Supramolecular Architectures of Nucleic Acid/Peptide Hybrids
Abstract
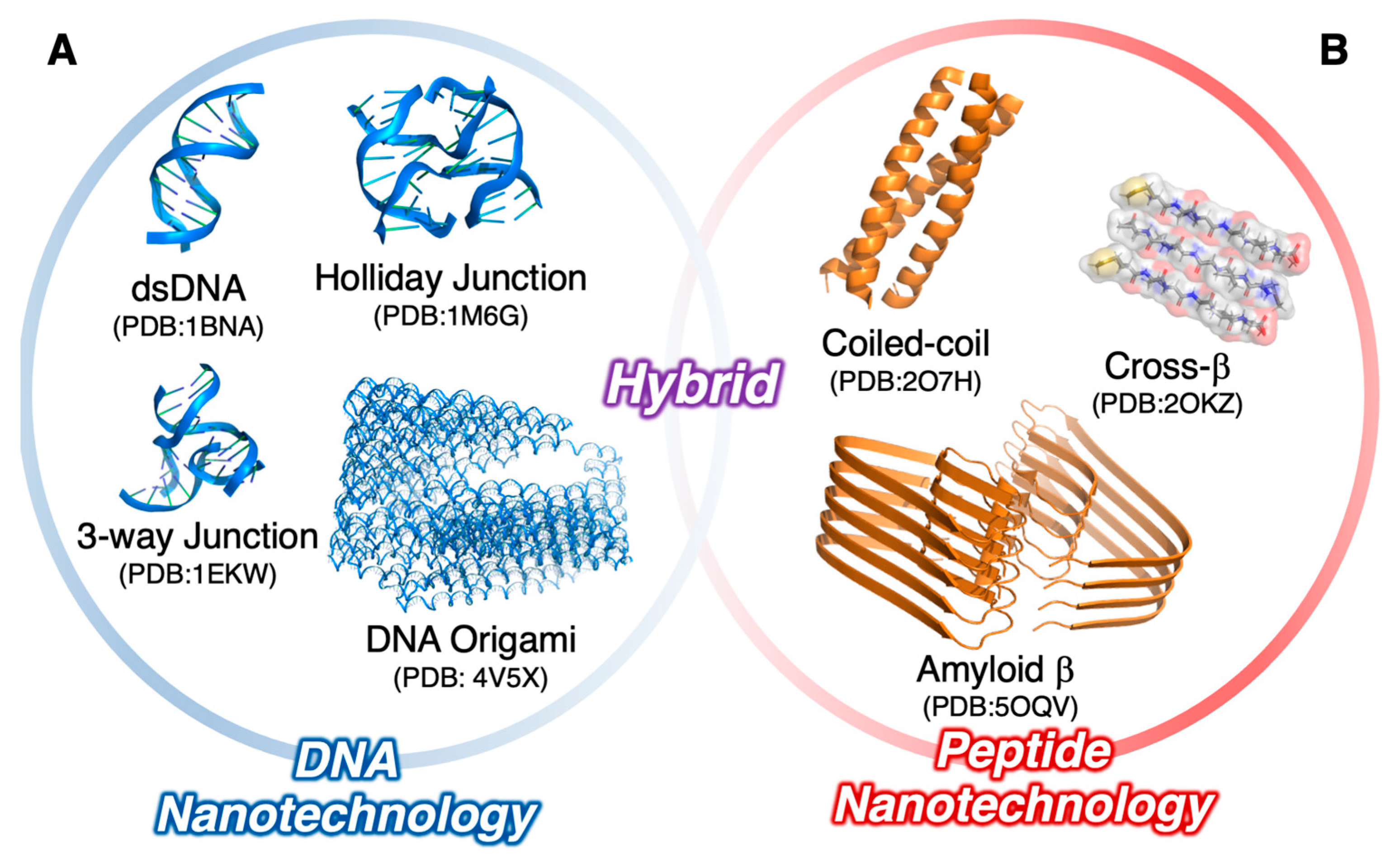
1. Introduction
2. Nucleic Acid/Oligopeptide Hybrids
2.1. Non-Covalently Conjugated Nucleic Acid/Oligopeptide Hybrids
2.1.1. Spherical Supramolecular Architectures
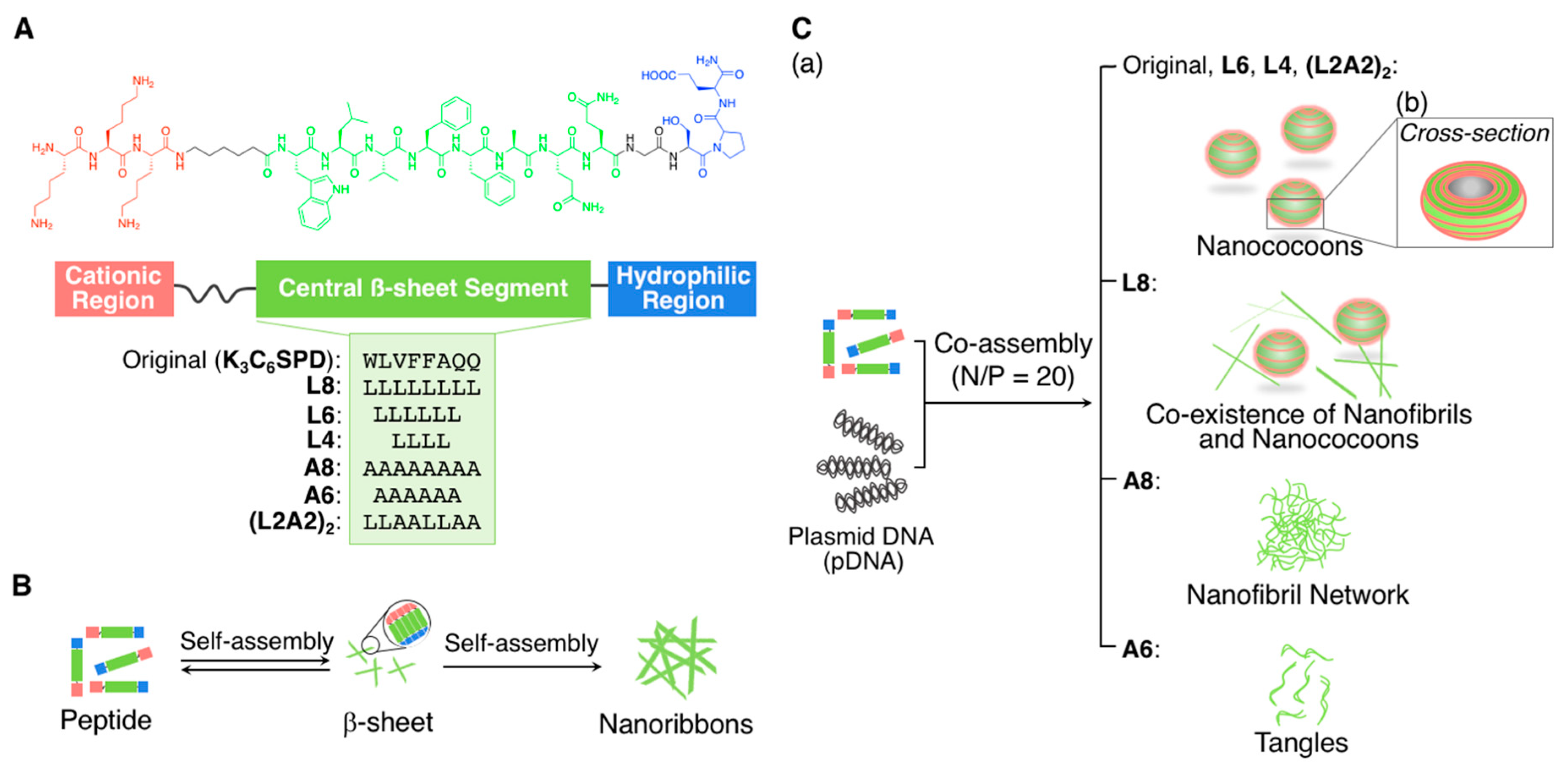
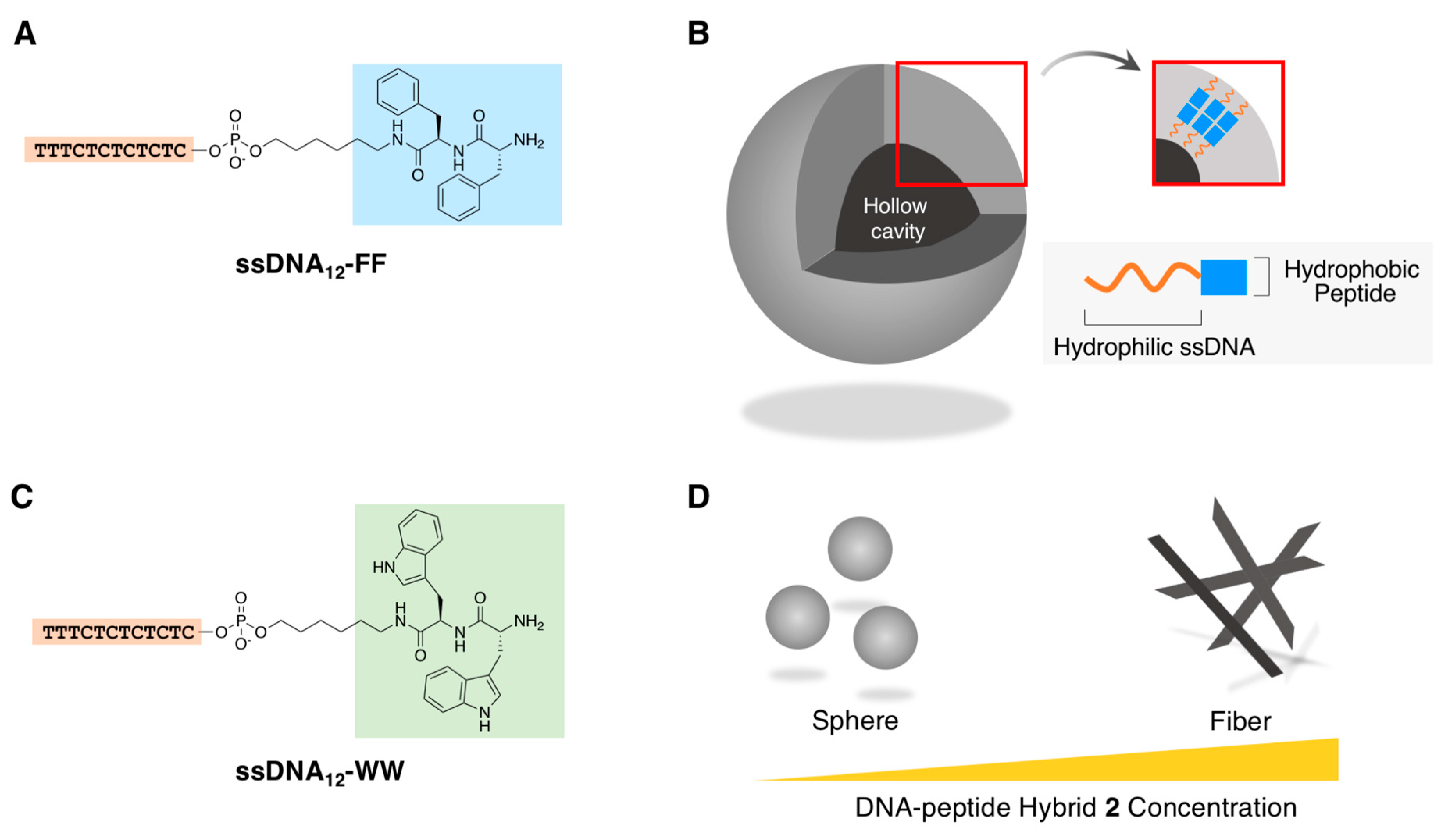
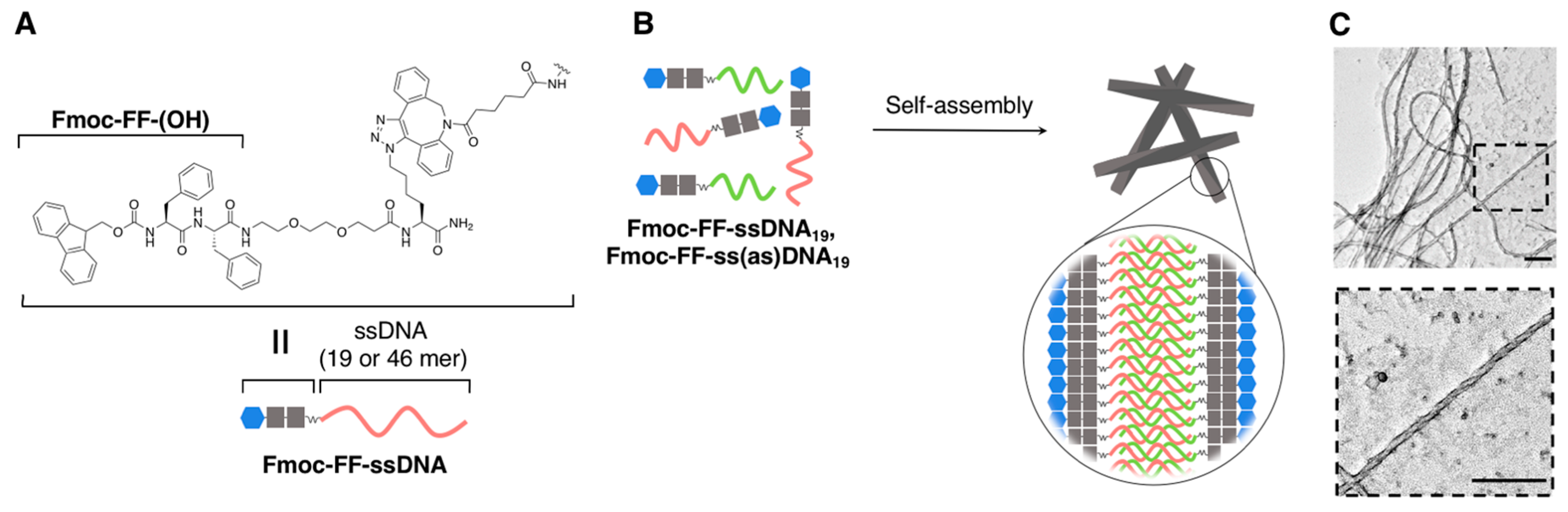
2.1.2. Fibrous Supramolecular Architectures
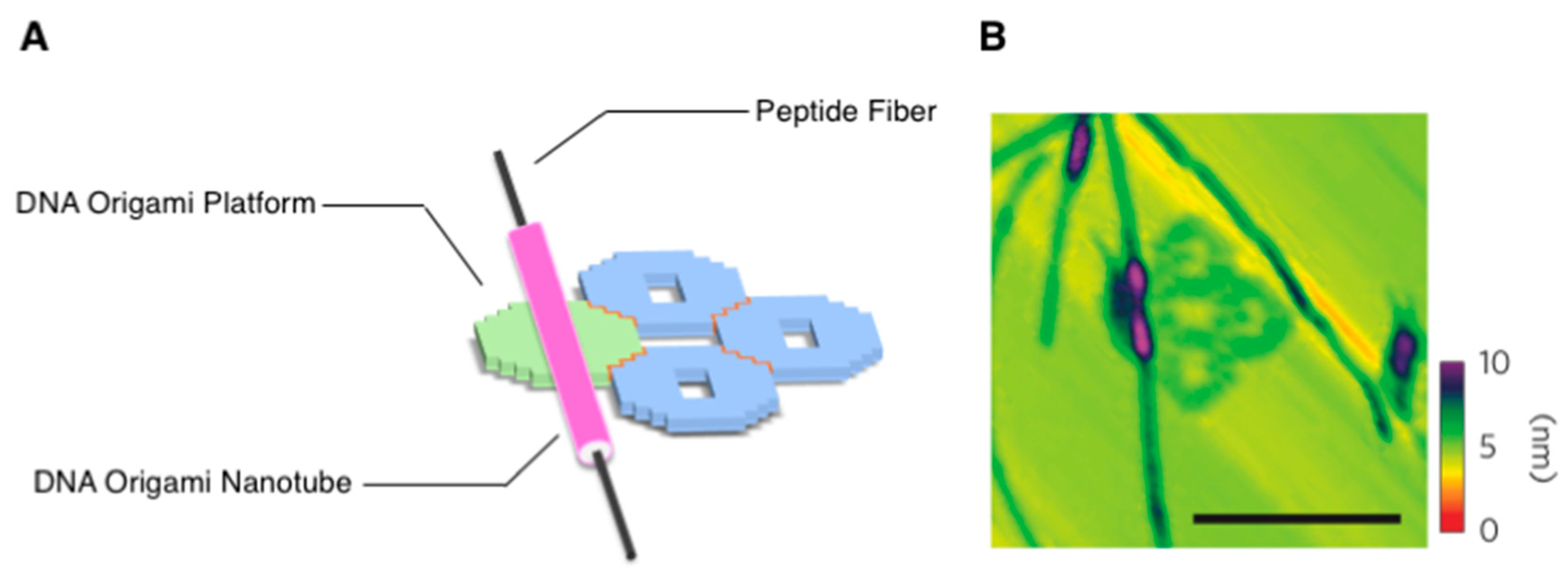
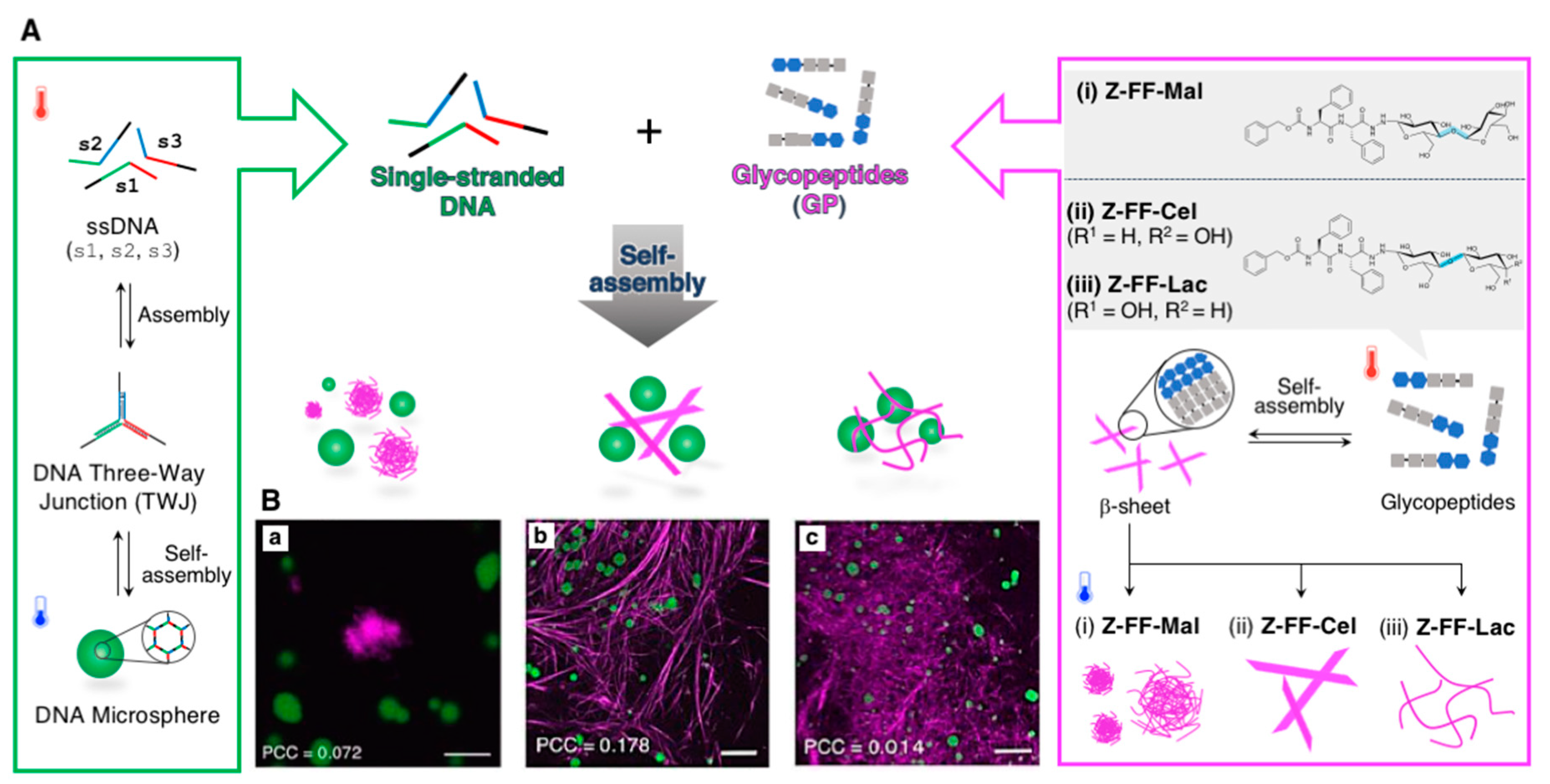
2.1.3. Orthogonal Supramolecular Architectures Without Random Co-Assembly
2.2. Covalently Conjugated Nucleic Acid-Oligopeptide Hybrids
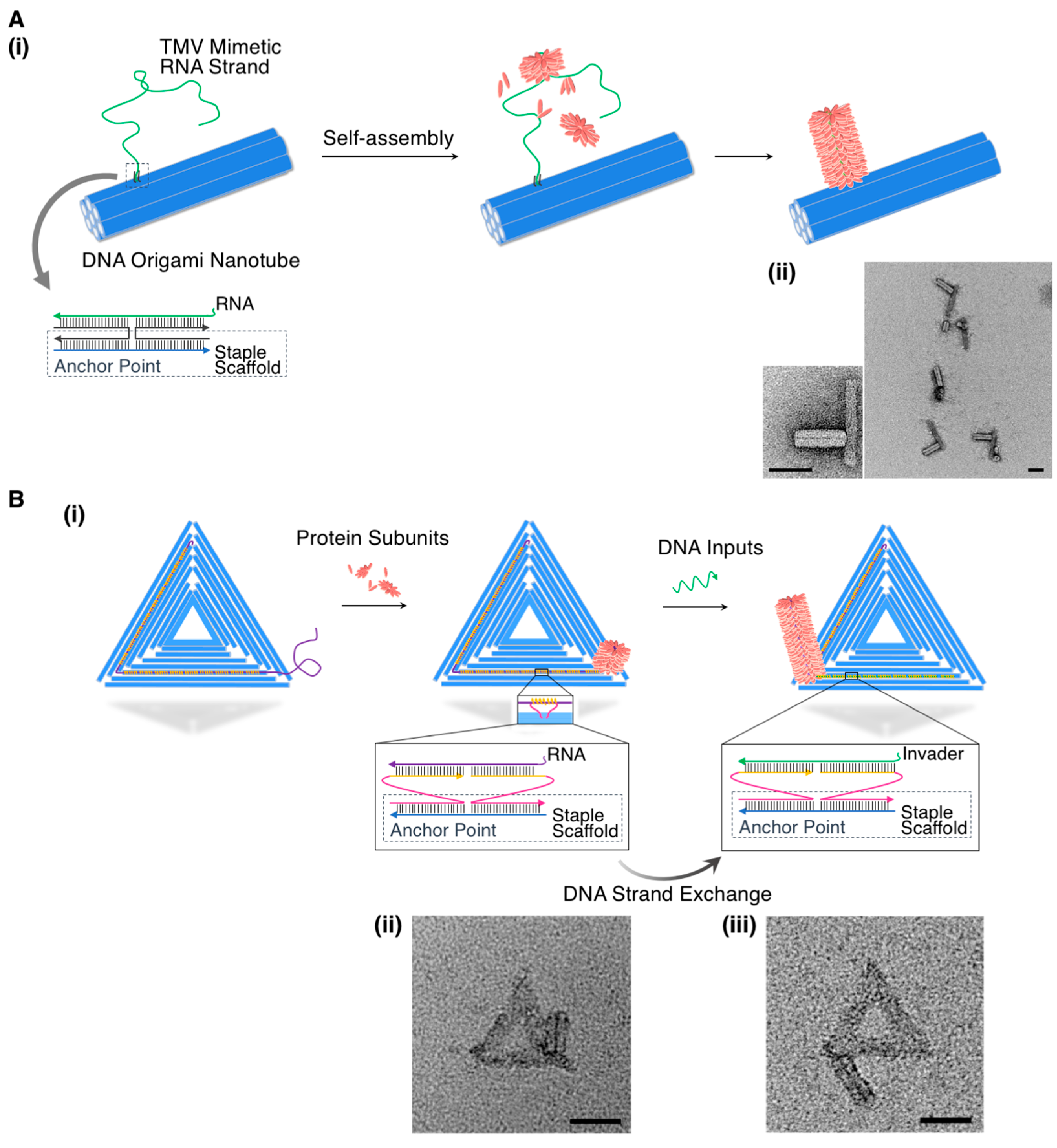
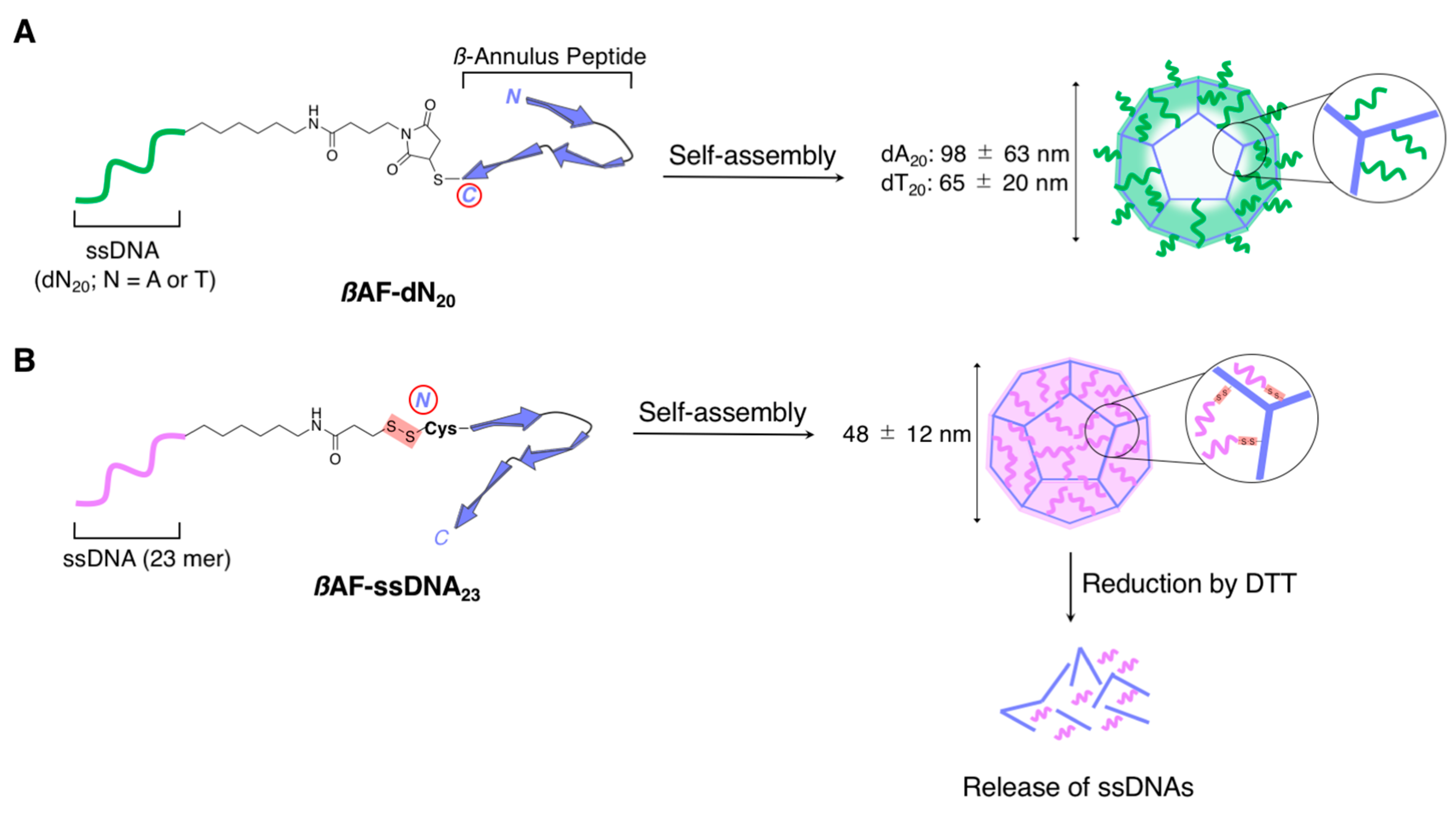
2.2.1. Spherical Supramolecular Architectures
2.2.2. Fibrous Supramolecular Architectures
3. Nucleic Acid/Polypeptide (Protein) Hybrids
3.1. Non-Covalently Conjugated Hybrids
3.2. Covalently Conjugated Hybrids
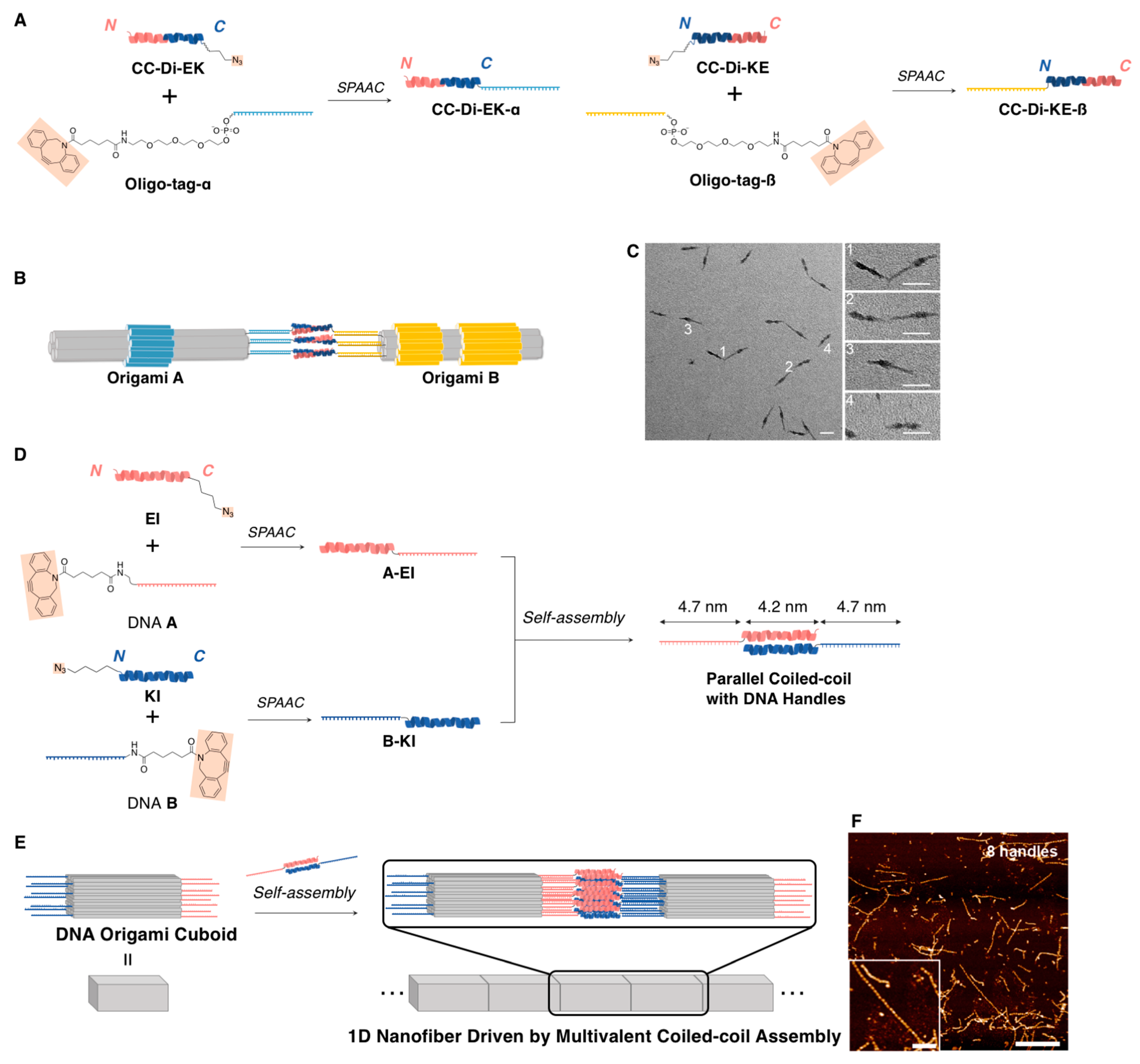
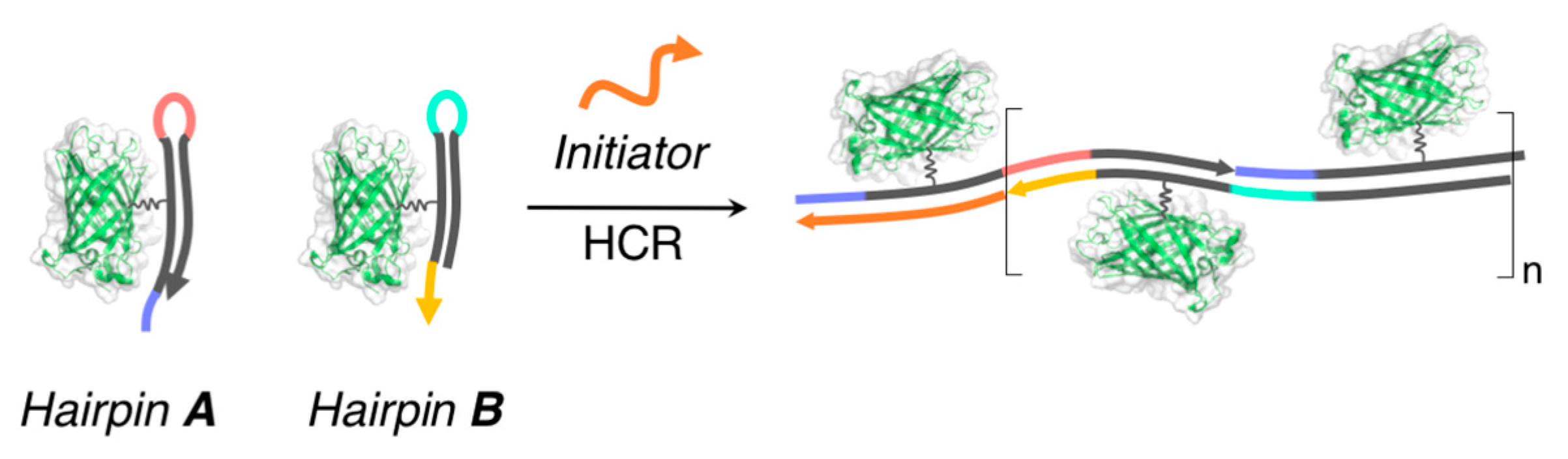
3.2.1. Linear Supramolecular Architectures
3.2.2. Cage-Like Supramolecular Architectures
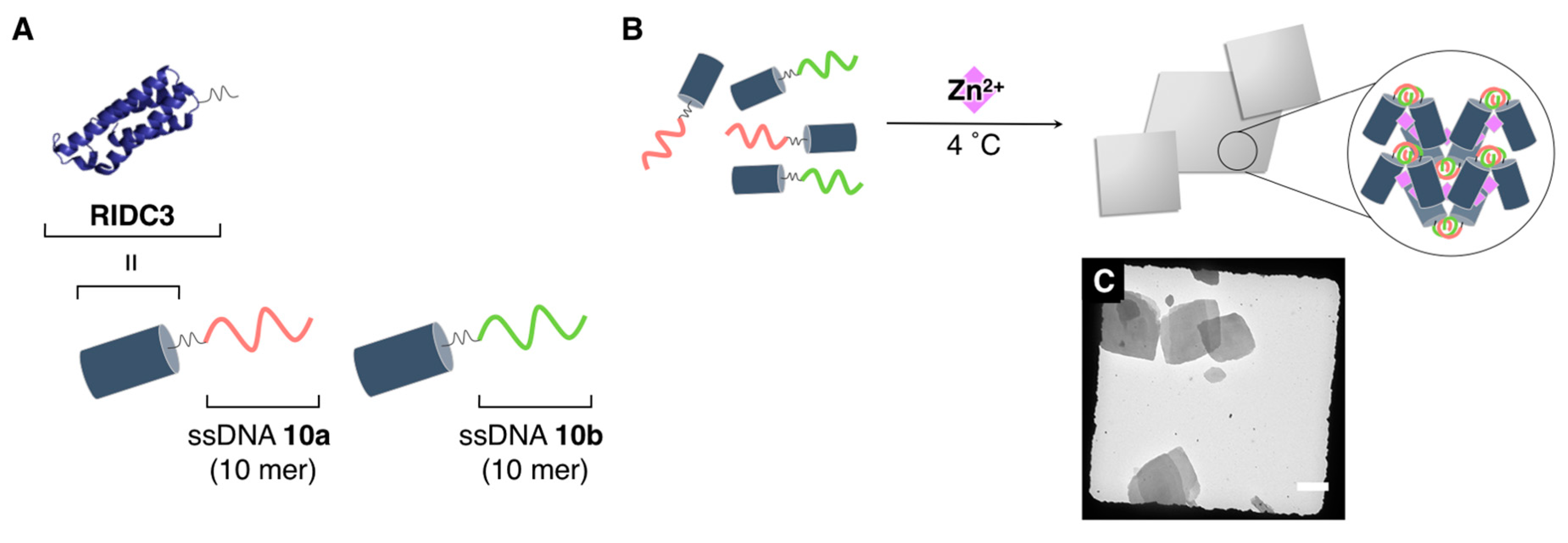
3.2.3. Crystalline Supramolecular Architectures
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Seeman, N.C.; Sleiman, H.F. DNA nanotechnology. Nat. Rev. Mater. 2018, 3, 17068. [Google Scholar] [CrossRef]
- Madsen, M.; Gothelf, K.V. Chemistries for DNA nanotechnology. Chem. Rev. 2019, 119, 6384–6458. [Google Scholar] [CrossRef] [PubMed]
- Heuer-Jungemann, A.; Liedl, T. From DNA tiles to functional DNA materials. Trends Chem. 2019, 1, 799–814. [Google Scholar] [CrossRef]
- Seeman, N.C. DNA in a material world. Nature 2003, 421, 427–431. [Google Scholar] [CrossRef]
- Seeman, N.C. Nucleic acid junctions and lattices. J. Theor. Biol. 1982, 99, 237–247. [Google Scholar] [CrossRef]
- Rothemund, P.W.K. Folding DNA to create nanoscale shapes and patterns. Nature 2006, 440, 297–302. [Google Scholar] [CrossRef]
- Hong, F.; Zhang, F.; Liu, Y.; Yan, H. DNA origami: Scaffolds for creating higher order structures. Chem. Rev. 2017, 117, 12584–12640. [Google Scholar] [CrossRef]
- Kallenbach, N.R.; Ma, R.-I.; Seeman, N.C. An immobile nucleic acid junction constructed from oligonucleotides. Nature 1983, 305, 829–831. [Google Scholar] [CrossRef]
- Fu, T.J.; Seeman, N.C. DNA double-crossover molecules. Biochemistry 1993, 32, 3211–3220. [Google Scholar] [CrossRef]
- Wang, Y.; Mueller, J.E.; Kemper, B.; Seeman, N.C. Assembly and characterization of five-arm and six-arm DNA branched junctions. Biochemistry 1991, 30, 5667–5674. [Google Scholar] [CrossRef]
- Wang, X.; Chandrasekaran, A.R.; Shen, Z.; Ohayon, Y.P.; Wang, T.; Kizer, M.E.; Sha, R.; Mao, C.; Yan, H.; Zhang, X.; et al. Paranemic crossover DNA: There and back again. Chem. Rev. 2018, 119, 6273–6289. [Google Scholar] [CrossRef] [PubMed]
- Winfree, E.; Liu, F.; Wenzler, L.A.; Seeman, N.C. Design and self-assembly of two-dimensional DNA crystals. Nature 1998, 394, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Yan, H. DNA-templated self-assembly of protein arrays and highly conductive nanowires. Science 2003, 301, 1882–1884. [Google Scholar] [CrossRef] [PubMed]
- Schulman, R.; Winfree, E. Synthesis of crystals with a programmable kinetic barrier to nucleation. Proc. Natl. Acad. Sci. USA 2007, 104, 15236–15241. [Google Scholar] [CrossRef] [PubMed]
- Rothemund, P.W.K.; Ekani-Nkodo, A.; Papadakis, N.; Kumar, A.; Fygenson, D.K.; Winfree, E. Design and characterization of programmable DNA nanotubes. J. Am. Chem. Soc. 2004, 126, 16344–16352. [Google Scholar] [CrossRef]
- Yin, P.; Hariadi, R.F.; Sahu, S.; Choi, H.M.T.; Park, S.H.; LaBean, T.H.; Reif, J.H. Programming DNA tube circumferences. Science 2008, 321, 824–826. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Y.; He, Y.; Ribbe, A.E.; Mao, C. Approaching the limit: Can one DNA oligonucleotide assemble into large nanostructures? Angew. Chem. Int. Ed. 2006, 45, 1942–1945. [Google Scholar] [CrossRef]
