Expression and Impact of Vaspin on In Vitro Oocyte Maturation through MAP3/1 and PRKAA1 Signalling Pathways
Abstract
1. Introduction
2. Results
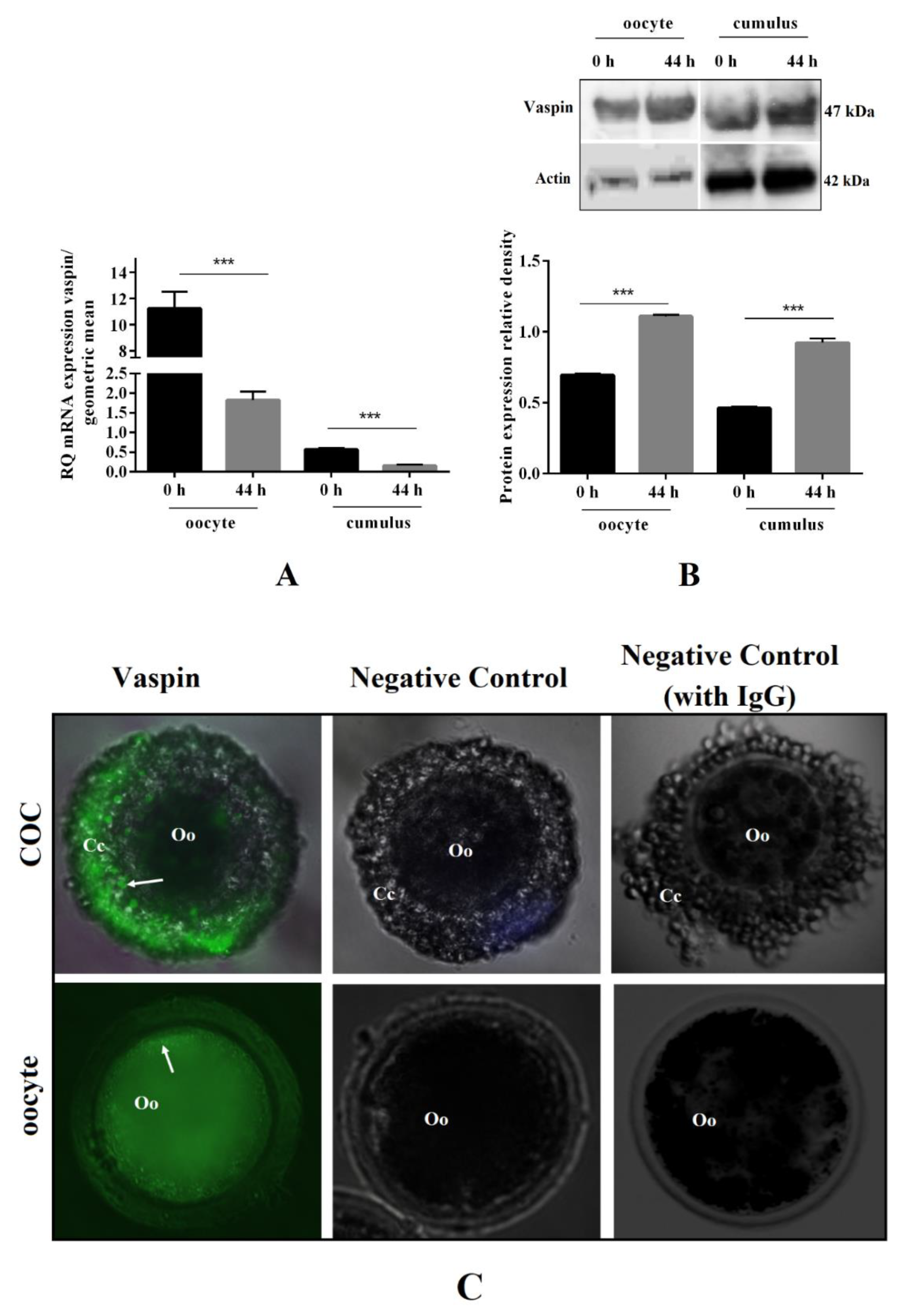
2.1. Vaspin mRNA and Protein Expression before and after In Vitro Oocyte Maturation, as well as Its Immunolocalisation in COCs
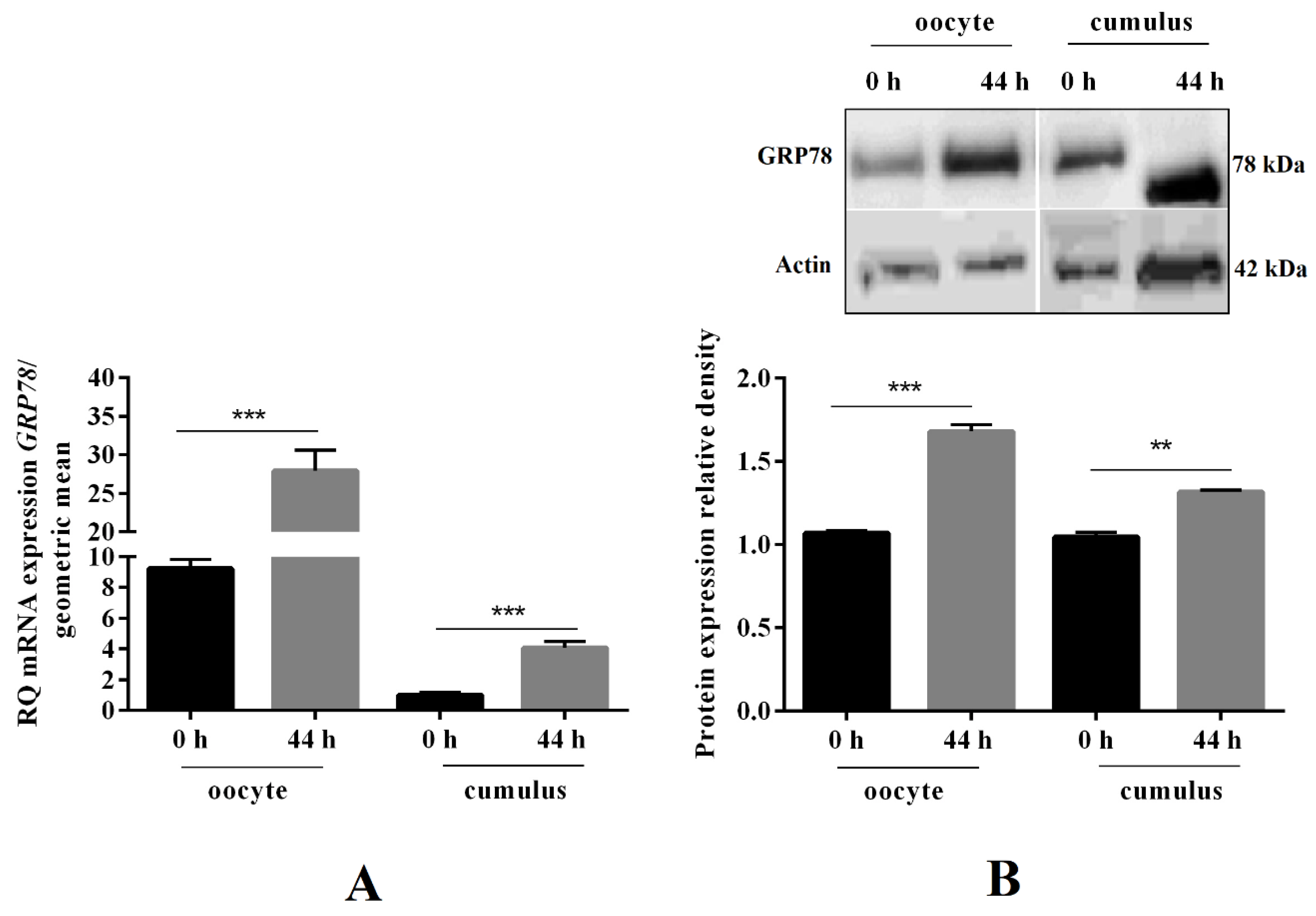
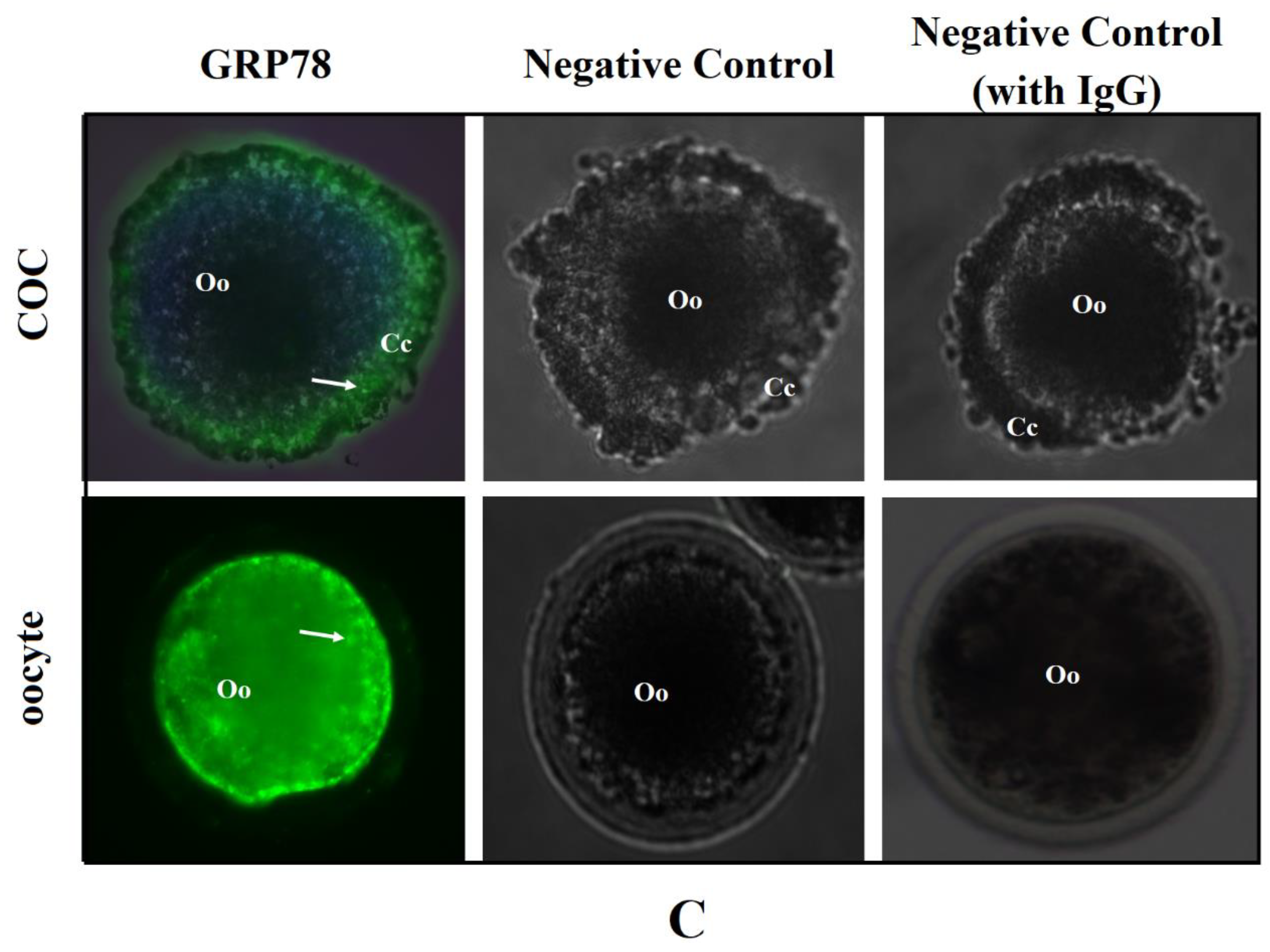
2.2. GRP78 mRNA and Protein Expression before and after Oocyte In Vitro Maturation, as well as Its Immunolocalisation in COCs
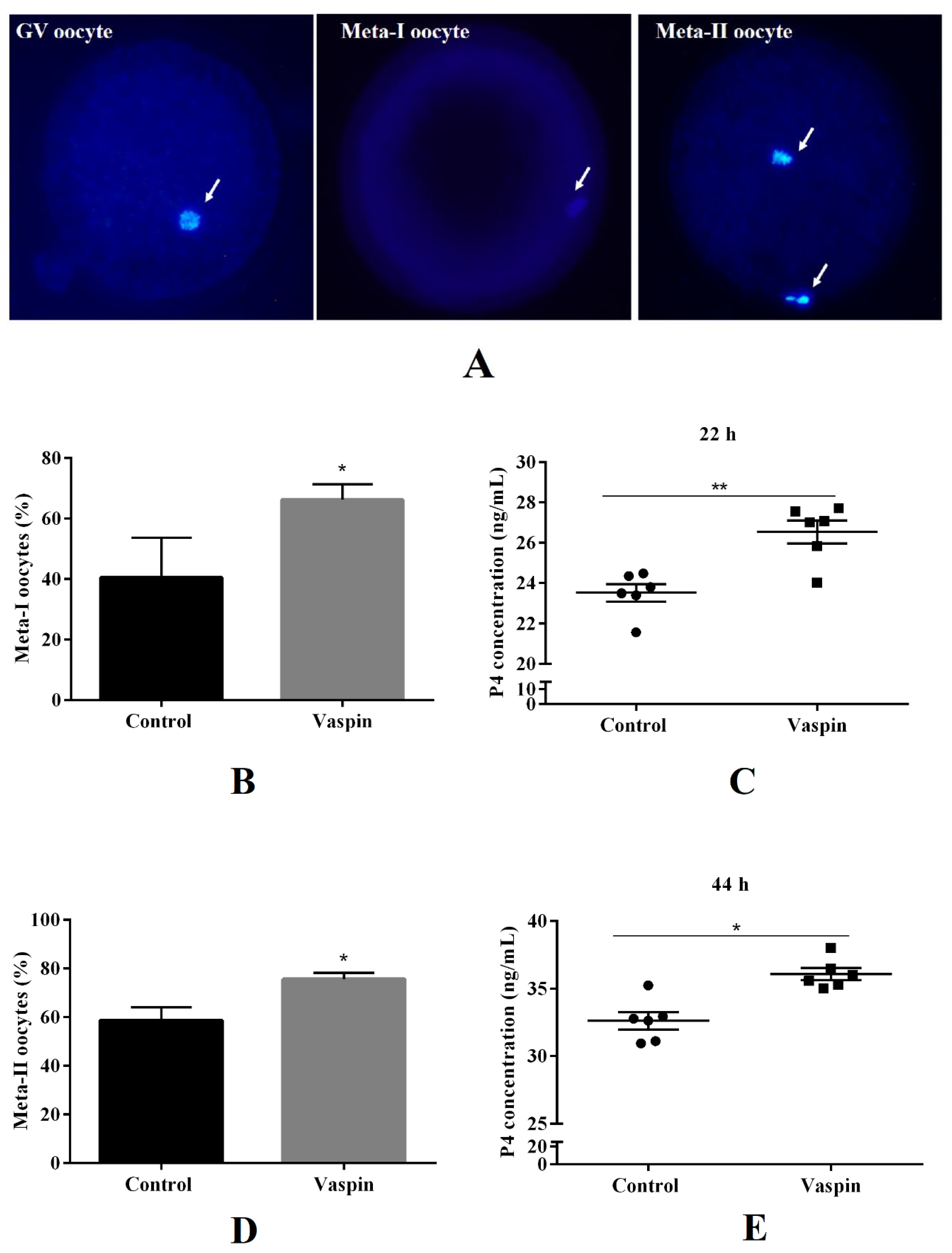
2.3. Effect of Vaspin on Nuclear In Vitro Oocyte Maturation and P4 Release in the In Vitro Oocyte Maturation Medium
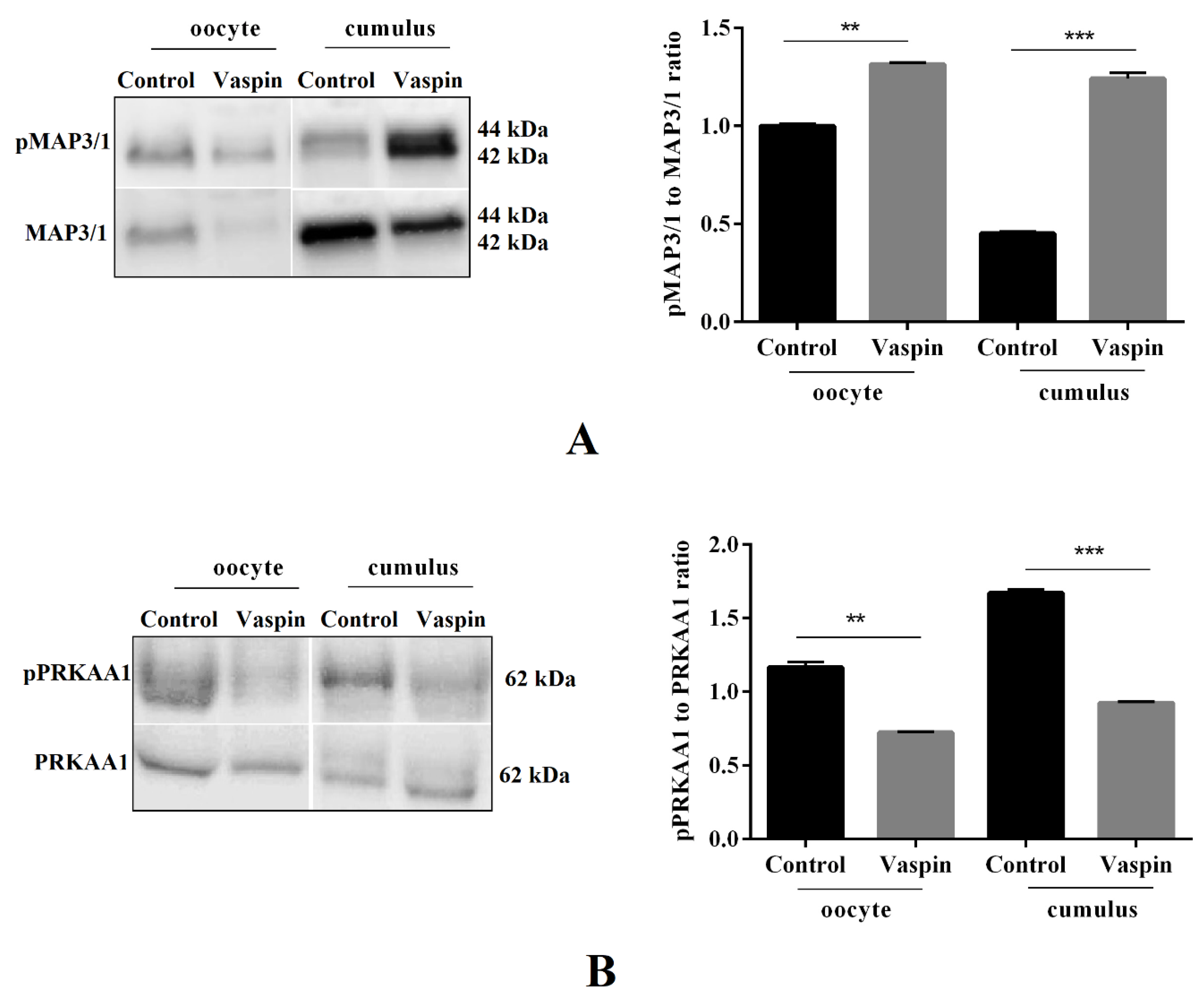
2.4. Effect of Vaspin on MAP3/1 and PRKAA1 Kinase Phosphorylation in Oocytes and Cumulus Cells after In Vitro Maturation
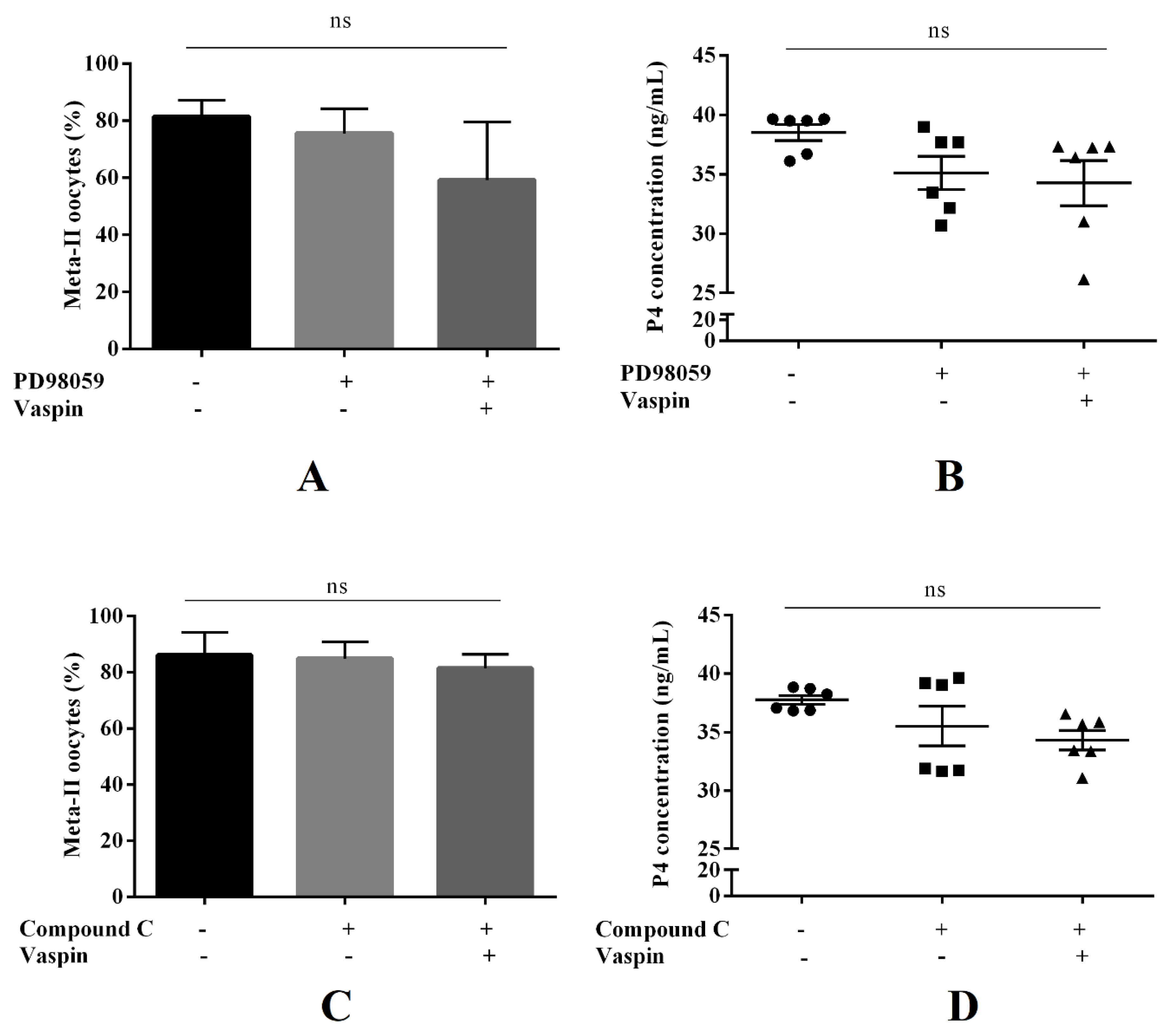
2.5. Involvement of the MAP3/1 and PRKAA1 Kinases in the Effect of Vaspin on In Vitro Oocyte Maturation
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. COC Collection
4.3. Porcine Oocytes In Vitro Maturation and Experimental Procedure
4.4. Real-Time PCR
4.5. Western Blot Analysis
4.6. Immunofluorescence
4.7. DAPI and Hoechst 33342 Staining
4.8. ELISA Assay
4.9. Statistical Analysis
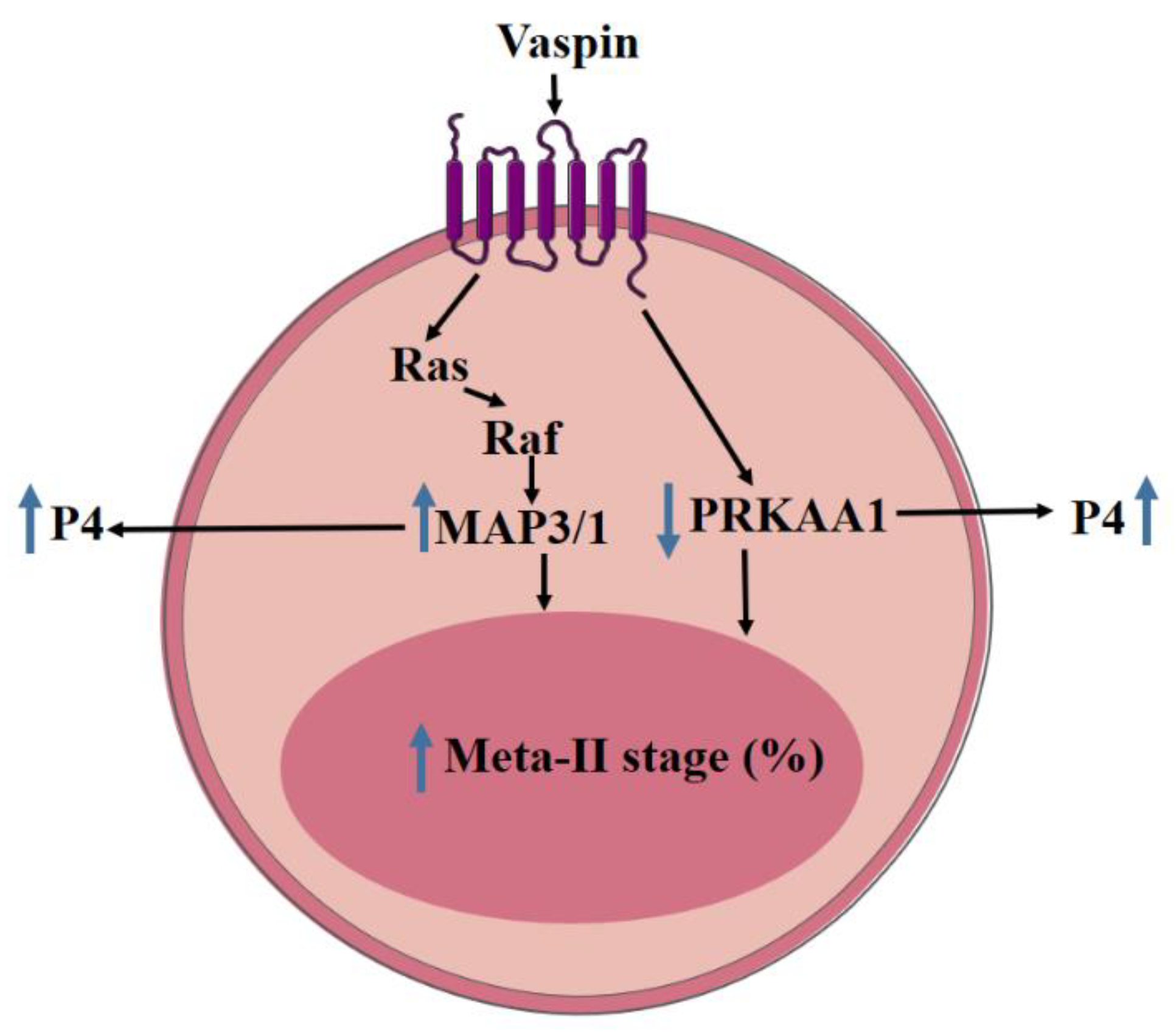
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| EGF | Epidermal growth factor |
| FGF | Fibroblast growth factor |
| GV | Germinal vehicle |
| LH | Luteinising hormone |
| FSH | Follicle stimulating hormone |
| FCS | Foetal calf serum |
| GRP78 | 78-kDa glucose-regulated protein |
| P4 | Progesterone |
| dbCAMP | Butryl AMP |
| PBS | Phosphate-buffered saline |
| COC | Cumulus–oocyte complex |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| MAP3/1 | Mitogen-activated kinase |
| PRKAA1 | AMP-activated kinase |
