Modulatory Effect of Myokines on Reactive Oxygen Species in Ischemia/Reperfusion
Abstract
1. Introduction
1.1. ROS in I/R Injury
1.2. Exercise-Induced Remote Organ Protection Against I/R
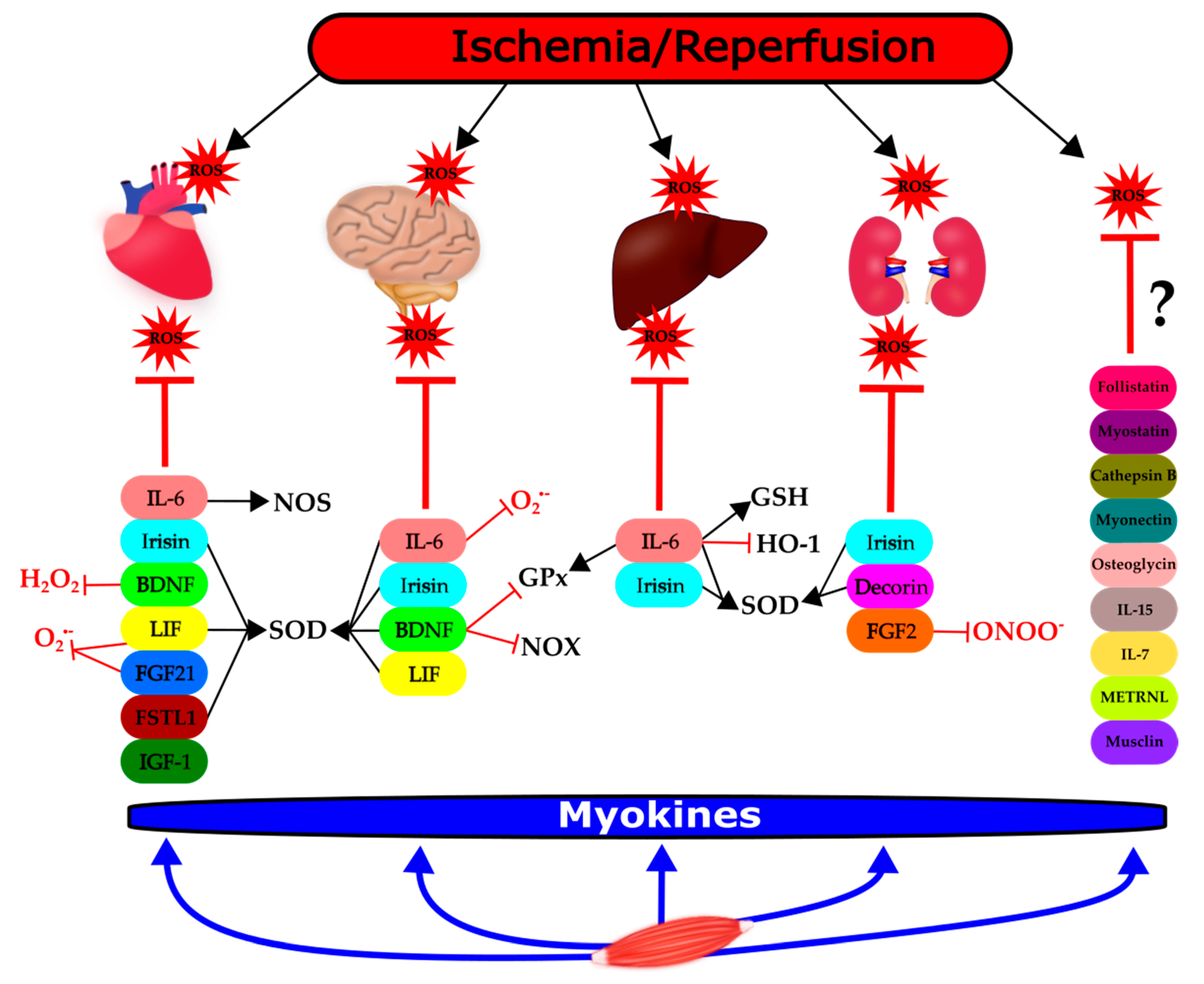
2. Modulatory Role of Myokines on ROS in the Context of I/R
2.1. Interleukin (IL) 6
2.2. Irisin
2.3. Brain-Derived Neurotrophic Factor (BDNF)
2.4. Follistatin-Like 1
2.5. Fibroblast Growth Factor-21
2.6. Decorin
2.7. Myonectin
2.8. Insulin-Like Growth Factor-1 (IGF-1)
2.9. Leukemia Inhibitory Factor (LIF)
2.10. Fibroblast Growth Factor 2 (FGF-2)
2.11. Other Myokines
3. Discussion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BDNF | brain-derived neurotrophic factor |
| FGF-2 | fibroblast growth factor 2 |
| FGF-21 | fibroblast growth factor-21 |
| FSTL-1 | follistatin-like |
| GPx | glutathione peroxidase |
| GSH | glutathione |
| HO-1 | heme oxygenase-1; |
| IGF-1 | insulin-like growth factor-1; |
| IL-6 | interleukin-6 |
| IL-7 | interleukin-7 |
| IL-15 | interleukin-15 |
| I/R | ischemia/reperfusion |
| LIF | leukemia inhibitory factor |
| METRLN | meteorin-like |
| NO | nitric oxide |
| NOS | NO synthase |
| NOS-1 | neuronal NOS (nNOS) |
| NOS-2 | inducible NOS (iNOS) |
| NOS-3 | endothelial NOS (eNOS) |
| NOX | NADPH oxidase |
| NRF-2 | nuclear factor erythroid 2–related factor 2 |
| RNS | reactive nitrogen species |
| ROI | reactive oxygen intermediates |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase (total) |
| SOD-1 | cytosolic CuZnSOD |
| SOD-2 | mitochondrial MnSOD |
| SOD-3 | extracellular SOD |
References
- Granger, D.N.; Kvietys, P.R. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015, 6, 524–551. [Google Scholar] [CrossRef] [PubMed]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Ischemia/Reperfusion. Compr. Physiol. 2016, 7, 113–170. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Biochemistry of oxidative stress. Biochem. Soc. Trans. 2007, 35, 1147–1150. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Zhou, T.; Pannell, B.K.; Ziegler, A.C.; Best, T.M. Biological and physiological role of reactive oxygen species--the good, the bad and the ugly. Acta Physiol. (Oxf. Engl.) 2015, 214, 329–348. [Google Scholar] [CrossRef]
- Csonka, C.; Sárközy, M.; Pipicz, M.; Dux, L.; Csont, T. Modulation of Hypercholesterolemia-Induced Oxidative/Nitrative Stress in the Heart. Oxidative Med. Cell. Longev. 2016, 2016, 3863726. [Google Scholar] [CrossRef]
- Wu, L.; Xiong, X.; Wu, X.; Ye, Y.; Jian, Z.; Zhi, Z.; Gu, L. Targeting Oxidative Stress and Inflammation to Prevent Ischemia-Reperfusion Injury. Front. Mol. Neurosci. 2020, 13. [Google Scholar] [CrossRef]
- Wu, M.Y.; Yiang, G.T.; Liao, W.T.; Tsai, A.P.Y.; Cheng, Y.L.; Cheng, P.W.; Li, C.Y.; Li, C.J. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell. Physiol. Biochem. 2018, 46, 1650–1667. [Google Scholar] [CrossRef]
- Raedschelders, K.; Ansley, D.M.; Chen, D.D.Y. The cellular and molecular origin of reactive oxygen species generation during myocardial ischemia and reperfusion. Pharmacol. Ther. 2012, 133, 230–255. [Google Scholar] [CrossRef]
- Davidson Sean, M.; Ferdinandy, P.; Andreadou, I.; Bøtker Hans, E.; Heusch, G.; Ibáñez, B.; Ovize, M.; Schulz, R.; Yellon Derek, M.; Hausenloy Derek, J.; et al. Multitarget Strategies to Reduce Myocardial Ischemia/Reperfusion Injury. J. Am. Coll. Cardiol. 2019, 73, 89–99. [Google Scholar] [CrossRef]
- Xiong, X.-Y.; Liu, L.; Yang, Q.-W. Refocusing Neuroprotection in Cerebral Reperfusion Era: New Challenges and Strategies. Front. Neurol. 2018, 9, 249. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Dong, F.; Zhang, J.; Zhang, F. Physical exercise-induced protection on ischemic cardiovascular and cerebrovascular diseases. Int. J. Clin. Exp. Med. 2015, 8, 19859–19866. [Google Scholar] [PubMed]
- Nystoriak, M.A.; Bhatnagar, A. Cardiovascular Effects and Benefits of Exercise. Front. Cardiovasc. Med. 2018, 5, 135. [Google Scholar] [CrossRef] [PubMed]
- Pinckard, K.; Baskin, K.K.; Stanford, K.I. Effects of Exercise to Improve Cardiovascular Health. Front. Cardiovasc. Med. 2019, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Lennon, S.L.; Quindry, J.; Mehta, J.L. Exercise and cardioprotection. Curr. Opin. Cardiol. 2002, 17, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Quindry, J.C.; Kavazis, A.N. Exercise-induced cardioprotection against myocardial ischemia-reperfusion injury. Free Radic. Biol. Med. 2008, 44, 193–201. [Google Scholar] [CrossRef]
- De Sousa, C.V.; Sales, M.M.; Rosa, T.S.; Lewis, J.E.; de Andrade, R.V.; Simões, H.G. The Antioxidant Effect of Exercise: A Systematic Review and Meta-Analysis. Sports Med. (Auckl. N. Z.) 2017, 47, 277–293. [Google Scholar] [CrossRef]
- Polley, K.R.; Jenkins, N.; O’Connor, P.; McCully, K. Influence of exercise training with resveratrol supplementation on skeletal muscle mitochondrial capacity. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2016, 41, 26–32. [Google Scholar] [CrossRef]
- Powers, S.K.; Sollanek, K.J.; Wiggs, M.P.; Demirel, H.A.; Smuder, A.J. Exercise-induced improvements in myocardial antioxidant capacity: The antioxidant players and cardioprotection. Free Radic. Res. 2014, 48, 43–51. [Google Scholar] [CrossRef]
- Ascensão, A.; Lumini-Oliveira, J.; Oliveira, P.J.; Magalhães, J. Mitochondria as a target for exercise-induced cardioprotection. Curr. Drug Targets 2011, 12, 860–871. [Google Scholar] [CrossRef]
- Powers, S.K. Exercise: Teaching myocytes new tricks. J. Appl. Physiol. 2017, 123, 460–472. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Steensberg, A.; Fischer, C.; Keller, C.; Keller, P.; Plomgaard, P.; Febbraio, M.; Saltin, B. Searching for the exercise factor: Is IL-6 a candidate? J. Muscle Res. Cell Motil. 2003, 24, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Penna, C.; Alloatti, G.; Crisafulli, A. Mechanisms Involved in Cardioprotection Induced by Physical Exercise. Antioxid. Redox Signal. 2020, 32, 1115–1134. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Fuentes, H.A.; Aragones, J.; Bernhagen, J.; Boening, A.; Boisvert, W.A.; Bøtker, H.E.; Bulluck, H.; Cook, S.; Di Lisa, F.; Engel, F.B.; et al. From basic mechanisms to clinical applications in heart protection, new players in cardiovascular diseases and cardiac theranostics: Meeting report from the third international symposium on “New frontiers in cardiovascular research”. Basic Res. Cardiol. 2016, 111, 69. [Google Scholar] [CrossRef][Green Version]
- Hausenloy, D.J.; Yellon, D.M. Ischaemic conditioning and reperfusion injury. Nat. Rev. Cardiol. 2016, 13, 193–209. [Google Scholar] [CrossRef]
- Heusch, G. Molecular basis of cardioprotection: Signal transduction in ischemic pre-, post-, and remote conditioning. Circ. Res. 2015, 116, 674–699. [Google Scholar] [CrossRef]
- Kleinbongard, P.; Skyschally, A.; Heusch, G. Cardioprotection by remote ischemic conditioning and its signal transduction. Pflug. Arch. Eur. J. Physiol. 2017, 469, 159–181. [Google Scholar] [CrossRef]
