Coacervate Thermoresponsive Polysaccharide Nanoparticles as Delivery System for Piroxicam
Abstract
1. Introduction
2. Results and Discusion
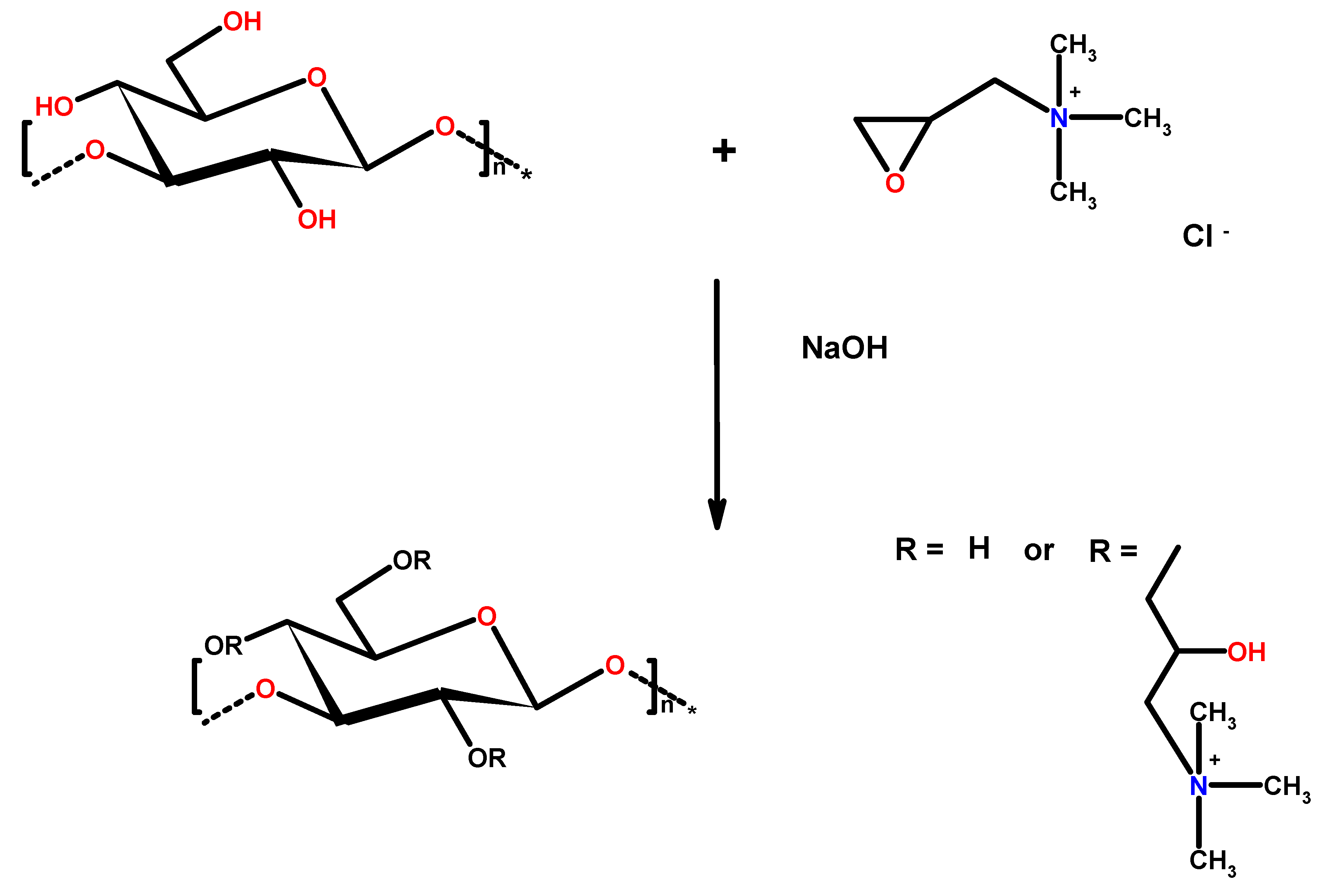
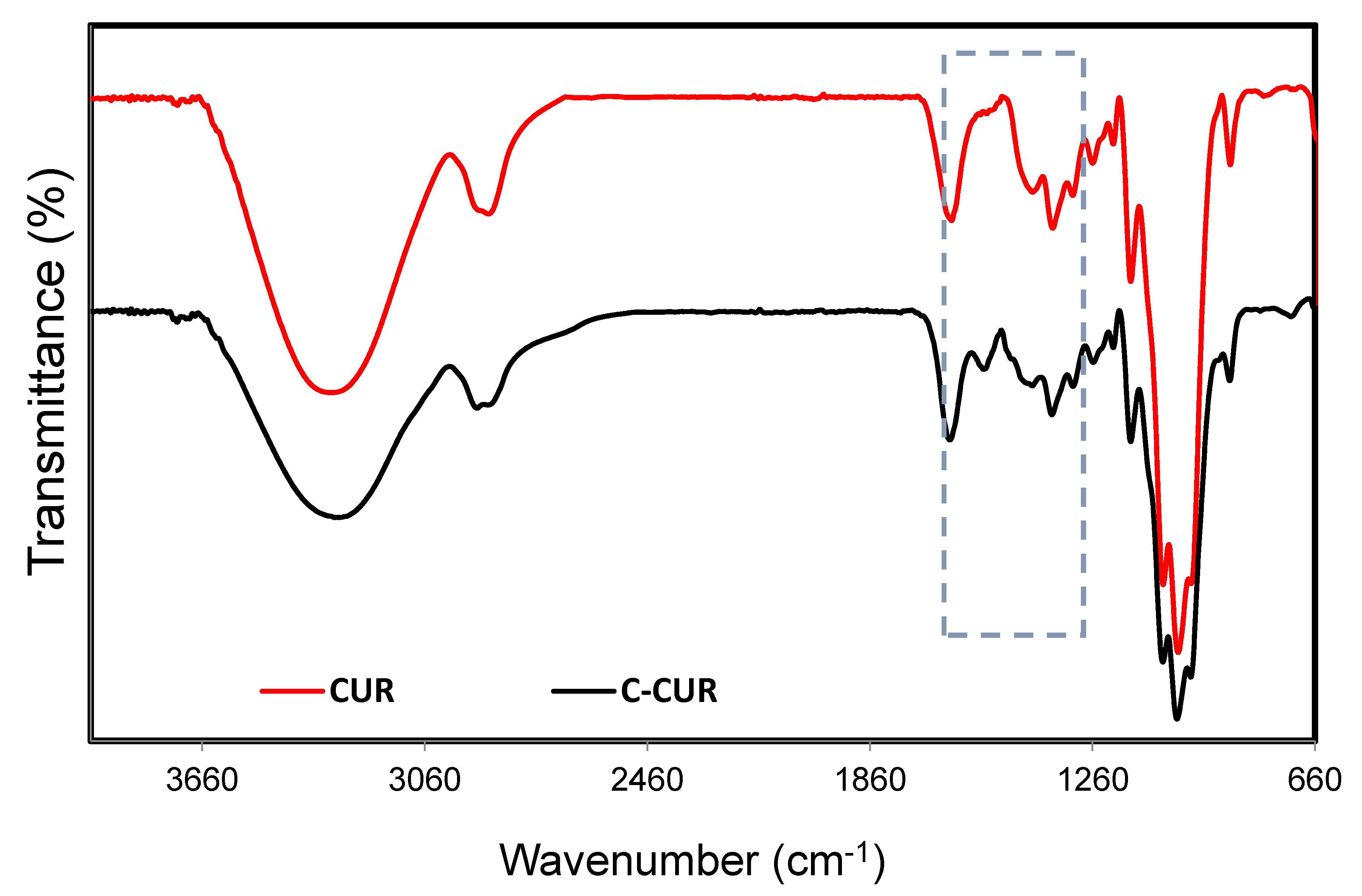
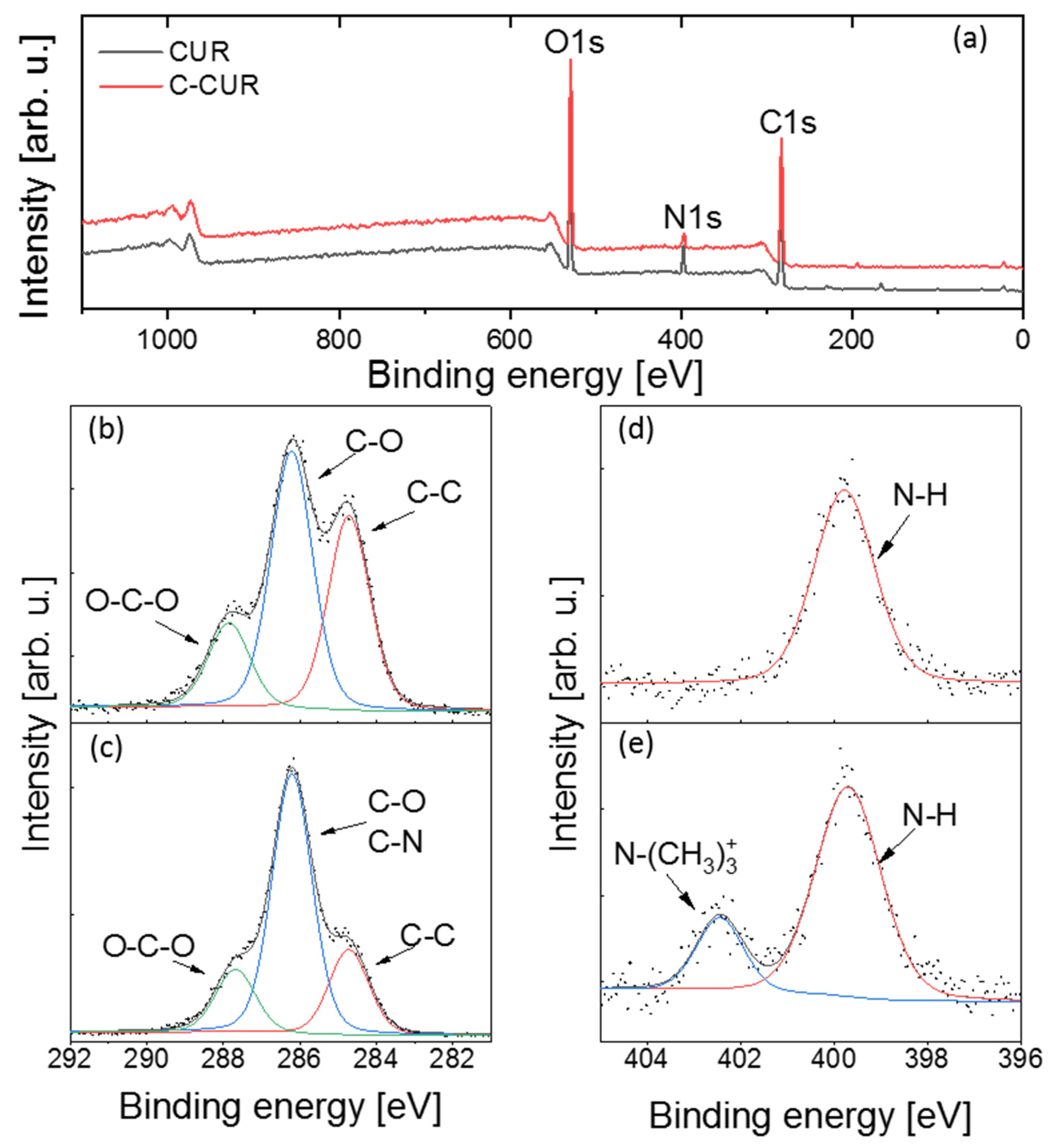
2.1. Cationic Curdlan—Synthesis and Characterization
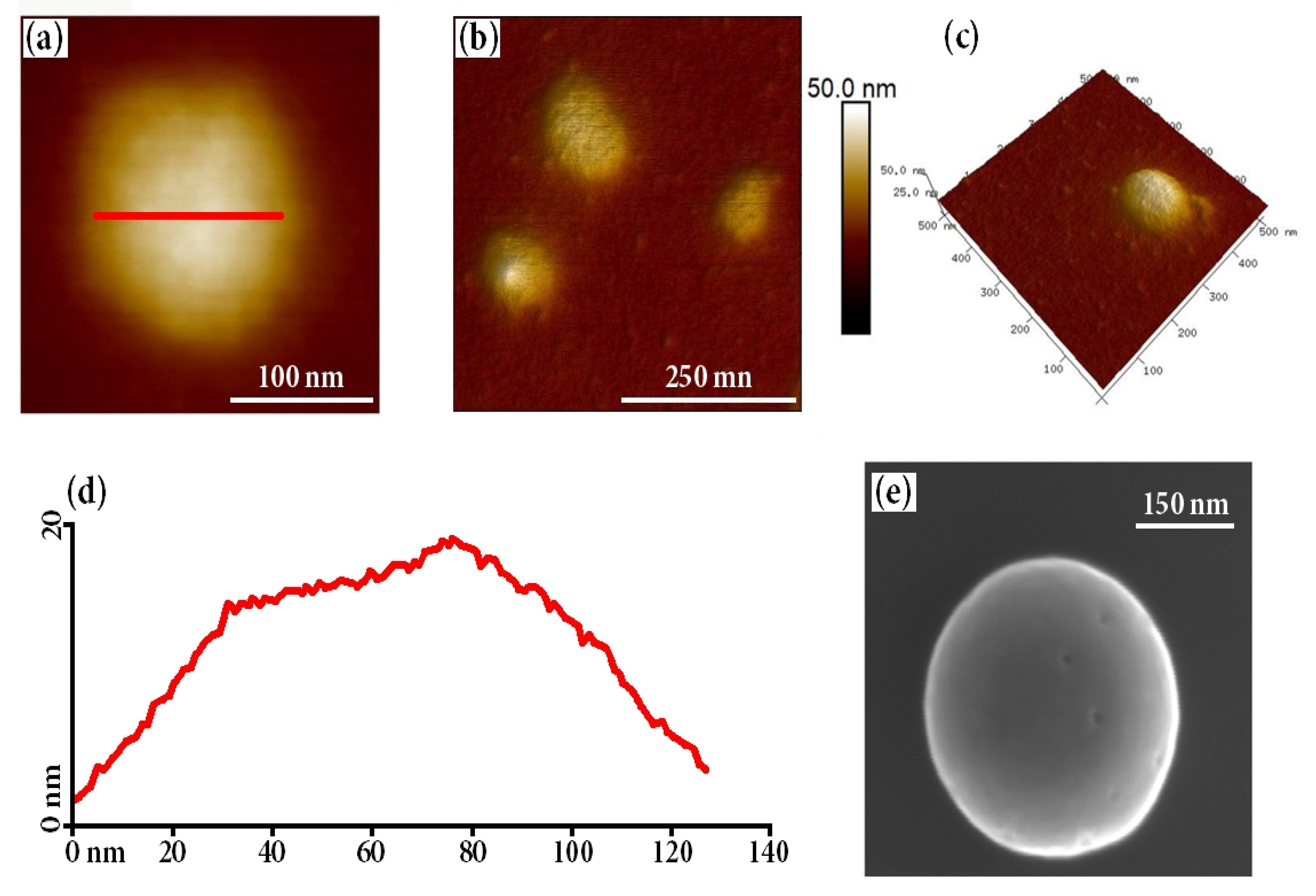
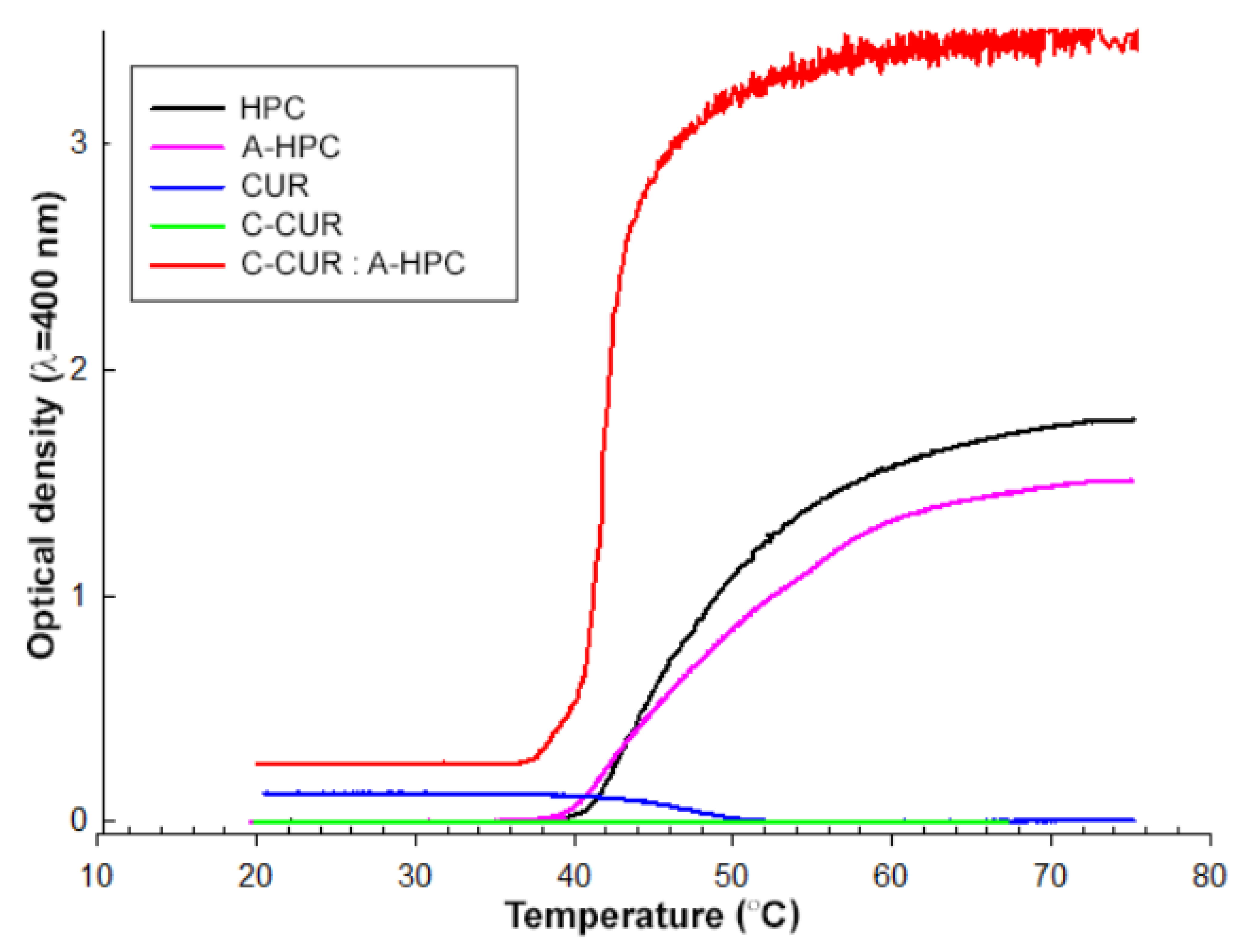
2.2. Preparation of Thermoresponsive Coacervate Polysaccharide Nanoparticles (CPNs)
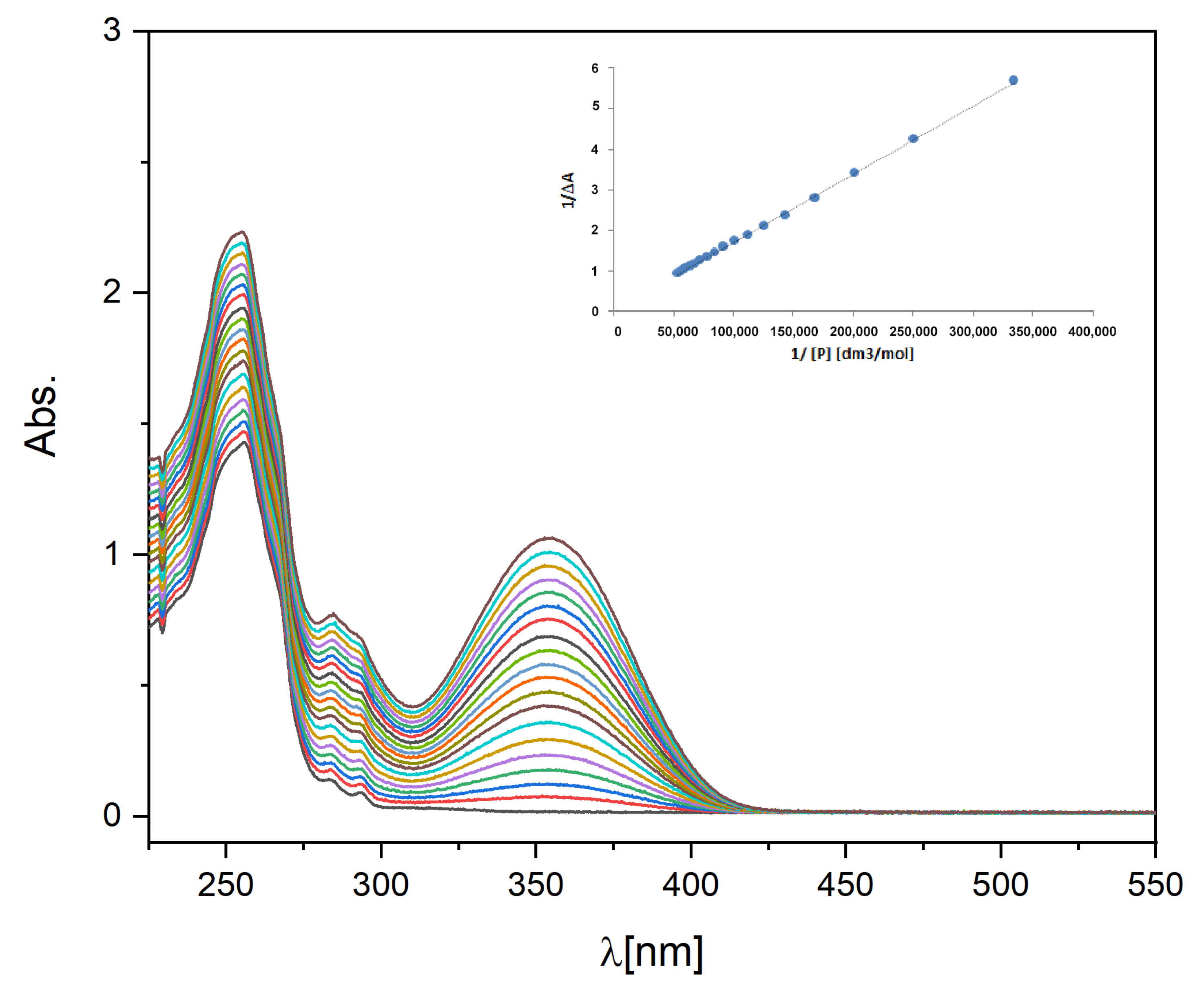
2.3. Binding Constant of Piroxicam to A-HPC
2.4. Piroxicam-Loaded C-CUR/A-HPC (1:25) Nanoparticles (CPNs-PIX)
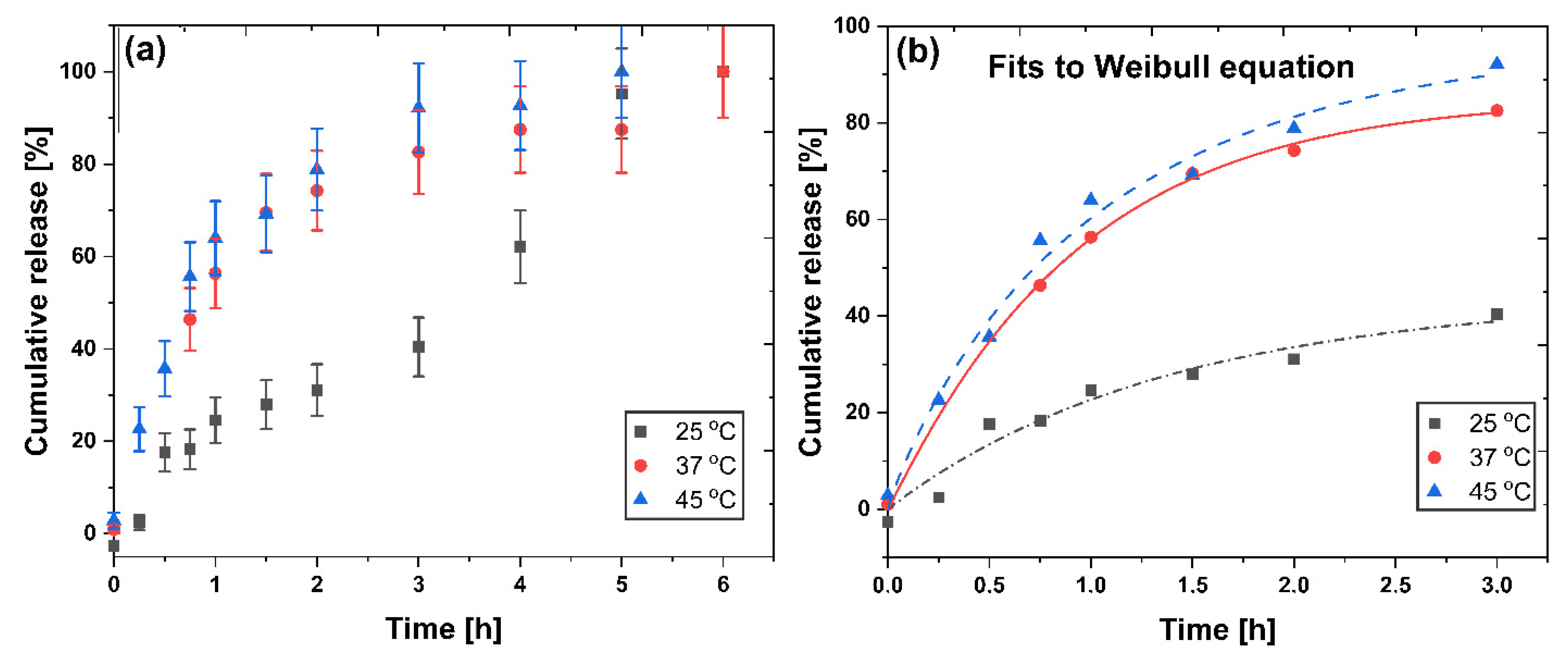
2.5. Piroxicam Release
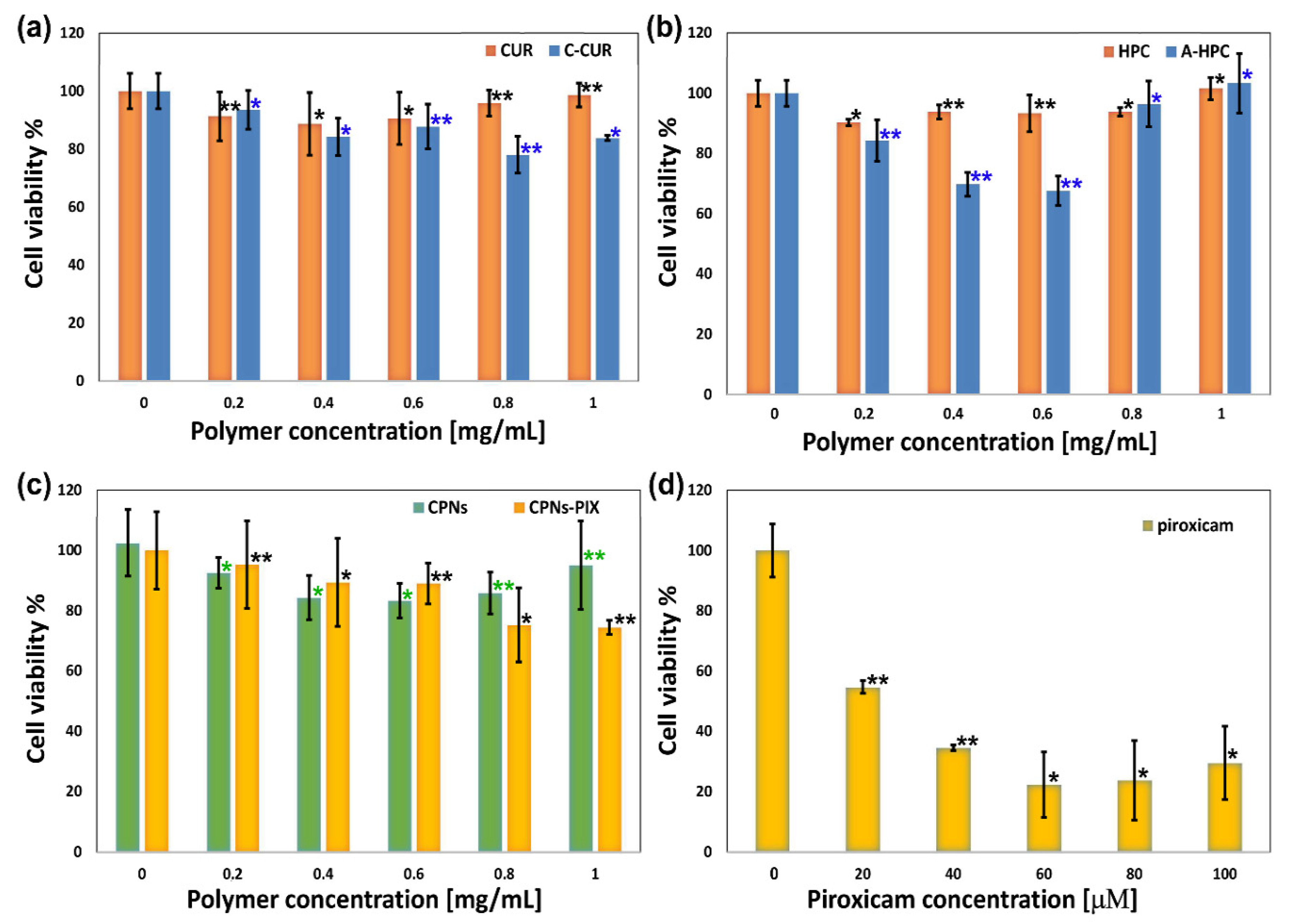
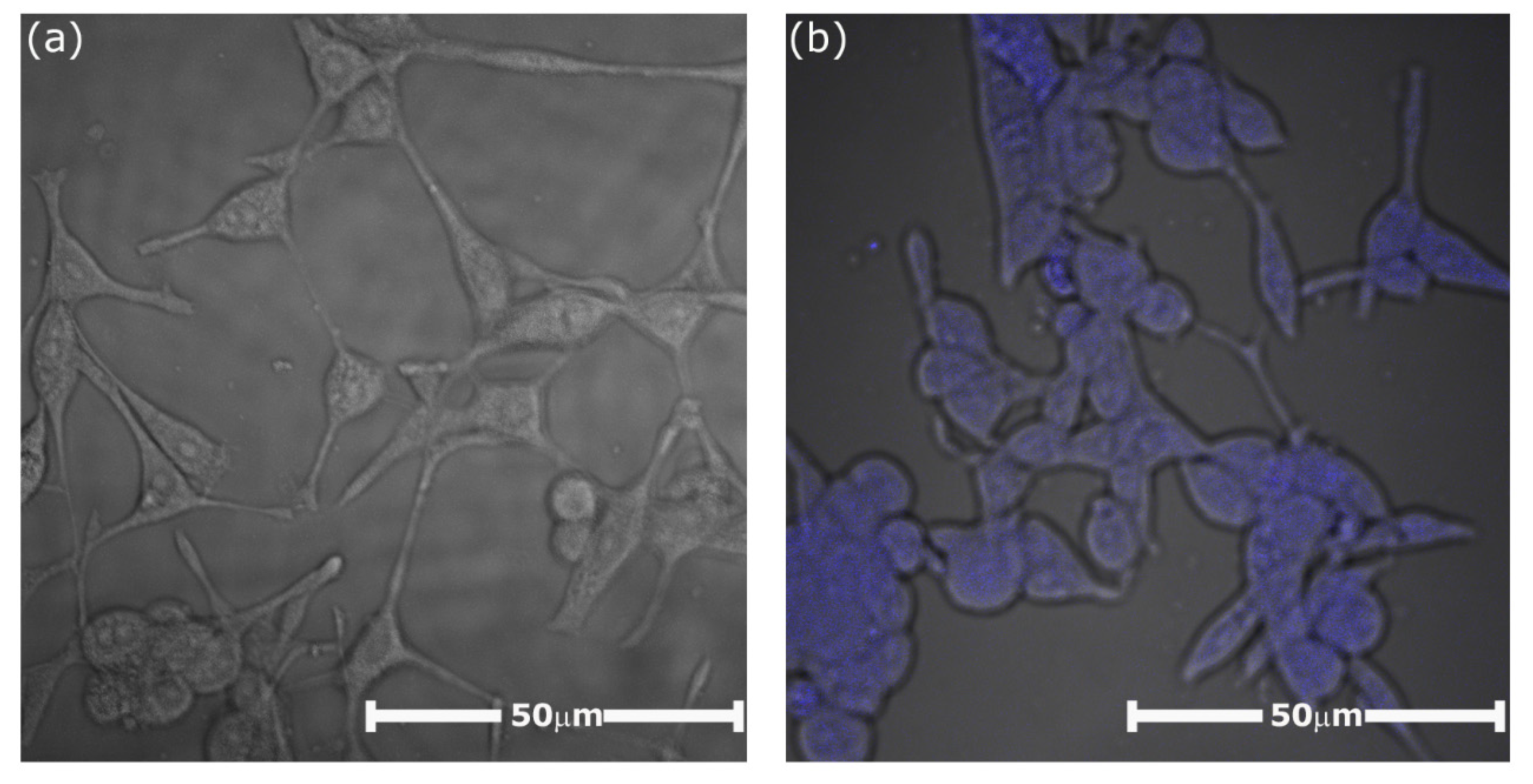
2.6. Biological Studies
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Ionic Polysaccharide Derivatives (C-CUR and A-HPC)
3.2.1. C-CUR
3.2.2. A-HPC
3.3. Characterization of Modified Polysaccharides
3.4. Preparation of C-CUR/A-HPC ThermoResponsive Nanoparticles (CPNs)
3.5. Preparation of the Piroxicam-Loaded C-CUR/A-HPC System (CPNs-PIX)
3.6. Morphology and Size of the Obtained Nanoparticles
3.7. Determination of the Piroxicam-A-HPC-Binding Constant
3.8. Determination of Piroxicam Entrapment Efficiency (EE) and Loading Efficiency (LE)
3.9. Piroxicam Release Profile from CPNs-PIX
3.10. Biological Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CUR | Curdlan |
| C-CUR | Cationic curdlan |
| HPC | Hydroxypropyl cellulose |
| A-HPC | Anionic hydroxypropyl cellulose |
| CPNs | Coacervate polysaccharide nanoparticles |
| PIROX | Piroxicam |
| DLS | Dynamic light scattering |
| AFM | Atomic force microscopy |
| SEM | Scanning electron microscopy |
| XPS | X-ray photoelectron spectroscopy |
References
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnology 2018, 16, 1–33. [Google Scholar] [CrossRef]