| PVP | Polyvinylpyrrolidone |
| CL | Corpus luteum |
| BSA | Bovine serum albumin |
| PKA | Protein kinase A |
| SEM | Standard error of the mean |
| STAT3 | Janus kinase |
| Oo | Oocyte |
| Cc | Cumulus cells |
| TBS | Tris-buffered saline |
| Meta-I | Metaphase-I |
| Meta-II | Metaphase-II |
| IVM | In vitro maturation |
| IgG | Immunoglobulin G |
References
- Yuan, Y.; Krisher, R.L. In vitro maturation (IVM) of porcine oocytes. Methods Mol. Biol. 2012, 825, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Pincus, G.; Enzmann, E.V. The comparative behavior of mammalian eggs in vivo and in vitro. II. The activation of tubal eggs of the rabbit. J. Exp. Zool. 1936, 73, 195–208. [Google Scholar] [CrossRef]
- McGaughey, R.W.; Montgomery, D.H.; Richter, J.D. Germinal vesicle configurations and patterns of polypeptide synthesis of porcine oocytes from antral follicles of different size, as related to their competency for spontaneous maturation. J. Exp. Zool. 1979, 209, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Sasaki, Y.; Shimizu, M.; Matsuzaki, M.; Hashizume, T.; Kuwayama, H. Ghrelin and leptin did not improve meiotic maturation of porcine oocytes cultured in vitro. Reprod. Domest. Anim. 2010, 45, 927–930. [Google Scholar] [CrossRef]
- Wehrend, A.; Meinecke, B. Kinetics of meiotic progression, M-phase promoting factor (MPF) and mitogen-activated protein kinase (MAP kinase) activities furing in vitro maturation of porcine and bovine oocytes. Anim. Reprod. Sci. 2001, 66, 175–184. [Google Scholar]
- Tosca, L.; Uzbekova, S.; Chabrolle, C.; Dupont, J. Possible role of 5′AMP-activated protein kinase in the metformin-mediated arrest of bovine oocytes at the germinal vesicle stage during in vitro maturation. Biol. Reprod. 2007, 77, 452–465. [Google Scholar] [CrossRef]
