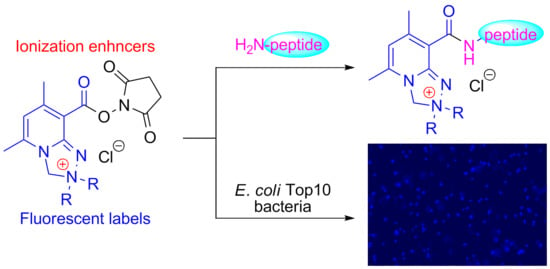
Application of Safirinium N-Hydroxysuccinimide Esters to Derivatization of Peptides for High-Resolution Mass Spectrometry, Tandem Mass Spectrometry, and Fluorescent Labeling of Bacterial Cells
Abstract
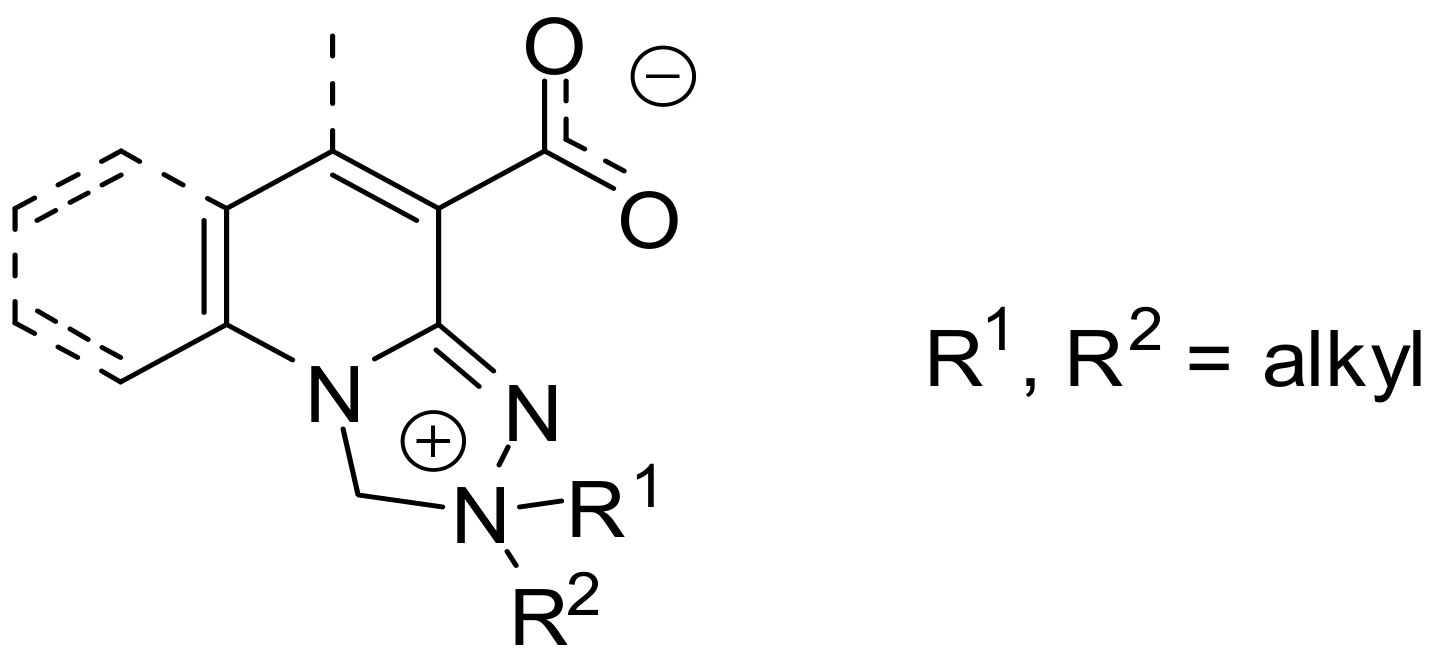
1. Introduction
2. Results and Discussion
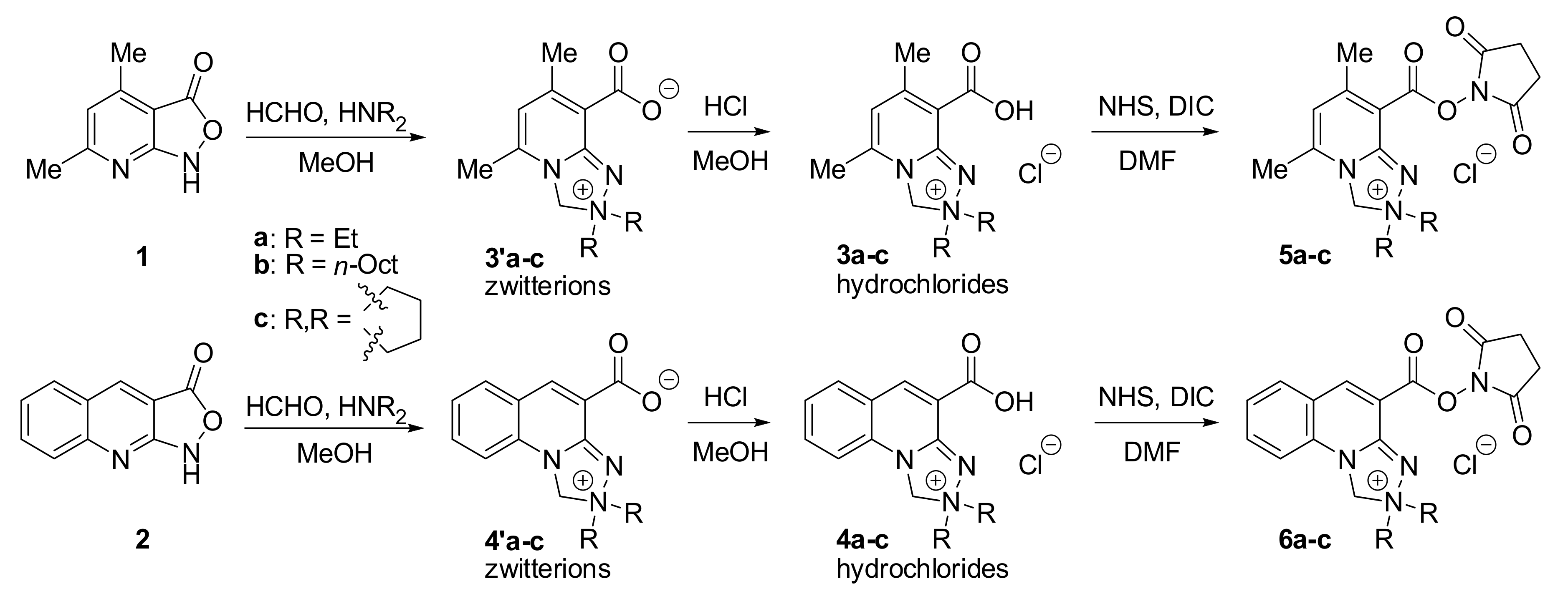
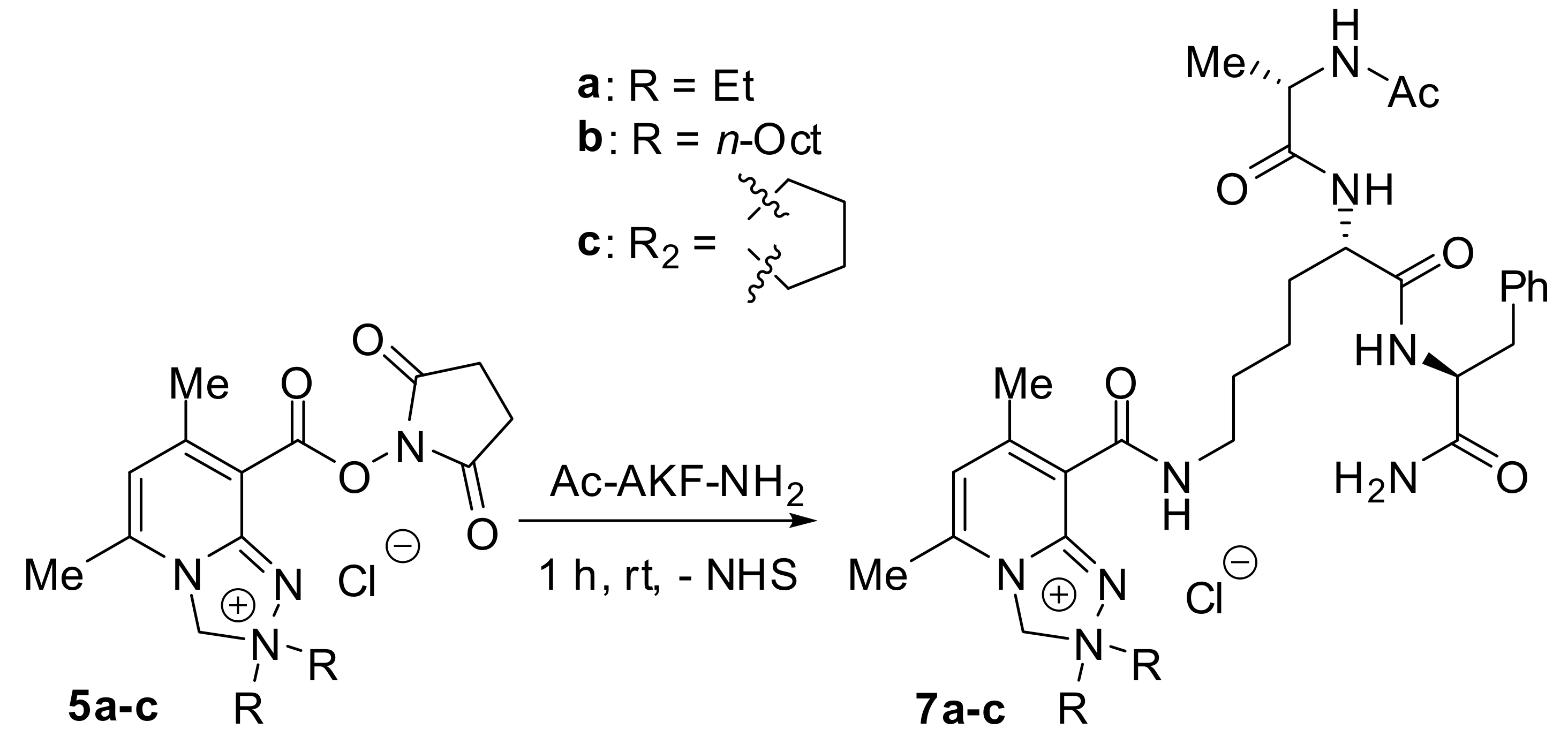
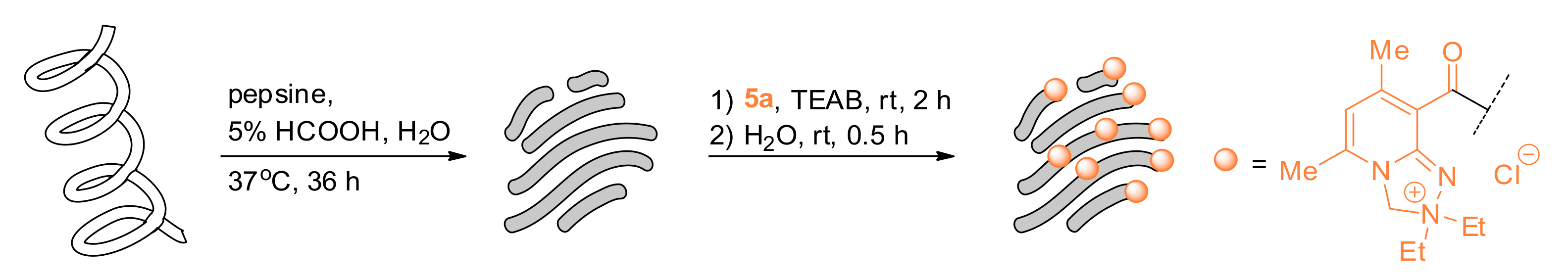
2.1. Chemistry
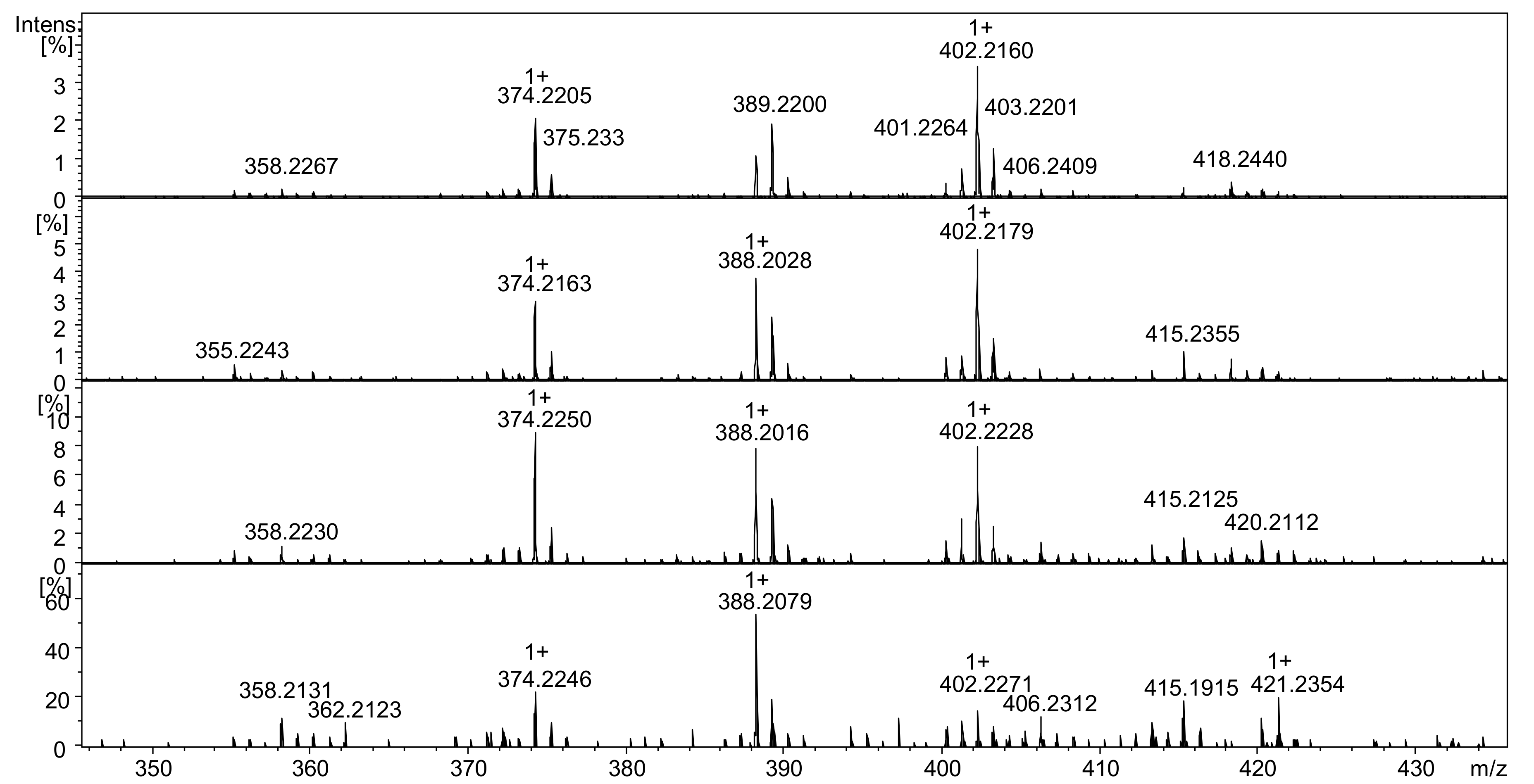
2.2. Mass Spectrometry Analysis
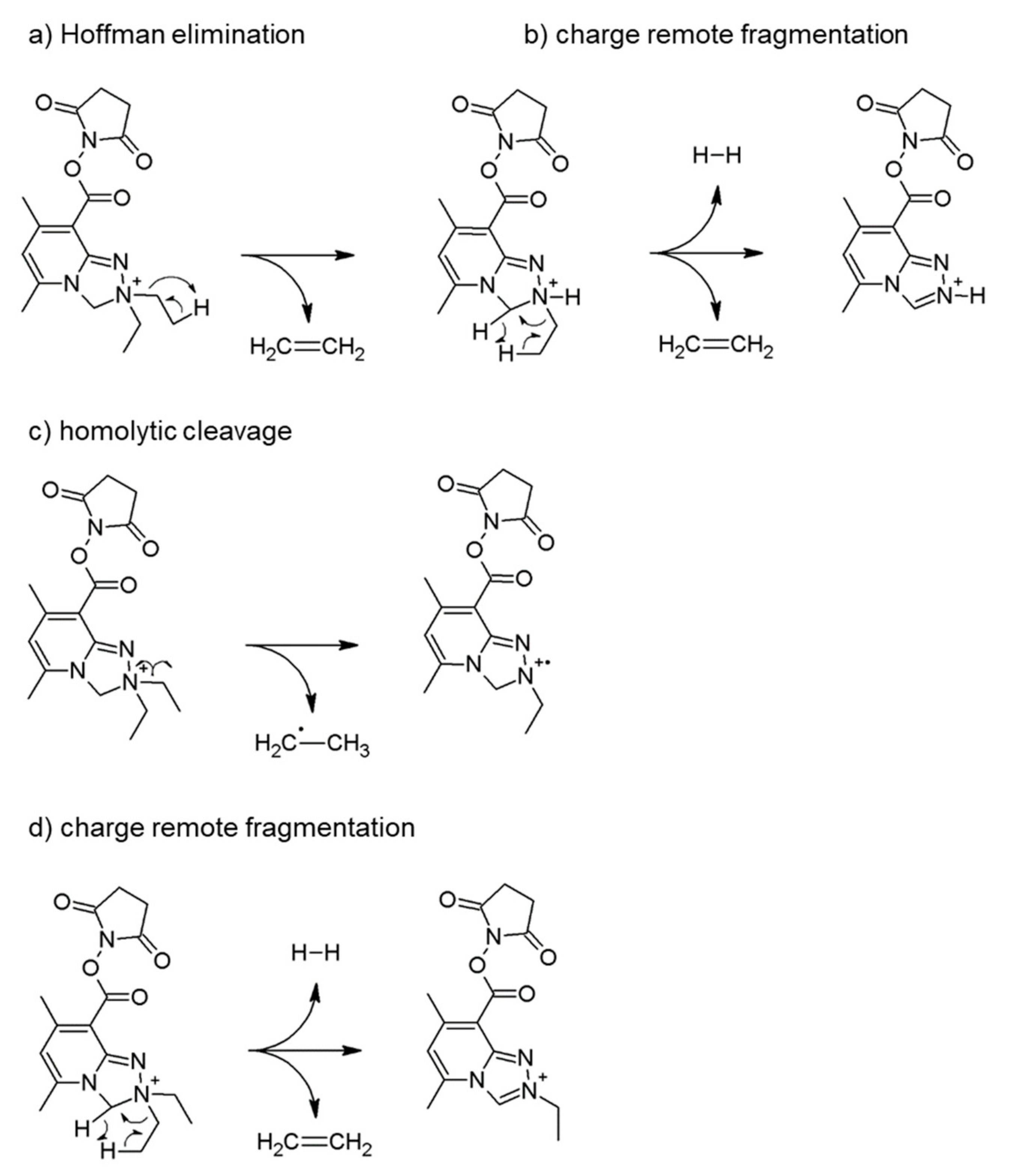
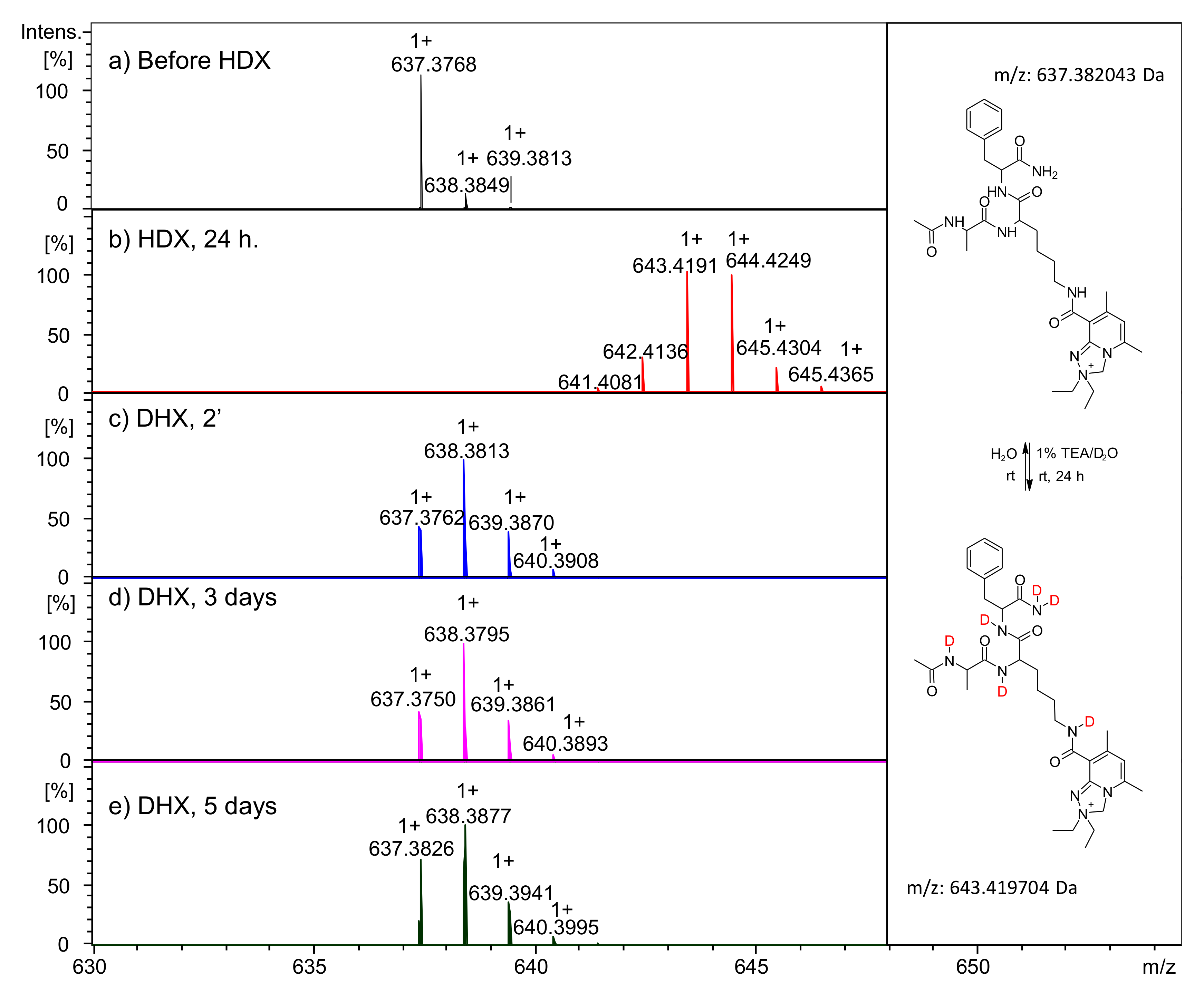
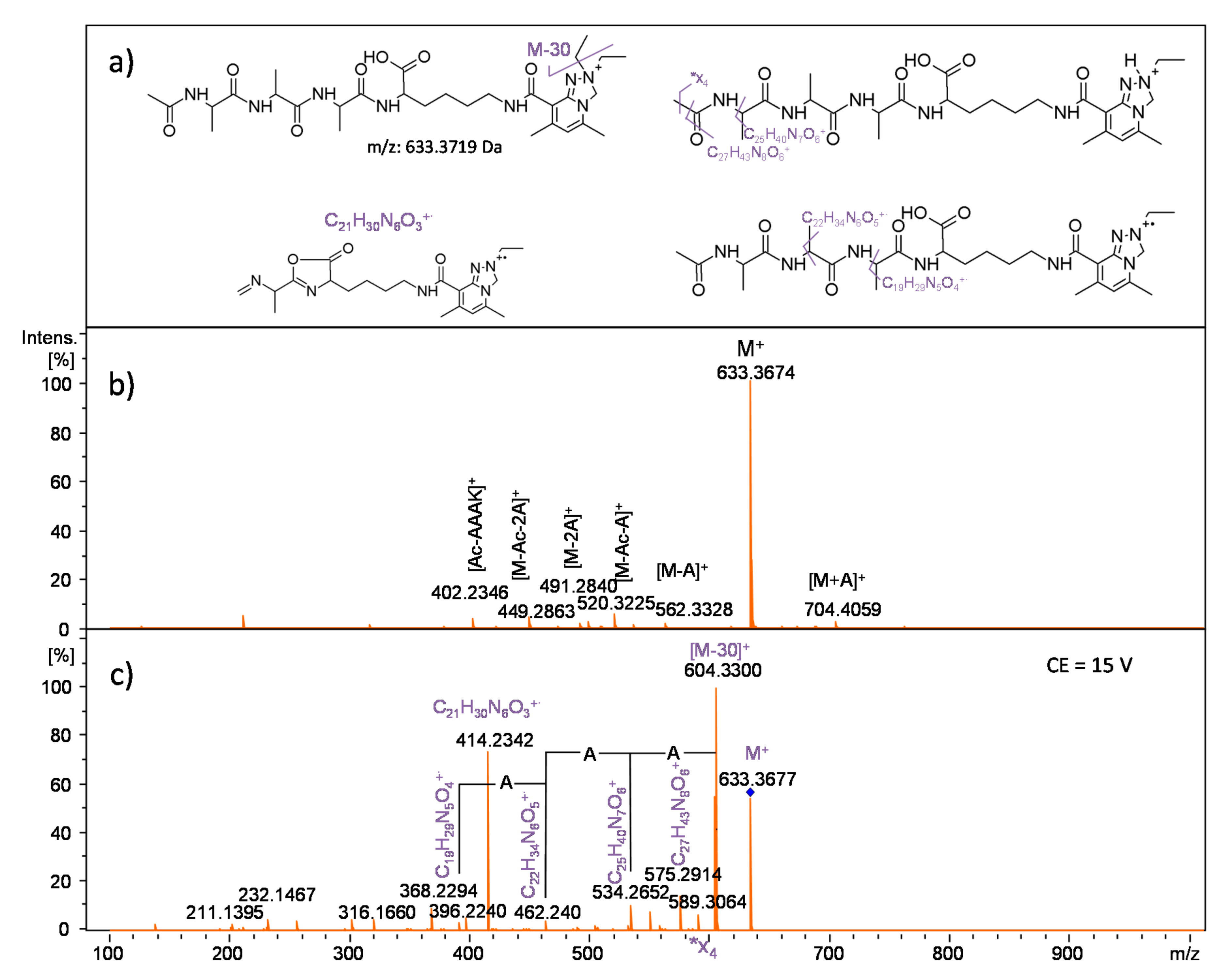
2.2.1. Analysis of Reactive NHS Ester 5a
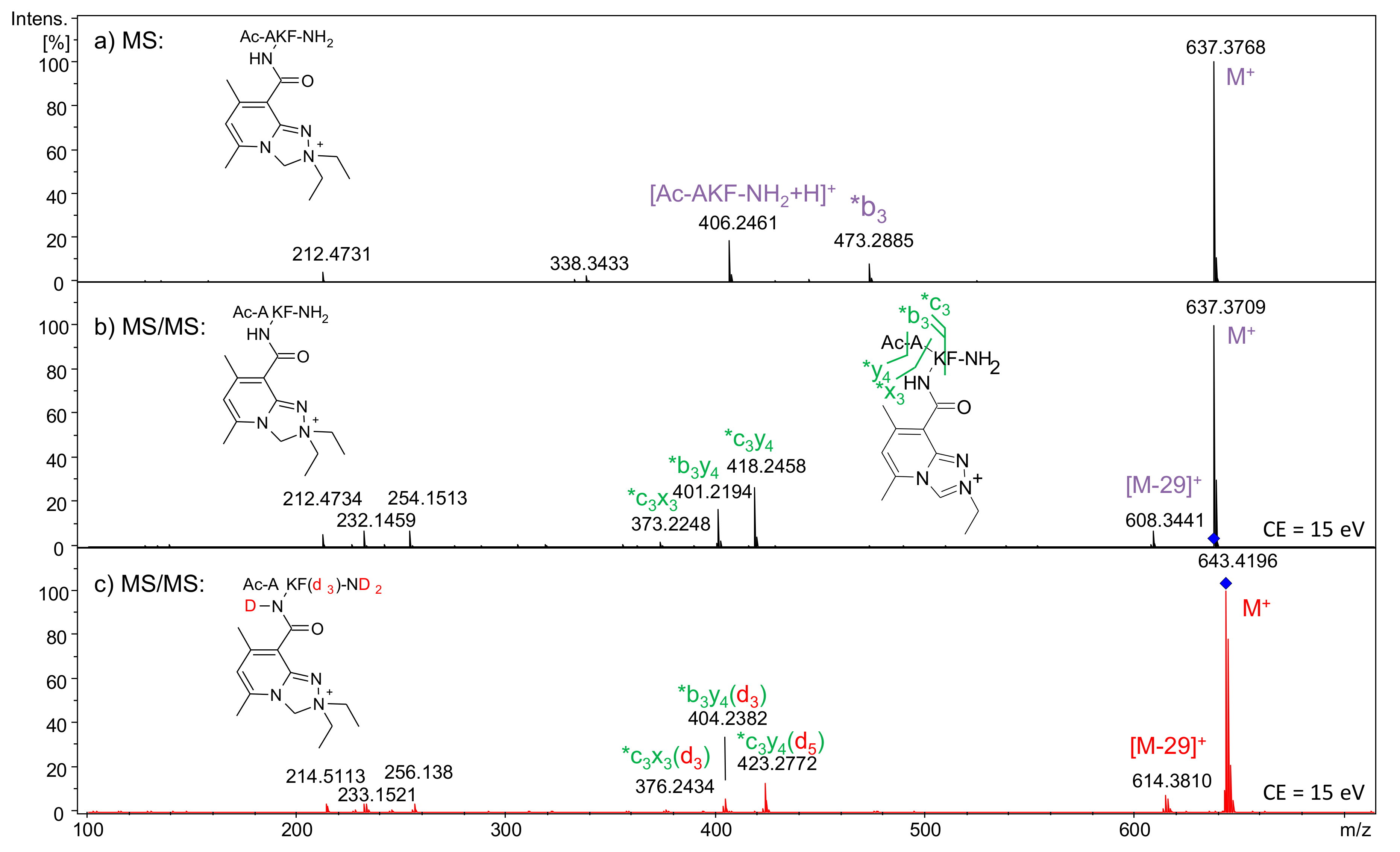
2.2.2. Analysis of Conjugate 7a, i.e., Peptide Ac-AKF-NH2 Tagged with Diethyl Derivative 5a
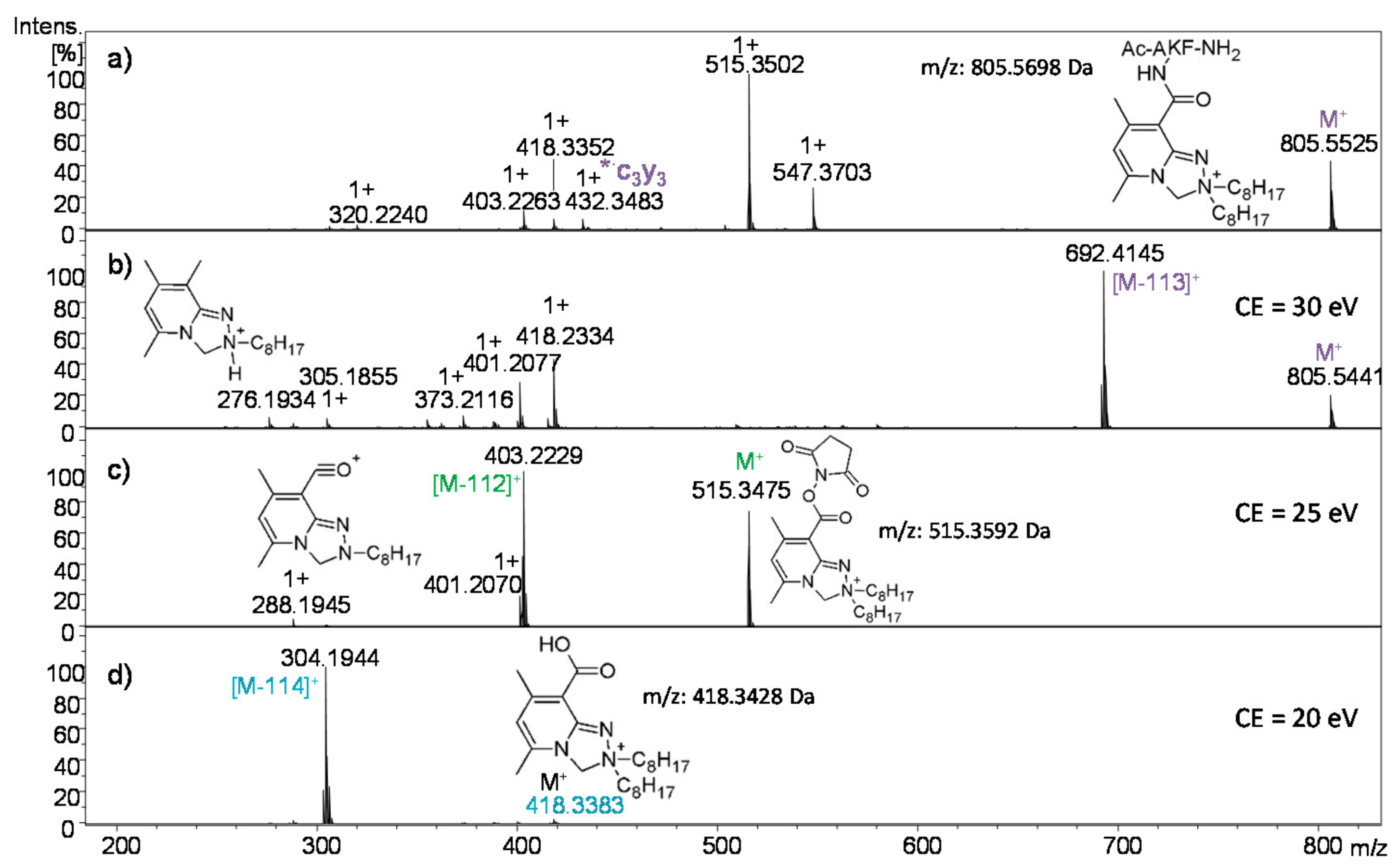
2.2.3. Analysis of Conjugate 7b, i.e., Peptide Ac-AKF-NH2 Tagged with Dioctyl Derivative 5b
2.2.4. Analysis of Conjugate 7c, i.e., Peptide Ac-AKF-NH2 Tagged with Pyrrolidine Derivative 5c
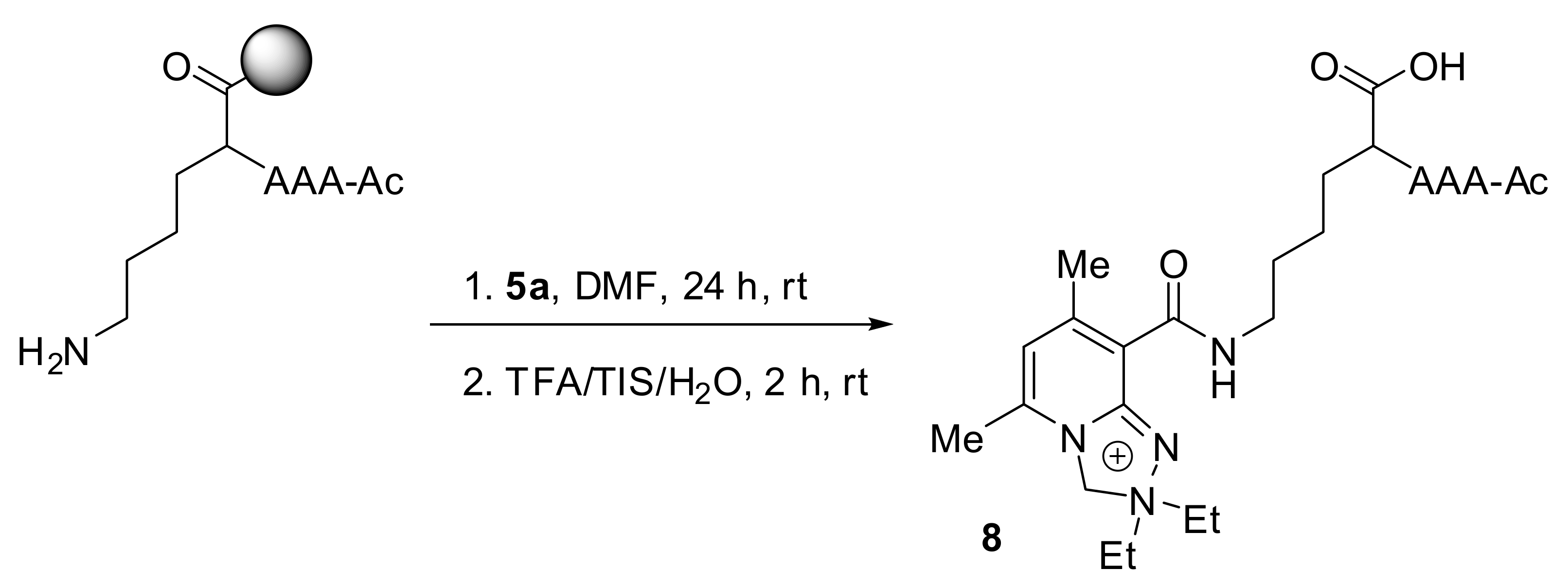
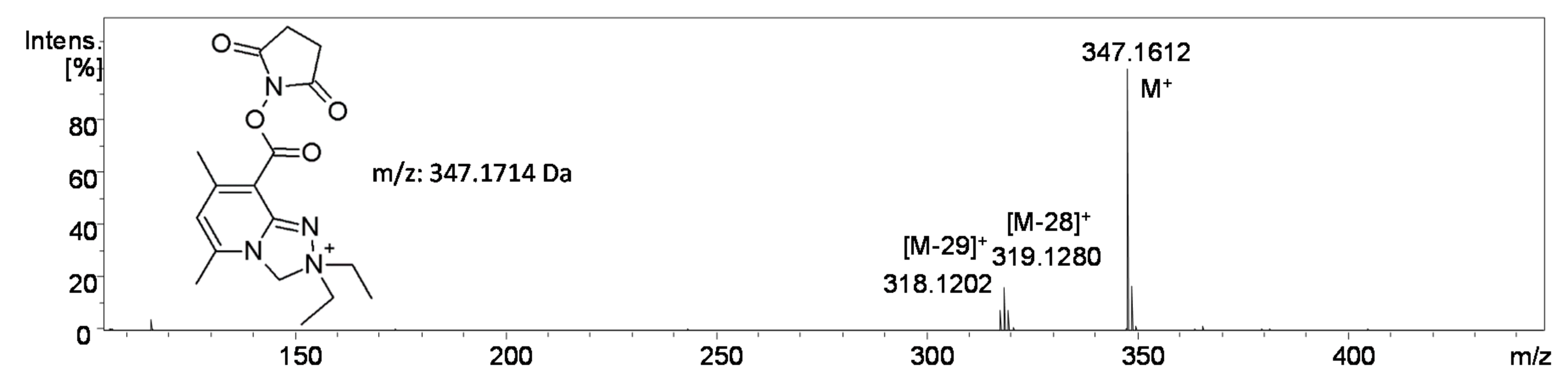
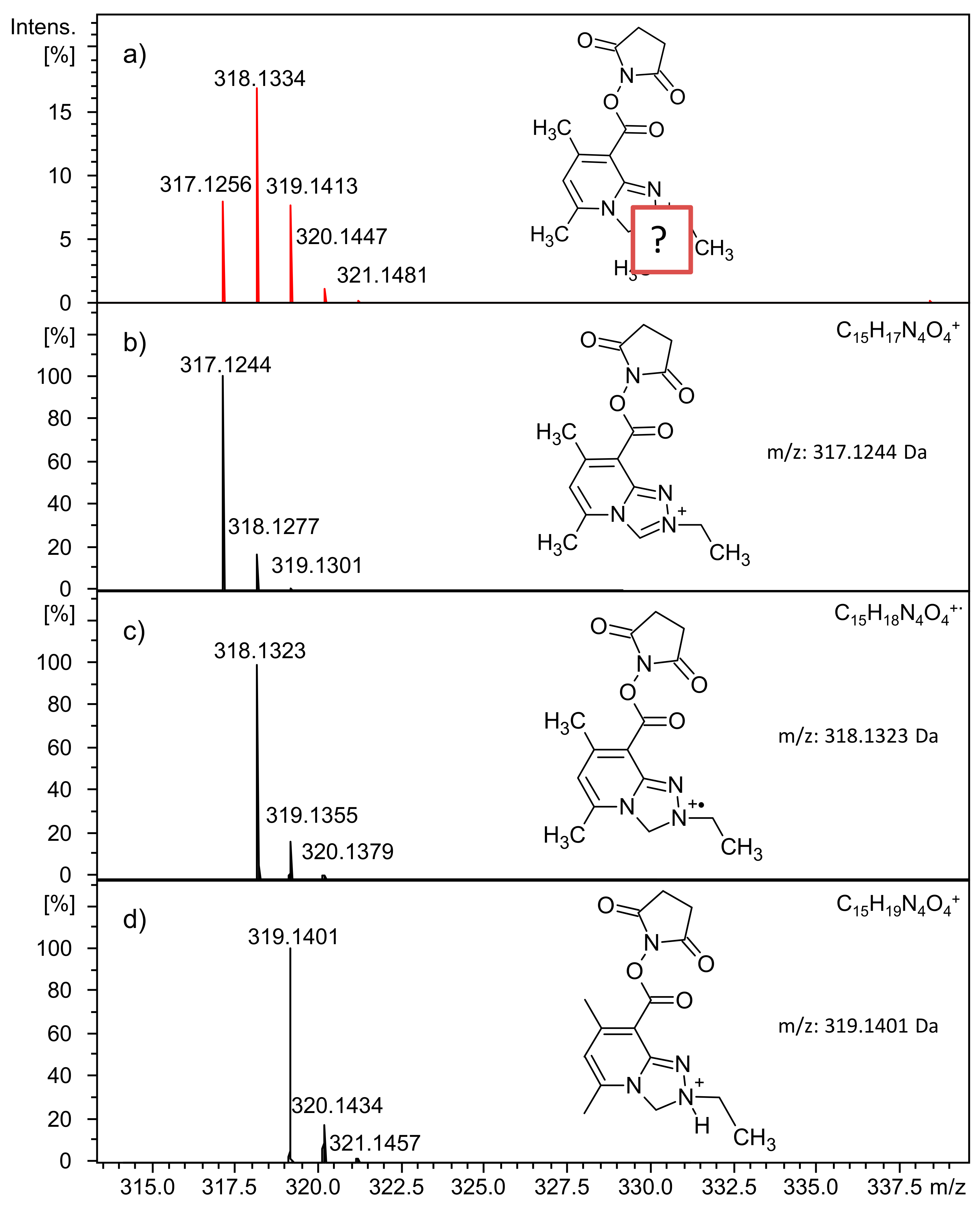
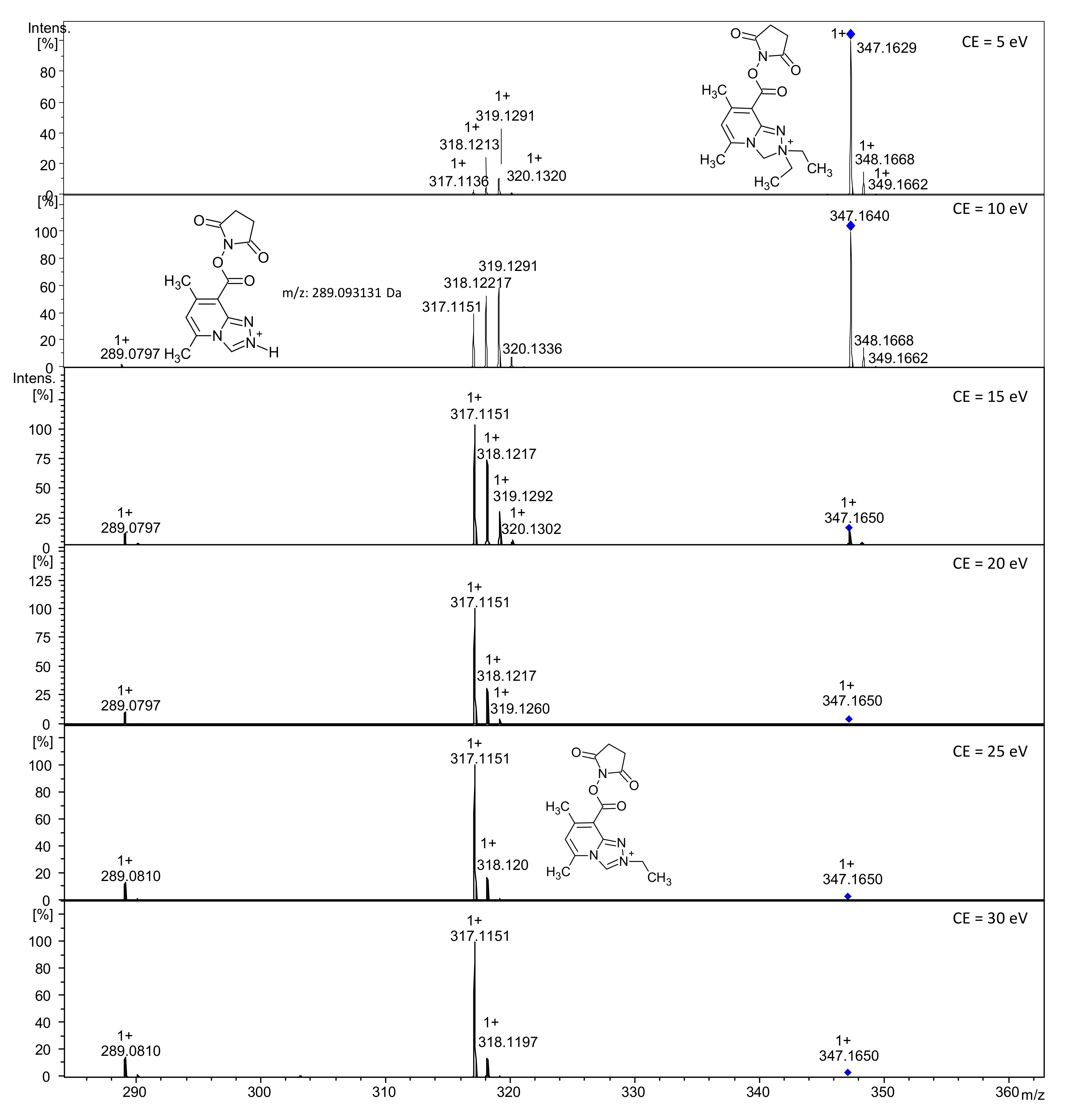
2.2.5. Analysis of Conjugate 8, i.e., Tetrapeptide Ac-AAAK Tagged with Diethyl Derivative 5a
2.3. Derivatization of Ubiquitin Hydrolysate with Reagent 5a
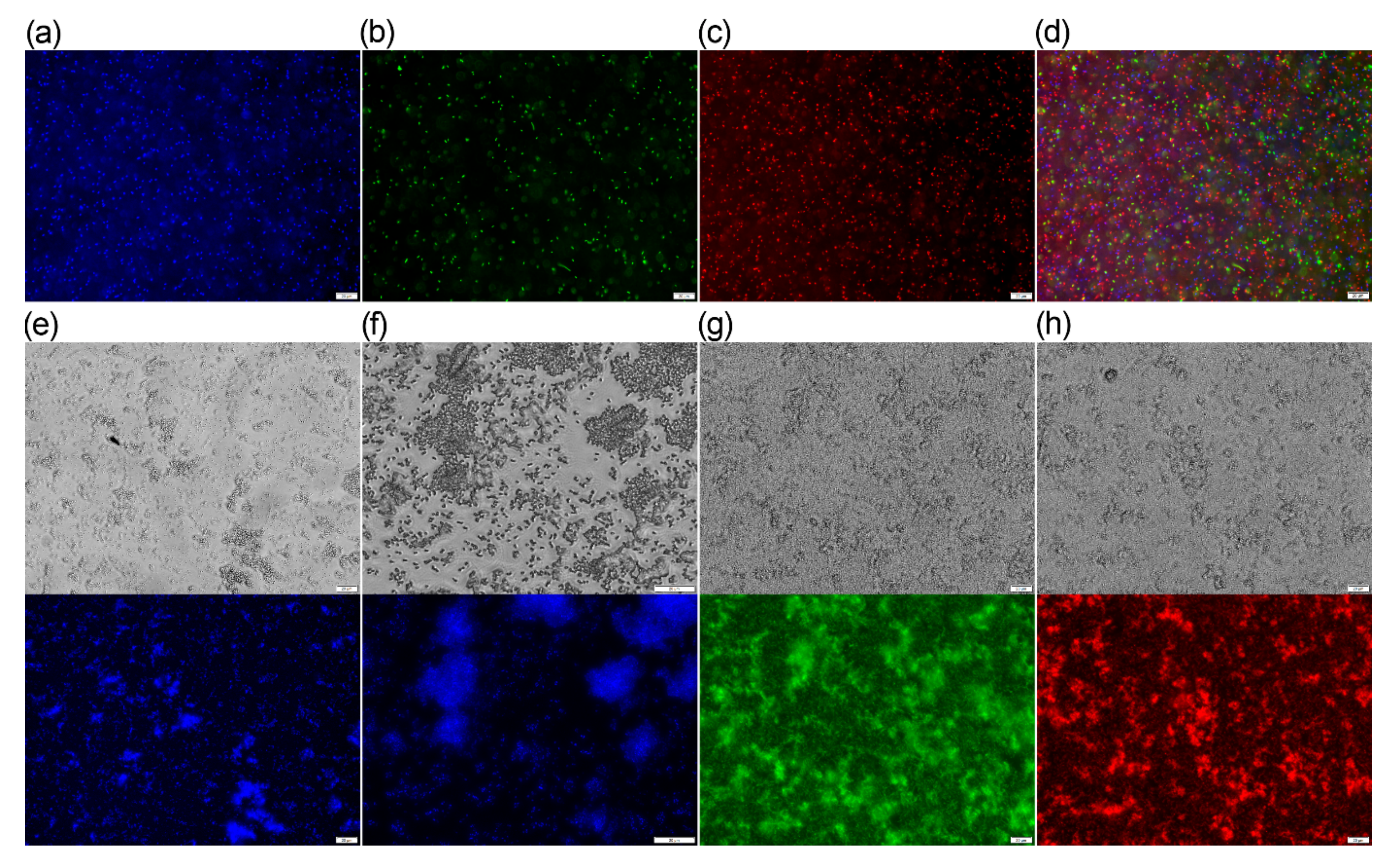
2.4. Fluorescence Microscopy
3. Materials and Methods
3.1. General Information
3.2. Chemical Synthesis
3.2.1. Synthesis of 8-Carboxy-5,7-Dimethyl-2,2-Dioctyl-2,3-Dihydro-[1,2,4]Triazolo[4,3-a]Pyridin-2-ium Chloride (3b)
3.2.2. Synthesis of Reactive Safirinium P (5b,c) and Q (6b,c) Probes
3.2.3. Synthesis of Peptide Conjugates 7a-c
3.2.4. Synthesis of the Derivatized Peptide 8
3.3. Tagging the Ubiquitin Hydrolysate with 5a
3.4. Fluorescence Microscopy of Bacteria
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CID | collision-induced dissociation |
| DCM | dichloromethane |
| EIC | extracted ion chromatogram |
| ESI | electrospray ionization |
| FT-ICR | Fourier-Transform Ion-Cyclotrone-Resonance |
| DIC | N,N-diisopropylcarbodiimide |
| DMF | dimethylformamide |
| DHX | deuterium-hydrogen-exchange |
| GFP | green fluorescence protein |
| HDX | hydrogen-deuterium exchange |
| HPLC | high performance liquid chromatography |
| ITs | ionization tags |
| LB | Luria broth |
| LC | liquid chromatography |
| MRM | multiple reaction monitoring |
| MS | mass spectrometry |
| MS/MS | tandem mass spectrometry |
| NHS | N-hydroxysuccinimide |
| NMR | nuclear magnetic resonance |
| OD | optical density |
| Oct | octyl |
| PAGE | polyacrylamide gel electrophoresis |
