Brassica Bioactives Could Ameliorate the Chronic Inflammatory Condition of Endometriosis
Abstract
1. Introduction
2. Etiology of Endometriosis
3. Inflammatory Background of Endometriosis
4. Endometriosis and Diet
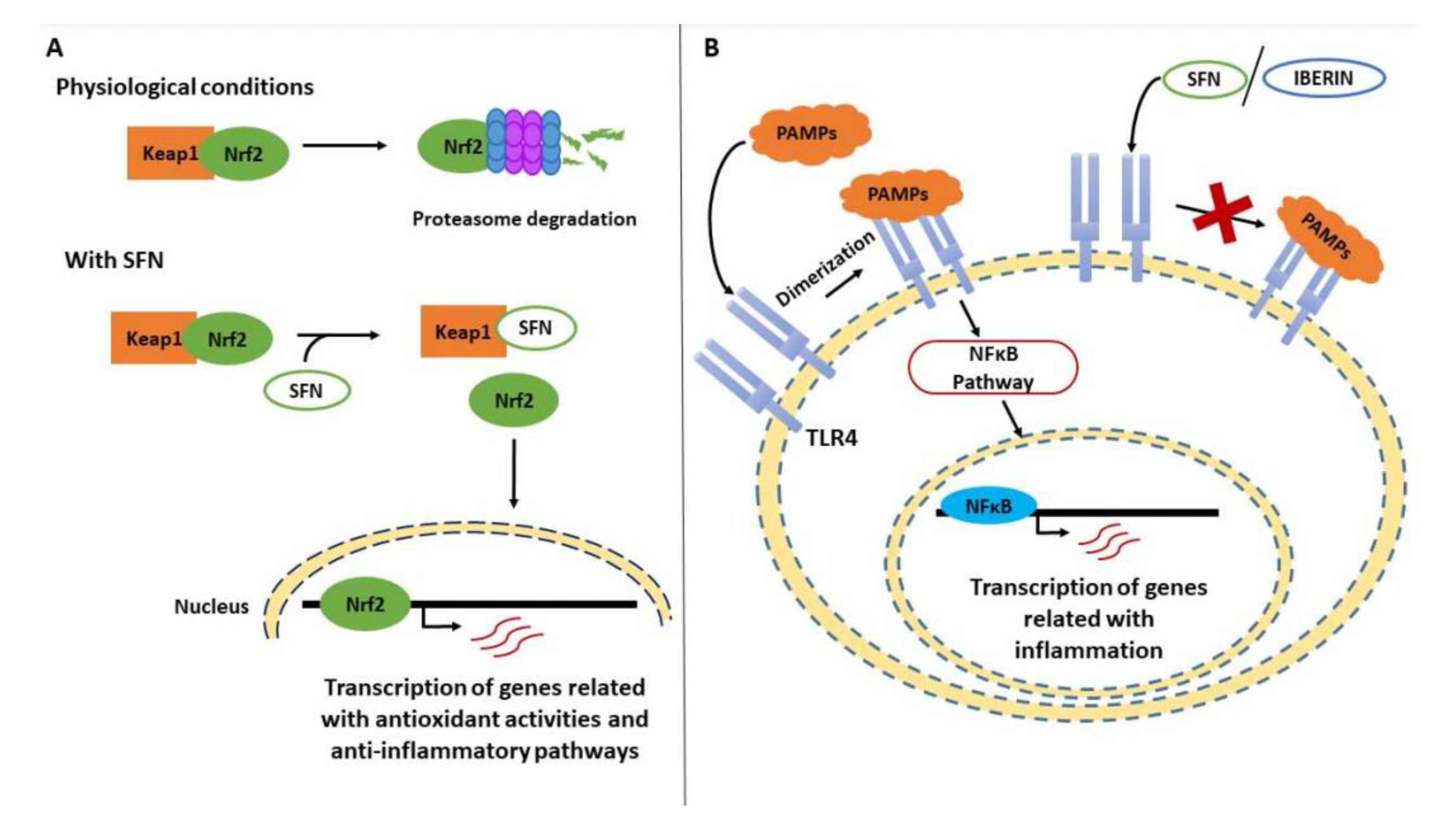
5. Brassicaceae and Their Bioactive Compounds in Inflammation
5.1. Aliphatic ITCs and Related Metabolites
5.2. Indoles and Related Compounds
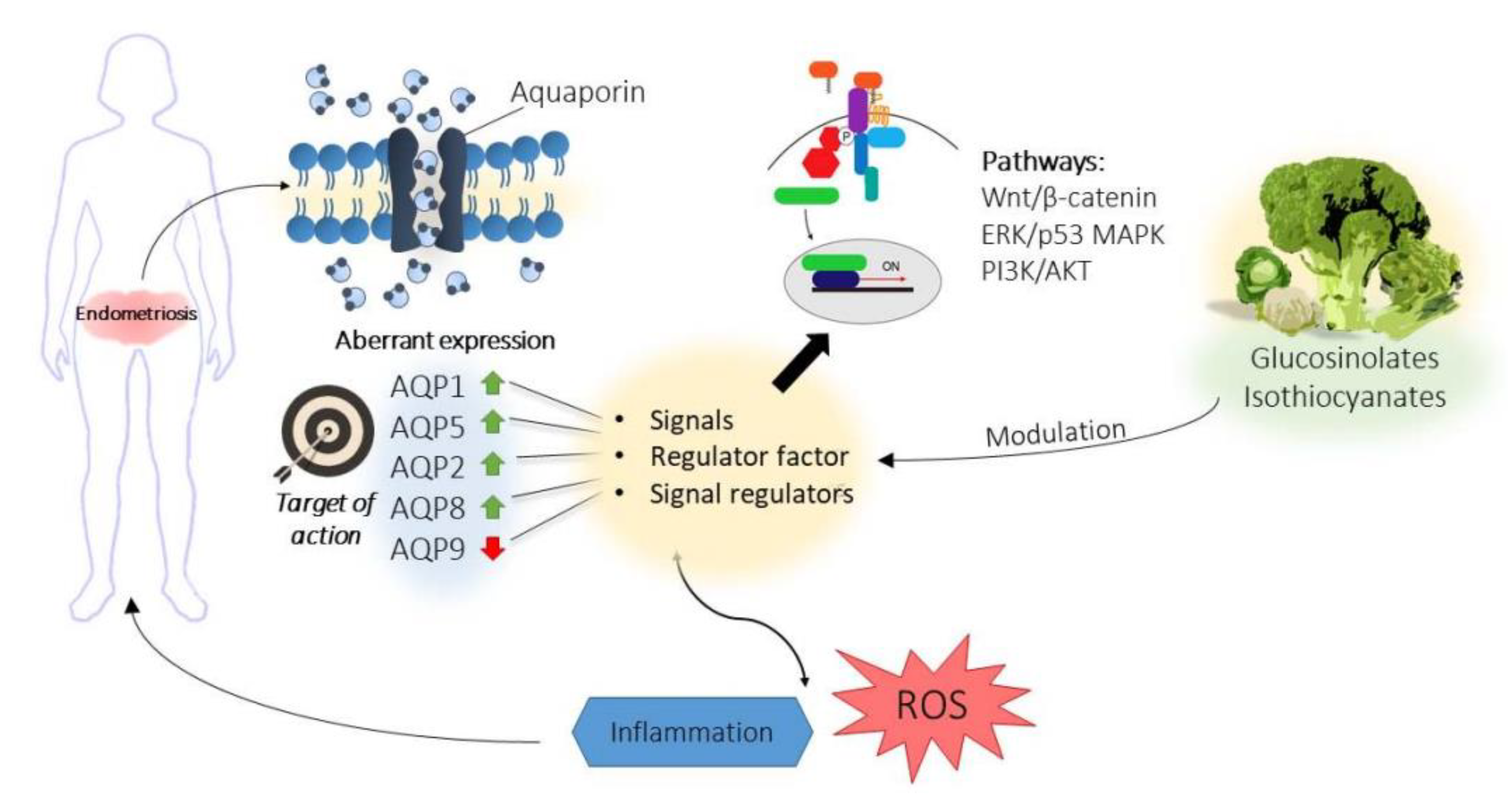
6. Aquaporins in Endometriosis
Modulation of Aquaporins by Bioactive Compounds: Further Targeted Therapies
7. Conclusions and Perspectives
8. Materials and Methods
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vercellini, P.; Viganò, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2014, 10, 261–275. [Google Scholar] [CrossRef]
- Zondervan, K.T.; Becker, C.M.; Koga, K.; Missmer, S.A.; Taylor, R.N.; Viganò, P. Endometriosis. Nat. Rev. Dis. Primers 2018, 4, 9. [Google Scholar] [CrossRef]
- As-Sanie, S.; Black, R.; Giudice, L.C.; Gray Valbrun, T.; Gupta, J.; Jones, B.; Laufer, M.R.; Milspaw, A.T.; Missmer, S.A.; Norman, A.; et al. Assessing research gaps and unmet needs in endometriosis. Am. J. Obstet. Gynecol. 2019, 221, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Denny, E.; Mann, C.H. A clinical overview of endometriosis: A misunderstood disease. Br. J. Nurs. 2007, 16, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, V.L.; de Franciscis, P.; Barra, F.; Schiattarella, A.; Tropea, A.; Tesarik, J.; Shah, M.; Kahramanoglu, I.; Marques Cerentini, T.; Ponta, M.; et al. Sexuality in women with endometriosis: A critical narrative review. Minerva Med. 2020, 111, 79–89. [Google Scholar] [PubMed]
- Jones, G.; Jenkinson, C.; Kennedy, S. The impact of endometriosis upon quality of life: A qualitative analysis. J. Psychosom. Obstet. Gynaecol. 2004, 25, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Aerts, L.; Grangier, L.; Streuli, I.; Dällenbach, P.; Marci, R.; Wenger, J.-M.; Pluchino, N. Psychosocial impact of endometriosis: From co-morbidity to intervention. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 50, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, T.A.; Templeton, A.A.; Thomson, L.; Fraser, C. Menstrual symptoms in women with pelvic endometriosis. Br. J. Obstet. Gynaecol. 1991, 98, 558–563. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, V.L.; De Franciscis, P.; Barra, F.; Schiattarella, A.; Török, P.; Shah, M.; Karaman, E.; Marques Cerentini, T.; Di Guardo, F.; Gullo, G.; et al. Quality of life in women with endometriosis: A narrative overview. Minerva Med. 2020, 111, 68–78. [Google Scholar] [CrossRef] [PubMed]
- López-Chillón, M.T.; Carazo-Díaz, C.; Prieto-Merino, D.; Zafrilla, P.; Moreno, D.A.; Villaño, D. Effects of long-term consumption of broccoli sprouts on inflammatory markers in overweight subjects. Clin. Nutr. 2019, 38, 745–752. [Google Scholar] [CrossRef]
- Finicelli, M.; Squillaro, T.; Di Cristo, F.; Di Salle, A.; Melone, M.A.B.; Galderisi, U.; Peluso, G. Metabolic syndrome, Mediterranean diet, and polyphenols: Evidence and perspectives. J. Cell. Physiol. 2019, 234, 5807–5826. [Google Scholar] [CrossRef] [PubMed]
- Soave, I.; Occhiali, T.; Wenger, J.M.; Pluchino, N.; Caserta, D.; Marci, R. Endometriosis and food habits: Can diet make the difference? J. Endometr. Pelvic Pain Disord. 2018, 10, 59–71. [Google Scholar] [CrossRef]
- Parazzini, F.; Esposito, G.; Tozzi, L.; Noli, S.; Bianchi, S. Epidemiology of endometriosis and its comorbidities. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 209, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Trabert, B.; Peters, U.; De Roos, A.J.; Scholes, D.; Holt, V.L. Diet and risk of endometriosis in a population-based case-control study. Br. J. Nutr. 2011, 105, 459–467. [Google Scholar] [CrossRef]
- Harris, H.R.; Eke, A.C.; Chavarro, J.E.; Missmer, S.A. Fruit and vegetable consumption and risk of endometriosis. Hum. Reprod. 2018, 33, 715–727. [Google Scholar] [CrossRef]
- Agic, A.; Djalali, S.; Diedrich, K.; Hornung, D. Apoptosis in Endometriosis. Gynecol. Obstet. Investig. 2009, 68, 217–223. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, M.A.; Lee, M.; Suh, D.H.; Kim, K.; No, J.H.; Chung, H.H.; Kim, Y.B.; Song, Y.S. Effect of endometriosis on the prognosis of ovarian clear cell carcinoma: A two-center cohortsStudy and meta-analysis. Ann. Surg. Oncol. 2015, 22, 2738–2745. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, T.H.; Chung, H.H.; Song, Y.S. Risk and prognosis of ovarian cancer in women with endometriosis: A meta-analysis. Br. J. Cancer 2014, 110, 1878–1890. [Google Scholar] [CrossRef]
- Machado-Linde, F.; Sánchez-Ferrer, M.L.; Cascales, P.; Torroba, A.; Orozco, R.; Silva Sánchez, Y.; Nieto, A.; Fiol, G. Prevalence of endometriosis in epithelial ovarian cancer. Analysis of the associated clinical features and study on molecular mechanisms involved in the possible causality. Eur. J. Gynaecol. Oncol. 2015, 36, 21–24. [Google Scholar]
- Pearce, C.L.; Templeman, C.; Rossing, M.A.; Lee, A.; Near, A.M.; Webb, P.M.; Nagle, C.M.; Doherty, J.A.; Cushing-Haugen, K.L.; Wicklund, K.G.; et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: A pooled analysis of case-control studies. Lancet. Oncol. 2012, 13, 385–394. [Google Scholar] [CrossRef]
- Wei, J.-J.; William, J.; Bulun, S. Endometriosis and ovarian cancer: A review of clinical, pathologic, and molecular aspects. Int. J. Gynecol. Pathol. 2011, 30, 553–568. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.A. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am. J. Pathol. 1927, 3, 93–110.43. [Google Scholar]
- Sourial, S.; Tempest, N.; Hapangama, D.K. Theories on the pathogenesis of endometriosis. Int. J. Reprod. Med. 2014, 2014, 179515. [Google Scholar] [CrossRef] [PubMed]