- Craig, J.; Zhu, H.; Dyce, P.W.; Petrik, J.; Li, J. Leptin enhances oocyte nuclear and cytoplasmic maturation via the mitogen-activated protein kinase pathway. Endocrinology 2004, 145, 5355–5363. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Bezáková, A.; Laurinčík, J.; Matejovičová, B. Involvement of the metabolic hormones leptin, ghrelin, obestatin, IGF-I and of MAP kinase in control of porcine oocyte maturation. Animal 2011, 5, 94–99. [Google Scholar] [CrossRef]
- Chappaz, E.; Albornoz, M.S.; Campos, D.; Che, L.; Palin, M.F.; Murphy, B.D.; Bordignon, V. Adiponectin enhances in vitro development of swine embryos. Domest. Anim. Endocrinol. 2008, 35, 198–207. [Google Scholar] [CrossRef]
- Gaughan, J.B.; Cameron, R.D.A.; Dryden, G.M.L.; Young, B.A. Effect of Body Composition at Selection on Reproductive Development in Large White Gilts. J. Anim. Sci. 1997, 75, 1764–1772. [Google Scholar] [CrossRef]
- Dupont, J.Ë.; Reverchon, M.; Cloix, L.; Froment, P.; Ramé, C. Involvement of adipokines, AMPK, PI3K and the PPAR signaling pathways in ovarian follicle development and cancer. Int. J. Dev. Biol. 2012, 56, 959–967. [Google Scholar] [CrossRef]
- Jia, Z.; Zhang, J.; Wu, Z.; Tian, J. Leptin enhances maturation and development of calf oocytes in vitro. Reprod. Domest. Anim. 2012, 47, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, M.; Bertoldo, M.J.; Ramé, C.; Froment, P.; Dupont, J. CHEMERIN (RARRES2) decreases in vitro granulosa cell steroidogenesis and blocks oocyte meiotic progression in bovine species. Biol. Reprod. 2014, 90, 15. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.; Ramé, C.; Reverchon, M.; Mellouk, N.; Rak, A.; Froment, P.; Dupont, J. Apelin (APLN) regulates progesterone secretion and oocyte maturation in bovine ovarian cells. Reproduction 2017, 153, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Gomes, E.T.; Costa, J.A.S.; Silva, D.M.F.; Al