Skin Resident γδ T Cell Function and Regulation in Wound Repair
Abstract
:1. Introduction
2. Epidermal T Cells, TCR Activation and Wound Repair
2.1. Impact of γδ T Cell Costimulation on Wound Repair
2.2. Cytokine and Chemokine Regulation of Skin γδ T Cells
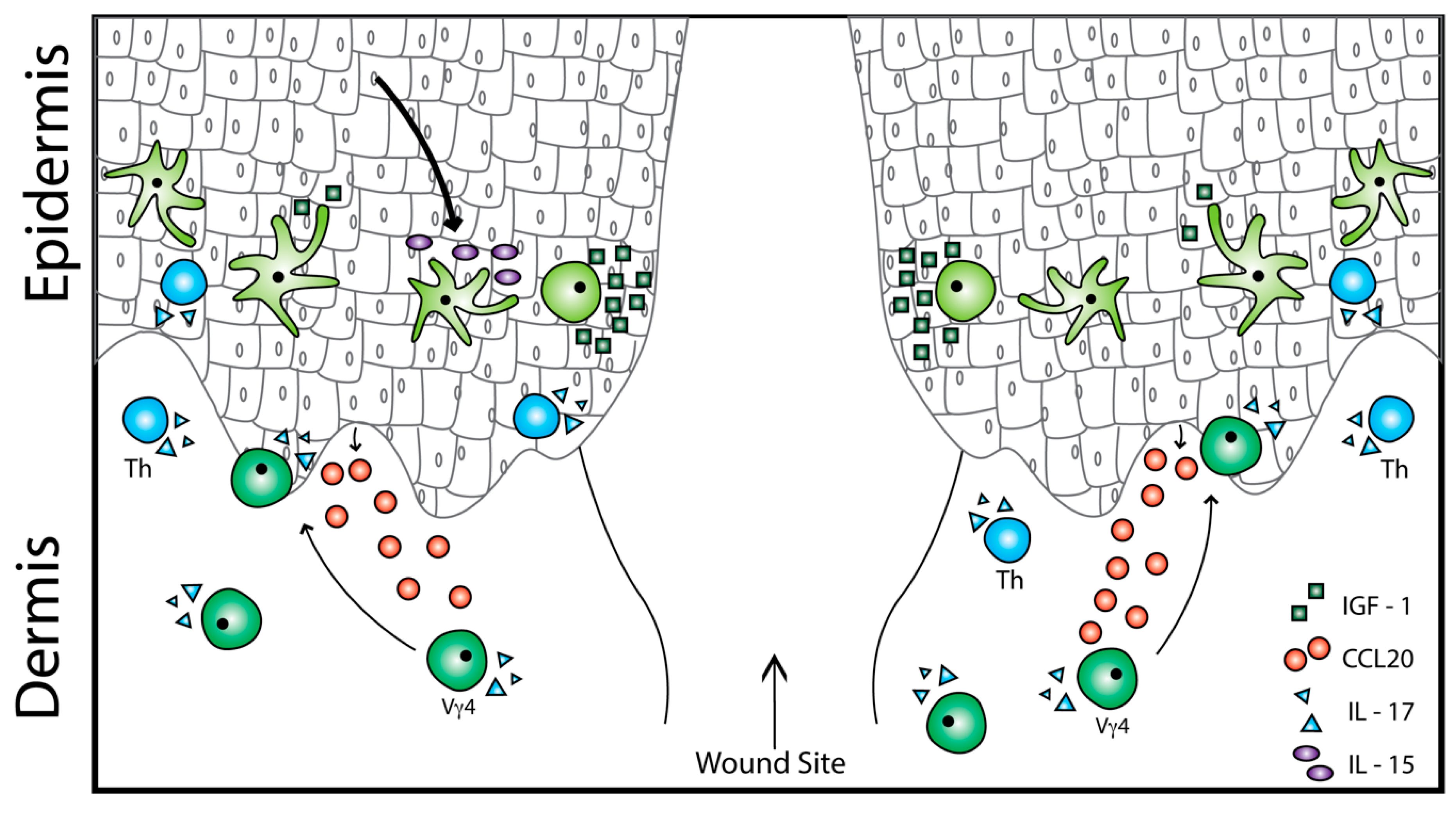
2.3. Epidermal γδ T Cell Function in Wound Repair
3. Dermal γδ T Cell Activation in Inflammation and Repair
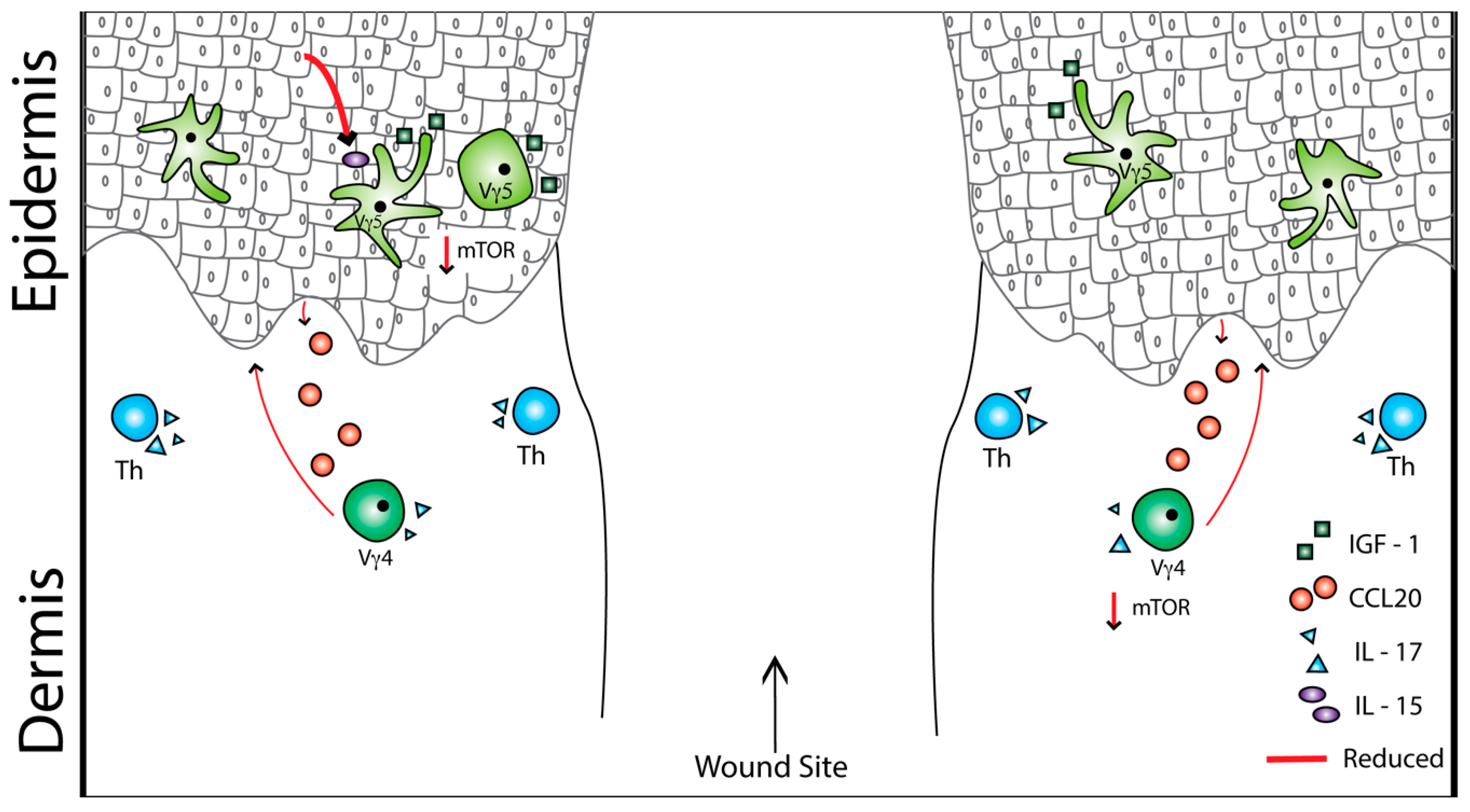
4. Obesity, Type 2 Diabetes, and Skin T Cells
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sutoh, Y.; Mohamed, R.H.; Kasahara, M. Origin and Evolution of Dendritic Epidermal T Cells. Front. Immunol. 2018, 9, 1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz, M.S.; Diamond, A.; Russell, A.; Jameson, J.M. Human αβ and γδ T Cells in Skin Immunity and Disease. Front. Immunol. 2018, 9, 1304. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.M.; Witherden, D.A.; Havran, W.L. γδ T cells in homeostasis and host defence of epithelial barrier tissues. Nat. Rev. Immunol. 2017, 17, 733–745. [Google Scholar] [CrossRef]
- Jameson, J. A Role for Skin gamma delta T Cells in Wound Repair. Science 2002, 296, 747–749. [Google Scholar] [CrossRef] [PubMed]
- Toulon, A.; Breton, L.; Taylor, K.R.; Tenenhaus, M.; Bhavsar, D.; Lanigan, C.; Rudolph, R.; Jameson, J.; Havran, W.L. A role for human skin-resident T cells in wound healing. J. Exp. Med. 2009, 206, 743–750. [Google Scholar] [CrossRef] [Green Version]
- McDonald, B.D.; Jabri, B.; Bendelac, A. Diverse developmental pathways of intestinal intraepithelial lymphocytes. Nat. Rev. Immunol. 2018, 18, 514–525. [Google Scholar] [CrossRef]
- Sharp, L.L.; Jameson, J.M.; Cauvi, G.; Havran, W.L. Dendritic epidermal T cells regulate skin homeostasis through local production of insulin-like growth factor 1. Nat. Immunol. 2005, 6, 73–79. [Google Scholar] [CrossRef]
- Johnson, M.D.; Witherden, D.A.; Havran, W.L. The Role of Tissue-resident γδ T Cells in Stress Surveillance and Tissue Maintenance. Cells 2020, 9, 686. [Google Scholar] [CrossRef] [Green Version]
- Jameson, J.M.; Sharp, L.L.; Witherden, D.A.; Havran, W.L. Regulation of skin cell homeostasis by gamma delta T cells. Front. Biosci. J. Virtual Libr. 2004, 9, 2640–2651. [Google Scholar] [CrossRef] [Green Version]
- Jameson, J.M.; Cauvi, G.; Witherden, D.A.; Havran, W.L. A keratinocyte-responsive gamma delta TCR is necessary for dendritic epidermal T cell activation by damaged keratinocytes and maintenance in the epidermis. J. Immunol. Baltim. Md. 1950 2004, 172, 3573–3579. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wu, J.; Luo, G.; He, W. Functions of Vγ4 T Cells and Dendritic Epidermal T Cells on Skin Wound Healing. Front. Immunol. 2018, 9, 1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, P.; Fu, X.; Xiao, N.; Guo, Y.; Pei, Q.; Peng, Y.; Zhang, Y.; Yao, M. Involvements of γδT Lymphocytes in Acute and Chronic Skin Wound Repair. Inflammation 2017, 40, 1416–1427. [Google Scholar] [CrossRef] [PubMed]
- Heilig, J.S.; Tonegawa, S. Diversity of murine gamma genes and expression in fetal and adult T lymphocytes. Nature 1986, 322, 836–840. [Google Scholar] [CrossRef] [PubMed]
- Havran, W.L.; Jameson, J.M. Epidermal T Cells and Wound Healing. J. Immunol. 2010, 184, 5423–5428. [Google Scholar] [CrossRef]
- Ramirez, K.; Witherden, D.A.; Havran, W.L. All hands on DE(T)C: Epithelial-resident γδ T cells respond to tissue injury. Cell. Immunol. 2015, 296, 57–61. [Google Scholar] [CrossRef] [Green Version]
- Havran, W.; Chien, Y.; Allison, J. Recognition of self antigens by skin-derived T cells with invariant gamma delta antigen receptors. Science 1991, 252, 1430–1432. [Google Scholar] [CrossRef]
- Havran, W.L.; Allison, J.P. Origin of Thy-1+ dendritic epidermal cells of adult mice from fetal thymic precursors. Nature 1990, 344, 68–70. [Google Scholar] [CrossRef]
- Havran, W.L.; Allison, J.P. Developmentally ordered appearance of thymocytes expressing different T-cell antigen receptors. Nature 1988, 335, 443–445. [Google Scholar] [CrossRef]
- MacLeod, A.S.; Hemmers, S.; Garijo, O.; Chabod, M.; Mowen, K.; Witherden, D.A.; Havran, W.L. Dendritic epidermal T cells regulate skin antimicrobial barrier function. J. Clin. Invest. 2013, 123, 4364–4374. [Google Scholar] [CrossRef]