| PBS | phosphate-buffered saline |
| QAS | quaternary ammonium salts |
| RP | reverse-phase |
| TEA | triethylamine |
| TEAB | triethylammonium bicarbonate |
| TFA | trifuoroacetic acid |
| TIC | total ion chromatogram |
| TIS | triisopropylsilane |
| TRITC | tetramethylrhodamine-isothiocyanate |
References
- Guzmán-Flores, J.M.; Flores-Pérez, E.C.; Hernández-Ortiz, M.; Vargas-Ortiz, K.; Ramírez-Emiliano, J.; Encarnación-Guevara, S.; Pérez-Vázquez, V. Protein Expression Profile of Twenty-Week-Old Diabetic db/db and Non-Diabetic Mice Livers: A Proteomic and Bioinformatic Analysis. Biomolecules 2018, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, A.; Zhou, M.; Nguyen, A.Y.; Liberton, M.; Kedia, K.; Shi, T.; Piehowski, P.; Shukla, A.; Fillmore, T.L.; Nicora, C.; et al. Proteomic Insights into Phycobilisome Degradation, A Selective and Tightly Controlled Process in The Fast-Growing Cyanobacterium Synechococcus elongatus UTEX 2973. Biomolecules 2019, 9, 374. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.-L.; Liu, Y.-N.; Liu, R.; Ren, A.; Ma, H.-Y.; Shu, L.-B.; Shi, L.; Zhu, J.; Zhao, M.-W. Integrated Proteomics and Metabolomics Analysis Provides Insights into Ganoderic Acid Biosynthesis in Response to Methyl Jasmonate in Ganoderma Lucidum. Int. J. Mol. Sci. 2019, 20, 6116. [Google Scholar] [CrossRef] [PubMed]
- L’Hocine, L.; Pitre, M.; Achouri, A. Detection and Identification of Allergens from Canadian Mustard Varieties of Sinapis alba and Brassica juncea. Biomolecules 2019, 9, 489. [Google Scholar] [CrossRef]
- Soboleva, A.; Modzel, M.; Didio, A.; Płóciennik, H.; Kijewska, M.; Grischina, T.; Karonova, T.; Bilova, T.; Stefanov, V.; Stefanowicz, P.; et al. Quantification of prospective type 2 diabetes mellitus biomarkers by stable isotope dilution with bi-labeled standard glycated peptides. Anal. Methods 2017, 9, 409–418. [Google Scholar] [CrossRef]
- Verrastro, I.; Pasha, S.; Jensen, K.T.; Pitt, A.R.; Spickett, C.M. Mass Spectrometry-Based Methods for Identifying Oxidized Proteins in Disease: Advances and Challenges. Biomolecules 2015, 5, 378–411. [Google Scholar] [CrossRef]
- Jamnongkan, W.; Lebrilla, C.B.; Barboza, M.; Techasen, A.; Loilome, W.; Sithithaworn, P.; Khuntikeo, N.; Pairojkul, C.; Chamadol, N.; Thanan, R.; et al. Discovery of Serotransferrin Glycoforms: Novel Markers for Diagnosis of Liver Periductal Fibrosis and Prediction of Cholangiocarcinoma. Biomolecules 2019, 9, 538. [Google Scholar] [CrossRef]
- Cebo, M.; Kielmas, M.; Adamczyk, J.; Cebrat, M.; Szewczuk, Z.; Stefanowicz, P. Hydrogen–deuterium exchange in imidazole as a tool for studying histidine phosphorylation. Anal. Bioanal. Chem. 2014, 406, 8013–8020. [Google Scholar] [CrossRef]
