A Review on the Fate of Legacy and Alternative Antimicrobials and Their Metabolites during Wastewater and Sludge Treatment
Abstract
1. Introduction
1.1. Introduction to Emerging Contaminants
1.2. Importance of Identification of Antimicrobials during Wastewater Treatment
2. Legacy Antimicrobial Triclosan
2.1. Background
2.2. Transformation/Degradation Products and Metabolites of Triclosan
2.3. Fate of Triclosan and Its Transformation/Degradation Products and Metabolites during Wastewater Treatment
2.4. Fate and Impacts of Triclosan and Triclosan-Related Compounds in the Environment
3. Legacy Antimicrobial Triclocarban
3.1. Background
3.2. Transformation/Degradation Products and Metabolites of Triclocarban
3.3. Fate of Triclocarban and Its Transformation/Degradation Products and Metabolites during Wastewater Treatment
3.4. Fate and Impacts of Triclocarban and Triclocarban-Related Compounds in the Environment
4. Emerging Alternative Antimicrobials
4.1. Introduction to Emerging Alternative Antimicrobials
4.2. Benzalkonium Chlorides as Emerging Alternative Antimicrobials
4.3. Degradation Byproducts/Metabolites of Benzalkonium Chlorides
4.4. Benzalkonium Chlorides in Sludge and Biosolids
4.5. Fate, Transformation and Impacts of Benzalkonium Chlorides in the Environment
4.6. Future Challenges and Concerns around Benzalkonium Chlorides
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 2,4-DCP | 2,4-dichlorophenol |
| 2,3,4-TCP | 2,3,4-trichlorophenol |
| 3,4-DCA | 3,4-dichloroaniline |
| 4-CA | 4-chloroaniline |
| 4-CC | 4-chlorocatechol |
| 4-Cl-TCC | 3,3′,4,4′-tetrachlorocarbanilide |
| BACs | Benzalkonium chlorides |
| BAC-8 | Benzalkonium chlorides containing eight carbon atoms in the alkyl chain |
| BAC-10 | Benzalkonium chlorides containing ten carbon atoms in the alkyl chain |
| BAC-12 | Benzalkonium chlorides containing twelve carbon atoms in the alkyl chain |
| BAC-14 | Benzalkonium chlorides containing fourteen carbon atoms in the alkyl chain |
| BAC-16 | Benzalkonium chlorides containing sixteen carbon atoms in the alkyl chain |
| BAC-18 | Benzalkonium chlorides containing eighteen carbon atoms in the alkyl chain |
| BNR | Biological nutrient removal |
| CAS | Chemical abstract service |
| CYP | Cytochrome P450 enzymes |
| DBPs | Disinfection byproducts |
| DCC | Dichlorocarbanilide |
| DCDDs | Dichlorodibenzo-p-dioxins |
| DL | Detection limit |
| dw | Dry weight |
| E. coli | Escherichia coli |
| IC25 | 25% inhibition concentration |
| LC50 | Median lethal concentration |
| LD50 | Lethal dose at which 50 percent of the population cannot survive |
| Log Kow | Logarithmic octanol-water partitioning coefficients |
| MCC | Monochlorocarbanilide |
| MeTCS | Methyl triclosan |
| MH | Mesophilic |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| NCC | Carbanilide |
| NDMA | N-Nitrosodimethylamine |
| NR | Not reported |
| OLR | Organic loading rate |
| pKa | Dissociation constant |
| PPCPs | Pharmaceuticals and personal care products |
| QACs | Quaternary ammonium compounds |
| SRT | Sludge retention time/solids retention time |
| TCC | Triclocarban |
| TCS | Triclosan |
| TCS-gluc | Glucuronidated triclosan |
| TCS-SO4 | Triclosan sulfate |
| TH | Thermophilic |
| TH-AD | Cambi Thermal Hydrolysis followed by anaerobic digestion |
| U.S. | United States |
| U.S. EPA | United States Environmental Protecion Agency |
| U.S. FDA | United States Food and Drug Administration |
| UV | Ultraviolet |
| WWTPs | Wastewater treatment plants |
References
- Zuloaga, O.; Navarro, P.; Bizkarguenaga, E.; Iparraguirre, A.; Vallejo, A.; Olivares, M.; Prieto, A. Overview of extraction, clean-up and detection techniques for the determination of organic pollutants in sewage sludge: A review. Anal. Chim. Acta 2012, 736, 7–29. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Meador, J.P.; Yeh, A.; Young, G.; Gallagher, E.P. Contaminants of emerging concern in a large temperate estuary. Environ. Pollut. 2016, 213, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Tijani, J.O.; Fatoba, O.O.; Petrik, L.F. A review of pharmaceuticals and endocrine-disrupting compounds: Sources, effects, removal, and detections. Water. Air. Soil Pollut. 2013, 224. [Google Scholar] [CrossRef]
- Venkatesan, A.K.; Halden, R.U. Wastewater treatment plants as chemical observatories to forecast ecological and human health risks of manmade chemicals. Sci. Rep. 2014, 4, 3731. [Google Scholar] [CrossRef] [PubMed]
- Diamond, J.M.; Latimer, H.A.; Munkittrick, K.R.; Thornton, K.W.; Bartell, S.M.; Kidd, K.A. Prioritizing contaminants of emerging concern for ecological screening assessments. Environ. Toxicol. Chem. 2011, 30, 2385–2394. [Google Scholar] [CrossRef]
- Verlicchi, P.; Al Aukidy, M.; Zambello, E. Occurrence of pharmaceutical compounds in urban wastewater: Removal, mass load and environmental risk after a secondary treatment-A review. Sci. Total Environ. 2012, 429, 123–155. [Google Scholar] [CrossRef]
- Samaras, V.G.; Stasinakis, A.S.; Mamais, D.; Thomaidis, N.S.; Lekkas, T.D. Fate of selected pharmaceuticals and synthetic endocrine disrupting compounds during wastewater treatment and sludge anaerobic digestion. J. Hazard. Mater. 2013, 244–245, 259–267. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Hu, A.; Rashid, A.; Ashfaq, M.; Wang, Y.; Wang, H.; Luo, H.; Yu, C.P.; Sun, Q. Monitoring, mass balance and fate of pharmaceuticals and personal care products in seven wastewater treatment plants in Xiamen City, China. J. Hazard. Mater. 2018, 354, 81–90. [Google Scholar] [CrossRef]
- Carballa, M.; Fink, G.; Omil, F.; Lema, J.M.; Ternes, T. Determination of the solid-water distribution coefficient (Kd) for pharmaceuticals, estrogens and musk fragrances in digested sludge. Water Res. 2008, 42, 287–295. [Google Scholar] [CrossRef]
- Gonzalez-Gil, L.; Papa, M.; Feretti, D.; Ceretti, E.; Mazzoleni, G.; Steimberg, N.; Pedrazzani, R.; Bertanza, G.; Lema, J.M.; Carballa, M. Is anaerobic digestion effective for the removal of organic micropollutants and biological activities from sewage sludge? Water Res. 2016, 102, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B.O.; Smith, S.R. Review of “emerging” organic contaminants in biosolids and assessment of international research priorities for the agricultural use of biosolids. Environ. Int. 2011, 37, 226–247. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.; Healy, M.G.; Fenton, O.; Cummins, E. Quantitative risk assessment of antimicrobials in biosolids applied on agricultural land and potential translocation into food. Food Res. Int. 2018, 106, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Macherius, A.; Lapen, D.R.; Reemtsma, T.; Römbke, J.; Topp, E.; Coors, A. Triclocarban, triclosan and its transformation product methyl triclosan in native earthworm species four years after a commercial-scale biosolids application. Sci. Total Environ. 2014, 472, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Spongberg, A.L.; Witter, J.D.; Fang, M.; Ames, A.; Czajkowski, K.P. Detection of pharmaceuticals and personal care products in agricultural soils receiving biosolids application. Clean Soil, Air, Water 2010, 38, 230–237. [Google Scholar] [CrossRef]
- Reid, B.J.; Jones, K.C.; Semple, K.T. Bioavailability of persistent organic pollutants in soils and sediments - A perspective on mechanisms, consequences and assessment. Environ. Pollut. 2000, 108, 103–112. [Google Scholar] [CrossRef]
- Harrison, E.Z.; Oakes, S.R.; Hysell, M.; Hay, A. Organic chemicals in sewage sludges. Sci. Total Environ. 2006, 367, 481–497. [Google Scholar] [CrossRef]
- McClellan, K.; Halden, R.U. Pharmaceuticals and personal care products in archived U.S. biosolids from the 2001 EPA national sewage sludge survey. Water Res. 2010, 44, 658–668. [Google Scholar] [CrossRef]
- Walters, E.; McClellan, K.; Halden, R.U. Occurrence and loss over three years of 72 pharmaceuticals and personal care products from biosolids–soil mixtures in outdoor mesocosms. Water Res. 2010, 44, 6011–6020. [Google Scholar] [CrossRef]
- Balakrishna, K.; Rath, A.; Praveenkumarreddy, Y.; Guruge, K.S.; Subedi, B. A review of the occurrence of pharmaceuticals and personal care products in Indian water bodies. Ecotoxicol. Environ. Saf. 2017, 137, 113–120. [Google Scholar] [CrossRef]
- Jiang, J.Q.; Zhou, Z.; Sharma, V.K. Occurrence, transportation, monitoring and treatment of emerging micro-pollutants in waste water—A review from global views. Microchem. J. 2013, 110, 292–300. [Google Scholar] [CrossRef]
- Brausch, J.M.; Rand, G.M. A review of personal care products in the aquatic environment: Environmental concentrations and toxicity. Chemosphere 2011, 82, 1518–1532. [Google Scholar] [CrossRef] [PubMed]
- Verlicchi, P.; Zambello, E. Pharmaceuticals and personal care products in untreated and treated sewage sludge: Occurrence and environmental risk in the case of application on soil—A critical review. Sci. Total Environ. 2015, 538, 750–767. [Google Scholar] [CrossRef]
- Jamil, K. Health effects of pharmaceuticals and personal care products. In Pharmaceuticals and Personal Care Products: Waste Management and Treatment Technology; Majeti Narasimha Vara, P., Meththika, V., Atya, K., Eds.; Butterworth-Heinemann: Oxford, UK, 2019; pp. 115–128. ISBN 9780128161890. [Google Scholar]
- Muñoz, I.; Gómez-Ramos, M.J.; Agüera, A.; Fernández-Alba, A.R.; García-Reyes, J.F.; Molina-Díaz, A. Chemical evaluation of contaminants in wastewater effluents and the environmental risk of reusing effluents in agriculture. TrAC - Trends Anal. Chem. 2009, 28, 676–694. [Google Scholar] [CrossRef]
- Deblonde, T.; Cossu-Leguille, C.; Hartemann, P. Emerging pollutants in wastewater: A review of the literature. Int. J. Hydrogen Environ. Health 2011, 214, 442–448. [Google Scholar] [CrossRef]
- Li, W.C. Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ. Pollut. 2014, 187, 193–201. [Google Scholar] [CrossRef]
- Prosser, R.S.; Sibley, P.K. Human health risk assessment of pharmaceuticals and personal care products in plant tissue due to biosolids and manure amendments, and wastewater irrigation. Environ. Int. 2015, 75, 223–233. [Google Scholar] [CrossRef]
- Juksu, K.; Zhao, J.L.; Liu, Y.S.; Yao, L.; Sarin, C.; Sreesai, S.; Klomjek, P.; Jiang, Y.X.; Ying, G.G. Occurrence, fate and risk assessment of biocides in wastewater treatment plants and aquatic environments in Thailand. Sci. Total Environ. 2019, 690, 1110–1119. [Google Scholar] [CrossRef]
- Fu, Q.; Sanganyado, E.; Ye, Q.; Gan, J. Meta-analysis of biosolid effects on persistence of triclosan and triclocarban in soil. Environ. Pollut. 2016, 210, 137–144. [Google Scholar] [CrossRef]
- Halden, R.U. On the need and speed of regulating triclosan and triclocarban in the United States. Environ. Sci. Technol. 2014, 48, 3603–3611. [Google Scholar] [CrossRef]
- Liu, W.R.; Yang, Y.Y.; Liu, Y.S.; Zhang, L.J.; Zhao, J.L.; Zhang, Q.Q.; Zhang, M.; Zhang, J.N.; Jiang, Y.X.; Ying, G.G. Biocides in wastewater treatment plants: Mass balance analysis and pollution load estimation. J. Hazard. Mater. 2017, 329, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Andrade, N.A.; Lozano, N.; McConnell, L.L.; Torrents, A.; Rice, C.P.; Ramirez, M. Long-term trends of PBDEs, triclosan, and triclocarban in biosolids from a wastewater treatment plant in the Mid-Atlantic region of the US. J. Hazard. Mater. 2015, 282, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Schebb, N.H.; Inceoglu, B.; Ahn, K.C.; Morisseau, C.; Gee, S.J.; Hammock, B.D. Investigation of human exposure to triclocarban after showering and preliminary evaluation of its biological effects. Environ. Sci. Technol. 2011, 45, 3109–3115. [Google Scholar] [CrossRef] [PubMed]