- Asghari, S.; Valizadeh, A.; Aghebati-Maleki, L.; Nouri, M.; Yousefi, M. Endometriosis: Perspective, lights, and shadows of etiology. Biomed. Pharmacother. 2018, 106, 163–174. [Google Scholar] [CrossRef]
- García-Peñarrubia, P.; Ruiz-Alcaraz, A.J.; Martínez-Esparza, M.; Marín, P.; Machado-Linde, F. Hypothetical roadmap towards endometriosis: Prenatal endocrine-disrupting chemical pollutant exposure, anogenital distance, gut-genital microbiota and subclinical infections. Hum. Reprod. Update 2020, 26, 214–246. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Chen, X.; Zhao, Y.; Cao, B.; Zhao, W. Effects of prenatal environmental exposures on the development of endometriosis in female offspring. Reprod. Sci. 2016, 23, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Missmer, S.A.; Hankinson, S.E.; Spiegelman, D.; Barbieri, R.L.; Michels, K.B.; Hunter, D.J. In utero exposures and the incidence of endometriosis. Fertil. Steril. 2004, 82, 1501–1508. [Google Scholar] [CrossRef]
- Upson, K.; Sathyanarayana, S.; Scholes, D.; Holt, V.L. Early-life factors and endometriosis risk. Fertil. Steril. 2015, 104, 964–971.e5. [Google Scholar] [CrossRef]
- Parasar, P.; Ozcan, P.; Terry, K.L. Endometriosis: Epidemiology, diagnosis and clinical management. Curr. Obstet. Gynecol. Rep. 2017, 6, 34–41. [Google Scholar] [CrossRef]
- Shafrir, A.L.; Farland, L.V.; Shah, D.K.; Harris, H.R.; Kvaskoff, M.; Zondervan, K.; Missmer, S.A. Risk for and consequences of endometriosis: A critical epidemiologic review. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 1–15. [Google Scholar] [CrossRef]
- Backonja, U.; Hediger, M.L.; Chen, Z.; Lauver, D.R.; Sun, L.; Peterson, C.M.; Buck Louis, G.M. Beyond body mass index: Using anthropometric measures and body composition indicators to assess odds of an endometriosis diagnosis. J. Womens. Health (Larchmt) 2017, 26, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Hediger, M.L.; Hartnett, H.J.; Buck Louis, G.M. Association of endometriosis with body size and figure. Fertil. Steril. 2005, 84, 1366–1374. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Allebeck, P.; Mishra, G.D.; Koupil, I. Developmental origins of endometriosis: A Swedish cohort study. J. Epidemiol. Community Health 2019, 73, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Cano-Sancho, G.; Ploteau, S.; Matta, K.; Adoamnei, E.; Louis, G.B.; Mendiola, J.; Darai, E.; Squifflet, J.; Le Bizec, B.; Antignac, J.-P. Human epidemiological evidence about the associations between exposure to organochlorine chemicals and endometriosis: Systematic review and meta-analysis. Environ. Int. 2019, 123, 209–223. [Google Scholar] [CrossRef]
- Hunt, P.A.; Sathyanarayana, S.; Fowler, P.A.; Trasande, L. Female reproductive disorders, diseases, and costs of exposure to endocrine disrupting chemicals in the European Union. J. Clin. Endocrinol. Metab. 2016, 101, 1562–1570. [Google Scholar] [CrossRef]
- Smarr, M.M.; Kannan, K.; Buck Louis, G.M. Endocrine disrupting chemicals and endometriosis. Fertil. Steril. 2016, 106, 959–966. [Google Scholar] [CrossRef]
- Wen, X.; Xiong, Y.; Qu, X.; Jin, L.; Zhou, C.; Zhang, M.; Zhang, Y. The risk of endometriosis after exposure to endocrine-disrupting chemicals: A meta-analysis of 30 epidemiology studies. Gynecol. Endocrinol. 2019, 35, 645–650. [Google Scholar] [CrossRef]
- Matalliotakis, I.M.; Cakmak, H.; Fragouli, Y.G.; Goumenou, A.G.; Mahutte, N.G.; Arici, A. Epidemiological characteristics in women with and without endometriosis in the Yale series. Arch. Gynecol. Obstet. 2008, 277, 389–393. [Google Scholar] [CrossRef]
- Heilier, J.-F.; Donnez, J.; Nackers, F.; Rousseau, R.; Verougstraete, V.; Rosenkranz, K.; Donnez, O.; Grandjean, F.; Lison, D.; Tonglet, R. Environmental and host-associated risk factors in endometriosis and deep endometriotic nodules: A matched case–control study. Environ. Res. 2007, 103, 121–129. [Google Scholar] [CrossRef]
- Parazzini, F.; Cipriani, S.; Bravi, F.; Pelucchi, C.; Chiaffarino, F.; Ricci, E.; Viganò, P. A metaanalysis on alcohol consumption and risk of endometriosis. Am. J. Obstet. Gynecol. 2013, 209, 106.e1–106.e10. [Google Scholar] [CrossRef]
- Saha, R.; Kuja-Halkola, R.; Tornvall, P.; Marions, L. Reproductive and lifestyle factors associated with endometriosis in a large cross-sectional population sample. J. Womens. Health (Larchmt) 2017, 26, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Rižner, T.L. Estrogen metabolism and action in endometriosis. Mol. Cell. Endocrinol. 2009, 307, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, S.; Remorgida, V.; Maganza, C.; Venturini, P.L.; Salvatore, S.; Papaleo, E.; Candiani, M.; Leone Roberti Maggiore, U. Aromatase and endometriosis: Estrogens play a role. Ann. N. Y. Acad. Sci. 2014, 1317, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Simoens, S.; Hummelshoj, L.; D’Hooghe, T. Endometriosis: Cost estimates and methodological perspective. Hum. Reprod. Update 2007, 13, 395–404. [Google Scholar] [CrossRef]
- Klemmt, P.A.B.; Starzinski-Powitz, A. Molecular and cellular pathogenesis of endometriosis. Curr. Women’s Health Rev. 2018, 14, 106–116. [Google Scholar] [CrossRef]
- Miller, J.E.; Ahn, S.H.; Monsanto, S.P.; Khalaj, K.; Koti, M.; Tayade, C. Implications of immune dysfunction on endometriosis associated infertility. Oncotarget 2017, 8, 7138–7147. [Google Scholar] [CrossRef]
- Patel, B.G.; Lenk, E.E.; Lebovic, D.I.; Shu, Y.; Yu, J.; Taylor, R.N. Pathogenesis of endometriosis: Interaction between Endocrine and inflammatory pathways. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 50, 50–60. [Google Scholar] [CrossRef]
- Zhang, T.; De Carolis, C.; Man, G.C.W.; Wang, C.C. The link between immunity, autoimmunity and endometriosis: A literature update. Autoimmun. Rev. 2018, 17, 945–955. [Google Scholar] [CrossRef]