- Lu, Z.-S.; Chen, Q.-S.; Zheng, Q.-X.; Shen, J.-J.; Luo, Z.-P.; Fan, K.; Xu, S.-H.; Shen, Q.; Liu, P.-P. Proteomic and Phosphoproteomic Analysis in Tobacco Mosaic Virus-Infected Tobacco (Nicotiana tabacum). Biomolecules 2019, 9, 39. [Google Scholar] [CrossRef]
- Kozuka-Hata, H.; Kitamura, A.; Hiroki, T.; Aizawa, A.; Tsumoto, K.; Inoue, J.-I.; Oyama, M. System-Wide Analysis of Protein Acetylation and Ubiquitination Reveals a Diversified Regulation in Human Cancer Cells. Biomolecules 2020, 10, 411. [Google Scholar] [CrossRef]
- Purushothaman, K.; Das, P.P.; Presslauer, C.; Lim, T.K.; Johansen, S.D.; Lin, Q.; Babiak, I. Proteomics Analysis of Early Developmental Stages of Zebrafish Embryos. Int. J. Mol. Sci. 2019, 20, 6359. [Google Scholar] [CrossRef] [PubMed]
- Kęska, P.; Wójciak, K.M.; Stadnik, J. Effect of Marination Time on the Antioxidant Properties of Peptides Extracted from Organic Dry-Fermented Beef. Biomolecules 2019, 9, 614. [Google Scholar] [CrossRef] [PubMed]
- Proaño-Bolaños, C.; Blasco-Zúñiga, A.; Almeida, J.R.; Wang, L.; Llumiquinga, M.A.; Rivera, M.; Zhou, M.; Chen, T.; Shaw, C. Unravelling the Skin Secretion Peptides of the Gliding Leaf Frog, Agalychnis spurrelli (Hylidae). Biomolecules 2019, 9, 667. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Fishman, M.A.; Castro, L.M.; Tashima, A.K.; Ferro, E.S.; Fricker, L.D. Effect of Protein Denaturation and Enzyme Inhibitors on Proteasomal-Mediated Production of Peptides in Human Embryonic Kidney Cells. Biomolecules 2019, 9, 207. [Google Scholar] [CrossRef] [PubMed]
- Setner, B.; Stefanowicz, P.; Szewczuk, Z. Quaternary ammonium isobaric tag for a relative and absolute quantification of peptides. J. Mass Spectrom. 2018, 53, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Bąchor, R.; Waliczek, M.; Stefanowicz, P.; Szewczuk, Z. Trends in the Design of New Isobaric Labeling Reagents for Quantitative Proteomics. Molecules 2019, 24, 701. [Google Scholar] [CrossRef]
- Waliczek, M.; Bąchor, R.; Kijewska, M.; Gąszczyk, D.; Panek-Laszczyńska, K.; Konieczny, A.; Dąbrowska, K.; Witkiewicz, W.; Marek-Bukowiec, K.; Tracz, J.; et al. Isobaric duplex based on a combination of 16O/18O enzymatic exchange and labeling with pyrylium salts. Anal. Chim. Acta 2019, 1048, 96–104. [Google Scholar] [CrossRef]
- Bąchor, R.; Dębowski, D.; Łęgowska, A.; Stefanowicz, P.; Rolka, K.; Szewczuk, Z. Convenient preparation of deuterium-labeled analogs of peptides containing N-substituted glycines for a stable isotope dilution LC-MS quantitative analysis. J. Pept. Sci. 2015, 21, 819–825. [Google Scholar] [CrossRef]
