Airway Redox Homeostasis and Inflammation Gone Awry: From Molecular Pathogenesis to Emerging Therapeutics in Respiratory Pathology
Abstract
1. Introduction
2. Reactive Oxygen Species Production in the Airways
3. Respiratory Surface: Antioxidant Defenses
4. Inflammation and Oxidative Stress in Pulmonary Diseases
4.1. Acute Pulmonary Inflammation
Acute Lung Injury (ALI) and Acute Respiratory Distress Syndrome (ARDS)
4.2. Chronic Pulmonary Inflammation
4.2.1. Asthma
4.2.2. Chronic Obstructive Pulmonary Disease (COPD)
4.2.3. Pulmonary Fibrosis
4.3. Cystic Fibrosis
5. Prospective Therapeutic Strategies
5.1. ALI/ARDS
Preclinical Studies
5.2. Asthma
Preclinical Studies
5.3. COPD
Preclinical Studies
5.4. Idiopathic Pulmonary Fibrosis (IPF)
Preclinical Studies
5.5. Cystic Fibrosis (CF)
Preclinical Studies
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Moldoveanu, B.; Otmishi, P.; Jani, P.; Walker, J.; Sarmiento, X.; Guardiola, J.; Saad, M.; Yu, J. Inflammatory mechanisms in the lung. J. Inflamm. Res. 2009, 2, 1–11. [Google Scholar] [PubMed]
- García-Bellmunt, L.; Sibilia, O. Mecanismos de defensa pulmonar. Med. Respir. 2013, 6, 15–24. [Google Scholar]
- LeMessurier, K.S.; Tiwary, M.; Morin, N.P.; Samarasinghe, A.E. Respiratory Barrier as a Safeguard and Regulator of Defense Against Influenza A Virus and Streptococcus pneumoniae. Front. Immunol. 2020, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Comstock, A.T.; Sajjan, U.S. Barrier function of airway tract epithelium. Tissue Barriers 2013, 1, e24997. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, H.; Turkeli, A. Airway epithelial barrier dysfunction in the pathogenesis and prognosis of respiratory tract diseases in childhood and adulthood. Tissue Barriers 2017, 5, e1367458. [Google Scholar] [CrossRef] [PubMed]
- Andrani, F.; Aiello, M.; Bertorelli, G.; Crisafulli, E.; Chetta, A. Cough, a vital reflex. Mechanisms, determinants and measurements. Acta Biomed. 2018, 89, 477–480. [Google Scholar]
- Nawroth, J.C.; Van Der Does, A.M.; Ryan, A.; Kanso, E. Multiscale mechanics of mucociliary clearance in the lung. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190160. [Google Scholar] [CrossRef]
- Twigg, H.L. Humoral immune defense (antibodies): Recent advances. Proc. Am. Thorac. Soc. 2005, 2, 417–421. [Google Scholar] [CrossRef]
- Sheehan, G.; Garvey, A.; Croke, M.; Kavanagh, K. Innate humoral immune defences in mammals and insects: The same, with differences? Virulence 2018, 9, 1625–1639. [Google Scholar] [CrossRef]
- Hartl, D.; Tirouvanziam, R.; Laval, J.; Greene, C.M.; Habiel, D.; Sharma, L.; Yildirim, A.Ö.; Dela Cruz, C.S.; Hogaboam, C.M. Innate Immunity of the Lung: From Basic Mechanisms to Translational Medicine. J. Innate Immun. 2018, 10, 487–501. [Google Scholar] [CrossRef]
- Hiemstra, P.S.; McCray, P.B.; Bals, R. The innate immune function of airway epithelial cells in inflammatory lung disease. Eur. Respir. J. 2015, 45, 1150–1162. [Google Scholar] [CrossRef] [PubMed]
- Aulakh, G.K. Neutrophils in the lung: “The first responders”. Cell Tissue Res. 2018, 371, 577–588. [Google Scholar] [CrossRef]
- Ramirez, G.A.; Yacoub, M.-R.; Ripa, M.; Mannina, D.; Cariddi, A.; Saporiti, N.; Ciceri, F.; Castagna, A.; Colombo, G.; Dagna, L. Eosinophils from Physiology to Disease: A Comprehensive Review. BioMed Res. Int. 2018, 2018, 9095275. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.; Bharat, A. Role of monocytes and macrophages in regulating immune response following lung transplantation. Curr. Opin. Organ Transplant. 2016, 21, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Byrne, A.J.; Mathie, S.A.; Gregory, L.G.; Lloyd, C.M. Pulmonary macrophages: Key players in the innate defence of the airways. Thorax 2015, 70, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Cong, J.; Wei, H. Natural killer cells in the lungs. Front. Immunol. 2019, 10, 1416. [Google Scholar] [CrossRef] [PubMed]
- Erjefält, J.S. Mast cells in human airways: The culprit? Eur. Respir. Rev. 2014, 23, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.; Peters, K.; Bufe, A. Regulation of lung immunity by dendritic cells: Implications for asthma, chronic obstructive pulmonary disease and infectious disease. Innate Immun. 2019, 25, 326–336. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, C.H.; Kim, M.J.; Ryu, J.H.; Seong, S.Y.; Kim, S.; Lim, S.J.; Holtzman, M.J.; Yoon, J.H. The induction of pattern-recognition receptor expression against influenza a virus through Duox2-derived reactive oxygen species in nasal mucosa. Am. J. Respir. Cell Mol. Biol. 2015, 53, 525–535. [Google Scholar] [CrossRef]
- Tengroth, L.; Millrud, C.R.; Kvarnhammar, A.M.; Georén, S.K.; Latif, L.; Cardell, L.O. Functional effects of Toll-Like Receptor (TLR)3, 7, 9, RIG-I and MDA-5 stimulation in nasal epithelial cells. PLoS ONE 2014, 9, e98239. [Google Scholar] [CrossRef]
- El-Zayat, S.R.; Sibaii, H.; Mannaa, F.A. Toll-like receptors activation, signaling, and targeting: An overview. Bull. Natl. Res. Cent. 2019, 43, 1–12. [Google Scholar] [CrossRef]
- Reynolds, J.M.; Dong, C. Toll-like receptor regulation of effector T lymphocyte function. Trends Immunol. 2013, 34, 511–519. [Google Scholar] [CrossRef]
- Taher, T.E.; Bystrom, J.; Ong, V.H.; Isenberg, D.A.; Renaudineau, Y.; Abraham, D.J.; Mageed, R.A. Intracellular B Lymphocyte Signalling and the Regulation of Humoral Immunity and Autoimmunity. Clin. Rev. Allergy Immunol. 2017, 53, 237–264. [Google Scholar] [CrossRef] [PubMed]
- Gomes, E.C.; Florida-James, G. Lung Inflammation, Oxidative Stress and Air Pollution. In Lung Inflammation; IntechOpen: London, UK, 2014. [Google Scholar]
- Lodovici, M.; Bigagli, E. Oxidative Stress and Air Pollution Exposure. J. Toxicol. 2011, 2011, 487074. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, A.; Ogun, M. Biochemistry of Reactive Oxygen and Nitrogen Species. In Basic Principles and Clinical Significance of Oxidative Stress; IntechOpen: London, UK, 2015. [Google Scholar]
- Dröse, S.; Brandt, U. Molecular mechanisms of superoxide production by the mitochondrial respiratory chain. Adv. Exp. Med. Biol. 2012, 748, 145–169. [Google Scholar] [PubMed]
- Lenaz, G. Mitochondria and reactive oxygen species. Which role in physiology and pathology? Adv. Exp. Med. Biol. 2012, 942, 93–136. [Google Scholar] [PubMed]
- Del Río, L.A.; López-Huertas, E. ROS generation in peroxisomes and its role in cell signaling. Plant Cell Physiol. 2016, 57, 1364–1376. [Google Scholar] [CrossRef]
- Bae, Y.S.; Oh, H.; Rhee, S.G.; Yoo, Y. Do Regulation of reactive oxygen species generation in cell signaling. Mol. Cells 2011, 32, 491–509. [Google Scholar] [CrossRef]
- Tejero, J.; Shiva, S.; Gladwin, M.T. Sources of vascular nitric oxide and reactive oxygen species and their regulation. Physiol. Rev. 2019, 99, 311–379. [Google Scholar] [CrossRef]
- Chelombitko, M.A. Role of Reactive Oxygen Species in Inflammation: A Minireview. Mosc. Univ. Biol. Sci. Bull. 2018, 73, 199–202. [Google Scholar] [CrossRef]
- Segal, B.H.; Grimm, M.J.; Khan, A.N.H.; Han, W.; Blackwell, T.S. Regulation of innate immunity by NADPH oxidase. Free Radic. Biol. Med. 2012, 53, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- van der Vliet, A.; Danyal, K.; Heppner, D.E. Dual oxidase: A novel therapeutic target in allergic disease. Br. J. Pharmacol. 2018, 175, 1401–1418. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.T.; Huang, Y.H.; Yang, G.W. Mini review: Immunologic functions of dual oxidases in mucosal systems of vertebrates. Braz. J. Biol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H. Mechanisms and function of DUOX in epithelia of the lung. Antioxid. Redox Signal. 2009, 11, 2453–2465. [Google Scholar] [CrossRef]
- Hu, L.; Zachariae, E.D.; Larsen, U.G.; Vilhardt, F.; Petersen, S.V. The dynamic uptake and release of SOD3 from intracellular stores in macrophages modulates the inflammatory response. Redox Biol. 2019, 26, 101268. [Google Scholar] [CrossRef]
- Kinnula, V.L.; Crapo, J.D. Superoxide dismutases in the lung and human lung diseases. Am. J. Respir. Crit. Care Med. 2003, 167, 1600–1619. [Google Scholar] [CrossRef]
- Ganguly, K.; Depner, M.; Fattman, C.; Bein, K.; Oury, T.D.; Wesselkamper, S.C.; Borchers, M.T.; Schreiber, M.; Gao, F.; Von Mutius, E.; et al. Superoxide dismutase 3, extracellular (SOD3) variants and lung function. Physiol. Genom. 2009, 37, 260–267. [Google Scholar] [CrossRef][Green Version]
- Petersen, S.V.; Enghild, J.J. Extracellular superoxide dismutase: Structural and functional considerations of a protein shaped by two different disulfide bridge patterns. Biomed. Pharmacother. 2005, 59, 175–182. [Google Scholar] [CrossRef]
- Rahman, I.; Biswas, S.K.; Kode, A. Oxidant and antioxidant balance in the airways and airway diseases. Eur. J. Pharmacol. 2006, 533, 222–239. [Google Scholar] [CrossRef]
- Han, W.; Fessel, J.P.; Sherrill, T.; Kocurek, E.G.; Yull, F.E.; Blackwell, T.S. Enhanced Expression of Catalase in Mitochondria Modulates NF-κB–Dependent Lung Inflammation through Alteration of Metabolic Activity in Macrophages. J. Immunol. 2020, 205, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Yatmaz, S.; Seow, H.J.; Gualano, R.C.; Wong, Z.X.; Stambas, J.; Selemidis, S.; Crack, P.J.; Bozinovski, S.; Anderson, G.P.; Vlahos, R. Glutathione peroxidase-1 reduces influenza A virus-induced lung inflammation. Am. J. Respir. Cell Mol. Biol. 2013, 48, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Elko, E.A.; Cunniff, B.; Seward, D.J.; Chia, S.B.; Aboushousha, R.; Van De Wetering, C.; Van Der Velden, J.; Manuel, A.; Shukla, A.; Heintz, N.H.; et al. Peroxiredoxins and Beyond; Redox Systems Regulating Lung Physiology and Disease. Antioxid. Redox Signal. 2019, 31, 1070–1091. [Google Scholar] [CrossRef] [PubMed]
- Schremmer, B.; Manevich, Y.; Feinstein, S.I.; Fisher, A.B. Peroxiredoxins in the lung with emphasis on peroxiredoxin VI. Subcell. Biochem. 2007, 44, 317–344. [Google Scholar]
- Kinnula, V.L.; Lehtonen, S.; Kaarteenaho-Wiik, R.; Lakari, E.; Pääkkö, P.; Kang, S.W.; Rhee, S.G.; Soini, Y. Cell specific expression of peroxiredoxins in human lung and pulmonary sarcoidosis. Thorax 2002, 57, 157–164. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, Y.S.; Lee, H.L.; Shim, J.Y.; Lee, K.S.; Oh, Y.J.; Shin, S.S.; Choi, Y.H.; Park, K.J.; Park, R.W.; et al. Expression of peroxiredoxin and thioredoxin in human lung cancer and paired normal lung. Respirology 2006, 11, 269–275. [Google Scholar] [CrossRef]
- Xu, J.; Li, T.; Wu, H.; Xu, T. Role of thioredoxin in lung disease. Pulm. Pharmacol. Ther. 2012, 25, 154–162. [Google Scholar] [CrossRef]
- Netto, L.E.S.; Antunes, F. The Roles of peroxiredoxin and thioredoxin in hydrogen peroxide sensing and in signal transduction. Mol. Cells 2016, 39, 65–71. [Google Scholar]
- Nakamura, T.; Nakamura, H.; Hoshino, T.; Ueda, S.; Wada, H.; Yodoi, J. Redox regulation of lung inflammation by thioredoxin. Antioxid. Redox Signal. 2005, 7, 60–71. [Google Scholar] [CrossRef]
- Shao, R.; Yang, Y.; Zhang, Y.; Zhao, S.; Zheng, Z.; Chen, G. The expression of thioredoxin-1 and inflammatory cytokines in patients with sepsis. Immunopharmacol. Immunotoxicol. 2020, 42, 280–285. [Google Scholar] [CrossRef]
- Janssen-Heininger, Y. Glutathione, Glutaredoxin And S-Glutathionylation In Lung Disease. Free Radic. Biol. Med. 2017, 112, 3. [Google Scholar] [CrossRef]
- Chia, S.B.; Elko, E.A.; Aboushousha, R.; Manuel, A.M.; van de Wetering, C.; Druso, J.E.; van der Velden, J.; Seward, D.J.; Anathy, V.; Irvin, C.G.; et al. Dysregulation of the glutaredoxin/S-glutathionylation redox axis in lung diseases. Am. J. Physiol. Cell Physiol. 2020, 318, C304–C327. [Google Scholar] [CrossRef]
- Hemilä, H.; Louhiala, P. Vitamin C may affect lung infections. J. R. Soc. Med. 2007, 100, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.O. Antioxidants and respiratory disease: The uric acid paradox. Thorax 2014, 69, 978–979. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fitzpatrick, A.M.; Jones, D.P.; Brown, L.A.S. Glutathione redox control of asthma: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2012, 17, 375–408. [Google Scholar] [CrossRef]
- Gould, N.S.; Min, E.; Gauthier, S.; Martin, R.J.; Day, B.J. Lung glutathione adaptive responses to cigarette smoke exposure. Respir. Res. 2011, 12, 133. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Burdeos, G.C.; Itaya, M.; Nakagawa, K.; Miyazawa, T. Vitamin E: Regulatory Redox Interactions. IUBMB Life 2019, 71, 430–441. [Google Scholar] [CrossRef]
- Cross, C.E.; van der Vliet, A.; O’Neill, C.A.; Louie, S.; Halliwell, B. Oxidants, antioxidants, and respiratory tract lining fluids. Environ. Health Perspect. 1994, 102, 185–191. [Google Scholar]
- Behndig, A.F.; Blomberg, A.; Helleday, R.; Duggan, S.T.; Kelly, F.J.; Mudway, I.S. Antioxidant responses to acute ozone challenge in the healthy human airway. Inhal. Toxicol. 2009, 21, 933–942. [Google Scholar] [CrossRef]
- Mudway, I.S.; Blomberg, A.; Frew, A.J.; Holgate, S.T.; Sandström, T.; Kelly, F.J. Antioxidant consumption and repletion kinetics in nasal lavage fluid following exposure of healthy human volunteers to ozone. Eur. Respir. J. 1999, 13, 1429–1438. [Google Scholar] [CrossRef]
- Barthelemy, J.; Sanchez, K.; Miller, M.R.; Khreis, H. New opportunities to mitigate the burden of disease caused by traffic related air pollution: Antioxidant-rich diets and supplements. Int. J. Environ. Res. Public Health 2020, 17, 630. [Google Scholar] [CrossRef] [PubMed]
- Ballinger, C.A.; Cueto, R.; Squadrito, G.; Coffin, J.F.; Velsor, L.W.; Pryor, W.A.; Postlethwait, E.M. Antioxidant-mediated augmentation of ozone-induced membrane oxidation. Free Radic. Biol. Med. 2005, 38, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.Y.; Reddy, S.P.; Kleeberger, S.R. Nrf2 defends the lung from oxidative stress. Antioxid. Redox Signal. 2006, 8, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Kavian, N.; Mehlal, S.; Jeljeli, M.; Saidu, N.E.B.; Nicco, C.; Cerles, O.; Chouzenoux, S.; Cauvet, A.; Camus, C.; Ait-Djoudi, M.; et al. The Nrf2-antioxidant response element signaling pathway controls fibrosis and autoimmunity in scleroderma. Front. Immunol. 2018, 9, 1896. [Google Scholar] [CrossRef]
- Müller, T.; Hengstermann, A. Nrf2: Friend and Foe in preventing cigarette smoking-dependent lung disease. Chem. Res. Toxicol. 2012, 25, 1805–1824. [Google Scholar] [CrossRef]
- Osburn, W.O.; Kensler, T.W. Nrf2 signaling: An adaptive response pathway for protection against environmental toxic insults. Mutat. Res. Rev. Mutat. Res. 2008, 659, 31–39. [Google Scholar] [CrossRef]
- Aghasafari, P.; George, U.; Pidaparti, R. A review of inflammatory mechanism in airway diseases. Inflamm. Res. 2019, 68, 59–74. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, S.R.; Lee, Y.C. Impact of oxidative stress on lung diseases. Respirology 2009, 14, 27–38. [Google Scholar] [CrossRef]
- Matthay, M.A.; Zemans, R.L.; Zimmerman, G.A.; Arabi, Y.M.; Beitler, J.R.; Mercat, A.; Herridge, M.; Randolph, A.G.; Calfee, C.S. Acute respiratory distress syndrome. Nat. Rev. Dis. Prim. 2018, 5, 18. [Google Scholar] [CrossRef]
- Fernando, S.M.; Cardinal, P.; Brindley, P.G. Hypoxemic Respiratory Failure from Acute Respiratory Distress Syndrome Secondary to Leptospirosis. Case Rep. Crit. Care 2017, 2017, 1–4. [Google Scholar] [CrossRef]
- Ashbaugh, D.G.; Bigelow, D.B.; Petty, T.L.; Levine, B.E. Acute respiratory distress in adults. Lancet 1967, 2, 319–323. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin definition. JAMA J. Am. Med. Assoc. 2012, 307, 2526–2533. [Google Scholar]
- Cardinal-Fernandez, P.; Lorente, J.A.; Ballen-Barragan, A.; Matute-Bello, G. Acute respiratory distress syndrome and diffuse alveolar damage new insights on a complex relationship. Ann. Am. Thorac. Soc. 2017, 14, 844–850. [Google Scholar] [CrossRef]
- Chiumello, D.; Coppola, S.; Froio, S.; Gotti, M. What’s next after ARDS: Long-term outcomes. Respir. Care 2016, 61, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, V.; Ranieri, V.M. Mechanisms and clinical consequences of acute lung injury. Ann. Am. Thorac. Soc. 2015, 12, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Kellner, M.; Noonepalle, S.; Lu, Q.; Srivastava, A.; Zemskov, E.; Black, S.M. ROS signaling in the pathogenesis of Acute Lung Injury (ALI) and Acute Respiratory Distress Syndrome (ARDS). In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2017; Volume 967, pp. 105–137. [Google Scholar]
- Zemans, R.L.; Matthay, M.A. What drives neutrophils to the alveoli in ARDS? Thorax 2017, 72, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Potey, P.M.; Rossi, A.G.; Lucas, C.D.; Dorward, D.A. Neutrophils in the initiation and resolution of acute pulmonary inflammation: Understanding biological function and therapeutic potential. J. Pathol. 2019, 247, 672–685. [Google Scholar] [CrossRef] [PubMed]
- Allardet-Servent, J.; Forel, J.M.; Roch, A.; Guervilly, C.; Chiche, L.; Castanier, M.; Embriaco, N.; Gainnier, M.; Papazian, L. Fio2 and acute respiratory distress syndrome definition during lung protective ventilation. Crit. Care Med. 2009, 37, 202–207. [Google Scholar] [CrossRef]
- Bellani, G.; Laffey, J.G.; Pham, T.; Madotto, F.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; Bumbasirevic, V.; Piquilloud, L.; et al. Noninvasive Ventilation of Patients with Acute Respiratory Distress Syndrome: Insights from the LUNG SAFE Study. Am. J. Respir. Crit. Care Med. 2017, 195, 67–77. [Google Scholar] [CrossRef]
- Elicker, B.M.; Jones, K.T.; Naeger, D.M.; Frank, J.A. Imaging of Acute Lung Injury. Radiol. Clin. N. Am. 2016, 54, 1119–1132. [Google Scholar] [CrossRef]
- Pesenti, A.; Musch, G.; Lichtenstein, D.; Mojoli, F.; Amato, M.B.P.; Cinnella, G.; Gattinoni, L.; Quintel, M. Imaging in acute respiratory distress syndrome. Intensive Care Med. 2016, 42, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.S.; Bozza, F.A.; Hanrahan, C.J.; Wang, L.M.; Wu, Q.; Hoffman, J.M.; Zimmerman, G.A.; Morton, K.A. 18F-fluoro-2-deoxyglucose PET informs neutrophil accumulation and activation in lipopolysaccharide-induced acute lung injury. Nucl. Med. Biol. 2017, 48, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ge, A.; Zhu, W.; Liu, Y.N.; Ji, N.F.; Zha, W.J.; Zhang, J.X.; Zeng, X.N.; Huang, M. Morin attenuates ovalbumin-induced airway inflammation by modulating oxidative stress-responsive MAPK signaling. Oxid. Med. Cell. Longev. 2016, 2016, 5843672. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y. Pneumonia, acute respiratory distress syndrome, and early immune-modulator therapy. Int. J. Mol. Sci. 2017, 18, 388. [Google Scholar] [CrossRef]
- Keddissi, J.I.; Youness, H.A.; Jones, K.R.; Kinasewitz, G.T. Fluid management in Acute Respiratory Distress Syndrome: A narrative review. Can. J. Respir. Ther. 2019, 50, 1–8. [Google Scholar] [CrossRef]
- Quirt, J.; Hildebrand, K.J.; Mazza, J.; Noya, F.; Kim, H. Asthma. Allergy Asthma Clin. Immunol. 2018, 14, 50. [Google Scholar] [CrossRef]
- Mims, J.W. Asthma: Definitions and pathophysiology. Int. Forum Allergy Rhinol. 2015, 5, S2–S6. [Google Scholar] [CrossRef]
- Bush, A. Pathophysiological mechanisms of asthma. Front. Pediatr. 2019, 7, 68. [Google Scholar] [CrossRef]
- Fahy, J.V. Type 2 inflammation in asthma-present in most, absent in many. Nat. Rev. Immunol. 2015, 15, 57–65. [Google Scholar] [CrossRef]
- Teran, L.M. CCL chemokines and asthma. Immunol. Today 2000, 21, 235–242. [Google Scholar] [CrossRef]
- Xue, L.; Fergusson, J.; Salimi, M.; Panse, I.; Ussher, J.E.; Hegazy, A.N.; Vinall, S.L.; Jackson, D.G.; Hunter, M.G.; Pettipher, R.; et al. Prostaglandin D2 and leukotriene E4 synergize to stimulate diverse TH2 functions and TH2 cell/neutrophil crosstalk. J. Allergy Clin. Immunol. 2015, 135, 1358–1366.e11. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhou, J.; Bi, H.; Li, L.; Gao, W.; Huang, M.; Adcock, I.M.; Barnes, P.J.; Yao, X. CCL11 as a potential diagnostic marker for asthma? J. Asthma 2014, 51, 847–854. [Google Scholar] [CrossRef] [PubMed]
- McBrien, C.N.; Menzies-Gow, A. The biology of eosinophils and their role in asthma. Front. Med. 2017, 4, 93. [Google Scholar] [CrossRef] [PubMed]
- Bowler, R.P.; Crapo, J.D. Oxidative stress in allergic respiratory diseases. J. Allergy Clin. Immunol. 2002, 110, 349–356. [Google Scholar] [CrossRef]