- Schebb, N.H.; Ahn, K.C.; Dong, H.; Gee, S.J.; Hammock, B.D. Whole blood is the sample matrix of choice for monitoring systemic triclocarban levels. Chemosphere 2012, 87, 825–827. [Google Scholar] [CrossRef] [PubMed]
- Schebb, N.H.; Muvvala, J.B.; Morin, D.; Buckpitt, A.R.; Hammock, B.D.; Rice, R.H. Metabolic activation of the antibacterial agent triclocarban by cytochrome P450 1A1 yielding glutathione adducts. Drug Metab. Dispos. 2014, 42, 1098–1102. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Wong, L.Y.; Dwivedi, P.; Zhou, X.; Jia, T.; Calafat, A.M. Urinary concentrations of the antibacterial agent triclocarban in United States residents: 2013−2014 national health and nutrition examination survey. Environ. Sci. Technol. 2016, 50, 13548–13554. [Google Scholar] [CrossRef]
- Allmyr, M.; McLachlan, M.S.; Sandborgh-Englund, G.; Adolfsson-Erici, M. Determination of triclosan as its pentafluorobenzoyl ester in human plasma and milk using electron capture negative ionization mass spectrometry. Anal. Chem. 2006, 78, 6542–6546. [Google Scholar] [CrossRef]
- Heidler, J.; Halden, R.U. Mass balance assessment of triclosan removal during conventional sewage treatment. Chemosphere 2007, 66, 362–369. [Google Scholar] [CrossRef]
- Bester, K. Triclosan in a sewage treatment process—balances and monitoring data. Water Res. 2003, 37, 3891–3896. [Google Scholar] [CrossRef]
- McAvoy, D.C.; Schatowitz, B.; Jacob, M.; Hauk, A.; Eckhoff, W.S. Measurement of triclosan in wastewater treatment systems. Environ. Toxicol. Chem. 2002, 21, 1323–1329. [Google Scholar] [CrossRef]
- Lozano, N.; Rice, C.P.; Ramirez, M.; Torrents, A. Fate of Triclocarban, Triclosan and Methyltriclosan during wastewater and biosolids treatment processes. Water Res. 2013, 47, 4519–4527. [Google Scholar] [CrossRef] [PubMed]
- Hydromantis Inc. Emerging Substances of Concern in Biosolids: Concentrations and Effects of Treatment Processes; Canadian Council of Ministers of the Environment: Hamilton, ON, Canada, 2010. [Google Scholar]
- Kor-Bicakci, G.; Abbott, T.; Ubay-Cokgor, E.; Eskicioglu, C. Occurrence and fate of antimicrobial triclocarban and its transformation products in municipal sludge during advanced anaerobic digestion using microwave pretreatment. Sci. Total Environ. 2020, 705. [Google Scholar] [CrossRef]
- Chen, F.; Ying, G.G.; Ma, Y.B.; Chen, Z.F.; Lai, H.J.; Peng, F.J. Field dissipation and risk assessment of typical personal care products TCC, TCS, AHTN and HHCB in biosolid-amended soils. Sci. Total Environ. 2014, 470–471, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- James, M.O.; Marth, C.J.; Rowland-Faux, L. Slow O-demethylation of methyl triclosan to triclosan, which is rapidly glucuronidated and sulfonated in channel catfish liver and intestine. Aquat. Toxicol. 2012, 124–125, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Xia, K.; Hundal, L.S.; Kumar, K.; Armbrust, K.; Cox, A.E.; Granato, T.C. Triclocarban, triclosan, polybrominated diphenyl ethers and 4-nonylphenol in biosolids and in soil receiving 33-year biosolids application. Environ. Toxicol. Chem. 2010, 29, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Aryal, N.; Reinhold, D.M. Phytoaccumulation of antimicrobials from biosolids: Impacts on environmental fate and relevance to human exposure. Water Res. 2011, 45, 5545–5552. [Google Scholar] [CrossRef] [PubMed]
- Riemenschneider, C.; Al-Raggad, M.; Moeder, M.; Seiwert, B.; Salameh, E.; Reemtsma, T. Pharmaceuticals, Their Metabolites, and Other Polar Pollutants in Field-Grown Vegetables Irrigated with Treated Municipal Wastewater. J. Agric. Food Chem. 2016, 64, 5784–5792. [Google Scholar] [CrossRef]
- Sabourin, L.; Duenk, P.; Bonte-Gelok, S.; Payne, M.; Lapen, D.R.; Topp, E. Uptake of pharmaceuticals, hormones and parabens into vegetables grown in soil fertilized with municipal biosolids. Sci. Total Environ. 2012, 431, 233–236. [Google Scholar] [CrossRef]
- Mathews, S.; Henderson, S.; Reinhold, D. Uptake and accumulation of antimicrobials, triclocarban and triclosan, by food crops in a hydroponic system. Environ. Sci. Pollut. Res. 2014, 21, 6025–6033. [Google Scholar] [CrossRef]
- Prosser, R.S.; Lissemore, L.; Topp, E.; Sibley, P.K. Bioaccumulation of triclosan and triclocarban in plants grown in soils amended with municipal dewatered biosolids. Environ. Toxicol. Chem. 2014, 33, 975–984. [Google Scholar] [CrossRef]
- Wu, C.; Spongberg, A.L.; Witter, J.D.; Fang, M.; Czajkowski, K.P. Uptake of pharmaceutical and personal care products by soybean plants from soils applied with biosolids and irrigated with contaminated water. Environ. Sci. Technol. 2010, 44, 6157–6161. [Google Scholar] [CrossRef] [PubMed]
- Schaider, L.A.; Rudel, R.A.; Ackerman, J.M.; Dunagan, S.C.; Brody, J.G. Pharmaceuticals, perfluorosurfactants, and other organic wastewater compounds in public drinking water wells in a shallow sand and gravel aquifer. Sci. Total Environ. 2014, 468–469, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Lapworth, D.J.; Baran, N.; Stuart, M.E.; Ward, R.S. Emerging organic contaminants in groundwater: A review of sources, fate and occurrence. Environ. Pollut. 2012, 163, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Pycke, B.F.G.; Roll, I.B.; Brownawell, B.J.; Kinney, C.A.; Furlong, E.T.; Kolpin, D.W.; Halden, R.U. Transformation products and human metabolites of triclocarban and triclosan in sewage sludge across the United States. Environ. Sci. Technol. 2014, 48, 7881–7890. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Casas, M.E.; Nielsen, J.L.; Wimmer, R.; Bester, K. Identification of Triclosan-O-Sulfate and other transformation products of Triclosan formed by activated sludge. Sci. Total Environ. 2015, 505, 39–46. [Google Scholar] [CrossRef]
- Armstrong, D.L.; Lozano, N.; Rice, C.P.; Ramirez, M.; Torrents, A. Fate of triclosan, triclocarban, and their transformation products in wastewater under nitrifying conditions. J. Water Process Eng. 2019, 28, 144–151. [Google Scholar] [CrossRef]
- Fang, J.-L.; Stingley, R.L.; Beland, F.A.; Harrouk, W.; Lumpkins, D.L.; Howard, P. Occurrence, Efficacy, Metabolism, and Toxicity of Triclosan. J. Environ. Sci. Heal. Part C 2010, 28, 147–171. [Google Scholar] [CrossRef]
- Bhargava, H.N.; Leonard, P.A. Triclosan: Applications and safety. Am. J. Infect. Control 1996, 24, 209–218. [Google Scholar] [CrossRef]
- Patel, M.; Coogan, M.M. Antifungal activity of the plant Dodonaea viscosa var. angustifolia on Candida albicans from HIV-infected patients. J. Ethnopharmacol. 2008, 118, 173–176. [Google Scholar] [CrossRef]
- Twanabasu, B.R.; Stevens, K.J.; Venables, B.J. The effects of triclosan on spore germination and hyphal growth of the arbuscular mycorrhizal fungus Glomus intraradices. Sci. Total Environ. 2013, 454–455, 51–60. [Google Scholar] [CrossRef]
- Alliance for the Prudent Use of Antibiotics Triclosan White Paper; Alliance for the Prudent Use of Antibiotics: Boston, MA, USA, 2011.
- Russell, A.D. Whither triclosan? J. Antimicrob. Chemother. 2004, 53, 693–695. [Google Scholar] [CrossRef] [PubMed]
- Westfall, C.; Flores-Mireles, A.L.; Robinson, J.I.; Lynch, A.J.L.; Hultgren, S.; Henderson, J.P.; Levin, P.A. The widely used antimicrobial triclosan induces high levels of antibiotic tolerance in vitro and reduces antibiotic efficacy up to 100-fold in vivo. Antimicrob. Agents Chemother. 2019, 63, 1–11. [Google Scholar] [CrossRef] [PubMed]
- McMurry, L.M.; Oethinger, M.; Levy, S.B. Triclosan targets lipid synthesis. Nature 1998, 394, 531–532. [Google Scholar] [CrossRef] [PubMed]
- Levy, C.W.; Roujeinikova, A.; Sedelnikova, S.; Baker, P.J.; Stuitje, A.R.; Slabas, A.R.; Rice, D.W.; Rafferty, J.B. Molecular basis of triclosan activity. Nature 1999, 398, 383–384. [Google Scholar] [CrossRef]
- Jones, R.D.; Jampani, H.B.; Newman, J.L.; Lee, A.S. Triclosan: A review of effectiveness and safety in health care settings. Am. J. Infect. Control 2000, 28, 184–196. [Google Scholar] [CrossRef]
- Yueh, M.F.; Taniguchi, K.; Chen, S.; Evans, R.M.; Hammock, B.D.; Karin, M.; Tukey, R.H. The commonly used antimicrobial additive triclosan is a liver tumor promoter. Proc. Natl. Acad. Sci. USA 2014, 111, 17200–17205. [Google Scholar] [CrossRef]
- Bester, K. Fate of triclosan and triclosan-methyl in sewage treatment plants and surface waters. Arch. Environ. Contam. Toxicol. 2005, 49, 9–17. [Google Scholar] [CrossRef]
- European Commission Directorate-General for Health & Consumers. Opinion on Triclosan; European Commission: Brussels, Belgium, 2011. [Google Scholar]
- Allmyr, M.; Adolfsson-Erici, M.; McLachlan, M.S.; Sandborgh-Englund, G. Triclosan in plasma and milk from Swedish nursing mothers and their exposure via personal care products. Sci. Total Environ. 2006, 372, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Health Canada Triclosan—Canada.ca. Available online: https://www.canada.ca/en/health-canada/services/chemicals-product-safety/triclosan.html (accessed on 21 November 2019).
- Clarivate Web of Science. Available online: https://clarivate.com/webofsciencegroup (accessed on 24 November 2019).
- Rogers, H.R. Sources, behaviour and fate of organic contaminants during sewage treatment and in sewage sludges. Sci. Total Environ. 1996, 185, 3–26. [Google Scholar] [CrossRef]
- PubChem Compound Summary—2,3,4-Trichlorophenol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/trichlorophenol (accessed on 10 October 2019).
- Heidler, J.; Halden, R.U. Meta-analysis of mass balances examining chemical fate during wastewater treatment. Environ. Sci. Technol. 2008, 42, 6324–6332. [Google Scholar] [CrossRef]
- Buth, J.M.; McNeill, K.; Steen, P.O.; Arnold, W.A.; Sueper, C.; Blumentritt, D.; Vikesland, P.J. Dioxin photoproducts of triclosan and its chlorinated derivatives in sediment cores. Environ. Sci. Technol. 2010, 44, 4545–4551. [Google Scholar] [CrossRef] [PubMed]
- Loraine, G.A.; Pettigrove, M.E. Seasonal variations in concentrations of pharmaceuticals and personal care products in drinking water and reclaimed wastewater in Southern California. Environ. Sci. Technol. 2006, 40, 687–695. [Google Scholar] [CrossRef]
- Abbott, T.; Eskicioglu, C. Comparison of anaerobic, cycling aerobic/anoxic, and sequential anaerobic/aerobic/anoxic digestion to remove triclosan and triclosan metabolites from municipal biosolids. Sci. Total Environ. 2020, 745, 140953. [Google Scholar] [CrossRef]
- Fu, Q.; Liao, C.; Du, X.; Schlenk, D.; Gan, J. Back Conversion from Product to Parent: Methyl Triclosan to Triclosan in Plants. Environ. Sci. Technol. Lett. 2018, 5, 181–185. [Google Scholar] [CrossRef]
- Wu, J.L.; Leung, K.F.; Tong, S.F.; Lam, C.W. Organochlorine isotopic pattern-enhanced detection and quantification of triclosan and its metabolites in human serum by ultra-high-performance liquid chromatography/quadrupole time-of-flight/mass spectrometry. Rapid Commun. Mass Spectrom. 2012, 26, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Sacco, J.C.; James, M.O. Sulfonation of environmental chemicals and their metabolites in the polar bear (Ursus maritimus). Pharmacology 2005, 33, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Trenholm, R.A.; Vanderford, B.J.; Lakshminarasimman, N.; McAvoy, D.C.; Dickenson, E.R.V. Identification of Transformation Products for Benzotriazole, Triclosan, and Trimethoprim by Aerobic and Anoxic-Activated Sludge. J. Environ. Eng. 2020, 146, 04020094. [Google Scholar] [CrossRef]
- Chen, X.; Nielsen, J.L.; Furgal, K.; Liu, Y.; Lolas, I.B.; Bester, K. Biodegradation of triclosan and formation of methyl-triclosan in activated sludge under aerobic conditions. Chemosphere 2011, 84, 452–456. [Google Scholar] [CrossRef]
- US EPA The Estimation Programs Interface (EPI) Suite V4.1 2012; US EPA: Washington, DC, USA, 2012.