- Aoki, M.M.; Kisiala, A.B.; Li, S.; Stock, N.L.; Brunetti, C.R.; Huber, R.J.; Emery, R.J.N. Cytokinin Detection during the Dictyostelium discoideum Life Cycle: Profiles Are Dynamic and Affect Cell Growth and Spore Germination. Biomolecules 2019, 9, 702. [Google Scholar] [CrossRef]
- Modzel, M.; Płóciennik, H.; Kielmas, M.; Szewczuk, Z.; Stefanowicz, P. A synthesis of new, bi-labeled peptides for quantitative proteomics. J. Proteom. 2015, 115, 1–7. [Google Scholar] [CrossRef]
- Bąchor, R.; Kluczyk, A.; Stefanowicz, P.; Szewczuk, Z. Preparation of novel deuterated cyclosporin A standards for quantitative LC-MS analysis. J. Mass Spectrom. 2017, 52, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Slavata, L.; Chmelík, J.; Kavan, D.; Filandrová, R.; Fiala, J.; Rosůlek, M.; Mrázek, H.; Kukačka, Z.; Vališ, K.; Man, P.; et al. MS-Based Approaches Enable the Structural Characterization of Transcription Factor/DNA Response Element Complex. Biomolecules 2019, 9, 535. [Google Scholar] [CrossRef] [PubMed]
- Bąchor, R.; Cydzik, M.; Rudowska, M.; Kluczyk, A.; Stefanowicz, P.; Szewczuk, Z. Sensitive electrospray mass spectrometry analysis of one-bead-one-compound peptide libraries labeled by quaternary ammonium salts. Mol. Divers. 2012, 16, 613–618. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bąchor, R.; Mielczarek, P.; Rudowska, M.; Silberring, J.; Szewczuk, Z. Sensitive detection of charge derivatized peptides at the attomole level using nano-LC-ESI–MRM analysis. Int. J. Mass Spectrom. 2014, 362, 32–38. [Google Scholar] [CrossRef]
- Kijewska, M.; Kuc, A.; Kluczyk, A.; Waliczek, M.; Man-Kupisinska, A.; Lukasiewicz, J.; Stefanowicz, P.; Szewczuk, Z. Selective Detection of Carbohydrates and Their Peptide Conjugates by ESI-MS Using Synthetic Quaternary Ammonium Salt Derivatives of Phenylboronic Acids. J. Am. Soc. Mass Spectrom. 2014, 25, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Cydzik, M.; Rudowska, M.; Stefanowicz, P.; Szewczuk, Z. Derivatization of peptides as quaternary ammonium salts for sensitive detection by ESI-MS. J. Pept. Sci. 2011, 17, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Setner, B.; Wierzbicka, M.; Jerzykiewicz, L.; Lisowski, M.; Szewczuk, Z. The unexpected racemization and hydrogen–deuterium exchange of the hydrogen at the α-carbon of proline analogs containing the 5-azoniaspiro[4.4]nonyl-group. Org. Biomol. Chem. 2018, 16, 825–831. [Google Scholar] [CrossRef]
- Setner, B.; Rudowska, M.; Klem, E.; Cebrat, M.; Szewczuk, Z. Peptides derivatized with bicyclic quaternary ammonium ionization tags. Sequencing via tandem mass spectrometry. J. Mass Spectrom. 2014, 49, 995–1001. [Google Scholar] [CrossRef]