- Mishra, V.; Banga, J.; Silveyra, P. Oxidative stress and cellular pathways of asthma and inflammation: Therapeutic strategies and pharmacological targets. Pharmacol. Ther. 2018, 181, 169–182. [Google Scholar] [CrossRef]
- Antczak, A.; Kurmanowska, Z.; Kasielski, M.; Nowak, D. Inhaled glucocorticosteroids decrease hydrogen peroxide level in expired air condensate in asthmatic patients. Respir. Med. 2000, 94, 416–421. [Google Scholar] [CrossRef][Green Version]
- Sahiner, U.M.; Birben, E.; Erzurum, S.; Sackesen, C.; Kalayci, O. Oxidative stress in asthma. World Allergy Organ. J. 2011, 4, 151–158. [Google Scholar] [CrossRef]
- Gerke, A.K.; Hunninghake, G. The Immunology of Sarcoidosis. Clin. Chest Med. 2008, 29, 379–390. [Google Scholar] [CrossRef]
- Lan, N.; Luo, G.; Yang, X.; Cheng, Y.; Zhang, Y.; Wang, X.; Wang, X.; Xie, T.; Li, G.; Liu, Z.; et al. 25-hydroxyvitamin D3-deficiency enhances oxidative stress and corticosteroid resistance in severe asthma exacerbation. PLoS ONE 2014, 9, e111599. [Google Scholar] [CrossRef] [PubMed]
- Dworski, R. Oxidant stress in asthma. Thorax 2000, 55, S51–S53. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.; Ansari, S.H. Role of Various Mediators in Inflammation of Asthmatic Airways. In Asthma—Biological Evidences; IntechOpen: London, UK, 2019. [Google Scholar]
- Qu, J.; Li, Y.; Zhong, W.; Gao, P.; Hu, C. Recent developments in the role of reactive oxygen species in allergic asthma. J. Thorac. Dis. 2017, 9, E32–E43. [Google Scholar] [CrossRef] [PubMed]
- Henricks, P.A.J.; Nijkamp, F.P. Reactive oxygen species as mediators in asthma. Pulm. Pharmacol. Ther. 2001, 14, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, B.N.; Hammad, H.; Fahy, J.V. The Cytokines of Asthma. Immunity 2019, 50, 975–991. [Google Scholar] [CrossRef] [PubMed]
- Rambacher, K.M.; Moniri, N.H. The β2-adrenergic receptor-ROS signaling axis: An overlooked component of β2AR function? Biochem. Pharmacol. 2020, 171, 113690. [Google Scholar] [CrossRef] [PubMed]
- Llano-Diez, M.; Sinclair, J.; Yamada, T.; Zong, M.; Fauconnier, J.; Zhang, S.J.; Katz, A.; Jardemark, K.; Westerblad, H.; Andersson, D.C.; et al. The role of reactive oxygen species in β-adrenergic signaling in cardiomyocytes from mice with the metabolic syndrome. PLoS ONE 2016, 11, e0167090. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhao, C.; Dai, C.; Ren, Y.; An, N.; Wen, H.; Pan, L.; Cheng, M.; Zhang, Y. Suppression of the increasing level of acetylcholine-stimulated intracellular Ca2+ in guinea pig airway smooth muscle cells by mabuterol. Biomed. Rep. 2015, 3, 778–786. [Google Scholar] [CrossRef][Green Version]
- Tang, W. Role of Airway Smooth Muscle Cells in Asthma Pathology. In Asthma—Biological Evidences; IntechOpen: London, UK, 2019. [Google Scholar]
- Jesenak, M.; Zelieskova, M.; Babusikova, E. Oxidative stress and bronchial asthma in children-causes or consequences? Front. Pediatr. 2017, 5, 162. [Google Scholar] [CrossRef]
- Cho, Y.S.; Moon, H.B. The role of oxidative stress in the pathogenesis of asthma. Allergy Asthma Immunol. Res. 2010, 2, 183–187. [Google Scholar] [CrossRef]
- Rao, R. Oxidative stress-induced disruption of epithelial and endothelial tight junctions. Front. Biosci. 2008, 13, 7210–7226. [Google Scholar] [CrossRef]
- He, P.; Talukder, M.A.H.; Gao, F. Oxidative Stress and Microvessel Barrier Dysfunction. Front. Physiol. 2020, 11, 472. [Google Scholar] [CrossRef]
- Wan, W.Y.H.; Hollins, F.; Haste, L.; Woodman, L.; Hirst, R.A.; Bolton, S.; Gomez, E.; Sutcliffe, A.; Desai, D.; Chachi, L.; et al. NADPH Oxidase-4 Overexpression Is Associated with Epithelial Ciliary Dysfunction in Neutrophilic Asthma. Chest 2016, 149, 1445–1459. [Google Scholar] [CrossRef] [PubMed]
- Nociari, M.; Ocheretina, O.; Schoggins, J.W.; Falck-Pedersen, E. Sensing Infection by Adenovirus: Toll-Like Receptor-Independent Viral DNA Recognition Signals Activation of the Interferon Regulatory Factor 3 Master Regulator. J. Virol. 2007, 81, 4145–4157. [Google Scholar] [CrossRef] [PubMed]
- Guillot, L.; Le Goffic, R.; Bloch, S.; Escriou, N.; Akira, S.; Chignard, M.; Si-Tahar, M. Involvement of Toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J. Biol. Chem. 2005, 280, 5571–5580. [Google Scholar] [CrossRef]
- Diebold, S.S.; Kaisho, T.; Hemmi, H.; Akira, S.; Reis E Sousa, C. Innate Antiviral Responses by Means of TLR7-Mediated Recognition of Single-Stranded RNA. Science 2004, 303, 1529–1531. [Google Scholar] [CrossRef] [PubMed]
- Kurt-Jones, E.A.; Popova, L.; Kwinn, L.; Haynes, L.M.; Jones, L.P.; Tripp, R.A.; Walsh, E.E.; Freeman, M.W.; Golenbock, D.T.; Anderson, L.J.; et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 2000, 1, 398–401. [Google Scholar] [CrossRef]
- Yao, H.; Yang, S.R.; Kode, A.; Rajendrasozhan, S.; Caito, S.; Adenuga, O.; Henry, R.; Edirisinghe, I.; Rahman, I. Redox regulation of lung inflammation: Role of NADPH oxidase and NF-κB signalling. Biochem. Soc. Trans. 2007, 35, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Woodland, D.L. Cell-mediated immunity to respiratory virus infections. Curr. Opin. Immunol. 2003, 15, 430–435. [Google Scholar] [CrossRef]
- Barrera-Mendoza, C.C.; Ayala-Mata, F.; Cortés-Rojo, C.; García-Pérez, M.E.; Rodríguez-Orozco, A.R. Vitaminas antioxidantes en asma. Rev. Alerg. México 2018, 65, 61. [Google Scholar] [CrossRef]
- Comhair, S.; Khan, A.; Erzurum, S. Superoxide dismutase as a longitudinal biomarker of lung function in asthma. Eur. Respir. J. 2011, 38. [Google Scholar]
- Janssen-Heininger, Y.; Ckless, K.; Reynaert, N.; Van Der Vliet, A. SOD inactivation in asthma: Bad news or NO news? Am. J. Pathol. 2005, 166, 649–652. [Google Scholar] [CrossRef]
- Endaryanto, A.; Hikmah, Z.; Harsono, A. The use of superoxide dismutase in accelerating symptom relief in asthmatic and house dust mite allergic children receiving house dust mite immunotherapy: Double blind randomized controlled clinical trial. Int. J. Integr. Health Sci. 2015, 3, 72–78. [Google Scholar] [CrossRef]
- Ghosh, S.; Willard, B.; Comhair, S.A.A.; Dibello, P.; Xu, W.; Shiva, S.; Aulak, K.S.; Kinter, M.; Erzurum, S.C. Disulfide bond as a switch for copper-zinc superoxide dismutase activity in asthma. Antioxid. Redox Signal. 2013, 18, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Pittet, J.F.; Griffiths, M.J.D.; Geiser, T.; Kaminski, N.; Dalton, S.L.; Huang, X.; Brown, L.A.S.; Gotwals, P.J.; Koteliansky, V.E.; Matthay, M.A.; et al. TGF-β is a critical mediator of acute lung injury. J. Clin. Investig. 2001, 107, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Comhair, S.A.A.; Xu, W.; Ghosh, S.; Thunnissen, F.B.J.M.; Almasan, A.; Calhoun, W.J.; Janocha, A.J.; Zheng, L.; Hazen, S.L.; Erzurum, S.C. Superoxide dismutase inactivation in pathophysiology of asthmatic airway remodeling and reactivity. Am. J. Pathol. 2005, 166, 663–674. [Google Scholar] [CrossRef]
- Comhair, S.A.A.; Erzurum, S.C. Redox control of asthma: Molecular mechanisms and therapeutic opportunities. Antioxid. Redox Signal. 2010, 12, 93–124. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Janocha, A.J.; Aronica, M.A.; Swaidani, S.; Comhair, S.A.A.; Xu, W.; Zheng, L.; Kaveti, S.; Kinter, M.; Hazen, S.L.; et al. Nitrotyrosine Proteome Survey in Asthma Identifies Oxidative Mechanism of Catalase Inactivation. J. Immunol. 2006, 176, 5587–5597. [Google Scholar] [CrossRef]
- Bozinovski, S.; Seow, H.J.; Crack, P.J.; Anderson, G.P.; Vlahos, R. Glutathione peroxidase-1 primes pro-inflammatory cytokine production after LPS challenge in vivo. PLoS ONE 2012, 7, e33172. [Google Scholar] [CrossRef]
- Won, H.Y.; Sohn, J.H.; Min, H.J.; Lee, K.; Woo, H.A.; Ho, Y.S.; Park, J.W.; Rhee, S.G.; Hwang, E.S. Glutathione peroxidase 1 deficiency attenuates allergen-induced airway inflammation by suppressing th2 and th17 cell development. Antioxid. Redox Signal. 2010, 13, 575–587. [Google Scholar] [CrossRef]
- Iorio, A.; Velocci, M.; Graziano, M.E.; Piacentini, S.; Polimanti, R.; Manfellotto, D.; Fuciarelli, M. GPX1*Pro198Leu AND GPX3 rs2070593 as genetic risk markers for Italian asthmatic patients. Clin. Exp. Pharmacol. Physiol. 2016, 43, 277–279. [Google Scholar] [CrossRef]
- Comhair, S.A.A.; Erzurum, S.C. The regulation and role of extracellular glutathione peroxidase. Antioxid. Redox Signal. 2005, 7, 72–79. [Google Scholar] [CrossRef]
- Callister, M.E.; Burke-Gaffney, A.; Quinlan, G.J.; Nicholson, A.G.; Florio, R.; Nakamura, H.; Yodoi, J.; Evans, T.W. Extracellular thioredoxin levels are increased in patients with acute lung injury. Thorax 2006, 61, 521–527. [Google Scholar] [CrossRef]
- Yamada, Y.; Nakamura, H.; Adachi, T.; Sannohe, S.; Oyamada, H.; Kayaba, H.; Yodoi, J.; Chihara, J. Elevated serum levels of thioredoxin in patients with acute exacerbation of asthma. Immunol. Lett. 2003, 86, 199–205. [Google Scholar] [CrossRef]
- Aesif, S.W.; Anathy, V.; Kuipers, I.; Guala, A.S.; Reiss, J.N.; Ho, Y.S.; Janssen-Heininger, Y.M.W. Ablation of glutaredoxin-1 attenuates lipopolysaccharide-induced lung inflammation and alveolar macrophage activation. Am. J. Respir. Cell Mol. Biol. 2011, 44, 491–499. [Google Scholar] [CrossRef]
- Kuipers, I.; Louis, R.; Manise, M.; Dentener, M.A.; Irvin, C.G.; Janssen-Heininger, Y.M.W.; Brightling, C.E.; Wouters, E.F.M.; Reynaert, N.L. Increased glutaredoxin-1 and decreased protein S-glutathionylation in sputum of asthmatics. Eur. Respir. J. 2013, 41, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, S.M.; Tully, J.E.; Lahue, K.G.; Anathy, V.; Nolin, J.D.; Guala, A.S.; van der Velden, J.L.J.; Ho, Y.S.; Aliyeva, M.; Daphtary, N.; et al. Genetic ablation of glutaredoxin-1 causes enhanced resolution of airways hyperresponsiveness and mucus metaplasia in mice with allergic airways disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 303, L528–L538. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chung, S.; Sundar, I.K.; Yao, H.; Ho, Y.S.; Rahman, I. Glutaredoxin 1 regulates cigarette smoke-mediated lung inflammation through differential modulation of IκB kinases in mice: Impact on histone acetylation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010, 299, L192–L203. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, I.; Bracke, K.R.; Brusselle, G.G.; Aesif, S.W.; Krijgsman, R.; Arts, I.C.; Wouters, E.F.M.; Reynaert, N.L. Altered cigarette smoke-induced lung inflammation due to Ablation of Grx1. PLoS ONE 2012, 7, e38984. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hoffman, S.; Nolin, J.; McMillan, D.; Wouters, E.; Janssen-Heininger, Y.; Reynaert, N. Thiol redox chemistry: Role of protein cysteine oxidation and altered redox homeostasis in allergic inflammation and asthma. J. Cell. Biochem. 2015, 116, 884–892. [Google Scholar] [CrossRef]
- Fitzpatrick, A.M.; Stephenson, S.T.; Hadley, G.R.; Burwell, L.; Penugonda, M.; Simon, D.M.; Hansen, J.; Jones, D.P.; Brown, L.A.S. Thiol redox disturbances in children with severe asthma are associated with posttranslational modification of the transcription factor nuclear factor (erythroid-derived 2)-like 2. J. Allergy Clin. Immunol. 2011, 127, 1604–1611. [Google Scholar] [CrossRef]
- Malhotra, D.; Thimmulappa, R.; Navas-Acien, A.; Sandford, A.; Elliott, M.; Singh, A.; Chen, L.; Zhuang, X.; Hogg, J.; Pare, P.; et al. Decline in NRF2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. Am. J. Respir. Crit. Care Med. 2008, 178, 592–604. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, Y.; Ci, X. Role of Nrf2 and its activators in respiratory diseases. Oxid. Med. Cell. Longev. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Fergeson, J.E.; Patel, S.S.; Lockey, R.F.; Fla, T. Acute asthma, prognosis, and treatment. J. Allergy Clin. Immunol. 2017, 139, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.R.; Peters, S.P.; Busse, W.W. Asthma Exacerbations: Pathogenesis, Prevention, and Treatment. J. Allergy Clin. Immunol. Pract. 2017, 5, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Keskin, O.; Uluca, Ü.; Birben, E.; Coşkun, Y.; Ozkars, M.Y.; Keskin, M.; Kucukosmanoglu, E.; Kalayci, O. Genetic associations of the response to inhaled corticosteroids in children during an asthma exacerbation. Pediatr. Allergy Immunol. 2016, 27, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Ökrös, Z.; Endreffy, E.; Novak, Z.; Maroti, Z.; Monostori, P.; Varga, I.S.; Király, A.; Turi, S. Changes in NADPH oxidase mRNA level can be detected in blood at inhaled corticosteroid treated asthmatic children. Life Sci. 2012, 91, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Tashkin, D.P.; Lipworth, B.; Brattsand, R. Benefit:Risk Profile of Budesonide in Obstructive Airways Disease. Drugs 2019, 79, 1757–1775. [Google Scholar] [CrossRef]
- Gibson, P.G.; Saltos, N.; Borgas, T. Airway mast cells and eosinophils correlate with clinical severity and airway hyperresponsiveness in corticosteroid-treated asthma. J. Allergy Clin. Immunol. 2000, 105, 752–759. [Google Scholar] [CrossRef]
- Brightling, C.E.; Green, R.H.; Pavord, I.D. Biomarkers predicting response to corticosteroid therapy in asthma. Treat. Respir. Med. 2005, 4, 309–316. [Google Scholar] [CrossRef]
- Austin, D.; Pouliquen, I.; Keene, O.; Yancey, S. Blood eosinophil dose response to oral corticosteroids in a population of patients with severe asthma. Eur. Respir. J. 2016, 48, PA1110. [Google Scholar]
- Domingo, C.; Rello, J.; Sogo, A. As-needed ICS-LABA in Mild Asthma: What Does the Evidence Say? Drugs 2019, 79, 1729–1737. [Google Scholar] [CrossRef]
- Johnston, N.W.; Mandhane, P.J.; Dai, J.; Duncan, J.M.; Greene, J.M.; Lambert, K.; Sears, M.R. Attenuation of the September epidemic of asthma exacerbations in children: A randomized, controlled trial of montelukast added to usual therapy. Pediatrics 2007, 120, e702–e712. [Google Scholar] [CrossRef] [PubMed]
- Vogelberg, C.; Szefler, S.J.; Vrijlandt, E.J.L.E.; Boner, A.L.; Engel, M.; El Azzi, G.; Vulcu, S.D.; Moroni-Zentgraf, P.M.; Eickmeier, O.; Hamelmann, E.H. Tiotropium add-on therapy is safe and reduces seasonal worsening in paediatric asthma patients. Eur. Respir. J. 2019, 53, 1801824. [Google Scholar] [CrossRef] [PubMed]
- Sterling, Y.M. Impact of the Environment on Asthma Control. J. Community Health Nurs. 2012, 29, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Donohue, J.F.; Wise, R.; Busse, W.W.; Garfinkel, S.; Zubek, V.B.; Ghafouri, M.; Manuel, R.C.; Schlenker-Herceg, R.; Bleecker, E.R. Efficacy and safety of ipratropium bromide/albuterol compared with albuterol in patients with moderate-to-severe asthma: A randomized controlled trial. BMC Pulm. Med. 2016, 16, 65. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rodrigo, G.J.; Castro-Rodriguez, J.A. Anticholinergics in the treatment of children and adults with acute asthma: A systematic review with meta-analysis. Thorax 2005, 60, 740–746, Erratum in Thorax 2010, 65, 1118. [Google Scholar] [CrossRef] [PubMed]
- Zorc, J.J.; Pusic, M.V.; Ogborn, C.J.; Lebet, R.; Duggan, A.K. Ipratropium bromide added to asthma treatment in the pediatric emergency department. Pediatrics 1999, 103, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, G.J. Rapid effects of inhaled corticosteroids in acute asthma: An evidence-based evaluation. Chest 2006, 130, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Rowe, B.H.; Edmonds, M.L.; Spooner, C.H.; Diner, B.; Camargo, C.A. Corticosteroid therapy for acute asthma. Respir. Med. 2004, 98, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Corlateanu, A.; Mendez, Y.; Wang, Y.; de Garnica, R.J.A.; Botnaru, V.; Siafakas, N. Chronic obstructive pulmonary disease and phenotypes: A state-of-the-art. Pulmonology 2020, 26, 95–100. [Google Scholar] [CrossRef]
- Rovina, N.; Koutsoukou, A.; Koulouris, N.G. Inflammation and immune response in COPD: Where do we stand? Mediat. Inflamm. 2013, 2013, 413735. [Google Scholar] [CrossRef]
- Donnelly, L.E.; Barnes, P.J. Chemokine receptors as therapeutic targets in chronic obstructive pulmonary disease. Trends Pharmacol. Sci. 2006, 27, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Cellular and molecular mechanisms of asthma and COPD. Clin. Sci. 2017, 131, 1541–1558. [Google Scholar] [CrossRef] [PubMed]
- Traynor, T.R.; Herring, A.C.; Dorf, M.E.; Kuziel, W.A.; Toews, G.B.; Huffnagle, G.B. Differential Roles of CC Chemokine Ligand 2/Monocyte Chemotactic Protein-1 and CCR2 in the Development of T1 Immunity. J. Immunol. 2002, 168, 4659–4666. [Google Scholar] [CrossRef] [PubMed]
- Gilowska, I.; Kasper, Ł.; Bogacz, K.; Szczegielniak, J.; Szymasek, T.; Kasper, M.; Czerwinski, M.; Sładek, K.; Majorczyk, E. Impact of Matrix Metalloproteinase 9 on COPD Development in Polish Patients: Genetic Polymorphism, Protein Level, and Their Relationship with Lung Function. BioMed Res. Int. 2018, 2018, 6417415. [Google Scholar] [CrossRef] [PubMed]
- Dey, T.; Kalita, J.; Weldon, S.; Taggart, C. Proteases and Their Inhibitors in Chronic Obstructive Pulmonary Disease. J. Clin. Med. 2018, 7, 244. [Google Scholar] [CrossRef] [PubMed]
- Ponce-Gallegos, M.A.; Ramírez-Venegas, A.; Falfán-Valencia, R. Th17 profile in COPD exacerbations. Int. J. COPD 2017, 12, 1857–1865. [Google Scholar] [CrossRef]
- Lane, N.; Robins, R.A.; Corne, J.; Fairclough, L. Regulation in chronic obstructive pulmonary disease: The role of regulatory T-cells and Th17 cells. Clin. Sci. 2010, 119, 75–86. [Google Scholar] [CrossRef]
- Di Stefano, A.; Sangiorgi, C.; Gnemmi, I.; Casolari, P.; Brun, P.; Ricciardolo, F.L.M.; Contoli, M.; Papi, A.; Maniscalco, P.; Ruggeri, P.; et al. TGF-β Signaling Pathways in Different Compartments of the Lower Airways of Patients With Stable COPD. Chest 2018, 153, 851–862. [Google Scholar] [CrossRef]
- Verhamme, F.M.; Bracke, K.R.; Joos, G.F.; Brusselle, G.G. Transforming growth factor-β superfamily in obstructive lung diseases: More suspects than TGF-β alone. Am. J. Respir. Cell Mol. Biol. 2015, 52, 653–662. [Google Scholar] [CrossRef]
- Yan, F.; Gao, H.; Zhao, H.; Bhatia, M.; Zeng, Y. Roles of airway smooth muscle dysfunction in chronic obstructive pulmonary disease. J. Transl. Med. 2018, 16, 262. [Google Scholar] [CrossRef]
- Zhuan, B.; Yu, Y.; Yang, Z.; Zhao, X.; Li, P. Mechanisms of oxidative stress effects of the NADPH oxidase-ROS-NF-κB transduction pathway and VPO1 on patients with chronic obstructive pulmonary disease combined with pulmonary hypertension. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 3459–3464. [Google Scholar] [PubMed]
- Meijer, M.; Rijkers, G.T.; Van Overveld, F.J. Neutrophils and emerging targets for treatment in chronic obstructive pulmonary disease. Expert Rev. Clin. Immunol. 2013, 9, 1055–1068. [Google Scholar] [CrossRef] [PubMed]
- Klimanov, I.A.; Khaletskaya, A.; Kuznechov, A.; Kontorschikova, K.; Kubysheva, N.; Leonova, D.; Bobkova, A.; Soodaeva, S. Lipid peroxidation in patients with COPD and chronic heart failure. Eur. Respir. J. 2018, 52, PA931. [Google Scholar]
- Nicks, M.E.; O’Brien, M.M.; Bowler, R.P. Plasma antioxidants are associated with impaired lung function and COPD exacerbations in smokers. COPD J. Chronic Obstr. Pulm. Dis. 2011, 8, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Lomas, D.A. Does protease-antiprotease imbalance explain Chronic obstructive pulmonary disease? Ann. Am. Thorac. Soc. 2016, 13, S130–S137. [Google Scholar]
- Fischer, B.M.; Pavlisko, E.; Voynow, J.A. Pathogenic triad in COPD: Oxidative stress, protease-antiprotease imbalance, and inflammation. Int. J. COPD 2011, 6, 413–421. [Google Scholar] [CrossRef]
- Turgut, T.; Ilhan, N.; Deveci, F.; Akpolat, N.; Erden, E.S.; Muz, M.H. Glutathione and nitrite levels in induced sputum at COPD patients and healthy smokers. J. Thorac. Dis. 2014, 6, 765–771. [Google Scholar]
- Harju, T.; Kaarteenaho-Wiik, R.; Sirviö, R.; Pääkkö, P.; Crapo, J.D.; Oury, T.D.; Soini, Y.; Kinnula, V.L. Manganese superoxide dismutase is increased in the airways of smokers’ lungs. Eur. Respir. J. 2004, 24, 765–771. [Google Scholar] [CrossRef]
- McGuinness, A.; Sapey, E. Oxidative Stress in COPD: Sources, Markers, and Potential Mechanisms. J. Clin. Med. 2017, 6, 21. [Google Scholar] [CrossRef]
- Bentley, A.R.; Emrani, P.; Cassano, P.A. Genetic variation and gene expression in antioxidant related enzymes and risk of COPD: A systematic review. Thorax 2008, 63, 956–961. [Google Scholar] [CrossRef]
