Potential for Protein Kinase Pharmacological Regulation in Flaviviridae Infections
Abstract
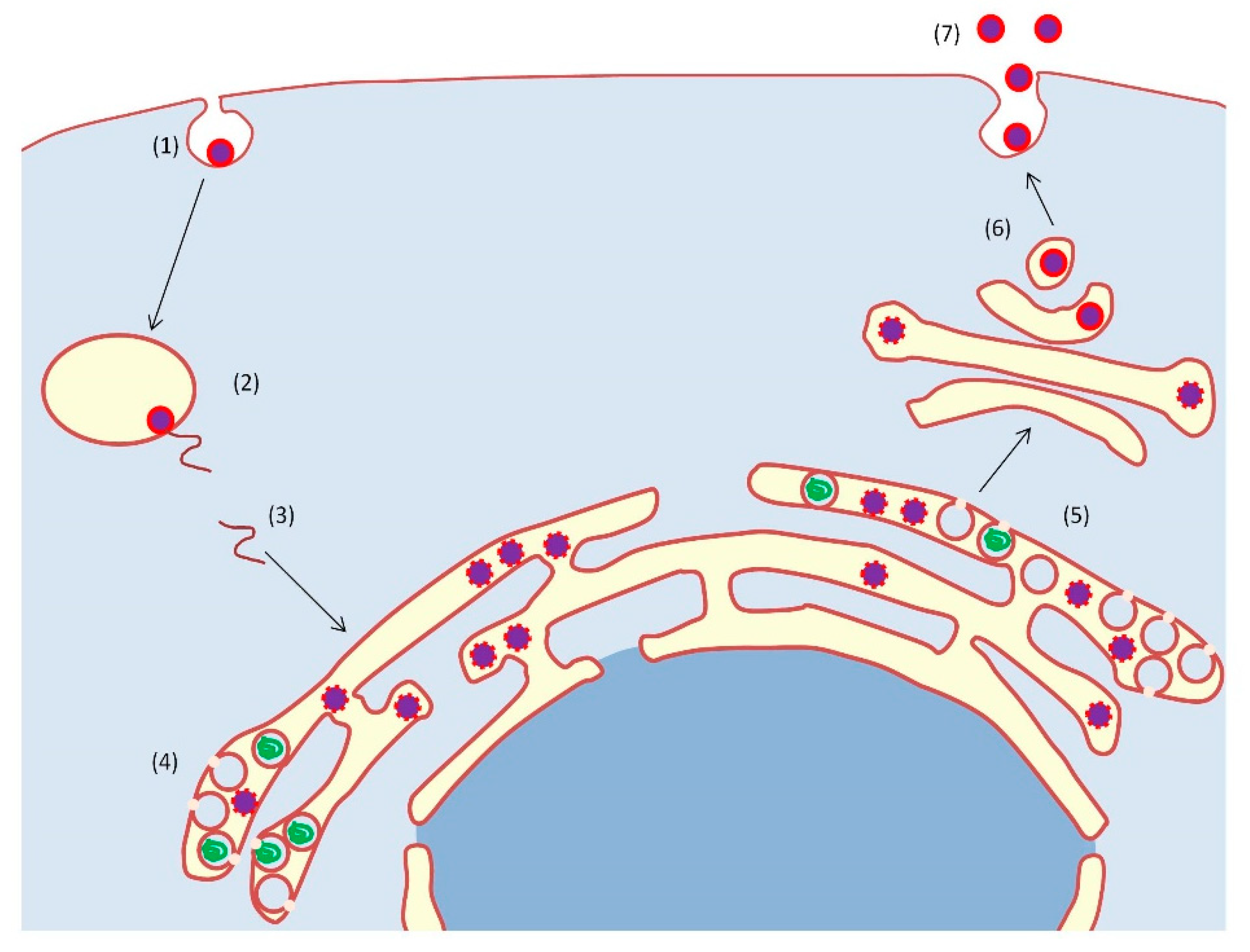
1. Introduction
2. Protein Kinase Targets in the Control of Virus of the Flaviviridae Family
2.1. The AGC Kinase
2.2. Calcium Calmodulin Dependent Kinases (CAMK)
2.3. Casein Kinase 1 (CK1)
2.4. CMGC Kinases
2.5. Tyrosine Kinases (TKs)
2.6. Tyrosine Kinase-Like (TKL)
2.7. Other PKs
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- The Nobel Prize. Available online: https://www.nobelprize.org/prizes/medicine/1992/press-release/ (accessed on 30 November 2020).
- Wu, P.; Nielsen, T.E.; Clausen, M.H. FDA-approved small-molecule kinase inhibitors. Trends Pharmacol. Sci. 2015, 36, 422–439. [Google Scholar] [CrossRef]
- Samudrala, R. Faculty Opinions recommendation of a comprehensive update of the sequence and structure classification of kinases. Fac. Opin.–Post-Publ. Peer Rev. Biomed. Lit. 2005, 320, 855–881. [Google Scholar]
- Genomics, Evolution and Function of Protein Kinases. Available online: http://kinase.com/web/current/ (accessed on 30 November 2020).