- Pepe, G.; Locati, M.; Della Torre, S.; Mornata, F.; Cignarella, A.; Maggi, A.; Vegeto, E. The estrogen-macrophage interplay in the homeostasis of the female reproductive tract. Hum. Reprod. Update 2018, 24, 652–672. [Google Scholar] [CrossRef]
- Capobianco, A.; Rovere-Querini, P. Endometriosis, a disease of the macrophage. Front. Immunol. 2013, 4, 9. [Google Scholar] [CrossRef]
- Bacci, M.; Capobianco, A.; Monno, A.; Cottone, L.; Di Puppo, F.; Camisa, B.; Mariani, M.; Brignole, C.; Ponzoni, M.; Ferrari, S.; et al. Macrophages are alternatively activated in patients with endometriosis and required for growth and vascularization of lesions in a mouse model of disease. Am. J. Pathol. 2009, 175, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xie, H.; Yao, S.; Liang, Y. Macrophage and nerve interaction in endometriosis. J. Neuroinflamm. 2017, 14, 53. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, D.; Yuan, M.; Li, Q.; Zhen, Q.; Li, N.; Wang, G. Macrophages alternatively activated by endometriosis-exosomes contribute to the development of lesions in mice. Mol. Hum. Reprod. 2019, 25, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Liu, X.; Wang, H.; Guo, S.-W. The M2a macrophage subset may be critically involved in the fibrogenesis of endometriosis in mice. Reprod. Biomed. Online 2018, 37, 254–268. [Google Scholar] [CrossRef]
- Ono, Y.; Yoshino, O.; Hiraoka, T.; Akiyama, I.; Sato, E.; Ito, M.; Kobayashi, M.; Nakashima, A.; Wada, S.; Onda, T.; et al. IL-33 Exacerbates endometriotic lesions via polarizing peritoneal macrophages to M2 subtype. Reprod. Sci. 2020, 27, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Hudson, Q.J.; Ashjaei, K.; Perricos, A.; Kuessel, L.; Husslein, H.; Wenzl, R.; Yotova, I. Endometriosis Patients Show an Increased M2 Response in the Peritoneal CD14+low/CD68+low Macrophage Subpopulation Coupled with an Increase in the T-helper 2 and T-regulatory Cells. Reprod. Sci. 2020, 27, 1920–1931. [Google Scholar] [CrossRef]
- Takebayashi, A.; Kimura, F.; Kishi, Y.; Ishida, M.; Takahashi, A.; Yamanaka, A.; Wu, D.; Zheng, L.; Takahashi, K.; Suginami, H.; et al. Subpopulations of macrophages within eutopic endometrium of endometriosis patients. Am. J. Reprod. Immunol. 2015, 73, 221–231. [Google Scholar] [CrossRef]
- Ruiz-Alcaraz, A.J.; Martínez-Banaclocha, H.; Marín-Sánchez, P.; Carmona-Martínez, V.; Iniesta-Albadalejo, M.A.; Tristán-Manzano, M.; Tapia-Abellán, A.; García-Peñarrubia, P.; Machado-Linde, F.; Pelegrín, P.; et al. Isolation of functional mature peritoneal macrophages from healthy humans. Immunol. Cell Biol. 2020, 98, 114–126. [Google Scholar] [CrossRef]
- Yu, J.-J.; Sun, H.-T.; Zhang, Z.-F.; Shi, R.-X.; Liu, L.-B.; Shang, W.-Q.; Wei, C.-Y.; Chang, K.-K.; Shao, J.; Wang, M.-Y.; et al. IL15 promotes growth and invasion of endometrial stromal cells and inhibits killing activity of NK cells in endometriosis. Reproduction 2016, 152, 151–160. [Google Scholar] [CrossRef]
- Wu, M.Y.; Yang, J.H.; Chao, K.H.; Hwang, J.L.; Yang, Y.S.; Ho, H.N. Increase in the expression of killer cell inhibitory receptors on peritoneal natural killer cells in women with endometriosis. Fertil. Steril. 2000, 74, 1187–1191. [Google Scholar] [CrossRef]
- Chuang, P.-C.; Wu, M.-H.; Shoji, Y.; Tsai, S.-J. Downregulation of CD36 results in reduced phagocytic ability of peritoneal macrophages of women with endometriosis. J. Pathol. 2009, 219, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Hanada, T.; Tsuji, S.; Nakayama, M.; Wakinoue, S.; Kasahara, K.; Kimura, F.; Mori, T.; Ogasawara, K.; Murakami, T. Suppressive regulatory T cells and latent transforming growth factor-β-expressing macrophages are altered in the peritoneal fluid of patients with endometriosis. Reprod. Biol. Endocrinol. 2018, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Christodoulakos, G.; Augoulea, A.; Lambrinoudaki, I.; Sioulas, V.; Creatsas, G. Pathogenesis of endometriosis: The role of defective “immunosurveillance”. Eur. J. Contracept. Reprod. Health Care 2007, 12, 194–202. [Google Scholar] [CrossRef]
- Gogacz, M.; Winkler, I.; Bojarska-Junak, A.; Tabarkiewicz, J.; Semczuk, A.; Rechberger, T.; Adamiak, A. T regulatory lymphocytes in patients with endometriosis. Mol. Med. Rep. 2014, 10, 1072–1076. [Google Scholar] [CrossRef]
- de Barros, I.B.L.; Malvezzi, H.; Gueuvoghlanian-Silva, B.Y.; Piccinato, C.A.; Rizzo, L.V.; Podgaec, S. What do we know about regulatory T cells and endometriosis? A systematic review. J. Reprod. Immunol. 2017, 120, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Q.; Zhou, W.-J.; Luo, X.-Z.; Tao, Y.; Li, D.-J. Synergistic effect of regulatory T cells and proinflammatory cytokines in angiogenesis in the endometriotic milieu. Hum. Reprod. 2017, 32, 1304–1317. [Google Scholar] [CrossRef] [PubMed]
- Elkabets, M.; Ribeiro, V.S.G.; Dinarello, C.A.; Ostrand-Rosenberg, S.; Di Santo, J.P.; Apte, R.N.; Vosshenrich, C.A.J. IL-1β regulates a novel myeloid-derived suppressor cell subset that impairs NK cell development and function. Eur. J. Immunol. 2010, 40, 3347–3357. [Google Scholar] [CrossRef]
- Gou, Y.; Li, X.; Li, P.; Zhang, H.; Xu, T.; Wang, H.; Wang, B.; Ma, X.; Jiang, X.; Zhang, Z. Estrogen receptor β upregulates CCL2 via NF-κB signaling in endometriotic stromal cells and recruits macrophages to promote the pathogenesis of endometriosis. Hum. Reprod. 2019, 34, 646–658. [Google Scholar] [CrossRef]
- Mu, F.; Harris, H.R.; Rich-Edwards, J.W.; Hankinson, S.E.; Rimm, E.B.; Spiegelman, D.; Missmer, S.A. A Prospective Study of Inflammatory Markers and Risk of Endometriosis. Am. J. Epidemiol. 2018, 187, 515–522. [Google Scholar] [CrossRef]
- Nothnick, W.; Alali, Z. Recent advances in the understanding of endometriosis: The role of inflammatory mediators in disease pathogenesis and treatment. F1000Research 2016, 5, 186. [Google Scholar] [CrossRef]