- Jahangirian, H.; Lemraski, E.G.; Webster, T.J.; Rafiee-Moghaddam, R.; Abdollahi, Y. A review of drug delivery systems based on nanotechnology and green chemistry: Green nanomedicine. Int. J. Nanomed. 2017, 12, 2957–2978. [Google Scholar] [CrossRef]
- Parveen, S.; Misra, R.; Sahoo, S.K. Nanoparticles: A boon to drug delivery, therapeutics, diagnostics and imaging. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 147–166. [Google Scholar] [CrossRef] [PubMed]
- Tariq, A.; Bhawani, S.A.; Moheman, A. Nanoparticles for drug delivery. In Advanced Structured Materials; Springer: Berlin/Heidelberg, Germany, 2019; Volume 118, pp. 175–197. [Google Scholar]
- Jacob, S.; Nair, A.B.; Shah, J. Emerging role of nanosuspensions in drug delivery systems. Biomater. Res. 2020, 24, 3. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Tolba, E.; Wang, S.; Neufurth, M.; Lieberwirth, I.; Ackermann, M.; Schröder, H.C.; Wang, X. Nanoparticle-directed and ionically forced polyphosphate coacervation: A versatile and reversible core–shell system for drug delivery. Sci. Rep. 2020, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kaloti, M.; Bohidar, H.B. Kinetics of coacervation transition versus nanoparticle formation in chitosan-sodium tripolyphosphate solutions. Colloids Surf. B Biointerfaces 2010, 81, 165–173. [Google Scholar] [CrossRef]
- Dutta, L.P.; Das, M. Coacervation—A Method for Drug Delivery; Springer: New Delhi, India, 2015; pp. 379–386. [Google Scholar]
- Blocher McTigue, W.C.; Perry, S.L. Design rules for encapsulating proteins into complex coacervates. Soft Matter 2019, 15, 3089–3103. [Google Scholar] [CrossRef]
- Ortony, J.H.; Choi, S.H.; Spruell, J.M.; Hunt, J.N.; Lynd, N.A.; Krogstad, D.V.; Urban, V.S.; Hawker, C.J.; Kramer, E.J.; Han, S. Fluidity and water in nanoscale domains define coacervate hydrogels. Chem. Sci. 2014, 5, 58–67. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Wang, S.; Ackermann, M.; Gerich, T.; Neufurth, M.; Wiens, M.; Schröder, H.C.; Wang, X. Biologization of Allogeneic Bone Grafts with Polyphosphate: A Route to a Biomimetic Periosteum. Adv. Funct. Mater. 2019, 29, 1905220. [Google Scholar] [CrossRef]
