.; Zivin, J.A.; et al. Stroke Prevention by Aggressive Reduction in Cholesterol Levels Investigators. Results of the stroke prevention by aggressive reduction in cholesterol levels (SPARCL) trial by stroke subtypes. Stroke 2009, 40, 1405–1409. [Google Scholar] [CrossRef] [PubMed]
- Stuehr, D.J.; Fasehun, O.A.; Kwon, N.S.; Gross, S.S.; Gonzalez, J.A.; Levi, R.; Nathan, C.F. Inhibition of macrophage and endothelial cell nitric oxide synthase by diphenyleneiodonium and its analogs. FASEB J. 1991, 5, 98–103. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, B.V.; Tew, D.G.; Jones, O.T.; England, P.J. Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem. J. 1993, 290 Pt 1, 41–49. [Google Scholar] [CrossRef]
- Vejrazka, M.; Micek, R.; Stipek, S. Apocynin inhibits NADPH oxidase in phagocytes but stimulates ROS production in non-phagocytic cells. Biochim. Biophys. Acta 2005, 1722, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Rey, F.E.; Cifuentes, M.E.; Kiarash, A.; Quinn, M.T.; Pagano, P.J. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O(2)(-) and systolic blood pressure in mice. Circ. Res. 2001, 89, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.A.; Hess, D.T.; Wang, B.; Miyagi, M.; Stamler, J.S. Off-target thiol alkylation by the NADPH oxidase inhibitor 3-benzyl-7-(2-benzoxazolyl)thio-1,2,3-triazolo[4,5-d]pyrimidine (VAS2870). Free Radic. Biol. Med. 2012, 52, 1897–1902. [Google Scholar] [CrossRef]
- Laleu, B.; Gaggini, F.; Orchard, M.; Fioraso-Cartier, L.; Cagnon, L.; Houngninou-Molango, S.; Gradia, A.; Duboux, G.; Merlot, C.; Heitz, F.; et al. First in class, potent, and orally bioavailable NADPH oxidase isoform 4 (Nox4) inhibitors for the treatment of idiopathic pulmonary fibrosis. J. Med. Chem. 2010, 53, 7715–7730. [Google Scholar] [CrossRef]