- Huang, F.; Cao, J.; Liu, Q.; Zou, Y.; Li, H.; Yin, T. MAPK/ERK signal pathway involved expression of COX-2 and VEGF by IL-1β induced in human endometriosis stromal cells in vitro. Int. J. Clin. Exp. Pathol. 2013, 6, 2129–2136. [Google Scholar] [PubMed]
- McLaren, J. Vascular endothelial growth factor and endometriotic angiogenesis. Hum. Reprod. Update 2000, 6, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-J.; Jeung, I.C.; Park, A.; Park, Y.-J.; Jung, H.; Kim, T.-D.; Lee, H.G.; Choi, I.; Yoon, S.R. An increased level of IL-6 suppresses NK cell activity in peritoneal fluid of patients with endometriosis via regulation of SHP-2 expression. Hum. Reprod. 2014, 29, 2176–2189. [Google Scholar] [CrossRef] [PubMed]
- Sikora, J.; Smycz-Kubańska, M.; Mielczarek-Palacz, A.; Kondera-Anasz, Z. Abnormal peritoneal regulation of chemokine activation-The role of IL-8 in pathogenesis of endometriosis. Am. J. Reprod. Immunol. 2017, 77, e12622. [Google Scholar] [CrossRef]
- Ulukus, M.; Ulukus, E.C.; Tavmergen Goker, E.N.; Tavmergen, E.; Zheng, W.; Arici, A. Expression of interleukin-8 and monocyte chemotactic protein 1 in women with endometriosis. Fertil. Steril. 2009, 91, 687–693. [Google Scholar] [CrossRef]
- Chang, K.-K.; Liu, L.-B.; Jin, L.-P.; Zhang, B.; Mei, J.; Li, H.; Wei, C.-Y.; Zhou, W.-J.; Zhu, X.-Y.; Shao, J.; et al. IL-27 triggers IL-10 production in Th17 cells via a c-Maf/RORγt/Blimp-1 signal to promote the progression of endometriosis. Cell Death Dis. 2017, 8, e2666. [Google Scholar] [CrossRef]
- Lee, M.-Y.; Kim, S.H.; Oh, Y.S.; Heo, S.-H.; Kim, K.-H.; Chae, H.D.; Kim, C.-H.; Kang, B.M. Role of interleukin-32 in the pathogenesis of endometriosis: In vitro, human and transgenic mouse data. Hum. Reprod. 2018, 33, 807–816. [Google Scholar] [CrossRef]
- Urata, Y.; Osuga, Y.; Akiyama, I.; Nagai, M.; Izumi, G.; Takamura, M.; Hasegawa, A.; Harada, M.; Hirata, T.; Hirota, Y.; et al. Interleukin-4 and prostaglandin E2 synergistically up-regulate 3β-hydroxysteroid dehydrogenase type 2 in endometrioma stromal cells. J. Clin. Endocrinol. Metab. 2013, 98, 1583–1590. [Google Scholar] [CrossRef]
- Veillat, V.; Carli, C.; Metz, C.N.; Al-Abed, Y.; Naccache, P.H.; Akoum, A. Macrophage migration inhibitory factor elicits an angiogenic phenotype in human ectopic endometrial cells and triggers the production of major angiogenic factors via CD44, CD74, and MAPK signaling pathways. J. Clin. Endocrinol. Metab. 2010, 95, E403–E412. [Google Scholar] [CrossRef]
- Tan, A.; Luo, R.; Liang, H.; Li, M.; Ruan, P. Bioinformatics approach reveals the key role of C-X-C motif chemokine receptor 2 in endometriosis development. Mol. Med. Rep. 2018, 18, 2841–2849. [Google Scholar] [CrossRef]
- Kuessel, L.; Wenzl, R.; Proestling, K.; Balendran, S.; Pateisky, P.; Yotova, I.; Yerlikaya, G.; Streubel, B.; Husslein, H. Soluble VCAM-1/soluble ICAM-1 ratio is a promising biomarker for diagnosing endometriosis. Hum. Reprod. 2017, 32, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-Q.; Li, H.-P.; Meng, Y.-H.; Wang, X.-Q.; Zhu, X.-Y.; Mei, J.; Li, D.-J. Chemokine CCL2 enhances survival and invasiveness of endometrial stromal cells in an autocrine manner by activating Akt and MAPK/Erk1/2 signal pathway. Fertil. Steril. 2012, 97, 919–929.e1. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.-D.; Chen, S.-L.; Chen, X.; Ye, D.-S.; Zhou, X.-Y.; Zhe, J.; Zhang, J. Down-regulation of long non-coding RNA MALAT1 inhibits granulosa cell proliferation in endometriosis by up-regulating P21 via activation of the ERK/MAPK pathway. Mol. Hum. Reprod. 2019, 25, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Darcha, C. Co-operation between the AKT and ERK signaling pathways may support growth of deep endometriosis in a fibrotic microenvironment in vitro. Hum. Reprod. 2015, 30, 1606–1616. [Google Scholar] [CrossRef]
- Aznaurova, Y.B.; Zhumataev, M.B.; Roberts, T.K.; Aliper, A.M.; Zhavoronkov, A.A. Molecular aspects of development and regulation of endometriosis. Reprod. Biol. Endocrinol. 2014, 12, 50. [Google Scholar] [CrossRef]
- McKinnon, B.D.; Kocbek, V.; Nirgianakis, K.; Bersinger, N.A.; Mueller, M.D. Kinase signalling pathways in endometriosis: Potential targets for non-hormonal therapeutics. Hum. Reprod. Update 2016, 22, 382–403. [Google Scholar] [CrossRef]
- Riccio, L.d.G.C.; Santulli, P.; Marcellin, L.; Abrão, M.S.; Batteux, F.; Chapron, C. Immunology of endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 50, 39–49. [Google Scholar] [CrossRef]
- Parazzini, F.; Viganò, P.; Candiani, M.; Fedele, L. Diet and endometriosis risk: A literature review. Reprod. Biomed. Online 2013, 26, 323–336. [Google Scholar] [CrossRef]
- Jurkiewicz-Przondziono, J.; Lemm, M.; Kwiatkowska-Pamuła, A.; Ziółko, E.; Wójtowicz, M.K. Influence of diet on the risk of developing endometriosis. Ginekol. Pol. 2017, 88, 96–102. [Google Scholar] [CrossRef]
- Yamamoto, A.; Harris, H.R.; Vitonis, A.F.; Chavarro, J.E.; Missmer, S.A. A prospective cohort study of meat and fish consumption and endometriosis risk. Am. J. Obstet. Gynecol. 2018, 219, 178.e1–178.e10. [Google Scholar] [CrossRef]
- Simmen, R.C.M.; Kelley, A.S. Seeing red: Diet and endometriosis risk. Ann. Transl. Med. 2018, 6, S119. [Google Scholar] [CrossRef] [PubMed]
- Nodler, J.L.; Harris, H.R.; Chavarro, J.E.; Frazier, A.L.; Missmer, S.A. Dairy consumption during adolescence and endometriosis risk. Am. J. Obstet. Gynecol. 2019, 222, 257. [Google Scholar] [CrossRef] [PubMed]
- Huijs, E.; Nap, A. The effects of nutrients on symptoms in women with endometriosis: A systematic review. Reprod. Biomed. Online 2020, 41, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Hallmann, E.; Kazimierczak, R.; Marszałek, K.; Drela, N.; Kiernozek, E.; Toomik, P.; Matt, D.; Luik, A.; Rembiałkowska, E. The nutritive value of organic and conventional white cabbage (Brassica oleracea L. var. capitata) and anti-apoptotic activity in gastric adenocarcinoma cells of sauerkraut juice produced therof. J. Agric. Food Chem. 2017, 65, 8171–8183. [Google Scholar] [CrossRef]