- Setner, B.; Rudowska, M.; Kluczyk, A.; Stefanowicz, P.; Szewczuk, Z. The 5-azoniaspiro[4.4]nonyl group for improved MS peptide analysis: A novel non-fragmenting ionization tag for mass spectrometric sensitive sequencing of peptides. Anal. Chim. Acta 2017, 986, 71–81. [Google Scholar] [CrossRef]
- Setner, B.; Szewczuk, Z. New ionization tags based on the structure of the 5-azoniaspiro[4.4]nonyl tag for a sensitive peptide sequencing by mass spectrometry. Anal Bioanal Chem. 2014, 410, 1311–1321. [Google Scholar] [CrossRef]
- Fink, J.; Pathak, H.; Smith, J.; Achat-Mendes, C.; Haining, R.L. Development of a Competition-Binding Assay to Determine Binding Affinity of Molecules to Neuromelanin via Fluorescence Spectroscopy. Biomolecules 2019, 9, 175. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.C.B.; Mariz, I.d.F.A.; Maçoas, E.M.S.; Tonelli, R.R.; Martinho, J.M.G.; Quina, F.H.; Bastos, E.L. Bioinspired water-soluble two-photon fluorophores. Dyes Pigment. 2018, 150, 105–111. [Google Scholar] [CrossRef]
- Bodio, E.; Goze, C. Investigation of B-F substitution on BODIPY and aza-BODIPY dyes: Development of B-O and B-C BODIPYs. Dyes Pigment. 2019, 160, 700–710. [Google Scholar] [CrossRef]
- Bernhard, Y.; Richard, P.; Decreau, R.A. Addressing subphthalocyanines and subnaphthalocyanines features relevant to fluorescence imaging. Tetrahedron 2018, 74, 1047–1052. [Google Scholar] [CrossRef]
- Rezende, L.C.D.; Melo, S.M.G.; Boodts, S.; Verbelen, B.; Emery, F.S.; Dehaen, W. Thiocyanation of 3-substituted and 3,5-disubstituted BODIPYs and its application for the synthesis of new fluorescent sensors. Dyes Pigment. 2018, 154, 155–163. [Google Scholar] [CrossRef]
- Pidluzhna, A.; Ivaniuk, K.; Stakhira, P.; Hotra, Z.; Chapran, M.; Ulanski, J.; Tynkevych, O.; Khalavka, Y.; Baryshnikov, G.V.; Minaev, B.F.; et al. Multi-channel electroluminescence of CdTe/CdS core-shell quantum dots implemented into a QLED device. Dyes Pigment. 2019, 162, 647–653. [Google Scholar] [CrossRef]
- Oliveira, E.; Santos, H.M.; Jorge, S.; Rodríguez-González, B.; Novio, F.; Lorenzo, J.; Ruiz-Molina, D.; Luis Capelo, J.; Lodeiro, C. Sustainable synthesis of luminescent CdTe quantum dots coated with modified silica mesoporous nanoparticles: Towards new protein scavengers and smart drug delivery carriers. Dyes Pigment. 2019, 161, 360–369. [Google Scholar] [CrossRef]
- Zahid, M.; Feldman, K.S.; Garcia-Borrero, G.; Feinstein, T.N.; Pogodzinski, N.; Xu, X.; Yurko, R.; Czachowski, M.; Wu, Y.L.; Mason, N.S.; et al. Cardiac Targeting Peptide, a Novel Cardiac Vector: Studies in Bio-Distribution, Imaging Application, and Mechanism of Transduction. Biomolecules 2018, 8, 147. [Google Scholar] [CrossRef]