- Young, R.P.; Hopkins, R.; Black, P.N.; Eddy, C.; Wu, L.; Gamble, G.D.; Mills, G.D.; Garrett, J.E.; Eaton, T.E.; Rees, M.I. Functional variants of antioxidant genes in smokers with COPD and in those with normal lung function. Thorax 2006, 61, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.D.; Wang, C.X.; Wu, J.L.; Fukunaga, A.; Cheng, Z.S.; Wang, J.Q.; Yamauchi, A.; Yodoi, J.; Tian, H. Anti-allergic and anti-inflammatory effects and molecular mechanisms of thioredoxin on respiratory system diseases. Antioxid. Redox Signal. 2020, 32, 785–801. [Google Scholar] [CrossRef] [PubMed]
- Peltoniemi, M.J.; Rytilä, P.H.; Harju, T.H.; Soini, Y.M.; Salmenkivi, K.M.; Ruddock, L.W.; Kinnula, V.L. Modulation of glutaredoxin in the lung and sputum of cigarette smokers and chronic obstructive pulmonary disease. Respir. Res. 2006, 7, 133. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Estornut, C.; Roger, I.; Ballester, B.; Ribera, P.; Cortijo, J. Activation of nuclear factor erythroid 2-related (Nrf2) system as a novel therapeutic approach in COPD. Eur. Respir. J. 2019, 54, PA4216. [Google Scholar]
- Okpechi, S.C.; Ghonim, M.A.; Lammi, M.R. Advances in chronic obstructive pulmonary disease therapy: A vascular-targeted approach. Clin. Med. Insights Ther. 2017, 9, 1179559X1771912. [Google Scholar] [CrossRef]
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am. J. Respir. Crit. Care Med. 2017, 195, 557–582. [Google Scholar] [CrossRef]
- Barratt, S.; Creamer, A.; Hayton, C.; Chaudhuri, N. Idiopathic Pulmonary Fibrosis (IPF): An Overview. J. Clin. Med. 2018, 7, 201. [Google Scholar] [CrossRef]
- Futosi, K.; Fodor, S.; Mócsai, A. Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int. Immunopharmacol. 2013, 17, 638–650. [Google Scholar] [CrossRef]
- Mayadas, T.N.; Cullere, X.; Lowell, C.A. The multifaceted functions of neutrophils. Annu. Rev. Pathol. Mech. Dis. 2014, 9, 181–218. [Google Scholar] [CrossRef]
- Mills, C.D. M1 and M2 macrophages: Oracles of health and disease. Crit. Rev. Immunol. 2012, 32, 463–488. [Google Scholar] [CrossRef]
- Schupp, J.C.; Binder, H.; Jäger, B.; Cillis, G.; Zissel, G.; Müller-Quernheim, J.; Prasse, A. Macrophage activation in acute exacerbation of idiopathic pulmonary fibrosis. PLoS ONE 2015, 10, e0116775. [Google Scholar] [CrossRef] [PubMed]
- Misharin, A.V.; Morales-Nebreda, L.; Reyfman, P.A.; Cuda, C.M.; Walter, J.M.; McQuattie-Pimentel, A.C.; Chen, C.I.; Anekalla, K.R.; Joshi, N.; Williams, K.J.N.; et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J. Exp. Med. 2017, 214, 2387–2404. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, M.; Maher, T.M. Can monocytes predict prognosis of idiopathic pulmonary fibrosis? Lancet Respir. Med. 2019, 7, 467–469. [Google Scholar] [CrossRef]
- Desai, O.; Winkler, J.; Minasyan, M.; Herzog, E.L. The role of immune and inflammatory cells in idiopathic pulmonary fibrosis. Front. Med. 2018, 5, 43. [Google Scholar] [CrossRef] [PubMed]
- Heukels, P.; van Hulst, J.A.C.; van Nimwegen, M.; Boorsma, C.E.; Melgert, B.N.; van den Toorn, L.M.; Boomars, K.A.T.; Wijsenbeek, M.S.; Hoogsteden, H.; von der Thüsen, J.H.; et al. Fibrocytes are increased in lung and peripheral blood of patients with idiopathic pulmonary fibrosis. Respir. Res. 2018, 19, 90. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, S.; Shimbori, C.; Kolb, M. Fibrocytes in pulmonary fibrosis: A brief synopsis. Eur. Respir. Rev. 2013, 22, 552–557. [Google Scholar] [CrossRef]
- Overed-Sayer, C.; Rapley, L.; Mustelin, T.; Clarke, D.L. Are mast cells instrumental for fibrotic diseases? Front. Pharmacol. 2014, 4, 174. [Google Scholar] [CrossRef]
- Komi, D.E.A.; Mortaz, E.; Amani, S.; Tiotiu, A.; Folkerts, G.; Adcock, I.M. The Role of Mast Cells in IgE-Independent Lung Diseases. Clin. Rev. Allergy Immunol. 2020, 58, 377–387. [Google Scholar] [CrossRef]
- Marchal-Sommé, J.; Uzunhan, Y.; Marchand-Adam, S.; Valeyre, D.; Soumelis, V.; Crestani, B.; Soler, P. Cutting Edge: Nonproliferating Mature Immune Cells Form a Novel Type of Organized Lymphoid Structure in Idiopathic Pulmonary Fibrosis. J. Immunol. 2006, 176, 5735–5739. [Google Scholar] [CrossRef]
- Nuovo, G.J.; Hagood, J.S.; Magro, C.M.; Chin, N.; Kapil, R.; Davis, L.; Marsh, C.B.; Folcik, V.A. The distribution of immunomodulatory cells in the lungs of patients with idiopathic pulmonary fibrosis. Mod. Pathol. 2012, 25, 416–433. [Google Scholar] [CrossRef]
- Todd, N.W.; Scheraga, R.G.; Galvin, J.R.; Iacono, A.T.; James Britt, E.; Luzina, I.G.; Burke, A.P.; Atamas, S.P. Lymphocyte aggregates persist and accumulate in the lungs of patients with idiopathic pulmonary fibrosis. J. Inflamm. Res. 2013, 6, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Fibrotic disease and the TH1/TH2 paradigm. Nat. Rev. Immunol. 2004, 4, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.A.; McKenzie, A.N.J. TH2 cell development and function. Nat. Rev. Immunol. 2018, 18, 121–133. [Google Scholar] [CrossRef]
- Saito, A.; Okazaki, H.; Sugawara, I.; Yamamoto, K.; Takizawa, H. Potential action of IL-4 and IL-13 as fibrogenic factors on lung fibroblasts in vitro. Int. Arch. Allergy Immunol. 2003, 132, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Keane, M.P.; Belperio, J.A.; Burdick, M.D.; Strieter, R.M. IL-12 attenuates bleomycin-induced pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 281, L92–L97. [Google Scholar] [CrossRef] [PubMed]
- Rottoli, P.; Magi, B.; Perari, M.G.; Liberatori, S.; Nikiforakis, N.; Bargagli, E.; Cianti, R.; Bini, L.; Pallini, V. Cytokine profile and proteome analysis in bronchoalveolar lavage of patients with sarcoidosis, pulmonary fibrosis associated with systematic sclerosis and idiopathic pulmonary fibrosis. Proteomics 2005, 5, 1423–1430. [Google Scholar] [CrossRef]
- Moore, M.W.; Herzog, E.L. Regulatory T Cells in Idiopathic Pulmonary Fibrosis: Too Much of a Good Thing? Am. J. Pathol. 2016, 186, 1978–1981. [Google Scholar] [CrossRef]
- Cheresh, P.; Kim, S.J.; Tulasiram, S.; Kamp, D.W. Oxidative stress and pulmonary fibrosis. Biochim. Biophys. Acta Mol. Basis Dis. 2013, 1832, 1028–1040. [Google Scholar] [CrossRef]
- Richter, K.; Kietzmann, T. Reactive oxygen species and fibrosis: Further evidence of a significant liaison. Cell Tissue Res. 2016, 365, 591–605. [Google Scholar] [CrossRef]
- Yue, X.; Shan, B.; Lasky, J.A. TGF-β: Titan of Lung Fibrogenesis. Curr. Enzym. Inhib. 2010, 6, 1–20. [Google Scholar] [CrossRef]
- Liu, R.M.; Desai, L.P. Reciprocal regulation of TGF-β and reactive oxygen species: A perverse cycle for fibrosis. Redox Biol. 2015, 6, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Cho, M. Activation of NADPH oxidase subunit NCF4 induces ROS-mediated EMT signaling in HeLa cells. Cell. Signal. 2014, 26, 784–796. [Google Scholar] [CrossRef] [PubMed]
- Amara, N.; Goven, D.; Prost, F.; Muloway, R.; Crestani, B.; Boczkowski, J. NOX4/NADPH oxidase expression is increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates TGFβ1-induced fibroblast differentiation into myofibroblasts. Thorax 2010, 65, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Hecker, L.; Vittal, R.; Jones, T.; Jagirdar, R.; Luckhardt, T.R.; Horowitz, J.C.; Pennathur, S.; Martinez, F.J.; Thannickal, V.J. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat. Med. 2009, 15, 1077–1081. [Google Scholar] [CrossRef]
- Sturrock, A.; Cahill, B.; Norman, K.; Huecksteadt, T.P.; Hill, K.; Sanders, K.; Karwande, S.V.; Stringham, J.C.; Bull, D.A.; Gleich, M.; et al. Transforming growth factor-β1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 290, L661–L673. [Google Scholar] [CrossRef]
- Waghray, M.; Cui, Z.; Horowitz, J.C.; Subramanian, I.M.; Martinez, F.J.; Toews, G.B.; Thannickal, V.J. Hydrogen peroxide is a diffusible paracrine signal for the induction of epithelial cell death by activated myofibroblasts. FASEB J. 2005, 19, 1–16. [Google Scholar] [CrossRef]
- Koli, K.; Myllärniemi, M.; Keski-Oja, J.; Kinnula, V.L. Transforming growth factor-β activation in the lung: Focus on fibrosis and reactive oxygen species. Antioxid. Redox Signal. 2008, 10, 333–342. [Google Scholar] [CrossRef]
- Herrera, B.; Murillo, M.M.; Álvarez-Barrientos, A.; Beltrán, J.; Fernández, M.; Fabregat, I. Source of early reactive oxygen species in the apoptosis induced by transforming growth factor-β in fetal rat hepatocytes. Free Radic. Biol. Med. 2004, 36, 16–26. [Google Scholar] [CrossRef]
- Fois, A.G.; Paliogiannis, P.; Sotgia, S.; Mangoni, A.A.; Zinellu, E.; Pirina, P.; Carru, C.; Zinellu, A. Evaluation of oxidative stress biomarkers in idiopathic pulmonary fibrosis and therapeutic applications: A systematic review. Respir. Res. 2018, 19, 51. [Google Scholar] [CrossRef]
- Muramatsu, Y.; Sugino, K.; Ishida, F.; Tatebe, J.; Morita, T.; Homma, S. Effect of inhaled N-acetylcysteine monotherapy on lung function and redox balance in idiopathic pulmonary fibrosis. Respir. Investig. 2016, 54, 170–178. [Google Scholar] [CrossRef]
- Bowler, R.P.; Nicks, M.; Warnick, K.; Crapo, J.D. Role of extracellular superoxide dismutase in bleomycin-induced pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 282, L719–L726. [Google Scholar] [CrossRef] [PubMed]
- Fattman, C.L.; Chang, L.Y.; Termin, T.A.; Petersen, L.; Enghild, J.J.; Oury, T.D. Enhanced bleomycin-induced pulmonary damage in mice lacking extracellular superoxide dismutase. Free Radic. Biol. Med. 2003, 35, 763–771. [Google Scholar] [CrossRef]
- Zelko, I.N.; Zhu, J.; Roman, J. Role of SOD3 in silica-related lung fibrosis and pulmonary vascular remodeling. Respir. Res. 2018, 19, 221. [Google Scholar] [CrossRef] [PubMed]
- Mouradian, G.C.; Gaurav, R.; Pugliese, S.; El Kasmi, K.; Hartman, B.; Hernandez-Lagunas, L.; Stenmark, K.R.; Bowler, R.P.; Nozik-Grayck, E. Superoxide dismutase 3 R213G single-nucleotide polymorphism blocks murine bleomycin-induced fibrosis and promotes resolution of inflammation. Am. J. Respir. Cell Mol. Biol. 2017, 56, 362–371. [Google Scholar] [CrossRef]
- Kinnula, V.L.; Hodgson, U.A.; Lakari, E.K.; Tan, R.J.; Sormunen, R.T.; Soini, Y.M.; Kakko, S.J.; Laitinen, T.H.; Oury, T.D.; Pääkkö, P.K. Extracellular superoxide dismutase has a highly specific localization in idiopathic pulmonary fibrosis/usual interstitial pneumonia. Histopathology 2006, 49, 66–74. [Google Scholar] [CrossRef]
- Tiitto, L.; Kaarteenaho-Wiik, R.; Sormunen, R.; Holmgren, A.; Pääkkö, P.; Soini, Y.; Kinnula, V.L. Expression of the thioredoxin system in interstitial lung disease. J. Pathol. 2003, 201, 363–370. [Google Scholar] [CrossRef]
- Iwata, Y.; Okamoto, M.; Hoshino, T.; Kitasato, Y.; Sakazaki, Y.; Tajiri, M.; Matsunaga, K.; Azuma, K.; Kawayama, T.; Kinoshita, T.; et al. Elevated levels of thioredoxin 1 in the lungs and sera of idiopathic pulmonary fibrosis, non-specific interstitial pneumonia and cryptogenic organizing pneumonia. Intern. Med. 2010, 49, 2393–2400. [Google Scholar] [CrossRef][Green Version]
- Richeldi, L.; Davies, H.R.H.R.; Spagnolo, P.; Luppi, F. Corticosteroids for idiopathic pulmonary fibrosis. Cochrane Database Syst. Rev. 2003, CD002880. [Google Scholar] [CrossRef]
- Martinez, F.J.; De Andrade, J.A.; Anstrom, K.J.; King, T.E.; Raghu, G. Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2093–2101. [Google Scholar]
- Raghu, G.; Anstrom, K.J.; King, T.E.; Lasky, J.A.; Martinez, F.J. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N. Engl. J. Med. 2012, 366, 1968–1977. [Google Scholar]
- Richeldi, L.; Du Bois, R.M.; Raghu, G.; Azuma, A.; Brown, K.K.; Costabel, U.; Cottin, V.; Flaherty, K.R.; Hansell, D.M.; Inoue, Y.; et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2071–2082. [Google Scholar] [CrossRef] [PubMed]
- King, T.E.; Bradford, W.Z.; Castro-Bernardini, S.; Fagan, E.A.; Glaspole, I.; Glassberg, M.K.; Gorina, E.; Hopkins, P.M.; Kardatzke, D.; Lancaster, L.; et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2083–2092. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.Y.; Park, S.; Kim, D.S.; Song, J.W. Efficacy and safety of nintedanib in advanced idiopathic pulmonary fibrosis. Respir. Res. 2018, 19, 203. [Google Scholar] [CrossRef] [PubMed]
- Kistler, K.D.; Nalysnyk, L.; Rotella, P.; Esser, D. Lung transplantation in idiopathic pulmonary fibrosis: A systematic review of the literature. BMC Pulm. Med. 2014, 14, 139. [Google Scholar] [CrossRef] [PubMed]
- Heukels, P.; Moor, C.C.; von der Thüsen, J.H.; Wijsenbeek, M.S.; Kool, M. Inflammation and immunity in IPF pathogenesis and treatment. Respir. Med. 2019, 147, 79–91. [Google Scholar] [CrossRef]
- Vianello, A.; Arcaro, G.; Molena, B.; Turato, C.; Braccioni, F.; Paladini, L.; Vio, S.; Ferrarese, S.; Peditto, P.; Gallan, F.; et al. High-flow nasal cannula oxygen therapy to treat acute respiratory failure in patients with acute exacerbation of idiopathic pulmonary fibrosis. Ther. Adv. Respir. Dis. 2019, 13, 1753466619847130. [Google Scholar] [CrossRef]
- Galli, F.; Battistoni, A.; Gambari, R.; Pompella, A.; Bragonzi, A.; Pilolli, F.; Iuliano, L.; Piroddi, M.; Dechecchi, M.C.; Cabrini, G. Oxidative stress and antioxidant therapy in cystic fibrosis. Biochim. Biophys. Acta Mol. Basis Dis. 2012, 1822, 690–713. [Google Scholar] [CrossRef]
- Chatterjee, S. Oxidative Stress, Inflammation, and Disease. In Oxidative Stress and Biomaterials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 35–58. ISBN 9780128032701. [Google Scholar]
- Koch, C.; Hoiby, N. Pathogenesis of cystic fibrosis. Lancet 1993, 341, 1065–1069. [Google Scholar] [CrossRef]
- Cantin, A.M.; Hartl, D.; Konstan, M.W.; Chmiel, J.F. Inflammation in cystic fibrosis lung disease: Pathogenesis and therapy. J. Cyst. Fibros. 2015, 14, 419–430. [Google Scholar] [CrossRef]
- Parkins, M.D.; Somayaji, R.; Waters, V.J. Epidemiology, Biology, and Impact of Clonal Pseudomonas aeruginosa Infections in Cystic Fibrosis. Clin. Microbiol. Rev. 2018, 31, e00019-18. [Google Scholar] [CrossRef]
- Huang, Y.J.; LiPuma, J.J. The Microbiome in Cystic Fibrosis. Clin. Chest Med. 2016, 37, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Turkovic, L.; Caudri, D.; Rosenow, T.; Hall, G.; Stick, S. Presence of mucus plugging is predictive of long term lung function in children with cystic fibrosis. Eur. Respir. J. 2017, 50, OA4401. [Google Scholar]
- Rieber, N.; Hector, A.; Carevic, M.; Hartl, D. Current concepts of immune dysregulation in cystic fibrosis. Int. J. Biochem. Cell Biol. 2014, 52, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.P.; Chmiel, J.F. Inflammation and its genesis in cystic fibrosis. Pediatr. Pulmonol. 2015, 50, S39–S56. [Google Scholar] [CrossRef] [PubMed]
- Sarr, D.; Tóth, E.; Gingerich, A.; Rada, B. Antimicrobial actions of dual oxidases and lactoperoxidase. J. Microbiol. 2018, 56, 373–386. [Google Scholar] [CrossRef]
- Rada, B.; Leto, T. Oxidative innate immune defenses by Nox/Duox Family NADPH oxidases. Contrib. Microbiol. 2008, 15, 164–187. [Google Scholar]
- Moreau-Marquis, S.; Coutermarsh, B.; Stanton, B.A. Combination of hypothiocyanite and lactoferrin (ALX-109) enhances the ability of tobramycin and aztreonam to eliminate Pseudomonas aeruginosa biofilms growing on cystic fibrosis airway epithelial cells. J. Antimicrob. Chemother. 2015, 70, 160–166. [Google Scholar] [CrossRef]
- El-Fakharany, E.M.; Uversky, V.N.; Redwan, E.M. Comparative Analysis of the Antiviral Activity of Camel, Bovine, and Human Lactoperoxidases Against Herpes Simplex Virus Type 1. Appl. Biochem. Biotechnol. 2017, 182, 294–310. [Google Scholar] [CrossRef]
- Moskwa, P.; Lorentzen, D.; Excoffon, K.J.D.A.; Zabner, J.; McCray, P.B.; Nauseef, W.M.; Dupuy, C.; Bánfi, B. A novel host defense system of airways is defective in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2007, 175, 174–183. [Google Scholar] [CrossRef]
- de Winter-de Groot, K.M.; van der Ent, C.K. Nitric oxide in cystic fibrosis. J. Cyst. Fibros. 2005, 4, 25–29. [Google Scholar] [CrossRef]
- Grasemann, H.; Michler, E.; Wallot, M.; Ratjen, F. Decreased concentration of exhaled nitric oxide (NO) in patients with cystic fibrosis. Pediatr. Pulmonol. 1997, 24, 173–177. [Google Scholar] [CrossRef]
- Causer, A.J.; Shute, J.K.; Cummings, M.H.; Shepherd, A.I.; Gruet, M.; Costello, J.T.; Bailey, S.; Lindley, M.; Pearson, C.; Connett, G.; et al. Circulating biomarkers of antioxidant status and oxidative stress in people with cystic fibrosis: A systematic review and meta-analysis. Redox Biol. 2020, 101436. [Google Scholar] [CrossRef] [PubMed]
- Fomenko, D.E.; Koc, A.; Agisheva, N.; Jacobsen, M.; Kaya, A.; Malinouski, M.; Rutherford, J.C.; Siu, K.L.; Jin, D.Y.; Winge, D.R.; et al. Thiol peroxidases mediate specific genome-wide regulation of gene expression in response to hydrogen peroxide. Proc. Natl. Acad. Sci. USA 2011, 108, 2729–2734. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I. Antioxidant therapies in COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2006, 1, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Leir, S.H.; Harris, A. Oxidative stress regulates CFTR gene expression in human airway epithelial cells through a distal antioxidant response element. Am. J. Respir. Cell Mol. Biol. 2015, 52, 387–396. [Google Scholar] [CrossRef]
- Chen, J.; Kinter, M.; Shank, S.; Cotton, C.; Kelley, T.J.; Ziady, A.G. Dysfunction of Nrf-2 in CF epithelia leads to excess intracellular H2O2 and inflammatory cytokine production. PLoS ONE 2008, 3, e3367. [Google Scholar] [CrossRef]
- Hudson, V.M. Rethinking cystic fibrosis pathology: The critical role of abnormal reduced glutathione (GSH) transport caused by CFTR mutation. Free Radic. Biol. Med. 2001, 30, 1440–1461. [Google Scholar] [CrossRef]
- Ghezzi, P. Role of glutathione in immunity and inflammation in the lung. Int. J. Gen. Med. 2011, 4, 105–113. [Google Scholar] [CrossRef]
- de Bari, L.; Favia, M.; Bobba, A.; Lassandro, R.; Guerra, L.; Atlante, A. Aberrant GSH reductase and NOX activities concur with defective CFTR to pro-oxidative imbalance in cystic fibrosis airways. J. Bioenerg. Biomembr. 2018, 50, 117–129. [Google Scholar] [CrossRef]
- Kettle, A.J.; Turner, R.; Gangell, C.L.; Harwood, D.T.; Khalilova, I.S.; Chapman, A.L.; Winterbourn, C.C.; Sly, P.D. Oxidation contributes to low glutathione in the airways of children with cystic fibrosis. Eur. Respir. J. 2014, 44, 122–129. [Google Scholar] [CrossRef]
- Sathe, M.N.; Patel, A.S. Update in pediatrics: Focus on fat-soluble vitamins. Nutr. Clin. Pract. 2010, 25, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Traber, M.G. The ABCs of vitamin E and β-carotene absorption. Am. J. Clin. Nutr. 2004, 80, 3–4. [Google Scholar] [CrossRef]
- Back, E.I.; Frindt, C.; Nohr, D.; Frank, J.; Ziebach, R.; Stern, M.; Ranke, M.; Biesalski, H.K. Antioxidant deficiency in cystic fibrosis: When is the right time to take action? Am. J. Clin. Nutr. 2004, 80, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Wood, Z.A.; Schröder, E.; Harris, J.R.; Poole, L.B. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 2003, 28, 32–40. [Google Scholar] [CrossRef]
- Iuliano, L.; Monticolo, R.; Straface, G.; Zullo, S.; Galli, F.; Boaz, M.; Quattrucci, S. Association of cholesterol oxidation and abnormalities in fatty acid metabolism in cystic fibrosis. Am. J. Clin. Nutr. 2009, 90, 477–484. [Google Scholar] [CrossRef]
- Gunasekara, L.; Al-Saiedy, M.; Green, F.; Pratt, R.; Bjornson, C.; Yang, A.; Michael Schoel, W.; Mitchell, I.; Brindle, M.; Montgomery, M.; et al. Pulmonary surfactant dysfunction in pediatric cystic fibrosis: Mechanisms and reversal with a lipid-sequestering drug. J. Cyst. Fibros. 2017, 16, 565–572. [Google Scholar] [CrossRef]
- Cheer, S.M.; Waugh, J.; Noble, S. Inhaled Tobramycin (TOBI®): A Review of its Use in the Management of Pseudomonas aeruginosa Infections in Patients with Cystic Fibrosis. Drugs 2003, 63, 2501–2520. [Google Scholar] [CrossRef]
- Chmiel, J.F.; Konstan, M.W.; Elborn, J.S. Antibiotic and anti-inflammatory therapies for cystic fibrosis. Cold Spring Harb. Perspect. Med. 2013, 3, a009779. [Google Scholar] [CrossRef]
- Nichols, D.P.; Durmowicz, A.G.; Field, A.; Flume, P.A.; VanDevanter, D.R.; Mayer-Hamblett, N. Developing inhaled antibiotics in cystic fibrosis: Current challenges and opportunities. Ann. Am. Thorac. Soc. 2019, 16, 534–539. [Google Scholar] [CrossRef]
- Moss, R.B. Long-term benefits of inhaled tobramycin in adolescent patients with cystic fibrosis. Chest 2002, 121, 55–63. [Google Scholar] [CrossRef]