- Wang, S.; Poon, K.; Cai, Z. Removal and metabolism of triclosan by three different microalgal species in aquatic environment. J. Hazard. Mater. 2018, 342, 643–650. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Poon, K.; Wang, Y.; Li, S.; Liu, H.; Lin, S.; Cai, Z. Removal and reductive dechlorination of triclosan by Chlorella pyrenoidosa. Chemosphere 2013, 92, 1498–1505. [Google Scholar] [CrossRef]
- Hay, A.G.; Dees, P.M.; Sayler, G.S. Growth of a bacterial consortium on triclosan. FEMS Microbiol. Ecol. 2001, 36, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Mulla, S.I.; Wang, H.; Sun, Q.; Hu, A.; Yu, C.P. Characterization of triclosan metabolism in Sphingomonas sp. strain YL-JM2C. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Mulla, S.I.; Asefi, B.; Bharagava, R.N.; Saratale, G.D.; Li, J.; Huang, C.L.; Yu, C.P. Processes for the removal of triclosan in the environment and engineered systems: A review. Environ. Rev. 2020, 28, 55–66. [Google Scholar] [CrossRef]
- Kim, Y.M.; Nam, I.H.; Murugesan, K.; Schmidt, S.; Crowley, D.E.; Chang, Y.S. Biodegradation of diphenyl ether and transformation of selected brominated congeners by Sphingomonas sp. PH-07. Appl. Microbiol. Biotechnol. 2007, 77, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Murugesan, K.; Schmidt, S.; Bokare, V.; Jeon, J.R.; Kim, E.J.; Chang, Y.S. Triclosan susceptibility and co-metabolism - A comparison for three aerobic pollutant-degrading bacteria. Bioresour. Technol. 2011, 102, 2206–2212. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.G.; Zhao, F.; Rezenom, Y.H.; Russell, D.H.; Chu, K.H. Biodegradation of triclosan by a wastewater microorganism. Water Res. 2012, 46, 4226–4234. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.G.; Chu, K.H. Effects of growth substrate on triclosan biodegradation potential of oxygenase-expressing bacteria. Chemosphere 2013, 93, 1904–1911. [Google Scholar] [CrossRef] [PubMed]
- Gangadharan, P.; Vijaya, A.; Bhasi, A.; Khan, S.; Bhaskaran, K. Degradation of triclosan under aerobic, anoxic, and anaerobic conditions. Appl. Biochem. Biotechnol. 2012, 167, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- Noutsopoulos, C.; Mamais, D.; Samaras, V.; Bouras, T.; Marneri, M.; Antoniou, K. Effect of wastewater chlorination on endocrine disruptor removal. Water Sci. Technol. 2013, 67, 1551–1556. [Google Scholar] [CrossRef]
- Fiss, E.M.; Rule, K.L.; Vikesland, P.J. Formation of chloroform and other chlorinated byproducts by chlorination of triclosan-containing antibacterial products. Environ. Sci. Technol. 2007, 41, 2387–2394. [Google Scholar] [CrossRef]
- Rule, K.L.; Ebbett, V.R.; Vikesland, P.J. Formation of chloroform and chlorinated organics by free-chlorine-mediated oxidation of triclosan. Environ. Sci. Technol. 2005, 39, 3176–3185. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Song, Q.; Cao, G.; Chen, Y. Photolytic degradation of triclosan in the presence of surfactants. Chem. Pap. 2008, 62, 608–615. [Google Scholar] [CrossRef]
- Kanetoshi, A.; Ogawa, H.; Katsura, E.; Kaneshima, H. Chlorination of irgasan DP300 and formation of dioxins from its chlorinated derivatives. J. Chromatogr. A 1987, 389, 139–153. [Google Scholar] [CrossRef]
- Tixier, C.; Singer, H.P.; Canonica, S.; Müller, S.R. Phototransformation of triclosan in surface waters: A relevant elimination process for this widely used biocide - Laboratory studies, field measurements, and modeling. Environ. Sci. Technol. 2002, 36, 3482–3489. [Google Scholar] [CrossRef] [PubMed]
- Aranami, K.; Readman, J.W. Photolytic degradation of triclosan in freshwater and seawater. Chemosphere 2007, 66, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Prado, L.; Llompart, M.; Lores, M.; García-Jares, C.; Bayona, J.M.; Cela, R. Monitoring the photochemical degradation of triclosan in wastewater by UV light and sunlight using solid-phase microextraction. Chemosphere 2006, 65, 1338–1347. [Google Scholar] [CrossRef]
- Wong-Wah-Chung, P.; Rafqah, S.; Voyard, G.; Sarakha, M. Photochemical behaviour of triclosan in aqueous solutions: Kinetic and analytical studies. J. Photochem. Photobiol. A Chem. 2007, 191, 201–208. [Google Scholar] [CrossRef]
- Latch, D.E.; Packer, J.L.; Arnold, W.A.; McNeill, K. Photochemical conversion of triclosan to 2,8-dichlorodibenzo-p-dioxin in aqueous solution. J. Photochem. Photobiol. A Chem. 2003, 158, 63–66. [Google Scholar] [CrossRef]
- Buth, J.M.; Grandbois, M.; Vikesland, P.J.; McNeill, K.; Arnold, W.A. Aquatic photochemistry of chlorinated triclosan derivatives: Potential source of polychlorodibenzo-p-dioxins. Environ. Toxicol. Chem. 2009, 28, 2555–2563. [Google Scholar] [CrossRef]
- Latch, D.E.; Packer, J.L.; Stender, B.L.; VanOverbeke, J.; Arnold, W.A.; McNeill, K. Aqueous photochemistry of triclosan: Formation of 2,4-dichlorophenol, 2,8-dichlorodibenzo-p-dioxin, and oligomerization products. Environ. Toxicol. Chem. 2005, 24, 517–525. [Google Scholar] [CrossRef]
- Ben, W.; Sun, P.; Huang, C.H. Effects of combined UV and chlorine treatment on chloroform formation from triclosan. Chemosphere 2016, 150, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.L.; Ji, F.; Zhang, H.; Hu, C.; Wong, M.H.; Hu, D.; Cai, Z. Formation of dioxins from triclosan with active chlorine: A potential risk assessment. J. Hazard. Mater. 2019, 367, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Guerra, P.; Teslic, S.; Shah, A.; Albert, A.; Gewurtz, S.B.; Smyth, S.A. Occurrence and removal of triclosan in Canadian wastewater systems. Environ. Sci. Pollut. Res. 2019, 31873–31886. [Google Scholar] [CrossRef]
- Lindström, A.; Buerge, I.J.; Poiger, T.; Bergqvist, P.A.; Müller, M.D.; Buser, H.R. Occurrence and environmental behavior of the bactericide triclosan and its methyl derivative in surface waters and in wastewater. Environ. Sci. Technol. 2002, 36, 2322–2329. [Google Scholar] [CrossRef] [PubMed]
- Kantiani, L.; Farré, M.; Asperger, D.; Rubio, F.; González, S.; López de Alda, M.J.; Petrović, M.; Shelver, W.L.; Barceló, D. Triclosan and methyl-triclosan monitoring study in the northeast of Spain using a magnetic particle enzyme immunoassay and confirmatory analysis by gas chromatography-mass spectrometry. J. Hydrol. 2008, 361, 1–9. [Google Scholar] [CrossRef]
- Tohidi, F.; Cai, Z. GC/MS analysis of triclosan and its degradation by-products in wastewater and sludge samples from different treatments. Environ. Sci. Pollut. Res. 2015, 22, 11387–11400. [Google Scholar] [CrossRef]
- Singer, H.; Müller, S.; Tixier, C.; Pillonel, L. Triclosan: Occurrence and fate of a widely used biocide in the aquatic environment: Field measurements in wastewater treatment plants, surface waters, and lake sediments. Environ. Sci. Technol. 2002, 36, 4998–5004. [Google Scholar] [CrossRef]
- Ying, G.G.; Kookana, R.S.; Kolpin, D.W. Occurrence and removal of pharmaceutically active compounds in sewage treatment plants with different technologies. J. Environ. Monit. 2009, 11, 1498–1505. [Google Scholar] [CrossRef]
- Narumiya, M.; Nakada, N.; Yamashita, N.; Tanaka, H. Phase distribution and removal of pharmaceuticals and personal care products during anaerobic sludge digestion. J. Hazard. Mater. 2013, 260, 305–312. [Google Scholar] [CrossRef]
- Nakada, N.; Yasojima, M.; Okayasu, Y.; Komori, K.; Suzuki, Y. Mass balance analysis of triclosan, diethyltoluamide, crotamiton and carbamazepine in sewage treatment plants. Water Sci. Technol. 2010, 61, 1739–1747. [Google Scholar] [CrossRef]
- Heidler, J.; Sapkota, A.; Halden, R.U. Partitioning, persistence, and accumulation in digested sludge of the topical antiseptic triclocarban during wastewater treatment. Environ. Sci. Technol. 2006, 40, 3634–3639. [Google Scholar] [CrossRef] [PubMed]
- Federle, T.W.; Kaiser, S.K.; Nuck, B.A. Fate and effects of triclosan in activated sludge. Environ. Toxicol. Chem. 2002, 21, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Guerra, P.; Kleywegt, S.; Payne, M.; Svoboda, M.L.; Lee, H.B.; Reiner, E.; Kolic, T.; Metcalfe, C.; Smyth, S.A. Occurrence and Fate of Trace Contaminants during Aerobic and Anaerobic Sludge Digestion and Dewatering. J. Environ. Qual. 2015, 44, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Kor-Bicakci, G. Effect of microwave pretreatment on fate of antimicrobials and conventional pollutants during anaerobic sludge digestion and biosolids quality for land application. Ph. D. Thesis, Istanbul Technical University, Istanbul, Turkey, 2018. [Google Scholar]
- Lee, H.B.; Peart, T.E. Organic contaminants in Canadian municipal sewage sludge. Part I. Toxic or endocrine-disrupting phenolic compounds. Water Qual. Res. J. Canada 2002, 37, 681–696. [Google Scholar] [CrossRef]
- Kor-Bicakci, G.; Abbott, T.; Ubay-Cokgor, E.; Eskicioglu, C. Occurrence of the persistent antimicrobial triclosan in microwave pretreated and anaerobically digested municipal sludges under various process conditions. Molecules 2020, 25, 310. [Google Scholar] [CrossRef]
- US EPA Targeted National Sewage Sludge Survey Sampling and Analysis; Technical Report; US EPA: Washington, DC, USA, 2009.