- Das, D.K.; Graham, Z.A.; Cardozo, C.P. Myokines in skeletal muscle physiology and metabolism: Recent advances and future perspectives. Acta Physiol. 2020, 228, e13367. [Google Scholar] [CrossRef]
- Severinsen, M.C.K.; Schéele, C.; Pedersen, B.K. Exercise and browning of white adipose tissue—A translational perspective. Curr. Opin. Pharmacol. 2020, 52, 18–24. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef]
- Pipicz, M.; Demján, V.; Sárközy, M.; Csont, T. Effects of Cardiovascular Risk Factors on Cardiac STAT3. Int. J. Mol. Sci. 2018, 19, 3572. [Google Scholar] [CrossRef] [PubMed]
- Harhous, Z.; Booz, G.W.; Ovize, M.; Bidaux, G.; Kurdi, M. An Update on the Multifaceted Roles of STAT3 in the Heart. Front. Cardiovasc. Med. 2019, 6, 150. [Google Scholar] [CrossRef] [PubMed]
- Marasco, M.R.; Conteh, A.M.; Reissaus, C.A.; Cupit, J.E.t.; Appleman, E.M.; Mirmira, R.G.; Linnemann, A.K. Interleukin-6 Reduces beta-Cell Oxidative Stress by Linking Autophagy With the Antioxidant Response. Diabetes 2018, 67, 1576–1588. [Google Scholar] [CrossRef] [PubMed]
- Hernández, J.; Hidalgo, J. Endotoxin and intracerebroventricular injection of IL-1 and IL-6 induce rat brain metallothionein-I and -II. Neurochem. Int. 1998, 32, 369–373. [Google Scholar] [CrossRef]
- Penkowa, M.; Hidalgo, J. IL-6 deficiency leads to reduced metallothionein-I+II expression and increased oxidative stress in the brain stem after 6-aminonicotinamide treatment. Exp. Neurol. 2000, 163, 72–84. [Google Scholar] [CrossRef]
- McGinnis, G.R.; Ballmann, C.; Peters, B.; Nanayakkara, G.; Roberts, M.; Amin, R.; Quindry, J.C. Interleukin-6 mediates exercise preconditioning against myocardial ischemia reperfusion injury. Am. J. Physiol. Circ. Physiol. 2015, 308, H1423–H1433. [Google Scholar] [CrossRef]
- Matsushita, K.; Iwanaga, S.; Oda, T.; Kimura, K.; Shimada, M.; Sano, M.; Umezawa, A.; Hata, J.-I.; Ogawa, S. Interleukin-6/soluble interleukin-6 receptor complex reduces infarct size via inhibiting myocardial apoptosis. Lab. Investig. 2005, 85, 1210–1223. [Google Scholar] [CrossRef][Green Version]
- Smart, N.; Mojet, M.H.; Latchman, D.S.; Marber, M.S.; Duchen, M.R.; Heads, R.J. IL-6 induces PI 3-kinase and nitric oxide-dependent protection and preserves mitochondrial function in cardiomyocytes. Cardiovasc. Res. 2006, 69, 164–177. [Google Scholar] [CrossRef]
- Dawn, B.; Xuan, Y.-T.; Guo, Y.; Rezazadeh, A.; Stein, A.B.; Hunt, G.; Wu, W.-J.; Tan, W.; Bolli, R. IL-6 plays an obligatory role in late preconditioning via JAK-STAT signaling and upregulation of iNOS and COX-2. Cardiovasc. Res. 2004, 64, 61–71. [Google Scholar] [CrossRef]
- Jung, J.E.; Kim, G.S.; Chan, P.H. Neuroprotection by interleukin-6 is mediated by signal transducer and activator of transcription 3 and antioxidative signaling in ischemic stroke. Stroke 2011, 42, 3574–3579. [Google Scholar] [CrossRef]
- Loddick, S.A.; Turnbull, A.V.; Rothwell, N.J. Cerebral interleukin-6 is neuroprotective during permanent focal cerebral ischemia in the rat. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 1998, 18, 176–179. [Google Scholar] [CrossRef] [PubMed]
- Ali, C.; Nicole, O.; Docagne, F.; Lesne, S.; MacKenzie, E.T.; Nouvelot, A.; Buisson, A.; Vivien, D. Ischemia-induced interleukin-6 as a potential endogenous neuroprotective cytokine against NMDA receptor-mediated excitotoxicity in the brain. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2000, 20, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Sawamoto, K.; Suzuki, S.; Suzuki, N.; Adachi, K.; Kawase, T.; Mihara, M.; Ohsugi, Y.; Abe, K.; Okano, H. Blockade of interleukin-6 signaling aggravates ischemic cerebral damage in mice: Possible involvement of Stat3 activation in the protection of neurons. J. Neurochem. 2005, 94, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Sakata, H.; Narasimhan, P.; Niizuma, K.; Maier, C.M.; Wakai, T.; Chan, P.H. Interleukin 6-preconditioned neural stem cells reduce ischaemic injury in stroke mice. Brain 2012, 135, 3298–3310. [Google Scholar] [CrossRef]
- Tiberio, L.; Tiberio, G.A.; Bardella, L.; Cervi, E.; Cerea, K.; Dreano, M.; Garotta, G.; Fra, A.; Montani, N.; Ferrari-Bravo, A.; et al. Mechanisms of interleukin-6 protection against ischemia-reperfusion injury in rat liver. Cytokine 2006, 34, 131–142. [Google Scholar] [CrossRef]
- Terui, K.; Enosawa, S.; Haga, S.; Zhang, H.Q.; Kuroda, H.; Kouchi, K.; Matsunaga, T.; Yoshida, H.; Engelhardt, J.F.; Irani, K.; et al. Stat3 confers resistance against hypoxia/reoxygenation-induced oxidative injury in hepatocytes through upregulation of Mn-SOD. J. Hepatol. 2004, 41, 957–965. [Google Scholar] [CrossRef]
- Tacchini, L.; Cairo, G.; De Ponti, C.; Massip, M.; Rosellò-Catafau, J.; Peralta, C. Up regulation of IL-6 by ischemic preconditioning in normal and fatty rat livers: Association with reduction of oxidative stress. Free Radic. Res. 2006, 40, 1206–1217. [Google Scholar] [CrossRef]
- Guo, S.; Wharton, W.; Moseley, P.; Shi, H. Heat shock protein 70 regulates cellular redox status by modulating glutathione-related enzyme activities. Cell Stress Chaperones 2007, 12, 245–254. [Google Scholar] [CrossRef]
- Hecksteden, A.; Wegmann, M.; Steffen, A.; Kraushaar, J.; Morsch, A.; Ruppenthal, S.; Kaestner, L.; Meyer, T. Irisin and exercise training in humans-results from a randomized controlled training trial. BMC Med. 2013, 11, 235. [Google Scholar] [CrossRef]
- Korta, P.; Pocheć, E. Irisin as a Multifunctional Protein: Implications for Health and Certain Diseases. Medicina (Kaunas) 2019, 55, 485. [Google Scholar] [CrossRef]
- Colaianni, G.; Cinti, S.; Colucci, S.; Grano, M. Irisin and musculoskeletal health. Ann. N. Y. Acad. Sci. 2017, 1402, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Perakakis, N.; Triantafyllou, G.A.; Fernández-Real, J.M.; Huh, J.Y.; Park, K.H.; Seufert, J.; Mantzoros, C.S. Physiology and role of irisin in glucose homeostasis. Nat. Rev. Endocrinol. 2017, 13, 324–337. [Google Scholar] [CrossRef] [PubMed]
- Briganti, S.I.; Gaspa, G.; Tabacco, G.; Naciu, A.M.; Cesareo, R.; Manfrini, S.; Palermo, A. Irisin as a regulator of bone and glucose metabolism. J. Obes. 2018, 43, 489–500. [Google Scholar] [CrossRef]
- Rabiee, F.; Lachinani, L.; Ghaedi, S.; Nasr-Esfahani, M.H.; Megraw, T.L.; Ghaedi, K. New insights into the cellular activities of Fndc5/Irisin and its signaling pathways. Cell Biosci. 2020, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, Y.T.; Zhang, S.; Dubielecka, P.M.; Du, J.; Yano, N.; Chin, Y.E.; Zhuang, S.; Qin, G.; Zhao, T.C. Irisin plays a pivotal role to protect the heart against ischemia and reperfusion injury. J. Cell. Physiol. 2017, 232, 3775–3785. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, K.; Han, Y.; Zhu, H.; Zhou, X.; Tan, T.; Zeng, J.; Zhang, J.; Liu, Y.; Li, Y.; et al. Irisin Protects Heart Against Ischemia-Reperfusion Injury Through a SOD2-Dependent Mitochondria Mechanism. J. Cardiovasc. Pharmacol. 2018, 72, 259–269. [Google Scholar] [CrossRef]