- Cayatte, A.J.; Rupin, A.; Oliver-Krasinski, J.; Maitland, K.; Sansilvestri-Morel, P.; Boussard, M.F.; Wierzbicki, M.; Verbeuren, T.J.; Cohen, R.A. S17834, a new inhibitor of cell adhesion and atherosclerosis that targets NADPH oxidase. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1577–1584. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Sano, K.; Takakura, K.; Saito, I.; Shinohara, Y.; Asano, T.; Yasuhara, H. Ebselen in acute ischemic stroke: A placebo-controlled, double-blind clinical trial. Ebselen Study Group Stroke 1998, 29, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Ulivi, L.; Maccarrone, M.; Giannini, N.; Ferrari, E.; Caselli, M.C.; Montano, V.; Chico, L.; Casani, A.; Navari, E.; Cerchiai, N.; et al. Oxidative Stress in Cerebral Small Vessel Disease Dizziness Patients, Basally and after Polyphenol Compound Supplementation. Curr. Mol. Med. 2018, 18, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, T.; Weiner, H.L. CNS inflammation and neurodegeneration. J. Clin Investig. 2017, 127, 3577–3587. [Google Scholar] [CrossRef] [PubMed]
- Kiechle, T.; Dedeoglu, A.; Kubilus, J.; Kowall, N.W.; Beal, M.F.; Friedlander, R.M.; Hersch, S.M.; Ferrante, R.J. Cytochrome C and caspase-9 expression in Huntington’s disease. Neuromol. Med. 2002, 1, 183–195. [Google Scholar] [CrossRef]
- Pasinelli, P.; Houseweart, M.K.; Brown, R.H.; Cleveland, D.W. Caspase-1 and -3 are sequentially activated in motor neuron death in Cu, Zn superoxide dismutase-mediated familial amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA 1998, 95, 13901–13906. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Ofengeim, D.; Najafov, A.; Das, S.; Saberi, S.; Li, Y.; Hitomi, J.; Zhu, H.; Chen, H.; Mayo, L.; et al. RIPK1 mediates axonal degeneration by promoting inflammation and necroptosis in ALS. Science 2016, 353, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Ofengeim, D.; Ito, Y.; Najafov, A.; Zhang, Y.; Shan, B.; DeWitt, J.P.; Ye, J.; Zhang, X.; Chang, A.; Vakifahmetoglu-Norberg, H.; et al. Activation of necroptosis in multiple sclerosis. Cell Rep. 2015, 10, 1836–1849. [Google Scholar] [CrossRef]
- Shi, Z.W.; Sha Ge, L.; Li, Y.C. The Role of Necroptosis in Cardiovascular Disease. Front. Pharmacol. 2018, 9, 721. [Google Scholar] [CrossRef]
