LDOC1 as Negative Prognostic Marker for Vulvar Cancer Patients
Abstract
1. Introduction
2. Results
2.1. Study Group and Clinical Data
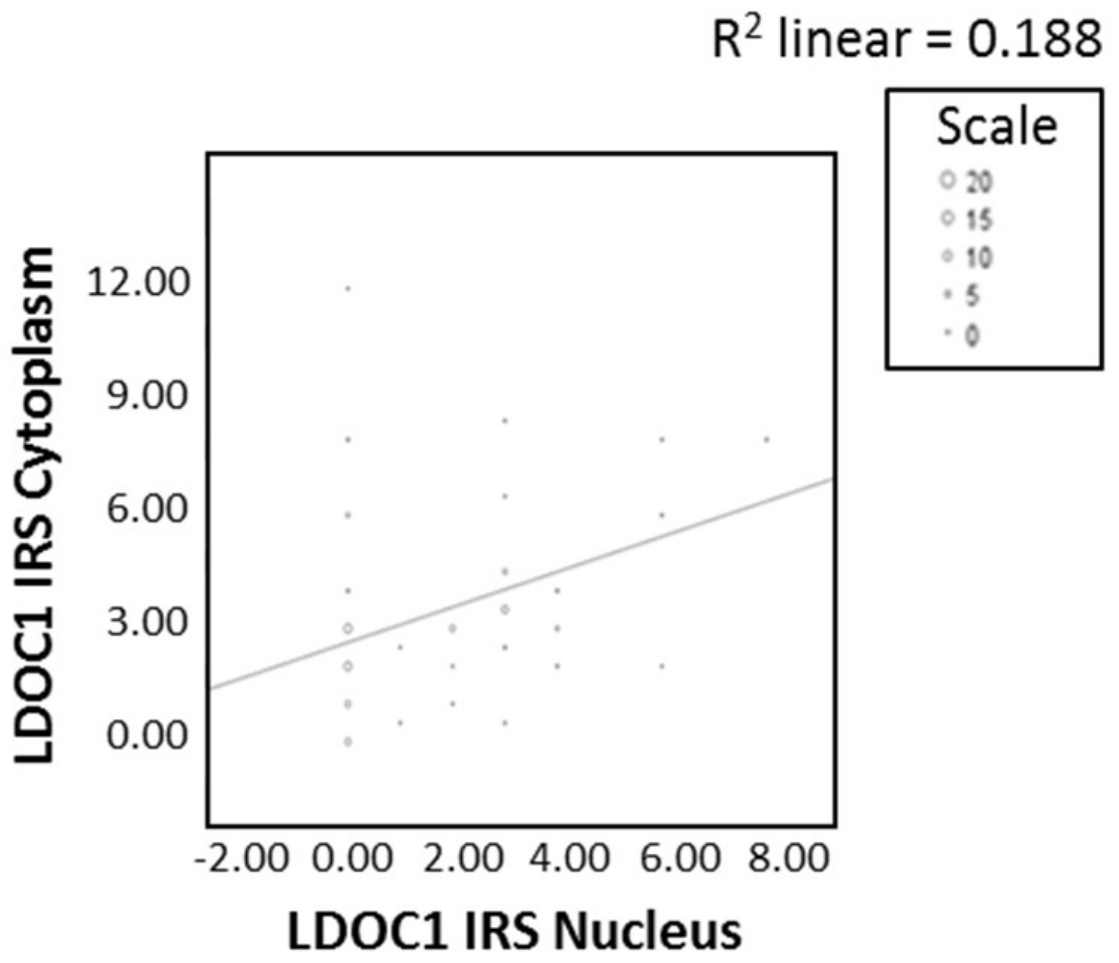
2.2. LDOC1 Expression in Vulvar Cancer Tissue
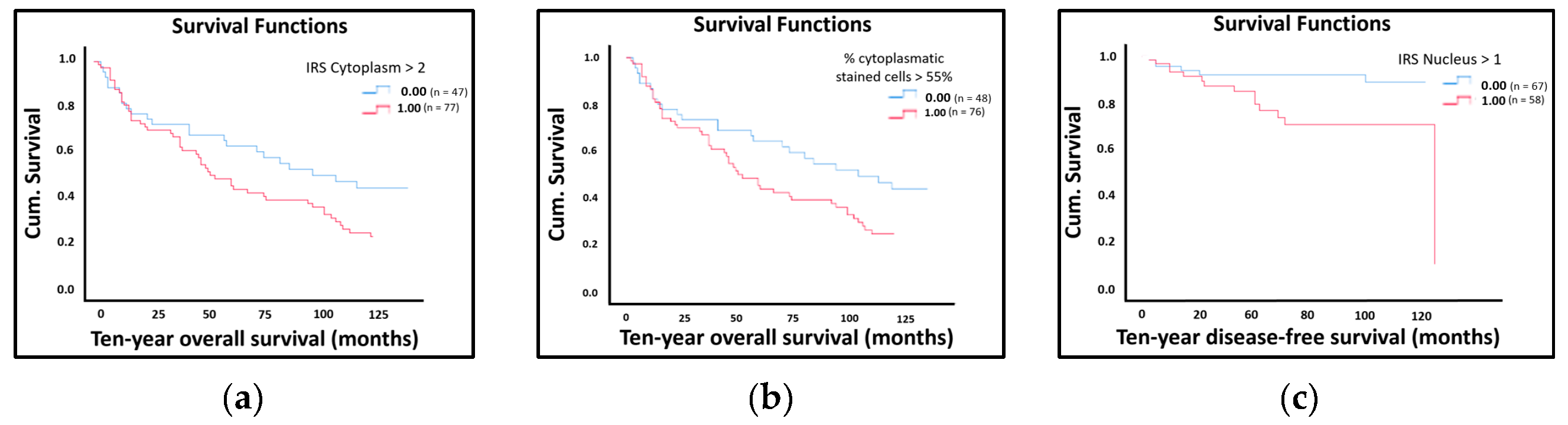
2.3. LDOC1 Expression and Patients’ Survival
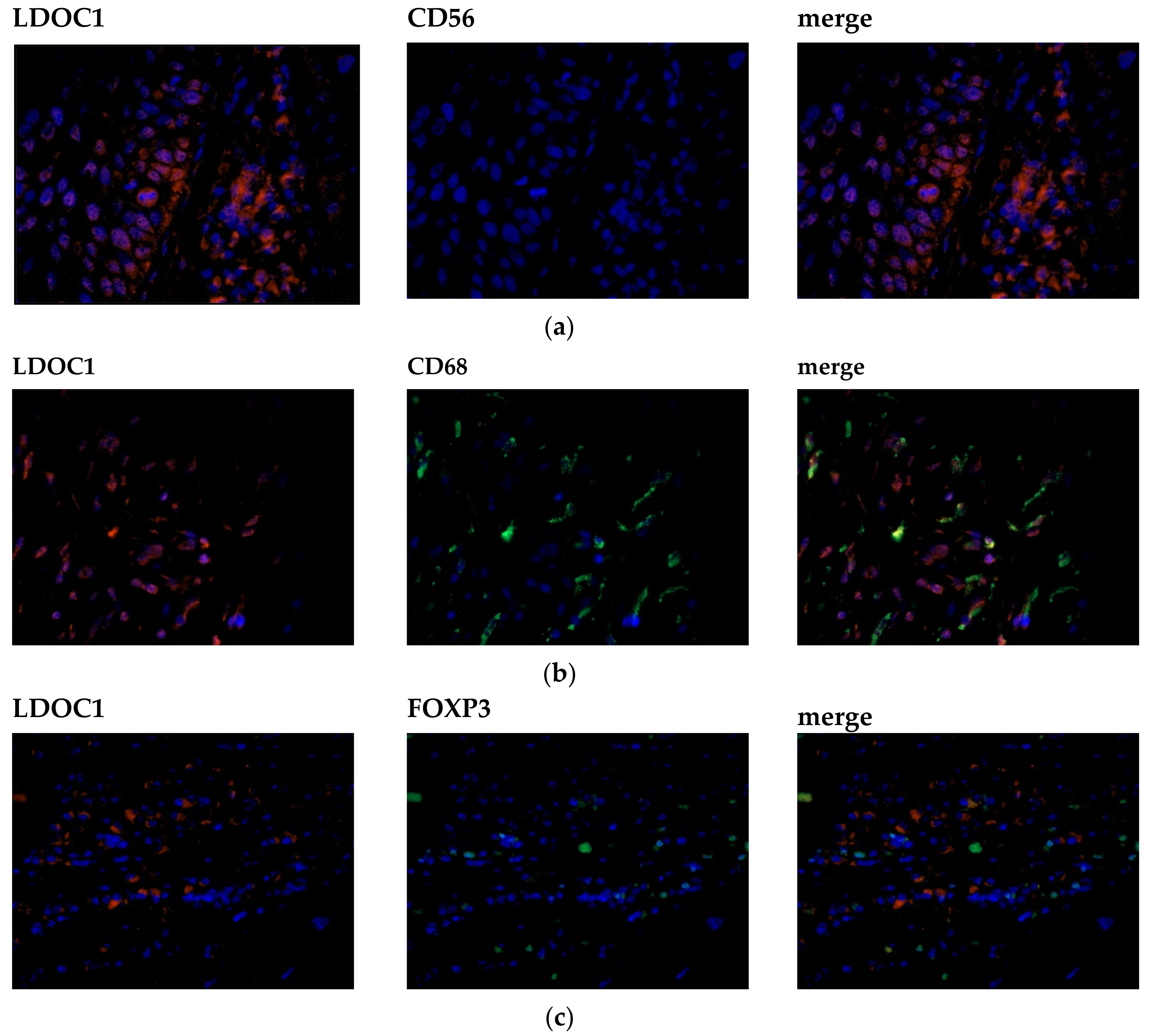
2.4. LDOC1 Expression on Infiltrating Immune Cells
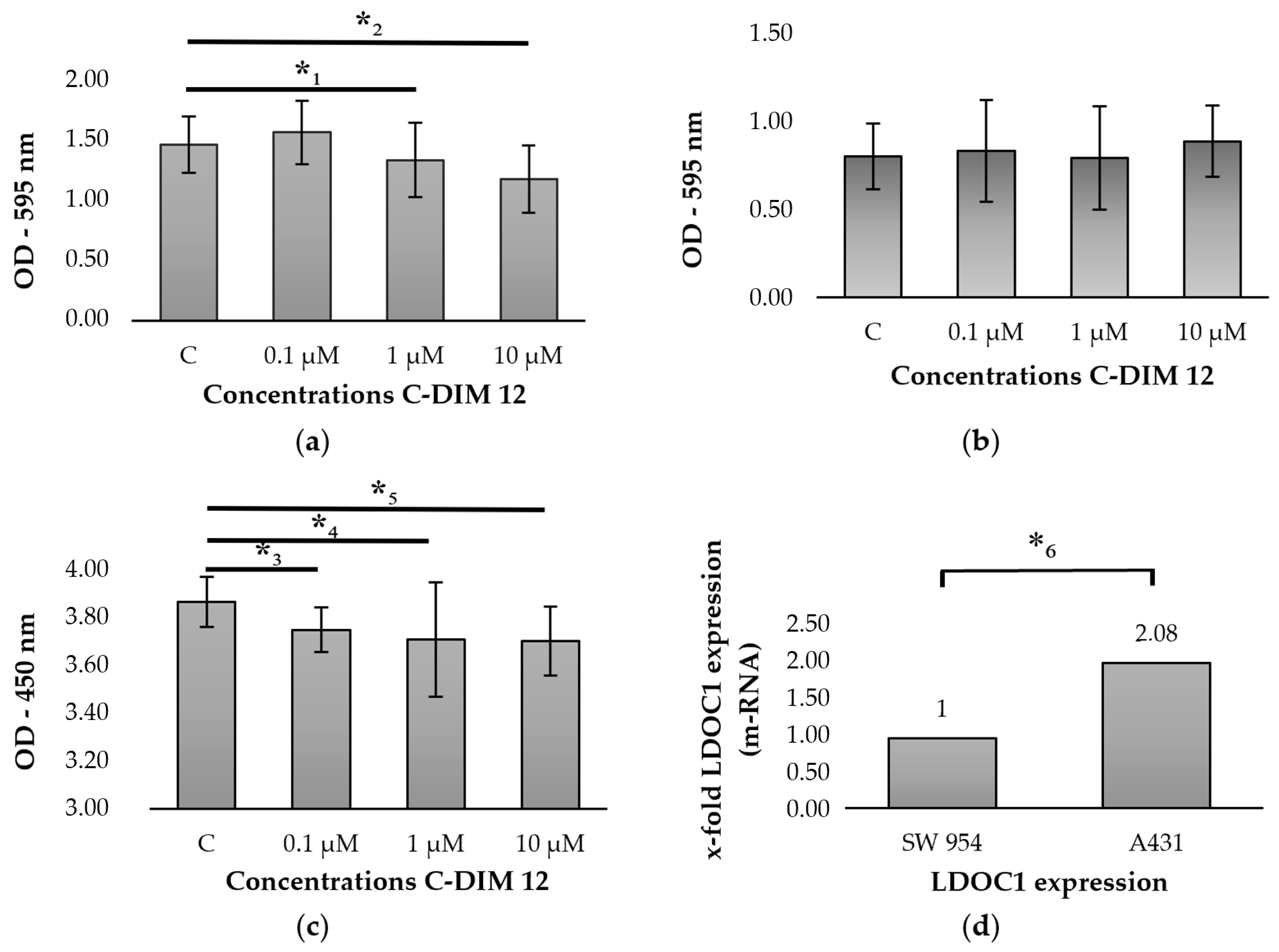
2.5. Regulation of LDOC1 by Stimulation with C-DIM 12
3. Discussion
4. Materials and Methods
4.1. Study Group and Clinical Data
4.2. Ethical Approval
4.3. Immunohistochemistry
4.4. Immunofluorescence