- Konstan, M.W.; Flume, P.A.; Kappler, M.; Chiron, R.; Higgins, M.; Brockhaus, F.; Zhang, J.; Angyalosi, G.; He, E.; Geller, D.E. Safety, efficacy and convenience of tobramycin inhalation powder in cystic fibrosis patients: The EAGER trial. J. Cyst. Fibros. 2011, 10, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Hengzhuang, W.; Green, K.; Pressler, T.; Skov, M.; Katzenstein, T.L.; Wu, X.; Høiby, N. Optimization of colistin dosing regimen for cystic fibrosis patients with chronic Pseudomonas aeruginosa biofilm lung infections. Pediatr. Pulmonol. 2019, 54, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Konstan, M.W. Ibuprofen therapy for cystic fibrosis lung disease: Revisited. Curr. Opin. Pulm. Med. 2008, 14, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Lands, L.C.; Stanojevic, S. Oral non-steroidal anti-inflammatory drug therapy for cystic fibrosis. Cochrane Database Syst. Rev. 1999, CD001505. [Google Scholar] [CrossRef]
- Köhler, E.; Sollich, V.; Schuster-Wonka, R.; Jorch, G. Lung deposition after electronically breath-controlled inhalation and manually triggered conventional inhalation in cystic fibrosis patients. J. Aerosol Med. Depos. Clear. Eff. Lung 2005, 18, 386–395. [Google Scholar] [CrossRef]
- Salvatore, D.; D’Andria, M. Effects of salmeterol on arterial oxyhemoglobin saturations in patients with cystic fibrosis. Pediatr. Pulmonol. 2002, 34, 11–15. [Google Scholar] [CrossRef]
- Robinson, M.; Regnis, J.A.; Bailey, D.L.; King, M.; Bautovich, G.J.; Bye, P.T.P. Effect of hypertonic saline, amiloride, and cough on mucociliary clearance in patients with cystic fibrosis. Am. J. Respir. Crit. Care Med. 1996, 153, 1503–1509. [Google Scholar] [CrossRef]
- Quan, J.M.; Tiddens, H.A.W.M.; Sy, J.P.; McKenzie, S.G.; Montgomery, M.D.; Robinson, P.J.; Wohl, M.E.B.; Konstan, M.W. A two-year randomized, placebo-controlled trial of dornase alfa in young patients with cystic fibrosis with mild lung function abnormalities. J. Pediatr. 2001, 139, 813–820. [Google Scholar] [CrossRef]
- Yang, C.; Montgomery, M. Dornase alfa for cystic fibrosis. Cochrane Database Syst. Rev. 2018, 2018, CD001127. [Google Scholar] [CrossRef]
- Shak, S.; Capon, D.J.; Hellmiss, R.; Marsters, S.A.; Baker, C.L. Recombinant human DNase I reduces the viscosity of cystic fibrosis sputum. Proc. Natl. Acad. Sci. USA 1990, 87, 9188–9192. [Google Scholar] [CrossRef]
- Aitken, M.L.; Burke, W.; McDonald, G.; Shak, S.; Montgomery, A.B.; Smith, A. Recombinant human DNase inhalation in normal subjects and patients with cystic fibrosis. A phase 1 study. JAMA 1992, 267, 1947–1951. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, R.C.; Mcelvaney, N.G.; Birrer, P.; Robinson, W.W.; Jolley, C.; Crystal, R.G.; Shak, S.; Wu, M.; Chernick, M.S. A preliminary study of aerosolized recombinant human deoxyribonuclease i in the treatment of cystic fibrosis. N. Engl. J. Med. 1992, 326, 812–815. [Google Scholar] [CrossRef] [PubMed]
- Radtke, T.; Nevitt, S.J.; Hebestreit, H.; Kriemler, S. Physical exercise training for cystic fibrosis. Cochrane Database Syst. Rev. 2017, 2017, CD002768. [Google Scholar] [CrossRef] [PubMed]
- Morrison, L.; Milroy, S. Oscillating devices for airway clearance in people with cystic fibrosis. Cochrane Database Syst. Rev. 2020, 2020, CD006842. [Google Scholar]
- Shaw, T.D.; McAuley, D.F.; O’Kane, C.M. Emerging drugs for treating the acute respiratory distress syndrome. Expert Opin. Emerg. Drugs 2019, 24, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Villar, J.; Ferrando, C.; Martínez, D.; Ambrós, A.; Muñoz, T.; Soler, J.A.; Aguilar, G.; Alba, F.; González-Higueras, E.; Conesa, L.A.; et al. Dexamethasone treatment for the acute respiratory distress syndrome: A multicentre, randomised controlled trial. Lancet Respir. Med. 2020, 8, 267–276. [Google Scholar] [CrossRef]
- Villar, J.; Belda, J.; Añón, J.M.; Blanco, J.; Pérez-Méndez, L.; Ferrando, C.; Martínez, D.; Soler, J.A.; Ambrós, A.; Muñoz, T.; et al. Evaluating the efficacy of dexamethasone in the treatment of patients with persistent acute respiratory distress syndrome: Study protocol for a randomized controlled trial. Trials 2016, 17, 342. [Google Scholar] [CrossRef]
- Festic, E.; Carr, G.E.; Cartin-Ceba, R.; Hinds, R.F.; Banner-Goodspeed, V.; Bansal, V.; Asuni, A.T.; Talmor, D.; Rajagopalan, G.; Frank, R.D.; et al. Randomized clinical trial of a combination of an inhaled corticosteroid and beta agonist in patients at risk of developing the acute respiratory distress syndrome. Crit. Care Med. 2017, 45, 798–805. [Google Scholar] [CrossRef]
- Matthay, M.A.; McAuley, D.F.; Ware, L.B. Clinical trials in acute respiratory distress syndrome: Challenges and opportunities. Lancet Respir. Med. 2017, 5, 524–534. [Google Scholar] [CrossRef]
- NCT03096314 Vitamin D to Improve Outcomes by Leveraging Early Treatment. 2017. Available online: https://clinicaltrials.gov/show/NCT03096314 (accessed on 2 May 2020).
- Parekh, D.; Dancer, R.C.A.; Scott, A.; D’Souza, V.K.; Howells, P.A.; Mahida, R.Y.; Tang, J.C.Y.; Cooper, M.S.; Fraser, W.D.; Tan, L.C.; et al. Vitamin D to Prevent Lung Injury Following Esophagectomy-A Randomized, Placebo-Controlled Trial. Crit. Care Med. 2018, 46, e1128–e1135. [Google Scholar] [CrossRef]
- Ginde, A.A.; Brower, R.G.; Caterino, J.M.; Finck, L.; Banner-Goodspeed, V.M.; Grissom, C.K.; Hayden, D.; Hough, C.L.; Hyzy, R.C.; Khan, A.; et al. Early high-dose Vitamin D3 for critically ill, Vitamin D–deficient patients. N. Engl. J. Med. 2019, 381, 2529–2540. [Google Scholar] [PubMed]
- Toner, P.; McAuley, D.F.; Shyamsundar, M. Aspirin as a potential treatment in sepsis or acute respiratory distress syndrome. Crit. Care 2015, 19, 374. [Google Scholar] [CrossRef] [PubMed]
- Harr, J.N.; Moore, E.E.; Johnson, J.; Chin, T.L.; Wohlauer, M.V.; Maier, R.; Cuschieri, J.; Sperry, J.; Banerjee, A.; Silliman, C.C.; et al. Antiplatelet therapy is associated with decreased transfusion-associated risk of lung dysfunction, multiple organ failure, and mortality in trauma patients. Crit. Care Med. 2013, 41, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Hamid, U.; Krasnodembskaya, A.; Fitzgerald, M.; Shyamsundar, M.; Kissenpfennig, A.; Scott, C.; Lefrancais, E.; Looney, M.R.; Verghis, R.; Scott, J.; et al. Aspirin reduces lipopolysaccharide-induced pulmonary inflammation in human models of ARDS. Thorax 2017, 72, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Matthay, M.A.; Calfee, C.S.; Zhuo, H.; Thompson, B.T.; Wilson, J.G.; Levitt, J.E.; Rogers, A.J.; Gotts, J.E.; Wiener-Kronish, J.P.; Bajwa, E.K.; et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): A randomised phase 2a safety trial. Lancet Respir. Med. 2019, 7, 154–162. [Google Scholar] [CrossRef]
- Fisher, B.J.; Kraskauskas, D.; Martin, E.J.; Farkas, D.; Wegelin, J.A.; Brophy, D.; Ward, K.R.; Voelkel, N.F.; Fowler, A.A.; Natarajan, R. Mechanisms of attenuation of abdominal sepsis induced acute lung injury by ascorbic acid. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 303, L20–L32. [Google Scholar] [CrossRef] [PubMed]
- NCT02106975 Vitamin C Infusion for Treatment in Sepsis Induced Acute Lung Injury—Full Text View—ClinicalTrials.gov. 2019. Available online: https://clinicaltrials.gov/show/NCT02106975 (accessed on 20 September 2020).
- Fowler, A.A.; Truwit, J.D.; Hite, R.D.; Morris, P.E.; Dewilde, C.; Priday, A.; Fisher, B.; Thacker, L.R.; Natarajan, R.; Brophy, D.F.; et al. Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients with Sepsis and Severe Acute Respiratory Failure: The CITRIS-ALI Randomized Clinical Trial. JAMA J. Am. Med. Assoc. 2019, 322, 1261–1270. [Google Scholar] [CrossRef]
- Dixon, B.; Schultz, M.J.; Smith, R.; Fink, J.B.; Santamaria, J.D.; Campbell, D.J. Nebulized heparin is associated with fewer days of mechanical ventilation in critically ill patients: A randomized controlled trial. Crit. Care 2010, 14, R180. [Google Scholar] [CrossRef]
- ACTRN12612000418875 A Multi-Centre Randomised, Placebo Controlled Trial of Nebulised Heparin in Patients with or at Risk of Developing Acute Respiratory Distress Syndrome, to Determine if Nebulised Heparin Improves Long Term Physical Function. Australian New Zealand Clinical Trials Registry. 2012. Available online: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=362354 (accessed on 25 September 2020).
- Morris, P.E.; Steingrub, J.S.; Huang, B.Y.; Tang, S.; Liu, P.M.; Rhode, P.R.; Wong, H.C. A phase I study evaluating the pharmacokinetics, safety and tolerability of an antibody-based tissue factor antagonist in subjects with acute lung injury or acute respiratory distress syndrome. BMC Pulm. Med. 2012, 12, 5. [Google Scholar] [CrossRef]
- NCT00879606 Anti-TF Antibody (ALT-836) to Treat Septic Patients With Acute Lung Injury or Acute Respiratory Distress Syndrome—Full Text View—ClinicalTrials.gov. 2015. Available online: https://clinicaltrials.gov/show/NCT00879606 (accessed on 2 May 2020).
- Denham, W.; Yang, J.; Norman, J.; Wang, H.; Botchkina, G.; Tracey, K.J. Inhibition of p38 mitogen activate kinase attenuates the severity of pancreatitis-induced adult respiratory distress syndrome. Crit. Care Med. 2000, 28, 2567–2572. [Google Scholar] [CrossRef]
- Christie, J.D.; Vaslef, S.; Chang, P.K.; May, A.K.; Gunn, S.R.; Yang, S.; Hardes, K.; Kahl, L.; Powley, W.M.; Lipson, D.A.; et al. A Randomized Dose-Escalation Study of the Safety and Anti-Inflammatory Activity of the p38 Mitogen-Activated Protein Kinase Inhibitor Dilmapimod in Severe Trauma Subjects at Risk for Acute Respiratory Distress Syndrome. Crit. Care Med. 2015, 43, 1859–1869. [Google Scholar] [CrossRef] [PubMed]
- Tagami, T.; Tosa, R.; Omura, M.; Fukushima, H.; Kaneko, T.; Endo, T.; Rinka, H.; Murai, A.; Yamaguchi, J.; Yoshikawa, K.; et al. Effect of a selective neutrophil elastase inhibitor on mortality and ventilator-free days in patients with increased extravascular lung water: A post hoc analysis of the PiCCO Pulmonary Edema Study. J. Intensive Care 2014, 2, 67. [Google Scholar] [CrossRef] [PubMed]
- Kido, T.; Muramatsu, K.; Yatera, K.; Asakawa, T.; Otsubo, H.; Kubo, T.; Fujino, Y.; Matsuda, S.; Mayumi, T.; Mukae, H. Efficacy of early sivelestat administration on acute lung injury and acute respiratory distress syndrome. Respirology 2017, 22, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.; Wang, D.; Liu, D.; Zhao, Y.; Qi, D.; He, J.; Zhou, G. Effect of sivelestat sodium in patients with acute lung injury or acute respiratory distress syndrome: A meta-analysis of randomized controlled trials. BMC Pulm. Med. 2017, 17, 148. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.Z.; Tu, Y.Y.; Chen, X.; Wang, B.L.; Zhong, Y.X.; Liu, M.H. Protective effect of Ulinastatin against murine models of sepsis: Inhibition of TNF-α and IL-6 and augmentation of IL-10 and IL-13. Exp. Toxicol. Pathol. 2012, 64, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.-X. Ulinastatin for acute lung injury and acute respiratory distress syndrome: A systematic review and meta-analysis. World J. Crit. Care Med. 2014, 3, 34. [Google Scholar] [CrossRef]
- NCT02895191 The Safety and Dose Response Relationship of Ulinastatin for Acute Respiratory Distress Syndrome(ARDS)—Full Text View—ClinicalTrials.gov. 2018. Available online: https://clinicaltrials.gov/show/NCT02895191 (accessed on 2 May 2020).
- Paine, R.; Standiford, T.J.; Dechert, R.E.; Moss, M.; Martin, G.S.; Rosenberg, A.L.; Thannickal, V.J.; Burnham, E.L.; Brown, M.B.; Hyzy, R.C. A randomized Trial of recombinant human granulocyte-macrophage colony stimulating factor for Patients with acute lung injury. Crit. Care Med. 2012, 40, 90–97. [Google Scholar] [CrossRef]
- Frevert, C.W.; Matute-Bello, G.; Skerrett, S.J.; Goodman, R.B.; Kajikawa, O.; Sittipunt, C.; Martin, T.R. Effect of CD14 Blockade in Rabbits with Escherichia coli Pneumonia and Sepsis. J. Immunol. 2000, 164, 5439–5445. [Google Scholar] [CrossRef]
- NCT03017547 A Phase 2 Study of IC14 in Acute Respiratory Distress Syndrome—Full Text View—ClinicalTrials.gov. 2019. Available online: https://clinicaltrials.gov/show/NCT03017547 (accessed on 2 May 2020).
- Fuller, B.M.; Mohr, N.M.; Skrupky, L.; Fowler, S.; Kollef, M.H.; Carpenter, C.R. The use of inhaled prostaglandins in patients with ARDS: A systematic review and meta-analysis. Chest 2015, 147, 1510–1522. [Google Scholar] [CrossRef]
- Bosmann, M.; Grailer, J.J.; Ruemmler, R.; Russkamp, N.F.; Zetoune, F.S.; Sarma, J.V.; Standiford, T.J.; Ward, P.A. Extracellular histones are essential effectors of C5aR- and C5L2-mediated tissue damage and inflammation in acute lung injury. FASEB J. 2013, 27, 5010–5021. [Google Scholar] [CrossRef]
- Wildhagen, K.C.A.A.; De Frutos, P.G.; Reutelingsperger, C.P.; Schrijver, R.; Aresté, C.; Ortega-Gómez, A.; Deckers, N.M.; Hemker, H.C.; Soehnlein, O.; Nicolaes, G.A.F. Nonanticoagulant heparin prevents histone-mediated cytotoxicity in vitro and improves survival in sepsis. Blood 2014, 123, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Kalliolias, G.D.; Liossis, S.N.C. The future of the IL-1 receptor antagonist anakinra: From rheumatoid arthritis to adult-onset still’s disease and systemic-onset juvenile idiopathic arthritis. Expert Opin. Investig. Drugs 2008, 17, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Lon, H.K.; DuBois, D.C.; Almon, R.R.; Jusko, W.J. Population pharmacokinetic-pharmacodynamic-disease progression model for effects of anakinra in Lewis rats with collagen-induced arthritis. J. Pharmacokinet. Pharmacodyn. 2011, 38, 769–786. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Patel, V.J.; Biswas Roy, S.; Mehta, H.J.; Joo, M.; Sadikot, R.T. Alternative and Natural Therapies for Acute Lung Injury and Acute Respiratory Distress Syndrome. Biomed Res. Int. 2018, 2018, 2476824. [Google Scholar] [CrossRef]
- Wechsler, M.E. Current and emerging biologic therapies for asthma and copd. Respir. Care 2018, 63, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, K.H. Asthma treatments: New and emerging therapies. Int. Forum Allergy Rhinol. 2015, 5, S76–S81. [Google Scholar] [CrossRef]
- Durham, A.L.; Caramori, G.; Chung, K.F.; Adcock, I.M. Targeted anti-inflammatory therapeutics in asthma and chronic obstructive lung disease. Transl. Res. 2016, 167, 192–203. [Google Scholar] [CrossRef]
- Tashkin, D.P.; Wechsler, M.E. Role of eosinophils in airway inflammation of chronic obstructive pulmonary disease. Int. J. COPD 2018, 13, 335–349. [Google Scholar] [CrossRef]
- Corren, J.; Casale, T.; Deniz, Y.; Ashby, M. Omalizumab, a recombinant humanized anti-IgE antibody, reduces asthma-related emergency room visits and hospitalizations in patients with allergic asthma. J. Allergy Clin. Immunol. 2003, 111, 87–90. [Google Scholar] [CrossRef]
- Cowan, D.C.; Taylor, D.R.; Peterson, L.E.; Cowan, J.O.; Palmay, R.; Williamson, A.; Hammel, J.; Erzurum, S.C.; Hazen, S.L.; Comhair, S.A.A. Biomarker-based asthma phenotypes of corticosteroid response. J. Allergy Clin. Immunol. 2015, 135, 877–883.e1. [Google Scholar] [CrossRef]
- Fajt, M.L.; Wenzel, S.E. Biologic therapy in asthma: Entering the new age of personalized medicine. J. Asthma 2014, 51, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Apter, A.J. Advances in adult asthma diagnosis and treatment in 2014. J. Allergy Clin. Immunol. 2015, 135, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Ortega, H.G.; Liu, M.C.; Pavord, I.D.; Brusselle, G.G.; FitzGerald, J.M.; Chetta, A.; Humbert, M.; Katz, L.E.; Keene, O.N.; Yancey, S.W.; et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N. Engl. J. Med. 2014, 371, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Albers, F.C.; Hozawa, S.; Bratton, D.J.; Yancey, S.W.; Prazma, C.M.; Humbert, M.; Liu, M.C. Update: Mepolizumab treatment in patients with severe eosinophilic asthma and prior omalizumab use. Allergy Eur. J. Allergy Clin. Immunol. 2020, 75, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Flood-Page, P.T.; Menzies-Gow, A.N.; Kay, A.B.; Robinson, D.S. Eosinophil’s role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am. J. Respir. Crit. Care Med. 2003, 167, 199–204. [Google Scholar] [CrossRef]
- Haldar, P.; Brightling, C.E.; Singapuri, A.; Hargadon, B.; Gupta, S.; Monteiro, W.; Bradding, P.; Green, R.H.; Wardlaw, A.J.; Ortega, H.; et al. Outcomes after cessation of mepolizumab therapy in severe eosinophilic asthma: A 12-month follow-up analysis. J. Allergy Clin. Immunol. 2014, 133, 921–923. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.; Zangrilli, J.; Wechsler, M.E.; Bateman, E.D.; Brusselle, G.G.; Bardin, P.; Murphy, K.; Maspero, J.F.; O’Brien, C.; Korn, S. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: Results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir. Med. 2015, 3, 355–366. [Google Scholar] [CrossRef]
- Castro, M.; King, T.S.; Kunselman, S.J.; Cabana, M.D.; Denlinger, L.; Holguin, F.; Kazani, S.D.; Moore, W.C.; Moy, J.; Sorkness, C.A.; et al. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: The VIDA randomized clinical trial. JAMA J. Am. Med. Assoc. 2014, 311, 2083–2091. [Google Scholar] [CrossRef]
- Ibrahim, H.; O’Sullivan, R.; Casey, D.; Murphy, J.; MacSharry, J.; Plant, B.J.; Murphy, D.M. The effectiveness of Reslizumab in severe asthma treatment: A real-world experience. Respir. Res. 2019, 20, 289. [Google Scholar] [CrossRef]
- Christian Virchow, J.; McDonald, M.; Garin, M.; Korn, S. Reslizumab as add-on therapy in patients with refractory asthma. BMJ Open Respir. Res. 2020, 7, e000494. [Google Scholar] [CrossRef]
- Markham, A. Benralizumab: First Global Approval. Drugs 2018, 78, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Saco, T.V.; Pepper, A.N.; Lockey, R.F. Benralizumab for the treatment of asthma. Expert Rev. Clin. Immunol. 2017, 13, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Bleecker, E.R.; FitzGerald, J.M.; Chanez, P.; Papi, A.; Weinstein, S.F.; Barker, P.; Sproule, S.; Gilmartin, G.; Aurivillius, M.; Werkström, V.; et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): A randomised, multicentre, placebo-controlled phase 3 trial. Lancet 2016, 388, 2115–2127. [Google Scholar] [CrossRef]
- Nair, P.; Wenzel, S.; Rabe, K.F.; Bourdin, A.; Lugogo, N.L.; Kuna, P.; Barker, P.; Sproule, S.; Ponnarambil, S.; Goldman, M. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N. Engl. J. Med. 2017, 376, 2448–2458. [Google Scholar] [CrossRef] [PubMed]
- Shirley, M. Dupilumab: First Global Approval. Drugs 2017, 77, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K.F.; Nair, P.; Brusselle, G.; Maspero, J.F.; Castro, M.; Sher, L.; Zhu, H.; Hamilton, J.D.; Swanson, B.N.; Khan, A.; et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N. Engl. J. Med. 2018, 378, 2475–2485. [Google Scholar] [CrossRef]
- Castro, M.; Corren, J.; Pavord, I.D.; Maspero, J.; Wenzel, S.; Rabe, K.F.; Busse, W.W.; Ford, L.; Sher, L.; FitzGerald, J.M.; et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N. Engl. J. Med. 2018, 378, 2486–2496. [Google Scholar] [CrossRef]
- Hanania, N.A.; Korenblat, P.; Chapman, K.R.; Bateman, E.D.; Kopecky, P.; Paggiaro, P.; Yokoyama, A.; Olsson, J.; Gray, S.; Holweg, C.T.J.; et al. Efficacy and safety of lebrikizumab in patients with uncontrolled asthma (LAVOLTA I and LAVOLTA II): Replicate, phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir. Med. 2016, 4, 781–796. [Google Scholar] [CrossRef]
- Brightling, C.E.; Chanez, P.; Leigh, R.; O’Byrne, P.M.; Korn, S.; She, D.; May, R.D.; Streicher, K.; Ranade, K.; Piper, E. Efficacy and safety of tralokinumab in patients with severe uncontrolled asthma: A randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir. Med. 2015, 3, 692–701. [Google Scholar] [CrossRef]
- Korenblat, P.; Kerwin, E.; Leshchenko, I.; Yen, K.; Holweg, C.T.J.; Anzures-Cabrera, J.; Martin, C.; Putnam, W.S.; Governale, L.; Olsson, J.; et al. Efficacy and safety of lebrikizumab in adult patients with mild-to-moderate asthma not receiving inhaled corticosteroids. Respir. Med. 2018, 134, 143–149. [Google Scholar] [CrossRef]
- Busse, W.W.; Brusselle, G.G.; Korn, S.; Kuna, P.; Magnan, A.; Cohen, D.; Bowen, K.; Piechowiak, T.; Wang, M.M.; Colice, G. Tralokinumab did not demonstrate oral corticosteroid-sparing effects in severe asthma. Eur. Respir. J. 2019, 53, 1800948. [Google Scholar] [CrossRef] [PubMed]
- NCT03927157 Study to Evaluate Tezepelumab in Adults With Severe Uncontrolled Asthma—Full Text View—ClinicalTrials.gov. 2020. Available online: https://clinicaltrials.gov/show/NCT03927157 (accessed on 2 May 2020).