- Wilner, O.I.; Orbach, R.; Henning, A.; Teller, C.; Yehezkeli, O.; Mertig, M.; Harries, D.; Willner, I. Self-assembly of DNA nanotubes with controllable diameters. Nat. Commun. 2011, 2, 540. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Hariadi, R.F.; Choi, H.M.T.; Winfree, E. Integrating DNA strand-displacement circuitry with DNA tile self-assembly. Nat. Commun. 2013, 4, 1965. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, Y.; Liu, P.; Wang, L.; Lin, J.; Fan, C. Biomimetic DNA nanotubes: Nanoscale channel design and applications. Angew. Chem. Int. Ed. 2019, 58, 8996–9011. [Google Scholar] [CrossRef]
- He, Y.; Ye, T.; Su, M.; Zhang, C.; Ribbe, A.E.; Jiang, W.; Mao, C. Hierarchical self-assembly of DNA into symmetric supramolecular polyhedra. Nature 2008, 452, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; He, Y.; Su, M.; Ko, S.H.; Ye, T.; Leng, Y.; Sun, X.; Ribbe, A.E.; Jiang, W.; Mao, C. DNA self-assembly: From 2D to 3D. Faraday Discuss. 2009, 143, 221. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Li, X.; Liu, Z.; Jiang, W.; Wang, G.; Mao, C. Directed self-assembly of DNA tiles into complex nanocages. Angew. Chem. Int. Ed. 2014, 53, 8041–8044. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Yang, Y.R.; Johnson-Buck, A.; Liu, M.; Liu, Y.; Walter, N.G.; Woodbury, N.W.; Yan, H. Multi-enzyme complexes on DNA scaffolds capable of substrate channelling with an artificial swinging arm. Nat. Nanotechnol. 2014, 9, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Linko, V.; Eerikäinen, M.; Kostiainen, M.A. A modular DNA origami-based enzyme cascade nanoreactor. Chem. Commun. 2015, 51, 5351–5354. [Google Scholar] [CrossRef]
- Yu, Y.; Jin, B.; Li, Y.; Deng, Z. Stimuli-responsive DNA self-assembly: From principles to applications. Chem. Eur. J. 2019, 25, 9785–9798. [Google Scholar] [CrossRef]
- Xiao, M.; Lai, W.; Man, T.; Chang, B.; Li, L.; Chandrasekaran, A.R.; Pei, H. Rationally engineered nucleic acid architectures for biosensing applications. Chem. Rev. 2019, 119, 11631–11717. [Google Scholar] [CrossRef]
- Douglas, S.M.; Bachelet, I.; Church, G.M. A logic-gated nanorobot for targeted transport of molecular payloads. Science 2012, 335, 831–834. [Google Scholar] [CrossRef]
- Li, S.; Jiang, Q.; Liu, S.; Zhang, Y.; Tian, Y.; Song, C.; Wang, J.; Zou, Y.; Anderson, G.J.; Han, J.-Y.; et al. A DNA nanorobot functions as a cancer therapeutic in response to a molecular trigger in vivo. Nat. Biotechnol. 2018, 36, 258–264. [Google Scholar] [CrossRef]
- Kopperger, E.; List, J.; Madhira, S.; Rothfischer, F.; Lamb, D.C.; Simmel, F.C. A self-assembled nanoscale robotic arm controlled by electric fields. Science 2018, 359, 296–301. [Google Scholar] [CrossRef]
- Nummelin, S.; Shen, B.; Piskunen, P.; Liu, Q.; Kostiainen, M.A.; Linko, V. Robotic DNA nanostructures. ACS Synth. Biol. 2020, 9, 1923–1940. [Google Scholar] [CrossRef] [PubMed]
- Mei, Q.; Wei, X.; Su, F.; Liu, Y.; Youngbull, C.; Johnson, R.; Lindsay, S.; Yan, H.; Meldrum, D. Stability of DNA origami nanoarrays in cell lysate. Nano Lett. 2011, 11, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Hahn, J.; Wickham, S.F.J.; Shih, W.M.; Perrault, S.D. Addressing the instability of DNA nanostructures in tissue culture. ACS Nano 2014, 8, 8765–8775. [Google Scholar] [CrossRef] [PubMed]
- Kielar, C.; Xin, Y.; Shen, B.; Kostiainen, M.A.; Grundmeier, G.; Linko, V.; Keller, A. On the stability of DNA origami nanostructures in low-magnesium buffers. Angew. Chem. Int. Ed. 2018, 57, 9470–9474. [Google Scholar] [CrossRef]
- Mikkilä, J.; Eskelinen, A.-P.; Niemelä, E.H.; Linko, V.; Frilander, M.J.; Törmä, P.; Kostiainen, M.A. Virus-encapsulated DNA origami nanostructures for cellular delivery. Nano Lett. 2014, 14, 2196–2200. [Google Scholar] [CrossRef]