4.5. Cells and Cell Culture
4.6. Stimulation with C-DIM 12
4.7. MTT-Assay
4.8. BrdU-Assay
4.9. RNA Isolation and cDNA Synthesis
4.10. Real-Time PCR
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ATCC | American Type Culture Collection |
| BrdU | Bromodeoxyuridine |
| C-DIM 12 | 3,3′-[(4-chlorophenyl)methylene]bis[1H-indole] |
| CLL | Chronic lymphatic leukemia |
| Ct | Cycle threshold |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DMSO | Dimethyl sulfoxide |
| DNA | Deoxyribonucleic acid |
| ECACC | European Collection of Cell Cultures |
| FFPE | Formalin-fixed paraffin-embedded |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| HIBEC | Human intrahepatic biliary epithelial cells |
| HPV | Human papillomavirus |
| IRS | Immunoreactive score |
| LDOC1 | Leucine zipper downregulated in cancer 1 |
| NF-κB | Nuclear factor ‘kappa-light-chain-enhancer’ of activated B-cells |
| PBS | Phosphate buffered saline |
| PCR | Polymerase chain reaction |
| PD-L1 | Programmed death-ligand 1 |
| RNA | Ribonucleic acid |
| TAM | Tumor-associated macrophages |
| VIN | Vulvar intraepithelial neoplasia |
| VSCC | Vulvar squamous cell carcinoma |
References
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Joura, E.A.; Losch, A.; Haider-Angeler, M.G.; Breitenecker, G.; Leodolter, S. Trends in vulvar neoplasia. Increasing incidence of vulvar intraepithelial neoplasia and squamous cell carcinoma of the vulva in young women. J. Reprod Med. 2000, 45, 613–615. [Google Scholar] [PubMed]
- Judson, P.L.; Habermann, E.B.; Baxter, N.N.; Durham, S.B.; Virnig, B.A. Trends in the incidence of invasive and in situ vulvar carcinoma. Obstet. Gynecol. 2006, 107, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Zentrum für Krebsregisterdaten. Krebs in Deutschland. Vulva. Available online: https://www.krebsdaten.de/Krebs/DE/Content/Publikationen/Krebs_in_Deutschland/kid_2017/kid_2017_c51_vulva.pdf?__blob=publicationFile (accessed on 4 September 2019).