- Shen, K.; Hines, A.C.; Schwarzer, D.; Pickin, K.A.; Cole, P.A. Protein kinase structure and function analysis with chemical tools. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2005, 1754, 65–78. [Google Scholar] [CrossRef]
- Johnson, L.N. Protein kinase inhibitors: Contributions from structure to clinical compounds. Q. Rev. Biophys. 2009, 42, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P. Protein kinases—The major drug targets of the twenty-first century? Nat. Rev. Drug Discov. 2002, 1, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Burnett, G.; Kennedy, E.P. The enzymatic phosphorylation of proteins. J. Biol. Chem. 1954, 211, 969–980. [Google Scholar] [PubMed]
- Walsh, D.A.; Perkins, J.P.; Krebs, E.G. An adenosine 3′,5′-monophosphate-dependant protein kinase from rabbit skeletal muscle. J. Biol. Chem. 1968, 243, 3763–3765. [Google Scholar]
- Soderling, T.R.; Hickenbottom, J.P.; Reimann, E.M.; Hunkeler, F.L.; Walsh, D.A.; Krebs, E.G. Inactivation of glycogen synthetase and activation of phosphorylase kinase by muscle adenosine 3′,5′-monophosphate-dependent protein kinases. J. Biol. Chem. 1970, 245, 6317–6328. [Google Scholar]
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The protein kinase complement of the human genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef]
- Dissmeyer, N.; Schnittger, A. The age of protein kinases. Methods Mol. Biol. 2011, 779, 7–52. [Google Scholar]
- Fabbro, D.; Cowan-Jacob, S.W.; Moebitz, H. Ten things you should know about protein kinases: IUPHAR Review 14. Br. J. Pharmacol. 2015, 172, 2675–2700. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, P.S.L.; Ferraz, F.A.N.; Pena, D.A.; Pramio, D.T.; Morais, F.A.; Schechtman, D. Revisiting protein kinase–substrate interactions: Toward therapeutic development. Sci. Signal. 2016, 9, re3. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R. A historical overview of protein kinases and their targeted small molecule inhibitors. Pharmacol. Res. 2015, 100, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R. Properties of FDA-approved small molecule protein kinase inhibitors. Pharmacol. Res. 2019, 144, 19–50. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R. Properties of FDA-approved small molecule protein kinase inhibitors: A 2020 update. Pharmacol. Res. 2020, 152, 104609. [Google Scholar] [CrossRef] [PubMed]
- Bekerman, E.; Neveu, G.; Shulla, A.; Brannan, J.M.; Pu, S.-Y.; Wang, S.; Xiao, F.; Barouch-Bentov, R.; Bakken, R.R.; Mateo, R.; et al. Anticancer kinase inhibitors impair intracellular viral trafficking and exert broad-spectrum antiviral effects. J. Clin. Investig. 2017, 127, 1338–1352. [Google Scholar] [CrossRef]
- Nousiainen, L.; Sillanpää, M.; Jiang, M.; Thompson, J.; Taipale, J.; Julkunen, I. Human kinome analysis reveals novel kinases contributing to virus infection and retinoic-acid inducible gene I-induced type I and type III IFN gene expression. Innate Immun. 2013, 19, 516–530. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Best, S.M.; Perera, R.; Kuhn, R.J.; Striker, R. Protein Kinase G Phosphorylates Mosquito-Borne Flavivirus NS5. J. Virol. 2009, 83, 9195–9205. [Google Scholar] [CrossRef]
- Colpitts, C.C.; Lupberger, J.; Doerig, C.; Baumert, T.F. Host cell kinases and the hepatitis C virus life cycle. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2015, 1854, 1657–1662. [Google Scholar] [CrossRef]
- Meineke, R.; Rimmelzwaan, G.F.; Elbahesh, H. Influenza Virus Infections and Cellular Kinases. Viruses 2019, 11, 171. [Google Scholar] [CrossRef]
- Schor, S.; Einav, S. Repurposing of Kinase Inhibitors as Broad-Spectrum Antiviral Drugs. DNA Cell Biol. 2018, 37, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, P.; Becher, P.; Bukh, J.; Gould, E.A.; Meyers, G.; Monath, T.; Muerhoff, S.; Pletnev, A.; Rico-Hesse, R.; Smith, D.B.; et al. ICTV Virus Taxonomy Profile: Flaviviridae. J. Gen. Virol. 2017, 98, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-Y.; Qin, C.-F. Structure and function of cis-acting RNA elements of flavivirus. Rev. Med. Virol. 2019, 30, e2092. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.Y.; Fibriansah, G.; Lok, S.-M. Capsid protein is central to the birth of flavivirus particles. PLoS Pathog. 2020, 16, e1008542. [Google Scholar] [CrossRef] [PubMed]
- Martín-Acebes, M.A.; Saiz, J.C. West Nile virus: A re-emerging pathogen revisited. World J. Virol. 2012, 1, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, E.; Penin, F.; Kallis, S.; Patel, A.H.; Bartenschlager, R.; Pietschmann, T. Hepatitis C Virus p7 Protein Is Crucial for Assembly and Release of Infectious Virions. PLoS Pathog. 2007, 3, e103. [Google Scholar] [CrossRef]
- Saiz, J.C.; Vázquez-Calvo, Á.; Blázquez, A.B.; Merino-Ramos, T.; Escribano-Romero, E.; Martín-Acebes, M.A. Zika Virus: The Latest Newcomer. Front. Microbiol. 2016, 7, 496. [Google Scholar] [CrossRef]
- Atoom, A.M.; Taylor, N.G.; Russell, R.S. The elusive function of the hepatitis C virus p7 protein. Virology 2014, 462, 377–387. [Google Scholar] [CrossRef]
- Leung, J.Y.; Pijlman, G.P.; Kondratieva, N.; Hyde, J.; MacKenzie, J.M.; Khromykh, A.A. Role of Nonstructural Protein NS2A in Flavivirus Assembly. J. Virol. 2008, 82, 4731–4741. [Google Scholar] [CrossRef]
- The ICTV Report on Virus Classification and Taxon Nomenclature. Genus Flavivirus. Available online: https://talk.ictvonline.org/ictv-reports/ictv_online_report/positive-sense-rna-viruses/w/flaviviridae/360/genus-flavivirus (accessed on 28 November 2020).
- Pierson, T.C.; Diamond, M.S. The continued threat of emerging flaviviruses. Nat. Microbiol. 2020, 5, 796–812. [Google Scholar] [CrossRef]
- De Oya, N.J.; Escribano-Romero, E.; Blázquez, A.-B.; Martín-Acebes, M.A.; Saiz, J.-C. Current Progress of Avian Vaccines against West Nile Virus. Vaccines 2019, 7, 126. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K. Faculty Opinions recommendation of Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Fac. Opin.–Post-Publ. Peer Rev. Biomed. Lit. 2003, 9, 311–322. [Google Scholar]
- Satchidanandam, V. Japanese Encephalitis Vaccines. Curr. Treat. Options Infect. Dis. 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pandit, P.; Doyle, M.M.; Smart, K.M.; Young, C.; Drape, G.W.; Kreuder-Johnson, C. Predicting wildlife reservoirs and global vulnerability to zoonotic Flaviviruses. Nat. Commun. 2018, 9, 5425. [Google Scholar] [CrossRef]
- World Health Organization. Dengue and Severe Dengue. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 7 October 2020).