- Marone, G.; Spadaro, G.; Braile, M.; Poto, R.; Criscuolo, G.; Pahima, H.; Loffredo, S.; Levi-Schaffer, F.; Varricchi, G. Tezepelumab: A novel biological therapy for the treatment of severe uncontrolled asthma. Expert Opin. Investig. Drugs 2019, 28, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Zou, G.L.; Zhang, W.; Lai, X.N.; Chen, H.W.; Xiong, L.X. Interleukin-33: Its emerging role in allergic diseases. Molecules 2018, 23, 1665. [Google Scholar] [CrossRef] [PubMed]
- Busse, W.W.; Holgate, S.; Kerwin, E.; Chon, Y.; Feng, J.; Lin, J.; Lin, S. Study of Brodalumab, a Human Anti – IL-17 Receptor Monoclonal Antibody, in Moderate to Severe Asthma. Am. J. Respir. Crit. Care Med. 2013, 188, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.; Gaga, M.; Zervas, E.; Alagha, K.; Hargreave, F.E.; O’Byrne, P.M.; Stryszak, P.; Gann, L.; Sadeh, J.; Chanez, P. Safety and efficacy of a CXCR2 antagonist in patients with severe asthma and sputum neutrophils: A randomized, placebo-controlled clinical trial. Clin. Exp. Allergy 2012, 42, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- NCT00632502 Neutrophilic Asthma Study With Navarixin (MK-7123, SCH 527123) (MK-7123-017)(COMPLETED)—Full Text View—ClinicalTrials.gov. 2019. Available online: https://clinicaltrials.gov/show/NCT00632502 (accessed on 2 May 2020).
- Imaoka, H.; Campbell, H.; Babirad, I.; Watson, R.M.; Mistry, M.; Sehmi, R.; Gauvreau, G.M. TPI ASM8 reduces eosinophil progenitors in sputum after allergen challenge. Clin. Exp. Allergy 2011, 41, 1740–1746. [Google Scholar] [CrossRef]
- Cahill, K.N.; Katz, H.R.; Cui, J.; Lai, J.; Kazani, S.; Crosby-Thompson, A.; Garofalo, D.; Castro, M.; Jarjour, N.; DiMango, E.; et al. KIT inhibition by imatinib in patients with severe refractory asthma. N. Engl. J. Med. 2017, 376, 1911–1920. [Google Scholar] [CrossRef]
- Howarth, P.H.; Babu, K.S.; Arshad, H.S.; Lau, L.; Buckley, M.; McConnell, W.; Beckett, P.; Al Ali, M.; Chauhan, A.; Wilson, S.J.; et al. Tumour necrosis factor (TNFα) as a novel therapeutic target in symptomatic corticosteroid dependent asthma. Thorax 2005, 60, 1012–1018. [Google Scholar] [CrossRef]
- Oliveri, C.; Polosa, R. Etanercept in chronic severe asthma. Thorax 2006, 61, 640. [Google Scholar]
- Holgate, S.T.; Noonan, M.; Chanez, P.; Busse, W.; Dupont, L.; Pavord, I.; Hakulinen, A.; Paolozzi, L.; Wajdula, J.; Zang, C.; et al. Efficacy and safety of etanercept in moderate-to-severe asthma: A randomised, controlled trial. Eur. Respir. J. 2011, 37, 1352–1359. [Google Scholar] [CrossRef]
- Morjaria, J.B.; Chauhan, A.J.; Babu, K.S.; Polosa, R.; Davies, D.E.; Holgate, S.T. The role of a soluble TNFα receptor fusion protein (etanercept) in corticosteroid refractory asthma: A double blind, randomised, placebo controlled trial. Thorax 2008, 63, 584–591. [Google Scholar] [CrossRef]
- Zhu, L.-Y.; Ni, Z.-H.; Luo, X.-M.; Wang, X.-B. Advance of antioxidants in asthma treatment. World J. Respirol. 2017, 7, 17. [Google Scholar] [CrossRef]
- Bray, T.M.; Bettger, W.J. The physiological role of zinc as an antioxidant. Free Radic. Biol. Med. 1990, 8, 281–291. [Google Scholar] [CrossRef]
- Machlin, L.J.; Bendich, A. Free radical tissue damage: Protective role of antioxidant nutrients. FASEB J. 1987, 1, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.G.; Garg, M.L.; Smart, J.M.; Scott, H.A.; Barker, D.; Gibson, P.G. Manipulating antioxidant intake in asthma: A randomized controlled trial1-3. Am. J. Clin. Nutr. 2012, 96, 534–543. [Google Scholar] [CrossRef]
- Kurti, S.P.; Rosenkranz, S.K.; Chapes, S.K.; Teeman, C.S.; Cull, B.J.; Emerson, S.R.; Levitt, M.H.; Smith, J.R.; Harms, C.A. Does chronic physical activity level modify the airway inflammatory response to an acute bout of exercise in the postprandial period? Appl. Physiol. Nutr. Metab. 2017, 42, 173–180. [Google Scholar] [CrossRef]
- Eftekhari, P.; Hajizadeh, S.; Reza Raoufy, M.; Reza Masjedi, M.; Yang, M.; Hansbro, N.; Li, J.J.; Foster, P.S. Preventive effect of N-acetylcysteine in a mouse model of steroid resistant acute exacerbation of asthma. EXCLI J. 2013, 12, 184–192. [Google Scholar]
- NCT02605824 Clinical Trial of NAC in Asthma—Full Text View—ClinicalTrials.gov. 2019. Available online: https://clinicaltrials.gov/show/NCT02605824 (accessed on 2 May 2020).
- Sakoda, C.P.P.; de Toledo, A.C.; Perini, A.; Pinheiro, N.M.; Hiyane, M.I.; dos Grecco, S.S.; de Fátima Lopes Calvo Tibério, I.; Câmara, N.O.S.; de Arruda Martins, M.; Lago, J.H.G.; et al. Sakuranetin reverses vascular peribronchial and lung parenchyma remodeling in a murine model of chronic allergic pulmonary inflammation. Acta Histochem. 2016, 118, 615–624. [Google Scholar] [CrossRef]
- Toledo, A.C.; Sakoda, C.P.P.; Perini, A.; Pinheiro, N.M.; Magalhães, R.M.; Grecco, S.; Tibério, I.F.L.C.; Câmara, N.O.; Martins, M.A.; Lago, J.H.G.; et al. Flavonone treatment reverses airway inflammation and remodelling in an asthma murine model. Br. J. Pharmacol. 2013, 168, 1736–1749. [Google Scholar] [CrossRef]
- Cho, I.H.; Choi, Y.J.; Gong, J.H.; Shin, D.; Kang, M.K.; Kang, Y.H. Astragalin inhibits autophagy-associated airway epithelial fibrosis. Respir. Res. 2015, 16, 51. [Google Scholar] [CrossRef]
- Cho, I.H.; Gong, J.H.; Kang, M.K.; Lee, E.J.; Park, J.H.Y.; Park, S.J.; Kang, Y.H. Astragalin inhibits airway eotaxin-1 induction and epithelial apoptosis through modulating oxidative stress-responsive MAPK signaling. BMC Pulm. Med. 2014, 14, 122. [Google Scholar] [CrossRef] [PubMed]
- André, D.M.; Calixto, M.C.; Sollon, C.; Alexandre, E.C.; Leiria, L.O.; Tobar, N.; Anhê, G.F.; Antunes, E. Therapy with resveratrol attenuates obesity-associated allergic airway inflammation in mice. Int. Immunopharmacol. 2016, 38, 298–305. [Google Scholar] [CrossRef] [PubMed]
- André, D.M.; Calixto, M.C.; Sollon, C.; Alexandre, E.C.; Tavares, E.B.G.; Naime, A.C.A.; Anhê, G.F.; Antunes, E. High-fat diet-induced obesity impairs insulin signaling in lungs of allergen-challenged mice: Improvement by resveratrol. Sci. Rep. 2017, 7, 17296. [Google Scholar] [CrossRef] [PubMed]
- Kandhare, A.D.; Liu, Z.; Mukherjee, A.A.; Bodhankar, S.L. Therapeutic Potential of Morin in Ovalbumin-induced Allergic Asthma Via Modulation of SUMF2/IL-13 and BLT2/NF-kB Signaling Pathway. Curr. Mol. Pharmacol. 2019, 12, 122–138. [Google Scholar] [CrossRef]
- Bokhari, J.; Khan, M.R. Evaluation of anti-asthmatic and antioxidant potential of Boerhavia procumbens in toluene diisocyanate (TDI) treated rats. J. Ethnopharmacol. 2015, 172, 377–385. [Google Scholar] [CrossRef]
- Wang, L.; Xu, M.L.; Liu, J.; Wang, Y.; Hu, J.H.; Wang, M.H. Sonchus asper extract inhibits LPS-induced oxidative stress and pro-inflammatory cytokine production in RAW264.7 macrophages. Nutr. Res. Pract. 2015, 9, 579–585. [Google Scholar] [CrossRef]
- Ci, X.; Zhong, W.; Ren, H.; Wen, Z.; Li, D.; Peng, L. Esculentoside a attenuates allergic airway inflammation via activation of the Nrf-2 pathway. Int. Arch. Allergy Immunol. 2015, 167, 280–290. [Google Scholar] [CrossRef]
- Jang, H.Y.; Kim, S.M.; Yuk, J.E.; Kwon, O.K.; Oh, S.R.; Lee, H.K.; Jeong, H.; Ahn, K.S. Capsicum annuum L. methanolic extract inhibits ovalbumin-induced airway inflammation and oxidative stress in a mouse model of asthma. J. Med. Food 2011, 14, 1144–1151. [Google Scholar] [CrossRef]
- Sahreen, S.; Khan, M.R.; Khan, R.A.; Alkreathy, H.M. Cardioprotective role of leaves extracts of Carissa opaca against CCl 4 induced toxicity in rats. BMC Res. Notes 2014, 7, 224. [Google Scholar] [CrossRef]
- Bouch, S.; Harding, R.; O’Reilly, M.; Wood, L.G.; Sozo, F. Impact of dietary tomato juice on changes in pulmonary oxidative stress, inflammation and structure induced by neonatal hyperoxia in mice (Mus musculus). PLoS ONE 2016, 11, e0159633. [Google Scholar] [CrossRef]
- Pigati, P.A.; Righetti, R.F.; Possa, S.S.; Romanholo, B.S.; Rodrigues, A.P.D.; dos Santos, A.S.A.; Xisto, D.G.; Antunes, M.A.; Prado, C.M.; Leick, E.A.; et al. Y-27632 is associated with corticosteroid-potentiated control of pulmonary remodeling and inflammation in guinea pigs with chronic allergic inflammation. BMC Pulm. Med. 2015, 15, 85. [Google Scholar] [CrossRef] [PubMed]
- Righetti, R.F.; da Pigati, P.A.S.; Possa, S.S.; Habrum, F.C.; Xisto, D.G.; Antunes, M.A.; Leick, E.A.; Prado, C.M.; de Martins, M.A.; Rocco, P.R.M.; et al. Effects of Rho-kinase inhibition in lung tissue with chronic inflammation. Respir. Physiol. Neurobiol. 2014, 192, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Aristoteles, L.R.C.R.B.; Righetti, R.F.; Pinheiro, N.M.; Franco, R.B.; Starling, C.M.; da Silva, J.C.P.; Pigati, P.A.; Caperuto, L.C.; Prado, C.M.; Dolhnikoff, M.; et al. Modulation of the oscillatory mechanics of lung tissue and the oxidative stress response induced by arginase inhibition in a chronic allergic inflammation model. BMC Pulm. Med. 2013, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Campos, D.; Ravagnani, F.G.; Gurgueira, S.A.; Vercesi, A.E.; Teixeira, S.A.; Costa, S.K.P.; Muscará, M.N.; Ferreira, H.H.A. Increased glutathione levels contribute to the beneficial effects of hydrogen sulfide and inducible nitric oxide inhibition in allergic lung inflammation. Int. Immunopharmacol. 2016, 39, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, L.; Pini, A.; Gerace, E.; Pellicciari, R.; Masini, E.; Moroni, F. Poly(ADP-ribose) polymerase inhibition with HYDAMTIQ reduces allergen-induced asthma-like reaction, bronchial hyper-reactivity and airway remodelling. J. Cell. Mol. Med. 2014, 18, 468–479. [Google Scholar] [CrossRef]
- Dhawale, V.S.; Amara, V.R.; Karpe, P.A.; Malek, V.; Patel, D.; Tikoo, K. Activation of angiotensin-converting enzyme 2 (ACE2) attenuates allergic airway inflammation in rat asthma model. Toxicol. Appl. Pharmacol. 2016, 306, 17–26. [Google Scholar] [CrossRef]
- Ho, C.Y.; Lu, C.C.; Weng, C.J.; Yen, G.C. Protective Effects of Diallyl Sulfide on Ovalbumin-Induced Pulmonary Inflammation of Allergic Asthma Mice by MicroRNA-144, -34a, and -34b/c-Modulated Nrf2 Activation. J. Agric. Food Chem. 2016, 64, 151–160. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Hong, G.H.; Kwon, H.S.; Park, S.; Park, S.Y.; Shin, B.; Kim, T.B.; Moon, H.B.; Cho, Y.S. S-adenosylmethionine reduces airway inflammation and fibrosis in a murine model of chronic severe asthma via suppression of oxidative stress. Exp. Mol. Med. 2016, 48, e236. [Google Scholar] [CrossRef]
- Tlili, M.; Rouatbi, S.; Sriha, B.; Ben Rhouma, K.; Sakly, M.; Vaudry, D.; Wurtz, O.; Tebourbi, O. Pituitary Adenylate Cyclase-Activating Polypeptide Reverses Ammonium Metavanadate-Induced Airway Hyperresponsiveness in Rats. Oxid. Med. Cell. Longev. 2015, 2015, 787561. [Google Scholar] [CrossRef]
- Gong, J.H.; Shin, D.; Han, S.Y.; Park, S.H.; Kang, M.K.; Kim, J.L.; Kang, Y.H. Blockade of airway inflammation by kaempferol via disturbing Tyk-STAT signaling in airway epithelial cells and in asthmatic mice. Evid. Based Complement. Altern. Med. 2013, 2013, 250725. [Google Scholar] [CrossRef]
- Liu, H.; Xue, J.X.; Li, X.; Ao, R.; Lu, Y. Quercetin liposomes protect against radiation-induced pulmonary injury in a murine model. Oncol. Lett. 2013, 6, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhong, T.; Wu, H. Quercetin protects against lipopolysaccharide-induced acute lung injury in rats through suppression of inflammation and oxidative stress. Arch. Med. Sci. 2015, 11, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Yasui, M.; Matsushima, M.; Omura, A.; Mori, K.; Ogasawara, N.; Kodera, Y.; Shiga, M.; Ito, K.; Kojima, S.; Kawabe, T. The Suppressive Effect of Quercetin on Toll-Like Receptor 7-Mediated Activation in Alveolar Macrophages. Pharmacology 2015, 96, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lin, J.M.; Li, X.S.; Li, J. Quercetin ameliorates LPS-induced inflammation in human peripheral blood mononuclear cells by inhibition of the TLR2-NF-κB pathway. Genet. Mol. Res. 2016, 15, 15028297. [Google Scholar] [CrossRef]
- Takeda, K.; Miyahara, N.; Matsubara, S.; Taube, C.; Kitamura, K.; Hirano, A.; Tanimoto, M.; Gelfand, E.W. Immunomodulatory effects of ambroxol on airway hyperresponsiveness and inflammation. Immune Netw. 2016, 16, 165–175. [Google Scholar] [CrossRef]
- Raju, K.R.S.; Kumar, M.N.S.; Gupta, S.; Naga, S.T.; Shankar, J.K.; Murthy, V.; Madhunapanthula, S.R.V.; Mulukutla, S.; Ambhore, N.S.; Tummala, S.; et al. 5-Aminosalicylic Acid attenuates allergen-induced airway inflammation and oxidative stress in asthma. Pulm. Pharmacol. Ther. 2014, 29, 209–216. [Google Scholar] [CrossRef]
- Nader, M.A. Inhibition of airway inflammation and remodeling by sitagliptin in murine chronic asthma. Int. Immunopharmacol. 2015, 29, 761–769. [Google Scholar] [CrossRef]
- Abdel-Fattah, M.M.; Messiha, B.A.S.; Salama, A.A.A. Assessment of the Mechanistic Role of Cinnarizine in Modulating Experimentally-Induced Bronchial Asthma in Rats. Pharmacology 2015, 96, 167–174. [Google Scholar] [CrossRef]
- Pavord, I.D.; Chanez, P.; Criner, G.J.; Kerstjens, H.A.M.; Korn, S.; Lugogo, N.; Martinot, J.B.; Sagara, H.; Albers, F.C.; Bradford, E.S.; et al. Mepolizumab for eosinophilic chronic obstructive pulmonary disease. N. Engl. J. Med. 2017, 377, 1613–1629. [Google Scholar] [CrossRef]
- Fernandez Romero, G.A.; Beros, J.; Criner, G. Mepolizumab for the prevention of chronic obstructive pulmonary disease exacerbations. Expert Rev. Respir. Med. 2019, 13, 125–132. [Google Scholar] [CrossRef]
- Criner, G.J.; Celli, B.R.; Brightling, C.E.; Agusti, A.; Papi, A.; Singh, D.; Sin, D.D.; Vogelmeier, C.F.; Sciurba, F.C.; Bafadhel, M.; et al. Benralizumab for the prevention of COPD exacerbations. N. Engl. J. Med. 2019, 381, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Brightling, C.E.; Bleecker, E.R.; Panettieri, R.A.; Bafadhel, M.; She, D.; Ward, C.K.; Xu, X.; Birrell, C.; van der Merwe, R. Benralizumab for chronic obstructive pulmonary disease and sputum eosinophilia: A randomised, double-blind, placebo-controlled, phase 2a study. Lancet Respir. Med. 2014, 2, 891–901. [Google Scholar] [CrossRef]
- NCT02138916 Benralizumab Efficacy in Moderate to Very Severe Chronic Obstructive Pulmonary Disease (COPD) With Exacerbation History—Full Text View—ClinicalTrials.gov. 2019. Available online: https://clinicaltrials.gov/show/NCT02138916 (accessed on 2 May 2020).
- Barnes, P.J. New anti-inflammatory targets for chronic obstructive pulmonary disease. Nat. Rev. Drug Discov. 2013, 12, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Chronic Obstructive Lung Disease: Pocket Guide To COPD Diagnosis, Management, and Prevention, A Guide for Health Care Professionals. 2017 Report. Available online: https://goldcopd.org/wp-content/uploads/2016/12/wms-GOLD-2017-Pocket-Guide.pdf (accessed on 25 September 2020).