- Xin, T.; Lu, C. Irisin activates Opa1-induced mitophagy to protect cardiomyocytes against apoptosis following myocardial infarction. Aging 2020, 12, 4474–4488. [Google Scholar] [CrossRef]
- Chen, K.; Xu, Z.; Liu, Y.; Wang, Z.; Li, Y.; Xu, X.; Chen, C.; Xia, T.; Liao, Q.; Yao, Y.; et al. Irisin protects mitochondria function during pulmonary ischemia/reperfusion injury. Sci. Transl. Med. 2017, 9, eaao6298. [Google Scholar] [CrossRef]
- Peng, J.; Deng, X.; Huang, W.; Yu, J.-H.; Wang, J.-X.; Wang, J.-P.; Yang, S.-B.; Liu, X.; Wang, L.; Zhang, Y.; et al. Irisin protects against neuronal injury induced by oxygen-glucose deprivation in part depends on the inhibition of ROS-NLRP3 inflammatory signaling pathway. Mol. Immunol. 2017, 91, 185–194. [Google Scholar] [CrossRef]
- Li, D.J.; Li, Y.H.; Yuan, H.B.; Qu, L.F.; Wang, P. The novel exercise-induced hormone irisin protects against neuronal injury via activation of the Akt and ERK1/2 signaling pathways and contributes to the neuroprotection of physical exercise in cerebral ischemia. Metab. Clin. Exp. 2017, 68, 31–42. [Google Scholar] [CrossRef]
- Du, J.; Fan, X.; Yang, B.; Chen, Y.; Liu, K.X.; Zhou, J. Irisin pretreatment ameliorates intestinal ischemia/reperfusion injury in mice through activation of the Nrf2 pathway. Int. Immunopharmacol. 2019, 73, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Zhang, J.; Ren, Y.; Du, Z.; Li, T.; Wang, T.; Zhang, L.; Wang, M.; Wu, Z.; Lv, Y.; et al. Irisin reverses intestinal epithelial barrier dysfunction during intestinal injury via binding to the integrin alphaVbeta5 receptor. J. Cell. Mol. Med. 2020, 24, 996–1009. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Zhang, J.; Ren, Y.; Du, Z.; Li, Q.; Wang, Y.; Wei, S.; Yang, L.; Zhang, J.; Liu, C.; et al. Irisin alleviates liver ischemia-reperfusion injury by inhibiting excessive mitochondrial fission, promoting mitochondrial biogenesis and decreasing oxidative stress. Redox Biol. 2019, 20, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Yang, L.; Wang, T.; Zhang, J.; Li, T.; Ren, Y.; Wang, M.; Chen, X.; Lv, Y.; Wu, R. Irisin Improves Autophagy of Aged Hepatocytes via Increasing Telomerase Activity in Liver Injury. Oxidative Med. Cell. Longev. 2020, 2020, 6946037. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ren, Y.; Bi, J.; Wang, M.; Zhang, L.; Wang, T.; Wei, S.; Mou, X.; Lv, Y.; Wu, R. Involvement of kindlin-2 in irisin’s protection against ischaemia reperfusion-induced liver injury in high-fat diet-fed mice. J. Cell. Mol. Med. 2020. [Google Scholar] [CrossRef]
- Zhang, J.; Bi, J.; Ren, Y.; Du, Z.; Li, T.; Wang, T.; Zhang, L.; Wang, M.; Wei, S.; Lv, Y.; et al. Involvement of GPX4 in irisin’s protection against ischemia reperfusion-induced acute kidney injury. J. Cell. Physiol. 2020. [Google Scholar] [CrossRef]
- Zhang, R.; Ji, J.; Zhou, X.; Li, R. Irisin Pretreatment Protects Kidneys against Acute Kidney Injury Induced by Ischemia/Reperfusion via Upregulating the Expression of Uncoupling Protein 2. BioMed Res. Int. 2020, 2020, 6537371. [Google Scholar] [CrossRef]
- Arnao, V.; Di Raimondo, D.; Tuttolomondo, A.; Pinto, A. Neurotrophic and Neuroprotective Effects of Muscle Contraction. Curr. Pharm. Des. 2016, 22, 3749–3763. [Google Scholar] [CrossRef]
- Brigadski, T.; Leßmann, V. The physiology of regulated BDNF release. Cell Tissue Res. 2020, 382, 15–45. [Google Scholar] [CrossRef]
- Pius-Sadowska, E.; Machaliński, B. BDNF—A key player in cardiovascular system. J. Mol. Cell. Cardiol. 2017, 110, 54–60. [Google Scholar] [CrossRef]
- Kermani, P.; Hempstead, B. BDNF Actions in the Cardiovascular System: Roles in Development, Adulthood and Response to Injury. Front. Physiol. 2019, 10, 455. [Google Scholar] [CrossRef]
- González-Rodríguez, P.; Ugidos, I.F.; Pérez-Rodríguez, D.; Anuncibay-Soto, B.; Santos-Galdiano, M.; Font-Belmonte, E.; Gonzalo-Orden, J.M.; Fernández-López, A. Brain-derived neurotrophic factor alleviates the oxidative stress induced by oxygen and glucose deprivation in an ex vivo brain slice model. J. Cell. Physiol. 2019, 234, 9592–9604. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, T.; Satoh, T.; Ishikawa, Y.; Nakatani, A.; Yamada, M.; Ikeuchi, T.; Hatanaka, H. Brain-derived neurotropic factor prevents superoxide anion-induced death of PC12h cells stably expressing TrkB receptor via modulation of reactive oxygen species. Neurosci. Res. 1999, 35, 9–17. [Google Scholar] [CrossRef]
- Kim, S.H.; Won, S.J.; Sohn, S.; Kwon, H.J.; Lee, J.Y.; Park, J.H.; Gwag, B.J. Brain-derived neurotrophic factor can act as a pronecrotic factor through transcriptional and translational activation of NADPH oxidase. J. Cell Biol. 2002, 159, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Usui, T.; Naruo, A.; Okada, M.; Hayabe, Y.; Yamawaki, H. Brain-derived neurotrophic factor promotes angiogenic tube formation through generation of oxidative stress in human vascular endothelial cells. Acta Physiol. (Oxf. Engl.) 2014, 211, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, S.P.; Shao, Q.; Li, P.F.; Sun, Y.; Luo, L.Z.; Yan, X.Q.; Fan, Z.Y.; Hu, J.; Zhao, J.; et al. Brain-derived neurotrophic factor mimetic, 7,8-dihydroxyflavone, protects against myocardial ischemia by rebalancing optic atrophy 1 processing. Free Radic. Biol. Med. 2019, 145, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.L.; Jin, H.; Han, X.Q.; Xia, Y.; Liu, N.F. Involvement of brain-derived neurotrophic factor in exercise-induced cardioprotection of post-myocardial infarction rats. J. Cell. Physiol. 2018, 42, 2867–2880. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, C.; Li, Y.; Chen, L.; Xiang, J. Tenacissoside H Promotes Neurological Recovery of Cerebral Ischemia-reperfusion Injury in Mice by Modulating Inflammation and Oxidative stress via TrkB Pathway. Clin. Exp. Pharmacol. Physiol. 2020. [Google Scholar] [CrossRef]
- Taliyan, R.; Ramagiri, S. Delayed neuroprotection against cerebral ischemia reperfusion injury: Putative role of BDNF and GSK-3β. J. Recept. Signal Transduct. 2016, 36, 402–410. [Google Scholar] [CrossRef]
- Okuyama, S.; Morita, M.; Sawamoto, A.; Terugo, T.; Nakajima, M.; Furukawa, Y. Edaravone enhances brain-derived neurotrophic factor production in the ischemic mouse brain. Pharmaceuticals (Basel Switz.) 2015, 8, 176–185. [Google Scholar] [CrossRef]