- Xia, J.; Dubin, P.L. Protein-polyelectrolyte complexes. In Macromolecular Complexes in Chemistry and Biology; Springer: Berlin/Heidelberg, Germany, 1994; pp. 247–271. [Google Scholar]
- Schmitt, C.; Sanchez, C.; Thomas, F.; Hardy, J. Complex coacervation between β-lactoglobulin and acacia gum in aqueous medium. Food Hydrocoll. 1999, 13, 483–496. [Google Scholar] [CrossRef]
- Jin, K.M.; Kim, Y.H. Injectable, thermo-reversible and complex coacervate combination gels for protein drug delivery. J. Control. Release 2008, 127, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Qi, A.; Hoo, S.P.; Friend, J.; Yeo, L.; Yue, Z.; Chan, P.P.Y. Hydroxypropyl Cellulose Methacrylate as a Photo-Patternable and Biodegradable Hybrid Paper Substrate for Cell Culture and Other Bioapplications. Adv. Healthc. Mater. 2014, 3, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Weißenborn, E.; Braunschweig, B. Hydroxypropyl cellulose as a green polymer for thermo-responsive aqueous foams. Soft Matter 2019, 15, 2876–2883. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zhao, S.; Jia, X.; Wang, H.; Zhang, H.; Liu, Q.; Kong, B. Thermal gelling properties and structural properties of myofibrillar protein including thermo-reversible and thermo-irreversible curdlan gels. Food Chem. 2020, 311, 126018. [Google Scholar] [CrossRef]
- Yunus Basha, R.; Venkatachalam, G.; Sampath Kumar, T.S.; Doble, M. Dimethylaminoethyl modified curdlan nanoparticles for targeted siRNA delivery to macrophages. Mater. Sci. Eng. C 2020, 108, 110379. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, K.H.; Lee, H.K.; Kim, J.S.; Kim, Y.G.; Lee, J.H.; Kim, K.H.; Yun, J.; Hwang, B.Y.; Hong, J.T.; et al. Curdlan activates dendritic cells through dectin-1 and toll-like receptor 4 signaling. Int. Immunopharmacol. 2016, 39, 71–78. [Google Scholar] [CrossRef]