Shebli, W.; Azevedo, M.L.; Monteiro, P.L.J.; Araújo Silva, R.A.J.; Santos Filho, A.S.; Guerra, M.M.P.; Bartolomeu, C.C.; et al. Effects of adiponectin during in vitro maturation of goat oocytes: MEK 1/2 pathway and gene expression pattern. Reprod. Domest. Anim. 2018, 53, 1323–1329. [Google Scholar] [CrossRef]
- Klöting, N.; Kovacs, P.; Kern, M.; Heiker, J.T.; Fasshauer, M.; Schön, M.R.; Stumvoll, M.; Beck-Sickinger, A.G.; Blüher, M. Central vaspin administration acutely reduces food intake and has sustained blood glucose-lowering effects. Diabetologia 2011, 54, 1819–1823. [Google Scholar] [CrossRef]
- Liu, S.; Duan, R.; Wu, Y.; Du, F.; Zhang, J.; Li, X.; Guo, S.; Wang, M.; Zhang, Q.; Li, Y.; et al. Effects of Vaspin on Insulin Resistance in Rats and Underlying Mechanisms. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Hida, K.; Wada, J.; Eguchi, J.; Zhang, H.; Baba, M.; Seida, A.; Hashimoto, I.; Okada, T.; Yasuhara, A.; Nakatsuka, A.; et al. Visceral adipose tissue-derived serine protease inhibitor: A unique insulin-sensitizing adipocytokine in obesity. Proc. Natl. Acad. Sci. USA 2005, 102, 10610–10615. [Google Scholar] [CrossRef]
- Kurowska, P.; Mlyczynska, E.; Barbe, A.; Staub, C.; Gregoraszczuk, E.; Dupont, J.; Rak, A. Vaspin in the pig ovarian follicles: Expression and regulation by different hormones. Reproduction 2019, 158, 135–146. [Google Scholar] [CrossRef]
- Kurowska, P.; Mlyczynska, E.; Dawid, M.; Grzesiak, M.; Dupont, J.; Rak, A. The role of vaspin in porcine corpus luteum. J. Endocrinol. 2020, 247, 201–212. [Google Scholar] [CrossRef]
- Barbe, A.; Kurowska, P.; Mlyczyńska, E.; Ramé, C.; Staub, C.; Venturi, E.; Billon, Y.; Rak, A.; Dupont, J. Adipokines expression profiles in both plasma and peri renal adipose tissue in Large White and Meishan sows: A possible involvement in the fattening and the onset of puberty. Gen. Comp. Endocrinol. 2020, 299, 113584. [Google Scholar] [CrossRef]
