Investigation on the Interactions between Self-Assembled β-Sheet Peptide Nanofibers and Model Cell Membranes
Abstract
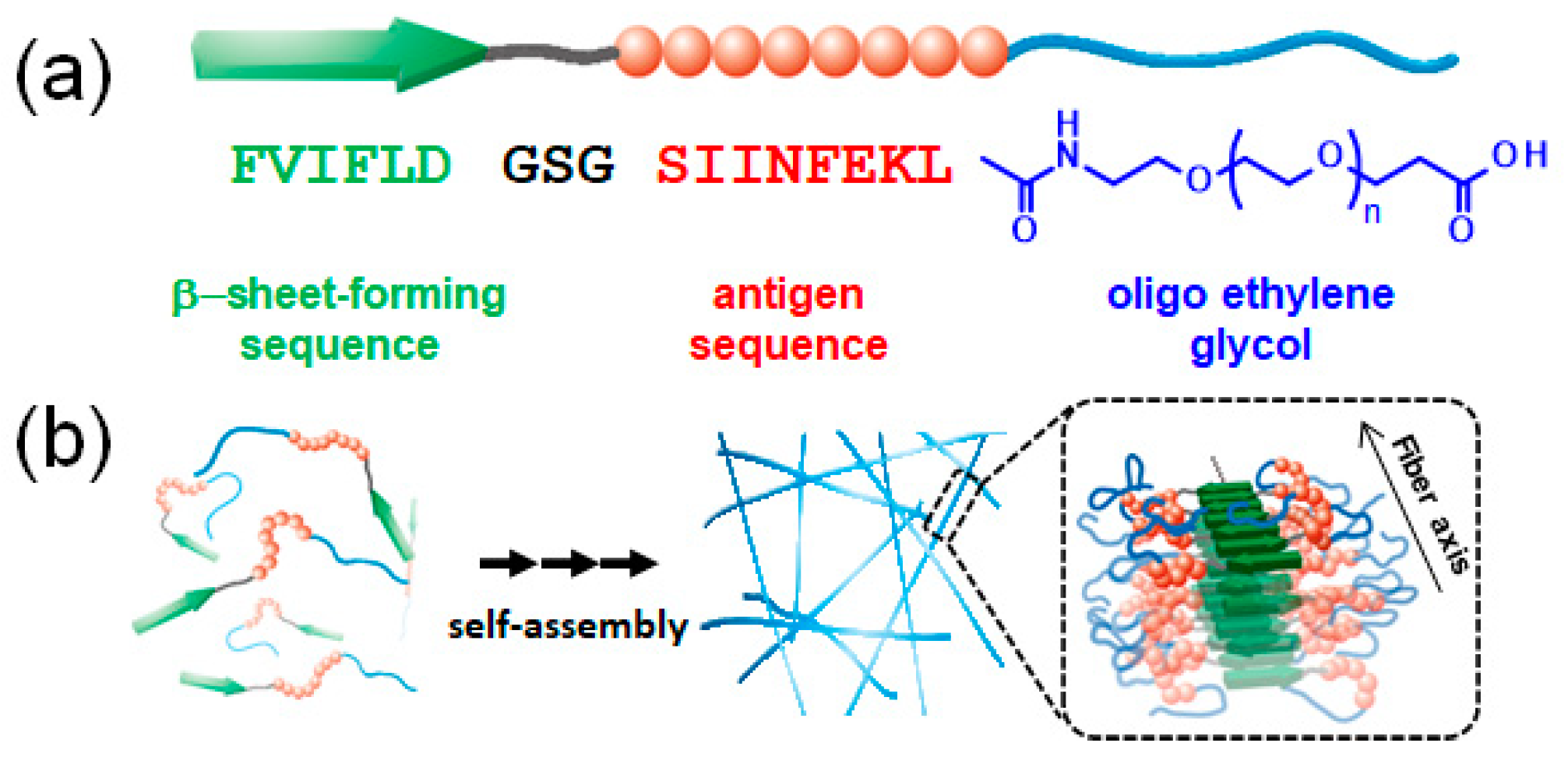
1. Introduction
2. Results
2.1. Interactions of NFs with the DPPC Membrane
2.2. Effect of NFs on the π–A Isotherm of the DPPC Membrane
2.3. Interaction of EGn Peptides with the DPPC Membrane
2.4. Effect of EGn Peptides on the π–A Isotherm of the DPPC Membrane
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Experimental Methods
4.2.1. Synthesis of Building Block Molecules
4.2.2. Preparation of Antigen-Loaded Peptide NFs
4.2.3. Interaction of EGn NFs with the DPPC Membrane
4.2.4. Compression Isotherm of DPPC in the Presence of EGn NFs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Allen, T.M.; Cullis, P.R. Drug delivery systems: Entering the mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Stellacci, F. Effect of surface properties on nanoparticle–cell interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Nie, G.; Meng, H.; Xia, T.; Nel, A.; Zhao, Y. Physicochemical properties determine nanomaterial cellular uptake, transport, and fate. Acc. Chem. Res. 2013, 46, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Jiang, G.; Chen, L.; Zhou, H.; Fourches, D.; Tropsha, A.; Yan, B. Chemical basis of interactions between engineered nanoparticles and biological systems. Chem. Rev. 2014, 114, 7740–7781. [Google Scholar] [CrossRef]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Mecke, A.; Majoros, I.J.; Patri, A.K.; Baker Jr, J.R.; Banaszak Holl, M.M.; Orr, B.G. Lipid bilayer disruption by polycationic polymers: The roles of size and chemical functional group. Langmuir 2005, 21, 10348–10354. [Google Scholar] [CrossRef]
- Hong, S.; Leroueil, P.R.; Janus, E.K.; Peters, J.L.; Kober, M.M.; Islam, M.T.; Orr, B.G.; Baker, J.R., Jr.; Banaszak Holl, M.M. Interaction of polycationic polymers with supported lipid bilayers and cells: Nanoscale hole formation and enhanced membrane permeability. Bioconjug. Chem. 2006, 17, 728–734. [Google Scholar] [CrossRef]
- Hirano, A.; Uda, K.; Maeda, Y.; Akasaka, T.; Shiraki, K. One-dimensional protein-based nanoparticles induce lipid bilayer disruption: Carbon nanotube conjugates and amyloid fibrils. Langmuir 2010, 26, 17256–17259. [Google Scholar] [CrossRef]
- Larios, C.; Miñones, J.; Haro, I.; Alsina, M.A.; Busquets, M.A.; Miñones Trillo, J. Study of adsorption and penetration of E2 (279–298) peptide into Langmuir phospholipid monolayers. J. Phys. Chem. B 2006, 110, 23292–23299. [Google Scholar] [CrossRef]
- Nakahara, H.; Nakamura, S.; Hiranita, T.; Kawasaki, H.; Lee, S.; Sugihara, G.; Shibata, O. Mode of Interaction of Amphiphilic α-Helical Peptide with Phosphatidylcholines at the Air− Water Interface. Langmuir 2006, 22, 1182–1192. [Google Scholar] [CrossRef]
- Gromelski, S.; Brezesinski, G. DNA condensation and interaction with zwitterionic phospholipids mediated by divalent cations. Langmuir 2006, 22, 6293–6301. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.A.; García-Morales, V.; Roozeman, R.J.; Manzanares, J.A.; Kontturi, K. Interfacial interaction between dextran sulfate and lipid monolayers: An electrochemical study. Langmuir 2005, 21, 5475–5484. [Google Scholar] [CrossRef] [PubMed]