- Daniel, T.; Thobe, B.M.; Chaudry, I.H.; Choudhry, M.A.; Hubbard, W.J.; Schwacha, M.G. Regulation of The Postburn Wound Inflammatory Response By γδ T-cells. Shock 2007, 28, 278–283. [Google Scholar] [CrossRef]
- Girardi, M. Regulation of Cutaneous Malignancy by gamma delta T Cells. Science 2001, 294, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.A.; Chong, B.; Mirchandani, N.; Brinster, N.K.; Yamanaka, K.; Dowgiert, R.K.; Kupper, T.S. The Vast Majority of CLA + T Cells Are Resident in Normal Skin. J. Immunol. 2006, 176, 4431–4439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCully, M.L.; Ladell, K.; Hakobyan, S.; Mansel, R.E.; Price, D.A.; Moser, B. Epidermis instructs skin homing receptor expression in human T cells. Blood 2012, 120, 4591–4598. [Google Scholar] [CrossRef] [Green Version]
- Donia, M.; Ellebaek, E.; Andersen, M.H.; Straten, P.T.; Svane, I.M. Analysis of Vδ1 T cells in clinical grade melanoma-infiltrating lymphocytes. OncoImmunology 2012, 1, 1297–1304. [Google Scholar] [CrossRef] [Green Version]
- Malhotra, N.; Narayan, K.; Cho, O.H.; Sylvia, K.E.; Yin, C.; Melichar, H.; Rashighi, M.; Lefebvre, V.; Harris, J.E.; Berg, L.J.; et al. A network of high-mobility group box transcription factors programs innate interleukin-17 production. Immunity 2013, 38, 681–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Y.; Xue, F.; Fleming, C.; Yang, J.; Ding, C.; Ma, Y.; Liu, M.; Zhang, H.; Zheng, J.; Xiong, N.; et al. Differential developmental requirement and peripheral regulation for dermal Vγ4 and Vγ6T17 cells in health and inflammation. Nat. Commun. 2014, 5, 3986. [Google Scholar] [CrossRef]
- Ramírez-Valle, F.; Gray, E.E.; Cyster, J.G. Inflammation induces dermal Vγ4 + γδT17 memory-like cells that travel to distant skin and accelerate secondary IL-17–driven responses. Proc. Natl. Acad. Sci. USA 2015, 112, 8046–8051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, R.L.; Born, W.K. Dermal γδ T cells—What have we learned? Cell. Immunol. 2015, 296, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Bos, J.D.; Teunissen, M.B.M.; Cairo, I.; Krieg, S.R.; Kapsenberg, M.L.; Das, P.K.; Borst, J. T-Cell Receptor γδ Bearing Cells in Normal Human Skin. J. Invest. Dermatol. 1990, 94, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Danilenko, D.M.; Valdez, P.; Kasman, I.; Eastham-Anderson, J.; Wu, J.; Ouyang, W. Interleukin-22, a TH17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 2007, 445, 648–651. [Google Scholar] [CrossRef]
- Hawkes, J.E.; Yan, B.Y.; Chan, T.C.; Krueger, J.G. Discovery of the IL-23/IL-17 Signaling Pathway and the Treatment of Psoriasis. J. Immunol. 2018, 201, 1605–1613. [Google Scholar] [CrossRef] [PubMed]
- Kusuba, N.; Kitoh, A.; Dainichi, T.; Honda, T.; Otsuka, A.; Egawa, G.; Nakajima, S.; Miyachi, Y.; Kabashima, K. Inhibition of IL-17–committed T cells in a murine psoriasis model by a vitamin D analogue. J. Allergy Clin. Immunol. 2018, 141, 972–981.e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boismenu, R.; Havran, W. Modulation of epithelial cell growth by intraepithelial gamma delta T cells. Science 1994, 266, 1253–1255. [Google Scholar] [CrossRef] [PubMed]
- Schwacha, M.G.; Ayala, A.; Chaudry, I.H. Insights into the role of gammadelta T lymphocytes in the immunopathogenic response to thermal injury. J. Leukoc. Biol. 2000, 67, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Jameson, J.M.; Cauvi, G.; Sharp, L.L.; Witherden, D.A.; Havran, W.L. γδ T cell–induced hyaluronan production by epithelial cells regulates inflammation. J. Exp. Med. 2005, 201, 1269–1279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werner, S.; Smola, H.; Liao, X.; Longaker, M.; Krieg, T.; Hofschneider, P.; Williams, L. The function of KGF in morphogenesis of epithelium and reepithelialization of wounds. Science 1994, 266, 819–822. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, Y.; Li, Y.; Liang, G.; Jiang, Y.; Liu, Z.; Liu, M.; Hao, J.; Zhang, X.; Hu, X.; et al. IL-15 Enhances Activation and IGF-1 Production of Dendritic Epidermal T Cells to Promote Wound Healing in Diabetic Mice. Front. Immunol. 2017, 8, 1557. [Google Scholar] [CrossRef] [Green Version]
- Ando, Y.; Jensen, P.J. Epidermal Growth Factor and Insulin-Like Growth Factor I Enhance Keratinocyte Migration. J. Invest. Dermatol. 1993, 100, 633–639. [Google Scholar] [CrossRef] [Green Version]