- Tohidi, F.; Cai, Z. Fate and mass balance of triclosan and its degradation products: Comparison of three different types of wastewater treatments and aerobic/anaerobic sludge digestion. J. Hazard. Mater. 2017, 323, 329–340. [Google Scholar] [CrossRef]
- Armstrong, D.L.; Rice, C.P.; Ramirez, M.; Torrents, A. Influence of thermal hydrolysis-anaerobic digestion treatment of wastewater solids on concentrations of triclosan, triclocarban, and their transformation products in biosolids. Chemosphere 2017, 171, 609–616. [Google Scholar] [CrossRef]
- Butler, E.; Whelan, M.J.; Sakrabani, R.; Van Egmond, R. Fate of triclosan in field soils receiving sewage sludge. Environ. Pollut. 2012, 167, 101–109. [Google Scholar] [CrossRef]
- Abbott, T. Fate and removal of organic pollutants, recalcitrant nutrients, and antimicrobials during single-stage and sequential sludge digestion. PhD Thesis, University of British Columbia, Kelowna, BC, Canada, 2000. [Google Scholar]
- Sánchez-Brunete, C.; Miguel, E.; Albero, B.; Tadeo, J.L. Determination of triclosan and methyl triclosan in environmental solid samples by matrix solid-phase dispersion and gas chromatography-mass spectrometry. J. Sep. Sci. 2010, 33, 2768–2775. [Google Scholar] [CrossRef]
- Lozano, N.; Torrents, A.; Rice, C.P.; Ramirez, M. Fate of triclosan in agricultural soils after biosolid applications. Chemosphere 2010, 78, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Lozano, N.; Rice, C.P.; Ramirez, M.; Torrents, A. Fate of Triclosan and Methyltriclosan in soil from biosolids application. Environ. Pollut. 2012, 160, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Samaras, V.G.; Stasinakis, A.S.; Thomaidis, N.S.; Mamais, D.; Lekkas, T.D. Fate of selected emerging micropollutants during mesophilic, thermophilic and temperature co-phased anaerobic digestion of sewage sludge. Bioresour. Technol. 2014, 162, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Tomei, M.C.; Mosca Angelucci, D.; Mascolo, G.; Kunkel, U. Post-aerobic treatment to enhance the removal of conventional and emerging micropollutants in the digestion of waste sludge. Waste Manag. 2019, 96, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Dann, A.B.; Hontela, A. Triclosan: Environmental exposure, toxicity and mechanisms of action. J. Appl. Toxicol. 2011, 31, 285–311. [Google Scholar] [CrossRef] [PubMed]
- Thilakan, A.P.; Kumar, K.; Shukla, S.P.; Kumar, S.; Saharan, N.; Karmakar, S. Occurrences of triclosan in Versova creek of Mumbai, India and its toxicity on selected aquatic organisms. J. Exp. Zool. India 2019, 22, 737–742. [Google Scholar]
- Araújo, C.V.M.; Gómez, L.; Silva, D.C.V.R.; Pintado-Herrera, M.G.; Lara-Martín, P.A.; Hampel, M.; Blasco, J. Risk of triclosan based on avoidance by the shrimp Palaemon varians in a heterogeneous contamination scenario: How sensitive is this approach? Chemosphere 2019, 235, 126–135. [Google Scholar] [CrossRef]
- Orvos, D.R.; Versteeg, D.J.; Inauen, J.; Capdevielle, M.; Rothenstein, A.; Cunningham, V. Aquatic toxicity of triclosan. Environ. Toxicol. Chem. 2002, 21, 1338–1349. [Google Scholar] [CrossRef]
- Gao, Y.; Ji, Y.; Li, G.; An, T. Mechanism, kinetics and toxicity assessment of OH-initiated transformation of triclosan in aquatic environments. Water Res. 2014, 49, 360–370. [Google Scholar] [CrossRef]
- Chalew, T.E.A.; Halden, R.U. Environmental exposure of aquatic and terrestrial biota to triclosan and triclocarban. J. Am. Water Resour. Assoc. 2009, 45, 4–13. [Google Scholar] [CrossRef]
- Tatarazako, N.; Ishibashi, H.; Teshima, K.; Kishi, K.; Arizono, K. Effects of triclosan on various aquatic organisms. Environ. Sci. 2004, 11, 133–140. [Google Scholar]
- Adolfsson-Erici, M.; Pettersson, M.; Parkkonen, J.; Sturve, J. Triclosan, a commonly used bactericide found in human milk and in the aquatic environment in Sweden. Chemosphere 2002, 46, 1485–1489. [Google Scholar] [CrossRef]
- Ying, G.G.; Yu, X.Y.; Kookana, R.S. Biological degradation of triclocarban and triclosan in a soil under aerobic and anaerobic conditions and comparison with environmental fate modelling. Environ. Pollut. 2007, 150, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Spongberg, A.L.; Witter, J.D. Adsorption and degradation of triclosan and triclocarban in soils and biosolids-amended soils. J. Agric. Food Chem. 2009, 57, 4900–4905. [Google Scholar] [CrossRef] [PubMed]
- Waller, N.J.; Kookana, R.S. Effect of triclosan on microbial activity in Australian soils. Environ. Toxicol. Chem. 2009, 28, 65–70. [Google Scholar] [CrossRef]
- Liu, F.; Ying, G.G.; Yang, L.H.; Zhou, Q.X. Terrestrial ecotoxicological effects of the antimicrobial agent triclosan. Ecotoxicol. Environ. Saf. 2009, 72, 86–92. [Google Scholar] [CrossRef]
- Bever, C.S.; Rand, A.A.; Nording, M.; Taft, D.; Kalanetra, K.M.; Mills, D.A.; Breck, M.A.; Smilowitz, J.T.; German, J.B.; Hammock, B.D. Effects of triclosan in breast milk on the infant fecal microbiome. Chemosphere 2018, 203, 467–473. [Google Scholar] [CrossRef]
- Adgent, M.A.; Rogan, W.J. Triclosan and prescription antibiotic exposures and enterolactone production in adults. Environ. Res. 2015, 142, 66–71. [Google Scholar] [CrossRef]
- Cullinan, M.P.; Palmer, J.E.; Carle, A.D.; West, M.J.; Westerman, B.; Seymour, G.J. The influence of a triclosan toothpaste on adverse events in patients with cardiovascular disease over 5-years. Sci. Total Environ. 2015, 508, 546–552. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, H.J.; Lee, S.; Kang, H.G.; Jeong, P.S.; Park, S.H.; Park, Y.H.; Lee, J.H.; Lim, K.S.; Lee, S.H.; et al. Effect of triclosan exposure on developmental competence in parthenogenetic porcine embryo during preimplantation. Int. J. Mol. Sci. 2020, 21, 5790. [Google Scholar] [CrossRef]
- Park, H.J.; Song, B.S.; Kim, J.W.; Yang, S.G.; Kim, S.U.; Koo, D.B. Exposure of triclosan in porcine oocyte leads to superoxide production and mitochondrial-mediated apoptosis during in vitro maturation. Int. J. Mol. Sci. 2020, 21, 3050. [Google Scholar] [CrossRef]
- Tran, D.N.; Jung, E.M.; Yoo, Y.M.; Lee, J.H.; Jeung, E.B. Perinatal exposure to triclosan results in abnormal brain development and behavior in mice. Int. J. Mol. Sci. 2020, 21, 4009. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.N.; Nolan, G.T.; Hood, S.R. Lignans, bacteriocides and organochlorine compounds activate the human pregnane X receptor (PXR). Toxicol. Appl. Pharmacol. 2005, 209, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shao, X.; Zhao, H.; Li, X.; Wei, J.; Yang, C.; Cai, Z. Integration of Metabolomics and Lipidomics Reveals Metabolic Mechanisms of Triclosan-Induced Toxicity in Human Hepatocytes. Environ. Sci. Technol. 2019, 53, 5406–5415. [Google Scholar] [CrossRef]
- Pycke, B.; Geer, L.A.; Dalloul, M.; Abulafia, O.; Jenck, A.M.; Halden, R.U. Human fetal exposure to triclosan and triclocarban in an urban population from Brooklyn, New York. Environ. Sci. Technol. 2014, 48, 8831–8838. [Google Scholar] [CrossRef] [PubMed]
- Sandborgh-Englund, G.; Adolfsson-Erici, M.; Odham, G.; Ekstrand, J. Pharmacokinetics of triclosan following oral ingestion in humans. J. Toxicol. Environ. Health 2006, 69, 1861–1873. [Google Scholar] [CrossRef] [PubMed]
- Juric, A.; Singh, K.; Hu, X.F.; Chan, H.M. Exposure to triclosan among the Canadian population: Results of the Canadian Health Measures Survey (2009–2013). Environ. Int. 2019, 123, 29–38. [Google Scholar] [CrossRef]
- Dayan, A.D. Risk assessment of triclosan [Irgasan®] in human breast milk. Food Chem. Toxicol. 2007, 45, 125–129. [Google Scholar] [CrossRef]
- Nassan, F.L.; Mínguez-Alarcón, L.; Williams, P.L.; Dadd, R.; Petrozza, J.C.; Ford, J.B.; Calafat, A.M.; Hauser, R. Urinary triclosan concentrations and semen quality among men from a fertility clinic. Environ. Res. 2019, 177, 108633. [Google Scholar] [CrossRef]
- Hartmann, E.M.; Hickey, R.; Hsu, T.; Betancourt Román, C.M.; Chen, J.; Schwager, R.; Kline, J.; Brown, G.Z.; Halden, R.U.; Huttenhower, C.; et al. Antimicrobial Chemicals Are Associated with Elevated Antibiotic Resistance Genes in the Indoor Dust Microbiome. Environ. Sci. Technol. 2016, 50, 9807–9815. [Google Scholar] [CrossRef]
- Carey, D.E.; Zitomer, D.H.; Kappell, A.D.; Choi, M.J.; Hristova, K.R.; McNamara, P.J. Chronic exposure to triclosan sustains microbial community shifts and alters antibiotic resistance gene levels in anaerobic digesters. Environ. Sci. Process. Impacts 2016, 18, 1060–1067. [Google Scholar] [CrossRef]
- Venkatesan, A.K.; Pycke, B.F.G.; Barber, L.B.; Lee, K.E.; Halden, R.U. Occurrence of triclosan, triclocarban, and its lesser chlorinated congeners in Minnesota freshwater sediments collected near wastewater treatment plants. J. Hazard. Mater. 2012, 229–230, 29–35. [Google Scholar] [CrossRef]
- Musee, N. Environmental risk assessment of triclosan and triclocarban from personal care products in South Africa. Environ. Pollut. 2018, 242, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Ferrera, Z.; Mahugo-Santana, C.; Santana-Rodríguez, J.J. Analytical methodologies for the determination of endocrine disrupting compounds in biological and environmental samples. Biomed Res. Int. 2013, 2013, 23. [Google Scholar] [CrossRef] [PubMed]
- Gaume, B.; Bourgougnon, N.; Auzoux-Bordenave, S.; Roig, B.; Le Bot, B.; Bedoux, G. In vitro effects of triclosan and methyl-triclosan on the marine gastropod Haliotis tuberculata. Comp. Biochem. Physiol. 2012, 156, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Tan, Y.X.R.; Gong, Z.; Bae, S. The toxic effect of triclosan and methyl-triclosan on biological pathways revealed by metabolomics and gene expression in zebrafish embryos. Ecotoxicol. Environ. Saf. 2020, 189, 110039. [Google Scholar] [CrossRef] [PubMed]
- Farré, M.; Asperger, D.; Kantiani, L.; González, S.; Petrovic, M.; Barceló, D. Assessment of the acute toxicity of triclosan and methyl triclosan in wastewater based on the bioluminescence inhibition of Vibrio fischeri. Anal. Bioanal. Chem. 2008, 390, 1999–2007. [Google Scholar] [CrossRef]
- US Department of Health and Human Services Report on Carcinogens—2,4,6-trichlorophenol; US Department of Health and Human Services: Washington, DC, USA, 2009.
- Schweigert, N.; Zehnder, A.J.B.; Eggen, R.I.L. Chemical properties of catechols and their molecular modes of toxic action in cells, from microorganisms to mammals. Environ. Microbiol. 2001, 3, 81–91. [Google Scholar] [CrossRef]
- Mulla, S.I.; Hu, A.; Wang, Y.; Sun, Q.; Huang, S.L.; Wang, H.; Yu, C.P. Degradation of triclocarban by a triclosan-degrading Sphingomonas sp. strain YL-JM2C. Chemosphere 2016, 144, 292–296. [Google Scholar] [CrossRef]
- Ahn, K.C.; Zhao, B.; Chen, J.; Cherednichenko, G.; Sanmarti, E.; Denison, M.S.; Lasley, B.; Pessah, I.N.; Kültz, D.; Chang, D.P.Y.; et al. In vitro biologic activities of the antimicrobials triclocarban, its analogs, and triclosan in bioassay screens: Receptor-based bioassay screens. Environ. Health Perspect. 2008, 116, 1203–1210. [Google Scholar] [CrossRef]
- Ye, X.; Zhou, X.; Furr, J.; Ahn, K.C.; Hammock, B.D.; Gray, E.L.; Calafat, A.M. Biomarkers of exposure to triclocarban in urine and serum. Toxicology 2011, 286, 69–74. [Google Scholar] [CrossRef]
- Yun, H.; Liang, B.; Kong, D.; Li, X.; Wang, A. Fate, risk and removal of triclocarban: A critical review. J. Hazard. Mater. 2020, 387, 121944. [Google Scholar] [CrossRef] [PubMed]
- The TCC Consortium High Production Volume (HPV) Chemical Challenge Program Data Availability and Screening Level Assessment for Triclocarban; CAS#: 101-20-2; American Cleaning Institute: Washington, DC, USA, 2002.