- Chen, N.N.; Wang, J.P.; Liu, H.F.; Zhang, M.; Zhao, Y.Z.; Fu, X.J.; Yu, L. The bone marrow mononuclear cells reduce the oxidative stress of cerebral infarction through PI3K/AKT/NRF2 signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5729–5735. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Park, J.H.; Yoo, K.Y.; Choi, J.H.; Hwang, I.K.; Ryu, P.D.; Kim, D.H.; Kwon, Y.G.; Kim, Y.M.; Won, M.H. Pre- and post-treatments with escitalopram protect against experimental ischemic neuronal damage via regulation of BDNF expression and oxidative stress. Exp. Neurol. 2011, 229, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.O.; Nabinger, P.M.; Strapasson, A.C.; Nardin, P.; Gonçalves, C.A.; Siqueira, I.R.; Netto, C.A. Long-term effects of environmental stimulation following hypoxia-ischemia on the oxidative state and BDNF levels in rat hippocampus and frontal cortex. Brain Res. 2009, 1247, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, L.; Ma, J.; Zhang, L.; Niu, F.; Feng, T.; Li, C. Auricular vagus nerve stimulation promotes functional recovery and enhances the post-ischemic angiogenic response in an ischemia/reperfusion rat model. Neurochem. Int. 2016, 97, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Kiprianova, I.; Freiman, T.M.; Desiderato, S.; Schwab, S.; Galmbacher, R.; Gillardon, F.; Spranger, M. Brain-derived neurotrophic factor prevents neuronal death and glial activation after global ischemia in the rat. J. Neurosci. Res. 1999, 56, 21–27. [Google Scholar] [CrossRef]
- Ramagiri, S.; Taliyan, R. Protective effect of remote limb post conditioning via upregulation of heme oxygenase-1/BDNF pathway in rat model of cerebral ischemic reperfusion injury. Brain Res. 2017, 1669, 44–54. [Google Scholar] [CrossRef]
- Ya, B.L.; Liu, Q.; Li, H.F.; Cheng, H.J.; Yu, T.; Chen, L.; Wang, Y.; Yuan, L.L.; Li, W.J.; Liu, W.Y.; et al. Uric Acid Protects against Focal Cerebral Ischemia/Reperfusion-Induced Oxidative Stress via Activating Nrf2 and Regulating Neurotrophic Factor Expression. Oxid. Med. Cell. Longev. 2018, 2018, 6069150. [Google Scholar] [CrossRef]
- Fanaei, H.; Karimian, S.M.; Sadeghipour, H.R.; Hassanzade, G.; Kasaeian, A.; Attari, F.; Khayat, S.; Ramezani, V.; Javadimehr, M. Testosterone enhances functional recovery after stroke through promotion of antioxidant defenses, BDNF levels and neurogenesis in male rats. Brain Res. 2014, 1558, 74–83. [Google Scholar] [CrossRef]
- Otsuka, S.; Sakakima, H.; Sumizono, M.; Takada, S.; Terashi, T.; Yoshida, Y. The neuroprotective effects of preconditioning exercise on brain damage and neurotrophic factors after focal brain ischemia in rats. Behav. Brain Res. 2016, 303, 9–18. [Google Scholar] [CrossRef]
- Sukhanova, I.A.; Sebentsova, E.A.; Khukhareva, D.D.; Manchenko, D.M.; Glazova, N.Y.; Vishnyakova, P.A.; Inozemtseva, L.S.; Dolotov, O.V.; Vysokikh, M.Y.; Levitskaya, N.G. Gender-dependent changes in physical development, BDNF content and GSH redox system in a model of acute neonatal hypoxia in rats. Behav. Brain Res. 2018, 350, 87–98. [Google Scholar] [CrossRef]
- Ouchi, N.; Oshima, Y.; Ohashi, K.; Higuchi, A.; Ikegami, C.; Izumiya, Y.; Walsh, K. Follistatin-like 1, a Secreted Muscle Protein, Promotes Endothelial Cell Function and Revascularization in Ischemic Tissue through a Nitric-oxide Synthase-dependent Mechanism. J. Biol. Chem. 2008, 283, 32802–32811. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-Q.; Chen, H.; Zhai, C.-L.; Tu, J.-F.; Shen, Y.; Pang, L.-X.; Xu, Q.-R. Cardioprotective role of mesenchymal stem cell-secreted follistatin-like protein 1 in a rat model of ischemia/reperfusion injury. Int. J. Clin. Exp. Pathol. 2016, 9, 3083–3093. [Google Scholar]
- Cong, W.-T.; Ling, J.; Tian, H.-S.; Ling, R.; Wang, Y.; Huang, B.-B.; Zhao, T.; Duan, Y.-M.; Jin, L.-T.; Li, X.K. Proteomic study on the protective mechanism of fibroblast growth factor 21 to ischemia-reperfusion injury. Can. J. Physiol. Pharmacol. 2013, 91, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Alan, C.; Kocoglu, H.; AltIntas, R.; AlIcI, B.; Resit Ersay, A. Protective effect of decorin on acute ischaemia-reperfusion injury in the rat kidney. Arch. Med. Sci. 2011, 7, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Pi, Y.; Goldenthal, M.J.; Marín-García, J. Mitochondrial involvement in IGF-1 induced protection of cardiomyocytes against hypoxia/reoxygenation injury. Mol. Cell. Biochem. 2007, 301, 181–189. [Google Scholar] [CrossRef]
- Nelson, S.K.; Wong, G.H.; McCord, J.M. Leukemia inhibitory factor and tumor necrosis factor induce manganese superoxide dismutase and protect rabbit hearts from reperfusion injury. J. Mol. Cell. Cardiol. 1995, 27, 223–229. [Google Scholar] [CrossRef]
- Rowe, D.D.; Collier, L.A.; Seifert, H.A.; Chapman, C.B.; Leonardo, C.C.; Willing, A.E.; Pennypacker, K.R. Leukemia inhibitor factor promotes functional recovery and oligodendrocyte survival in rat models of focal ischemia. Eur. J. Neurosci. 2014, 40, 3111–3119. [Google Scholar] [CrossRef]
- Davis, S.M.; Collier, L.A.; Leonardo, C.C.; Seifert, H.A.; Ajmo, C.T.; Pennypacker, K.R. Leukemia Inhibitory Factor Protects Neurons from Ischemic Damage via Upregulation of Superoxide Dismutase 3. Mol. Neurobiol. 2017, 54, 608–622. [Google Scholar] [CrossRef]
- Tan, X.-H.; Zheng, X.-M.; Yu, L.-X.; He, J.; Zhu, H.-M.; Ge, X.-P.; Ren, X.-L.; Ye, F.-Q.; Bellusci, S.; Xiao, J.; et al. Fibroblast growth factor 2 protects against renal ischaemia/reperfusion injury by attenuating mitochondrial damage and proinflammatory signalling. J. Cell. Mol. Med. 2017, 21, 2909–2925. [Google Scholar] [CrossRef]
- Shibanuma, M.; Mashimo, J.; Mita, A.; Kuroki, T.; Nose, K. Cloning from a mouse osteoblastic cell line of a set of transforming-growth-factor-beta 1-regulated genes, one of which seems to encode a follistatin-related polypeptide. Eur. J. Biochem. 1993, 217, 13–19. [Google Scholar] [CrossRef]
- Görgens, S.W.; Raschke, S.; Holven, K.B.; Jensen, J.; Eckardt, K.; Eckel, J. Regulation of follistatin-like protein 1 expression and secretion in primary human skeletal muscle cells. Arch. Physiol. Biochem. 2013, 119, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Kon, M.; Ebi, Y.; Nakagaki, K. Effects of acute sprint interval exercise on follistatin-like 1 and apelin secretions. Arch. Physiol. Biochem. 2019, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Norheim, F.; Raastad, T.; Thiede, B.; Rustan, A.C.; Drevon, C.A.; Haugen, F. Proteomic identification of secreted proteins from human skeletal muscle cells and expression in response to strength training. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E1013–E1021. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Serpooshan, V.; Hurtado, C.; Diez-Cuñado, M.; Zhao, M.; Maruyama, S.; Zhu, W.; Fajardo, G.; Noseda, M.; Nakamura, K.; et al. Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature 2015, 525, 479–485. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, W.; Liu, J.; Li, J.; Wang, J.; Zhang, Y.; Zhang, Z.; Liu, Y.; Jin, Y.; Li, J.; et al. Follistatin-like 1 protects against hypoxia-induced pulmonary hypertension in mice. Sci. Rep. 2017, 7, 45820. [Google Scholar] [CrossRef]
- Yang, W.; Duan, Q.; Zhu, X.; Tao, K.; Dong, A. Follistatin-Like 1 Attenuates Ischemia/Reperfusion Injury in Cardiomyocytes via Regulation of Autophagy. Available online: https://www.hindawi.com/journals/bmri/2019/9537382/ (accessed on 3 November 2020).