- Kiyama, M.; Iwano, S.; Otsuka, S.; Lu, S.W.; Obata, R.; Miyawaki, A.; Hirano, T.; Maki, S.A. Quantum yield improvement of red-light-emitting firefly luciferin analogues for in vivo bioluminescence imaging. Tetrahedron 2018, 74, 652–660. [Google Scholar] [CrossRef]
- Hanif, M.; Rafiq, M.; Yousuf, M.; Kotwica-Mojzych, K.; Saleem, M.; Mojzych, M. Organic small molecular receptors as fluorimetric/bioimaging probe for extracellular/intracellular zinc sensation. Bioorg. Chem. 2020, 94, 103398. [Google Scholar] [CrossRef]
- Váradi, J.; Hermenean, A.; Gesztelyi, R.; Jeney, V.; Balogh, E.; Majoros, L.; Malanga, M.; Fenyvesi, É.; Szente, L.; Bácskay, I.; et al. Pharmacokinetic Properties of Fluorescently Labelled Hydroxypropyl-Beta-Cyclodextrin. Biomolecules 2019, 9, 509. [Google Scholar] [CrossRef] [PubMed]
- Saczewski, J.; Hinc, K.; Obuchowski, M.; Gdaniec, M. The tandem Mannich–electrophilic amination reaction: A versatile platform for fluorescent probing and labelling. Chem. Eur. J. 2013, 19, 11531–11535. [Google Scholar] [CrossRef] [PubMed]
- Fedorowicz, J.; Sączewski, J.; Drażba, Z.; Wiśniewska, P.; Gdaniec, M.; Wicher, B.; Suwiński, G.; Jalińska, A. Synthesis and fluorescence of dihydro-[1,2,4]triazolo[4,3-a]pyridin-2-iumcarboxylates: An experimental and TD-DFT comparative study. Dyes Pigment. 2019, 161, 347–359. [Google Scholar] [CrossRef]
- Fedorowicz, J.; Sączewski, J.; Konopacka, A.; Waleron, K.; Lejnowski, D.; Ciura, K.; Tomasic, T.; Skok, Z.; Savijoki, K.; Morawska, M.; et al. Synthesis and biological evaluation of hybrid quinolone-based quaternary ammonium antibacterial agents. Eur. J. Med. Chem. 2019, 179, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Ciura, K.; Fedorowicz, J.; Andrić, F.; Greber, K.E.; Gurgielewicz, A.; Sawicki, W.; Sączewski, J. Lipophilicity Determination of Quaternary (Fluoro) Quinolones by Chromatographic and Theoretical Approaches. Int. J. Mol. Sci. 2019, 20, 5288. [Google Scholar] [CrossRef] [PubMed]
- Ciura, K.; Fedorowicz, J.; Kapica, H.; Adamkowska, A.; Sawicki, W.; Sączewski, J. Affinity of Fluoroquinolone–Safirinium Dye Hybrids to Phospholipids Estimated by IAM-HPLC. Processes 2020, 8, 1148. [Google Scholar] [CrossRef]
- Kraft, O.; Kozubek, M.; Hoenke, S.; Serbian, I.; Major, D.; Csuk, R. Cytotoxic triterpenoid-safirinium conjugates target the endoplasmic reticulum. Eur. J. Med. Chem. 2021, 209, 112920. [Google Scholar] [CrossRef]
- Fedorowicz, J.; Cebrat, M.; Wierzbicka, M.; Wiśniewska, P.; Jalińska, A.; Dziomba, S.; Gdaniec, M.; Jaremko, M.; Jaremko, Ł.; Chandra, K.; et al. Synthesis and evaluation of dihydro-[1,2,4]triazolo[4,3-a]pyridin-2-ium carboxylates as fixed charge fluorescent derivatization reagents for MEKC and MS proteomic analyses. J. Mol. Struct. 2020, 1217, 128426. [Google Scholar] [CrossRef]