- Bonneville, M.; O’Brien, R.L.; Born, W.K. γδ T cell effector functions: A blend of innate programming and acquired plasticity. Nat. Rev. Immunol. 2010, 10, 467–478. [Google Scholar] [CrossRef]
- Cai, Y.; Shen, X.; Ding, C.; Qi, C.; Li, K.; Li, X.; Jala, V.R.; Zhang, H.; Wang, T.; Zheng, J.; et al. Pivotal Role of Dermal IL-17-Producing γδ T Cells in Skin Inflammation. Immunity 2011, 35, 596–610. [Google Scholar] [CrossRef] [Green Version]
- Spidale, N.A.; Malhotra, N.; Frascoli, M.; Sylvia, K.; Miu, B.; Freeman, C.; Stadinski, B.D.; Huseby, E.; Kang, J. Neonatal-derived IL-17 producing dermal γδ T cells are required to prevent spontaneous atopic dermatitis. eLife 2020, 9, e51188. [Google Scholar] [CrossRef] [PubMed]
- Colpitts, S.L.; Puddington, L.; Lefrançois, L. IL-15 receptor α signaling constrains the development of IL-17–producing γδ T cells. Proc. Natl. Acad. Sci. USA 2015, 112, 9692–9697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witherden, D.A.; Watanabe, M.; Garijo, O.; Rieder, S.E.; Sarkisyan, G.; Cronin, S.J.F.; Verdino, P.; Wilson, I.A.; Kumanogoh, A.; Kikutani, H.; et al. The CD100 Receptor Interacts with Its Plexin B2 Ligand to Regulate Epidermal γδ T Cell Function. Immunity 2012, 37, 314–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witherden, D.A.; Verdino, P.; Rieder, S.E.; Garijo, O.; Mills, R.E.; Teyton, L.; Fischer, W.H.; Wilson, I.A.; Havran, W.L. The Junctional Adhesion Molecule JAML Is a Costimulatory Receptor for Epithelial T Cell Activation. Science 2010, 329, 1205–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whang, M.I.; Guerra, N.; Raulet, D.H. Costimulation of Dendritic Epidermal γδ T Cells by a New NKG2D Ligand Expressed Specifically in the Skin. J. Immunol. 2009, 182, 4557–4564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, S.; Mohamed, R.H.; Kajikawa, M.; Koizumi, J.; Tanaka, M.; Fugo, K.; Otsuka, N.; Maenaka, K.; Yagita, H.; Chiba, H.; et al. Involvement of an NKG2D Ligand H60c in Epidermal Dendritic T Cell-Mediated Wound Repair. J. Immunol. 2012, 188, 3972–3979. [Google Scholar] [CrossRef] [Green Version]
- Strid, J.; Roberts, S.J.; Filler, R.B.; Lewis, J.M.; Kwong, B.Y.; Schpero, W.; Kaplan, D.H.; Hayday, A.C.; Girardi, M. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat. Immunol. 2008, 9, 146–154. [Google Scholar] [CrossRef]
- Dillen, C.A.; Pinsker, B.L.; Marusina, A.I.; Merleev, A.A.; Farber, O.N.; Liu, H.; Archer, N.K.; Lee, D.B.; Wang, Y.; Ortines, R.V.; et al. Clonally expanded γδ T cells protect against Staphylococcus aureus skin reinfection. J. Clin. Invest. 2018, 128, 1026–1042. [Google Scholar] [CrossRef]
- Barral, A.; Barral-Netto, M.; Oliveira, F.; Cafe, V.; Rosato, A.B.; Costa, J.M.; Bafica, A.; Favali, C.B.F. Lesion Size Correlates with Leishmania Antigen-Stimulated TNF-Levels in Human Cutaneous Leishmaniasis. Am. J. Trop. Med. Hyg. 2011, 85, 70–73. [Google Scholar] [CrossRef]
- Witherden, D.; Garijo, O.D.; Kelly, R.; Komori, H.K.; Havran, W.L. TCR-ligand interactions are required for murine epidermal Vγ3Vδ1 T cell development. J. Immunol. 2019, 202, 53.4. [Google Scholar]
- Ferrero, I.; Wilson, A.; Beermann, F.; Held, W.; MacDonald, H.R. T Cell Receptor Specificity Is Critical for the Development of Epidermal γδ T Cells. J. Exp. Med. 2001, 194, 1473–1483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chodaczek, G.; Toporkiewicz, M.; Zal, M.A.; Zal, T. Epidermal T Cell Dendrites Serve as Conduits for Bidirectional Trafficking of Granular Cargo. Front. Immunol. 2018, 9, 1430. [Google Scholar] [CrossRef] [PubMed]
- Chodaczek, G.; Papanna, V.; Zal, M.A.; Zal, T. Body-barrier surveillance by epidermal γδ TCRs. Nat. Immunol. 2012, 13, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wu, J.; Jiao, Y.; Bock, C.; Dai, M.; Chen, B.; Chao, N.; Zhang, W.; Zhuang, Y. Differential Requirements of TCR Signaling in Homeostatic Maintenance and Function of Dendritic Epidermal T Cells. J. Immunol. 2015, 195, 4282–4291. [Google Scholar] [CrossRef] [Green Version]
- Di Marco Barros, R.; Roberts, N.A.; Dart, R.J.; Vantourout, P.; Jandke, A.; Nussbaumer, O.; Deban, L.; Cipolat, S.; Hart, R.; Iannitto, M.L.; et al. Epithelia Use Butyrophilin-like Molecules to Shape Organ-Specific γδ T Cell Compartments. Cell 2016, 167, 203–218.e17. [Google Scholar] [CrossRef] [Green Version]