- Halden, R.U. Lessons Learned from Probing for Impacts of Triclosan and Triclocarban on Human Microbiomes. mSphere 2016, 1, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Halden, R.U.; Paull, D.H. Co-occurrence of triclocarban and triclosan in U.S. water resources. Environ. Sci. Technol. 2005, 39, 1420–1426. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.Y.; Chang, M.S.; Yang, S.H.; Wu, G.J. Simultaneous determination of triclosan, triclocarban, and transformation products of triclocarban in aqueous samples using solid-phase micro-extraction-HPLC-MS/MS. J. Sep. Sci. 2012, 35, 2544–2552. [Google Scholar] [CrossRef]
- Vimalkumar, K.; Seethappan, S.; Pugazhendhi, A. Fate of Triclocarban (TCC) in aquatic and terrestrial systems and human exposure. Chemosphere 2019, 230, 201–209. [Google Scholar] [CrossRef]
- Hongsawat, P.; Vangnai, A.S. Biodegradation pathways of chloroanilines by Acinetobacter baylyi strain GFJ2. J. Hazard. Mater. 2011, 186, 1300–1307. [Google Scholar] [CrossRef]
- Miller, T.R.; Colquhoun, D.R.; Halden, R.U. Identification of wastewater bacteria involved in the degradation of triclocarban and its non-chlorinated congener. J. Hazard. Mater. 2010, 183, 766–772. [Google Scholar] [CrossRef]
- Yun, H.; Liang, B.; Kong, D.; Li, Z.; Qi, G.; Wang, A. Enhanced Biotransformation of Triclocarban by Ochrobactrum sp. TCC-1 Under Anoxic Nitrate Respiration Conditions. Curr. Microbiol. 2017, 74, 491–498. [Google Scholar] [CrossRef]
- Yun, H.; Liang, B.; Qiu, J.; Zhang, L.; Zhao, Y.; Jiang, J.; Wang, A. Functional Characterization of a Novel Amidase Involved in Biotransformation of Triclocarban and its Dehalogenated Congeners in Ochrobactrum sp. TCC-2. Environ. Sci. Technol. 2017, 51, 291–300. [Google Scholar] [CrossRef]
- Sipahutar, M.K.; Piapukiew, J.; Vangnai, A.S. Efficiency of the formulated plant-growth promoting Pseudomonas fluorescens MC46 inoculant on triclocarban treatment in soil and its effect on Vigna radiata growth and soil enzyme activities. J. Hazard. Mater. 2018, 344, 883–892. [Google Scholar] [CrossRef]
- Sipahutar, M.K.; Vangnai, A.S. Role of plant growth-promoting Ochrobactrum sp. MC22 on triclocarban degradation and toxicity mitigation to legume plants. J. Hazard. Mater. 2017, 329, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Taweetanawanit, P.; Ratpukdi, T.; Siripattanakul-Ratpukdi, S. Performance and kinetics of triclocarban removal by entrapped Pseudomonas fluorescens strain MC46. Bioresour. Technol. 2019, 274, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zheng, G.; Wang, X.; Wang, M.; Chen, T. Biodegradation of triclosan and triclocarban in sewage sludge during composting under three ventilation strategies. Front. Environ. Sci. Eng. 2019, 13. [Google Scholar] [CrossRef]
- Liang, B.; Yun, H.; Kong, D.; Ding, Y.; Li, X.; Vangnai, A.S.; Wang, A. Bioaugmentation of triclocarban and its dechlorinated congeners contaminated soil with functional degraders and the bacterial community response. Environ. Res. 2020, 180, 108840. [Google Scholar] [CrossRef]
- Kwon, J.W.; Xia, K. Fate of triclosan and triclocarban in soil columns with and without biosolids surface application. Environ. Toxicol. Chem. 2012, 31, 262–269. [Google Scholar] [CrossRef]
- Gledhill, W.E. Biodegradation of 3,4,4′-trichlorocarbanilide, TCC®, in sewage and activated sludge. Water Res. 1975, 9, 649–654. [Google Scholar] [CrossRef]
- Balajee, S.; Mahadevan, A. Utilization of aromatic substances by Azotobacter chroococcum. Res. Microbiol. 1990, 141, 577–584. [Google Scholar] [CrossRef]
- Noh, S.J.; Kim, Y.; Min, K.H.; Karegoudar, T.B.; Kim, C.K. Cloning and nucleotide sequence analysis of xylE gene responsible for meta-cleavage of 4-chlorocatechol from Pseudomonas sp. S-47. Mol. Cells 2000, 10, 475–479. [Google Scholar]
- Miller, T.R.; Heidler, J.; Chillrud, S.N.; Delaquil, A.; Ritchie, J.C.; Mihalic, J.N.; Bopp, R.; Halden, R.U. Fate of triclosan and evidence for reductive dechlorination of triclocarban in estuarine sediments. Environ. Sci. Technol. 2008, 42, 4570–4576. [Google Scholar] [CrossRef]
- Souchier, M.; Benali-Raclot, D.; Benanou, D.; Boireau, V.; Gomez, E.; Casellas, C.; Chiron, S. Screening triclocarban and its transformation products in river sediment using liquid chromatography and high resolution mass spectrometry. Sci. Total Environ. 2015, 502, 199–205. [Google Scholar] [CrossRef]
- Chiaia-Hernandez, A.C.; Krauss, M.; Hollender, J. Screening of lake sediments for emerging contaminants by liquid chromatography atmospheric pressure photoionization and electrospray ionization coupled to high resolution mass spectrometry. Environ. Sci. Technol. 2013, 47, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Souchier, M.; Casellas, C.; Ingrand, V.; Chiron, S. Insights into reductive dechlorination of triclocarban in river sediments: Field measurements and in vitro mechanism investigations. Chemosphere 2016, 144, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Jeffcoat, A.R.; Handy, R.W.; Francis, M.T.; Willis, S.; Wall, M.E.; Birch, C.G.; Hiles, R.A. The metabolism and toxicity of halogenated carbanilides. Biliary metabolites of 3,4,4’-trichlorocarbanilide and 3-trifluoromethyl-4,4’-dichlorocarbanilide in the rat. Drug Metab. Dispos. 1977, 5, 157–166. [Google Scholar] [PubMed]
- Warren, J.T.; Allen, R.; Carter, D.E. Identification of the metabolites of trichlorocarbanilide in the rat. Drug Metab. Dispos. 1978, 6, 38–44. [Google Scholar] [PubMed]
- Hiles, R.A.; Birch, C.G. The absorption, excretion, and biotransformation of 3,4,4’-trichlorocarbanilide in humans. Drug Metab. Dispos. 1978, 6, 177–183. [Google Scholar]
- Sun, Q.; Li, M.; Ma, C.; Chen, X.; Xie, X.; Yu, C.P. Seasonal and spatial variations of PPCP occurrence, removal and mass loading in three wastewater treatment plants located in different urbanization areas in Xiamen, China. Environ. Pollut. 2016, 208, 371–381. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, H. The fate and risk of selected pharmaceutical and personal care products in wastewater treatment plants and a pilot-scale multistage constructed wetland system. Environ. Sci. Pollut. Res. 2014, 21, 1466–1479. [Google Scholar] [CrossRef]
- Heidler, J.; Halden, R.U. Fate of organohalogens in US wastewater treatment plants and estimated chemical releases to soils nationwide from biosolids recycling. J. Environ. Monit. 2009, 11, 2207–2215. [Google Scholar] [CrossRef]
- Lehutso, R.F.; Daso, A.P.; Okonkwo, J.O. Occurrence and environmental levels of triclosan and triclocarban in selected wastewater treatment plants in Gauteng Province, South Africa. Emerg. Contam. 2017, 3, 107–114. [Google Scholar] [CrossRef]
- Kumar, K.S.; Priya, S.M.; Peck, A.M.; Sajwan, K.S. Mass loadings of triclosan and triclocarbon from four wastewater treatment plants to three rivers and landfill in Savannah, Georgia, USA. Arch. Environ. Contam. Toxicol. 2010, 58, 275–285. [Google Scholar] [CrossRef]
- Chari, B.P.; Halden, R.U. Predicting the concentration range of unmonitored chemicals in wastewater-dominated streams and in run-off from biosolids-amended soils. Sci. Total Environ. 2012, 440, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Guerra, P.; Kim, M.; Shah, A.; Alaee, M.; Smyth, S.A. Occurrence and fate of antibiotic, analgesic/anti-inflammatory, and antifungal compounds in five wastewater treatment processes. Sci. Total Environ. 2014, 473–474, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Meng, X.Z.; Bergman, A.; Halden, R.U. Nationwide reconnaissance of five parabens, triclosan, triclocarban and its transformation products in sewage sludge from China. J. Hazard. Mater. 2019, 365, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Subedi, B.; Lee, S.; Moon, H.-B.; Kannan, K. Emission of artificial sweeteners, select pharmaceuticals, and personal care products through sewage sludge from wastewater treatment plants in Korea. Environ. Int. 2014, 68, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Metcalfe, C.D. Simultaneous determination of triclocarban and triclosan in municipal biosolids by liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2007, 1164, 212–218. [Google Scholar] [CrossRef]
- Kor-Bicakci, G.; Eskicioglu, C. Recent developments on thermal municipal sludge pretreatment technologies for enhanced anaerobic digestion. Renew. Sustain. Energy Rev. 2019, 110, 423–443. [Google Scholar] [CrossRef]
- Yang, S.; Hai, F.I.; Price, W.E.; McDonald, J.; Khan, S.J.; Nghiem, L.D. Occurrence of trace organic contaminants in wastewater sludge and their removals by anaerobic digestion. Bioresour. Technol. 2016, 210, 153–159. [Google Scholar] [CrossRef]
- Yang, S.; McDonald, J.; Hai, F.I.; Price, W.E.; Khan, S.J.; Nghiem, L.D. Effects of thermal pre-treatment and recuperative thickening on the fate of trace organic contaminants during anaerobic digestion of sewage sludge. Int. Biodeterior. Biodegrad. 2017, 124, 146–154. [Google Scholar] [CrossRef]
- Tadeo, J.L.; Sánchez-Brunete, C.; Albero, B.; García-Valcárcel, A.I.; Pérez, R.A. Analysis of emerging organic contaminants in environmental solid samples. Cent. Eur. J. Chem. 2012, 10, 480–520. [Google Scholar] [CrossRef]
- Sapkota, A.; Heidler, J.; Halden, R.U. Detection of triclocarban and two co-contaminating chlorocarbanilides in US aquatic environments using isotope dilution liquid chromatography tandem mass spectrometry. Environ. Res. 2007, 103, 21–29. [Google Scholar] [CrossRef]
- Al-Rajab, A.J.; Sabourin, L.; Lapen, D.R.; Topp, E. Dissipation of triclosan, triclocarban, carbamazepine and naproxen in agricultural soil following surface or sub-surface application of dewatered municipal biosolids. Sci. Total Environ. 2015, 512–513, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Lozano, N.; Rice, C.P.; Ramirez, M.; Torrents, A. Fate of triclocarban in agricultural soils after biosolid applications. Environ. Sci. Pollut. Res. 2018, 25, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ki, C.A.; Gee, N.A.; Ahmed, M.I.; Duleba, A.J.; Zhao, L.; Gee, S.J.; Hammock, B.D.; Lasley, B.L. Triclocarban enhances testosterone action: A new type of endocrine disruptor? Endocrinology 2008, 149, 1173–1179. [Google Scholar] [CrossRef]
- Hinther, A.; Bromba, C.M.; Wulff, J.E.; Helbing, C.C. Effects of triclocarban, triclosan, and methyl triclosan on thyroid hormone action and stress in frog and mammalian culture systems. Environ. Sci. Technol. 2011, 45, 5395–5402. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Xu, H.; Wu, X.; Yang, L. Multiple bioanalytical method to reveal developmental biological responses in zebrafish embryos exposed to triclocarban. Chemosphere 2018, 193, 251–258. [Google Scholar] [CrossRef]
- Torres, T.; Cunha, I.; Martins, R.; Santos, M.M. Screening the toxicity of selected personal care products using embryo bioassays: 4-MBC, propylparaben and triclocarban. Int. J. Mol. Sci. 2016, 17, 1762. [Google Scholar] [CrossRef]
- Geer, L.A.; Pycke, B.F.G.; Waxenbaum, J.; Sherer, D.M.; Abulafia, O.; Halden, R.U. Association of birth outcomes with fetal exposure to parabens, triclosan and triclocarban in an immigrant population in Brooklyn, New York. J. Hazard. Mater. 2017, 323, 177–183. [Google Scholar] [CrossRef]
- Sood, S.; Choudhary, S.; Wang, H.C.R. Induction of human breast cell carcinogenesis by triclocarban and intervention by curcumin. Biochem. Biophys. Res. Commun. 2013, 438, 600–606. [Google Scholar] [CrossRef]