- Xi, Y.; Gong, D.-W.; Tian, Z. FSTL1 as a Potential Mediator of Exercise-Induced Cardioprotection in Post-Myocardial Infarction Rats. Sci. Rep. 2016, 6, 32424. [Google Scholar] [CrossRef]
- Xi, Y.; Hao, M.; Tian, Z. Resistance Exercise Increases the Regulation of Skeletal Muscle FSTL1 Consequently Improving Cardiac Angiogenesis in Rats with Myocardial Infarctions. J. Sci. Sport Exerc. 2019, 1, 78–87. [Google Scholar] [CrossRef]
- Oshima, Y.; Ouchi, N.; Sato, K.; Izumiya, Y.; Pimentel, D.R.; Walsh, K. Follistatin-Like 1 Is an Akt-Regulated Cardioprotective Factor That Is Secreted by the Heart. Circulation 2008, 117, 3099–3108. [Google Scholar] [CrossRef]
- Ogura, Y.; Ouchi, N.; Ohashi, K.; Shibata, R.; Kataoka, Y.; Kambara, T.; Kito, T.; Maruyama, S.; Yuasa, D.; Matsuo, K.; et al. Therapeutic Impact of Follistatin-Like 1 on Myocardial Ischemic Injury in Preclinical Models. Circulation 2012, 126, 1728–1738. [Google Scholar] [CrossRef]
- Hayakawa, S.; Ohashi, K.; Shibata, R.; Kataoka, Y.; Miyabe, M.; Enomoto, T.; Joki, Y.; Shimizu, Y.; Kambara, T.; Uemura, Y.; et al. Cardiac myocyte-derived follistatin-like 1 prevents renal injury in a subtotal nephrectomy model. J. Am. Soc. Nephrol. JASN 2015, 26, 636–646. [Google Scholar] [CrossRef]
- Maruyama, S.; Nakamura, K.; Papanicolaou, K.N.; Sano, S.; Shimizu, I.; Asaumi, Y.; van den Hoff, M.J.; Ouchi, N.; Recchia, F.A.; Walsh, K. Follistatin-like 1 promotes cardiac fibroblast activation and protects the heart from rupture. EMBO Mol. Med. 2016, 8, 949–966. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, J.; Zhang, W.; Peng, M.; Chen, J.; Zheng, L.; Zhang, X.; Yang, H.; Liu, Y. Follistatin-Like 1 Protects against Doxorubicin-Induced Cardiomyopathy through Upregulation of Nrf2. Oxid. Med. Cell. Longev. 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, S.; Ohashi, K.; Shibata, R.; Takahashi, R.; Otaka, N.; Ogawa, H.; Ito, M.; Kanemura, N.; Hiramatsu-Ito, M.; Ikeda, N.; et al. Association of Circulating Follistatin-Like 1 Levels with Inflammatory and Oxidative Stress Markers in Healthy Men. PLoS ONE 2016, 11, e0153619. [Google Scholar] [CrossRef] [PubMed]
- Kharitonenkov, A.; Shiyanova, T.L.; Koester, A.; Ford, A.M.; Micanovic, R.; Galbreath, E.J.; Sandusky, G.E.; Hammond, L.J.; Moyers, J.S.; Owens, R.A.; et al. FGF-21 as a novel metabolic regulator. J. Clin. Investig. 2005, 115, 1627–1635. [Google Scholar] [CrossRef]
- Nishimura, T.; Nakatake, Y.; Konishi, M.; Itoh, N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim. Biophys. Acta 2000, 1492, 203–206. [Google Scholar] [CrossRef]
- Izumiya, Y.; Bina, H.A.; Ouchi, N.; Akasaki, Y.; Kharitonenkov, A.; Walsh, K. FGF21 is an Akt-regulated myokine. FEBS Lett. 2008, 582, 3805–3810. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, S.H.; Min, Y.-K.; Yang, H.-M.; Lee, J.-B.; Lee, M.-S. Acute exercise induces FGF21 expression in mice and in healthy humans. PLoS ONE 2013, 8, e63517. [Google Scholar] [CrossRef]
- Cuevas-Ramos, D.; Almeda-Valdés, P.; Meza-Arana, C.E.; Brito-Córdova, G.; Gómez-Pérez, F.J.; Mehta, R.; Oseguera-Moguel, J.; Aguilar-Salinas, C.A. Exercise Increases Serum Fibroblast Growth Factor 21 (FGF21) Levels. PLoS ONE 2012, 7, e38022. [Google Scholar] [CrossRef]
- Ye, D.; Li, H.; Wang, Y.; Jia, W.; Zhou, J.; Fan, J.; Man, K.; Lo, C.; Wong, C.; Wang, Y.; et al. Circulating Fibroblast Growth Factor 21 Is A Sensitive Biomarker for Severe Ischemia/reperfusion Injury in Patients with Liver Transplantation. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Planavila, A.; Redondo-Angulo, I.; Ribas, F.; Garrabou, G.; Casademont, J.; Giralt, M.; Villarroya, F. Fibroblast growth factor 21 protects the heart from oxidative stress. Cardiovasc. Res. 2015, 106, 19–31. [Google Scholar] [CrossRef]
- Penela, P.; Inserte, J.; Ramos, P.; Rodriguez-Sinovas, A.; Garcia-Dorado, D.; Mayor, F. Degradation of GRK2 and AKT is an early and detrimental event in myocardial ischemia/reperfusion. EBioMedicine 2019, 48, 605–618. [Google Scholar] [CrossRef]
- Liu, S.Q.; Roberts, D.; Kharitonenkov, A.; Zhang, B.; Hanson, S.M.; Li, Y.C.; Zhang, L.-Q.; Wu, Y.H. Endocrine Protection of Ischemic Myocardium by FGF21 from the Liver and Adipose Tissue. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Q.; Tefft, B.J.; Roberts, D.T.; Zhang, L.-Q.; Ren, Y.; Li, Y.C.; Huang, Y.; Zhang, D.; Phillips, H.R.; Wu, Y.H. Cardioprotective proteins upregulated in the liver in response to experimental myocardial ischemia. Am. J. Physiology. Heart Circ. Physiol. 2012, 303, H1446–H1458. [Google Scholar] [CrossRef] [PubMed]
- Planavila, A.; Iglesias, R.; Giralt, M.; Villarroya, F. Sirt1 acts in association with PPARα to protect the heart from hypertrophy, metabolic dysregulation, and inflammation. Cardiovasc. Res. 2011, 90, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-P.; Zhai, P.; Yamamoto, T.; Maejima, Y.; Matsushima, S.; Hariharan, N.; Shao, D.; Takagi, H.; Oka, S.; Sadoshima, J. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation 2010, 122, 2170–2182. [Google Scholar] [CrossRef]
- Vinciguerra, M.; Santini, M.P.; Martinez, C.; Pazienza, V.; Claycomb, W.C.; Giuliani, A.; Rosenthal, N. mIGF-1/JNK1/SirT1 signaling confers protection against oxidative stress in the heart. Aging Cell 2012, 11, 139–149. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Zhang, Z.; Liu, Q.; Gu, J. Cardioprotective effects of fibroblast growth factor 21 against doxorubicin-induced toxicity via the SIRT1/LKB1/AMPK pathway. Cell Death Dis. 2017, 8, e3018. [Google Scholar] [CrossRef]
- Li, S.; Zhu, Z.; Xue, M.; Yi, X.; Liang, J.; Niu, C.; Chen, G.; Shen, Y.; Zhang, H.; Zheng, J.; et al. Fibroblast growth factor 21 protects the heart from angiotensin II-induced cardiac hypertrophy and dysfunction via SIRT1. Biochim. Biophys. Acta. Mol. Basis Dis. 2019, 1865, 1241–1252. [Google Scholar] [CrossRef]
- Furusawa, Y.; Uruno, A.; Yagishita, Y.; Higashi, C.; Yamamoto, M. Nrf2 induces fibroblast growth factor 21 in diabetic mice. Genes Cells 2014, 19, 864–878. [Google Scholar] [CrossRef]
- Yu, Y.; Bai, F.; Liu, Y.; Yang, Y.; Yuan, Q.; Zou, D.; Qu, S.; Tian, G.; Song, L.; Zhang, T.; et al. Fibroblast growth factor (FGF21) protects mouse liver against D-galactose-induced oxidative stress and apoptosis via activating Nrf2 and PI3K/Akt pathways. Mol. Cell. Biochem. 2015, 403, 287–299. [Google Scholar] [CrossRef]
- Yan, X.; Chen, J.; Zhang, C.; Zhou, S.; Zhang, Z.; Chen, J.; Feng, W.; Li, X.; Tan, Y. FGF21 deletion exacerbates diabetic cardiomyopathy by aggravating cardiac lipid accumulation. J. Cell. Mol. Med. 2015, 19, 1557–1568. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Feng, A.; Lin, S.; Yu, L.; Lin, X.; Yan, X.; Lu, X.; Zhang, C. Fibroblast growth factor-21 prevents diabetic cardiomyopathy via AMPK-mediated antioxidation and lipid-lowering effects in the heart. Cell Death Dis. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Huang, Z.; Gu, J.; Yan, X.; Lu, X.; Zhou, S.; Wang, S.; Shao, M.; Zhang, F.; Cheng, P.; et al. Fibroblast growth factor 21 protects the heart from apoptosis in a diabetic mouse model via extracellular signal-regulated kinase 1/2-dependent signalling pathway. Diabetologia 2015, 58, 1937–1948. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhang, J.; Guo, W.; Li, F.; Sun, W.; Chen, J.; Zhang, C.; Lu, X.; Tan, Y.; Feng, W.; et al. Up-regulation of Nrf2 is involved in FGF21 mediated fenofibrate protection against type 1 diabetic nephropathy. Free Radic. Biol. Med. 2016, 93, 94–109. [Google Scholar] [CrossRef]
- Hu, S.; Cao, S.; Liu, J. Role of angiopoietin-2 in the cardioprotective effect of fibroblast growth factor 21 on ischemia/reperfusion-induced injury in H9c2 cardiomyocytes. Exp. Ther. Med. 2017, 14, 771–779. [Google Scholar] [CrossRef]
- Hu, S.; Cao, S.; Tong, Z.; Liu, J. FGF21 protects myocardial ischemia-reperfusion injury through reduction of miR-145-mediated autophagy. Am. J. Transl. Res. 2018, 10, 3677–3688. [Google Scholar]