- Auvinen, H.; Zhang, H.; Nonappa; Kopilow, A.; Niemelä, E.H.; Nummelin, S.; Correia, A.; Santos, H.A.; Linko, V.; Kostiainen, M.A. Protein coating of DNA nanostructures for enhanced stability and immunocompatibility. Adv. Healthc. Mater. 2017, 6, 1700692. [Google Scholar] [CrossRef]
- Agarwal, N.P.; Matthies, M.; Gür, F.N.; Osada, K.; Schmidt, T.L. Block copolymer micellization as a protection strategy for DNA origami. Angew. Chem. Int. Ed. 2017, 56, 5460–5464. [Google Scholar] [CrossRef]
- Ponnuswamy, N.; Bastings, M.M.C.; Nathwani, B.; Ryu, J.H.; Chou, L.Y.T.; Vinther, M.; Li, W.A.; Anastassacos, F.M.; Mooney, D.J.; Shih, W.M. Oligolysine-based coating protects DNA nanostructures from low-salt denaturation and nuclease degradation. Nat. Commun. 2017, 8, 15654. [Google Scholar] [CrossRef]
- Ahmadi, Y.; Llano, E.D.; Barišić, I. (Poly)cation-induced protection of conventional and wireframe DNA origami nanostructures. Nanoscale 2018, 10, 7494–7504. [Google Scholar] [CrossRef]
- Wang, S.-T.; Gray, M.A.; Xuan, S.; Lin, Y.; Byrnes, J.; Nguyen, A.I.; Todorova, N.; Stevens, M.M.; Bertozzi, C.R.; Zuckermann, R.N.; et al. DNA origami protection and molecular interfacing through engineered sequence-defined peptoids. Proc. Natl. Acad. Sci. USA 2020, 117, 6339–6348. [Google Scholar] [CrossRef]
- Dobson, C.M. Protein folding and misfolding. Nature 2003, 426, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Riek, R.; Eisenberg, D.S. The activities of amyloids from a structural perspective. Nature 2016, 539, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Lou, S.; Wang, X.; Yu, Z.; Shi, L. Peptide tectonics: Encoded structural complementarity dictates programmable self-assembly. Adv. Sci. 2019, 6, 1802043. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, D.A.; Mullins, R.D. Cell mechanics and the cytoskeleton. Nature 2010, 463, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Ke, P.C.; Zhou, R.; Serpell, L.C.; Riek, R.; Knowles, T.P.J.; Lashuel, H.A.; Gazit, E.; Hamley, I.W.; Davis, T.P.; Fändrich, M.; et al. Half a century of amyloids: Past, present and future. Chem. Soc. Rev. 2020, 49, 5473–5509. [Google Scholar] [CrossRef] [PubMed]
- Knowles, T.P.J.; Vendruscolo, M.; Dobson, C.M. The amyloid state and its association with protein misfolding diseases. Nat. Rev. Mol. Cell Biol. 2014, 15, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Gremer, L.; Schölzel, D.; Schenk, C.; Reinartz, E.; Labahn, J.; Ravelli, R.B.G.; Tusche, M.; Lopez-Iglesias, C.; Hoyer, W.; Heise, H.; et al. Fibril structure of amyloid-β(1–42) by cryo–electron microscopy. Science 2017, 358, 116–119. [Google Scholar] [CrossRef]
- Tayeb-Fligelman, E.; Tabachnikov, O.; Moshe, A.; Goldshmidt-Tran, O.; Sawaya, M.R.; Coquelle, N.; Colletier, J.-P.; Landau, M. The cytotoxic Staphylococcus aureus PSMα3 reveals a cross-α amyloid-like fibril. Science 2017, 355, 831–833. [Google Scholar] [CrossRef]
- Engelberg, Y.; Landau, M. The Human LL-37(17-29) antimicrobial peptide reveals a functional supramolecular structure. Nat. Commun. 2020, 11, 3894. [Google Scholar] [CrossRef]
- Channon, K.; Bromley, E.H.C.; Woolfson, D.N. Synthetic biology through biomolecular design and engineering. Curr. Opin. Struct. Biol. 2008, 18, 491–498. [Google Scholar] [CrossRef]
- Robson Marsden, H.; Kros, A. Self-assembly of coiled coils in synthetic biology: Inspiration and progress. Angew. Chem. Int. Ed. 2010, 49, 2988–3005. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.M.; Boyle, A.L.; Bruning, M.; Bartlett, G.J.; Vincent, T.L.; Zaccai, N.R.; Armstrong, C.T.; Bromley, E.H.C.; Booth, P.J.; Brady, R.L.; et al. A basis set of de novo coiled-coil peptide oligomers for rational protein design and synthetic biology. ACS Synth. Biol. 2012, 1, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Caruthers, M. Synthesis of DNA/RNA and their analogs via phosphoramidite and H-phosphonate chemistries. Molecules 2013, 18, 14268–14284. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Qiao, J.; Qi, H. Current and emerging methods for the synthesis of single-stranded DNA. Genes 2020, 11, 116. [Google Scholar] [CrossRef] [PubMed]