- Corvis, Y.; Barzyk, W.; Brezesinski, G.; Mrabet, N.; Badis, M.; Hecht, S.; Rogalska, E. Interactions of a fungistatic antibiotic, griseofulvin, with phospholipid monolayers used as models of biological membranes. Langmuir 2006, 22, 7701–7711. [Google Scholar] [CrossRef] [PubMed]
- Maskarinec, S.A.; Lee, K.Y.C. Comparative study of poloxamer insertion into lipid monolayers. Langmuir 2003, 19, 1809–1815. [Google Scholar] [CrossRef]
- Frey, S.L.; Lee, K.Y.C. Temperature dependence of poloxamer insertion into and squeeze-out from lipid monolayers. Langmuir 2007, 23, 2631–2637. [Google Scholar] [CrossRef]
- Ortiz-Collazos, S.; Picciani, P.H.; Oliveira, O.N., Jr.; Pimentel, A.S.; Edler, K.J. Influence of levofloxacin and clarithromycin on the structure of DPPC monolayers. Biochim. et Biophys. Acta (BBA)-Biomembr. 2019, 1861, 182994. [Google Scholar] [CrossRef]
- Peetla, C.; Labhasetwar, V. Biophysical characterization of nanoparticle− endothelial model cell membrane interactions. Mol. Pharm. 2008, 5, 418–429. [Google Scholar] [CrossRef]
- Peetla, C.; Labhasetwar, V. Effect of molecular structure of cationic surfactants on biophysical interactions of surfactant-modified nanoparticles with a model membrane and cellular uptake. Langmuir 2009, 25, 2369–2377. [Google Scholar] [CrossRef]
- Peetla, C.; Rao, K.S.; Labhasetwar, V. Relevance of biophysical interactions of nanoparticles with a model membrane in predicting cellular uptake: Study with TAT peptide-conjugated nanoparticles. Mol. Pharm. 2009, 6, 1311–1320. [Google Scholar] [CrossRef]
- Schüer, J.J.; Arndt, A.; Wölk, C.; Pinnapireddy, S.R.; Bakowsky, U. Establishment of a synthetic in vitro lung surfactant model for particle interaction studies on a Langmuir film balance. Langmuir 2020, 36, 4808–4819. [Google Scholar] [CrossRef]
- Guzmán, E.; Santini, E.; Ferrari, M.; Liggieri, L.; Ravera, F. Interfacial properties of mixed DPPC–hydrophobic fumed silica nanoparticle layers. J. Phys. Chem. C 2015, 119, 21024–21034. [Google Scholar] [CrossRef]
- Guzmán, E.; Santini, E.; Ferrari, M.; Liggieri, L.; Ravera, F. Effect of the incorporation of nanosized titanium dioxide on the interfacial properties of 1, 2-dipalmitoyl-sn-glycerol-3-phosphocholine langmuir monolayers. Langmuir 2017, 33, 10715–10725. [Google Scholar] [CrossRef] [PubMed]
- Uehara, T.M.; Marangoni, V.S.; Pasquale, N.; Miranda, P.B.; Lee, K.B.; Zucolotto, V. A detailed investigation on the interactions between magnetic nanoparticles and cell membrane models. ACS Appl. Mater. Interfaces 2013, 5, 13063–13068. [Google Scholar] [CrossRef] [PubMed]
- Torrano, A.A.; Pereira, Â.S.; Oliveira, O.N., Jr.; Barros-Timmons, A. Probing the interaction of oppositely charged gold nanoparticles with DPPG and DPPC Langmuir monolayers as cell membrane models. Colloids Surf. B 2013, 108, 120–126. [Google Scholar] [CrossRef]