- King, C.A.; Wegman, A.D.; Endy, T.P. Mobilization and Activation of the Innate Immune Response to Dengue Virus. Front. Cell. Infect. Microbiol. 2020, 10, 574417. [Google Scholar] [CrossRef]
- Blázquez, A.-B.; Saiz, J.-C. Neurological manifestations of Zika virus infection. World J. Virol. 2016, 5, 135–143. [Google Scholar] [CrossRef]
- Saiz, J.-C. Therapeutic Advances against ZIKV: A Quick Response, a Long Way to Go. Pharmaceuticals 2019, 12, 127. [Google Scholar] [CrossRef]
- Su, Q.; Xie, Z.-X.; He, F.; Liu, Z.-C.; Song, X.-J.; Zhao, F.-C.; Li, D.; Che, F.-Y. Adults with severe Japanese encephalitis: A retrospective analysis of 9 cases in Linyi, China. Neurol. Sci. 2020, 1–7. [Google Scholar] [CrossRef]
- Bifani, A.M.; Ong, E.Z.; De Alwis, R. Vaccination and Therapeutics: Responding to the Changing Epidemiology of Yellow Fever. Curr. Treat. Options Infect. Dis. 2020, 1–12. [Google Scholar] [CrossRef]
- Li, H.-C. Hepatitis C virus: Virology, diagnosis and treatment. World J. Hepatol. 2015, 7, 1377–1389. [Google Scholar] [CrossRef]
- The Nobel Prize in Physiology or Medicine 2020. Available online: https://www.nobelprize.org/prizes/medicine/2020/summary/ (accessed on 25 November 2020).
- Chan, D.P.; Sun, H.-Y.; Wong, H.T.; Lee, S.-S.; Hung, C.C. Sexually acquired hepatitis C virus infection: A review. Int. J. Infect. Dis. 2016, 49, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Ganges, L.; Crooke, H.R.; Bohórquez, J.A.; Postel, A.; Sakoda, Y.; Becher, P.; Ruggli, N. Classical swine fever virus: The past, present and future. Virus Res. 2020, 289, 198151. [Google Scholar] [CrossRef]
- The ICTV Report on Virus Classification and Taxon Nomenclature. Genus Pegivirus. Available online: https://talk.ictvonline.org/ictv-reports/ictv_online_report/positive-sense-rna-viruses/w/flaviviridae/363/genus-pegivirus (accessed on 28 November 2020).
- Cloherty, A.P.; Olmstead, A.D.; Ribeiro, C.M.S.; Jean, F. Hijacking of Lipid Droplets by Hepatitis C, Dengue and Zika Viruses—From Viral Protein Moonlighting to Extracellular Release. Int. J. Mol. Sci. 2020, 21, 7901. [Google Scholar] [CrossRef]
- Aktepe, T.E.; MacKenzie, J.M. Shaping the flavivirus replication complex: It is curvaceous! Cell. Microbiol. 2018, 20, e12884. [Google Scholar] [CrossRef] [PubMed]
- Chong, H.Y.; Leow, C.H.; Majeed, A.B.A. Flavivirus infection—A review of immunopathogenesis, immunological response, and immunodiagnosis. Virus Res. 2019, 274, 197770. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; De Clercq, E. Current therapy for chronic hepatitis C: The role of direct-acting antivirals. Antivir. Res. 2017, 142, 83–122. [Google Scholar] [CrossRef] [PubMed]
- Martín-Acebes, M.A.; Vázquez-Calvo, Á.; Saiz, J.-C. Lipids and flaviviruses, present and future perspectives for the control of dengue, Zika, and West Nile viruses. Prog. Lipid Res. 2016, 64, 123–137. [Google Scholar] [CrossRef]
- Saiz, J.-C.; De Oya, N.J.; Blázquez, A.-B.; Escribano-Romero, E.; Martín-Acebes, M.A. Host-Directed Antivirals: A Realistic Alternative to Fight Zika Virus. Viruses 2018, 10, 453. [Google Scholar] [CrossRef]
- Sinigaglia, A.; Peta, E.; Riccetti, S.; Barzon, L. New avenues for therapeutic discovery against West Nile virus. Expert Opin. Drug Discov. 2020, 15, 333–348. [Google Scholar] [CrossRef]
- Bartenschlager, R.; Baumert, T.F.; Bukh, J.; Houghton, M.; Lemon, S.M.; Lindenbach, B.D.; Lohmann, V.; Moradpour, D.; Pietschmann, T.; Rice, C.M.; et al. Critical challenges and emerging opportunities in hepatitis C virus research in an era of potent antiviral therapy: Considerations for scientists and funding agencies. Virus Res. 2018, 248, 53–62. [Google Scholar] [CrossRef]
- Kok, W.M. New developments in flavivirus drug discovery. Expert Opin. Drug Discov. 2016, 11, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Sharma, S.; Kumar, R.; Tripathi, B.N.; Barua, S.; Ly, H.; Rouse, B.T. Host-Directed Antiviral Therapy. Clin. Microbiol. Rev. 2020, 33, e00168-19. [Google Scholar] [CrossRef] [PubMed]
- Manns, M.P.; Buti, M.; Gane, E.; Pawlotsky, J.M.; Razavi, H.; Terrault, N.; Younossi, Z. Hepatitis C virus infection. Nat. Rev. Dis. Primers 2017, 3, 17006. [Google Scholar] [CrossRef] [PubMed]
- Pawlotski, J.-M. Hepatitis C Virus Resistance to Direct-Acting Antiviral Drugs in Interferon-Free Regimens. Gastroenterology 2016, 151, 70–86. [Google Scholar] [CrossRef]