- Fan, B.; Li, J.; Wang, X.; Gao, X.; Chen, J.; Ai, S.; Li, W.; Huang, Y.; Liu, Z. Study of aquatic life criteria and ecological risk assessment for triclocarban (TCC). Environ. Pollut. 2019, 254, 112956. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Chen, F.; Yang, Q.; Li, Y.; Li, X.; Zeng, G. Effect of triclocarban on hydrogen production from dark fermentation of waste activated sludge. Bioresour. Technol. 2019, 279, 307–316. [Google Scholar] [CrossRef]
- Carey, D.E.; Zitomer, D.H.; Hristova, K.R.; Kappell, A.D.; McNamara, P.J. Triclocarban Influences Antibiotic Resistance and Alters Anaerobic Digester Microbial Community Structure. Environ. Sci. Technol. 2016, 50, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Higgins, C.P.; Paesani, Z.J.; Abbott Chalew, T.E.; Halden, R.U.; Hundal, L.S. Persistence of triclocarban and triclosan in soils after land application of biosolids and bioaccumulation in Eisenia foetida. Environ. Toxicol. Chem. 2011, 30, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Sherburne, J.J.; Anaya, A.M.; Fernie, K.J.; Forbey, J.S.; Furlong, E.T.; Kolpin, D.W.; Dufty, A.M.; Kinney, C.A. Occurrence of triclocarban and triclosan in an agro-ecosystem following application of biosolids. Environ. Sci. Technol. 2016, 50, 13206–13214. [Google Scholar] [CrossRef] [PubMed]
- Snyder, E.H.; O’Connor, G.A.; McAvoy, D.C. Toxicity and bioaccumulation of biosolids-borne triclocarban (TCC) in terrestrial organisms. Chemosphere 2011, 82, 460–467. [Google Scholar] [CrossRef]
- Kinney, C.A.; Furlong, E.T.; Kolpin, D.W.; Burkhardt, M.R.; Zaugg, S.D.; Werner, S.L.; Bossio, J.P.; Benotti, M.J. Bioaccumulation of pharmaceuticals and other anthropogenic waste indicators in earthworms from agricultural soil amended with biosolid or swine manure. Environ. Sci. Technol. 2008, 42, 1863–1870. [Google Scholar] [CrossRef] [PubMed]
- Coogan, M.A.; La Point, T.W. Snail bioaccumulation of triclocarban, triclosan, and methyltriclosan in a North Texas, USA, stream affected by wastewater treatment plant runoff. Environ. Toxicol. Chem. 2008, 27, 1788–1793. [Google Scholar] [CrossRef] [PubMed]
- Higgins, C.P.; Paesani, Z.J.; Chalew, T.E.A.; Halden, R.U. Bioaccumulation of triclocarban in Lumbriculus variegatus. Environ. Toxicol. 2009, 28, 2663–2670. [Google Scholar] [CrossRef]
- Schebb, N.H.; Flores, I.; Kurobe, T.; Franze, B.; Ranganathan, A.; Hammock, B.D.; Teh, S.J. Bioconcentration, metabolism and excretion of triclocarban in larval Qurt medaka (Oryzias latipes). Aquat. Toxicol. 2011, 105, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Coogan, M.A.; Edziyie, R.E.; La Point, T.W.; Venables, B.J. Algal bioaccumulation of triclocarban, triclosan, and methyl-triclosan in a North Texas wastewater treatment plant receiving stream. Chemosphere 2007, 67, 1911–1918. [Google Scholar] [CrossRef]
- Vimalkumar, K.; Arun, E.; Krishna-Kumar, S.; Poopal, R.K.; Nikhil, N.P.; Subramanian, A.; Babu-Rajendran, R. Occurrence of triclocarban and benzotriazole ultraviolet stabilizers in water, sediment, and fish from Indian rivers. Sci. Total Environ. 2018, 625, 1351–1360. [Google Scholar] [CrossRef]
- Huynh, K.; Banach, E.; Reinhold, D. Transformation, Conjugation, and Sequestration Following the Uptake of Triclocarban by Jalapeno Pepper Plants. J. Agric. Food Chem. 2018, 66, 4032–4043. [Google Scholar] [CrossRef] [PubMed]
- Halden, R.U.; Lindeman, A.E.; Aiello, A.E.; Andrews, D.; Arnold, W.A.; Fair, P.; Fuoco, R.E.; Geer, L.A.; Johnson, P.I.; Lohmann, R.; et al. The florence statement on triclosan and triclocarban. Environ. Health Perspect. 2017, 125, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ashrap, P.; Watkins, D.J.; Calafat, A.M.; Ye, X.; Rosario, Z.; Brown, P.; Vélez-Vega, C.M.; Alshawabkeh, A.; Cordero, J.F.; Meeker, J.D. Elevated concentrations of urinary triclocarban, phenol and paraben among pregnant women in Northern Puerto Rico: Predictors and trends. Environ. Int. 2018, 121, 990–1002. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wei, L.; Shi, Y.; Zhang, J.; Wu, Q.; Shao, B. Chinese population exposure to triclosan and triclocarban as measured via human urine and nails. Environ. Geochem. Health 2016, 38, 1125–1135. [Google Scholar] [CrossRef]
- Iyer, A.P.; Xue, J.; Honda, M.; Robinson, M.; Kumosani, T.A.; Abulnaja, K.; Kannan, K. Urinary levels of triclosan and triclocarban in several Asian countries, Greece and the USA: Association with oxidative stress. Environ. Res. 2018, 160, 91–96. [Google Scholar] [CrossRef]
- Wei, L.; Qiao, P.; Shi, Y.; Ruan, Y.; Yin, J.; Wu, Q.; Shao, B. Triclosan/triclocarban levels in maternal and umbilical blood samples and their association with fetal malformation. Clin. Chim. Acta 2017, 466, 133–137. [Google Scholar] [CrossRef]
- Groshart, C.; Okkerman, P.C. Towards the Establishment of a Priority List of Substances for Further Evaluation of Their Role in Endocrine Disruption-Preparation of a Candidate List of Substances as a Basis for Priority Setting; European Commission: Delft, The Netherlands, 2000. [Google Scholar]
- Office of Environmental Health Hazard Assessment (OEHHA) Safe Drinking Water and Toxic Enforcement Act of 1986: Chemicals Known to the State to Cause Cancer or Reproductive Toxicity; State of California Environmental Protection Agency: Sacramento, CA, USA; Office of Environmental Health Hazard Assessment: Sacramento, CA, USA, 2011.
- Yuan, Y.; Zhang, P.; Schäffer, A.; Schmidt, B. 3,4-Dichloroaniline revisited: A study on the fate of the priority pollutant in a sediment-water system derived from a rice growing region in Italy. Sci. Total Environ. 2017, 574, 1012–1020. [Google Scholar] [CrossRef]
- Vangnai, A.S.; Petchkroh, W. Biodegradation of 4-chloroaniline by bacteria enriched from soil. FEMS Microbiol. Lett. 2007, 268, 209–216. [Google Scholar] [CrossRef]
- Tasca, A.L.; Fletcher, A. State of the art of the environmental behaviour and removal techniques of the endocrine disruptor 3,4-dichloroaniline. J. Environ. Sci. Health 2018, 53, 260–270. [Google Scholar] [CrossRef]
- Ding, S.L.; Wang, X.K.; Jiang, W.Q.; Meng, X.; Zhao, R.S.; Wang, C.; Wang, X. Photodegradation of the antimicrobial triclocarban in aqueous systems under ultraviolet radiation. Environ. Sci. Pollut. Res. 2013, 20, 3195–3201. [Google Scholar] [CrossRef]
- Ding, S.L.; Wang, X.K.; Jiang, W.Q.; Zhao, R.S.; Shen, T.T.; Wang, C.; Wang, X. Influence of pH, inorganic anions, and dissolved organic matter on the photolysis of antimicrobial triclocarban in aqueous systems under simulated sunlight irradiation. Environ. Sci. Pollut. Res. 2015, 22, 5204–5211. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Xiong, S.; Ou, W.; Wang, Z.; Tan, J.; Jin, J.; Tang, C.; Liu, J.; Fan, Y. Persistence, temporal and spatial profiles of ultraviolet absorbents and phenolic personal care products in riverine and estuarine sediment of the Pearl River catchment, China. J. Hazard. Mater. 2017, 323, 139–146. [Google Scholar] [CrossRef] [PubMed]
- United States Food and Drug Administration Final Rule Safety and Effectiveness of Consumer Antiseptic Rubs. Topical Antimicrobial Drug Products for Over-the-Counter Human Use; Federal Register: Washington, DC, USA, 2019; Volume 84, pp. 14847–14864.
- United States Food and Drug Administration Safety and Effectiveness of Health Care Antiseptics. Topical Antimicrobial Drug Products for Over-the-Counter Human Use; Federal Register: Washington, DC, USA, 2019; Volume 82, pp. 60474–60503.
- United States Food and Drug Administration Safety and Effectiveness of Consumer Antiseptics. Topical Antimicrobial Drug Products for Over-the-Counter Human Use; Federal Register: Washington, DC, USA, 2016; Volume 81, pp. 61106–61130.
- Erickson, B. Consumer Safety: US FDA finalizes hand sanitizer rule. Chem. Eng. News 2019, 97. [Google Scholar]
- Pradhan, D.; Biswasroy, P.; Kumar Naik, P.; Ghosh, G.; Rath, G. A Review of Current Interventions for COVID-19 Prevention. Arch. Med. Res. 2020, 51. [Google Scholar] [CrossRef]
- Johansson, I.; Somasundaran, P. Handbook for Cleaning/decontamination of Surfaces; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 0080555535. [Google Scholar]
- Zhang, C.; Cui, F.; Zeng, G.M.; Jiang, M.; Yang, Z.Z.; Yu, Z.G.; Zhu, M.Y.; Shen, L.Q. Quaternary ammonium compounds (QACs): A review on occurrence, fate and toxicity in the environment. Sci. Total Environ. 2015, 518–519, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Pati, S.G.; Arnold, W.A. Comprehensive screening of quaternary ammonium surfactants and ionic liquids in wastewater effluents and lake sediments. Environ. Sci. Process. Impacts 2020, 22, 430–441. [Google Scholar] [CrossRef]
- Ruan, T.; Song, S.; Wang, T.; Liu, R.; Lin, Y.; Jiang, G. Identification and composition of emerging quaternary ammonium compounds in municipal sewage sludge in China. Environ. Sci. Technol. 2014, 48, 4289–4297. [Google Scholar] [CrossRef]
- Östman, M.; Fick, J.; Tysklind, M. Detailed mass flows and removal efficiencies for biocides and antibiotics in Swedish sewage treatment plants. Sci. Total Environ. 2018, 640–641, 327–336. [Google Scholar] [CrossRef]
- Dai, X.; Wang, C.; Lam, J.C.W.; Yamashita, N.; Yamazaki, E.; Horii, Y.; Chen, W.; Li, X. Accumulation of quaternary ammonium compounds as emerging contaminants in sediments collected from the Pearl River Estuary, China and Tokyo Bay, Japan. Mar. Pollut. Bull. 2018, 136, 276–281. [Google Scholar] [CrossRef]
- Van De Voorde, A.; Lorgeoux, C.; Gromaire, M.C.; Chebbo, G. Analysis of quaternary ammonium compounds in urban stormwater samples. Environ. Pollut. 2012, 164, 150–157. [Google Scholar] [CrossRef]
- Harrison, K.R.; Kappell, A.D.; McNamara, P.J. Benzalkonium chloride alters phenotypic and genotypic antibiotic resistance profiles in a source water used for drinking water treatment. Environ. Pollut. 2020, 257, 113472. [Google Scholar] [CrossRef] [PubMed]
- Östman, M.; Lindberg, R.H.; Fick, J.; Björn, E.; Tysklind, M. Screening of biocides, metals and antibiotics in Swedish sewage sludge and wastewater. Water Res. 2017, 115, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Tezel, U.; Pavlostathis, S.G. Transformation of benzalkonium chloride under nitrate reducing conditions. Environ. Sci. Technol. 2009, 43, 1342–1348. [Google Scholar] [CrossRef] [PubMed]
- Wieck, S.; Olsson, O.; Kümmerer, K. Not only biocidal products: Washing and cleaning agents and personal care products can act as further sources of biocidal active substances in wastewater. Environ. Int. 2018, 115, 247–256. [Google Scholar] [CrossRef]
- Li, W.L.; Zhang, Z.F.; Sparham, C.; Li, Y.F. Validation of sampling techniques and SPE-UPLC/MS/MS for home and personal care chemicals in the Songhua Catchment, Northeast China. Sci. Total Environ. 2020, 707, 136038. [Google Scholar] [CrossRef]
- Kim, S.; Ji, K.; Shin, H.; Park, S.; Kho, Y.; Park, K.; Kim, K.; Choi, K. Occurrences of benzalkonium chloride in streams near a pharmaceutical manufacturing complex in Korea and associated ecological risk. Chemosphere 2020, 256, 127084. [Google Scholar] [CrossRef]
- Uematsu, M.; Kumagami, T.; Shimoda, K.; Kusano, M.; Teshima, M.; Sasaki, H.; Kitaoka, T. Influence of alkyl chain length of benzalkonium chloride on acute corneal epithelial toxicity. Cornea 2010, 29, 1296–1301. [Google Scholar] [CrossRef]