- Liu, M.; Luo, F.; Ding, C.; Albeituni, S.; Hu, X.; Ma, Y.; Cai, Y.; McNally, L.; Sanders, M.A.; Jain, D.; et al. Dectin-1 Activation by a Natural Product β-Glucan Converts Immunosuppressive Macrophages into an M1-like Phenotype. J. Immunol. 2015, 195, 5055–5065. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, J.; Lan, H. Tumor-associated macrophages in tumor metastasis: Biological roles and clinical therapeutic applications. J. Hematol. Oncol. 2019, 12, 1–16. [Google Scholar] [CrossRef]
- Zhu, C.C.; Zhao, G.Q.; Lin, J.; Hu, L.T.; Xu, Q.; Peng, X.D.; Wang, X.; Qiu, S. Dectin-1 agonist curdlan modulates innate immunity to Aspergillus fumigatus in human corneal epithelial cells. Int. J. Ophthalmol. 2015, 8, 690–696. [Google Scholar] [CrossRef]
- Scuderi, B.; Driussi, G.B.; Chizzolini, M.; Salvetat, M.L.; Beltrame, G. Effectiveness and tolerance of piroxicam 0.5% and diclofenac sodium 0.1% in controlling inflammation after cataract surgery. Eur. J. Ophthalmol. 2003, 13, 536–540. [Google Scholar] [CrossRef]
- Campione, E.; Paternò, E.J.; Candi, E.; Falconi, M.; Costanza, G.; Diluvio, L.; Terrinoni, A.; Bianchi, L.; Orlandi, A. The relevance of piroxicam for the prevention and treatment of nonmelanoma skin cancer and its precursors. Drug Des. Devel. Ther. 2015, 9, 5843. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Arantes-Rodrigues, R.; Pinto-Leite, R.; Faustino-Rocha, A.I.; Fidalgo-Gonçalves, L.; Santos, L.; Oliveira, P.A. Synergistic Effect of Carboplatin and Piroxicam on Two Bladder Cancer Cell Lines. Anticancer Res. 2017, 37, 1737–1745. [Google Scholar] [CrossRef] [PubMed]