- Merrifield, R.B. Solid phase peptide synthesis. I. the synthesis of a tetrapeptide. J. Am. Chem. Soc. 1963, 85, 2149–2154. [Google Scholar] [CrossRef]
- Mäde, V.; Els-Heindl, S.; Beck-Sickinger, A.G. Automated solid-phase peptide synthesis to obtain therapeutic peptides. Beilstein J. Org. Chem. 2014, 10, 1197–1212. [Google Scholar] [CrossRef]
- Moss, G.P.; Smith, P.A.S.; Tavernier, D. Glossary of class names of organic compounds and reactivity intermediates based on structure (IUPAC Recommendations 1995). Pure Appl. Chem. 1995, 67, 1307–1375. [Google Scholar] [CrossRef]
- Ni, R.; Chau, Y. Structural mimics of viruses through peptide/DNA co-assembly. J. Am. Chem. Soc. 2014, 136, 17902–17905. [Google Scholar] [CrossRef]
- Ni, R.; Chau, Y. Tuning the inter-nanofibril interaction to regulate the morphology and function of peptide/DNA co-assembled viral mimics. Angew. Chem. Int. Ed. 2017, 56, 9356–9360. [Google Scholar] [CrossRef]
- Ni, R.; Chau, Y. Nanoassembly of oligopeptides and DNA mimics the sequential disassembly of a spherical virus. Angew. Chem. Int. Ed. 2020, 59, 3578–3584. [Google Scholar] [CrossRef]
- Lindsey, J.S. Self-assembly in synthetic routes to molecular devices. biological principles and chemical perspectives. New J. Chem. 1991, 15, 153–180. [Google Scholar]
- Philp, D.; Stoddart, J.F. Self-assembly in natural and unnatural systems. Angew. Chem. Int. Ed. 1996, 35, 1154–1196. [Google Scholar] [CrossRef]
- Klug, A. The tobacco mosaic virus particle: Structure and assembly. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1999, 354, 531–535. [Google Scholar] [CrossRef]
- Zhou, K.; Ke, Y.; Wang, Q. Selective in situ assembly of viral protein onto DNA origami. J. Am. Chem. Soc. 2018, 140, 8074–8077. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Zhou, Y.; Pan, V.; Wang, Q.; Ke, Y. Programming dynamic assembly of viral proteins with DNA origami. J. Am. Chem. Soc. 2020, 142, 5929–5932. [Google Scholar] [CrossRef] [PubMed]
- Ruff, Y.; Moyer, T.; Newcomb, C.J.; Demeler, B.; Stupp, S.I. Precision templating with DNA of a virus-like particle with peptide nanostructures. J. Am. Chem. Soc. 2013, 135, 6211–6219. [Google Scholar] [CrossRef]
- Kang, Z.; Meng, Q.; Liu, K. Peptide-based gene delivery vectors. J. Mater. Chem. B 2019, 7, 1824–1841. [Google Scholar] [CrossRef]
- Jiang, T.; Meyer, T.A.; Modlin, C.; Zuo, X.; Conticello, V.P.; Ke, Y. Structurally ordered nanowire formation from co-assembly of DNA origami and collagen-mimetic peptides. J. Am. Chem. Soc. 2017, 139, 14025–14028. [Google Scholar] [CrossRef]
- Jin, J.; Baker, E.G.; Wood, C.W.; Bath, J.; Woolfson, D.N.; Turberfield, A.J. Peptide assembly directed and quantified using megadalton DNA nanostructures. ACS Nano 2019, 13, 9927–9935. [Google Scholar] [CrossRef]
- Buchberger, A.; Simmons, C.R.; Fahmi, N.E.; Freeman, R.; Stephanopoulos, N. Hierarchical assembly of nucleic acid/coiled-coil peptide nanostructures. J. Am. Chem. Soc. 2020, 142, 1406–1416. [Google Scholar] [CrossRef]
- Brizard, A.M.; van Esch, J.H. Self-assembly approaches for the construction of cell architecture mimics. Soft Matter 2009, 5, 1320–1327. [Google Scholar] [CrossRef]
- Safont-Sempere, M.M.; Fernández, G.; Würthner, F. Self-sorting phenomena in complex supramolecular systems. Chem. Rev. 2011, 111, 5784–5814. [Google Scholar] [CrossRef] [PubMed]
- Draper, E.R.; Adams, D.J. How should multicomponent supramolecular gels be characterised? Chem. Soc. Rev. 2018, 47, 3395–3405. [Google Scholar] [CrossRef] [PubMed]
- Kubota, R.; Nakamura, K.; Torigoe, S.; Hamachi, I. The power of confocal laser scanning microscopy in supramolecular chemistry: In situ real-time imaging of stimuli-responsive multicomponent supramolecular hydrogels. ChemistryOpen 2020, 9, 67–79. [Google Scholar] [CrossRef]