- Kurowska, P.; Mlyczyńska, E.; Dawid, M.; Dupont, J.; Rak, A. Role of vaspin in porcine ovary: Effect on signaling pathways and steroid synthesis via GRP78 receptor and protein kinase A. Biol. Reprod. 2020, 102, 1290–1305. [Google Scholar] [CrossRef]
- Kurowska, P.; Mlyczyńska, E.; Dawid, M.; Opydo-Chanek, M.; Dupont, J.; Rak, A. In vitro effects of vaspin on porcine granulosa cell proliferation, cell cycle progression, and apoptosis by activation of grp78 receptor and several kinase signaling pathways including MAP3/1, AKT, and STAT3. Int. J. Mol. Sci. 2019, 20, 5816. [Google Scholar] [CrossRef] [PubMed]
- Kurowska, P.; Mlyczyńska, E.; Dupont, J.; Rak, A. Novel insights on the corpus luteum function: Role of vaspin on porcine luteal cell angiogenesis, proliferation and apoptosis by activation of GRP78 receptor and MAP3/1 kinase pathways. Int. J. Mol. Sci. 2020, 21, 6823. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wei, H.X.; Zhang, Y.H.; Wang, S.H.; Li, Y.; Dai, Y.P.; Li, N. Effects of ghrelin on in vitro development of porcine in vitro fertilized and parthenogenetic embryos. J. Reprod. Dev. 2007, 53, 647–653. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marchal, R.; Feugang, J.M.; Perreau, C.; Venturi, E.; Terqui, M.; Mermillod, P. Meiotic and developmental competence of prepubertal and adult swine oocytes. Theriogenology 2001, 56, 17–29. [Google Scholar] [CrossRef]
- Greenbaum, D.; Jansen, R.; Gerstein, M. Analysis of mRNA expression and protein abundance data: An approach for the comparison of the enrichment of features in the cellular population of proteins and transcripts. Bioinformatics 2002, 18, 585–596. [Google Scholar] [CrossRef]