- Ren, Z.; Xiao, W.; Zeng, Y.; Liu, M.-H.; Li, G.-H.; Tang, Z.-H.; Qu, S.-L.; Hao, Y.-M.; Yuan, H.-Q.; Jiang, Z.-S. Fibroblast growth factor-21 alleviates hypoxia/reoxygenation injury in H9c2 cardiomyocytes by promoting autophagic flux. Int. J. Mol. Med. 2019, 43, 1321–1330. [Google Scholar] [CrossRef]
- Liang, P.; Zhong, L.; Gong, L.; Wang, J.; Zhu, Y.; Liu, W.; Yang, J. Fibroblast growth factor 21 protects rat cardiomyocytes from endoplasmic reticulum stress by promoting the fibroblast growth factor receptor 1-extracellular signal-regulated kinase 1/2 signaling pathway. Int. J. Mol. Med. 2017, 40, 1477–1485. [Google Scholar] [CrossRef]
- Wan, X.-S.; Lu, X.-H.; Xiao, Y.-C.; Lin, Y.; Zhu, H.; Ding, T.; Yang, Y.; Huang, Y.; Zhang, Y.; Liu, Y.-L.; et al. ATF4- and CHOP-dependent induction of FGF21 through endoplasmic reticulum stress. BioMed Res. Int. 2014, 2014, 807874. [Google Scholar] [CrossRef]
- Kanzleiter, T.; Rath, M.; Görgens, S.W.; Jensen, J.; Tangen, D.S.; Kolnes, A.J.; Kolnes, K.J.; Lee, S.; Eckel, J.; Schürmann, A.; et al. The myokine decorin is regulated by contraction and involved in muscle hypertrophy. Biochem. Biophys. Res. Commun. 2014, 450, 1089–1094. [Google Scholar] [CrossRef]
- Brandan, E.; Fuentes, M.E.; Andrade, W. The proteoglycan decorin is synthesized and secreted by differentiated myotubes. Eur. J. Cell Biol. 1991, 55, 209–216. [Google Scholar] [PubMed]
- Marta, S.; Renata, G.; Kamilla, G.; Laszlo, D.; Csaba, C.; Tamas, C. Effects of Proteoglycans on Oxidative/Nitrative Stress. Curr. Org. Chem. 2017, 21, 2117–2124. [Google Scholar] [CrossRef][Green Version]
- Gáspár, R.; Gömöri, K.; Kiss, B.; Szántai, Á.; Pálóczi, J.; Varga, Z.V.; Pipis, J.; Váradi, B.; Ágg, B.; Csont, T.; et al. Decorin Protects Cardiac Myocytes against Simulated Ischemia/Reperfusion Injury. Molecules 2020, 25, 3426. [Google Scholar] [CrossRef]
- Özay, R.; Türkoğlu, E.; Gürer, B.; Dolgun, H.; Evirgen, O.; Ergüder, B.İ.; Hayırlı, N.; Gürses, L.; Şekerci, Z.; Yılmaz, E.R. Does Decorin Protect Neuronal Tissue via Its Antioxidant and Antiinflammatory Activity from Traumatic Brain Injury? An Experimental Study. World Neurosurg. 2017, 97, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Chen, F.; Chen, J.; Ruan, G.; He, M.; Chen, C.; Tang, J.; Wang, D.W. Overexpression of decorin promoted angiogenesis in diabetic cardiomyopathy via IGF1R-AKT-VEGF signaling. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Seldin, M.M.; Peterson, J.M.; Byerly, M.S.; Wei, Z.; Wong, G.W. Myonectin (CTRP15), a Novel Myokine That Links Skeletal Muscle to Systemic Lipid Homeostasis. J. Biol. Chem. 2012, 287, 11968–11980. [Google Scholar] [CrossRef] [PubMed]
- Pourranjbar, M.; Arabnejad, N.; Naderipour, K.; Rafie, F. Effects of Aerobic Exercises on Serum Levels of Myonectin and Insulin Resistance in Obese and Overweight Women. J. Med. Life 2018, 11, 381–386. [Google Scholar] [CrossRef]
- Otaka, N.; Shibata, R.; Ohashi, K.; Uemura, Y.; Kambara, T.; Enomoto, T.; Ogawa, H.; Ito, M.; Kawanishi, H.; Maruyama, S.; et al. Myonectin Is an Exercise-Induced Myokine That Protects the Heart From Ischemia-Reperfusion Injury. Circ. Res. 2018, 123, 1326–1338. [Google Scholar] [CrossRef]
- Suidasari, S.; Uragami, S.; Yanaka, N.; Kato, N. Dietary vitamin B6 modulates the gene expression of myokines, Nrf2-related factors, myogenin and HSP60 in the skeletal muscle of rats. Exp. Ther. Med. 2017, 14, 3239–3246. [Google Scholar] [CrossRef]
- Liu, M.; Stevens-Lapsley, J.E.; Jayaraman, A.; Ye, F.; Conover, C.; Walter, G.A.; Bose, P.; Thompson, F.J.; Borst, S.E.; Vandenborne, K. Impact of treadmill locomotor training on skeletal muscle IGF1 and myogenic regulatory factors in spinal cord injured rats. Eur. J. Appl. Physiol. 2010, 109, 709–720. [Google Scholar] [CrossRef]
- Dieli-Conwright, C.M.; Kiwata, J.L.; Tuzon, C.T.; Spektor, T.M.; Sattler, F.R.; Rice, J.C.; Schroeder, E.T. Acute Response of PGC-1α and IGF-1 Isoforms to Maximal Eccentric Exercise in Skeletal Muscle of Postmenopausal Women. J. Strength Cond. Res. 2016, 30, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Żebrowska, A.; Sikora, M.; Konarska, A.; Zwierzchowska, A.; Kamiński, T.; Robins, A.; Hall, B. Moderate intensity exercise in hypoxia increases IGF-1 bioavailability and serum irisin in individuals with type 1 diabetes. Ther. Adv. Endocrinol. 2020, 11, 2042018820925326. [Google Scholar] [CrossRef] [PubMed]
- Pena, G.S.; Paez, H.G.; Johnson, T.K.; Halle, J.L.; Carzoli, J.P.; Visavadiya, N.P.; Zourdos, M.C.; Whitehurst, M.A.; Khamoui, A.V. Hippocampal Growth Factor and Myokine Cathepsin B Expression Following Aerobic and Resistance Training in 3xTg-AD Mice. Available online: https://www.hindawi.com/journals/ijcd/2020/5919501/ (accessed on 21 October 2020).
- Ascenzi, F.; Barberi, L.; Dobrowolny, G.; Bacurau, A.V.N.; Nicoletti, C.; Rizzuto, E.; Rosenthal, N.; Scicchitano, B.M.; Musarò, A. Effects of IGF-1 isoforms on muscle growth and sarcopenia. Aging Cell 2019, 18, e12954. [Google Scholar] [CrossRef] [PubMed]
- Kajstura, J.; Fiordaliso, F.; Andreoli, A.M.; Li, B.; Chimenti, S.; Medow, M.S.; Limana, F.; Nadal-Ginard, B.; Leri, A.; Anversa, P. IGF-1 overexpression inhibits the development of diabetic cardiomyopathy and angiotensin II-mediated oxidative stress. Diabetes 2001, 50, 1414–1424. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Downs, L.C.; Mitschelen, M.; Sosnowska, D.; Toth, P.; Pinto, J.T.; Ballabh, P.; Valcarcel-Ares, M.N.; Farley, J.; Koller, A.; Henthorn, J.C.; et al. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: A novel model of vascular aging. J. Gerontol. Biol. Sci. Med. Sci. 2012, 67, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, X.; Sreejayan, N.; Ren, J. Insulin-like growth factor I deficiency prolongs survival and antagonizes paraquat-induced cardiomyocyte dysfunction: Role of oxidative stress. Rejuvenation Res. 2007, 10, 501–512. [Google Scholar] [CrossRef]
- Liao, Y.; Li, H.; Pi, Y.; Li, Z.; Jin, S. Cardioprotective effect of IGF-1 against myocardial ischemia/reperfusion injury through activation of PI3K/Akt pathway in rats in vivo. J. Int. Med. Res. 2019, 47, 3886–3897. [Google Scholar] [CrossRef]
- Song, C.-L.; Liu, B.; Diao, H.-Y.; Shi, Y.-F.; Zhang, J.-C.; Li, Y.-X.; Liu, N.; Yu, Y.-P.; Wang, G.; Wang, J.-P.; et al. Down-regulation of microRNA-320 suppresses cardiomyocyte apoptosis and protects against myocardial ischemia and reperfusion injury by targeting IGF-1. Oncotarget 2016, 7, 39740–39757. [Google Scholar] [CrossRef]
- Tang, S.; Zhong, H.; Xiong, T.; Yang, X.; Mao, Y.; Wang, D. MiR-489 aggravates H2O2-induced apoptosis of cardiomyocytes via inhibiting IGF1. Biosci. Rep. 2020, 40. [Google Scholar] [CrossRef]
- Tejada, T.; Tan, L.; Torres, R.A.; Calvert, J.W.; Lambert, J.P.; Zaidi, M.; Husain, M.; Berce, M.D.; Naib, H.; Pejler, G.; et al. IGF-1 degradation by mouse mast cell protease 4 promotes cell death and adverse cardiac remodeling days after a myocardial infarction. Proc. Natl. Acad. Sci. USA 2016, 113, 6949–6954. [Google Scholar] [CrossRef]
- Buerke, M.; Murohara, T.; Skurk, C.; Nuss, C.; Tomaselli, K.; Lefer, A.M. Cardioprotective effect of insulin-like growth factor I in myocardial ischemia followed by reperfusion. Proc. Natl. Acad. Sci. USA 1995, 92, 8031–8035. [Google Scholar] [CrossRef] [PubMed]