- Yang, I.A.; Clarke, M.S.; Sim, E.H.; Fong, K.M. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2012, 2012, CD002991. [Google Scholar] [CrossRef]
- Nannini, L.J.; Poole, P.; Milan, S.J.; Holmes, R.; Normansell, R. Combined corticosteroid and long-acting beta2-agonist in one inhaler versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2013, 2017, CD003794. [Google Scholar] [CrossRef]
- Calzetta, L.; Di Marco, F.; Blasi, F.; Cazzola, M.; Centanni, S.; Micheletto, C.; Rossi, A.; Rogliani, P. Impact of ICS/LABA and LABA/LAMA FDCs on functional and clinical outcomes in COPD: A network meta-analysis. Pulm. Pharmacol. Ther. 2019, 59, 101855. [Google Scholar] [CrossRef]
- Barnes, P.J. Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2013, 131, 636–645. [Google Scholar] [CrossRef]
- Rennard, S.I.; Dale, D.C.; Donohue, J.F.; Kanniess, F.; Magnussen, H.; Sutherland, E.R.; Watz, H.; Lu, S.; Stryszak, P.; Rosenberg, E.; et al. CXCR2 antagonist MK-7123 a phase 2 proof-of-concept trial for chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2015, 191, 1001–1011. [Google Scholar] [CrossRef]
- Barreiro, E.; Wang, X.; Tang, J. COPD: Preclinical models and emerging therapeutic targets. Expert Opin. Ther. Targets 2019, 23, 829–838. [Google Scholar] [CrossRef]
- Paul, T.; Salazar-Degracia, A.; Peinado, V.I.; Tura-Ceide, O.; Blanco, I.; Barreiro, E.; Barberà, J.A. Soluble guanylate cyclase stimulation reduces oxidative stress in experimental Chronic Obstructive Pulmonary Disease. PLoS ONE 2018, 13, e0190628. [Google Scholar] [CrossRef]
- Barreiro, E.; Puig-Vilanova, E.; Marin-Corral, J.; Chacón-Cabrera, A.; Salazar-Degracia, A.; Mateu, X.; Puente-Maestu, L.; García-Arumí, E.; Andreu, A.L.; Molina, L. Therapeutic Approaches in Mitochondrial Dysfunction, Proteolysis, and Structural Alterations of Diaphragm and Gastrocnemius in Rats With Chronic Heart Failure. J. Cell. Physiol. 2016, 231, 1495–1513. [Google Scholar] [CrossRef]
- Brasier, A.R. Therapeutic targets for inflammation-mediated airway remodeling in chronic lung disease. Expert Rev. Respir. Med. 2018, 12, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tian, B.; Chen, H.; Wang, P.; Brasier, A.R.; Zhou, J. Discovery of potent and selective BRD4 inhibitors capable of blocking TLR3-induced acute airway inflammation. Eur. J. Med. Chem. 2018, 151, 450–461. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, P.; Chen, H.; Wold, E.A.; Tian, B.; Brasier, A.R.; Zhou, J. Drug Discovery Targeting Bromodomain-Containing Protein 4. J. Med. Chem. 2017, 60, 4533–4558. [Google Scholar] [CrossRef] [PubMed]
- Broekman, W.; Khedoe, P.P.S.J.; Schepers, K.; Roelofs, H.; Stolk, J.; Hiemstra, P.S. Mesenchymal stromal cells: A novel therapy for the treatment of chronic obstructive pulmonary disease? Thorax 2018, 73, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G. Pharmacotherapy for idiopathic pulmonary fibrosis: Current landscape and future potential. Eur. Respir. Rev. 2017, 26, 170071. [Google Scholar] [CrossRef] [PubMed]
- Margaritopoulos, G.A.; Trachalaki, A.; Wells, A.U.; Vasarmidi, E.; Bibaki, E.; Papastratigakis, G.; Detorakis, S.; Tzanakis, N.; Antoniou, K.M. Pirfenidone improves survival in IPF: Results from a real-life study. BMC Pulm. Med. 2018, 18, 177. [Google Scholar] [CrossRef]
- Somogyi, V.; Chaudhuri, N.; Torrisi, S.E.; Kahn, N.; Müller, V.; Kreuter, M. The therapy of idiopathic pulmonary fibrosis: What is next? Eur. Respir. Rev. 2019, 28, 190021. [Google Scholar] [CrossRef]
- Lederer, D.J.; Martinez, F.J. Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2018, 378, 1811–1823. [Google Scholar] [CrossRef]
- Murray, L.A.; Rosada, R.; Moreira, A.P.; Joshi, A.; Kramer, M.S.; Hesson, D.P.; Argentieri, R.L.; Mathai, S.; Gulati, M.; Herzog, E.L.; et al. Serum amyloid P therapeutically attenuates murine bleomycin-induced pulmonary fibrosis via its effects on macrophages. PLoS ONE 2010, 5, e9683. [Google Scholar] [CrossRef]
- Santhiago, M.R.; Singh, V.; Barbosa, F.L.; Agrawal, V.; Wilson, S.E. Monocyte development inhibitor PRM-151 decreases corneal myofibroblast generation in rabbits. Exp. Eye Res. 2011, 93, 786–789. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van Den Blink, B.; Dillingh, M.R.; Ginns, L.C.; Morrison, L.D.; Moerland, M.; Wijsenbeek, M.; Trehu, E.G.; Bartholmai, B.J.; Burggraaf, J. Recombinant human pentraxin-2 therapy in patients with idiopathic pulmonary fibrosis: Safety, pharmacokinetics and exploratory efficacy. Eur. Respir. J. 2016, 47, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Van Den Blink, B.; Hamblin, M.J.; Whitney Brown, A.; Golden, J.A.; Ho, L.A.; Wijsenbeek, M.S.; Vasakova, M.; Pesci, A.; Antin-Ozerkis, D.E.; et al. Effect of recombinant human pentraxin 2 vs placebo on change in forced vital capacity in patients with idiopathic pulmonary fibrosis a randomized clinical trial. JAMA J. Am. Med. Assoc. 2018, 319, 2299–2307. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; van den Blink, B.; Hamblin, M.J.; Brown, A.W.; Golden, J.A.; Ho, L.A.; Wijsenbeek, M.S.; Vasakova, M.; Pesci, A.; Antin-Ozerkis, D.E.; et al. Long-term treatment with recombinant human pentraxin 2 protein in patients with idiopathic pulmonary fibrosis: An open-label extension study. Lancet Respir. Med. 2019, 7, 657–664. [Google Scholar] [CrossRef]
- NCT01890265 Evaluate the Safety and Efficacy of FG-3019 (Pamrevlumab) in Participants With Idiopathic Pulmonary Fibrosis (IPF)—Full Text View—ClinicalTrials.gov. 2020. Available online: https://clinicaltrials.gov/show/NCT01890265 (accessed on 20 September 2020).
- Gorina, E.; Richeldi, L.; Raghu, G.; Fernandez Perez, E.; Costabel, U.; Albera, C.; Lederer, D.; Flaherty, K.; Ettinger, N.; Bercz, P.; et al. PRAISE, a randomized, placebo-controlled, double-blind Phase 2 clinical trial of pamrevlumab (FG-3019) in IPF patients. Eur. Respir. J. 2017, 50, OA3400. [Google Scholar]
- Gagnon, L.; Leduc, M.; Thibodeau, J.F.; Zhang, M.Z.; Grouix, B.; Sarra-Bournet, F.; Gagnon, W.; Hince, K.; Tremblay, M.; Geerts, L.; et al. A Newly Discovered Antifibrotic Pathway Regulated by Two Fatty Acid Receptors: GPR40 and GPR84. Am. J. Pathol. 2018, 188, 1132–1148. [Google Scholar] [CrossRef]
- Khalil, N.; Manganas, H.; Ryerson, C.J.; Shapera, S.; Cantin, A.M.; Hernandez, P.; Turcotte, E.E.; Parker, J.M.; Moran, J.E.; Albert, G.R.; et al. Phase 2 clinical trial of PBI-4050 in patients with idiopathic pulmonary fibrosis. Eur. Respir. J. 2019, 53, 1800663. [Google Scholar] [CrossRef]
- NCT02738801 Study to Assess Safety, Tolerability, Pharmacokinetic and Pharmacodynamic Properties of GLPG1690. 2017. Available online: https://clinicaltrials.gov/show/NCT02738801 (accessed on 20 September 2020).
- Maher, T.M.; van der Aar, E.M.; Van de Steen, O.; Allamassey, L.; Desrivot, J.; Dupont, S.; Fagard, L.; Ford, P.; Fieuw, A.; Wuyts, W. Safety, tolerability, pharmacokinetics, and pharmacodynamics of GLPG1690, a novel autotaxin inhibitor, to treat idiopathic pulmonary fibrosis (FLORA): A phase 2a randomised placebo-controlled trial. Lancet Respir. Med. 2018, 6, 627–635. [Google Scholar] [CrossRef]
- Maher, T.M.; Kreuter, M.; Lederer, D.J.; Brown, K.K.; Wuyts, W.; Verbruggen, N.; Stutvoet, S.; Fieuw, A.; Ford, P.; Abi-Saab, W.; et al. Rationale, design and objectives of two phase III, randomised, placebo-controlled studies of GLPG1690, a novel autotaxin inhibitor, in idiopathic pulmonary fibrosis (ISABELA 1 and 2). BMJ Open Respir. Res. 2019, 6, e000422. [Google Scholar] [CrossRef]
- Peters-Golden, M.; Henderson, W.R. University of Michigan Health Sys-tem, 6301 MSRB III, 1150 W. Medical Cen-ter Dr. N. Engl. J. Med. 2007, 357, 1841–1854. [Google Scholar] [CrossRef]
- Izumo, T.; Kondo, M.; Nagai, A. Effects of a leukotriene B4 receptor antagonist on bleomycin-induced pulmonary fibrosis. Eur. Respir. J. 2009, 34, 1444–1451. [Google Scholar] [CrossRef] [PubMed]
- NCT02503657 Safety and Tolerability Study in Subjects With Idiopathic Pulmonary Fibrosis (IPF)—Full Text View—ClinicalTrials.gov. 2020. Available online: https://clinicaltrials.gov/show/NCT02503657 (accessed on 20 September 2020).
- NCT02688647 A Study to Evaluate the Safety, Tolerability, and Activity of KD025 in Subjects With Idiopathic Pulmonary Fibrosis. 2019. Available online: https://clinicaltrials.gov/show/NCT02688647 (accessed on 20 September 2020).
- Zanin-Zhorov, A.; Weiss, J.M.; Nyuydzefe, M.S.; Chen, W.; Scher, J.U.; Mo, R.; Depoil, D.; Rao, N.; Liu, B.; Wei, J.; et al. Selective oral ROCK2 inhibitor down-regulates IL-21 and IL-17 secretion in human T cells via STAT3-dependent mechanism. Proc. Natl. Acad. Sci. USA 2014, 111, 16814–16819. [Google Scholar] [CrossRef] [PubMed]
- NCT03142191 A Study to Evaluate the Efficacy and Safety of CC-90001 in Subjects With Idiopathic Pulmonary Fibrosis—Full Text View—ClinicalTrials.gov. 2020. Available online: https://clinicaltrials.gov/show/NCT03142191 (accessed on 20 September 2020).
- van der Velden, J.L.J.; Ye, Y.; Nolin, J.D.; Hoffman, S.M.; Chapman, D.G.; Lahue, K.G.; Abdalla, S.; Chen, P.; Liu, Y.; Bennett, B.; et al. JNK inhibition reduces lung remodeling and pulmonary fibrotic systemic markers. Clin. Transl. Med. 2016, 5, 36. [Google Scholar] [CrossRef] [PubMed]
- Horan, G.S.; Wood, S.; Ona, V.; Dan, J.L.; Lukashev, M.E.; Weinreb, P.H.; Simon, K.J.; Hahm, K.; Allaire, N.E.; Rinaldi, N.J.; et al. Partial inhibition of integrin αvβ6 prevents pulmonary fibrosis without exacerbating inflammation. Am. J. Respir. Crit. Care Med. 2008, 177, 56–65. [Google Scholar] [CrossRef] [PubMed]
- NCT01371305 STX-100 in Patients With Idiopathic Pulmonary Fibrosis (IPF)—Full Text View—ClinicalTrials.gov. 2020. Available online: https://clinicaltrials.gov/show/NCT01371305 (accessed on 20 September 2020).
- Zhang, X.L.; Xing, R.G.; Chen, L.; Liu, C.R.; Miao, Z.G. PI3K/Akt signaling is involved in the pathogenesis of bleomycin-induced pulmonary fibrosis via regulation of epithelial-mesenchymal transition. Mol. Med. Rep. 2016, 14, 5699–5706. [Google Scholar] [CrossRef] [PubMed]
- NCT01725139 A Proof of Mechanism Study With GSK2126458 in Patients With Idiopathic Pulmonary Fibrosis (IPF)—Full Text View—ClinicalTrials.gov. 2019. Available online: https://clinicaltrials.gov/show/NCT01725139 (accessed on 20 September 2020).
- Mercer, P.F.; Woodcock, H.V.; Eley, J.D.; Platé, M.; Sulikowski, M.G.; Durrenberger, P.F.; Franklin, L.; Nanthakumar, C.B.; Man, Y.; Genovese, F.; et al. Exploration of a potent PI3 kinase/mTOR inhibitor as a novel anti-fibrotic agent in IPF. Thorax 2016, 71, 701–711. [Google Scholar] [CrossRef]
- Lukey, P.T.; Harrison, S.A.; Yang, S.; Man, Y.; Holman, B.F.; Rashidnasab, A.; Azzopardi, G.; Grayer, M.; Simpson, J.K.; Bareille, P.; et al. A randomised, placebo-controlled study of omipalisib (PI3K/mTOR) in idiopathic pulmonary fibrosis. Eur. Respir. J. 2019, 53, 1801992. [Google Scholar] [CrossRef]
- NCT01462006 Double-blind Placebo-controlled Pilot Study of Sirolimus in Idiopathic Pulmonary Fibrosis (IPF). 2018. Available online: https://clinicaltrials.gov/show/NCT01462006 (accessed on 20 September 2020).
- NCT01969409 Autoantibody Reduction Therapy in Patients With Idiopathic Pulmonary Fibrosis. 2020. Available online: https://clinicaltrials.gov/show/NCT01969409 (accessed on 20 September 2020).
- NCT01266317 Combined PEX, Rituximab and Steroids in Acute Idiopathic Pulmonary Fibrosis Exacerbations—Full Text View—ClinicalTrials.gov. 2018. Available online: https://clinicaltrials.gov/show/NCT01266317 (accessed on 20 September 2020).
- Donahoe, M.; Valentine, V.G.; Chien, N.; Gibson, K.F.; Raval, J.S.; Saul, M.; Xue, J.; Zhang, Y.; Duncan, S.R. Autoantibody-targeted treatments for acute exacerbations of idiopathic pulmonary fibrosis. PLoS ONE 2015, 10, e0127771. [Google Scholar]
- NCT01777737 Study to Test the Validity of the Treatment of Idiopathic Pulmonary Fibrosis With Cotrimoxazole. 2017. Available online: https://clinicaltrials.gov/show/nct01777737 (accessed on 20 September 2020).
- NCT01872689 A Study of Lebrikizumab in Patients With Idiopathic Pulmonary Fibrosis—Full Text View—ClinicalTrials.gov. 2018; pp. 5–7. Available online: https://clinicaltrials.gov/show/NCT01872689 (accessed on 20 September 2020).
- NCT01629667 A Phase 2, Randomized Dose-ranging Study to Evaluate the Efficacy of Tralokinumab in Adults With Idiopathic Pulmonary Fibrosis—Full Text View—ClinicalTrials.gov. 2017. Available online: https://clinicaltrials.gov/show/NCT01629667 (accessed on 20 September 2020).
- NCT02173145 Azithromycin in Idiopathic Pulmonary Fibrosis—Full Text View—ClinicalTrials.gov. 2019. Available online: https://clinicaltrials.gov/show/NCT02173145 (accessed on 20 September 2020).
- Cazzola, M.; Matera, M.G.; Rogliani, P.; Calzetta, L. Senolytic drugs in respiratory medicine: Is it an appropriate therapeutic approach? Expert Opin. Investig. Drugs 2018, 27, 573–581. [Google Scholar] [CrossRef]
- Malayeri, A.R.; Hemmati, A.A.; Arzi, A.; Rezaie, A.; Ghafurian-Boroojerdnia, M.; Khalili, H.R. A comparison of the effects of quercetin hydrate with those of vitamin E on the levels of IL-13, PDGF, TNF-α, and INF- γ in bleomycin-induced pulmonary fibrosis in rats. Jundishapur J. Nat. Pharm. Prod. 2016, 11. [Google Scholar] [CrossRef]
- Schafer, M.J.; White, T.A.; Iijima, K.; Haak, A.J.; Ligresti, G.; Atkinson, E.J.; Oberg, A.L.; Birch, J.; Salmonowicz, H.; Zhu, Y.; et al. Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun. 2017, 8, 14532. [Google Scholar] [CrossRef]
- Lehmann, M.; Korfei, M.; Mutze, K.; Klee, S.; Skronska-Wasek, W.; Alsafadi, H.N.; Ota, C.; Costa, R.; Schiller, H.B.; Lindner, M.; et al. Senolytic drugs target alveolar epithelial cell function and attenuate experimental lung fibrosis ex vivo. Eur. Respir. J. 2017, 50, 1602367. [Google Scholar] [CrossRef] [PubMed]
- Barkauskas, C.E.; Cronce, M.J.; Rackley, C.R.; Bowie, E.J.; Keene, D.R.; Stripp, B.R.; Randell, S.H.; Noble, P.W.; Hogan, B.L.M. Type 2 alveolar cells are stem cells in adult lung. J. Clin. Investig. 2013, 123, 3025–3036. [Google Scholar] [CrossRef] [PubMed]
- Sisson, T.H.; Mendez, M.; Choi, K.; Subbotina, N.; Courey, A.; Cunningham, A.; Dave, A.; Engelhardt, J.F.; Liu, X.; White, E.S.; et al. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2010, 181, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Martyanov, V.; Kim, G.H.J.; Hayes, W.; Du, S.; Ganguly, B.J.; Sy, O.; Lee, S.K.; Bogatkevich, G.S.; Schieven, G.L.; Schiopu, E.; et al. Novel lung imaging biomarkers and skin gene expression subsetting in dasatinib treatment of systemic sclerosis-associated interstitial lung disease. PLoS ONE 2017, 12, e0187580. [Google Scholar] [CrossRef] [PubMed]
- Guignabert, C.; Phan, C.; Seferian, A.; Huertas, A.; Tu, L.; Thuillet, R.; Sattler, C.; Le Hiress, M.; Tamura, Y.; Jutant, E.M.; et al. Dasatinib induces lung vascular toxicity and predisposes to pulmonary hypertension. J. Clin. Investig. 2016, 126, 3207–3218. [Google Scholar] [CrossRef]
- Mailleux, A.A.; Crestani, B. Licence to kill senescent cells in idiopathic pulmonary fibrosis? Eur. Respir. J. 2017, 50, 1701360. [Google Scholar] [CrossRef]
- Banerjee, E.R.; Kar, S.; Konsam, S.; Hore, G.; Mitra, S.; Biswas, S.; Sinha, A.; Jana, N.R. Therapeutic use of fisetin, curcumin, and mesoporous carbon nanoparticle loaded fisetin in bleomycin-induced idiopathic pulmonary fibrosis. Biomed. Res. Ther. 2015, 2, 250–262. [Google Scholar] [CrossRef]
- Pan, J.; Li, D.; Xu, Y.; Zhang, J.; Wang, Y.; Chen, M.; Lin, S.; Huang, L.; Chung, E.J.; Citrin, D.E.; et al. Inhibition of Bcl-2/xl With ABT-263 Selectively Kills Senescent Type II Pneumocytes and Reverses Persistent Pulmonary Fibrosis Induced by Ionizing Radiation in Mice. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 353–361. [Google Scholar] [CrossRef]
- Justice, J.N.; Nambiar, A.M.; Tchkonia, T.; LeBrasseur, N.K.; Pascual, R.; Hashmi, S.K.; Prata, L.; Masternak, M.M.; Kritchevsky, S.B.; Musi, N.; et al. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine 2019, 40, 554–563. [Google Scholar] [CrossRef]
- Habib, A.R.R.; Kajbafzadeh, M.; Desai, S.; Yang, C.L.; Skolnik, K.; Quon, B.S. A Systematic Review of the Clinical Efficacy and Safety of CFTR Modulators in Cystic Fibrosis. Sci. Rep. 2019, 9, 7234. [Google Scholar] [CrossRef] [PubMed]
- Clancy, J.P.; Cotton, C.U.; Donaldson, S.H.; Solomon, G.M.; VanDevanter, D.R.; Boyle, M.P.; Gentzsch, M.; Nick, J.A.; Illek, B.; Wallenburg, J.C.; et al. CFTR modulator theratyping: Current status, gaps and future directions. J. Cyst. Fibros. 2019, 18, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Pacheco, M. CFTR Modulators: The Changing Face of Cystic Fibrosis in the Era of Precision Medicine. Front. Pharmacol. 2020, 10, 1662. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Pacheco, M. CFTR modulators: Shedding light on precision medicine for cystic fibrosis. Front. Pharmacol. 2016, 7, 275. [Google Scholar] [CrossRef]
- Li, H.; Pesce, E.; Sheppard, D.N.; Singh, A.K.; Pedemonte, N. Therapeutic approaches to CFTR dysfunction: From discovery to drug development. J. Cyst. Fibros. 2018, 17, S14–S21. [Google Scholar] [CrossRef]
- Van Goor, F.; Hadida, S.; Grootenhuis, P.D.J.; Burton, B.; Stack, J.H.; Straley, K.S.; Decker, C.J.; Miller, M.; McCartney, J.; Olson, E.R.; et al. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc. Natl. Acad. Sci. USA 2011, 108, 18843–18848. [Google Scholar] [CrossRef]