- Slimani, K.; Féret, A.; Pirotais, Y.; Maris, P.; Abjean, J.P.; Hurtaud-Pessel, D. Liquid chromatography-tandem mass spectrometry multiresidue method for the analysis of quaternary ammonium compounds in cheese and milk products: Development and validation using the total error approach. J. Chromatogr. A 2017, 1517, 86–96. [Google Scholar] [CrossRef]
- Martínez-Carballo, E.; Sitka, A.; González-Barreiro, C.; Kreuzinger, N.; Fürhacker, M.; Scharf, S.; Gans, O. Determination of selected quaternary ammonium compounds by liquid chromatography with mass spectrometry. Part I. Application to surface, waste and indirect discharge water samples in Austria. Environ. Pollut. 2007, 145, 489–496. [Google Scholar] [CrossRef]
- García, M.T.; Ribosa, I.; Guindulain, T.; Sánchez-Leal, J.; Vives-Rego, J. Fate and effect of monoalkyl quaternary ammonium surfactants in the aquatic environment. Environ. Pollut. 2001, 111, 169–175. [Google Scholar] [CrossRef]
- Hora, P.I.; Arnold, W.A. Photochemical fate of quaternary ammonium compounds in river water. Environ. Sci. Process. Impacts 2020, 22, 1368–1381. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Ge, F.; Zhu, R.; Wang, X.; Zheng, X. A DFT-based QSAR study of the toxicity of quaternary ammonium compounds on Chlorella vulgaris. Chemosphere 2010, 80, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Daull, P.; Lallemand, F.; Garrigue, J.S. Benefits of cetalkonium chloride cationic oil-in-water nanoemulsions for topical ophthalmic drug delivery. J. Pharm. Pharmacol. 2014, 66, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xia, Y.F.; Hong, J.M. Mechanism and toxicity research of benzalkonium chloride oxidation in aqueous solution by H2O2/Fe2+ process. Environ. Sci. Pollut. Res. 2016, 23, 17822–17830. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wang, W.L.; Zhang, D.Y.; Zhou, T.H.; Lee, M.Y.; Wu, Q.Y.; Hu, H.Y.; He, Z.M.; Huang, T.Y. Degradation of non-oxidizing biocide benzalkonium chloride and bulk dissolved organic matter in reverse osmosis concentrate by UV/chlorine oxidation. J. Hazard. Mater. 2020, 396. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Wang, T.; Wang, W.L.; Wu, Q.Y.; Li, A.; Hu, H.Y. UV/chlorine as an advanced oxidation process for the degradation of benzalkonium chloride: Synergistic effect, transformation products and toxicity evaluation. Water Res. 2017, 114, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Andrews, S.A. NDMA formation from amine-based pharmaceuticals - Impact from prechlorination and water matrix. Water Res. 2013, 47, 2446–2457. [Google Scholar] [CrossRef]
- West, D.M.; Wu, Q.; Donovan, A.; Shi, H.; Ma, Y.; Jiang, H.; Wang, J. N-nitrosamine formation by monochloramine, free chlorine, and peracetic acid disinfection with presence of amine precursors in drinking water system. Chemosphere 2016, 153, 521–527. [Google Scholar] [CrossRef]
- Selbes, M.; Kim, D.; Ates, N.; Karanfil, T. The roles of tertiary amine structure, background organic matter and chloramine species on NDMA formation. Water Res. 2013, 47, 945–953. [Google Scholar] [CrossRef]
- Wu, Q.; Shi, H.; Ma, Y.; Adams, C.; Eichholz, T.; Timmons, T.; Jiang, H. Determination of secondary and tertiary amines as N-nitrosamine precursors in drinking water system using ultra-fast liquid chromatography-tandem mass spectrometry. Talanta 2015, 131, 736–741. [Google Scholar] [CrossRef]
- Asami, M.; Oya, M.; Kosaka, K. A nationwide survey of NDMA in raw and drinking water in Japan. Sci. Total Environ. 2009, 407, 3540–3545. [Google Scholar] [CrossRef] [PubMed]
- Pottegård, A.; Kristensen, K.B.; Ernst, M.T.; Johansen, N.B.; Quartarolo, P.; Hallas, J. Use of N-nitrosodimethylamine (NDMA) contaminated valsartan products and risk of cancer: Danish nationwide cohort study. BMJ 2018, 362. [Google Scholar] [CrossRef] [PubMed]
- Kemper, J.M.; Walse, S.S.; Mitch, W.A. Quaternary amines as nitrosamine precursors: A role for consumer products? Environ. Sci. Technol. 2010, 44, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Ul’yanovskii, N.V.; Kosyakov, D.S.; Varsegov, I.S.; Popov, M.S.; Lebedev, A.T. Identification of novel disinfection byproducts in pool water: Chlorination of the algaecide benzalkonium chloride. Chemosphere 2020, 239, 124801. [Google Scholar] [CrossRef] [PubMed]
- Woods-Chabane, G.C.; Glover, C.M.; Marti, E.J.; Dickenson, E.R.V. A novel assay to measure tertiary and quaternary amines in wastewater: An indicator for NDMA wastewater precursors. Chemosphere 2017, 179, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Kim, J.M.; Kweon, O.; Kim, S.J.; Jones, R.C.; Woodling, K.; Gamboa da Costa, G.; LiPuma, J.J.; Hussong, D.; Marasa, B.S.; et al. Intrinsic resistance of Burkholderia cepacia complex to benzalkonium chloride. MBio 2016, 7, 1–11. [Google Scholar] [CrossRef]
- Dean Raymond, D.; Alexander, M. Bacterial metabolism of quaternary ammonium compounds. Appl. Environ. Microbiol. 1977, 33, 1037–1041. [Google Scholar] [CrossRef]
- Oh, S.; Kurt, Z.; Tsementzi, D.; Weigand, M.R.; Kim, M.; Hatt, J.K.; Tandukar, M.; Pavlostathis, S.G.; Spain, J.C.; Konstantinidis, K.T.; et al. Microbial community degradation of widely used quaternary ammonium disinfectants. Appl. Environ. Microbiol. 2014, 80, 5892–5900. [Google Scholar] [CrossRef]
- Tezel, U.; Pavlostathis, S.G. Quaternary ammonium disinfectants: Microbial adaptation, Degradation and ecology. Curr. Opin. Biotechnol. 2015, 33, 296–304. [Google Scholar] [CrossRef]
- Khan, A.H.; Topp, E.; Scott, A.; Sumarah, M.; Macfie, S.M.; Ray, M.B. Biodegradation of benzalkonium chlorides singly and in mixtures by a Pseudomonas sp. isolated from returned activated sludge. J. Hazard. Mater. 2015, 299, 595–602. [Google Scholar] [CrossRef]
- Seguin, R.P.; Herron, J.M.; Lopez, V.A.; Dempsey, J.L.; Xu, L. Metabolism of Benzalkonium Chlorides by Human Hepatic Cytochromes P450. Chem. Res. Toxicol. 2019, 32, 2466–2478. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Li, K.; Zhang, C.; Liu, D.; Sun, J. Biosorption of tetradecyl benzyl dimethyl ammonium chloride on activated sludge: Kinetic, thermodynamic and reaction mechanisms. Bioresour. Technol. 2011, 102, 3799–3804. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Tezel, U.; Li, K.; Liu, D.; Ren, R.; Du, J.; Pavlostathis, S.G. Evaluation and modeling of benzalkonium chloride inhibition and biodegradation in activated sludge. Water Res. 2011, 45, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Clara, M.; Scharf, S.; Scheffknecht, C.; Gans, O. Occurrence of selected surfactants in untreated and treated sewage. Water Res. 2007, 41, 4339–4348. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K.; Eitel, A.; Braun, U.; Hubner, P.; Daschner, F.; Mascart, G.; Milandri, M.; Reinthaler, F.; Verhoef, J. Analysis of benzalkonium chloride in the effluent from European hospitals by solid-phase extraction and high-performance liquid chromatography with post-column ion-pairing and fluorescence detection. J. Chromatogr. A 1997, 774, 281–286. [Google Scholar] [CrossRef]
- Khan, A.H.; Macfie, S.M.; Ray, M.B. Sorption and leaching of benzalkonium chlorides in agricultural soils. J. Environ. Manage. 2017, 196, 26–35. [Google Scholar] [CrossRef]
- Ismail, Z.Z.; Tezel, U.; Pavlostathis, S.G. Sorption of quaternary ammonium compounds to municipal sludge. Water Res. 2010, 44, 2303–2313. [Google Scholar] [CrossRef]
- Li, X.; Luo, X.; Mai, B.; Liu, J.; Chen, L.; Lin, S. Occurrence of quaternary ammonium compounds (QACs) and their application as a tracer for sewage derived pollution in urban estuarine sediments. Environ. Pollut. 2014, 185, 127–133. [Google Scholar] [CrossRef]
- Li, X.; Brownawell, B.J. Quaternary ammonium compounds in urban estuarine sediment environments - A class of contaminants in need of increased attention? Environ. Sci. Technol. 2010, 44, 7561–7568. [Google Scholar] [CrossRef]
- Ding, W.H.; Liao, Y.H. Determination of alkylbenzyldimethylammonium chlorides in river water and sewage effluent by solid-phase extraction and gas chromatography/mass spectrometry. Anal. Chem. 2001, 73, 36–40. [Google Scholar] [CrossRef]
- Sütterlin, H.; Trittler, R.; Bojanowski, S.; Stadlbauer, E.A.; Kümmerer, K. Fate of benzalkonium chloride in a sewage sludge low temperature conversion process investigated by LC-LC/ESI-MS/MS. Clean - Soil, Air, Water 2007, 35, 81–87. [Google Scholar] [CrossRef]
- He, Z.W.; Liu, W.Z.; Tang, C.C.; Liang, B.; Guo, Z.C.; Wang, L.; Ren, Y.X.; Wang, A.J. Performance and microbial community responses of anaerobic digestion of waste activated sludge to residual benzalkonium chlorides. Energy Convers. Manag. 2019, 202, 112211. [Google Scholar] [CrossRef]
- Li, X.; Doherty, A.C.; Brownawell, B.; Lara-Martin, P.A. Distribution and diagenetic fate of synthetic surfactants and their metabolites in sewage-impacted estuarine sediments. Environ. Pollut. 2018, 242, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Ndabambi, M.; Kwon, J.H. Benzalkonium ion sorption to peat and clays: Relative contributions of ion exchange and van der Waals interactions. Chemosphere 2020, 247, 125924. [Google Scholar] [CrossRef] [PubMed]
- Kreuzinger, N.; Fuerhacker, M.; Scharf, S.; Uhl, M.; Gans, O.; Grillitsch, B. Methodological approach towards the environmental significance of uncharacterized substances - quaternary ammonium compounds as an example. Desalination 2007, 215, 209–222. [Google Scholar] [CrossRef]
- Sanidad, K.Z.; Yang, H.; Wang, W.; Ozay, E.I.; Yang, J.; Gu, M.; Karner, E.; Zhang, J.; Kim, D.; Minter, L.M. Effects of consumer antimicrobials benzalkonium chloride, benzethonium chloride, and chloroxylenol on colonic inflammation and colitis-associated colon tumorigenesis in mice. Toxicol. Sci. 2018, 163, 490–499. [Google Scholar] [CrossRef]
- Chen, Y.; Geurts, M.; Sjollema, S.B.; Kramer, N.I.; Hermens, J.L.M.; Droge, S.T.J. Acute toxicity of the cationic surfactant C12-benzalkonium in different bioassays: How test design affects bioavailability and effect concentrations. Environ. Toxicol. Chem. 2014, 33, 606–615. [Google Scholar] [CrossRef]
- Ferk, F.; Mišík, M.; Hoelzl, C.; Uhl, M.; Fuerhacker, M.; Grillitsch, B.; Parzefall, W.; Nersesyan, A.; Mičieta, K.; Grummt, T. Benzalkonium chloride (BAC) and dimethyldioctadecyl-ammonium bromide (DDAB), two common quaternary ammonium compounds, cause genotoxic effects in mammalian and plant cells at environmentally relevant concentrations. Mutagenesis 2007, 22, 363–370. [Google Scholar] [CrossRef]
- Luz, A.; DeLeo, P.; Pechacek, N.; Freemantle, M. Human health hazard assessment of quaternary ammonium compounds: Didecyl dimethyl ammonium chloride and alkyl (C12–C16) dimethyl benzyl ammonium chloride. Regul. Toxicol. Pharmacol. 2020, 116. [Google Scholar] [CrossRef]
- Lim, X. Do we know enough about the safety of quat disinfectants? Chem. Eng. News 2020, 98, 28. [Google Scholar]
- Wieck, S.; Olsson, O.; Kümmerer, K. Possible underestimations of risks for the environment due to unregulated emissions of biocides from households to wastewater. Environ. Int. 2016, 94, 695–705. [Google Scholar] [CrossRef] [PubMed]
- National Iinstitute for Occupational Safety and Health (NIOSH) Registry of Toxic Effects of Chemical Substances—Benzalkonium Chloride. Available online: https://www.cdc.gov/niosh-rtecs/bo3010b0.html (accessed on 25 November 2020).