- Colpitts, C.C.; Baumert, T.F. Addressing the Challenges of Hepatitis C Virus Resistance and Treatment Failure. Viruses 2016, 8, 226. [Google Scholar] [CrossRef]
- Kaufmann, S.H.E.; Dorhoi, A.; Hotchkiss, R.S.; Bartenschlager, R. Host-directed therapies for bacterial and viral infections. Nat. Rev. Drug Discov. 2018, 17, 35–56. [Google Scholar] [CrossRef]
- Troost, B.; Smit, J.M. Recent advances in antiviral drug development towards dengue virus. Curr. Opin. Virol. 2020, 43, 9–21. [Google Scholar] [CrossRef]
- Radi, M. Drug repurposing approaches to fight Dengue virus infection and related diseases. Front. Biosci. 2018, 23, 997–1019. [Google Scholar] [CrossRef]
- Martín-Acebes, M.A.; De Oya, N.J.; Saiz, J.-C. Lipid Metabolism as a Source of Druggable Targets for Antiviral Discovery against Zika and Other Flaviviruses. Pharmaceuticals 2019, 12, 97. [Google Scholar] [CrossRef]
- Mendes, É.A.; de Pilger, D.R.; Nastri, A.C.; de Mello Malta, F.; dos Santos Pascoalino, B.; D’Albuquerque, L.A.; Balan, A.; de Freitas, L.H., Jr.; Durigon, E.L.; Carrilho, F.J.; et al. Sofosbuvir inhibits yellow fever virus in vitro and in patients with acute liver failure. Ann. Hepatol. 2019, 18, 816–824. [Google Scholar] [CrossRef]
- Cusinato, J.; Cau, Y.; Calvani, A.M.; Mori, M. Repurposing drugs for the management of COVID-19. Expert Opin. Ther. Patents 2020. [Google Scholar] [CrossRef] [PubMed]
- Kotta, S.; Aldawsari, H.M.; Badr-Eldin, S.M.; Alhakamy, N.A.; Md, S.; Nair, A.B.; Deb, P.K. Combating the Pandemic COVID-19: Clinical Trials, Therapies and Perspectives. Front. Mol. Biosci. 2020, 7, 606393. [Google Scholar] [CrossRef] [PubMed]
- De Wispelaere, M.; Lacroix, A.J.; Yang, P.L. The Small Molecules AZD0530 and Dasatinib Inhibit Dengue Virus RNA Replication via Fyn Kinase. J. Virol. 2013, 87, 7367–7381. [Google Scholar] [CrossRef] [PubMed]
- Felicetti, T.; Manfroni, G.; Cecchetti, V.; Cannalire, R. Broad-Spectrum Flavivirus Inhibitors: A Medicinal Chemistry Point of View. ChemMedChem 2020. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Da Silva, S.R.; Huang, I.-C.; Jung, J.U.; Gao, S.-J. Suppression of Zika virus infection and replication in endothelial cells and astrocytes by PKA inhibitor PKI 14-22. J. Virol. 2017, 92, JVI.02019–17. [Google Scholar] [CrossRef] [PubMed]
- Noppakunmongkolchai, W.; Poyomtip, T.; Jittawuttipoka, T.; Luplertlop, N.; Sakuntabhai, A.; Chimnaronk, S.; Jirawatnotai, S.; Tohtong, R. Inhibition of protein kinase C promotes dengue virus replication. Virol. J. 2016, 13, 1–13. [Google Scholar] [CrossRef]
- Anwar, A.; Hosoya, T.; Leong, K.M.; Onogi, H.; Okuno, Y.; Hiramatsu, T.; Koyama, H.; Suzuki, M.; Hagiwara, M.; Garcia-Blanco, M.A. The Kinase Inhibitor SFV785 Dislocates Dengue Virus Envelope Protein from the Replication Complex and Blocks Virus Assembly. PLoS ONE 2011, 6, e23246. [Google Scholar] [CrossRef]
- De Oya, N.J.; Blázquez, A.-B.; Casas, J.; Saiz, J.-C.; Martín-Acebes, M.A. Direct Activation of Adenosine Monophosphate-Activated Protein Kinase (AMPK) by PF-06409577 Inhibits Flavivirus Infection through Modification of Host Cell Lipid Metabolism. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Ceballos-Olvera, I.; Chávez-Salinas, S.; Medina, F.; Ludert, J.E.; Del Ángel, R.M. JNK phosphorylation, induced during dengue virus infection, is important for viral infection and requires the presence of cholesterol. Virology 2010, 396, 30–36. [Google Scholar] [CrossRef]
- Fu, Y.; Yip, A.; Seah, P.G.; Blasco, F.; Shi, P.-Y.; Hervé, M. Modulation of inflammation and pathology during dengue virus infection by p38 MAPK inhibitor SB203580. Antivir. Res. 2014, 110, 151–157. [Google Scholar] [CrossRef]
- Sreekanth, G.P.; Chuncharunee, A.; Sirimontaporn, A.; Panaampon, J.; Noisakran, S.; Yenchitsomanus, P.-T.; Limjindaporn, T. SB203580 Modulates p38 MAPK Signaling and Dengue Virus-Induced Liver Injury by Reducing MAPKAPK2, HSP27, and ATF2 Phosphorylation. PLoS ONE 2016, 11, e0149486. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, M.; Miyamoto, Y.; Suzuki, T.; Otani, M.; Inuki, S.; Esaki, T.; Nagao, C.; Mizuguchi, K.; Ohno, H.; Yoneda, Y.; et al. Novel anti-flavivirus drugs targeting the nucleolar distribution of core protein. Virology 2020, 541, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.J.H.; Yang, P.L. c-Src protein kinase inhibitors block assembly and maturation of dengue virus. Proc. Natl. Acad. Sci. USA 2007, 104, 3520–3525. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.J.; Miduturu, C.; Schmidt, A.G.; Zhu, X.; Pitts, J.D.; Wang, J.; Potisopon, S.; Zhang, J.; Wojciechowski, A.; Chu, J.J.H.; et al. GNF-2 Inhibits Dengue Virus by Targeting Abl Kinases and the Viral E Protein. Cell Chem. Biol. 2016, 23, 443–452. [Google Scholar] [CrossRef]