- Suflet, D.M.; Popescu, I.; Pelin, I.M.; Nicolescu, A.; Hitruc, G. Cationic curdlan: Synthesis, characterization and application of quaternary ammonium salts of curdlan. Carbohydr. Polym. 2015, 123, 396–405. [Google Scholar] [CrossRef]
- Nichifor, M.; Stanciu, M.C.; Simionescu, B.C. New cationic hydrophilic and amphiphilic polysaccharides synthesized by one pot procedure. Carbohydr. Polym. 2010, 82, 965–975. [Google Scholar] [CrossRef]
- Cho, J.; Grant, J.; Piquette-Miller, M.; Allen, C. Synthesis and physicochemical and dynamic mechanical properties of a water-soluble chitosan derivative as a biomaterial. Biomacromolecules 2006, 7, 2845–2855. [Google Scholar] [CrossRef]
- Gieroba, B.; Sroka-Bartnicka, A.; Kazimierczak, P.; Kalisz, G.; Lewalska-Graczyk, A.; Vivcharenko, V.; Nowakowski, R.; Pieta, I.S.; Przekora, A. Spectroscopic studies on the temperature-dependent molecular arrangements in hybrid chitosan/1,3-β-D-glucan polymeric matrices. Int. J. Biol. Macromol. 2020, 159, 911–921. [Google Scholar] [CrossRef]
- Jiang, L. Effect of nitrogen source on curdlan production by Alcaligenes faecalis ATCC. Int. J. Biol. Macromol. 2013, 52, 218–220. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D.; Chichester, W. The Scienta ESCA300 Database; John Wiley & Sons: Hoboken, NJ, USA, 1992; p. 2205. ISBN 0471 935921. [Google Scholar]
- Bielska, D.; Karewicz, A.; Kamiński, K.; Kiełkowicz, I.; Lachowicz, T.; Szczubiałka, K.; Nowakowska, M. Self-organized thermo-responsive hydroxypropyl cellulose nanoparticles for curcumin delivery. Eur. Polym. J. 2013, 49, 2485–2494. [Google Scholar] [CrossRef]
- Kępczyński, M.; Pandian, R.P.; Smith, K.M.; Ehrenberg, B. Do Liposome-binding Constants of Porphyrins Correlate with Their Measured and Predicted Partitioning Between Octanol and Water? Photochem. Photobiol. 2007, 76, 127–134. [Google Scholar] [CrossRef]
- Pitha, J.; Hoshino, T.; Torres-Labandeira, J.; Irie, T. Preparation of drug: Hydroxypropylcyclodextrin complexes by a method using ethanol or aqueous ammonium hydroxide as co-solubilizers. Int. J. Pharm. 1992, 80, 253–258. [Google Scholar] [CrossRef]