- Wieczorek, M.N.; Walczak, M.; Skrzypczak-Zielińska, M.; Jeleń, H.H. Bitter taste of Brassica vegetables: The role of genetic factors, receptors, isothiocyanates, glucosinolates, and flavor context. Crit. Rev. Food Sci. Nutr. 2018, 58, 3130–3140. [Google Scholar] [CrossRef]
- Russo, M.; Spagnuolo, C.; Russo, G.L.; Skalicka-Woźniak, K.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Nrf2 targeting by sulforaphane: A potential therapy for cancer treatment. Crit. Rev. Food Sci. Nutr. 2018, 58, 1391–1405. [Google Scholar] [CrossRef]
- Bayat Mokhtari, R.; Baluch, N.; Homayouni, T.S.; Morgatskaya, E.; Kumar, S.; Kazemi, P.; Yeger, H. The role of Sulforaphane in cancer chemoprevention and health benefits: A mini-review. J. Cell Commun. Signal. 2018, 12, 91–101. [Google Scholar] [CrossRef]
- Burčul, F.; Generalić Mekinić, I.; Radan, M.; Rollin, P.; Blažević, I. Isothiocyanates: Cholinesterase inhibiting, antioxidant, and anti-inflammatory activity. J. Enzyme Inhib. Med. Chem. 2018, 33, 577–582. [Google Scholar] [CrossRef]
- Agerbirk, N.; Olsen, C.E. Glucosinolate structures in evolution. Phytochemistry 2012, 77, 16–45. [Google Scholar] [CrossRef]
- Bhat, R.; Vyas, D. Myrosinase: Insights on structural, catalytic, regulatory, and environmental interactions. Crit. Rev. Biotechnol. 2019, 39, 508–523. [Google Scholar] [CrossRef]
- Nugrahedi, P.Y.; Verkerk, R.; Widianarko, B.; Dekker, M. A Mechanistic Perspective on Process-Induced Changes in Glucosinolate Content in Brassica Vegetables: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 823–838. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.; Carrascosa, C.; Raposo, A. Influence of Different Cooking Methods on the Concentration of Glucosinolates and Vitamin C in Broccoli. Food Bioprocess Technol. 2017, 10, 1387–1411. [Google Scholar] [CrossRef]
- Bessler, H.; Djaldetti, M. Broccoli and human health: Immunomodulatory effect of sulforaphane in a model of colon cancer. Int. J. Food Sci. Nutr. 2018, 69, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Houghton, C.A.; Fassett, R.G.; Coombes, J.S. Sulforaphane and other nutrigenomic Nrf2 activators: Can the clinician’s expectation be matched by the reality? Oxid. Med. Cell. Longev. 2016, 2016, 785186. [Google Scholar] [CrossRef] [PubMed]
- Eren, E.; Tufekci, K.U.; Isci, K.B.; Tastan, B.; Genc, K.; Genc, S. Sulforaphane inhibits lipopolysaccharide-induced inflammation, cytotoxicity, oxidative stress, and miR-155 expression and switches to mox phenotype through activating extracellular signal-regulated kinase 1/2-nuclear factor erythroid 2-related factor 2/An. Front. Immunol. 2018, 23, 9–36. [Google Scholar]
- Greaney, A.J.; Maier, N.K.; Leppla, S.H.; Moayeri, M. Sulforaphane inhibits multiple inflammasomes through an Nrf2-independent mechanism. J. Leukoc. Biol. 2016, 99, 189–199. [Google Scholar] [CrossRef]
- Okamura, Y.; Watari, M.; Jerud, E.S.; Young, D.W.; Ishizaka, S.T.; Rose, J.; Chow, J.C.; Strauss, J.F. The extra domain A of fibronectin activates Toll-like Receptor 4. J. Biol. Chem. 2001, 276, 10229–10233. [Google Scholar] [CrossRef]
- O’Callaghan, P.; Zhang, X.; Li, J.P. Heparan sulfate proteoglycans as relays of neuroinflammation. J. Histochem. Cytochem. 2018, 66, 305–319. [Google Scholar] [CrossRef]
- Youn, H.S.; Kim, Y.S.; Park, Z.Y.; Kim, S.Y.; Choi, N.Y.; Joung, S.M.; Seo, J.A.; Lim, K.-M.; Kwak, M.-K.; Hwang, D.H.; et al. Sulforaphane Suppresses Oligomerization of TLR4 in a Thiol-Dependent Manner. J. Immunol. 2010, 184, 305–319. [Google Scholar] [CrossRef]
- Mazarakis, N.; Snibson, K.; Licciardi, P.V.; Karagiannis, T.C. The potential use of L-sulforaphane for the treatment of chronic inflammatory diseases: A review of the clinical evidence. Clin. Nutr. 2019, 39, 664–675. [Google Scholar] [CrossRef]
- Zhao, Z.; Liao, G.; Zhou, Q.; Lv, D.; Holthfer, H.; Zou, H. Sulforaphane attenuates contrast-induced nephropathy in rats via Nrf2/HO-1 pathway. Oxid. Med. Cell. Longev. 2016, 2016, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Hong, Y.; Lv, Y. Sulforaphane attenuates endometriosis in rat models through inhibiting PI3K/Akt signaling pathway. Dose-Response 2019, 17, 1559325819855538. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Z.; Lu, X.; Meng, J.; Qin, X.; Jiang, J. Anti-nociceptive and anti-inflammatory effects of sulforaphane on sciatic endometriosis in a rat model. Neurosci. Lett. 2020, 723, 134858. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; Piegholdt, S.; Schloesser, A.; Moreno, D.A.; García-Viguera, C.; Rimbach, G.; Wagner, A.E. Metabolic activity of radish sprouts derived isothiocyanates in drosophila melanogaster. Int. J. Mol. Sci. 2016, 17, 251. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, A.; Wała, M.; Hać, A.; Felczykowska, A.; Herman-Antosiewicz, A. Sulforaphene, an isothiocyanate present in radish plants, inhibits proliferation of human breast cancer cells. Phytomedicine 2017, 29, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pocasap, P.; Weerapreeyakul, N.; Barusrux, S. Cancer preventive effect of Thai rat-tailed radish (Raphanus sativus L. var. caudatus Alef). J. Funct. Foods 2013, 5, 1372–1381. [Google Scholar] [CrossRef]
- Baenas, N.; Suárez-Martínez, C.; García-Viguera, C.; Moreno, D.A. Bioavailability and new biomarkers of cruciferous sprouts consumption. Food Res. Int. 2017. [Google Scholar] [CrossRef]
- Westphal, A.; Riedl, K.M.; Cooperstone, J.L.; Kamat, S.; Balasubramaniam, V.M.; Schwartz, S.J.; Böhm, V. High-pressure processing of broccoli sprouts: Influence on bioactivation of glucosinolates to isothiocyanates. J. Agric. Food Chem. 2017, 65, 8578–8585. [Google Scholar] [CrossRef]