- Vincetti, P.; Caporuscio, F.; Kaptein, S.; Gioiello, A.; Mancino, V.; Suzuki, Y.; Yamamoto, N.; Crespan, E.; Lossani, A.; Maga, G.; et al. Discovery of Multitarget Antivirals Acting on Both the Dengue Virus NS5-NS3 Interaction and the Host Src/Fyn Kinases. J. Med. Chem. 2015, 58, 4964–4975. [Google Scholar] [CrossRef]
- Tsai, Y.-T.; Chen, Y.-H.; Chang, D.-M.; Chen, P.-C.; Lai, J.-H. Janus kinase/signal transducer and activator of transcription 3 signaling pathway is crucial in chemokine production from hepatocytes infected by dengue virus. Exp. Biol. Med. 2011, 236, 1156–1165. [Google Scholar] [CrossRef]
- De Wispelaere, M.; Carocci, M.; Liang, Y.; Liu, Q.; Sun, E.; Vetter, M.L.; Wang, J.; Gray, N.S.; Yang, P.L. Discovery of host-targeted covalent inhibitors of dengue virus. Antivir. Res. 2017, 139, 171–179. [Google Scholar] [CrossRef][Green Version]
- Wongtrakul, J.; Thongtan, T.; Pannengpetch, S.; Wikan, N.; Kantamala, D.; Kumrapich, B.; Suwan, W.; Smith, D.R. Phosphoproteomic analysis of dengue virus infected U937 cells and identification of pyruvate kinase M2 as a differentially phosphorylated phosphoprotein. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Pérez-Olais, J.H.; Ruíz-Jiménez, F.; Calderon-Garcia, E.J.; De Jesús-González, L.A.; Hernández-Rivas, R.; Del Ángel, R.M.; De Jesus-González, L.A. The activity of Aurora kinase B is required for dengue virus release. Virus Res. 2019, 274, 197777. [Google Scholar] [CrossRef]
- Singh, S.; Singh, P.K.; Suhail, H.; Arumugaswami, V.; Pellett, P.E.; Giri, S.; Kumar, A. AMP-Activated Protein Kinase Restricts Zika Virus Replication in Endothelial Cells by Potentiating Innate Antiviral Responses and Inhibiting Glycolysis. J. Immunol. 2020, 204, 1810–1824. [Google Scholar] [CrossRef]
- Zhu, S.; Luo, H.; Liu, H.; Ha, Y.; Mays, E.R.; Lawrence, R.E.; Winkelmann, E.R.; Barrett, A.D.; Smith, S.B.; Wang, M.; et al. p38MAPK plays a critical role in induction of a pro-inflammatory phenotype of retinal Müller cells following Zika virus infection. Antivir. Res. 2017, 145, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; DeLalio, L.J.; Isakson, B.E.; Wang, T.T. AXL-Mediated Productive Infection of Human Endothelial Cells by Zika Virus. Circ. Res. 2016, 119, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Daniels, B.P.; Kofman, S.B.; Smith, J.R.; Norris, G.T.; Snyder, A.G.; Kolb, J.P.; Gao, X.; Locasale, J.W.; Martinez, J.; Gale, M.; et al. The Nucleotide Sensor ZBP1 and Kinase RIPK3 Induce the Enzyme IRG1 to Promote an Antiviral Metabolic State in Neurons. Immunity 2019, 50, 64–76.e4. [Google Scholar] [CrossRef] [PubMed]
- Kolpikova, E.P.; Tronco, A.R.; Hartigh, A.B.D.; Jackson, K.J.; Iwawaki, T.; Fink, S.L. IRE1α Promotes Zika Virus Infection via XBP1. Viruses 2020, 12, 278. [Google Scholar] [CrossRef]
- Besson, B.; Basset, J.; Gatellier, S.; Chabrolles, H.; Chaze, T.; Hourdel, V.; Matondo, M.; Pardigon, N.; Choumet, V. Comparison of a human neuronal model proteome upon Japanese encephalitis or West Nile Virus infection and potential role of mosquito saliva in neuropathogenesis. PLoS ONE 2020, 15, e0232585. [Google Scholar] [CrossRef]
- Blázquez, A.-B.; Vázquez-Calvo, Á.; Martín-Acebes, M.A.; Saiz, J.-C. Pharmacological Inhibition of Protein Kinase C Reduces West Nile Virus Replication. Viruses 2018, 10, 91. [Google Scholar] [CrossRef]
- Hirsch, A.J.; Medigeshi, G.R.; Meyers, H.L.; DeFilippis, V.; Früh, K.; Briese, T.; Lipkin, W.I.; Nelson, J.A. The Src Family Kinase c-Yes Is Required for Maturation of West Nile Virus Particles. J. Virol. 2005, 79, 11943–11951. [Google Scholar] [CrossRef]
- Li, J.; Ding, S.C.; Cho, H.; Chung, B.C.; Gale, M.; Chanda, S.K.; Diamond, M.S. A Short Hairpin RNA Screen of Interferon-Stimulated Genes Identifies a Novel Negative Regulator of the Cellular Antiviral Response. mBio 2013, 4, e00385-13. [Google Scholar] [CrossRef]