- Ellederova, Z.; Halada, P.; Man, P.; Kubelka, M.; Motlik, J.; Kovarova, H. Protein patterns of pig oocytes during in vitro maturation. Biol. Reprod. 2004, 71, 1533–1539. [Google Scholar] [CrossRef]
- Arias-Alvarez, M.; Bermejo-Alvarez, P.; Gutierrez-Adan, A.; Rizos, D.; Lorenzo, P.L.; Lonergan, P. Effect of leptin supplementation during in vitro oocyte maturation and embryo culture on bovine embryo development and gene expression patterns. Theriogenology 2011, 75, 887–896. [Google Scholar] [CrossRef]
- Maillard, V.; Uzbekova, S.; Guignot, F.; Perreau, C.; Ramé, C.; Coyral-Castel, S.; Dupont, J. Effect of adiponectin on bovine granulosa cell steroidogenesis, oocyte maturation and embryo development. Reprod. Biol. Endocrinol. 2010, 8, 1–15. [Google Scholar] [CrossRef]
- Reverchon, M.; Rame, C.; Bunel, A.; Chen, W.; Froment, P.; Dupont, J. VISFATIN (NAMPT) improves in vitro IGF1-induced steroidogenesis and IGF1 receptor signaling through SIRT1 in bovine granulosa cells. Biol. Reprod. 2016, 94, 54. [Google Scholar] [CrossRef]
- Poniedziałek-Kempny, K.; Gajda, B.; Rajska, I.; Gajda, L.; Smorąg, Z. Piglets produced by transfer of embryos obtained by in vitro fertilization of oocytes matured in vitro with thymosin: A case report. Med. Weter. 2019. [Google Scholar] [CrossRef]
- Sirotkin, A.V. Involvement of steroid hormones in bovine oocytes maturation in vitro. J. Steroid Biochem. Mol. Biol. 1992, 41, 855–858. [Google Scholar] [CrossRef]
- Dode, M.A.N.; Graves, C. Involvement of steroid hormones on in vitro maturation of pig oocytes. Theriogenology 2002, 57, 811–821. [Google Scholar] [CrossRef]
- Galeati, G.; Vallorani, C.; Bucci, D.; Bernardini, C.; Tamanini, C.; Parmeggiani, A.; Spinaci, M. Daidzein does affect progesterone secretion by pig cumulus cells but it does not impair oocytes IVM. Theriogenology 2010, 74, 451–457. [Google Scholar] [CrossRef]