- Udomprasert, A.; Bongiovanni, M.N.; Sha, R.; Sherman, W.B.; Wang, T.; Arora, P.S.; Canary, J.W.; Gras, S.L.; Seeman, N.C. Amyloid fibrils nucleated and organized by DNA origami constructions. Nat. Nanotechnol. 2014, 9, 537–541. [Google Scholar] [CrossRef]
- Higashi, S.L.; Shibata, A.; Kitamura, Y.; Hirosawa, K.M.; Suzuki, K.G.N.; Matsuura, K.; Ikeda, M. Hybrid soft nanomaterials composed of DNA microspheres and supramolecular nanostructures of semi-artificial glycopeptides. Chem. Eur. J. 2019, 25, 11955–11962. [Google Scholar] [CrossRef]
- Higashi, S.L.; Hirosawa, K.M.; Suzuki, K.G.N.; Matsuura, K.; Ikeda, M. One-pot construction of multicomponent supramolecular materials comprising self-sorted supramolecular architectures of DNA and semi-artificial glycopeptides. ACS Appl. Bio Mater. 2020. [Google Scholar] [CrossRef]
- Reches, M.; Gazit, E. Casting metal nanowires within discrete self-assembled peptide nanotubes. Science 2003, 300, 625–627. [Google Scholar] [CrossRef]
- Görbitz, C.H. Nanotube formation by hydrophobic dipeptides. Chem. Eur. J. 2001, 7, 5153–5159. [Google Scholar] [CrossRef]
- Du, X.; Zhou, J.; Xu, B. Supramolecular hydrogels made of basic biological building blocks. Chem. Asian J. 2014, 9, 1446–1472. [Google Scholar] [CrossRef]
- Adler-Abramovich, L.; Gazit, E. The physical properties of supramolecular peptide assemblies: From building block association to technological applications. Chem. Soc. Rev. 2014, 43, 6881–6893. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.; Ulijn, R.V. Design of nanostructures based on aromatic peptide amphiphiles. Chem. Soc. Rev. 2014, 43, 8150–8177. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Makam, P.; Aizen, R.; Gazit, E. Self-assembling peptide semiconductors. Science 2017, 358. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, T.; Kabumoto, M.; Arakawa, H.; Ikeda, M. The effect of carbohydrate structures on the hydrogelation ability and morphology of self-assembled structures of peptide–carbohydrate conjugates in water. Org. Biomol. Chem. 2017, 15, 4595–4600. [Google Scholar] [CrossRef]
- Makam, P.; Gazit, E. Minimalistic peptide supramolecular co-assembly: Expanding the conformational space for nanotechnology. Chem. Soc. Rev. 2018, 47, 3406–3420. [Google Scholar] [CrossRef]
- Ikeda, M. Stimuli-responsive supramolecular systems guided by chemical reactions. Polym. J. 2019, 51, 371–380. [Google Scholar] [CrossRef]
- Draper, E.R.; Adams, D.J. Controlling the assembly and properties of low-molecular-weight hydrogelators. Langmuir 2019, 35, 6506–6521. [Google Scholar] [CrossRef]
- Sugiura, T.; Kanada, T.; Mori, D.; Sakai, H.; Shibata, A.; Kitamura, Y.; Ikeda, M. Chemical stimulus-responsive supramolecular hydrogel formation and shrinkage of a hydrazone-containing short peptide derivative. Soft Matter 2020, 16, 899–906. [Google Scholar] [CrossRef]
- Ohtomi, T.; Higashi, S.L.; Mori, D.; Shibata, A.; Kitamura, Y.; Ikeda, M. Effect of side chain phenyl group on the self-assembled morphology of dipeptide hydrazides. Pept. Sci. 2020, e24200. [Google Scholar] [CrossRef]
- Lampel, A. Biology-inspired supramolecular peptide systems. Chem 2020, 6, 1222–1236. [Google Scholar] [CrossRef]
- Gour, N.; Kedracki, D.; Safir, I.; Ngo, K.X.; Vebert-Nardin, C. Self-assembling DNA–peptide hybrids: Morphological consequences of oligonucleotide grafting to a pathogenic amyloid fibrils forming dipeptide. Chem. Commun. 2012, 48, 5440–5442. [Google Scholar] [CrossRef] [PubMed]
- Gour, N.; Abraham, J.N.; Chami, M.; Castillo, A.; Verma, S.; Vebert-Nardin, C. Label-free, optical sensing of the supramolecular assembly into fibrils of a ditryptophan–DNA hybrid. Chem. Commun. 2014, 50, 6863–6865. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Yamada, S.; Nishikawa, S.; Matsuura, K. DNA-modified artificial viral capsids self-assembled from DNA-conjugated β-annulus peptide. J. Pept. Sci. 2017, 23, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Watanabe, K.; Matsuzaki, T.; Sakurai, K.; Kimizuka, N. Self-assembled synthetic viral capsids from a 24-mer viral peptide fragment. Angew. Chem. Int. Ed. 2010, 49, 9662–9665. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Inaba, H.; Matsuura, K. Construction of artificial viral capsids encapsulating short DNAs via disulfide bonds and controlled release of DNAs by reduction. Chem. Lett. 2019, 48, 544–546. [Google Scholar] [CrossRef]