- Guzmán, E.; Santini, E.; Ferrari, M.; Liggieri, L.; Ravera, F. Interaction of Particles with Langmuir Monolayers of 1, 2-Dipalmitoyl-Sn-Glycero-3-Phosphocholine: A Matter of Chemistry? Coatings 2020, 10, 469. [Google Scholar] [CrossRef]
- Guzmán, E.; Liggieri, L.; Santini, E.; Ferrari, M.; Ravera, F. Effect of hydrophilic and hydrophobic nanoparticles on the surface pressure response of DPPC monolayers. J. Phys. Chem. C 2011, 115, 21715–21722. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Yang, S. Studies of dipalmitoylphosphatidylcholine (DPPC) monolayers embedded with endohedral metallofullerene (Dy@ C82). Langmuir 2009, 25, 12968–12973. [Google Scholar] [CrossRef]
- Guzmán, E.; Liggieri, L.; Santini, E.; Ferrari, M.; Ravera, F. Influence of silica nanoparticles on phase behavior and structural properties of DPPC—Palmitic acid Langmuir monolayers. Colloids Surf. A Physicochem. Eng. Asp. 2012, 413, 280–287. [Google Scholar] [CrossRef]
- Baoukina, S.; Monticelli, L.; Marrink, S.J.; Tieleman, D.P. Pressure− area isotherm of a lipid monolayer from molecular dynamics simulations. Langmuir 2007, 23, 12617–12623. [Google Scholar] [CrossRef]
- Yuba, E. Development of functional liposomes by modification of stimuli-responsive materials and their biomedical applications. J. Mater. Chem. B 2020, 8, 1093–1107. [Google Scholar] [CrossRef]
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.R.; Rudzinski, W.E. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release 2001, 70, 1–20. [Google Scholar] [CrossRef]
- Osada, K.; Christie, R.J.; Kataoka, K. Polymeric micelles from poly (ethylene glycol)–poly (amino acid) block copolymer for drug and gene delivery. J. R. Soc. Interface 2009, 6, S325–S339. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kaplan, J.A.; Sun, Y.; Shieh, A.; Sun, H.L.; Croce, C.M.; Grinstaff, M.W.; Parquette, J.R. The self-assembly of anticancer camptothecin–dipeptide nanotubes: A minimalistic and high drug loading approach to increased efficacy. Chem. Eur. J. 2015, 21, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Cheetham, A.G.; Zhang, P.; Lin, Y.A.; Lock, L.L.; Cui, H. Supramolecular nanostructures formed by anticancer drug assembly. J. Am. Chem. Soc. 2013, 135, 2907–2910. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cheetham, A.G.; Angacian, G.; Su, H.; Xie, L.; Cui, H. Peptide–drug conjugates as effective prodrug strategies for targeted delivery. Adv. Drug Deliv. Rev. 2017, 110, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Waku, T.; Tanaka, N. Recent advances in nanofibrous assemblies based on β-sheet-forming peptides for biomedical applications. Polym. Int. 2017, 66, 277–288. [Google Scholar] [CrossRef]
- Waku, T.; Kitagawa, Y.; Kawabata, K.; Nishigaki, S.; Kunugi, S.; Tanaka, N. Self-assembled β-sheet peptide nanofibers for efficient antigen delivery. Chem. Lett. 2013, 42, 1441–1443. [Google Scholar] [CrossRef]