- Boyden, L.M.; Lewis, J.M.; Barbee, S.D.; Bas, A.; Girardi, M.; Hayday, A.C.; Tigelaar, R.E.; Lifton, R.P. Skint1, the prototype of a newly identified immunoglobulin superfamily gene cluster, positively selects epidermal γδ T cells. Nat. Genet. 2008, 40, 656–662. [Google Scholar] [CrossRef] [Green Version]
- Jandke, A.; Melandri, D.; Monin, L.; Ushakov, D.S.; Laing, A.G.; Vantourout, P.; East, P.; Nitta, T.; Narita, T.; Takayanagi, H.; et al. Butyrophilin-like proteins display combinatorial diversity in selecting and maintaining signature intraepithelial γδ T cell compartments. Nat. Commun. 2020, 11, 3769. [Google Scholar] [CrossRef]
- Barbee, S.D.; Woodward, M.J.; Turchinovich, G.; Mention, J.-J.; Lewis, J.M.; Boyden, L.M.; Lifton, R.P.; Tigelaar, R.; Hayday, A.C. Skint-1 is a highly specific, unique selecting component for epidermal T cells. Proc. Natl. Acad. Sci. USA 2011, 108, 3330–3335. [Google Scholar] [CrossRef] [Green Version]
- Keyes, B.E.; Liu, S.; Asare, A.; Naik, S.; Levorse, J.; Polak, L.; Lu, C.P.; Nikolova, M.; Pasolli, H.A.; Fuchs, E. Impaired Epidermal to Dendritic T Cell Signaling Slows Wound Repair in Aged Skin. Cell 2016, 167, 1323–1338.e14. [Google Scholar] [CrossRef] [Green Version]
- Bas, A.; Swamy, M.; Abeler-Dörner, L.; Williams, G.; Pang, D.J.; Barbee, S.D.; Hayday, A.C. Butyrophilin-like 1 encodes an enterocyte protein that selectively regulates functional interactions with T lymphocytes. Proc. Natl. Acad. Sci. USA 2011, 108, 4376–4381. [Google Scholar] [CrossRef] [Green Version]
- Witherden, D.A.; Johnson, M.D.; Havran, W.L. Coreceptors and Their Ligands in Epithelial γδ T Cell Biology. Front. Immunol. 2018, 9, 731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byeseda, S.E.; Burns, A.R.; Dieffenbaugher, S.; Rumbaut, R.E.; Smith, C.W.; Li, Z. ICAM-1 Is Necessary for Epithelial Recruitment of γδ T Cells and Efficient Corneal Wound Healing. Am. J. Pathol. 2009, 175, 571–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagaoka, T.; Kaburagi, Y.; Hamaguchi, Y.; Hasegawa, M.; Takehara, K.; Steeber, D.A.; Tedder, T.F.; Sato, S. Delayed Wound Healing in the Absence of Intercellular Adhesion Molecule-1 or L-Selectin Expression. Am. J. Pathol. 2000, 157, 237–247. [Google Scholar] [CrossRef] [Green Version]
- Gay, A.N.; Mushin, O.P.; Lazar, D.A.; Naik-Mathuria, B.J.; Yu, L.; Gobin, A.; Smith, C.W.; Olutoye, O.O. Wound Healing Characteristics of ICAM-1 Null Mice Devoid of All Isoforms of ICAM-1. J. Surg. Res. 2011, 171, e1–e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerwenka, A.; Lanier, L.L. NKG2D ligands: Unconventional MHC class I-like molecules exploited by viruses and cancer: Cerwenka & Lanier: NKG2D ligands. Tissue Antigens 2003, 61, 335–343. [Google Scholar] [CrossRef]
- Jamieson, A.M.; Diefenbach, A.; McMahon, C.W.; Xiong, N.; Carlyle, J.R.; Raulet, D.H. The Role of the NKG2D Immunoreceptor in Immune Cell Activation and Natural Killing. Immunity 2002, 17, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.; Hsiung, B.; Pestal, K.; Procyk, E.; Raulet, D.H. RAE-1 ligands for the NKG2D receptor are regulated by E2F transcription factors, which control cell cycle entry. J. Exp. Med. 2012, 209, 2409–2422. [Google Scholar] [CrossRef] [Green Version]
- Nitahara, A.; Shimura, H.; Ito, A.; Tomiyama, K.; Ito, M.; Kawai, K. NKG2D Ligation without T Cell Receptor Engagement Triggers Both Cytotoxicity and Cytokine Production in Dendritic Epidermal T Cells. J. Invest. Dermatol. 2006, 126, 1052–1058. [Google Scholar] [CrossRef] [Green Version]
- MacLeod, A.S.; Havran, W.L. Functions of skin-resident γδ T cells. Cell. Mol. Life Sci. 2011, 68, 2399–2408. [Google Scholar] [CrossRef] [Green Version]
- Ye, S.-K.; Maki, K.; Lee, H.-C.; Ito, A.; Kawai, K.; Suzuki, H.; Mak, T.W.; Chien, Y.; Honjo, T.; Ikuta, K. Differential Roles of Cytokine Receptors in the Development of Epidermal γδ T Cells. J. Immunol. 2001, 167, 1929–1934. [Google Scholar] [CrossRef] [Green Version]
- Sharp, L.L.; Jameson, J.M.; Witherden, D.A.; Komori, H.K.; Havran, W.L. Dendritic Epidermal T-Cell Activation. Crit. Rev. Immunol. 2005, 25, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Edelbaum, D.; Mohamadzadeh, M.; Bergstresser, P.R.; Sugamura, K.; Takashima, A. Interleukin (IL)-15 Promotes the Growth of Murine Epidermal γδ T Cells by a Mechanism Involving the β- and γc-Chains of the IL-2 Receptor. J. Invest. Dermatol. 1995, 105, 837–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudo, T.; Nishikawa, S.; Ohno, N.; Akiyama, N.; Tamakoshi, M.; Yoshida, H.; Nishikawa, S. Expression and function of the interleukin 7 receptor in murine lymphocytes. Proc. Natl. Acad. Sci. USA 1993, 90, 9125–9129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, X.; Shores, E.W.; Hu-Li, J.; Anver, M.R.; Kelsail, B.L.; Russell, S.M.; Drago, J.; Noguchi, M.; Grinberg, A.; Bloom, E.T.; et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor γ chain. Immunity 1995, 2, 223–238. [Google Scholar] [CrossRef] [Green Version]