- Davani, E.Y.; Brumme, Z.; Singhera, G.K.; Côté, H.C.F.; Harrigan, P.R.; Dorscheid, D.R. Insulin-like growth factor-1 protects ischemic murine myocardium from ischemia/reperfusion associated injury. Crit. Care 2003, 7, R176–R183. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Kajstura, J.; Discher, D.J.; Wasserlauf, B.J.; Bishopric, N.H.; Anversa, P.; Webster, K.A. Reperfusion-activated Akt kinase prevents apoptosis in transgenic mouse hearts overexpressing insulin-like growth factor-1. Circ. Res. 2001, 88, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Heinen, A.; Nederlof, R.; Panjwani, P.; Spychala, A.; Tschaidse, T.; Reffelt, H.; Boy, J.; Raupach, A.; Gödecke, S.; Petzsch, P.; et al. IGF1 Treatment Improves Cardiac Remodeling after Infarction by Targeting Myeloid Cells. Mol. Ther. 2019, 27, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Vinciguerra, M.; Santini, M.P.; Claycomb, W.C.; Ladurner, A.G.; Rosenthal, N. Local IGF-1 isoform protects cardiomyocytes from hypertrophic and oxidative stresses via SirT1 activity. Aging (Albany NY) 2009, 2, 43–62. [Google Scholar] [CrossRef] [PubMed]
- Broholm, C.; Mortensen, O.H.; Nielsen, S.; Akerstrom, T.; Zankari, A.; Dahl, B.; Pedersen, B.K. Exercise induces expression of leukaemia inhibitory factor in human skeletal muscle. J. Physiol. 2008, 586, 2195–2201. [Google Scholar] [CrossRef]
- Kanda, M.; Nagai, T.; Takahashi, T.; Liu, M.L.; Kondou, N.; Naito, A.T.; Akazawa, H.; Sashida, G.; Iwama, A.; Komuro, I.; et al. Leukemia Inhibitory Factor Enhances Endogenous Cardiomyocyte Regeneration after Myocardial Infarction. PLoS ONE 2016, 11, e0156562. [Google Scholar] [CrossRef]
- Tian, L.; Zhu, W.; Liu, Y.; Gong, Y.; Lv, A.; Wang, Z.; Ding, X.; Li, S.; Fu, Y.; Lin, Y.; et al. Neural Stem Cells Transfected with Leukemia Inhibitory Factor Promote Neuroprotection in a Rat Model of Cerebral Ischemia. Neurosci. Bull. 2019, 35, 901–908. [Google Scholar] [CrossRef]
- Han, Y.; Xu, J.; Li, Z.; Yang, Z. Neuroprotective Effect of Leukemia Inhibitory Factor on Antimycin A-Induced Oxidative Injury in Differentiated PC12 Cells. J. Mol. Neurosci. 2013, 50, 577–585. [Google Scholar] [CrossRef]
- Xu, J.; Li, Z.; Xu, P.; Yang, Z. Protective effects of leukemia inhibitory factor against oxidative stress during high glucose-induced apoptosis in podocytes. Cell Stress Chaperones 2012, 17, 485–493. [Google Scholar] [CrossRef]
- Mignatti, P.; Morimoto, T.; Rifkin, D.B. Basic fibroblast growth factor, a protein devoid of secretory signal sequence, is released by cells via a pathway independent of the endoplasmic reticulum-Golgi complex. J. Cell. Physiol. 1992, 151, 81–93. [Google Scholar] [CrossRef]
- Florkiewicz, R.Z.; Majack, R.A.; Buechler, R.D.; Florkiewicz, E. Quantitative export of FGF-2 occurs through an alternative, energy-dependent, non-ER/Golgi pathway. J. Cell. Physiol. 1995, 162, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.S.; Khakee, R.; McNeil, P.L. Loss of cytoplasmic basic fibroblast growth factor from physiologically wounded myofibers of normal and dystrophic muscle. J. Cell Sci. 1993, 106 Pt 1, 121–133. [Google Scholar]
- Choi, J.S.; Yoon, H.I.; Lee, K.S.; Choi, Y.C.; Yang, S.H.; Kim, I.-S.; Cho, Y.W. Exosomes from differentiating human skeletal muscle cells trigger myogenesis of stem cells and provide biochemical cues for skeletal muscle regeneration. J. Control. Release 2016, 222, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, M.W.; McNeil, P.L.; Patterson, S.L. Role of muscle-derived growth factors in bone formation. J. Musculoskelet. Neuronal Interact. 2010, 10, 64–70. [Google Scholar] [PubMed]
- Breen, E.C.; Johnson, E.C.; Wagner, H.; Tseng, H.M.; Sung, L.A.; Wagner, P.D. Angiogenic growth factor mRNA responses in muscle to a single bout of exercise. J. Appl. Physiol. 1996, 81, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, F.; Sontag, D.P.; Fandrich, R.R.; Kardami, E.; Cattini, P.A. Overexpression of FGF-2 increases cardiac myocyte viability after injury in isolated mouse hearts. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H1039–H1050. [Google Scholar] [CrossRef]
- House, S.L.; Bolte, C.; Zhou, M.; Doetschman, T.; Klevitsky, R.; Newman, G.; Schultz, J.E.J. Cardiac-specific overexpression of fibroblast growth factor-2 protects against myocardial dysfunction and infarction in a murine model of low-flow ischemia. Circulation 2003, 108, 3140–3148. [Google Scholar] [CrossRef]
- House, S.L.; Branch, K.; Newman, G.; Doetschman, T.; Schultz, J.E.J. Cardioprotection induced by cardiac-specific overexpression of fibroblast growth factor-2 is mediated by the MAPK cascade. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H2167–H2175. [Google Scholar] [CrossRef]
- House, S.L.; Melhorn, S.J.; Newman, G.; Doetschman, T.; Schultz, J.E.J. The protein kinase C pathway mediates cardioprotection induced by cardiac-specific overexpression of fibroblast growth factor-2. Am. J. Physiology. Heart Circ. Physiol. 2007, 293, H354–H365. [Google Scholar] [CrossRef]
- Liao, S.; Porter, D.; Scott, A.; Newman, G.; Doetschman, T.; Schultz, J.E.J. The cardioprotective effect of the low molecular weight isoform of fibroblast growth factor-2: The role of JNK signaling. J. Mol. Cell. Cardiol. 2007, 42, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Bodmer, J.R.; Azhar, M.; Newman, G.; Coffin, J.D.; Doetschman, T.; Schultz, J.E.J. The influence of FGF2 high molecular weight (HMW) isoforms in the development of cardiac ischemia-reperfusion injury. J. Mol. Cell. Cardiol. 2010, 48, 1245–1254. [Google Scholar] [CrossRef]
- Manning, J.R.; Perkins, S.O.; Sinclair, E.A.; Gao, X.; Zhang, Y.; Newman, G.; Pyle, W.G.; Schultz, J.E.J. Low molecular weight fibroblast growth factor-2 signals via protein kinase C and myofibrillar proteins to protect against postischemic cardiac dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1382–H1396. [Google Scholar] [CrossRef]
- Wang, J.; Nachtigal, M.W.; Kardami, E.; Cattini, P.A. FGF-2 protects cardiomyocytes from doxorubicin damage via protein kinase C-dependent effects on efflux transporters. Cardiovasc. Res. 2013, 98, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Koleini, N.; Nickel, B.E.; Edel, A.L.; Fandrich, R.R.; Ravandi, A.; Kardami, E. Non-mitogenic FGF2 protects cardiomyocytes from acute doxorubicin-induced toxicity independently of the protein kinase CK2/heme oxygenase-1 pathway. Cell Tissue Res. 2018, 374, 607–617. [Google Scholar] [CrossRef]
- Koleini, N.; Nickel, B.E.; Wang, J.; Roveimiab, Z.; Fandrich, R.R.; Kirshenbaum, L.A.; Cattini, P.A.; Kardami, E. Fibroblast growth factor-2-mediated protection of cardiomyocytes from the toxic effects of doxorubicin requires the mTOR/Nrf-2/HO-1 pathway. Oncotarget 2017, 8, 87415–87430. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Tao, Q.; Li, G.; Xiang, L.; Zheng, X.; Zhang, T.; Wu, C.; Li, D. Fibroblast Growth Factor 2 Attenuates Renal Ischemia-Reperfusion Injury via Inhibition of Endoplasmic Reticulum Stress. Front. Cell. Dev. Biol. 2020, 8, 147. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lu, Y.; Ding, D.; Ma, Z.; Xing, X.; Hua, X.; Xu, J. Fibroblast growth factor 2 contributes to the effect of salidroside on dendritic and synaptic plasticity after cerebral ischemia/reperfusion injury. Aging (Albany NY) 2020, 12, 10951–10968. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Xu, X.; Shi, H.; Yu, X.; Wang, X.; Yan, Y.; Fu, X.; Hu, H.; Li, X.; et al. bFGF inhibits ER stress induced by ischemic oxidative injury via activation of the PI3K/Akt and ERK1/2 pathways. Toxicol. Lett. 2012, 212, 137–146. [Google Scholar] [CrossRef]
| Myokine | +Myokine | +Ischemia | +Ischemia +ROS | +Ischemia +ROI | +Ischemia +Oxidative | +Ischemia +Antiox | +Ischemia +Redox | +Ischemia +Nitric | +Ischemia +Stress | |
|---|---|---|---|---|---|---|---|---|---|---|
| BDNF | 25,624 | 77 | 1092 | 16 | 0 | 127 | 101 | 14 | 41 | 145 |
| CTSB | 6098 | 6 | 146 | 2 | 0 | 13 | 17 | 5 | 4 | 13 |
| decorin | 2883 | 23 | 23 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| FGF2 | 17,563 | 7 | 851 | 4 | 0 | 69 | 28 | 0 | 38 | 51 |
| FGF21 | 1914 | 55 | 30 | 1 | 0 | 7 | 2 | 0 | 1 | 5 |
| follistatin | 2510 | 42 | 49 | 0 | 0 | 4 | 0 | 0 | 2 | 7 |
| FSTL-1 | 386 | 12 | 18 | 0 | 0 | 2 | 0 | 0 | 1 | 6 |
| IGF-1 | 44,896 | 32 | 716 | 4 | 0 | 67 | 34 | 10 | 28 | 72 |