- Faber, M.T.; Sand, F.L.; Albieri, V.; Norrild, B.; Kjaer, S.K.; Verdoodt, F. Prevalence and type distribution of human papillomavirus in squamous cell carcinoma and intraepithelial neoplasia of the vulva. Int. J. Cancer 2017, 141, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Hoang, L.N.; Park, K.J.; Soslow, R.A.; Murali, R. Squamous precursor lesions of the vulva: Current classification and diagnostic challenges. Pathology 2016, 48, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Preti, M.; Igidbashian, S.; Costa, S.; Cristoforoni, P.; Mariani, L.; Origoni, M.; Sandri, M.T.; Boveri, S.; Spolti, N.; Spinaci, L.; et al. VIN usual type-from the past to the future. Ecancermedicalscience 2015, 9, 531. [Google Scholar] [CrossRef]
- Sopori, M. Effects of cigarette smoke on the immune system. Nat. Rev. Immunol. 2002, 2, 372–377. [Google Scholar] [CrossRef]
- Janni, W.; de Gregorio, N.; Schwenter, L.; Kürzl, R.H. Erkrankungen der Vulva; De Gruyter: Berlin, Germany, 2016. [Google Scholar]
- Satmary, W.; Holschneider, C.H.; Brunette, L.L.; Natarajan, S. Vulvar intraepithelial neoplasia: Risk factors for recurrence. Gynecol. Oncol. 2018, 148, 126–131. [Google Scholar] [CrossRef]
- Te Grootenhuis, N.C.; van der Zee, A.G.; van Doorn, H.C.; van der Velden, J.; Vergote, I.; Zanagnolo, V.; Baldwin, P.J.; Gaarenstroom, K.N.; van Dorst, E.B.; Trum, J.W.; et al. Sentinel nodes in vulvar cancer: Long-term follow-up of the GROningen INternational Study on Sentinel nodes in Vulvar cancer (GROINSS-V) I. Gynecol. Oncol. 2016, 140, 8–14. [Google Scholar] [CrossRef]
- Mahner, S.; Jueckstock, J.; Hilpert, F.; Neuser, P.; Harter, P.; de Gregorio, N.; Hasenburg, A.; Sehouli, J.; Habermann, A.; Hillemanns, P.; et al. Adjuvant therapy in lymph node-positive vulvar cancer: The AGO-CaRE-1 study. J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef]
- Lyford-Pike, S.; Peng, S.; Young, G.D.; Taube, J.M.; Westra, W.H.; Akpeng, B.; Bruno, T.C.; Richmon, J.D.; Wang, H.; Bishop, J.A.; et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013, 73, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Huang, Z.; Teng, F.; Xing, L.; Yu, J. Predictive biomarkers in PD-1/PD-L1 checkpoint blockade immunotherapy. Cancer Treat. Rev. 2015, 41, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Mezache, L.; Paniccia, B.; Nyinawabera, A.; Nuovo, G.J. Enhanced expression of PD L1 in cervical intraepithelial neoplasia and cervical cancers. Mod. Pathol. 2015, 28, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- Czogalla, B.; Pham, D.; Trillsch, F.; Rottmann, M.; Gallwas, J.; Burges, A.; Mahner, S.; Kirchner, T.; Jeschke, U.; Mayr, D.; et al. PD-L1 expression and survival in p16-negative and -positive squamous cell carcinomas of the vulva. J. Cancer Res. Clin. Oncol. 2020, 146, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Nagasaki, K.; Manabe, T.; Hanzawa, H.; Maass, N.; Tsukada, T.; Yamaguchi, K. Identification of a novel gene, LDOC1, down-regulated in cancer cell lines. Cancer Lett. 1999, 140, 227–234. [Google Scholar] [CrossRef]
- Thoompumkal, I.J.; Rehna, K.; Anbarasu, K.; Mahalingam, S. Leucine Zipper Down-regulated in Cancer-1 (LDOC1) interacts with Guanine nucleotide binding protein-like 3-like (GNL3L) to modulate Nuclear Factor-kappa B (NF-kappaB) signaling during cell proliferation. Cell Cycle 2016, 15, 3251–3267. [Google Scholar] [CrossRef]