- Kobayashi, K.; Sumitomo, H. Binding of Organic Solutes to a Polymer Containing Pendant Sugar Groups. Polym. J. 1981, 13, 517–519. [Google Scholar] [CrossRef]
- Aricov, L.; Angelescu, D.G.; Băran, A.; Leontieş, A.R.; Popa, V.T.; Precupaş, A.; Sandu, R.; Stîngă, G.; Anghel, D.-F. Interaction of piroxicam with bovine serum albumin investigated by spectroscopic, calorimetric and computational molecular methods. J. Biomol. Struct. Dyn. 2019, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bobokalonov, D.T.; Mukhidinov, Z.K.; Rakhimov, I.F.; Khodzhaeva, F.M.; Kasymova, G.F.; Liu, L.S. Piroxicam ex vivo release kinetics from zein/pectin delivery systems. Pharm. Chem. J. 2012, 46, 378–380. [Google Scholar] [CrossRef]
- Paul, D.R. Elaborations on the Higuchi model for drug delivery. Int. J. Pharm. 2011, 418, 13–17. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Papadopoulou, V.; Kosmidis, K.; Vlachou, M.; Macheras, P. On the use of the Weibull function for the discernment of drug release mechanisms. Int. J. Pharm. 2006, 309, 44–50. [Google Scholar] [CrossRef]
- Karewicz, A.; Zasada, K.; Szczubiałka, K.; Zapotoczny, S.; Lach, R.; Nowakowska, M. “Smart” alginate-hydroxypropylcellulose microbeads for controlled release of heparin. Int. J. Pharm. 2010, 385, 163–169. [Google Scholar] [CrossRef]
- Monnery, B.D.; Wright, M.; Cavill, R.; Hoogenboom, R.; Shaunak, S.; Steinke, J.H.G.; Thanou, M. Cytotoxicity of polycations: Relationship of molecular weight and the hydrolytic theory of the mechanism of toxicity. Int. J. Pharm. 2017, 521, 249–258. [Google Scholar] [CrossRef]
- Karewicz, A.; Bielska, D.; Loboda, A.; Gzyl-Malcher, B.; Bednar, J.; Jozkowicz, A.; Dulak, J.; Nowakowska, M. Curcumin-containing liposomes stabilized by thin layers of chitosan derivatives. Colloids Surf. B Biointerfaces 2013, 109, 307–316. [Google Scholar] [CrossRef]