- Inoue, M.; Naito, K.; Aoki, F.; Toyoda, Y.; Sato, E. Activation of mitogen-activated protein kinase during meiotic maturation in porcine ocrcytes. Zygote 1995, 3, 265–271. [Google Scholar] [CrossRef]
- Mayes, M.A.; Laforest, M.F.; Guillemette, C.; Gilchrist, R.B.; Richard, F.J. Adenosine 5′-monophosphate kinase-activated protein kinase (PRKA) activators delay meiotic resumption in porcine oocytes. Biol. Reprod. 2007, 76, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Bilodeau-Goeseels, S.; Magyara, N.; Collignon, C. Characterization of the effects of metformin on porcine oocyte meiosis and on AMP-activated protein kinase activation in oocytes and cumulus cells. Zygote 2014, 22, 275–285. [Google Scholar] [CrossRef]
- Dogan, K.; Ekin, M.; Helvacioğlu, Ç.; Yaşar, L. Can serum vaspin levels predict clomiphene resistance in infertile women with PCOS? Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 217, 6–11. [Google Scholar] [CrossRef]
- Nakatsuka, A.; Wada, J.; Iseda, I.; Teshigawara, S.; Higashio, K.; Murakami, K.; Kanzaki, M.; Inoue, K.; Terami, T.; Katayama, A.; et al. Vaspin is an adipokine ameliorating ER stress in obesity as a ligand for cell-surface GRP78/MTJ-1 complex. Diabetes 2012, 61, 2823–2832. [Google Scholar] [CrossRef]
- Nakatsuka, A.; Wada, J.; Iseda, I.; Teshigawara, S.; Higashio, K.; Murakami, K.; Kanzaki, M.; Inoue, K.; Terami, T.; Katayama, A.; et al. Visceral adipose tissue-derived serine proteinase inhibitor inhibits apoptosis of endothelial cells as a ligand for the cell-surface GRP78/voltage-dependent anion channel complex. Circ. Res. 2013, 112, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Rak, A.; Drwal, E.; Rame, C.; Knapczyk-Stwora, K.; Słomczyńska, M.; Dupont, J.; Gregoraszczuk, E.L. Expression of apelin and apelin receptor (APJ) in porcine ovarian follicles and in vitro effect of apelin on steroidogenesis and proliferation through APJ activation and different signaling pathways. Theriogenology 2017, 96, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3. [Google Scholar] [CrossRef] [PubMed]