- Jiang, J.; Li, Y.; Jiang, Z. Effects of LDOC1 on colorectal cancer cells via downregulation of the Wnt/beta-catenin signaling pathway. Oncol. Rep. 2019, 41, 3281–3291. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Q.; Li, Z.; Ma, X.; Wu, L.; Ji, H.; Qin, G. LDOC1 inhibits proliferation and promotes apoptosis by repressing NF-kappaB activation in papillary thyroid carcinoma. J. Exp. Clin. Cancer Res. 2015, 34, 146. [Google Scholar] [CrossRef]
- Buchholtz, M.L.; Juckstock, J.; Weber, E.; Mylonas, I.; Dian, D.; Bruning, A. Loss of LDOC1 expression by promoter methylation in cervical cancer cells. Cancer Investig. 2013, 31, 571–577. [Google Scholar] [CrossRef]
- Buchholtz, M.L.; Bruning, A.; Mylonas, I.; Juckstock, J. Epigenetic silencing of the LDOC1 tumor suppressor gene in ovarian cancer cells. Arch. Gynecol Obstet. 2014, 290, 149–154. [Google Scholar] [CrossRef]
- Duzkale, H.; Schweighofer, C.D.; Coombes, K.R.; Barron, L.L.; Ferrajoli, A.; O’Brien, S.; Wierda, W.G.; Pfeifer, J.; Majewski, T.; Czerniak, B.A.; et al. LDOC1 mRNA is differentially expressed in chronic lymphocytic leukemia and predicts overall survival in untreated patients. Blood 2011, 117, 4076–4084. [Google Scholar] [CrossRef] [PubMed]
- Salemi, M.; Giuffrida, D.; Giuffrida, M.C.; Soma, P.F.; Rolfo, A.; Cimino, L.; Condorelli, R.A.; Castiglione, R.; La Vignera, S.; Calogero, A.E. LDOC1 gene expression in two patients with head and neck squamous cell carcinomas and Parkinson’s disease. Tumori 2012, 98, 86e–88e. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Dong, R.; Zhao, R.; Zheng, S. Overexpression of LDOC1 in human biliary epithelial cells inhibits apoptosis through NF-kappaB signaling. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 713–717. [Google Scholar] [CrossRef]
- Nagasaki, K.; Schem, C.; von Kaisenberg, C.; Biallek, M.; Rosel, F.; Jonat, W.; Maass, N. Leucine-zipper protein, LDOC1, inhibits NF-kappaB activation and sensitizes pancreatic cancer cells to apoptosis. Int. J. Cancer 2003, 105, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, R.; Ishiguro, H.; Kuwabara, Y.; Kimura, M.; Mitsui, A.; Mori, Y.; Mori, R.; Tomoda, K.; Katada, T.; Harada, K.; et al. Identification of candidate genes involved in the radiosensitivity of esophageal cancer cells by microarray analysis. Dis. Esophagus 2008, 21, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, K.; Koike, D.; Suetsugu, S.; Takenawa, T. WAVE3 functions as a negative regulator of LDOC1. J. Biochem. 2005, 138, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Chen, J.H.; Hsia, S.M.; Liao, C.C.; Chang, H.W.; Shieh, T.M.; Shih, Y.H. Salivary LDOC1 is a gender-difference biomarker of oral squamous cell carcinoma. PeerJ 2019, 7, e6732. [Google Scholar] [CrossRef]
- Wu, T.; Dai, Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017, 387, 61–68. [Google Scholar] [CrossRef]
- Abdulrahman, Z.; Kortekaas, K.E.; De Vos Van Steenwijk, P.J.; Van Der Burg, S.H.; Van Poelgeest, M.I.E. The immune microenvironment in vulvar (pre)cancer: Review of literature and implications for immunotherapy. Expert Opin. Biol. Ther. 2018, 18, 1223–1233. [Google Scholar] [CrossRef]
- Van Overmeire, E.; Laoui, D.; Keirsse, J.; Van Ginderachter, J.; Sarukhan, A. Mechanisms Driving Macrophage Diversity and Specialization in Distinct Tumor Microenvironments and Parallelisms with Other Tissues. Front. Immunol. 2014, 5. [Google Scholar] [CrossRef]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Karin, M. NF-kappaB and cancer: Mechanisms and targets. Mol. Carcinog. 2006, 45, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Hoesel, B.; Schmid, J.A. The complexity of NF-kappaB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Seppanen, M.; Vihko, K.K. Activation of transcription factor NF-kappaB by growth inhibitory cytokines in vulvar carcinoma cells. Immunol. Lett. 2000, 74, 103–109. [Google Scholar] [CrossRef]