- Karewicz, A.; Bielska, D.; Gzyl-Malcher, B.; Kepczynski, M.; Lach, R.; Nowakowska, M. Interaction of curcumin with lipid monolayers and liposomal bilayers. Colloids Surf. B Biointerfaces 2011, 88, 231–239. [Google Scholar] [CrossRef]
- Lachowicz, D.; Mielczarek, P.; Wirecka, R.; Berent, K.; Karewicz, A.; Szuwarzyński, M.; Zapotoczny, S. Nanohydrogels Based on Self-Assembly of Cationic Pullulan and Anionic Dextran Derivatives for Efficient Delivery of Piroxicam. Pharmaceutics 2019, 11, 622. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, S.; Avinash Chunduri, L.A.; Sai Akilesh, M.; Srimadh Bhagavatham, S.; Kamisetti, V. Enhanced dissolution characteristics of piroxicam-Soluplus® nanosuspensions. J. Exp. Nanosci. 2016, 11, 916–929. [Google Scholar] [CrossRef]
- Ustün Alkan, F.; Ustüner, O.; Bakırel, T.; Erten, G.; Deniz, G. The Effects of Piroxicam and Deracoxib on Canine Mammary Tumour Cell Line. Sci. World J. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
| C-CUR: A-HPC (w/w) | dz (nm) | PDI | ζ (mV) |
|---|---|---|---|
| 1:1 | 908 ± 54 | 0.751 | 13.6 ± 0.5 |
| 1:2 | 856 ± 160 | 0.859 | 15.7 ± 0.8 |
| 1:3 | 519 ± 89 | 0.652 | 11.2 ± 0.6 |
| 1:4 | 351 ± 30 | 0.860 | 14.7 ± 1.5 |
| 1:5 | 909 ± 54 | 0.751 | 13.6 ± 0.5 |
| 1:10 | 421 ± 10 | 0.793 | 6.2 ± 0.9 |
| 1:14 | 405 ± 84 | 0.572 | −0.1 ± 0.3 |
| 1:20 | 315 ± 2 | 0.223 | −1.0 ± 0.3 |
| 1:25 | 293 ± 1 | 0.197 | −3.1 ± 0.4 |
| 1:30 | 260 ± 3 | 0.260 | −4.2 ± 0.4 |
| Polymeric System | LCST (L)/UCST (U) (°C) |
|---|---|
| HPC | 43 (L) |
| A-HPC | 43 (L) |
| CUR | 47 (U) |
| C-CUR | not observed |
| C-CUR/A-HPC (1:25) | 41 (L) |
| Temperature (°C) | dz (nm) | PDI |
|---|---|---|
| 21 | 265 ± 2 | 0.234 |
| 24 | 256 ± 1 | 0.218 |
| 25 | 268 ± 2 | 0.195 |
| 27 | 256 ± 1 | 0.207 |
| 30 | 254 ± 1 | 0.218 |
| 33 | 254 ± 1 | 0.192 |
| 36 | 249 ± 3 | 0.194 |