- Papotto, P.H.; Reinhardt, A.; Prinz, I.; Silva-Santos, B. Innately versatile: γδ17 T cells in inflammatory and autoimmune diseases. J. Autoimmun. 2018, 87, 26–37. [Google Scholar] [CrossRef]
- Hadian, Y.; Bagood, M.D.; Dahle, S.E.; Sood, A.; Isseroff, R.R. Interleukin-17: Potential Target for Chronic Wounds. Mediat. Inflamm. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Leclercq, G.; De Smedt, M.; Tison, B.; Plum, J. Preferential proliferation of T cell receptor V gamma 3-positive cells in IL-2-stimulated fetal thymocytes. J. Immunol. 1990, 145, 3992–3997. [Google Scholar]
- Matsue, H.; Bergstresser, P.R.; Takashima, A. Keratinocyte-derived IL-7 serves as a growth factor for dendritic epidermal T cells in mice. J. Immunol. 1993, 151, 6012–6019. [Google Scholar]
- Kondo, M.; Takeshita, T.; Ishii, N.; Nakamura, M.; Watanabe, S.; Arai, K.; Sugamura, K. Sharing of the interleukin-2 (IL-2) receptor gamma chain between receptors for IL-2 and IL-4. Science 1993, 262, 1874–1877. [Google Scholar] [CrossRef]
- Rani, M.; Zhang, Q.; Schwacha, M.G. Burn Wound γδ T-Cells Support a Th2 and Th17 Immune Response. J. Burn Care Res. 2014, 35, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, K.; White, A.J.; Parnell, S.M.; Lane, P.J.; Jenkinson, E.J.; Jenkinson, W.E.; Anderson, G. Differential Requirement for CCR4 in the Maintenance but Not Establishment of the Invariant Vγ5+ Dendritic Epidermal T-Cell Pool. PLoS ONE 2013, 8, e74019. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.J.; Haraldsen, G.; Pan, J.; Rottman, J.; Qin, S.; Ponath, P.; Andrew, D.P.; Warnke, R.; Ruffing, N.; Kassam, N.; et al. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature 1999, 400, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Paradis, T.J.; Cole, S.H.; Nelson, R.T.; Gladue, R.P. Essential role of CCR6 in directing activated T cells to the skin during contact hypersensitivity. J. Invest. Dermatol. 2008, 128, 628–633. [Google Scholar] [CrossRef] [Green Version]
- Anderson, L.S.; Yu, S.; Rivara, K.R.; Reynolds, M.B.; Hernandez, A.A.; Wu, X.; Yang, H.-Y.; Isseroff, R.R.; Miller, L.S.; Hwang, S.T.; et al. CCR6+ γδ T Cells Home to Skin Wounds and Restore Normal Wound Healing in CCR6-Deficient Mice. J. Invest. Dermatol. 2019, 139, 2061–2064.e2. [Google Scholar] [CrossRef] [PubMed]
- Lahl, K.; Sweere, J.; Pan, J.; Butcher, E. Orphan chemoattractant receptor GPR15 mediates dendritic epidermal T-cell recruitment to the skin: Immunomodulation. Eur. J. Immunol. 2014, 44, 2577–2581. [Google Scholar] [CrossRef] [Green Version]
- Homey, B.; Wang, W.; Soto, H.; Buchanan, M.E.; Wiesenborn, A.; Catron, D.; Müller, A.; McClanahan, T.K.; Dieu-Nosjean, M.-C.; Orozco, R.; et al. Cutting Edge: The Orphan Chemokine Receptor G Protein-Coupled Receptor-2 (GPR-2, CCR10) Binds the Skin-Associated Chemokine CCL27 (CTACK/ALP/ILC). J. Immunol. 2000, 164, 3465–3470. [Google Scholar] [CrossRef] [Green Version]
- Harper, E.G.; Guo, C.; Rizzo, H.; Lillis, J.V.; Kurtz, S.E.; Skorcheva, I.; Purdy, D.; Fitch, E.; Iordanov, M.; Blauvelt, A. Th17 Cytokines Stimulate CCL20 Expression in Keratinocytes In Vitro and In Vivo: Implications for Psoriasis Pathogenesis. J. Invest. Dermatol. 2009, 129, 2175–2183. [Google Scholar] [CrossRef] [Green Version]
- Fitzpatrick, S.; Lausch, R.; Barrington, R.A. CCR6-Positive γδ T Cells Provide Protection Against Intracorneal HSV-1 Infection. Investig. Opthalmology Vis. Sci. 2019, 60, 3952. [Google Scholar] [CrossRef] [Green Version]
- Mabuchi, T.; Singh, T.P.; Takekoshi, T.; Jia, G.; Wu, X.; Kao, M.C.; Weiss, I.; Farber, J.M.; Hwang, S.T. CCR6 Is Required for Epidermal Trafficking of γδ-T Cells in an IL-23-Induced Model of Psoriasiform Dermatitis. J. Invest. Dermatol. 2013, 133, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Laggner, U.; Di Meglio, P.; Perera, G.K.; Hundhausen, C.; Lacy, K.E.; Ali, N.; Smith, C.H.; Hayday, A.C.; Nickoloff, B.J.; Nestle, F.O. Identification of a Novel Proinflammatory Human Skin-Homing Vγ9Vδ2 T Cell Subset with a Potential Role in Psoriasis. J. Immunol. 2011, 187, 2783–2793. [Google Scholar] [CrossRef]