- Clarke, J.D.; Hsu, A.; Riedl, K.; Bella, D.; Schwartz, S.J.; Stevens, J.F.; Ho, E. Bioavailability and inter-conversion of sulforaphane and erucin in human subjects consuming broccoli sprouts or broccoli supplement in a cross-over study design. Pharmacol. Res. 2011, 64, 456–463. [Google Scholar] [CrossRef]
- Cho, H.J.; Lee, K.W.; Yoon Park, J.H. Erucin exerts anti-inflammatory properties in murine macrophages and mouse skin: Possible mediation through the inhibition of NFκB signaling. Int. J. Mol. Sci. 2013, 14, 20564–20577. [Google Scholar] [CrossRef]
- Luang-In, V.; Deeseenthum, S.; Udomwong, P.; Saengha, W.; Gregori, M. Formation of sulforaphane and iberin products from Thai cabbage fermented by myrosinase-positive bacteria. Molecules 2018, 23, 955. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; Marhuenda, J.; García-Viguera, C.; Zafrilla, P.; Moreno, D.A. Influence of cooking methods on glucosinolates and isothiocyanates content in novel cruciferous foods. Foods 2019, 8, 257. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Nakashima, F.; Honda, K.; Lu, Y.J.; Kondo, T.; Ushida, Y.; Aizawa, K.; Suganuma, H.; Oe, S.; Tanaka, H.; et al. Toll-like receptors as a target of food-derived anti-inflammatory compounds. J. Biol. Chem. 2014, 289, 32757–32772. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.J.; Chen, B.H.; Inbaraj, B.S.; Chien, J.T. Preparation of allyl isothiocyanate nanoparticles, their anti-inflammatory activity towards RAW 264.7 macrophage cells and anti-proliferative effect on HT1376 bladder cancer cells. J. Sci. Food Agric. 2019, 99, 3106–3116. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, T.; Pugalendhi, P.; Jayaganesh, R.; Ananthakrishnan, D.; Gunasekaran, K. Effect of allyl isothiocyanate on NF-κB signaling in 7,12-dimethylbenz(a)anthracene and N-methyl-N-nitrosourea-induced mammary carcinogenesis. Breast Cancer 2018, 25, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, T.; Pugalendhi, P.; Thilagavathi, S.; Ananthakrishnan, D.; Gunasekaran, K. Allyl isothiocyanate, a potent chemopreventive agent targets AhR/Nrf2 signaling pathway in chemically induced mammary carcinogenesis. Mol. Cell. Biochem. 2018, 437, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bessede, A.; Gargaro, M.; Pallotta, M.T.; Matino, D.; Servillo, G.; Brunacci, C.; Bicciato, S.; Mazza, E.M.C.; Macchiarulo, A.; Vacca, C.; et al. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature 2014, 511, 184–190. [Google Scholar] [CrossRef]
- Murray, I.A.; Patterson, A.D.; Perdew, G.H. Aryl hydrocarbon receptor ligands in cancer: Friend and foe. Nat. Rev. Cancer 2014, 14, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, Y.; de Dios Rosado, J.; Vega, L.; Elizondo, G.; Estrada-Muñiz, E.; Saavedra, R.; Juárez, I.; Rodríguez-Sosa, M. The unexpected role for the aryl hydrocarbon receptor on susceptibility to experimental toxoplasmosis. J. Biomed. Biotechnol. 2010, 2010, 505694. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.M.; Park, B.; Dang, Y.M.; Kim, S.Y.; Seo, H.Y. Simultaneous direct determination of 15 glucosinolates in eight Brassica species by UHPLC-Q-Orbitrap-MS. Food Chem. 2019, 282, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Grose, K.R.; Bjeldanes, L.F. Oligomerization of Indole-3-carbinol in aqueous acid. Chem. Res. Toxicol. 1992, 5, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.Y.; Pham, Q.; Kim, Y.S. Elucidating the role of CD84 and AHR in modulation of LPS-induced cytokines production by cruciferous vegetable-derived compounds indole-3-carbinol and 3,3′-diindolylmethane. Int. J. Mol. Sci. 2018, 19, 339. [Google Scholar] [CrossRef] [PubMed]
- Hajra, S.; Patra, A.R.; Basu, A.; Bhattacharya, S. Prevention of doxorubicin (DOX)-induced genotoxicity and cardiotoxicity: Effect of plant derived small molecule indole-3-carbinol (I3C) on oxidative stress and inflammation. Biomed. Pharmacother. 2018, 101, 228–243. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Memarian, A.; Sedighi, S.; Behnampour, N.; Yazdani, Y. Immunoregulatory effects of indole-3-carbinol on monocyte-derived macrophages in systemic lupus erythematosus: A crucial role for aryl hydrocarbon receptor. Autoimmunity 2018, 51, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Seon, M.R.; Lee, Y.M.; Kim, J.; Kim, J.-K.; Kim, S.G.; Park, J.H.Y. 3,3′-Diindolylmethane suppresses the inflammatory response to lipopolysaccharide in murine macrophages. J. Nutr. 2008, 138, 17–23. [Google Scholar] [CrossRef]
- Jeon, E.J.; Davaatseren, M.; Hwang, J.T.; Park, J.H.; Hur, H.J.; Lee, A.S.; Sung, M.J. Effect of oral administration of 3,3′-Diindolylmethane on dextran sodium sulfate-induced acute colitis in mice. J. Agric. Food Chem. 2016, 64, 7702–7709. [Google Scholar] [CrossRef]
- Morales-Prieto, D.M.; Herrmann, J.; Osterwald, H.; Kochhar, P.S.; Schleussner, E.; Markert, U.R.; Oettel, M. Comparison of dienogest effects upon 3,3′–diindolylmethane supplementation in models of endometriosis and clinical cases. Reprod. Biol. 2018, 18, 252–258. [Google Scholar] [CrossRef]
- Tai, A.; Fukunaga, K.; Ohno, A.; Ito, H. Antioxidative properties of ascorbigen in using multiple antioxidant assays. Biosci. Biotechnol. Biochem. 2014, 78, 1723–1730. [Google Scholar] [CrossRef][Green Version]
- Martinez-Villaluenga, C.; Peñas, E.; Sidro, B.; Ullate, M.; Frias, J.; Vidal-Valverde, C. White cabbage fermentation improves ascorbigen content, antioxidant and nitric oxide production inhibitory activity in LPS-induced macrophages. LWT Food Sci. Technol. 2012, 46, 77–83. [Google Scholar] [CrossRef]
- Magni, F.; Sarto, C.; Ticozzi, D.; Soldi, M.; Bosso, N.; Mocarelli, P.; Kienle, M.G. Proteomic knowledge of human aquaporins. Proteomics 2006, 6, 5637–5649. [Google Scholar] [CrossRef]