| 39 | 239 ± 2 | 0.192 |
| 42 | 269 ± 1 | 0.184 |
| 45 | 401 ± 12 | 0.114 |
| 46 | 861 ± 31 | 0.259 |
| Model | 25 °C | 37 °C | 45 °C |
|---|---|---|---|
| Higuchi | |||
| kH | 27.3 ± 1.43 | 22.1 ± 2.76 | 57.3 ± 2.57 |
| R2 | 0.9306 | 0.9411 | 0.9534 |
| Peppas | |||
| a | 21.4 ± 1.67 | 57.3 ± 2.33 | 53.3 ± 3.02 |
| n | 0.594 ± 0.097 | 0.304 ± 0.031 | 0.427 ± 0.048 |
| R2 | 0.8991 | 0.9377 | 0.9302 |
| Weibull | |||
| a | 44.1 ± 13.29 | 88.7 ± 1.27 | 97.4 ± 10.87 |
| b | 0.99 ± 0.335 | 0.94 ± 0.061 | 0.90 ± 0.153 |
| K | 0.72 ± 0.443 | 0.99 ± 0.034 | 0.96 ± 0.25 |
| R2 | 0.9399 | 0.9985 | 0.9819 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lachowicz, D.; Kaczyńska, A.; Bodzon-Kulakowska, A.; Karewicz, A.; Wirecka, R.; Szuwarzyński, M.; Zapotoczny, S. Coacervate Thermoresponsive Polysaccharide Nanoparticles as Delivery System for Piroxicam. Int. J. Mol. Sci. 2020, 21, 9664. https://doi.org/10.3390/ijms21249664
Lachowicz D, Kaczyńska A, Bodzon-Kulakowska A, Karewicz A, Wirecka R, Szuwarzyński M, Zapotoczny S. Coacervate Thermoresponsive Polysaccharide Nanoparticles as Delivery System for Piroxicam. International Journal of Molecular Sciences. 2020; 21(24):9664. https://doi.org/10.3390/ijms21249664
Chicago/Turabian StyleLachowicz, Dorota, Agnieszka Kaczyńska, Anna Bodzon-Kulakowska, Anna Karewicz, Roma Wirecka, Michał Szuwarzyński, and Szczepan Zapotoczny. 2020. "Coacervate Thermoresponsive Polysaccharide Nanoparticles as Delivery System for Piroxicam" International Journal of Molecular Sciences 21, no. 24: 9664. https://doi.org/10.3390/ijms21249664
APA StyleLachowicz, D., Kaczyńska, A., Bodzon-Kulakowska, A., Karewicz, A., Wirecka, R., Szuwarzyński, M., & Zapotoczny, S. (2020). Coacervate Thermoresponsive Polysaccharide Nanoparticles as Delivery System for Piroxicam. International Journal of Molecular Sciences, 21(24), 9664. https://doi.org/10.3390/ijms21249664