- Ratjen, F.; Hug, C.; Marigowda, G.; Tian, S.; Huang, X.; Stanojevic, S.; Milla, C.E.; Robinson, P.D.; Waltz, D.; Davies, J.C.; et al. Efficacy and safety of lumacaftor and ivacaftor in patients aged 6–11 years with cystic fibrosis homozygous for F508del-CFTR: A randomised, placebo-controlled phase 3 trial. Lancet Respir. Med. 2017, 5, 557–567. [Google Scholar] [CrossRef]
- Konstan, M.W.; McKone, E.F.; Moss, R.B.; Marigowda, G.; Tian, S.; Waltz, D.; Huang, X.; Lubarsky, B.; Rubin, J.; Millar, S.J.; et al. Assessment of safety and efficacy of long-term treatment with combination lumacaftor and ivacaftor therapy in patients with cystic fibrosis homozygous for the F508del-CFTR mutation (PROGRESS): A phase 3, extension study. Lancet Respir. Med. 2017, 5, 107–118. [Google Scholar] [CrossRef]
- Wainwright, C.E.; Elborn, J.S.; Ramsey, B.W.; Marigowda, G.; Huang, X.; Cipolli, M.; Colombo, C.; Davies, J.C.; De Boeck, K.; Flume, P.A.; et al. Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for phe508del CFTR. N. Engl. J. Med. 2015, 373, 220–231. [Google Scholar] [CrossRef]
- McElvaney, O.J.; Gunaratnam, C.; McElvaney, O.F.; Bagwe, I.; Reeves, E.P.; McElvaney, N.G. Emerging pharmacotherapies in cystic fibrosis. Expert Rev. Respir. Med. 2018, 12, 843–855. [Google Scholar] [CrossRef]
- Pedemonte, N.; Lukacs, G.L.; Du, K.; Caci, E.; Zegarra-Moran, O.; Galietta, L.J.V.; Verkman, A.S. Small-molecule correctors of defective ΔF508-CFTR cellular processing identified by high-throughput screening. J. Clin. Investig. 2005, 115, 2564–2571. [Google Scholar] [CrossRef]
- Phuan, P.W.; Veit, G.; Tan, J.A.; Finkbeiner, W.E.; Lukacs, G.L.; Verkman, A.S. Potentiators of defective DF508-CFTR gating that do not interfere with corrector action. Mol. Pharmacol. 2015, 88, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Shang, H.; Jordan, N.J.; Wong, E.; Mercadante, D.; Saltz, J.; Mahiou, J.; Bihler, H.J.; Mense, M. High-Throughput Screening for Readthrough Modulators of CFTR PTC Mutations. SLAS Technol. 2017, 22, 315–324. [Google Scholar] [CrossRef]
- Giuliano, K.A.; Wachi, S.; Drew, L.; Dukovski, D.; Green, O.; Bastos, C.; Cullen, M.D.; Hauck, S.; Tait, B.D.; Munoz, B.; et al. Use of a High-Throughput Phenotypic Screening Strategy to Identify Amplifiers, a Novel Pharmacological Class of Small Molecules That Exhibit Functional Synergy with Potentiators and Correctors. SLAS Discov. 2018, 23, 111–121. [Google Scholar] [CrossRef]
- Van Der Plas, S.E.; Kelgtermans, H.; De Munck, T.; Martina, S.L.X.; Dropsit, S.; Quinton, E.; De Blieck, A.; Joannesse, C.; Tomaskovic, L.; Jans, M.; et al. Discovery of N-(3-Carbamoyl-5,5,7,7-tetramethyl-5,7-dihydro-4H-thieno[2,3-c]pyran-2-yl)-lH-pyrazole-5-carboxamide (GLPG1837), a Novel Potentiator Which Can Open Class III Mutant Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Channels to a High. J. Med. Chem. 2018, 61, 1425–1435. [Google Scholar] [CrossRef]
- Veit, G.; Xu, H.; Dreano, E.; Avramescu, R.G.; Bagdany, M.; Beitel, L.K.; Roldan, A.; Hancock, M.A.; Lay, C.; Li, W.; et al. Structure-guided combination therapy to potently improve the function of mutant CFTRs. Nat. Med. 2018, 24, 1732–1742. [Google Scholar] [CrossRef]
- Wang, X.; Liu, B.; Searle, X.; Yeung, C.; Bogdan, A.; Greszler, S.; Singh, A.; Fan, Y.; Swensen, A.M.; Vortherms, T.; et al. Discovery of 4-[(2R,4R)-4-({[1-(2,2-Difluoro-1,3-benzodioxol-5-yl)cyclopropyl]carbonyl}amino)-7-(difluoromethoxy)-3,4-dihydro-2H-chromen-2-yl]benzoic Acid (ABBV/GLPG-2222), a Potent Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Corrector for. J. Med. Chem. 2018, 61, 1436–1449. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.; Hallowell, S.; Tibbetts, M.; Beasley, C.; Brown-Phillips, T.; Healy, A.; Pustilnik, L.; Doyonnas, R.; Pregel, M. High-Throughput Surface Liquid Absorption and Secretion Assays to Identify F508del CFTR Correctors Using Patient Primary Airway Epithelial Cultures. SLAS Discov. 2019, 24, 724–737. [Google Scholar] [CrossRef] [PubMed]
- De Wilde, G.; Gees, M.; Musch, S.; Verdonck, K.; Jans, M.; Wesse, A.S.; Singh, A.K.; Hwang, T.C.; Christophe, T.; Pizzonero, M.; et al. Identification of GLPG/ABBV-2737, a novel class of corrector, which exerts functional synergy with other CFTR modulators. Front. Pharmacol. 2019, 10, 514. [Google Scholar] [CrossRef] [PubMed]
- Merkert, S.; Schubert, M.; Olmer, R.; Engels, L.; Radetzki, S.; Veltman, M.; Scholte, B.J.; Zöllner, J.; Pedemonte, N.; Galietta, L.J.V.; et al. High-Throughput Screening for Modulators of CFTR Activity Based on Genetically Engineered Cystic Fibrosis Disease-Specific iPSCs. Stem Cell Rep. 2019, 12, 1389–1403. [Google Scholar] [CrossRef]
- Rafeeq, M.M.; Murad, H.A.S. Cystic fibrosis: Current therapeutic targets and future approaches. J. Transl. Med. 2017, 15, 84. [Google Scholar] [CrossRef]
- Cheng, K.; Ashby, D.; Smyth, R.L. Oral steroids for long-term use in cystic fibrosis. Cochrane Database Syst. Rev. 2015, 2015, CD000407. [Google Scholar] [CrossRef]
- Balfour-Lynn, I.M.; Welch, K.; Smith, S. Inhaled corticosteroids for cystic fibrosis. Cochrane Database Syst. Rev. 2019, 2019, CD001915. [Google Scholar] [CrossRef] [PubMed]
- Ross, K.R.; Chmiel, J.F.; Konstan, M.W. The role of inhaled corticosteroids in the management of cystic fibrosis. Pediatr. Drugs 2009, 11, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Lands, L.C.; Stanojevic, S. Oral non-steroidal anti-inflammatory drug therapy for lung disease in cystic fibrosis. Cochrane Database Syst. Rev. 2019, 2019, CD001505. [Google Scholar] [CrossRef] [PubMed]
- Konstan, M.W.; VanDevanter, D.R.; Sawicki, G.S.; Pasta, D.J.; Foreman, A.J.; Neiman, E.A.; Morgan, W.J. Association of high-dose ibuprofen use, lung function decline, and long-term survival in children with cystic fibrosis. Ann. Am. Thorac. Soc. 2018, 15, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Konstan, M.W.; Döring, G.; Heltshe, S.L.; Lands, L.C.; Hilliard, K.A.; Koker, P.; Bhattacharya, S.; Staab, A.; Hamilton, A. A randomized double blind, placebo controlled phase 2 trial of BIIL 284 BS (an LTB4 receptor antagonist) for the treatment of lung disease in children and adults with cystic fibrosis. J. Cyst. Fibros. 2014, 13, 148–155. [Google Scholar] [CrossRef]
- Elborn, J.S.; Bhatt, L.; Grosswald, R.; Ahuja, S.; Springman, E.B. Phase I Studies of Acebilustat: Pharmacokinetics, Pharmacodynamics, Food Effect, and CYP3A Induction. Clin. Transl. Sci. 2017, 10, 20–27. [Google Scholar] [CrossRef]
- Elborn, J.S.; Ahuja, S.; Springman, E.; Mershon, J.; Grosswald, R.; Rowe, S.M. EMPIRE-CF: A phase II randomized placebo-controlled trial of once-daily, oral acebilustat in adult patients with cystic fibrosis—Study design and patient demographics. Contemp. Clin. Trials 2018, 72, 86–94. [Google Scholar] [CrossRef]
- NCT02759562 Effect of Andecaliximab on FEV1 in Adults With Cystic Fibrosis—Full Text View—ClinicalTrials.gov. 2018. Available online: https://clinicaltrials.gov/show/NCT02759562 (accessed on 3 May 2020).
- NCT03748199 Clinical Study to Investigate Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of POL6014 in Patients with CF—Full Text View—ClinicalTrials.gov. 2018. Available online: https://clinicaltrials.gov/show/NCT03748199 (accessed on 3 May 2020).
- McElvaney, N.G. Alpha-1 antitrypsin therapy in cystic fibrosis and the lung disease associated with alpha-1 antitrypsin deficiency. Ann. Am. Thorac. Soc. 2016, 13, S191–S196. [Google Scholar] [PubMed]
- Elborn, J.S.; Perrett, J.; Forsman-Semb, K.; Marks-Konczalik, J.; Gunawardena, K.; Entwistle, N. Efficacy, safety and effect on biomarkers of AZD9668 in cystic fibrosis. Eur. Respir. J. 2012, 40, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Motwani, M.; Bennett, F.; Tepper, M.; White, B.; Norris, P.; MacAllister, R.; Serhan, C.; Gilroy, D. Anabasum (JBT-101) enhances resolution of inflammation in humans. Arthritis Rheumatol. 2017, 69 (Suppl. 10). Available online: https://acrabstracts.org/abstract/anabasum-jbt-101-enhances-resolution-of-inflammation-in-humans/ (accessed on 7 December 2020).
- NCT02465450 Safety, Tolerability, Pharmacokinetics, and Efficacy of JBT-101 (Lenabasum) in Cystic Fibrosis—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02465450 (accessed on 20 September 2020).
- Burstein, S.H. Ajulemic acid: Potential treatment for chronic inflammation. Pharmacol. Res. Perspect. 2018, 6, e00394. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. Endocannabinoids and their pharmacological actions. In Handbook of Experimental Pharmacology; Springer: New York, NY, USA, 2015; Volume 231, pp. 1–37. [Google Scholar]
- Gkoumassi, E.; Dekkers, B.G.J.; Dröge, M.J.; Elzinga, C.R.S.; Schmidt, M.; Meurs, H.; Zaagsma, J.; Nelemans, S.A. Virodhamine and CP55,940 modulate cAMP production and IL-8 release in human bronchial epithelial cells. Br. J. Pharmacol. 2007, 151, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- McElvaney, N.G.; Hubbard, R.C.; Birrer, P.; Crystal, R.G.; Chernick, M.S.; Frank, M.M.; Caplan, D.B. Aerosol α1 -antitrypsin treatment for cystic fibrosis. Lancet 1991, 337, 392–394. [Google Scholar] [CrossRef]
- Griese, M.; Latzin, P.; Kappler, M.; Weckerle, K.; Heinzimaier, T.; Bernhardt, T.; Hartl, D. α1-Antitrypsin inhalation reduces airway inflammation in cystic fibrosis patients. Eur. Respir. J. 2007, 29, 240–250. [Google Scholar] [CrossRef]
- Grimbert, D.; Vecellio, L.; Delépine, P.; Attucci, S.; Boissinot, E.; Poncin, A.; Gauthier, F.; Valat, C.; Saudubray, F.; Antonioz, P.; et al. Characteristics of EPI-hNE4 aerosol: A new elastase inhibitor for treatment of cystic fibrosis. J. Aerosol Med. Depos. Clear. Eff. Lung 2003, 16, 121–129. [Google Scholar] [CrossRef]
- Williams, B.; Robinette, M.; Slovis, B.; Deretci, V.; Perkett, E. Hydroxychloroquine—Pilot study of anti-inflammatory effects in cystic fibrosis. Pediatr. Pulmonol. 2008, 43, 314. [Google Scholar]
- Moss, R.B.; Mistry, S.J.; Konstan, M.W.; Pilewski, J.M.; Kerem, E.; Tal-Singer, R.; Lazaar, A.L. Safety and early treatment effects of the CXCR2 antagonist SB-656933 in patients with cystic fibrosis. J. Cyst. Fibros. 2013, 12, 241–248. [Google Scholar] [CrossRef]
- Ballmann, M.; Junge, S.; von der Hardt, H. Low-dose methotrexate for advanced pulmonary disease in patients with cystic fibrosis. Respir. Med. 2003, 97, 498–500. [Google Scholar] [CrossRef][Green Version]
- McElvaney, O.J.; McElvaney, N.G. Targeting IL-8 in cystic fibrosis: Enough but not too much. Am. J. Respir. Cell Mol. Biol. 2018, 59, 401–402. [Google Scholar] [CrossRef] [PubMed]
- McElvaney, O.J.; O’Reilly, N.; White, M.; Lacey, N.; Pohl, K.; Gerlza, T.; Bergin, D.A.; Kerr, H.; McCarthy, C.; O’Brien, M.E.; et al. The effect of the decoy molecule PA401 on CXCL8 levels in bronchoalveolar lavage fluid of patients with cystic fibrosis. Mol. Immunol. 2015, 63, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Karp, C.L.; Flick, L.M.; Park, K.W.; Softic, S.; Greer, T.M.; Keledjian, R.; Yang, R.; Uddin, J.; Guggino, W.B.; Atabani, S.F.; et al. Defective lipoxin-mediated anti-inflammatory activity in the cystic fibrosis airway. Nat. Immunol. 2004, 5, 388–392. [Google Scholar] [CrossRef] [PubMed]
- József, L.; Zouki, C.; Petasis, N.A.; Serhan, C.N.; Filep, J.G. Lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 inhibit peroxynitrite formation, NF-κB and AP-1 activation, and IL-8 gene expression in human leukocytes. Proc. Natl. Acad. Sci. USA 2002, 99, 13266–13271. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, I.; Tamura, H.; Hirata, M. An Antimicrobial Cathelicidin Peptide, Human CAP18/LL-37, Suppresses Neutrophil Apoptosis via the Activation of Formyl-Peptide Receptor-Like 1 and P2X 7. J. Immunol. 2006, 176, 3044–3052. [Google Scholar] [CrossRef]
- Herrera, B.S.; Hasturk, H.; Kantarci, A.; Freire, M.O.; Nguyen, O.; Kansal, S.; van Dyke, T.E. Impact of resolvin E1 on murine neutrophil phagocytosis in type 2 diabetes. Infect. Immun. 2015, 83, 792–801. [Google Scholar] [CrossRef][Green Version]
- Freire, M.O.; Dalli, J.; Serhan, C.N.; Van Dyke, T.E. Neutrophil Resolvin E1 Receptor Expression and Function in Type 2 Diabetes. J. Immunol. 2017, 198, 718–728. [Google Scholar] [CrossRef]
- Kurihara, T.; Jones, C.N.; Yu, Y.M.; Fischman, A.J.; Watada, S.; Tompkins, R.G.; Fagan, S.P.; Irimia, D. Resolvin D2 restores neutrophil directionality and improves survival after burns. FASEB J. 2013, 27, 2270–2281. [Google Scholar] [CrossRef]
- Schwab, J.M.; Chiang, N.; Arita, M.; Serhan, C.N. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 2007, 447, 869–874. [Google Scholar] [CrossRef]
- Hsiao, H.-M.; Thatcher, T.H.; Levy, E.P.; Fulton, R.A.; Owens, K.M.; Phipps, R.P.; Sime, P.J. Resolvin D1 Attenuates Polyinosinic-Polycytidylic Acid–Induced Inflammatory Signaling in Human Airway Epithelial Cells via TAK1. J. Immunol. 2014, 193, 4980–4987. [Google Scholar] [CrossRef]
- Ringholz, F.C.; Higgins, G.; Hatton, A.; Sassi, A.; Moukachar, A.; Fustero-Torre, C.; Hollenhorst, M.; Sermet-Gaudelus, I.; Harvey, B.J.; McNally, P.; et al. Resolvin D1 regulates epithelial ion transport and inflammation in cystic fibrosis airways. J. Cyst. Fibros. 2018, 17, 607–615. [Google Scholar] [CrossRef] [PubMed]
- NCT03265288 Study of LAU-7b in the Treatment of Cystic Fibrosis in Adults—Full Text View—ClinicalTrials.gov. 2020. Available online: https://clinicaltrials.gov/show/NCT03265288 (accessed on 3 May 2020).
- Kontoghiorghes, G.J.; Kontoghiorghe, C.N. Prospects for the introduction of targeted antioxidant drugs for the prevention and treatment of diseases related to free radical pathology. Expert Opin. Investig. Drugs 2019, 28, 593–603. [Google Scholar] [CrossRef]
- Cantin, A.M. Low-hanging fruit and antioxidant therapy in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2018, 198, 555–557. [Google Scholar] [CrossRef]
- Calabrese, C.; Tosco, A.; Abete, P.; Carnovale, V.; Basile, C.; Magliocca, A.; Quattrucci, S.; De Sanctis, S.; Alatri, F.; Mazzarella, G.; et al. Randomized, single blind, controlled trial of inhaled glutathione vs placebo in patients with cystic fibrosis. J. Cyst. Fibros. 2015, 14, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Skov, M.; Pressler, T.; Lykkesfeldt, J.; Poulsen, H.E.; Jensen, P.Ø.; Johansen, H.K.; Qvist, T.; Kræmer, D.; Høiby, N.; Ciofu, O. The effect of short-term, high-dose oral N-acetylcysteine treatment on oxidative stress markers in cystic fibrosis patients with chronic P. aeruginosa infection—A pilot study. J. Cyst. Fibros. 2015, 14, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Hector, A.; Griese, M.; Hartl, D. Oxidative stress in cystic fibrosis lung disease: An early event, but worth targeting? Eur. Respir. J. 2014, 44, 17–19. [Google Scholar] [CrossRef]
- Griese, M.; Kappler, M.; Eismann, C.; Ballmann, M.; Junge, S.; Rietschel, E.; Van Koningsbruggen-Rietschel, S.; Staab, D.; Rolinck-Werninghaus, C.; Mellies, U.; et al. Inhalation treatment with glutathione in patients with cystic fibrosis: A randomized clinical trial. Am. J. Respir. Crit. Care Med. 2013, 188, 83–89. [Google Scholar] [CrossRef]
- de Vries, J.J.V.; Chang, A.B.; Bonifant, C.M.; Shevill, E.; Marchant, J.M. Vitamin A and beta (β)-carotene supplementation for cystic fibrosis. Cochrane Database Syst. Rev. 2018, 2018, CD006751. [Google Scholar] [CrossRef]
- González Jiménez, D.; Díaz Martín, J.J.; Arias Llorente, R.P.; Bousoño García, C. Oxidative Stress in Cystic Fibrosis. In Cystic Fibrosis in the Light of New Research; IntechOpen: London, UK, 2015. [Google Scholar]
- Kontoghiorghe, C.N.; Kolnagou, A.; Kontoghiorghes, G.J. Antioxidant targeting by deferiprone in diseases related to oxidative damage. Front. Biosci. Landmark 2014, 19, 862–885. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. Prospects for introducing deferiprone as potent pharmaceutical antioxidant. Front. Biosci. Elit. 2009, 1, 161–178. [Google Scholar]
- Conrad, C.; Lymp, J.; Thompson, V.; Dunn, C.; Davies, Z.; Chatfield, B.; Nichols, D.; Clancy, J.; Vender, R.; Egan, M.E.; et al. Long-term treatment with oral N-acetylcysteine: Affects lung function but not sputum inflammation in cystic fibrosis subjects. A phase II randomized placebo-controlled trial. J. Cyst. Fibros. 2015, 14, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.A.; Kelly, F.J. Vitamin E supplementation in cystic fibrosis. J. Pediatr. Gastroenterol. Nutr. 1996, 22, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, A.; Stallings, V.A. Update on fat-soluble vitamins in cystic fibrosis. Curr. Opin. Pulm. Med. 2008, 14, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Galli, F.; Azzi, A. Present trends in vitamin E research. BioFactors 2010, 36, 33–42. [Google Scholar] [CrossRef]
- Hamahata, A.; Enkhbaatar, P.; Kraft, E.R.; Lange, M.; Leonard, S.W.; Traber, M.G.; Cox, R.A.; Schmalstieg, F.C.; Hawkins, H.K.; Whorton, E.B.; et al. γ-Tocopherol nebulization by a lipid aerosolization device improves pulmonary function in sheep with burn and smoke inhalation injury. Free Radic. Biol. Med. 2008, 45, 425–433. [Google Scholar] [CrossRef]
- Anais, J.P.; Razzouq, N.; Carvalho, M.; Fernandez, C.; Astier, A.; Paul, M.; Astier, A.; Fessi, H.; Lorino, A.M. Development of α-tocopherol acetate nanoparticles: Influence of preparative processes. Drug Dev. Ind. Pharm. 2009, 35, 216–223. [Google Scholar] [CrossRef]