- Daniels, B.P.; Snyder, A.G.; Olsen, T.M.; Orozco, S.; Oguin, T.H., 3rd; Tait, S.W.G.; Martinez, J.; Gale, M., Jr.; Loo, Y.M.; Oberst, A. RIPK3 Restricts Viral Pathogenesis via Cell Death-Independent Neuroinflammation. Cell 2017, 169, 301–313.e11. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Ansari, I.H.; Striker, R. The flaviviral methyltransferase is a substrate of Casein Kinase 1. Virus Res. 2009, 141, 101–104. [Google Scholar] [CrossRef]
- Chan, Y.-L.; Liao, C.-L.; Lin, Y.-L. Human Kinase/Phosphatase-Wide RNAi Screening Identified Checkpoint Kinase 2 as a Cellular Factor Facilitating Japanese Encephalitis Virus Infection. Front. Cell. Infect. Microbiol. 2018, 8, 142. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Allam, H.; Bader, T.; May, R.; Basalingappa, K.M.; Berry, W.L.; Chandrakesan, P.; Qu, D.; Weygant, N.; Bronze, M.S.; et al. Fluvastatin interferes with hepatitis C virus replication via microtubule bundling and a doublecortin-like kinase-mediated mechanism. PLoS ONE 2013, 8, e80304. [Google Scholar] [CrossRef]
- Lee, M.; Chen, W.-C.; Hsu, W.-H.; Chen, S.-C.; Lee, J.-C. Liraglutide Inhibits Hepatitis C Virus Replication Through an AMP Activated Protein Kinase Dependent Mechanism. Int. J. Mol. Sci. 2019, 20, 4569. [Google Scholar] [CrossRef]
- Tellinghuisen, T.L.; Foss, K.L.; Treadaway, J. Regulation of Hepatitis C Virion Production via Phosphorylation of the NS5A Protein. PLoS Pathog. 2008, 4, e1000032. [Google Scholar] [CrossRef]
- Menzel, N.; Fischl, W.; Hueging, K.; Bankwitz, D.; Frentzen, A.; Haid, S.; Gentzsch, J.; Kaderali, L.; Bartenschlager, R.; Pietschmann, T. MAP-Kinase Regulated Cytosolic Phospholipase A2 Activity Is Essential for Production of Infectious Hepatitis C Virus Particles. PLoS Pathog. 2012, 8, e1002829. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Sun, F.; Li, S.; Gao, M.; Wang, L.; Sarhan, M.; Abdel-Rahman, M.A.; Li, W.; Kwok, H.F.; Wu, Y.; et al. Inhibitory Activity of a Scorpion Defensin BmKDfsin3 against Hepatitis C Virus. Antibiotics 2020, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Karakama, Y.; Sakamoto, N.; Itsui, Y.; Nakagawa, M.; Tasaka-Fujita, M.; Nishimura-Sakurai, Y.; Kakinuma, S.; Oooka, M.; Azuma, S.; Tsuchiya, K.; et al. Inhibition of Hepatitis C Virus Replication by a Specific Inhibitor of Serine-Arginine-Rich Protein Kinase. Antimicrob. Agents Chemother. 2010, 54, 3179–3186. [Google Scholar] [CrossRef]
- Lupberger, J.; Zeisel, M.B.; Xiao, F.; Thumann, C.; Fofana, I.; Zona, L.; Davis, C.; Mee, C.J.; Turek, M.; Gorke, S.; et al. EGFR and EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. Nat. Med. 2011, 17, 589–595. [Google Scholar] [CrossRef]
- Lee, S.-H.; Moon, J.-S.; Pak, B.-Y.; Kim, G.-W.; Lee, W.; Cho, H.; Kim, S.; Kim, S.-J.; Oh, J.-W. HA1077 displays synergistic activity with daclatasvir against hepatitis C virus and suppresses the emergence of NS5A resistance-associated substitutions in mice. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Kovackova, S.; Chang, L.; Bekerman, E.; Neveu, G.; Barouch-Bentov, R.; Chaikuad, A.; Heroven, C.; Šála, M.; De Jonghe, S.; Knapp, S.; et al. Selective Inhibitors of Cyclin G Associated Kinase (GAK) as Anti-Hepatitis C Agents. J. Med. Chem. 2015, 58, 3393–3410. [Google Scholar] [CrossRef]
- Neveu, G.; Ziv-Av, A.; Barouch-Bentov, R.; Berkerman, E.; Mulholland, J.; Einav, S.; DeWitt, W.S.; Emerson, R.O.; Lindau, P.; Vignali, M.; et al. AP-2-Associated Protein Kinase 1 and Cyclin G-Associated Kinase Regulate Hepatitis C Virus Entry and Are Potential Drug Targets. J. Virol. 2015, 89, 4387–4404. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, M.B.; Crouchet, E.; Baumert, T.F.; Schuster, C. Host-Targeting Agents to Prevent and Cure Hepatitis C Virus Infection. Viruses 2015, 7, 5659–5685. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, C.; Tan, C.Y.; Horner, S.M. Hepatitis C Virus Infection Is Inhibited by a Noncanonical Antiviral Signaling Pathway Targeted by NS3-NS4A. J. Virol. 2019, 93. [Google Scholar] [CrossRef] [PubMed]
- Leroux, A.E.; Schulze, J.O.; Biondi, R.M. AGC kinases, mechanisms of regulation and innovative drug development. Semin. Cancer Biol. 2018, 48, 1–17. [Google Scholar] [CrossRef]
- Park, C.; Min, S.; Park, E.-M.; Lim, Y.-S.; Kang, S.; Suzuki, T.; Shin, E.-C.; Hwang, S.B. Pim Kinase Interacts with Nonstructural 5A Protein and Regulates Hepatitis C Virus Entry. J. Virol. 2015, 89, 10073–10086. [Google Scholar] [CrossRef]