- Karvinen, S.; Pasonen-Seppänen, S.; Hyttinen, J.M.T.; Pienimäki, J.-P.; Törrönen, K.; Jokela, T.A.; Tammi, M.I.; Tammi, R. Keratinocyte Growth Factor Stimulates Migration and Hyaluronan Synthesis in the Epidermis by Activation of Keratinocyte Hyaluronan Synthases 2 and 3. J. Biol. Chem. 2003, 278, 49495–49504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boismenu, R.; Feng, L.; Xia, Y.Y.; Chang, J.C.; Havran, W.L. Chemokine expression by intraepithelial gamma delta T cells. Implications for the recruitment of inflammatory cells to damaged epithelia. J. Immunol. Baltim. Md. 1950 1996, 157, 985–992. [Google Scholar]
- Taylor, K.R.; Mills, R.E.; Costanzo, A.E.; Jameson, J.M. γδ T Cells Are Reduced and Rendered Unresponsive by Hyperglycemia and Chronic TNFα in Mouse Models of Obesity and Metabolic Disease. PLoS ONE 2010, 5, e11422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Chou, K.; Fuchs, E.; Havran, W.L.; Boismenu, R. Protection of the intestinal mucosa by intraepithelial T cells. Proc. Natl. Acad. Sci. USA 2002, 99, 14338–14343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, M.M.; Lovato, P.; MacLeod, A.S.; Witherden, D.A.; Skov, L.; Dyring-Andersen, B.; Dabelsteen, S.; Woetmann, A.; Ødum, N.; Havran, W.L.; et al. IL-1β–Dependent Activation of Dendritic Epidermal T Cells in Contact Hypersensitivity. J. Immunol. 2014, 192, 2975–2983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niyonsaba, F.; Ushio, H.; Nakano, N.; Ng, W.; Sayama, K.; Hashimoto, K.; Nagaoka, I.; Okumura, K.; Ogawa, H. Antimicrobial Peptides Human β-Defensins Stimulate Epidermal Keratinocyte Migration, Proliferation and Production of Proinflammatory Cytokines and Chemokines. J. Invest. Dermatol. 2007, 127, 594–604. [Google Scholar] [CrossRef] [Green Version]
- Thorey, I.S.; Roth, J.; Regenbogen, J.; Halle, J.-P.; Bittner, M.; Vogl, T.; Kaesler, S.; Bugnon, P.; Reitmaier, B.; Durka, S.; et al. The Ca 2+ -binding Proteins S100A8 and S100A9 Are Encoded by Novel Injury-regulated Genes. J. Biol. Chem. 2001, 276, 35818–35825. [Google Scholar] [CrossRef] [Green Version]
- Lai, Y.; Li, D.; Li, C.; Muehleisen, B.; Radek, K.A.; Park, H.J.; Jiang, Z.; Li, Z.; Lei, H.; Quan, Y.; et al. The Antimicrobial Protein REG3A Regulates Keratinocyte Proliferation and Differentiation after Skin Injury. Immunity 2012, 37, 74–84. [Google Scholar] [CrossRef] [Green Version]
- Ha, H.-L.; Wang, H.; Pisitkun, P.; Kim, J.-C.; Tassi, I.; Tang, W.; Morasso, M.I.; Udey, M.C.; Siebenlist, U. IL-17 drives psoriatic inflammation via distinct, target cell-specific mechanisms. Proc. Natl. Acad. Sci. USA 2014, 111, E3422–E3431. [Google Scholar] [CrossRef] [Green Version]
- King, A.; Balaji, S.; Le, L.D.; Crombleholme, T.M.; Keswani, S.G. Regenerative Wound Healing: The Role of Interleukin-10. Adv. Wound Care 2014, 3, 315–323. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.; Sandrock, I.; Odak, I.; Aizenbud, Y.; Wilharm, A.; Barros-Martins, J.; Tabib, Y.; Borchers, A.; Amado, T.; Gangoda, L.; et al. Single-Cell Transcriptomics Identifies the Adaptation of Scart1+ Vγ6+ T Cells to Skin Residency as Activated Effector Cells. Cell Rep. 2019, 27, 3657–3671.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khairallah, C.; Chu, T.H.; Sheridan, B.S. Tissue Adaptations of Memory and Tissue-Resident Gamma Delta T Cells. Front. Immunol. 2018, 9, 2636. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Zhou, L.; Liu, M.; Liang, G.; Yan, R.; Jiang, Y.; Hao, J.; Zhang, X.; Hu, X.; et al. Vγ4 T Cells Inhibit the Pro-healing Functions of Dendritic Epidermal T Cells to Delay Skin Wound Closure Through IL-17A. Front. Immunol. 2018, 9, 240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwacha, M.G.; Rani, M.; Nicholson, S.E.; Lewis, A.M.; Holloway, T.L.; Sordo, S.; Cap, A.P. Dermal γδ T-Cells Can Be Activated by Mitochondrial Damage-Associated Molecular Patterns. PLoS ONE 2016, 11, e0158993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, P.F.; Porterfield, N.; Pannell, D.; Davis, T.A.; Elster, E.A. Trauma is danger. J. Transl. Med. 2011, 9, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manfredi, A.A.; Capobianco, A.; Bianchi, M.E.; Rovere-Querini, P. Regulation of Dendritic- and T-Cell Fate by Injury-Associated Endogenous Signals. Crit. Rev. Immunol. 2009, 29, 69–86. [Google Scholar] [CrossRef]