- Perry, V.H.; Cunningham, C.; Holmes, C. Systemic infections and inflammation affect chronic neurodegeneration. Nat. Rev. Immunol. 2007, 7, 161–167. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. Gut instincts: Microbiota as a key regulator of brain development, aging, and neurodegeneration. J. Physiol. 2017, 595, 489–503. [Google Scholar] [CrossRef]
- Quigley, E.M. Microbiota-brain-gut axis and neurodegenerative disease. Curr. Neurol. Neurosci. Rep. 2017, 17, 94. [Google Scholar] [CrossRef]
- Chen, I.; Chia, N.; Kalari, K.R.; Yao, J.Z.; Novotvna, M.; Soldan, M.M.; Luckey, D.H.; Marietta, E.V.; Jeraldo, P.R.; Chen, X.; et al. Multiple Sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci. Rep. 2016, 6, 28484. [Google Scholar] [CrossRef] [PubMed]
- Andreadou, E.; Pantazaki, A.A.; Danilidou, M.; Tsolaki, M. rhamnolipids, microbial virulence factors in Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 59, 209–222. [Google Scholar] [CrossRef]
- Goldman, S.M.; Kamel, F.; Rose, G.W.; Jewell, S.A.; Marras, C.; Hopper, J.A.; Umbach, D.M.; Bhudhikanok, G.S.; Meng, C.; Korell, M.; et al. Peptydologlycan recognition protein genes and risk of Parkinson’s Disease. Mov. Disord. 2014, 29, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging: An evolutionary perspective on immunoscence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef]
- Ynakner, B.A.; Lu, T.; Loerch, P. The aging brain. Ann. Rev. Pathol. 2008, 3, 41–66. [Google Scholar] [CrossRef]
- Rawji, K.S.; Mishra, M.K.; Michaels, N.J.; Rivest, S.; Stys, P.K.; Yong, V.W. Immunosenescence of microglia and macrophage impact on aging central nervous system. Brain 2016, 139 Pt 13, 653–661. [Google Scholar] [CrossRef]
- Chinta, S.J.; Woods, G.; Rane, A.; Demaria, M.; Campisi, J.; Andersen, J.K. Cellular senescence and the aging brain. Exp. Gerontol. 2015, 68, 3–7. [Google Scholar] [CrossRef]
- Flanary, O.E.; Sammons, N.W.; Nguyen, C.; Walker, D.; Streit, W.J. Evidence that aging and amyloid promote microglial cell senescence. Rejuvenation Res. 2007, 10, 61–74. [Google Scholar] [CrossRef]