- Rust, P.; Eichler, I.; Renner, S.; Elmadfa, I. Long-term oral β-carotene supplementation in patients with cystic fibrosis—Effects on antioxidative status and pulmonary function. Ann. Nutr. Metab. 2000, 44, 30–37. [Google Scholar] [CrossRef]
- Sagel, S.D.; Sontag, M.K.; Anthony, M.M.; Emmett, P.; Papas, K.A. Effect of an antioxidant-rich multivitamin supplement in cystic fibrosis. J. Cyst. Fibros. 2011, 10, 31–36. [Google Scholar] [CrossRef]
- Papas, K.A.; Sontag, M.K.; Pardee, C.; Sokol, R.J.; Sagel, S.D.; Accurso, F.J.; Wagener, J.S. A pilot study on the safety and efficacy of a novel antioxidant rich formulation in patients with cystic fibrosis. J. Cyst. Fibros. 2008, 7, 60–67. [Google Scholar] [CrossRef]
- Sagel, S.D.; Khan, U.; Jain, R.; Graff, G.; Daines, C.L.; Dunitz, J.M.; Borowitz, D.; Orenstein, D.M.; Abdulhamid, I.; Noe, J.; et al. Effects of an antioxidant-enriched multivitamin in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2018, 198, 639–647. [Google Scholar] [CrossRef]
- Foucaud, P.; Therond, P.; Marchand, M.; Brion, F.; Demelier, J.F.; Navarro, J. Selenium and vitamin E in cystic fibrosis. Arch. Fr. Pediatr. 1988, 45, 383–386. [Google Scholar] [PubMed]
- Tsavachidou, D.; McDonnell, T.J.; Wen, S.; Wang, X.; Vakar-Lopez, F.; Pisters, L.L.; Pettaway, C.A.; Wood, C.G.; Do, K.A.; Thall, P.F.; et al. Selenium and vitamin E: Cell type- and intervention-specific tissue effects in prostate cancer. J. Natl. Cancer Inst. 2009, 101, 306–320. [Google Scholar] [CrossRef] [PubMed]
- Winklhofer-Roob, B.M.; Ellemunter, H.; Frühwirth, M.; Schlegel-Haueter, S.E.; Khoschsorur, G.; Van’t Hof, M.A.; Shmerling, D.H. Plasma vitamin C concentrations in patients with cystic fibrosis: Evidence of associations with lung inflammation. Am. J. Clin. Nutr. 1997, 65, 1858–1866. [Google Scholar] [CrossRef] [PubMed]
- Van Biervliet, S.; Vande Velde, S.; Van Biervliet, J.P.; Robberecht, E. The effect of zinc supplements in cystic fibrosis patients. Ann. Nutr. Metab. 2008, 52, 152–156. [Google Scholar] [CrossRef]
- De Castro-Silva, C.; De Bruin, V.M.S.; Cunha, G.M.A.; Nunes, D.M.; Medeiros, C.A.M.; De Bruin, P.F.C. Melatonin improves sleep and reduces nitrite in the exhaled breath condensate in cystic fibrosis—A randomized, double-blind placebo-controlled study. J. Pineal Res. 2010, 48, 65–71. [Google Scholar] [CrossRef]
- Moss, R.B.; Mayer-Hamblett, N.; Wagener, J.; Daines, C.; Hale, K.; Ahrens, R.; Gibson, R.L.; Anderson, P.; Retsch-Bogart, G.; Nasr, S.Z.; et al. Randomized, double-blind, placebo-controlled, dose-escalating study of aerosolized interferon gamma-1b in patients with mild to moderate cystic fibrosis lung disease. Pediatr. Pulmonol. 2005, 39, 209–218. [Google Scholar] [CrossRef]
- Matsuse, T.; Teramoto, S.; Katayama, H.; Sudo, E.; Ekimoto, H.; Mitsuhashi, H.; Uejima, Y.; Fukuchi, Y.; Ouchi, Y. ICAM-1 mediates lung leukocyte recruitment but not pulmonary fibrosis in a murine model of bleomycin-induced lung injury. Eur. Respir. J. 1999, 13, 71–77. [Google Scholar] [CrossRef]
- Ferretti, S.; Bonneau, O.; Dubois, G.R.; Jones, C.E.; Trifilieff, A. IL-17, Produced by Lymphocytes and Neutrophils, Is Necessary for Lipopolysaccharide-Induced Airway Neutrophilia: IL-15 as a Possible Trigger. J. Immunol. 2003, 170, 2106–2112. [Google Scholar] [CrossRef]
- Ruan, H.; Pownall, H.J.; Lodish, H.F. Troglitazone antagonizes tumor necrosis factor-α-induced reprogramming of adipocyte gene expression by inhibiting the transcriptional regulatory functions of NF-κB. J. Biol. Chem. 2003, 278, 28181–28192. [Google Scholar] [CrossRef]
- Vanden Berghe, W.; Vermeulen, L.; Delerive, P.; De Bosscher, K.; Staels, B.; Haegeman, G. A paradigm for gene regulation: Inflammation, NF-κB and PPAR. Adv. Exp. Med. Biol. 2003, 544, 181–196. [Google Scholar]
- Zingarelli, B.; Sheehan, M.; Hake, P.W.; O’Connor, M.; Denenberg, A.; Cook, J.A. Peroxisome Proliferator Activator Receptor-γ Ligands, 15-Deoxy-Δ 12,14 -Prostaglandin J 2 and Ciglitazone, Reduce Systemic Inflammation in Polymicrobial Sepsis by Modulation of Signal Transduction Pathways. J. Immunol. 2003, 171, 6827–6837. [Google Scholar] [CrossRef] [PubMed]
- Perez, A.; Van Heeckeren, A.M.; Nichols, D.; Gupta, S.; Eastman, J.F.; Davis, P.B. Peroxisome proliferator-activated receptor-γ in cystic fibrosis lung epithelium. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 295, L303–L313. [Google Scholar] [CrossRef] [PubMed]
- Dunzendorfer, S.; Rothbucher, D.; Schratzberger, P.; Reinisch, N.; Kähler, C.M.; Wiedermann, C.J. Mevalonate-dependent inhibition of transendothelial migration and chemotaxis of human peripheral blood neutrophils by pravastatin. Circ. Res. 1997, 81, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Rezaie-Majd, A.; Maca, T.; Bucek, R.A.; Valent, P.; Müller, M.R.; Husslein, P.; Kashanipour, A.; Minar, E.; Baghestanian, M. Simvastatin reduces expression of cytokines interleukin-6, interleukin-8, and monocyte chemoattractant protein-1 in circulating monocytes from hypercholesterolemic patients. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Zelvyte, I.; Dominaitiene, R.; Crisby, M.; Janciauskiene, S. Modulation of inflammatory mediators and PPARγ and NFκB expression by pravastatin in response to lipoproteins in human monocytes in vitro. Pharmacol. Res. 2002, 45, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, N.; Gunning, Y.; O’Croinin, D.F.; Laffey, J.G.; McLoughlin, P. Anti-inflammatory effect of augmented nitric oxide production in chronic lung infection. J. Pathol. 2006, 209, 198–205. [Google Scholar] [CrossRef]
- Watson, H.; Stackhouse, C. Omega-3 fatty acid supplementation for cystic fibrosis. Cochrane Database Syst. Rev. 2020, 2020, CD002201. [Google Scholar] [CrossRef]
- Lee, M.; Kim, S.; Kwon, O.K.; Oh, S.R.; Lee, H.K.; Ahn, K. Anti-inflammatory and anti-asthmatic effects of resveratrol, a polyphenolic stilbene, in a mouse model of allergic asthma. Int. Immunopharmacol. 2009, 9, 418–424. [Google Scholar] [CrossRef]
- Wood, L.G.; Wark, P.A.B.; Garg, M.L. Antioxidant and anti-inflammatory effects of resveratrol in airway disease. Antioxidants Redox Signal. 2010, 13, 1535–1548. [Google Scholar] [CrossRef]
- Georgi, E.; Le Guellec, S.; Vecellio, L.; Fichant, E.; Stordeur, P.; Bordeau, P.; Perraudin, J. 68* Feasibility study of OSCN− and lactoferrin (Meveol®) nebulization for cystic fibrosis patients. J. Cyst. Fibros. 2011, 10, S18. [Google Scholar] [CrossRef][Green Version]
- Chandler, J.D.; Min, E.; Huang, J.; McElroy, C.S.; Dickerhof, N.; Mocatta, T.; Fletcher, A.A.; Evans, C.M.; Liang, L.; Patel, M.; et al. Antiinflammatory and antimicrobial effects of thiocyanate in a cystic fibrosis mouse model. Am. J. Respir. Cell Mol. Biol. 2015, 53, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Parkin, K.L. Induction of phase II enzyme activity by various selenium compounds. Nutr. Cancer 2006, 55, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Galli, F. Interactions of Polyphenolic Compounds with Drug Disposition and Metabolism. Curr. Drug Metab. 2007, 8, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Van Goor, F.; Hadida, S.; Grootenhuis, P.D.J.; Burton, B.; Cao, D.; Neuberger, T.; Turnbull, A.; Singh, A.; Joubran, J.; Hazlewood, A.; et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc. Natl. Acad. Sci. USA 2009, 106, 18825–18830. [Google Scholar] [CrossRef] [PubMed]
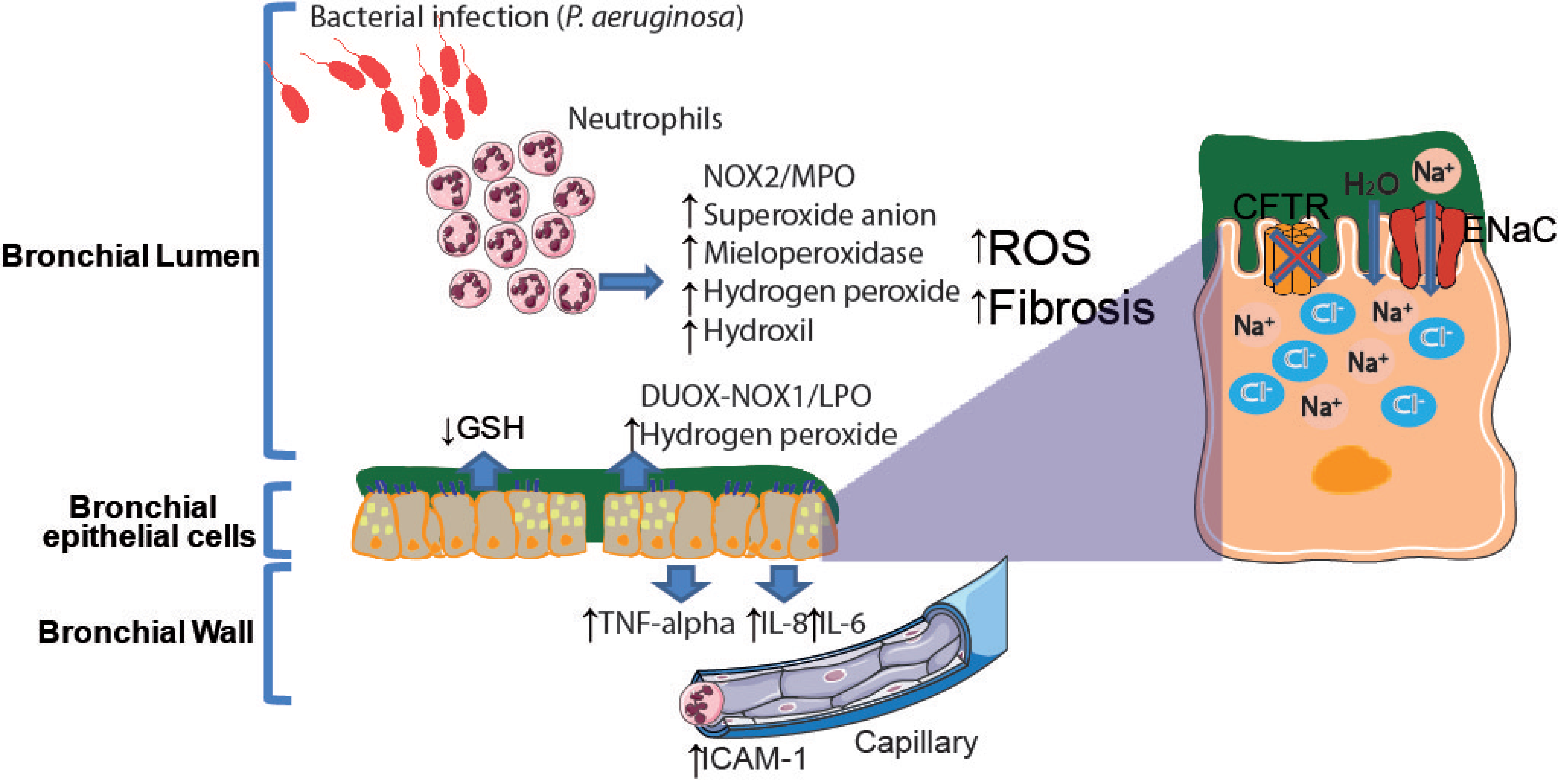
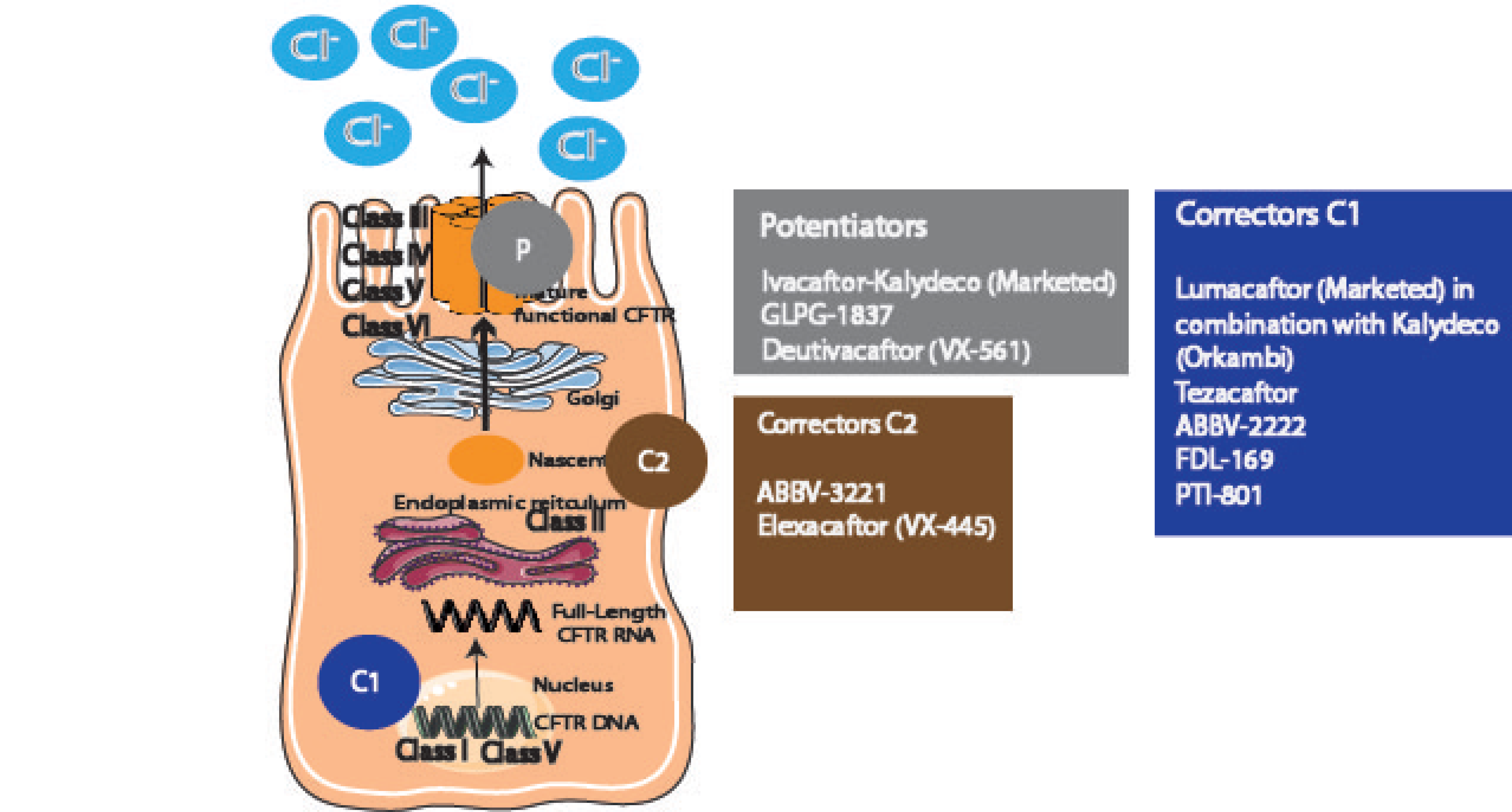
| Disease | Drug | Target | Biologic Function | Trial | Reference |
|---|---|---|---|---|---|
| ALI/ARDS | Corticosteroids (dexamethasone, budesonide and formoterol) | Corticosteroid receptors | Reduce the signs and symptoms of inflammatory conditions | Phase 3 | [292,293,294,295] |
| ALI/ARDS | Aspirin | Cox-1 and Cox-2 | Significant reduction in neutrophil infiltration into the alveolar space | Phase 2 | [299,300,301] |
| ALI/ARDS | MSCs and MPAs | - | Reduction in angiopoietin decreased 28-day mortality, higher ventilator-free days and higher ICU-free days | Phase 2 | [302] |
| ALI/ARDS | ALT-836 | Tissue factor (TF) or TF-factor VIIa | Blocks binding to coagulation factor VIIa and attenuation of sepsis-induced ALI | Phase 2 | [308,309] |
| ALI/ARDS | Dilmapimod | p38 MAPK Inhibitor | Reduces severity of ALI | Phase 2 | [310,311] |
| ALI/ARDS | Ulinastin | Physiological human inhibitor of neutrophil elastase | Effective in ameliorating ARDS | Phase 2 | [315,316,317] |
| ALI/ARDS | Anti-CD14 antibodies | Amti-CD-14 | Antibodies which protect against septic hypotension | Phase 2 | [319,320] |
| Asthma and COPD | Omalizumab | Anti-IgE | Binds to free human IgE, forming small-size immune complexes, blocking its interaction with the high-affinity IgE receptor and preventing its contact with mast cells and basophils | Approved | [331,332,333,334] |
| Asthma and COPD | Mepolizumab | Anti-IL-5 | Decreases eosinophils in blood and sputum; fewer asthma exacerbations, better asthma control, improved quality of life, and reduced proteins involved in airway remodeling | Approved for asthma; Phase 2 for COPD | Asthma: [335,336,337,338]/COPD: [404,405] |
| Asthma and COPD | Reslizumab | Anti-IL-5R | Decreases blood, sputum, and airway eosinophils, reduces asthma exacerbations, improves lung function, and reduces systemic corticosteroid dosing by as much as 75% | Approved | [339,340] |
| Asthma and COPD | Benralizumab | Anti-IL-5 | Positive results in asthma. Decrease airway eosinophilia | Approved | Asthma: [343,344,345,346]/COPD: [406,407,408] |
| Asthma | Depilumab | Anti-IL-4R | Positive results in asthma. Decrease airway eosinophilia | Approved | [347,348,349] |
| Asthma and COPD | Tezepelumab | Humanized monoclonal antibody | Binds thymic stromal lymphopoietin, an epithelial-cell–derived cytokine that drives allergic inflammatory responses | Phase 3 | [354,355] |
| Asthma and COPD | Navarixin | CXCR2 antagonist | Reduces sputum and blood neutrophils; no significant change in FEV1 | Phase 2 | Asthma: [358,359]/COPD: [409] |
| Asthma | Etanercept | TNF-α | Reduces bronchial hyperreactivity; small but significant increase in quality of life | Clinical | [362,363,364,365] |
| COPD | ICS + LABA | Corticosteroid receptors + β-adrenergic receptors | Significant reduction in the number of severe exacerbations and improvement in FEV1, quality of life, and respiratory symptoms in stable COPD patients | Approved | [412,413] |
| IPF | Pirfenidone and nintedanib | TGF-β and angiokinase | Significant reduction of respiratory deterioration in IPF and, perhaps, prolonged survival | Phase 3 | [236,237,424] |
| IPF | PRM-151 | Protein that binds to monocytes promoting epithelial healing and resolution of fibrosis | Ameliorates fibrosis in a bleomycin- and TGF-β-overexpressing animal model of fibrosis | Phase 2, heading for phase 3 | [427,429,430,431] |
| IPF | Pamrevlumab | CTGF | Reduction of lung function decline | Phase 2, heading for Phase 3 | [433] |
| IPF | PBI4050 | Analogue of a medium-chain fatty acid. Activates the GPR40 receptor, while it suppresses GPR84 activity, | Inhibition of endoplasmic reticulum stress and ROS production, epithelial–mesenchymal transition and fibrocyte/fibroblast recruitment, migration, proliferation, and differentiation | Phase 2 | [434,435] |
| IPF | GLPG1690 | Autotaxin | Selective autotaxin inhibitor. Enzyme increased in IPF and involved in cell apoptosis and endothelial cell damage, and LPA inhibitor | Phase 3 | [436,437,438] |
| IPF | Tipelukast | Leukotriene antagonists | Downregulation of genes that promote fibrosis, such as LOXL2, collagen type 1, and TIMP-1; and genes responsible for promoting inflammation like CCR2 and MCP-1. | Phase 2 | [441] |
| IPF | KD025 | Selective ROCK2 inhibitor | Downregulates the ability of T cells to secrete IL-21 and IL-17 in response to T-cell receptor stimulation in vitro; restores disrupted immune homeostasis | Phase 2 | [442,443] |
| IPF | CC-90001 | Second-generation JNK inhibitor | Reduces the development of fibrosis, as evidenced by a 48% reduction in collagen and a 53% reduction in α-smooth muscle actin | Phase 2 | [444] |
| IPF | BG00011 | Humanized monoclonal antibody targeting the alpha-v beta-6 (αvβ6) integrin receptor | TGF-β suppression as evidenced by reduction in pSMAD2 signaling and TGF-β dependent gene expression in bronchoalveolar lavage (BAL) cells; preclinical models have shown maximal fibrosis inhibition correlating with 70% pSMAD reduction. | Phase 2 | [447] |
| IPF | Omipalisib | PI3K/Akt pathway inhibitor | Halts fibrosing processes | Phase 1 | [451] |
| IPF | Sirolimus | mTOR | Reduces the number of circulating fibrocytes | Phase 2 | [452] |
| IPF | Rituximab | CD20 surface molecule of B lymphocytes | Reduction of autoantibodies, a favorable safety profile and, possibly, stabilization of lung function. | Phase 2 | [453,454,455] |
| IPF | Cotrimoxazole | Antibiotic | Antibacterial drug | Phase 3 | [456] |
| CF | Lumacaftor | CFTR corrector C1 | Increases the amount of F508del-CFTR that reaches the cell surface | Approved | [478,479] |
| CF | Ivacaftor | CFTR potentiator | CF patients possessing a G551D CFTR mutation | Approved | [571] |
| CF | Orkambi | CFTR corrector (C1) | For patients homozygous for F508del-CFTR; increases the amount of F508del-CFTR that reaches the cell surface | Approved | [480] |
| CF | Ibuprofen | Cox-1 and Cox-2 | Slows the progression of lung disease in children with CF | Approved | [279,280,496,497] |
| CF | Amelubant | LTB4 receptor antagonist | Eicosanoid modulator; anti-inflammatory activity | Phase 2 | [498] |
| CF | POL6014 | Neutrophil elastase function blocker | Clear inhibition of neutrophil elastase in the sputum of subjects with CF after single dosing | Phase 1 | [502] |
| CF | CTX-4430 | Leukotriene A4 hydrolase (LTA4H) inhibitor. Decreases the production of LTB4 | LTA4H and LTB4 are strongly associated with the development of many conditions involving inflammation, including CF | Phase 2 | [499,500] |
| CF | JBT-101 | Selective CB2 agonist. Decreases neutrophilic inflammation by inhibiting LTB4 and promotes resolution of inflammation by modulation of arachidonic acid metabolism | Reduction in some sputum inflammatory markers; reduction of exacerbations in response to lenabasum, with no serious adverse effects reported | Phase 2 heading for Phase 2b | [505,507] |
| CF | Thiazolidinediones (glitazones) | Inhibition of NF-κB activity through upregulation of peroxisome proliferator activating receptor (PPAR) | Reduce systemic inflammation in polymicrobial sepsis by modulation of signal transduction pathways | Approved | [556,557,558] |
| CF | Troglitazone and ciglitazone | PPAR activators | Reduce production of proinflammatory mediators in response to P. aeruginosa | Approved | [559] |
| CF | α1-antitrypsin | Serine protease inhibitor | Suppresses inflammatory markers, including free neutrophil elastase, proinflammatory cytokines and neutrophils | Phase 2 | [503] |
| CF | SB-656933 | CXCR2 antagonist | Promising modulator of airway inflammation | Phase 2 | [514] |
| CF | LAU-7B | Retinoids | Promotes extracellular matrix homeostasis; safe and well tolerated. | Phase 1b | [527] |
| CF | Lenabasum | Cannabinoid receptor type 2 (CB2) | CB2 is found primarily on the surfaces of activated immune cells; upon binding to the CB2 receptors, lenabasum triggers the production of proinflammatory mediators, reducing inflammation; reduces the number of inflammatory cells and inflammatory mediators found in the sputum; FEV1 was stable throughout the study for both lenabasum and placebo groups. | Phase 2, heading for 2b | [506] |
| CF | GSH | Endogenous antioxidant | Improves lung function and decreases oxidative stress | Phase 2 | [530,531,532,533] |
| CF | β-carotene | Natural antioxidant | Improves lung function and decreases oxidative stress | Phase 1 | [534,535] |
| CF | Deferiprone (L1) | Chelating drug/pharmaceutical antioxidant | Used as a main, alternative or adjuvant therapy in many pathological conditions | - | [536,537] |
| CF | N-acetyl cysteine | Antioxidant | Inhibits H2O2 and increases GSH | Phase 2b | [531,538] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Checa, J.; Aran, J.M. Airway Redox Homeostasis and Inflammation Gone Awry: From Molecular Pathogenesis to Emerging Therapeutics in Respiratory Pathology. Int. J. Mol. Sci. 2020, 21, 9317. https://doi.org/10.3390/ijms21239317
Checa J, Aran JM. Airway Redox Homeostasis and Inflammation Gone Awry: From Molecular Pathogenesis to Emerging Therapeutics in Respiratory Pathology. International Journal of Molecular Sciences. 2020; 21(23):9317. https://doi.org/10.3390/ijms21239317
Chicago/Turabian StyleCheca, Javier, and Josep M. Aran. 2020. "Airway Redox Homeostasis and Inflammation Gone Awry: From Molecular Pathogenesis to Emerging Therapeutics in Respiratory Pathology" International Journal of Molecular Sciences 21, no. 23: 9317. https://doi.org/10.3390/ijms21239317
APA StyleCheca, J., & Aran, J. M. (2020). Airway Redox Homeostasis and Inflammation Gone Awry: From Molecular Pathogenesis to Emerging Therapeutics in Respiratory Pathology. International Journal of Molecular Sciences, 21(23), 9317. https://doi.org/10.3390/ijms21239317