- Matsuura, K.; Watanabe, K.; Matsushita, Y.; Kimizuka, N. Guest-binding behavior of peptide nanocapsules self-assembled from viral peptide fragments. Polym. J. 2013, 45, 529–534. [Google Scholar] [CrossRef]
- Matsuura, K.; Ueno, G.; Fujita, S. Self-assembled artificial viral capsid decorated with gold nanoparticles. Polym. J. 2015, 47, 146–151. [Google Scholar] [CrossRef]
- Fujita, S.; Matsuura, K. Encapsulation of CdTe quantum dots into synthetic viral capsids. Chem. Lett. 2016, 45, 922–924. [Google Scholar] [CrossRef]
- Matsuura, K.; Nakamura, T.; Watanabe, K.; Noguchi, T.; Minamihata, K.; Kamiya, N.; Kimizuka, N. Self-assembly of Ni-NTA-modified β-annulus peptides into artificial viral capsids and encapsulation of His-tagged proteins. Org. Biomol. Chem. 2016, 14, 7869–7874. [Google Scholar] [CrossRef]
- Matsuura, K. Synthetic approaches to construct viral capsid-like spherical nanomaterials. Chem. Commun. 2018, 54, 8944–8959. [Google Scholar] [CrossRef]
- Inaba, H.; Matsuura, K. Peptide nanomaterials designed from natural supramolecular systems. Chem. Rec. 2019, 19, 843–858. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Ota, J.; Fujita, S.; Shiomi, Y.; Inaba, H. Construction of ribonuclease-decorated artificial virus-like capsid by peptide self-assembly. J. Org. Chem. 2020, 85, 1668–1673. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K. Dressing up artificial viral capsids self-assembled from C-terminal-modified β-annulus peptides. Polym. J. 2020, 52, 1035–1041. [Google Scholar] [CrossRef]
- Kye, M.; Lim, Y.-B. Reciprocal self-assembly of peptide-DNA conjugates into a programmable sub-10-nm supramolecular deoxyribonucleoprotein. Angew. Chem. Int. Ed. 2016, 55, 12003–12007. [Google Scholar] [CrossRef]
- Kye, M.; Lim, Y. Synthesis and purification of self-assembling peptide-oligonucleotide conjugates by solid-phase peptide fragment condensation. J. Pept. Sci. 2018, 24, e3092. [Google Scholar] [CrossRef]
- Basavalingappa, V.; Bera, S.; Xue, B.; Azuri, I.; Tang, Y.; Tao, K.; Shimon, L.J.W.; Sawaya, M.R.; Kolusheva, S.; Eisenberg, D.S.; et al. Mechanically rigid supramolecular assemblies formed from an Fmoc-guanine conjugated peptide nucleic acid. Nat. Commun. 2019, 10, 5256. [Google Scholar] [CrossRef]
- Basavalingappa, V.; Guterman, T.; Tang, Y.; Nir, S.; Lei, J.; Chakraborty, P.; Schnaider, L.; Reches, M.; Wei, G.; Gazit, E. Expanding the functional scope of the Fmoc-diphenylalanine hydrogelator by introducing a rigidifying and chemically active urea backbone modification. Adv. Sci. 2019, 6. [Google Scholar] [CrossRef]
- Arakawa, H.; Takeda, K.; Higashi, S.L.; Shibata, A.; Kitamura, Y.; Ikeda, M. Self-assembly and hydrogel formation ability of Fmoc-dipeptides comprising α-methyl-L-phenylalanine. Polym. J. 2020, 52, 923–930. [Google Scholar] [CrossRef]
- Daly, M.L.; Gao, Y.; Freeman, R. Encoding reversible hierarchical structures with supramolecular peptide–DNA materials. Bioconjug. Chem. 2019, 30, 1864–1869. [Google Scholar] [CrossRef]
- Freeman, R.; Han, M.; Álvarez, Z.; Lewis, J.A.; Wester, J.R.; Stephanopoulos, N.; McClendon, M.T.; Lynsky, C.; Godbe, J.M.; Sangji, H.; et al. Reversible self-assembly of superstructured networks. Science 2018, 362, 808–813. [Google Scholar] [CrossRef]