- Weller, R.O.; Sharp, M.M.; Christodoulides, M.; Carare, R.O.; Møllgård, K. The meninges as barriers and facilitators for the movement of fluid, cells and pathogens related to the rodent and human CNS. Acta Neuropathol. 2018, 135, 363–385. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, W.; Jia, L.; Qin, W.; Hou, T.; Wu, Q.; Li, H.; Tian, Y.; Jia, J. The Effect of Chronic Cerebral Hypoperfusion on Blood-Brain Barrier Permeability in a Transgenic Alzheimer’s Disease Mouse Model (PS1V97L). J. Alzheimers Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including ABa. Sci. Transl. Med. 2012, 4, ra111. [Google Scholar] [CrossRef] [PubMed]
- Shetty, A.K.; Zanirati, G. The Interstitial System of the Brain in Health and Disease. Aging Dis. 2020, 11, 200–211. [Google Scholar] [CrossRef]
- Rungta, R.L.; Chaigneau, E.; Osmanski, B.F.; Charpak, S. Vascular Compartmentalization of Functional Hyperemia from the Synapse to the Pia. Neuron 2018, 99, 362–375. [Google Scholar] [CrossRef]
- Xiong, L.; van Veluw, S.J.; Bounemia, N.; Charidimou, A.; Pasi, M.; Boulouis, G.; Reijmer, Y.D.; Giese, A.K.; Davidsdottir, S.; Fotiadis, P.; et al. Cerebral Cortical Microinfarcts on Magnetic Resonance Imaging and Their Association With Cognition in Cerebral Amyloid Angiopathy. Stroke 2018, 49, 2330–2336. [Google Scholar] [CrossRef]
- Shen, M.; Wei, G.; Cheng, M.; Jiang, H. Association between Enlarged Perivascular Spaces and Internal Carotid Artery Stenosis: A Study in Patients Diagnosed by Digital Subtraction Angiography. J. Stroke Cerebrovasc. Dis. 2020. [Google Scholar] [CrossRef]
- Jagtap, A.; Gawande, S.; Sharma, S. Biomarkers in vascular dementia: A recent update. Biomark. Genom. Med. 2015, 2, 43–56. [Google Scholar] [CrossRef]
- Wallin, A.; Kapaki, E.; Boban, M.; Engelborghs, S.; Hermann, D.M.; Huisa, B.; Jonsson, M.; Kramberger, M.G.; Lossi, L.; Malovic, B.; et al. Biochemical markers in vascular cognitive impairment associated with subcortical small vessel disease-A consensus report. BMC Neurol. 2017, 23, 102. [Google Scholar] [CrossRef]