- Zhou, Y.; He, C.; Yan, D.; Liu, F.; Liu, H.; Chen, J.; Cao, T.; Zuo, M.; Wang, P.; Ge, Y.; et al. The kinase CK1varepsilon controls the antiviral immune response by phosphorylating the signaling adaptor TRAF3. Nat. Immunol. 2016, 17, 397–405. [Google Scholar] [CrossRef]
- Kim, S.; Jin, B.; Choi, S.H.; Han, K.-H.; Ahn, S.H. Casein Kinase II Inhibitor Enhances Production of Infectious Genotype 1a Hepatitis C Virus (H77S). PLoS ONE 2014, 9, e113938. [Google Scholar] [CrossRef]
- Xu, M.; Lee, E.M.; Wen, Z.; Cheng, Y.; Huang, W.-K.; Qian, X.; Tcw, J.; Kouznetsova, J.; Ogden, S.C.; Hammack, C.; et al. Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat. Med. 2016, 22, 1101–1107. [Google Scholar] [CrossRef]
- Schoggins, J.W.; Dorner, M.; Feulner, M.; Imanaka, N.; Murphy, M.Y.; Ploss, A.; Rice, C. Dengue reporter viruses reveal viral dynamics in interferon receptor-deficient mice and sensitivity to interferon effectors in vitro. Proc. Natl. Acad. Sci. USA 2012, 109, 14610–14615. [Google Scholar] [CrossRef]
- Meyer, K.; Kwon, Y.-C.; Liu, S.; Hagedorn, C.H.; Ray, R.B.; Ray, R. Interferon-α inducible protein 6 impairs EGFR activation by CD81 and inhibits hepatitis C virus infection. Sci. Rep. 2015, 5, 9012. [Google Scholar] [CrossRef]
- Eng, V.V.; Wemyss, M.A.; Pearson, J.S. The diverse roles of RIP kinases in host-pathogen interactions. Semin. Cell Dev. Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bian, P.; Ye, C.; Zheng, X.; Luo, C.; Yang, J.; Li, M.; Wang, Y.; Yang, J.; Zhou, Y.; Zhang, F.; et al. RIPK3 Promotes JEV Replication in Neurons via Downregulation of IFI44L. Front. Microbiol. 2020, 11, 368. [Google Scholar] [CrossRef] [PubMed]
- Colpitts, C.C.; Ridewood, S.; Schneiderman, B.; Warne, J.; Tabata, K.; Ng, C.F.; Bartenschlager, R.; Selwood, D.L.; Towers, G.J. Hepatitis C virus exploits cyclophilin A to evade PKR. eLife 2020, 9, e52237. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xin, X.; Wang, T.; Wan, J.; Ou, Y.; Yang, Z.; Yu, Q.; Zhu, L.; Guo, Y.; Wu, Y.; et al. Japanese Encephalitis Virus Induces Apoptosis and Encephalitis by Activating the PERK Pathway. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Datan, E.; Roy, S.G.; Germain, G.; Zali, N.; McLean, J.E.; Golshan, G.; Harbajan, S.; Lockshin, R.A.; Zakeri, Z. Dengue-induced autophagy, virus replication and protection from cell death require ER stress (PERK) pathway activation. Cell Death Dis. 2016, 7, e2127. [Google Scholar] [CrossRef]
- Peña, J.; Harris, E. Dengue Virus Modulates the Unfolded Protein Response in a Time-dependent Manner. J. Biol. Chem. 2011, 286, 14226–14236. [Google Scholar] [CrossRef]
- Medigeshi, G.R.; Lancaster, A.M.; Hirsch, A.J.; Briese, T.; Lipkin, W.I.; DeFilippis, V.; Früh, K.; Mason, P.W.; Nikolich-Zugich, J.; Nelson, J.A. West Nile Virus Infection Activates the Unfolded Protein Response, Leading to CHOP Induction and Apoptosis. J. Virol. 2007, 81, 10849–10860. [Google Scholar] [CrossRef]
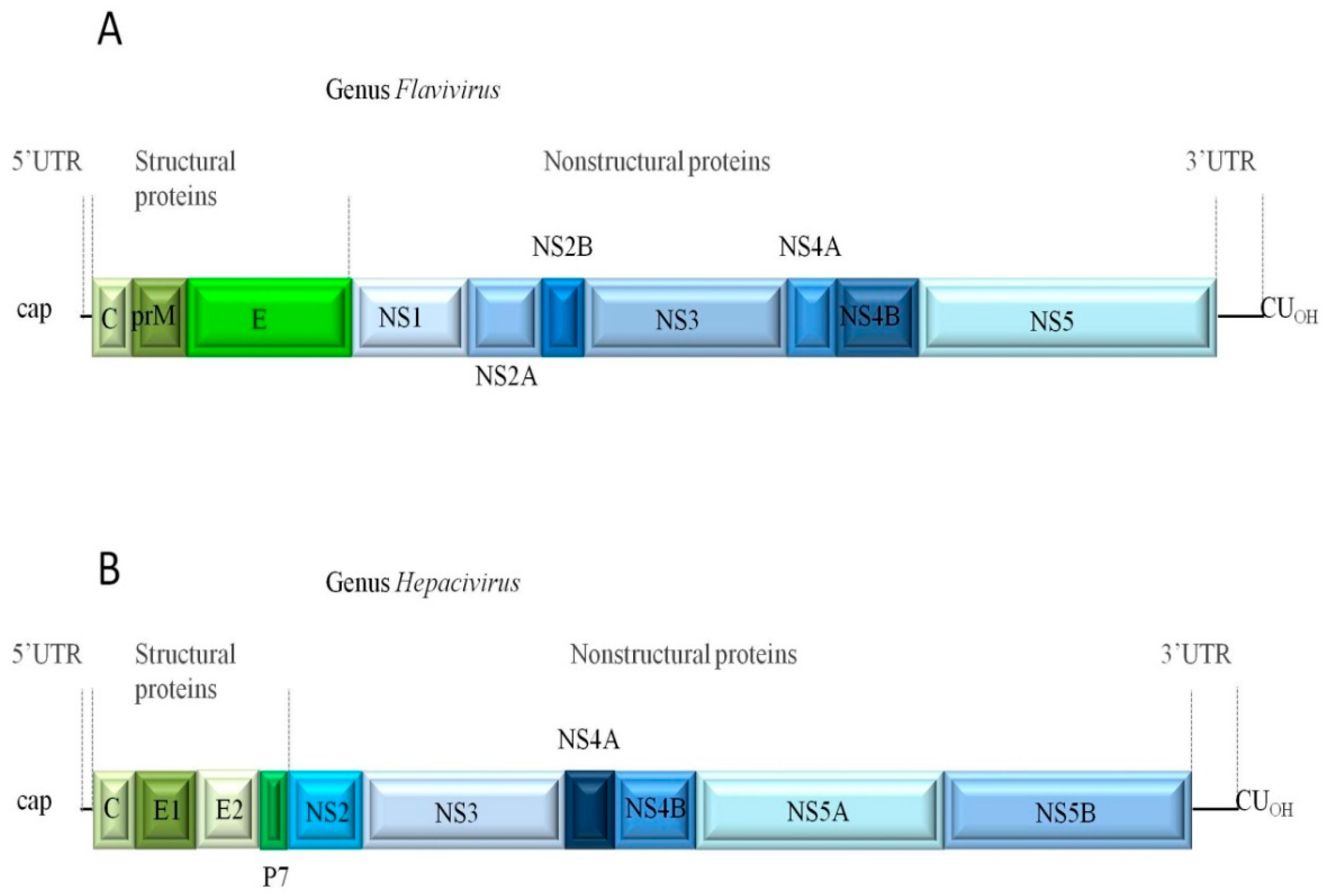
| Group | Representative Families of the Group |
|---|---|
| AGC | PKA (cAMP-dependent protein kinase), PKC (protein kinase C), PKG (cGMP-dependent protein kinase), PKN (protein kinase N), AKT (protein kinase B) |
| CAMK (Calcium Calmodulin dependent kinase) | PhK (phosphorylase kinase), CAMK (Ca2+/calmodulin-dependent protein kinase), MAPKAPK (mitogen-activated protein kinase-activated protein kinase), MLCK (myosin light-chain kinase) |
| CK1 (Casein Kinase 1) | TTBK (tau-tubulin kinase), VRK (vaccinia-related kinase) |
| CMGC | CDK (cyclin-dependent kinase), MAPK (mitogen-activated protein kinase), GSK (glycogen synthase kinase), CDKL (cyclin Dependent Kinase Like), JNK (c-Jun N-terminal kinase), p38 |
| STE | STE7 (sterile 7), STE11 (sterile 11), STE20 (sterile 20) |
| TK (Tyrosine kinase) | EGFR (epidermal growth factor receptor), PDGFR (platelet-derived growth factor receptors), JAK (Janus kinase), Eph (erythropoietin-producing human hepatocellular receptors), Fyn (proto-oncogene tyrosine-protein kinase), SFK (Src-family kinase), TRK (tropomyosin receptor kinase) |
| TKL (Tyrosine kinase-like) | MLK (mixed lineage kinase), LISK, IRAK (interleukin-1 receptor-associated kinase), RIPK (receptor-interacting serine/threonine-protein kinase) |
| RGC (Receptor guanylate cyclase) | RGC (receptor guanylate cyclase) |
| Others | MYT (membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase), ULK (Unc-51 like autophagy activating kinase), PLK (polo-like kinase), SCY, NKF (new kinase family), NAK (numb-associated kinase), PEK (pancreatic eukaryotic initiation factor-2alpha kinase) |