- Dajani, S.; Saripalli, A.; Sharma-Walia, N. Water transport proteins–aquaporins (AQPs) in cancer biology. Oncotarget 2018, 9, 36392–36405. [Google Scholar] [CrossRef] [PubMed]
- Agre, P.; Kozono, D. Aquaporin water channels: Molecular mechanisms for human diseases. FEBS Letters 2003, 555, 72–78. [Google Scholar] [CrossRef]
- Madeira, A.; Moura, T.F.; Soveral, G. Detecting aquaporin function and regulation. Front. Chem. 2016, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S. Aquaporins in clinical medicine. Annu. Rev. Med. 2012, 63, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Jiang, Z.; Bazer, F.W.; Johnson, G.A.; Burghardt, R.C.; Wu, G. Aquaporins in the female reproductive system of mammals. Front. Biosci. 2015, 20, 838–871. [Google Scholar]
- Zhang, D.; Tan, Y.J.; Qu, F.; Sheng, J.Z.; Huang, H.F. Functions of water channels in male and female reproductive systems. Mol. Aspects Med. 2012, 33, 676–690. [Google Scholar] [CrossRef]
- Meli, R.; Pirozzi, C.; Pelagalli, A. New perspectives on the potential role of aquaporins (AQPs) in the physiology of inflammation. Front. Physiol. 2018, 16, 101. [Google Scholar] [CrossRef]
- Riemma, G.; Laganà, A.S.; Schiattarella, A.; Garzon, S.; Cobellis, L.; Autiero, R.; Licciardi, F.; Corte, L.D.; La Verde, M.; De Franciscis, P. Ion channels in the pathogenesis of endometriosis: A cutting-edge point of view. Int. J. Mol. Sci. 2020, 21, 1114. [Google Scholar] [CrossRef]
- Shu, C.; Shu, Y.; Gao, Y.; Chi, H.; Han, J. Inhibitory effect of AQP1 silencing on adhesion and angiogenesis in ectopic endometrial cells of mice with endometriosis through activating the Wnt signaling pathway. Cell Cycle 2019, 18, 2026–2039. [Google Scholar] [CrossRef]
- Skowronski, M.T. Distribution and quantitative changes in amounts of aquaporin 1, 5 and 9 in the pig uterus during the estrous cycle and early pregnancy. Reprod. Biol. Endocrinol. 2010, 8, 1–11. [Google Scholar] [CrossRef]
- Jiang, X.X.; Wu, R.J.; Xu, K.H.; Zhou, C.Y.; Guo, X.Y.; Sun, Y.L.; Lin, J. Immunohistochemical detection of aquaporin expression in eutopic and ectopic endometria from women with endometriomas. Fertil. Steril. 2010, 94, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.X.; Fei, X.W.; Zhao, L.; Ye, X.L.; Xin, L.B.; Qu, Y.; Xu, K.H.; Wu, R.J.; Lin, J. Aquaporin 5 plays a role in estrogen-induced ectopic implantation of endometrial stromal cells in endometriosis. PLoS ONE 2015, 10, e0145290. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.X.; Xu, K.H.; Ma, J.Y.; Tian, Y.H.; Guo, X.Y.; Lin, J.; Wu, R.J. Reduced migration of Ishikawa cells associated with downregulation of aquaporin-5. Oncol. Lett. 2012, 4, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Park, J.H.; Yoon, J.-K.; Yoon, J.S.; Kim, J.S.; Lee, J.H.; Yun, B.H.; Park, J.H.; Seo, S.K.; Cho, S.; et al. Potential roles of aquaporin 9 in the pathogenesis of endometriosis. MHR Basic Sci. Reprod. Med. 2019, 125, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.G.; Li, C.F.; Liu, M.; Chen, X.F.; Shuai, K.; Kong, X.; Lv, L.; Mei, Z. chuan Aquaporin 9 is down-regulated in hepatocellular carcinoma and its over-expression suppresses hepatoma cell invasion through inhibiting epithelial-to-mesenchymal transition. Cancer Lett. 2016, 378, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Tyteca, D.; Nishino, T.; Debaix, H.; Van Smissen, P.D.; N’Kuli, F.; Hoffmann, D.; Cnops, Y.; Rabolli, V.; Van Loo, G.; Beyaert, R.; et al. Regulation of macrophage motility by the water channel aquaporin-1: Crucial role of M0/M2 phenotype switch. PLoS ONE 2015, 10, e0117398. [Google Scholar] [CrossRef]
- Li, B.; Liu, C.; Tang, K.; Dong, X.; Xue, L.; Su, G.; Zhang, W.; Jin, Y. Aquaporin-1 attenuates macrophage-mediated inflammatory responses by inhibiting p38 mitogen-activated protein kinase activation in lipopolysaccharide-induced acute kidney injury. Inflamm. Res. 2019, 68, 1035–1047. [Google Scholar] [CrossRef]
- Li, Y.; Lu, H.; Lv, X.; Tang, Q.; Li, W.; Zhu, H.; Long, Y. Blockade of Aquaporin 4 Inhibits Irradiation-Induced Pulmonary Inflammation and Modulates Macrophage Polarization in Mice. Inflammation 2018, 41, 2196–2203. [Google Scholar] [CrossRef]
- Kitchen, P.; Day, R.E.; Salman, M.M.; Conner, M.T.; Bill, R.M.; Conner, A.C. Beyond water homeostasis: Diverse functional roles of mammalian aquaporins. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 2410–2421. [Google Scholar] [CrossRef]
- Lin, Y.H.; Chen, Y.H.; Chang, H.Y.; Au, H.K.; Tzeng, C.R.; Huang, Y.H. Chronic niche inflammation in endometriosis-associated infertility: Current understanding and future therapeutic strategies. Int. J. Mol. Sci. 2018, 19, 2385. [Google Scholar] [CrossRef]
- Martínez-Ballesta, M.d.C.; Bou, G.; Carvajal, M. Aquaporins as targets of pharmacological plant-derived compounds. Phytochem. Rev. 2014, 13, 573–586. [Google Scholar] [CrossRef]
- Tanimura, Y.; Hiroaki, Y.; Fujiyoshi, Y. Acetazolamide reversibly inhibits water conduction by aquaporin-4. J. Struct. Biol. 2009, 166, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, H.; Huber, V.J.; Tsujita, M.; Nakada, T. Pretreatment with a novel aquaporin 4 inhibitor, TGN-020, significantly reduces ischemic cerebral edema. Neurol. Sci. 2011, 32, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Brooks, H.L.; Regan, J.W.; Yool, A.J. Inhibition of aquaporin-1 water permeability by tetraethylammonium: Involvement of the loop E pore region. Mol. Pharmacol. 2000, 57, 1021–1026. [Google Scholar] [PubMed]
- Detmers, F.J.M.; De Groot, B.L.; Müller, E.M.; Hinton, A.; Konings, I.B.M.; Sze, M.; Flitsch, S.L.; Grubmüller, H.; Deen, P.M.T. Quaternary ammonium compounds as water channel blockers: Specificity, potency, and site of action. J. Biol. Chem. 2006, 281, 14207–14214. [Google Scholar] [CrossRef] [PubMed]