- Campanholle, G.; Mittelsteadt, K.; Nakagawa, S.; Kobayashi, A.; Lin, S.-L.; Gharib, S.A.; Heinecke, J.W.; Hamerman, J.A.; Altemeier, W.A.; Duffield, J.S. TLR-2/TLR-4 TREM-1 Signaling Pathway Is Dispensable in Inflammatory Myeloid Cells during Sterile Kidney Injury. PLoS ONE 2013, 8, e68640. [Google Scholar] [CrossRef] [Green Version]
- Schwacha, M.G.; Rani, M.; Zhang, Q.; Nunez-Cantu, O.; Cap, A.P. Mitochondrial damage-associated molecular patterns activate γδ T-cells. Innate Immun. 2014, 20, 261–268. [Google Scholar] [CrossRef]
- Hartwig, T.; Pantelyushin, S.; Croxford, A.L.; Kulig, P.; Becher, B. Dermal IL-17-producing γδ T cells establish long-lived memory in the skin: HIGHLIGHTS. Eur. J. Immunol. 2015, 45, 3022–3033. [Google Scholar] [CrossRef]
- Pence, B.D.; Woods, J.A. Exercise, Obesity, and Cutaneous Wound Healing: Evidence from Rodent and Human Studies. Adv. Wound Care 2014, 3, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Seitz, O.; Schürmann, C.; Hermes, N.; Müller, E.; Pfeilschifter, J.; Frank, S.; Goren, I. Wound Healing in Mice with High-Fat Diet- or ob Gene-Induced Diabetes-Obesity Syndromes: A Comparative Study. Exp. Diabetes Res. 2010, 2010, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blakytny, R.; Jude, E.B.; Martin Gibson, J.; Boulton, A.J.; Ferguson, M.W. Lack of insulin-like growth factor 1 (IGF1) in the basal keratinocyte layer of diabetic skin and diabetic foot ulcers. J. Pathol. 2000, 190, 589–594. [Google Scholar] [CrossRef]
- Brown, D.L.; Kane, C.D.; Chernausek, S.D.; Greenhalgh, D.G. Differential expression and localization of insulin-like growth factors I and II in cutaneous wounds of diabetic and nondiabetic mice. Am. J. Pathol. 1997, 151, 715–724. [Google Scholar] [PubMed]
- Liu, Z.; Xu, Y.; Chen, L.; Xie, J.; Tang, J.; Zhao, J.; Shu, B.; Qi, S.; Chen, J.; Liang, G.; et al. Dendritic epidermal T cells facilitate wound healing in diabetic mice. Am. J. Transl. Res. 2016, 8, 2375–2384. [Google Scholar]
- Liu, Z.; Xu, Y.; Zhang, X.; Liang, G.; Chen, L.; Xie, J.; Tang, J.; Zhao, J.; Shu, B.; Qi, S.; et al. Defects in dermal Vγ4 γ δ T cells result in delayed wound healing in diabetic mice. Am. J. Transl. Res. 2016, 8, 2667–2680. [Google Scholar]
- Kerley-Hamilton, J.S.; Trask, H.W.; Ridley, C.J.A.; DuFour, E.; Ringelberg, C.S.; Nurinova, N.; Wong, D.; Moodie, K.L.; Shipman, S.L.; Moore, J.H.; et al. Obesity Is Mediated by Differential Aryl Hydrocarbon Receptor Signaling in Mice Fed a Western Diet. Environ. Health Perspect. 2012, 120, 1252–1259. [Google Scholar] [CrossRef] [Green Version]
- Costanzo, A.E.; Taylor, K.R.; Dutt, S.; Han, P.P.; Fujioka, K.; Jameson, J.M. Obesity Impairs γδ T Cell Homeostasis and Antiviral Function in Humans. PLoS ONE 2015, 10, e0120918. [Google Scholar] [CrossRef]
- Zubkiewicz-Kucharska, A.; Noczyńska, A.; Usnarska-Zubkiewicz, L. Abnormal Distribution of Gamma-Delta T Lymphocytes and Their Subsets in Type 1 Diabetes. Adv. Clin. Exp. Med. 2016, 25, 665–671. [Google Scholar] [CrossRef] [Green Version]
- Okonkwo, U.; DiPietro, L. Diabetes and Wound Angiogenesis. Int. J. Mol. Sci. 2017, 18, 1419. [Google Scholar] [CrossRef] [Green Version]
- Brem, H.; Tomic-Canic, M. Cellular and molecular basis of wound healing in diabetes. J. Clin. Invest. 2007, 117, 1219–1222. [Google Scholar] [CrossRef] [Green Version]
- Taylor, K.R.; Costanzo, A.E.; Jameson, J.M. Dysfunctional γδ T Cells Contribute to Impaired Keratinocyte Homeostasis in Mouse Models of Obesity. J. Invest. Dermatol. 2011, 131, 2409–2418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fay, N.S.; Larson, E.C.; Jameson, J.M. Chronic Inflammation and γδ T Cells. Front. Immunol. 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayassi, T.; Ladell, K.; Gudjonson, H.; McLaren, J.E.; Shaw, D.G.; Tran, M.T.; Rokicka, J.J.; Lawrence, I.; Grenier, J.-C.; van Unen, V.; et al. Chronic Inflammation Permanently Reshapes Tissue-Resident Immunity in Celiac Disease. Cell 2019, 176, 967–981.e19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.; Cheung, K.P.; Limon, N.; Costanzo, A.; Barba, C.; Miranda, N.; Gargas, S.; Johnson, A.M.F.; Olefsky, J.M.; Jameson, J.M. Obesity Modulates Intestinal Intraepithelial T Cell Persistence, CD103 and CCR9 Expression, and Outcome in Dextran Sulfate Sodium–Induced Colitis. J. Immunol. 2019, 203, 3427–3435. [Google Scholar] [CrossRef] [PubMed]