- Staszewski, J.; Piusinsks-MAcoch, R.; Brodacki, E.; Stepien, A. Il-6, PF-4, sCD40 L, and homocysteine are associated with the radiological progression of small vessel disease: A 2-year follow-up study. Clin. Interv. Ageing 2018, 13, 1135–1141. [Google Scholar] [CrossRef]
- Staszewski, J.; Piusinska-Macoch, R.; Brodacki, B.; Skrobowska, E.; Stepien, A. Association between hemostatic markers, serum lipid fractions and progression of cerebral small vessel disease: A 2-year follow-up study. Neurol. Neurochir. Pol. 2018, 52, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Low, A.; Mak, E.; Rowe, J.B.; Markus, H.S.; O’Brien, J.T. Inflammation and cerebral small vessel disease: A systematic review. Ageing Res. Rev. 2019, 53, 100916. [Google Scholar] [CrossRef] [PubMed]
- Moretti, R.; Peinkhofer, C.B. Vitamins and Fatty Acids: What Do They Share with Small Vessel Disease-Related Dementia? Int. J. Mol. Sci. 2019, 20, 5797. [Google Scholar] [CrossRef] [PubMed]
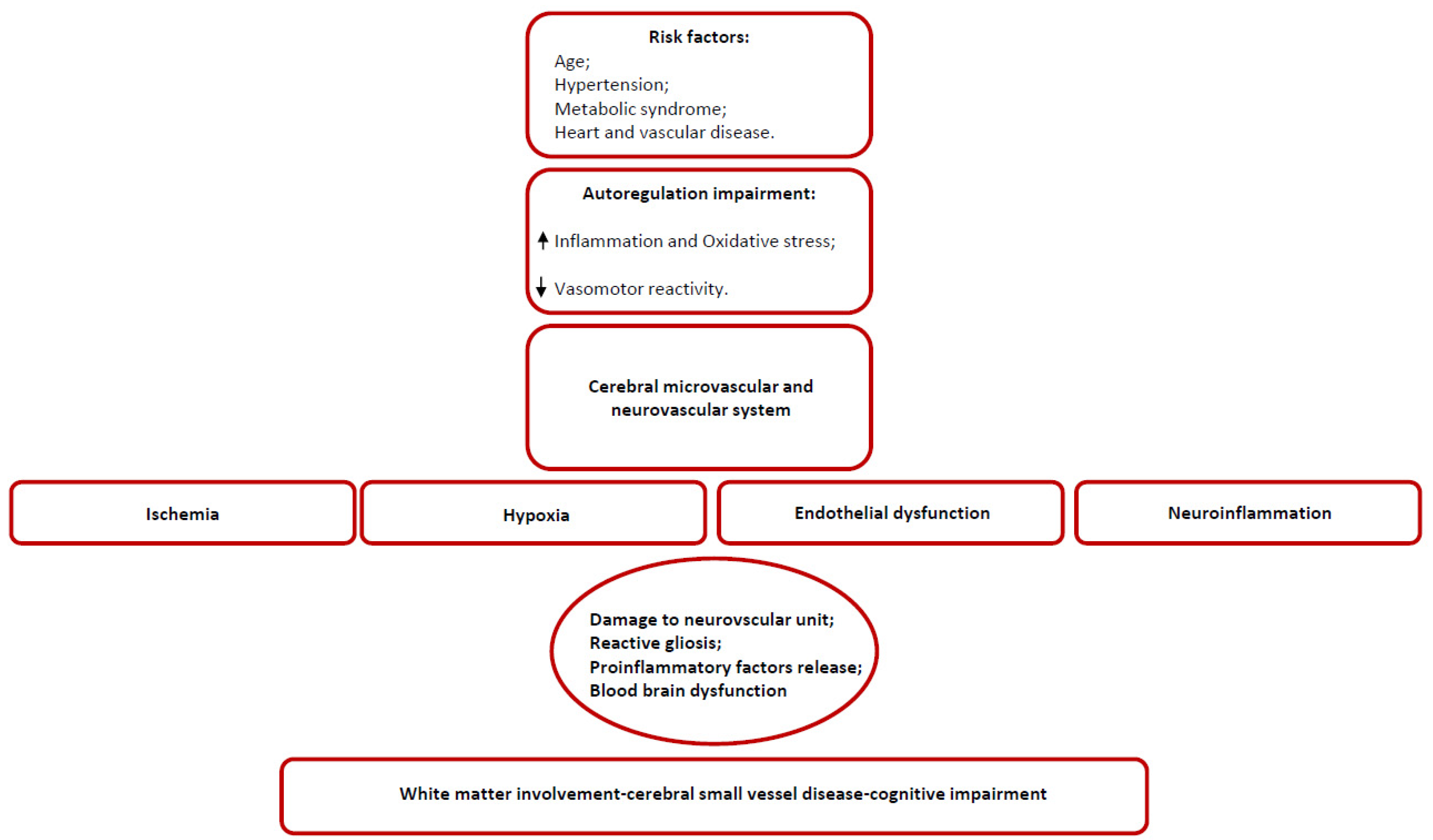
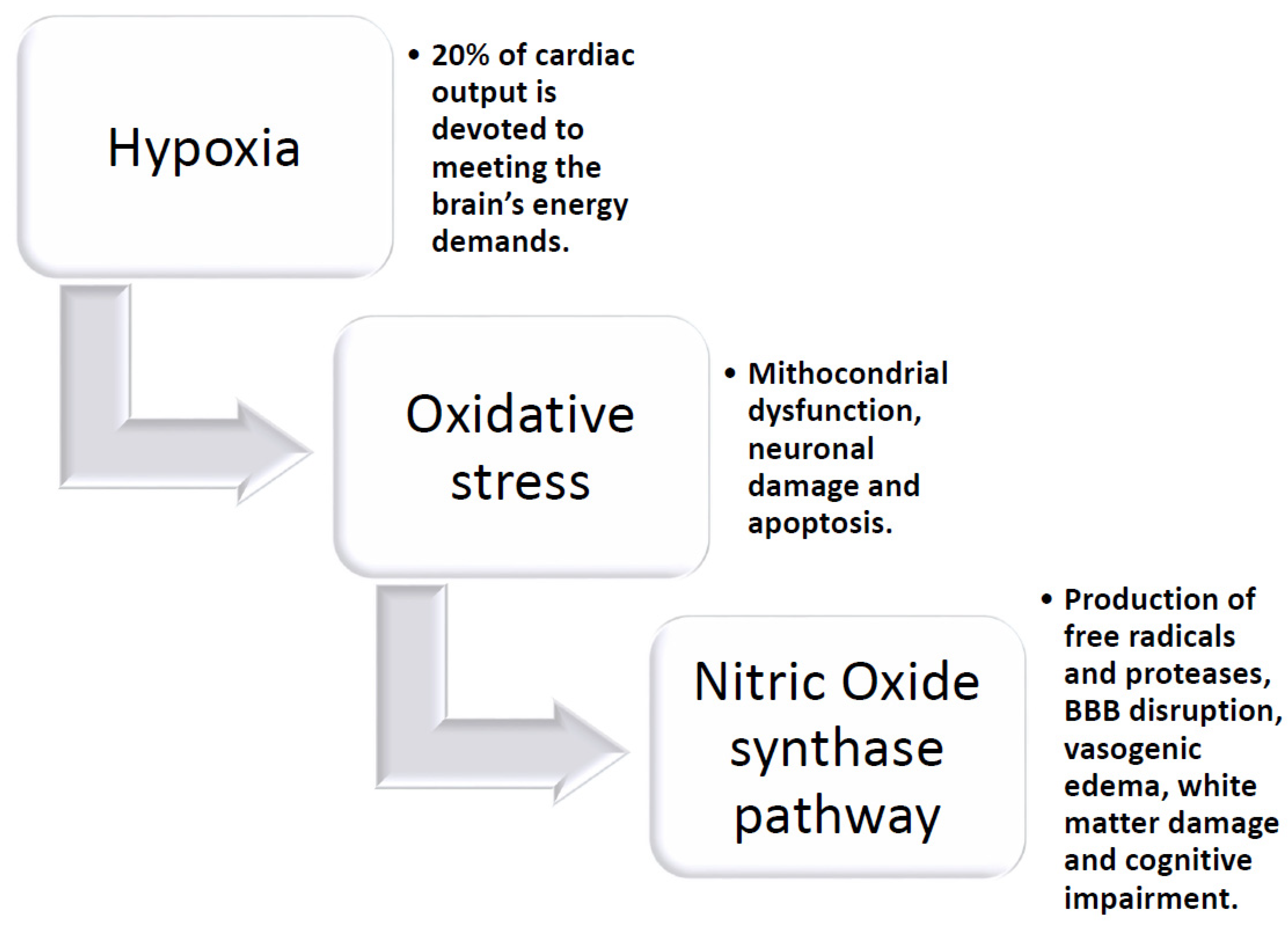
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moretti, R.; Caruso, P. Small Vessel Disease-Related Dementia: An Invalid Neurovascular Coupling? Int. J. Mol. Sci. 2020, 21, 1095. https://doi.org/10.3390/ijms21031095
Moretti R, Caruso P. Small Vessel Disease-Related Dementia: An Invalid Neurovascular Coupling? International Journal of Molecular Sciences. 2020; 21(3):1095. https://doi.org/10.3390/ijms21031095
Chicago/Turabian StyleMoretti, Rita, and Paola Caruso. 2020. "Small Vessel Disease-Related Dementia: An Invalid Neurovascular Coupling?" International Journal of Molecular Sciences 21, no. 3: 1095. https://doi.org/10.3390/ijms21031095
APA StyleMoretti, R., & Caruso, P. (2020). Small Vessel Disease-Related Dementia: An Invalid Neurovascular Coupling? International Journal of Molecular Sciences, 21(3), 1095. https://doi.org/10.3390/ijms21031095