- Yool, A.J.; Morelle, J.; Cnops, Y.; Verbavatz, J.M.; Campbell, E.M.; Beckett, E.A.H.; Booker, G.W.; Flynn, G.; Devuyst, O. AqF026 is a pharmacologic agonist of the water channel aquaporin-1. J. Am. Soc. Nephrol. 2013, 24, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, X.; Chang, Y.; Zhang, J.; Song, Q.; Yu, H.; Li, X. Acetazolamide inhibits osmotic water permeability by interaction with aquaporin-1. Anal. Biochem. 2006, 350, 165–170. [Google Scholar] [CrossRef]
- Huber, V.J.; Tsujita, M.; Yamazaki, M.; Sakimura, K.; Nakada, T. Identification of arylsulfonamides as Aquaporin 4 inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 1270–1273. [Google Scholar] [CrossRef]
- Huber, V.J.; Tsujita, M.; Kwee, I.L.; Nakada, T. Inhibition of Aquaporin 4 by antiepileptic drugs. Bioorg. Med. Chem. 2009, 17, 418–424. [Google Scholar] [CrossRef]
- Devuyst, O.; Yool, A.J. Aquaporin-1: New developments and perspectives for peritoneal dialysis. Perit. Dial. Int. 2010, 30, 135–141. [Google Scholar] [CrossRef]
- Szczepańska, M.; Koźlik, J.; Skrzypczak, J.; Mikołajczyk, M. Oxidative stress may be a piece in the endometriosis puzzle. Fertil. Steril. 2003, 79, 1288–1293. [Google Scholar] [CrossRef]
- Bestetti, S.; Medraño-Fernandez, I.; Galli, M.; Ghitti, M.; Bienert, G.P.; Musco, G.; Orsi, A.; Rubartelli, A.; Sitia, R. A persulfidation-based mechanism controls aquaporin-8 conductance. Sci. Adv. 2018, 4, eaar5770. [Google Scholar] [CrossRef] [PubMed]
- Prata, C.; Facchini, C.; Leoncini, E.; Lenzi, M.; Maraldi, T.; Angeloni, C.; Zambonin, L.; Hrelia, S.; Fiorentini, D. Sulforaphane modulates AQP8-linked redox signalling in leukemia cells. Oxid. Med. Cell. Longev. 2018, 2018, 4125297. [Google Scholar] [CrossRef]
- Kitawaki, J.; Kado, N.; Ishihara, H.; Koshiba, H.; Kitaoka, Y.; Honjo, H. Endometriosis: The pathophysiology as an estrogen-dependent disease. J. Steroid Biochem. Mol. Biol. 2002, 83, 149–155. [Google Scholar] [CrossRef]
- Oliveira, C.A.; Carnes, K.; França, L.R.; Hermo, L.; Hess, R.A. Aquaporin-1 and -9 are differentially regulated by oestrogen in the efferent ductule epithelium and initial segment of the epididymis. Biol. Cell 2005, 97, 385–395. [Google Scholar] [CrossRef]
- Zou, L.B.; Zhang, R.J.; Tan, Y.J.; Ding, G.L.; Shi, S.; Zhang, D.; He, R.H.; Liu, A.X.; Wang, T.T.; Leung, P.C.K.; et al. Identification of estrogen response element in the aquaporin-2 gene that mediates estrogen-induced cell migration and invasion in human endometrial carcinoma. J. Clin. Endocrinol. Metab. 2011, 96, E1399–E1408. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Wang, L.; Zhang, Z.; Chen, F.; Wu, Q.; Li, L. Sulforaphane-induced metabolomic responses with epigenetic changes in estrogen receptor positive breast cancer cells. FEBS Open Bio 2018, 8, 2022–2034. [Google Scholar] [CrossRef] [PubMed]
- Marconett, C.N.; Singhal, A.K.; Sundar, S.N.; Firestone, G.L. Indole-3-carbinol disrupts estrogen receptor-alpha dependent expression of insulin-like growth factor-1 receptor and insulin receptor substrate-1 and proliferation of human breast cancer cells. Mol. Cell. Endocrinol. 2012, 363, 74–84. [Google Scholar] [CrossRef]
- Meng, Q.; Yuan, F.; Goldberg, I.D.; Rosen, E.M.; Auborn, K.; Fan, S. Indole-3-Carbinol Is a Negative Regulator of Estrogen Receptor-α Signaling in Human Tumor Cells. J. Nutr. 2000, 130, 2927–2931. [Google Scholar] [CrossRef]
- Amare, D.E.; Bovee, T.F.H.; Mulder, P.P.J.; Hamers, A.; Hoogenboom, R.L.A.P. Acid condensation products of indole-3-carbinol and their in-vitro (anti)estrogenic, (anti)androgenic and aryl hydrocarbon receptor activities. Arab. J. Chem. 2020, 13, 7199–7211. [Google Scholar] [CrossRef]
- Zhao, J.; Moore, A.N.; Clifton, G.L.; Dash, P.K. Sulforaphane enhances aquaporin-4 expression and decreases cerebral edema following traumatic brain injury. J. Neurosci. Res. 2005, 82, 499–506. [Google Scholar] [CrossRef]
- Dash, P.K.; Zhao, J.; Orsi, S.A.; Zhang, M.; Moore, A.N. Sulforaphane improves cognitive function administered following traumatic brain injury. Neurosci. Lett. 2009, 460, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Gasparyan, A.Y.; Ayvazyan, L.; Blackmore, H.; Kitas, G.D. Writing a narrative biomedical review: Considerations for authors, peer reviewers, and editors. Rheumatol. Int. 2011, 31, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Saracci, C.; Mahamat, M.; Jacquérioz, F. How to write a narrative literature review article? Rev. Med. Suisse 2019, 15, 1694–1698. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Ibañez, P.; Yepes-Molina, L.; Ruiz-Alcaraz, A.J.; Martínez-Esparza, M.; Moreno, D.A.; Carvajal, M.; García-Peñarrubia, P. Brassica Bioactives Could Ameliorate the Chronic Inflammatory Condition of Endometriosis. Int. J. Mol. Sci. 2020, 21, 9397. https://doi.org/10.3390/ijms21249397
García-Ibañez P, Yepes-Molina L, Ruiz-Alcaraz AJ, Martínez-Esparza M, Moreno DA, Carvajal M, García-Peñarrubia P. Brassica Bioactives Could Ameliorate the Chronic Inflammatory Condition of Endometriosis. International Journal of Molecular Sciences. 2020; 21(24):9397. https://doi.org/10.3390/ijms21249397
Chicago/Turabian StyleGarcía-Ibañez, Paula, Lucía Yepes-Molina, Antonio J. Ruiz-Alcaraz, María Martínez-Esparza, Diego A. Moreno, Micaela Carvajal, and Pilar García-Peñarrubia. 2020. "Brassica Bioactives Could Ameliorate the Chronic Inflammatory Condition of Endometriosis" International Journal of Molecular Sciences 21, no. 24: 9397. https://doi.org/10.3390/ijms21249397
APA StyleGarcía-Ibañez, P., Yepes-Molina, L., Ruiz-Alcaraz, A. J., Martínez-Esparza, M., Moreno, D. A., Carvajal, M., & García-Peñarrubia, P. (2020). Brassica Bioactives Could Ameliorate the Chronic Inflammatory Condition of Endometriosis. International Journal of Molecular Sciences, 21(24), 9397. https://doi.org/10.3390/ijms21249397