- Cydzik, M.; Rudowska, M.; Stefanowicz, P.; Szewczuk, Z. The Competition of Charge Remote and Charge Directed Fragmentation Mechanisms in Quaternary Ammonium Salt Derivatized Peptides—An Isotopic Exchange Study. J. Am. Soc. Mass Spectrom. 2011, 22, 2103–2107. [Google Scholar] [CrossRef]
- Gross, M.L. Charge-remote fragmentations: Method, mechanism and applications. Int. J. Mass Spectrom. Ion Process. 1992, 118–119, 137–165. [Google Scholar] [CrossRef]
- Demarque, D.P.; Crotti, A.E.M.; Vessecchi, R.; Lopes, J.L.C.; Lopes, N.P. Fragmentation reactions using electrospray ionization mass spectrometry: An important tool for the structural elucidation and characterization of synthetic and natural products. Nat. Prod. Rep. 2016, 33, 432–455. [Google Scholar] [CrossRef] [PubMed]
- Setner, B.; Rudowska, M.; Wojewska, D.; Kluczyk, A.; Stefanowicz, P.; Szewczuk, Z. Peptides labeled by 5-azoniaspiro[4.4]nonyl group for sensitive sequencing by electrospray tandem mass spectrometry. J. Pept. Sci. 2014, 20, S64–S65. [Google Scholar] [CrossRef]
- Syka, J.E.P.; Coon, J.J.; Schroeder, M.J.; Shabanowitz, J.; Hunt, D.F. Peptide and protein sequence analysis by electron transfer dissociation mass spectrometry. Proc. Natl. Acad. Sci. USA 2004, 101, 9528–9533. [Google Scholar] [CrossRef] [PubMed]
- Jaremko, Ł.; Jaremko, M.; Pasikowski, P.; Cebrat, M.; Stefanowicz, P.; Lisowski, M.; Artym, J.; Zimecki, M.; Zhukov, I.; Szewczuk, Z. The immunosuppressive activity and solution structures of ubiquitin fragments. Biopolymers 2009, 91, 423–431. [Google Scholar] [CrossRef]
- Sączewski, J.; Fedorowicz, J.; Korcz, M.; Sączewski, F.; Wicher, B.; Gdaniec, M.; Konopacka, A. Experimental and theoretical studies on the tautomerism and reactivity of isoxazolo[3,4-b]quinolin-3(1H)-ones. Tetrahedron 2015, 71, 8975–8984. [Google Scholar] [CrossRef]

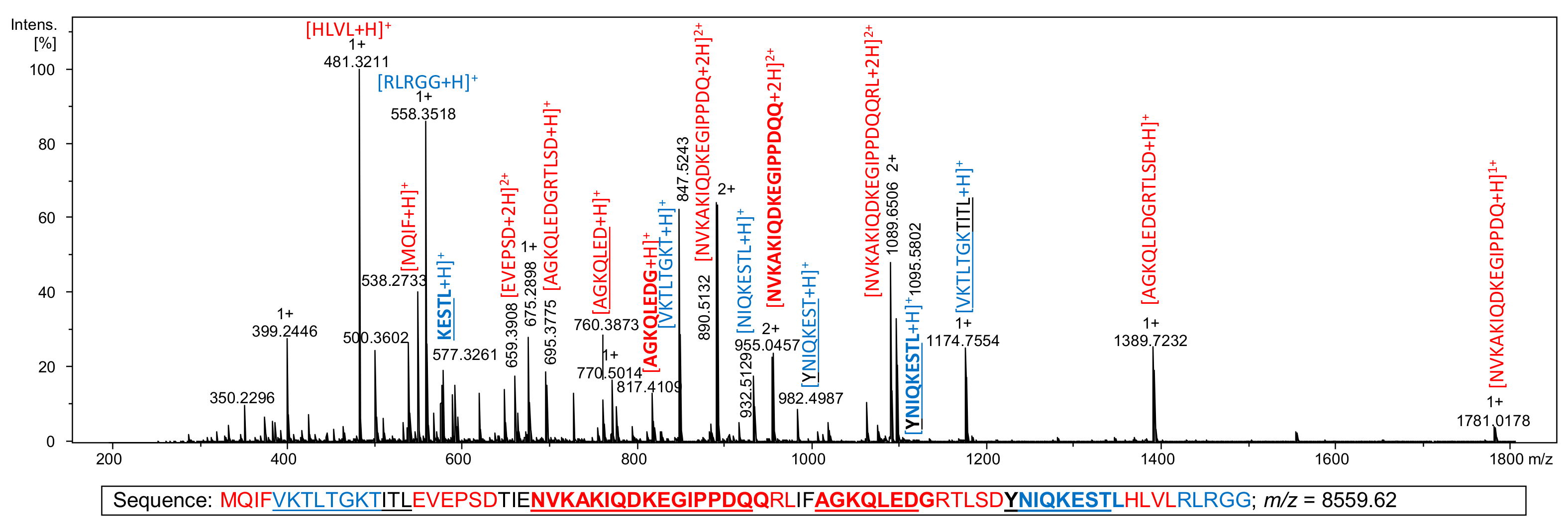
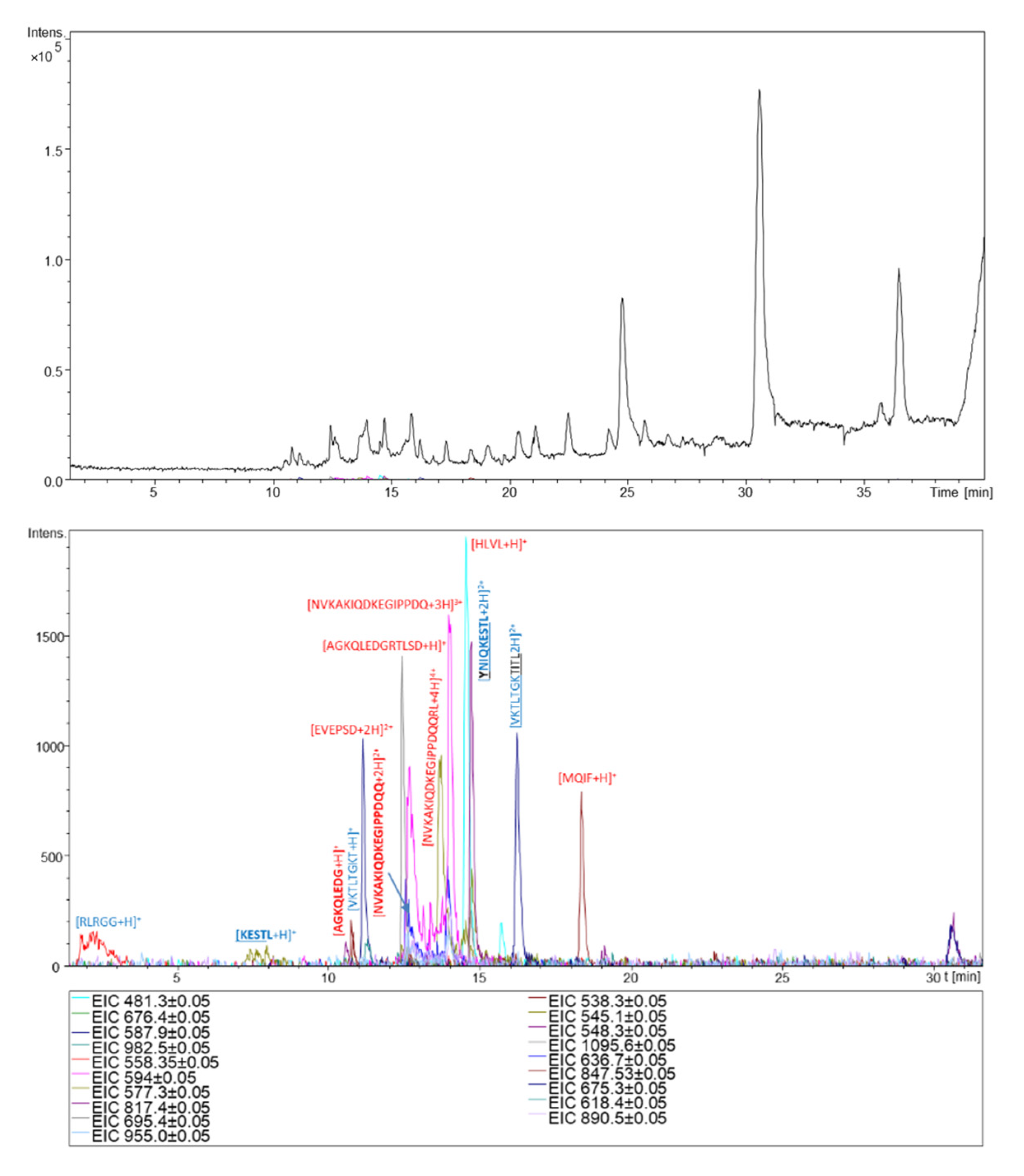

| No | Peptide Sequence | [M+H]+ | [M+Saf]1+ | [M+2H]2+ | [M+H+Saf]2+ | [M+2Saf]2+ | [M+3H]3+ | [M+2H+Saf]3+ | [M+H+2Saf]3+ | [M+3Saf]3+ | [M+4H]4+ | [M+4Saf]4+ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | NVKAKIQDKEGIPPDQQRL | - | - | - | - | 726.4061 | - | - | - | 545.0564 | - | |
| 2 | NVKAKIQDKEGIPPDQQ | - | 954.5129 | - | - | - | - | - | - | - | - | |
| 3 | NVKAKIQDKEGIPPDQ | - | 890.4837 | - | - | - | - | - | - | - | 676.8827 | |
| 4 | AGKQLEDGRTLSD | - | 695.3521 | - | 926.4893 | - | 540.9496 | 617.9953 | - | - | ||
| 5 | VKTLTGKTITL | - | 587.8740 | - | - | - | - | - | 623.3889 | - | - | |