- Kirschner, D.A.; Abraham, C.; Selkoe, D.J. X-ray diffraction from intraneuronal paired helical filaments and extraneuronal amyloid fibers in Alzheimer disease indicates cross-beta conformation. Proc. Natl. Acad. Sci. USA 1986, 83, 503–507. [Google Scholar] [CrossRef]
- Minami, T.; Matsumoto, S.; Sanada, Y.; Waku, T.; Tanaka, N.; Sakurai, K. Rod-like architecture and cross-sectional structure of an amyloid protofilament-like peptide supermolecule in aqueous solution. Polym. J. 2016, 48, 197–202. [Google Scholar] [CrossRef]
- Waku, T.; Nishigaki, S.; Kitagawa, Y.; Koeda, S.; Kawabata, K.; Kunugi, S.; Kobori, A.; Tanaka, N. Effect of the Hydrophilic-Hydrophobic Balance of Antigen-Loaded Peptide Nanofibers on Their Cellular Uptake, Cellular Toxicity, and Immune Stimulatory Properties. Int. J. Mol. Sci. 2019, 20, 3781. [Google Scholar] [CrossRef]
- Preetha, A.; Huilgol, N.; Banerjee, R. Comparison of paclitaxel penetration in normal and cancerous cervical model monolayer membranes. Colloids Surf. B 2006, 53, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, K.; Mattila, J.P.; Megli, F.M.; Kinnunen, P.K. Characterization of two oxidatively modified phospholipids in mixed monolayers with DPPC. Biophys. J. 2006, 90, 4488–4499. [Google Scholar] [CrossRef] [PubMed]
- Kaganer, V.M.; Möhwald, H.; Dutta, P. Structure and phase transitions in Langmuir monolayers. Rev. Mod. Phys. 1999, 71, 779–819. [Google Scholar] [CrossRef]
- Ma, G.; Allen, H.C. DPPC Langmuir monolayer at the air−water interface: Probing the tail and head groups by vibrational sum frequency generation spectroscopy. Langmuir 2006, 22, 5341–5349. [Google Scholar] [CrossRef] [PubMed]
- Rapaport, H.; Kjaer, K.; Jensen, T.R.; Leiserowitz, L.; Tirrell, D.A. Two-dimensional order in β-sheet peptide monolayers. J. Am. Chem. Soc. 2000, 122, 12523–12529. [Google Scholar] [CrossRef]
- Koga, T.; Taguchi, T.; Higashi, N. β-Sheet peptide-assisted polymerization of diacetylene at the air–water interface and thermochromic property. Polym. J. 2012, 44, 195–199. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waku, T.; Kasai, A.; Kobori, A.; Tanaka, N. Investigation on the Interactions between Self-Assembled β-Sheet Peptide Nanofibers and Model Cell Membranes. Int. J. Mol. Sci. 2020, 21, 9518. https://doi.org/10.3390/ijms21249518
Waku T, Kasai A, Kobori A, Tanaka N. Investigation on the Interactions between Self-Assembled β-Sheet Peptide Nanofibers and Model Cell Membranes. International Journal of Molecular Sciences. 2020; 21(24):9518. https://doi.org/10.3390/ijms21249518
Chicago/Turabian StyleWaku, Tomonori, Ayane Kasai, Akio Kobori, and Naoki Tanaka. 2020. "Investigation on the Interactions between Self-Assembled β-Sheet Peptide Nanofibers and Model Cell Membranes" International Journal of Molecular Sciences 21, no. 24: 9518. https://doi.org/10.3390/ijms21249518
APA StyleWaku, T., Kasai, A., Kobori, A., & Tanaka, N. (2020). Investigation on the Interactions between Self-Assembled β-Sheet Peptide Nanofibers and Model Cell Membranes. International Journal of Molecular Sciences, 21(24), 9518. https://doi.org/10.3390/ijms21249518