| IL-6 | 145,377 | 232 | 5070 | 151 | 2 | 1204 | 786 | 120 | 441 | 897 |
| IL-7 | 6671 | 12 | 23 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| IL-15 | 6248 | 95 | 22 | 0 | 0 | 1 | 0 | 0 | 1 | 5 |
| irisin | 1131 | 466 | 49 | 5 | 0 | 18 | 6 | 3 | 1 | 18 |
| LIF | 4576 | 11 | 69 | 2 | 0 | 10 | 12 | 4 | 2 | 11 |
| METRNL | 66 | 11 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| myonectin | 32 | 25 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| musclin | 35 | 8 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 |
| myostatin | 2997 | 112 | 22 | 0 | 0 | 3 | 1 | 0 | 1 | 3 |
| osteoglycin | 133 | 2 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Species | Organ/Cell | Model | IL-6 Dose, Treatment Time, Route | Effect of IL-6 on I/R Injury | Effect of IL-6 on Redox State | Ref. |
|---|---|---|---|---|---|---|
| control and IL-6−/− mice | heart | in vivo I/R | loss of function study | infarct size ↓, arrhythmia ↓ | NOS-2 ↔, COX-2 ↔ | [36] |
| rat | neonatal ventricular cardiomyocytes | in vitro sI/R | 10 ng/mL for 6 h, 24 h before SI/R | cell death ↓ | NOS-2 ↑, NOS-3 ↔, NOS-1 ↔ | [38] |
| mice | brain | in vivo I/R | 2 × 50 ng, 30 min before and 15 min after I/R, icv. | infarct size ↓ | SOD-2 ↑, protein oxidation ↓ | [40] |
| mice | primary cortical neurons | in vitro OGD | 50 ng/mL, 30 min before OGD | cell death ↓ | SOD-2 ↑, protein oxidation ↓ | [40] |
| mice | neural stem cells | in vitro OGD | 20 ng/mL 24 h before OGD | cell death ↓ | SOD-2 ↑, O2•−↓ (DHE) * | [44] |
| mice | brain transplanted with neural stem cells (NSC) | in vivo I/R | NSC cells treated with 20 ng/mL 24 h before transplantation | infarct size ↓, behavior ↑ | O2•−↓ (DHE) * | [44] |
| rat | liver | in vivo I/R | 2 × 10–500 µg/kg, 12 h and 6 h before I/R, ip. | cell death ↓ | HSP70 ↑, unfolded protein response ↓ | [45] |
| rat | primary cultured hepatocytes | in vitro sI/R | 10 ng/mL 24–72 h before H/R | cell death ↓ | SOD-2 ↑ | [46] |
| Zucker rat | fatty liver | in vivo I/R | 500 µg/kg, 12 h or 24 h before I/R, ip. | cell death ↓ | MDA ↓, GSH ↑, HO-1 ↓, NF-kB↓ | [47] |
| Species | Organ/Cell | Model | Irisin Dose, Treatment Time, Route | Effect of Irisin on I/R Injury | Effect of Irisin on Redox State | Ref. |
|---|---|---|---|---|---|---|
| mice | heart | ex vivo I/R | 100 µg/kg, 30 min before I/R, ip. | infarct size ↓, function ↑ | SOD-1 ↑ | [55] |
| rat | heart | in vivo I/R | 1 µg/kg immediately before reperfusion, iv. | infarct size ↓, function ↑ | total SOD ↑, SOD-1 ↔, SOD-2 ↑ | [56] |
| rat | H9c2 cardiomyoblasts | in vitro A/R | 100 ng/mL, during reoxygenation | cell death ↓ | ROS ↓ (DCFH-DA/DHE) * | [56] |
| N.A. | primary cardiomyocytes | in vitro H/R | N.A. | cell death ↓ | ROS ↓ (CM-H2-DCFDA) *, GSH ↑, total SOD ↑, GPx ↑ | [57] |
| control and UCP-2−/− mice | lung | in vivo I/R | 1 µg/kg immediately after ischemia, iv. | function ↑, edema ↓ | ROS ↓ (DHE) *, UCP-2 ↑ | [58] |
| human | A549 lung epithelial cell | in vitro A/R | 0.1 μg/mL immediately after anoxia | apoptosis ↓ | O2•−↓ (MitoSOX) * | [58] |
| rat | PC12 neuronal cells | in vitro OGD | 12.5–50 nmol/L before OGD | cell death ↓, apoptosis ↓ | ROS ↓ (ROS assay) *, MDA ↓, total SOD ↑ | [59] |
| mice | brain | in vivo I/R | 0.2 μg/g, 30 min after ischemia, iv. | infarct size ↓, inflammation ↓ | O2•− ↓ (superoxide assay) *, MDA ↔, 4-HNE ↓, nitrotyrosine ↓ | [60] |
| mice | intestine | in vivo I/R | 10ng/g or 100ng/g, 30 min before I/R, iv. | morphology ↑, inflammation ↓ | MDA ↓, MPO ↓, total SOD ↑, GPx ↑ | [61] |
| rat | IEC-6 intestinal epithelial cells | in vitro H/R | 10 ng/mL 24 h before hypoxia | cell death ↓ | MDA ↓, total SOD ↑, GPx ↑ | [61] |
| mice | intestine | in vivo I/R | 250 μg/kg, beginning of reperfusion, iv. | gut barrier function ↑ | 4-HNE ↓, MDA ↓, total SOD ↑, GPx ↑, XOR ↓ | [62] |
| human | Caco-2 colon cells | in vitro H/R | 10 nmol/L, beginning of reoxygenation | cell death ↓ | ROS ↓ (DHE) * | [62] |
| rat | liver | in vivo I/R | 250 μg/kg, beginning of reperfusion, iv. | cell death ↓, inflammation ↓ | MDA ↓, total SOD ↑, GPx ↑ | [63] |
| human | HL-7702 hepatocytes | in vitro H/R | 100 ng/mL, during reoxygenation | apoptosis ↓ | ROS ↓ (DHE) *, UCP-2 ↑ | [63] |
| rat | liver | in vivo I/R | 250 μg/kg, beginning of reperfusion, iv. | cell death ↓, inflammation ↓ | MDA ↓, total SOD ↑, GPx ↑ | [64] |
| mice | liver | in vivo I/R | 250 μg/kg, beginning of reperfusion, ip. | cell death ↓ | ROS ↓ (DHE) *, GPx ↑ | [65] |
| mice | kidney | in vivo I/R | 250 μg/kg, beginning of reperfusion, ip. | cell death ↓, function ↑, inflammation ↓ | ROS ↓ (DHE) *, total SOD ↑, GPx ↑ | [66] |
| mice | kidney | in vivo I/R | 10, 100, and 200 μg/kg/day for 14 days before I/R, ip. | tubular injury ↓, function ↑ | MDA ↓, UCP-2 ↑, MPO ↓, total SOD ↑ | [67] |
| Myokine | Species | Organ/Cell | Model | Myokine Dose, Treatment Time, Route | Effect of Myokine on I/R Injury | Effect of Myokine on Redox State | Ref. |
|---|---|---|---|---|---|---|---|
| BDNF | rat | brain hippocampus | ex vivo OGD | 50 ng/mL after OGD | cell death ↓ | ROS ↓ (CellRox) *, NOX ↓, GPx ↓, MDA+4-HNE ↓, TAC ↓, SOD-2 ↓ | [72] |
| rat | brain cortex | ex vivo OGD | 50 ng/mL after OGD | cell death ↓ | ROS ↔ (CellRox) *, NOX ↔, GPx ↔, MDA+4-HNE ↔, TAC↔, SOD-2↑ | [72] | |
| rat | H9c2 cardiomyoblasts | in vitro H2O2 1 | 100 µM2 24 h before H2O2 | cell death ↓ | mitochondrial O2•− ↓ (MitoSox) * | [76] | |
| FSTL-1 | mice | adductor muscle | hindlimb ischemia | skeletal muscle-derived transgenic FSTL-1 | endothelial cell function ↑, revascularization ↑ | NOS-3 ↑ | [91] |
| rat | heart | in vivo I/R | 1 × 106 mesenchymal stem cells injected into the abdomen under I/R | infarct size ↓, apoptosis ↓ | MDA ↓, total SOD ↑ | [92] | |
| FGF-21 | rat | H9C2 cardiomyoblasts | in vitro H/R | 0.25, 1 or 4 μg/mL during 6 h reoxygenation | cell death ↓, apoptosis ↓ | O2•−↓ (DHE) * | [93] |
| Decorin | rat | kidney | in vivo I/R | 100 μg/kg for 9 days after reperfusion, ip. | TGF-β1 ↓, apoptosis ↓ | lipid peroxidation ↓, total SOD ↑ | [94] |
| IGF-1 | rat | primary cardiomyocytes | in vitro H/R | 1 h pretreatment with 100 nM | mitochondrial function ↑ | MDA ↓ | [95] |
| LIF | rabbit | heart | ex vivo I/R | 5 × 107 U/mg, 24 h prior to heart isolation, iv. | tension recovery ↑ | protein carbonylation ↓, TBARS ↓, SOD-2 ↑ | [96] |
| rat | cultured oligodendrocytes | in vitro OGD | cotreatment with 200 ng/mL LIF during 24 h OGD | LDH release ↓ | Prdx4 ↑, total SOD ↓ | [97] | |
| rat | brain | in vivo I/R | 125 μg/kg at 6, 24, and 48 h after I/R, iv. | N.A. | SOD-3 ↑, total SOD ↑ | [98] | |
| rat | primary cortical neurons | in vitro OGD | pretreatment with 200 ng/mL | LDH release ↓ | SOD-3 ↑ | [98] | |
| FGF-2 | rat | kidney | in vivo I/R | 500 μg/kg 30 min prior to I/R, ip. | renal function ↑, apoptosis ↓, | 3NT ↓, 8-OHdG ↓ | [99] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabó, M.R.; Pipicz, M.; Csont, T.; Csonka, C. Modulatory Effect of Myokines on Reactive Oxygen Species in Ischemia/Reperfusion. Int. J. Mol. Sci. 2020, 21, 9382. https://doi.org/10.3390/ijms21249382
Szabó MR, Pipicz M, Csont T, Csonka C. Modulatory Effect of Myokines on Reactive Oxygen Species in Ischemia/Reperfusion. International Journal of Molecular Sciences. 2020; 21(24):9382. https://doi.org/10.3390/ijms21249382
Chicago/Turabian StyleSzabó, Márton Richárd, Márton Pipicz, Tamás Csont, and Csaba Csonka. 2020. "Modulatory Effect of Myokines on Reactive Oxygen Species in Ischemia/Reperfusion" International Journal of Molecular Sciences 21, no. 24: 9382. https://doi.org/10.3390/ijms21249382
APA StyleSzabó, M. R., Pipicz, M., Csont, T., & Csonka, C. (2020). Modulatory Effect of Myokines on Reactive Oxygen Species in Ischemia/Reperfusion. International Journal of Molecular Sciences, 21(24), 9382. https://doi.org/10.3390/ijms21249382