- Rha, A.K.; Das, D.; Taran, O.; Ke, Y.; Mehta, A.K.; Lynn, D.G. Electrostatic complementarity drives amyloid/nucleic acid co-assembly. Angew. Chem. Int. Ed. 2020, 59, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Mou, Y.; Yu, J.-Y.; Wannier, T.M.; Guo, C.-L.; Mayo, S.L. Computational design of co-assembling protein–DNA nanowires. Nature 2015, 525, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Clarke, N.D.; Kissinger, C.R.; Desjarlais, J.; Gilliland, G.L.; Pabo, C.O. Structural studies of the engrailed homeodomain. Protein Sci. 1994, 3, 1779–1787. [Google Scholar] [CrossRef] [PubMed]
- Dirks, R.M.; Pierce, N.A. Triggered amplification by hybridization chain reaction. Proc. Natl. Acad. Sci. USA 2004, 101, 15275–15278. [Google Scholar] [CrossRef]
- Fu, T.; Lyu, Y.; Liu, H.; Peng, R.; Zhang, X.; Ye, M.; Tan, W. DNA-based dynamic reaction networks. Trends Biochem. 2018, 43, 547–560. [Google Scholar] [CrossRef]
- McMillan, J.R.; Hayes, O.G.; Remis, J.P.; Mirkin, C.A. Programming protein polymerization with DNA. J. Am. Chem. Soc. 2018, 140, 15950–15956. [Google Scholar] [CrossRef]
- Figg, C.A.; Winegar, P.H.; Hayes, O.G.; Mirkin, C.A. Controlling the DNA hybridization chain reaction. J. Am. Chem. Soc. 2020, 142, 8596–8601. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, S.; Simmons, C.R.; Narayanan, R.P.; Zhang, F.; Aziz, A.-M.; Yan, H.; Stephanopoulos, N. Tunable nanoscale cages from self-assembling DNA and protein building blocks. ACS Nano 2019, 13, 3545–3554. [Google Scholar] [CrossRef]
- Subramanian, R.H.; Smith, S.J.; Alberstein, R.G.; Bailey, J.B.; Zhang, L.; Cardone, G.; Suominen, L.; Chami, M.; Stahlberg, H.; Baker, T.S.; et al. Self-assembly of a designed nucleoprotein architecture through multimodal interactions. ACS Cent. Sci. 2018, 4, 1578–1586. [Google Scholar] [CrossRef]
- Brodin, J.D.; Ambroggio, X.I.; Tang, C.; Parent, K.N.; Baker, T.S.; Tezcan, F.A. Metal-directed, chemically tunable assembly of one-, two- and three-dimensional crystalline protein arrays. Nat. Chem. 2012, 4, 375–382. [Google Scholar] [CrossRef]
- Brodin, J.D.; Carr, J.R.; Sontz, P.A.; Tezcan, F.A. Exceptionally stable, redox-active supramolecular protein assemblies with emergent properties. Proc. Natl. Acad. Sci. USA 2014, 111, 2897–2902. [Google Scholar] [CrossRef] [PubMed]
- Brodin, J.D.; Smith, S.J.; Carr, J.R.; Tezcan, F.A. Designed, helical protein nanotubes with variable diameters from a single building block. J. Am. Chem. Soc. 2015, 137, 10468–10471. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.; Linko, V. Challenges and perspectives of DNA nanostructures in biomedicine. Angew. Chem. Int. Ed. 2020, 59, 15818–15833. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Ohara, S.; Yamamoto, H. 3,4,5-trifluorobenzeneboronic acid as an extremely active amidation catalyst. J. Org. Chem. 1996, 61, 4196–4197. [Google Scholar] [CrossRef]
- Pattabiraman, V.R.; Bode, J.W. Rethinking amide bond synthesis. Nature 2011, 480, 471–479. [Google Scholar] [CrossRef]
- Opie, C.R.; Noda, H.; Shibasaki, M.; Kumagai, N. All non-carbon B3NO2 exotic heterocycles: Synthesis, dynamics, and catalysis. Chem. Eur. J. 2019, 25, 4648–4653. [Google Scholar] [CrossRef]
- Liu, Z.; Noda, H.; Shibasaki, M.; Kumagai, N. Catalytic oligopeptide synthesis. Org. Lett. 2018, 20, 612–615. [Google Scholar] [CrossRef]
- Todorovic, M.; Perrin, D.M. Recent developments in catalytic amide bond formation. Pept. Sci. 2020, 112. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002; ISBN 978-0-8153-3218-3. [Google Scholar]
- Suhanovsky, M.M.; Teschke, C.M. Nature’s favorite building block: Deciphering folding and capsid assembly of proteins with the HK97-fold. Virology 2015, 479–480, 487–497. [Google Scholar] [CrossRef]
- Wu, H.; Fuxreiter, M. The structure and dynamics of higher-order assemblies: Amyloids, signalosomes, and granules. Cell 2016, 165, 1055–1066. [Google Scholar] [CrossRef]