- Rak, A.; Drwal, E.; Wróbel, A.; Gregoraszczuk, E.Ł. Resistin is a survival factor for porcine ovarian follicular cells. Reproduction 2015, 150, 343–355. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurowska, P.; Mlyczyńska, E.; Estienne, A.; Barbe, A.; Rajska, I.; Soból, K.; Poniedziałek-Kempny, K.; Dupont, J.; Rak, A. Expression and Impact of Vaspin on In Vitro Oocyte Maturation through MAP3/1 and PRKAA1 Signalling Pathways. Int. J. Mol. Sci. 2020, 21, 9342. https://doi.org/10.3390/ijms21249342
Kurowska P, Mlyczyńska E, Estienne A, Barbe A, Rajska I, Soból K, Poniedziałek-Kempny K, Dupont J, Rak A. Expression and Impact of Vaspin on In Vitro Oocyte Maturation through MAP3/1 and PRKAA1 Signalling Pathways. International Journal of Molecular Sciences. 2020; 21(24):9342. https://doi.org/10.3390/ijms21249342
Chicago/Turabian StyleKurowska, Patrycja, Ewa Mlyczyńska, Anthony Estienne, Alix Barbe, Iwona Rajska, Katarzyna Soból, Katarzyna Poniedziałek-Kempny, Joelle Dupont, and Agnieszka Rak. 2020. "Expression and Impact of Vaspin on In Vitro Oocyte Maturation through MAP3/1 and PRKAA1 Signalling Pathways" International Journal of Molecular Sciences 21, no. 24: 9342. https://doi.org/10.3390/ijms21249342
APA StyleKurowska, P., Mlyczyńska, E., Estienne, A., Barbe, A., Rajska, I., Soból, K., Poniedziałek-Kempny, K., Dupont, J., & Rak, A. (2020). Expression and Impact of Vaspin on In Vitro Oocyte Maturation through MAP3/1 and PRKAA1 Signalling Pathways. International Journal of Molecular Sciences, 21(24), 9342. https://doi.org/10.3390/ijms21249342