| Group | Representative Families of the Group |
|---|---|
| alpha | ChaK (Channel kinase), eEF2K (Eukaryotic elongation factor 2 kinase) |
| PIKK (phosphatidyl inositol 3′ kinase-related kinase) | ATM (Ataxia telangiectasia mutated kinase), ATR (Ataxia telangiectasia and Rad3 related kinase), FRAP, SMG1 (Nonsense Mediated MRNA Decay Associated PI3K Related Kinase) |
| PDHK (pyruvate dehydrogenase kinase) | PHDK (pyruvate dehydrogenase kinase), BKCDK |
| RIO (right open reading frame) | RIOK (Right open reading frame protein kinase), SUDD (Right open reading frame protein kinase3) |
| Genus | Representative Members * | Genome Size (Kb) |
|---|---|---|
| Flavivirus | YFV, WNV, DENV, ZIKV, TBEV | 9.2–11 |
| Hepacivirus | HCV | 8.9–10.5 |
| Pestivirus | BVDV, CSFV | 12.3–13 |
| Pegivirus | GBV-A, HPgV | 8.9–11.3 |
| Virus | PK | Kinase Group | Kind of PK Regulation | Inhibitor/Activator Used | References |
|---|---|---|---|---|---|
| PKG | AGC | Inhibition | Rp-8-pCPT-cGMPS, TEA | [20] | |
| PKC | AGC | Activation | Phorbol 12-myristate 13-acetate (PMA) | [72] | |
| MAPKAPK5 | CAMK | Inhibition | SFV785 | [73] | |
| AMPK | CAMK | Activation | PF-06409577 | [74] | |
| JNK | CMGC | Inhibition | SP60025 | [75] | |
| P38 | CMGC | Inhibition | SB003580 | [75,76,77] | |
| CDK | CMGC | Inhibition | Alsterpaullone 2-cyanoethyl, Cdk1/2 inh III,Cdk2/9 inh | [78] | |
| DENV | SFK | TK | Inhibition | AZD0530, Dasatinib, GNF-2 | [69,79,80,81] |
| JAK | TK | Inhibition | WHI-P131 | [82] | |
| BTK | TK | Inhibition | QL-XII-47 | [83] | |
| NTRK1 | TK | Inhibition | SFV785 | [73] | |
| PKM2 | Other | Inhibition | PKM2 inhibitor | [84] | |
| AurKB | Other | Inhibition | ZM 447439 | [85] | |
| NAK | Other | Inhibition | Sunitinib, Erlotinib | [18] | |
| PKA | AGC | Inhibition | PKI 14-22 | [71] | |
| AMPK | CAMK | Activation | PF-06409577 AICAR, Metformin, GSK621 | [74,86] | |
| P38 | CMGC | Inhibition | SB203580, SB202190 | [71,87] | |
| ZIKV | AXL | TK | Inhibition | Cabozantinib, R428 | [88] |
| BTK | TK | Inhibition | QL-XII-47 | [83] | |
| RIPKs | TKL | Activation | AP1 | [89] | |
| IRE1 K | Other | Inhibition | KIRA 6 | [90] | |
| PKC | AGC | Inhibition | Calphostin C, Chelerythrine | [91,92] | |
| AMPK | CAMK | Activation | PF-06409577 | [74] | |
| SFK | TK | Inhibition | PP2 | [93] | |
| WNV | BTK | TK | Inhibition | QL-XII-47 | [83] |
| EGFR | TK | Inhibition | IFN-α inducible protein 6 | [94] | |
| RIPKs | TKL | Activation | AP1 | [95] | |
| MAPKAPK5 | CAMK | Inhibition | SFV785 | [73] | |
| YFV | CK1 | CK1 | Inhibition | D4776 | [96] |
| NTRK1 | TK | Inhibition | SFV785 | [73] | |
| JEV | CHK2 | CAMK | Inhibition | CHK2 inhibitor II | [97] |
| CDK | CMGC | Inhibition | Alsterpaullone 2-cyanoethyl, Cdk1/2 inh III,Cdk2/9 inh | [78] | |
| DCLK1 | CAMK | Inhibition | Fluvastatine | [98] | |
| AMPK | CAMK | Activation | Liraglutide | [99] | |
| CKII | CK1 | Inhibition | 2-dimethylamino-4,5,6,7-tetrabromo-1H-benzimidazole | [100] | |
| MAPK/ERK | CMGC | Inhibition | PD98059, Sorafenib | [101] | |
| HCV | P38/MAPK | CMGC | Inhibition | BmKDfsin3 | [102] |
| SRPK | CMGC | Inhibition | SRPIN340 | [103] | |
| EGFR | TK | Inhibition | Erlotinib, Dasatinib | [69,104] | |
| PKR | Other | Inhibition | HA1077 | [105] | |
| NAK | Other | Inhibition | Isothiazolo [5,4-b]pyridine, Sunitinib, PKC-412 | [106,107,108] | |
| TBK1/IKKε | Other | Inhibition | BX795 | [109] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blázquez, A.-B.; Saiz, J.-C. Potential for Protein Kinase Pharmacological Regulation in Flaviviridae Infections. Int. J. Mol. Sci. 2020, 21, 9524. https://doi.org/10.3390/ijms21249524
Blázquez A-B, Saiz J-C. Potential for Protein Kinase Pharmacological Regulation in Flaviviridae Infections. International Journal of Molecular Sciences. 2020; 21(24):9524. https://doi.org/10.3390/ijms21249524
Chicago/Turabian StyleBlázquez, Ana-Belén, and Juan-Carlos Saiz. 2020. "Potential for Protein Kinase Pharmacological Regulation in Flaviviridae Infections" International Journal of Molecular Sciences 21, no. 24: 9524. https://doi.org/10.3390/ijms21249524
APA StyleBlázquez, A.-B., & Saiz, J.-C. (2020). Potential for Protein Kinase Pharmacological Regulation in Flaviviridae Infections. International Journal of Molecular Sciences, 21(24), 9524. https://doi.org/10.3390/ijms21249524