- Inamoto, T.; Papineni, S.; Chintharlapalli, S.; Cho, S.D.; Safe, S.; Kamat, A.M. 1,1-Bis(3’-indolyl)-1-(p-chlorophenyl)methane activates the orphan nuclear receptor Nurr1 and inhibits bladder cancer growth. Mol. Cancer 2008, 7, 3825–3833. [Google Scholar] [CrossRef]
- Remmele, W.; Stegner, H. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 1987, 8, 138–140. [Google Scholar]
- Hammond, S.L.; Safe, S.; Tjalkens, R.B. A novel synthetic activator of Nurr1 induces dopaminergic gene expression and protects against 6-hydroxydopamine neurotoxicity in vitro. Neurosci. Lett. 2015, 607, 83–89. [Google Scholar] [CrossRef]
- De Miranda, B.R.; Popichak, K.A.; Hammond, S.L.; Jorgensen, B.A.; Phillips, A.T.; Safe, S.; Tjalkens, R.B. The Nurr1 Activator 1,1-Bis(3’-Indolyl)-1-(p-Chlorophenyl)Methane Blocks Inflammatory Gene Expression in BV-2 Microglial Cells by Inhibiting Nuclear Factor kappaB. Mol. Pharmacol. 2015, 87, 1021–1034. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]

| Clinicopathologic Parameters | n | Percentage (%) | |
|---|---|---|---|
| Histology | |||
| Keratinizing | 160 | 90.4 | |
| Warty/basaloid | 17 | 9.6 | |
| Tumor size | |||
| T1 | 69 | 39 | |
| T2 | 92 | 52 | |
| T3 | 9 | 5.1 | |
| data unavailable | 7 | 3.9 | |
| Nodal status | |||
| N0 | 78 | 44.1 | |
| N1 | 38 | 21.5 | |
| N2 | 12 | 6.8 | |
| data unavailable | 49 | 27.6 | |
| Metastasis | |||
| M0 | 8 | 4.5 | |
| data unavailable | 169 | 95.5 | |
| FIGO | |||
| I | 61 | 34.4 | |
| II | 54 | 30.5 | |
| III | 47 | 26.6 | |
| IV | 9 | 5.1 | |
| data unavailable | 6 | 3.4 | |
| Grading | |||
| G1 | 29 | 16.4 | |
| G2 | 108 | 61 | |
| G3 | 39 | 22 | |
| data unavailable | 1 | 0.6 | |
| P16 status | |||
| Positive | 38 | 21.5 | |
| Negative | 57 | 32.2 | |
| data unavailable | 82 | 46.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wanka, G.; Schmoeckel, E.; Mayr, D.; Fuerst, S.; Kuhn, C.; Mahner, S.; Knabl, J.; Karsten, M.M.; Dannecker, C.; Heidegger, H.H.; et al. LDOC1 as Negative Prognostic Marker for Vulvar Cancer Patients. Int. J. Mol. Sci. 2020, 21, 9287. https://doi.org/10.3390/ijms21239287
Wanka G, Schmoeckel E, Mayr D, Fuerst S, Kuhn C, Mahner S, Knabl J, Karsten MM, Dannecker C, Heidegger HH, et al. LDOC1 as Negative Prognostic Marker for Vulvar Cancer Patients. International Journal of Molecular Sciences. 2020; 21(23):9287. https://doi.org/10.3390/ijms21239287
Chicago/Turabian StyleWanka, Giulia, Elisa Schmoeckel, Doris Mayr, Sophie Fuerst, Christina Kuhn, Sven Mahner, Julia Knabl, Maria Margarete Karsten, Christian Dannecker, Helene H. Heidegger, and et al. 2020. "LDOC1 as Negative Prognostic Marker for Vulvar Cancer Patients" International Journal of Molecular Sciences 21, no. 23: 9287. https://doi.org/10.3390/ijms21239287
APA StyleWanka, G., Schmoeckel, E., Mayr, D., Fuerst, S., Kuhn, C., Mahner, S., Knabl, J., Karsten, M. M., Dannecker, C., Heidegger, H. H., Vattai, A., Jeschke, U., & Jueckstock, J. (2020). LDOC1 as Negative Prognostic Marker for Vulvar Cancer Patients. International Journal of Molecular Sciences, 21(23), 9287. https://doi.org/10.3390/ijms21239287