| 6 | YNIQKESTL | 1095.5681 | - | 548.2877 | - | 779.4249 | - | - | - | 596.9981 | - | - |
| 7 | YNIQKEST | 982.4840 | - | - | - | 722.8829 | - | - | - | - | - | - |
| 8 | NIQKESTL | - | - | - | 582.3246 | 697.8932 | - | - | - | - | - | - |
| 9 | VKTLTGKT | 847.5248 | - | 424.2661 | - | - | - | - | - | 514.3170 | - | - |
| 10 | AGKQLEDG | 817.4051 | - | - | - | 640.3434 | - | - | - | - | - | - |
| 11 | AGKQLED | - | - | - | - | 611.8326 | - | - | - | - | - | - |
| 12 | QRLIF | 676.4141 | - | - | - | - | - | - | - | - | - | - |
| 13 | EVEPSD | 675.2832 | 906.4204 | - | - | - | - | - | - | - | - | - |
| 14 | VKTLTG | 618.3821 | - | - | - | 540.8319 | - | - | - | - | - | - |
| 15 | KESTL | 577.3192 | - | - | - | 520.3004 | - | - | - | - | - | - |
| 16 | RLRGG | 558.3471 | - | - | - | - | - | - | - | - | - | - |
| 17 | MQIF | 538.2694 | 769.4066 | - | - | - | - | - | - | - | - | - |
| 18 | HLVL | 481.3133 | 712.4505 | - | - | 472.2975 | - | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedorowicz, J.; Wierzbicka, M.; Cebrat, M.; Wiśniewska, P.; Piątek, R.; Zalewska-Piątek, B.; Szewczuk, Z.; Sączewski, J. Application of Safirinium N-Hydroxysuccinimide Esters to Derivatization of Peptides for High-Resolution Mass Spectrometry, Tandem Mass Spectrometry, and Fluorescent Labeling of Bacterial Cells. Int. J. Mol. Sci. 2020, 21, 9643. https://doi.org/10.3390/ijms21249643
Fedorowicz J, Wierzbicka M, Cebrat M, Wiśniewska P, Piątek R, Zalewska-Piątek B, Szewczuk Z, Sączewski J. Application of Safirinium N-Hydroxysuccinimide Esters to Derivatization of Peptides for High-Resolution Mass Spectrometry, Tandem Mass Spectrometry, and Fluorescent Labeling of Bacterial Cells. International Journal of Molecular Sciences. 2020; 21(24):9643. https://doi.org/10.3390/ijms21249643
Chicago/Turabian StyleFedorowicz, Joanna, Magdalena Wierzbicka, Marek Cebrat, Paulina Wiśniewska, Rafał Piątek, Beata Zalewska-Piątek, Zbigniew Szewczuk, and Jarosław Sączewski. 2020. "Application of Safirinium N-Hydroxysuccinimide Esters to Derivatization of Peptides for High-Resolution Mass Spectrometry, Tandem Mass Spectrometry, and Fluorescent Labeling of Bacterial Cells" International Journal of Molecular Sciences 21, no. 24: 9643. https://doi.org/10.3390/ijms21249643
APA StyleFedorowicz, J., Wierzbicka, M., Cebrat, M., Wiśniewska, P., Piątek, R., Zalewska-Piątek, B., Szewczuk, Z., & Sączewski, J. (2020). Application of Safirinium N-Hydroxysuccinimide Esters to Derivatization of Peptides for High-Resolution Mass Spectrometry, Tandem Mass Spectrometry, and Fluorescent Labeling of Bacterial Cells. International Journal of Molecular Sciences, 21(24), 9643. https://doi.org/10.3390/ijms21249643