- Hoffman, D.P.; Shtengel, G.; Xu, C.S.; Campbell, K.R.; Freeman, M.; Wang, L.; Milkie, D.E.; Pasolli, H.A.; Iyer, N.; Bogovic, J.A.; et al. Correlative three-dimensional super-resolution and block-face electron microscopy of whole vitreously frozen cells. Science 2020, 367. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Brangwynne, C.P. Liquid phase condensation in cell physiology and disease. Science 2017, 357. [Google Scholar] [CrossRef] [PubMed]
- Kroschwald, S.; Alberti, S. Gel or die: Phase separation as a survival strategy. Cell 2017, 168, 947–948. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, T.; Nozawa, R.-S.; Jia, T.Z.; Saio, T.; Mori, E. Biological phase separation: Cell biology meets biophysics. Biophys. Rev. 2020, 12, 519–539. [Google Scholar] [CrossRef]
- Trivedi, P.; Palomba, F.; Niedzialkowska, E.; Digman, M.A.; Gratton, E.; Stukenberg, P.T. The inner centromere is a biomolecular condensate scaffolded by the chromosomal passenger complex. Nat. Cell Biol. 2019, 21, 1127–1137. [Google Scholar] [CrossRef]
- Herzel, L.; Ottoz, D.S.M.; Alpert, T.; Neugebauer, K.M. Splicing and transcription touch base: Co-transcriptional spliceosome assembly and function. Nat. Rev. Mol. Cell Biol. 2017, 18, 637–650. [Google Scholar] [CrossRef]
- Sanulli, S.; Trnka, M.J.; Dharmarajan, V.; Tibble, R.W.; Pascal, B.D.; Burlingame, A.L.; Griffin, P.R.; Gross, J.D.; Narlikar, G.J. HP1 reshapes nucleosome core to promote phase separation of heterochromatin. Nature 2019, 575, 390–394. [Google Scholar] [CrossRef]
- Guo, Y.E.; Manteiga, J.C.; Henninger, J.E.; Sabari, B.R.; Dall’Agnese, A.; Hannett, N.M.; Spille, J.-H.; Afeyan, L.K.; Zamudio, A.V.; Shrinivas, K.; et al. Pol II phosphorylation regulates a switch between transcriptional and splicing condensates. Nature 2019, 572, 543–548. [Google Scholar] [CrossRef]
- Du, Y.; Dong, S. Nucleic acid biosensors: Recent advances and perspectives. Anal. Chem. 2017, 89, 189–215. [Google Scholar] [CrossRef]
- Rubert Pérez, C.M.; Stephanopoulos, N.; Sur, S.; Lee, S.S.; Newcomb, C.; Stupp, S.I. The powerful functions of peptide-based bioactive matrices for regenerative medicine. Ann. Biomed. Eng. 2015, 43, 501–514. [Google Scholar] [CrossRef]
- Gelain, F.; Luo, Z.; Zhang, S. Self-assembling peptide EAK16 and RADA16 nanofiber scaffold hydrogel. Chem. Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.P.K.; Keane, T.J.; Roques, A.C.; Patrick, P.S.; Mooney, C.M.; Kuan, W.-L.; Pisupati, V.; Oreffo, R.O.C.; Stuckey, D.J.; Watt, F.M.; et al. A blueprint for translational regenerative medicine. Sci. Transl. Med. 2020, 12, eaaz2253. [Google Scholar] [CrossRef] [PubMed]
- Cutler, J.I.; Auyeung, E.; Mirkin, C.A. Spherical nucleic acids. J. Am. Chem. Soc. 2012, 134, 1376–1391. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Jia, F.; Wang, P.; Zhang, K. Nucleic acid-based drug delivery strategies. J. Control. Release 2020, 323, 240–252. [Google Scholar] [CrossRef]
- Jiang, Z.; Thayumanavan, S. Noncationic material design for nucleic acid delivery. Adv. Therap. 2020, 3, 1900206. [Google Scholar] [CrossRef]
- Roberts, T.C.; Langer, R.; Wood, M.J.A. Advances in oligonucleotide drug delivery. Nat. Rev. Drug Discov. 2020, 19, 673–694. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higashi, S.L.; Rozi, N.; Hanifah, S.A.; Ikeda, M. Supramolecular Architectures of Nucleic Acid/Peptide Hybrids. Int. J. Mol. Sci. 2020, 21, 9458. https://doi.org/10.3390/ijms21249458
Higashi SL, Rozi N, Hanifah SA, Ikeda M. Supramolecular Architectures of Nucleic Acid/Peptide Hybrids. International Journal of Molecular Sciences. 2020; 21(24):9458. https://doi.org/10.3390/ijms21249458
Chicago/Turabian StyleHigashi, Sayuri L., Normazida Rozi, Sharina Abu Hanifah, and Masato Ikeda. 2020. "Supramolecular Architectures of Nucleic Acid/Peptide Hybrids" International Journal of Molecular Sciences 21, no. 24: 9458. https://doi.org/10.3390/ijms21249458
APA StyleHigashi, S. L., Rozi, N., Hanifah, S. A., & Ikeda, M. (2020). Supramolecular Architectures of Nucleic Acid/Peptide Hybrids. International Journal of Molecular Sciences, 21(24), 9458. https://doi.org/10.3390/ijms21249458