- Merches, K.; Schiavi, A.; Weighardt, H.; Steinwachs, S.; Teichweyde, N.; Förster, I.; Hochrath, K.; Schumak, B.; Ventura, N.; Petzsch, P.; et al. AHR Signaling Dampens Inflammatory Signature in Neonatal Skin γδ T Cells. Int. J. Mol. Sci. 2020, 21, 2249. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Liang, G.; Gui, L.; Li, Y.; Liu, M.; Bai, Y.; Zhang, X.; Hu, X.; Chen, J.; Huang, C.; et al. Weakened IL-15 Production and Impaired mTOR Activation Alter Dendritic Epidermal T Cell Homeostasis in Diabetic Mice. Sci. Rep. 2017, 7, 6028. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Rodero, M.P.; Patel, J.; Moi, D.; Mazzieri, R.; Khosrotehrani, K. Interleukin-23 regulates interleukin-17 expression in wounds, and its inhibition accelerates diabetic wound healing through the alteration of macrophage polarization. FASEB J. 2018, 32, 2086–2094. [Google Scholar] [CrossRef] [Green Version]
- Balaji, S.; LeSaint, M.; Bhattacharya, S.S.; Moles, C.; Dhamija, Y.; Kidd, M.; Le, L.D.; King, A.; Shaaban, A.; Crombleholme, T.M.; et al. Adenoviral-mediated gene transfer of insulin-like growth factor 1 enhances wound healing and induces angiogenesis. J. Surg. Res. 2014, 190, 367–377. [Google Scholar] [CrossRef] [Green Version]
| Factors/Cytokines/Receptors | Function in Wound Repair | Reference |
|---|---|---|
| Keratinocyte Growth Factor-1 (KGF-1) | Induces keratinocyte proliferation, migration, and differentiation. | [33,36] |
| Insulin-like Growth Factor-1 (IGF-1) | Facilitates wound closure by mediating keratinocyte survival and limiting differentiation along with enhancing migration. | [36,37,38] |
| Tumor Necrosis Factor (TNF) | Induces inflammation. | [39] |
| Interleukin-17A (IL-17A) | Induces inflammation and the proliferation and differentiation of keratinocytes. | [40,41,42] |
| CD100 | Regulates activation through ligation of plexin B2. | [43] |
| Junction Adhesion Molecule-like Protein (JAML) | Costimulates proliferation and cytokine/growth factor production. | [44] |
| Natural Killer Group 2D (NKG2D) | Recognizes receptors upregulated by stressed keratinocytes and induces cytolysis. | [45] |
| H60 | Upregulated upon stress and ligates NKG2D to activate γδ T cells. | [46] |
| Retinoic Acid Early Inducible 1 (Rae-1) | Ligates NKG2D to costimulate epidermal γδ T cell degranulation, Il-2 production, and proliferation. | [47] |
| Cytokine/Chemokine/Receptor | Function in Wound Repair | Reference |
|---|---|---|
| Interleukin-2 (IL-2) | Induces proliferation of epidermal γδ T cells | [77] |
| IL-7 | Induces proliferation of epidermal γδ T cells and TCR-γ chain rearrangement | [78] |
| IL-4 | Influences number and growth of γδ T cells | [79] |
| IL-15 | Regulates growth and survival of γδ T cells and production of IGF-1 | [37] |
| IL-23 | Recruits dermal γδ T cells, stimulates IL-17 production, and inhibits IGF-1 production | [31] |
| IL-10 | Reduces inflammatory responses | [80] |
| Chemokine Receptor 4 (CCR4) | Recruits γδ T cells to wound site or site of infection | [81,82] |
| CCR6 | Binds CCL20 to recruit γδ T cells from the dermis to the epidermis | [83,84] |
| CCR10 | Binds CCL27 for homing of γδ T cells to the epidermis | [85,86] |
| Chemokine Ligand 20 (CCL20) | Binds CCR6 to induce γδ T cell recruitment from the dermis to epidermis. | [87] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munoz, L.D.; Sweeney, M.J.; Jameson, J.M. Skin Resident γδ T Cell Function and Regulation in Wound Repair. Int. J. Mol. Sci. 2020, 21, 9286. https://doi.org/10.3390/ijms21239286
Munoz LD, Sweeney MJ, Jameson JM. Skin Resident γδ T Cell Function and Regulation in Wound Repair. International Journal of Molecular Sciences. 2020; 21(23):9286. https://doi.org/10.3390/ijms21239286
Chicago/Turabian StyleMunoz, Luis D., Michael J. Sweeney, and Julie M. Jameson. 2020. "Skin Resident γδ T Cell Function and Regulation in Wound Repair" International Journal of Molecular Sciences 21, no. 23: 9286. https://doi.org/10.3390/ijms21239286
APA StyleMunoz, L. D., Sweeney, M. J., & Jameson, J. M. (2020). Skin Resident γδ T Cell Function and Regulation in Wound Repair. International Journal of Molecular Sciences, 21(23), 9286. https://doi.org/10.3390/ijms21239286