- Song, X.; Cvelbar, U.; Strazar, P.; Vossebein, L.; Zille, A. Antimicrobial Efficiency and Surface Interactions of Quaternary Ammonium Compound Absorbed on Dielectric Barrier Discharge (DBD) Plasma Treated Fiber-Based Wiping Materials. ACS Appl. Mater. Interfaces 2020, 12, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.; Morgan, N.; Humphreys, G.J.; Amézquita, A.; Mistry, H.; McBain, A.J. Loss of function in Escherichia coli exposed to environmentally relevant concentrations of benzalkonium chloride. Appl. Environ. Microbiol. 2019, 85, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Weigand, M.R.; Oh, S.; Hatt, J.K.; Krishnan, R.; Tezel, U.; Pavlostathis, S.G.; Konstantinidis, K.T. Widely used benzalkonium chloride disinfectants can promote antibiotic resistance. Appl. Environ. Microbiol. 2018, 84, 1–14. [Google Scholar] [CrossRef]
- Buffet-Bataillon, S.; Tattevin, P.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Emergence of resistance to antibacterial agents: The role of quaternary ammonium compounds - A critical review. Int. J. Antimicrob. Agents 2012, 39, 381–389. [Google Scholar] [CrossRef]
- Braoudaki, M.; Hilton, A.C. Mechanisms of resistance in Salmonella enterica adapted to erythromycin, benzalkonium chloride and triclosan. Int. J. Antimicrob. Agents 2005, 25, 31–37. [Google Scholar] [CrossRef]
- Kanno, S.; Hirano, S.; Kato, H.; Fukuta, M.; Mukai, T.; Aoki, Y. Benzalkonium chloride and cetylpyridinium chloride induce apoptosis in human lung epithelial cells and alter surface activity of pulmonary surfactant monolayers. Chem. Biol. Interact. 2020, 317, 108962. [Google Scholar] [CrossRef]
- Buffet-Bataillon, S.; Tattevin, P.; Maillard, J.Y.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Efflux pump induction by quaternary ammonium compounds and fluoroquinolone resistance in bacteria. Future Microbiol. 2016, 11, 81–92. [Google Scholar] [CrossRef]
- Rogov, A.G.; Goleva, T.N.; Sukhanova, E.I.; Epremyan, K.K.; Trendeleva, T.A.; Ovchenkova, A.P.; Aliverdieva, D.A.; Zvyagilskaya, R.A. Mitochondrial Dysfunctions May Be One of the Major Causative Factors Underlying Detrimental Effects of Benzalkonium Chloride. Oxid. Med. Cell. Longev. 2020, 2020. [Google Scholar] [CrossRef]
- Kim, S.H.; Kwon, D.; Lee, S.; Son, S.W.; Kwon, J.T.; Kim, P.J.; Lee, Y.H.; Jung, Y.S. Concentration-and time-dependent effects of benzalkonium chloride in human lung epithelial cells: Necrosis, apoptosis, or epithelial mesenchymal transition. Toxics 2020, 8, 17. [Google Scholar] [CrossRef]
- Lavorgna, M.; Russo, C.; D’Abrosca, B.; Parrella, A.; Isidori, M. Toxicity and genotoxicity of the quaternary ammonium compound benzalkonium chloride (BAC) using Daphnia magna and Ceriodaphnia dubia as model systems. Environ. Pollut. 2016, 210, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.; Kwon, J.T.; Lim, Y.M.; Shim, I.; Kim, E.; Lee, D.H.; Yoon, B.I.; Kim, P.; Kim, H.M. Inhalation toxicity of benzalkonium chloride and triethylene glycol mixture in rats. Toxicol. Appl. Pharmacol. 2019, 378, 114609. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Kundi, M.; Lavorgna, M.; Parrella, A.; Isidori, M. Benzalkonium Chloride and Anticancer Drugs in Binary Mixtures: Reproductive Toxicity and Genotoxicity in the Freshwater Crustacean Ceriodaphnia dubia. Arch. Environ. Contam. Toxicol. 2018, 74, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Sreevidya, V.S.; Lenz, K.A.; Svoboda, K.R.; Ma, H. Benzalkonium chloride, benzethonium chloride, and chloroxylenol - Three replacement antimicrobials are more toxic than triclosan and triclocarban in two model organisms. Environ. Pollut. 2018, 235, 814–824. [Google Scholar] [CrossRef]
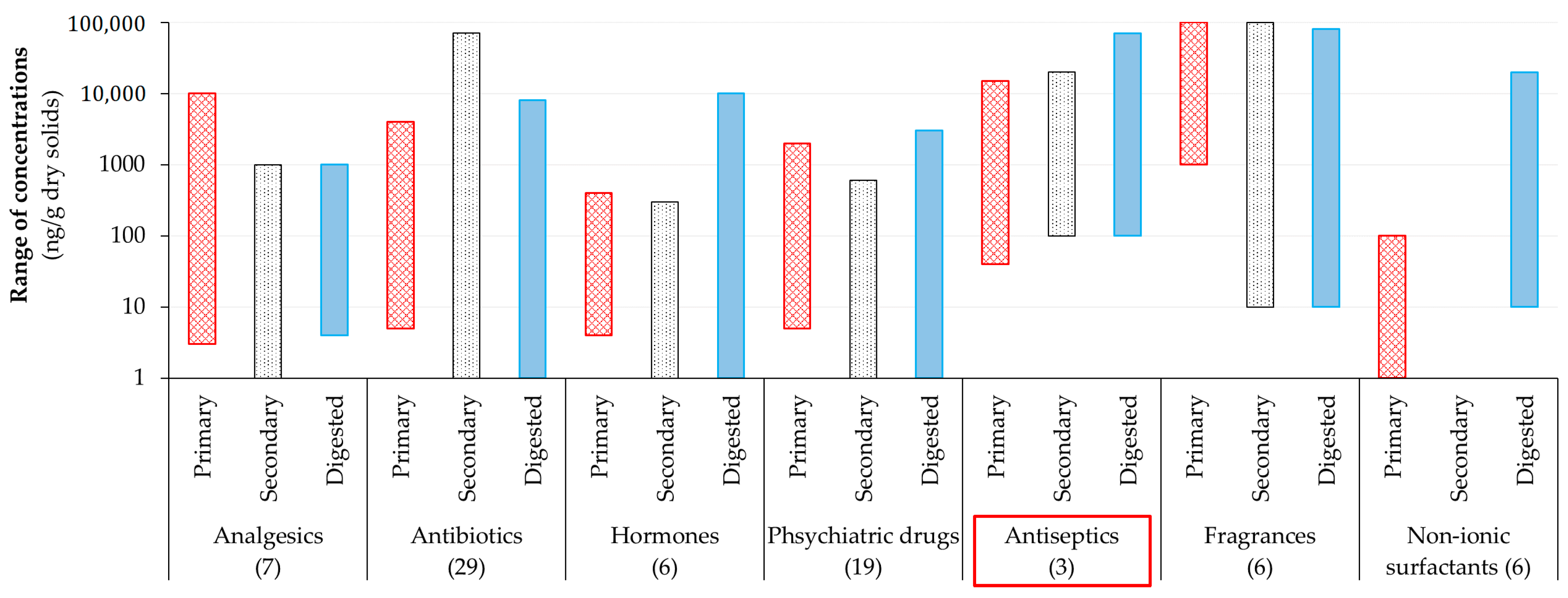
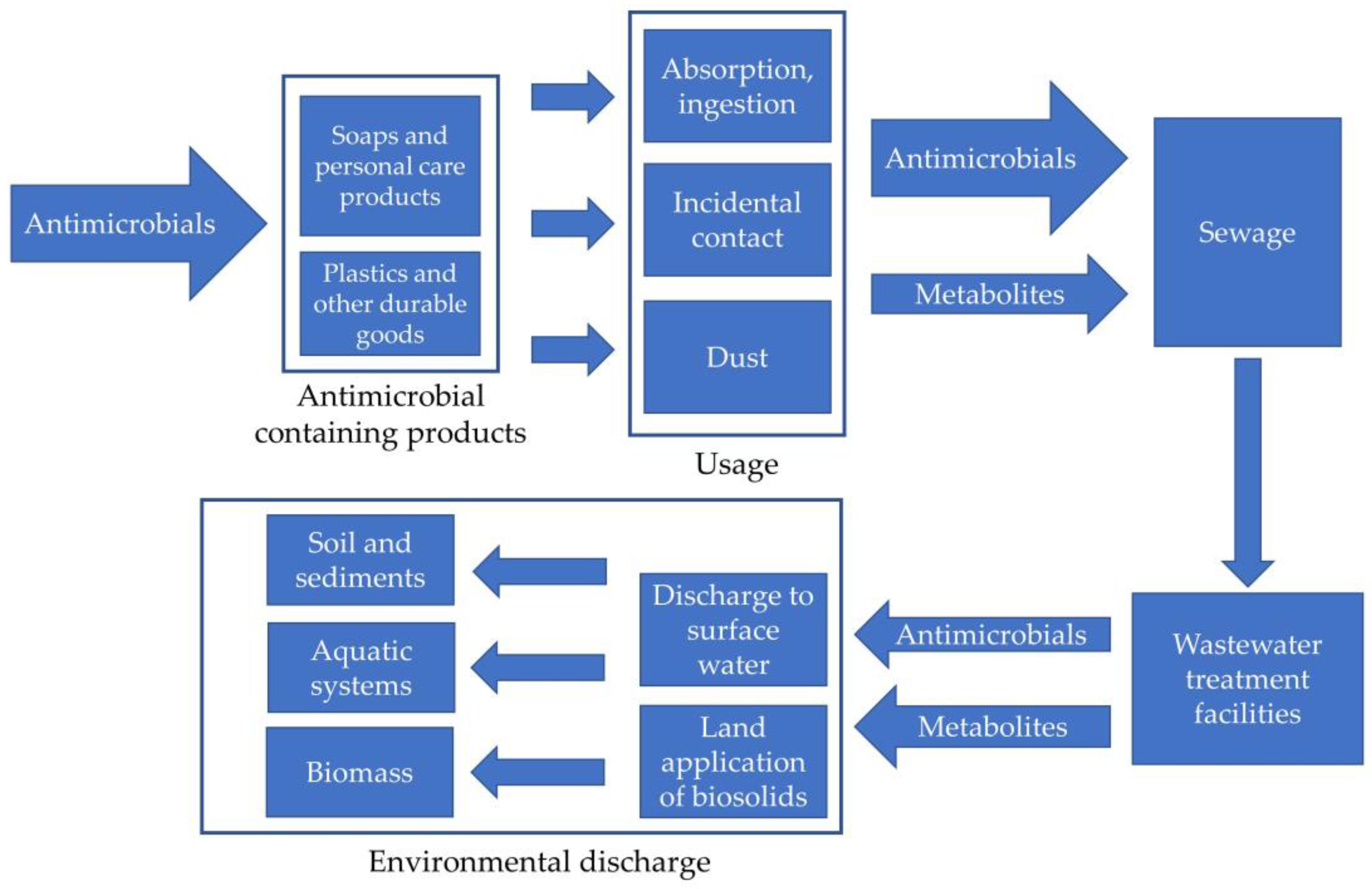
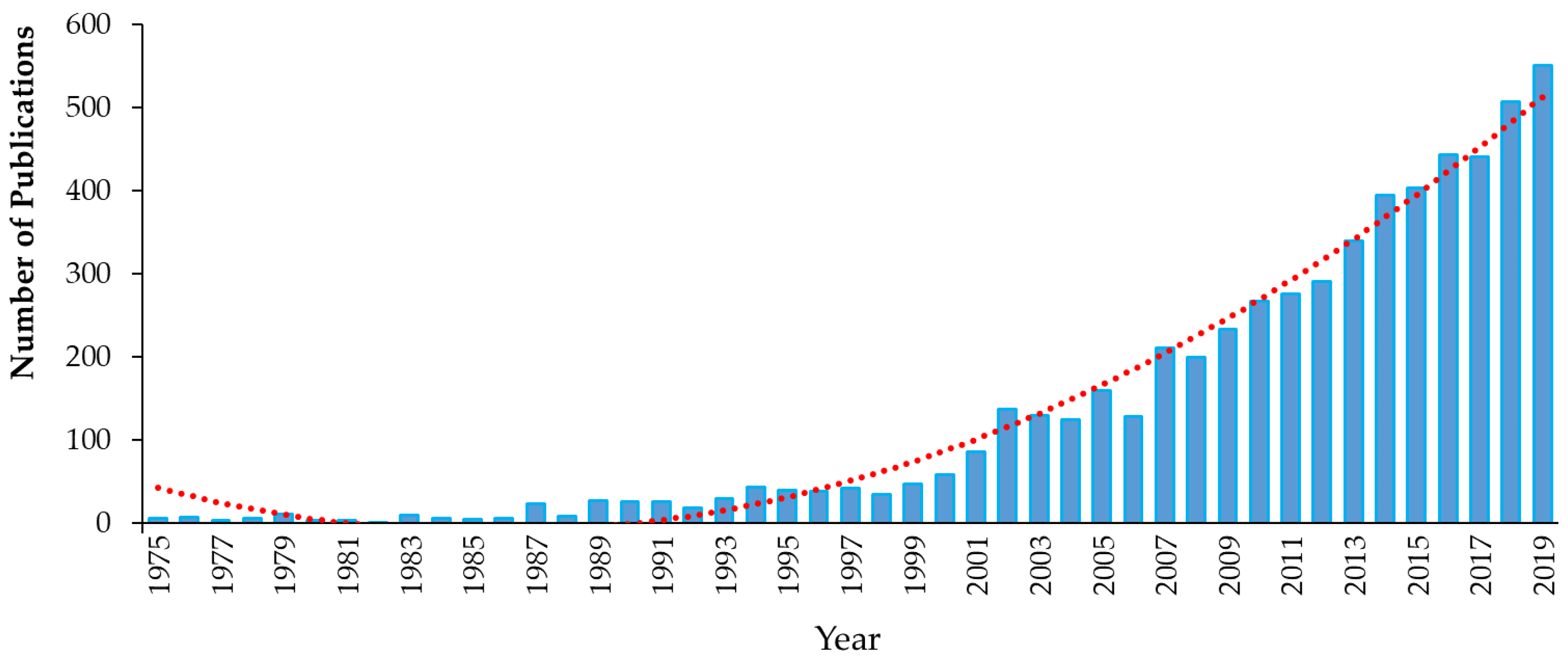
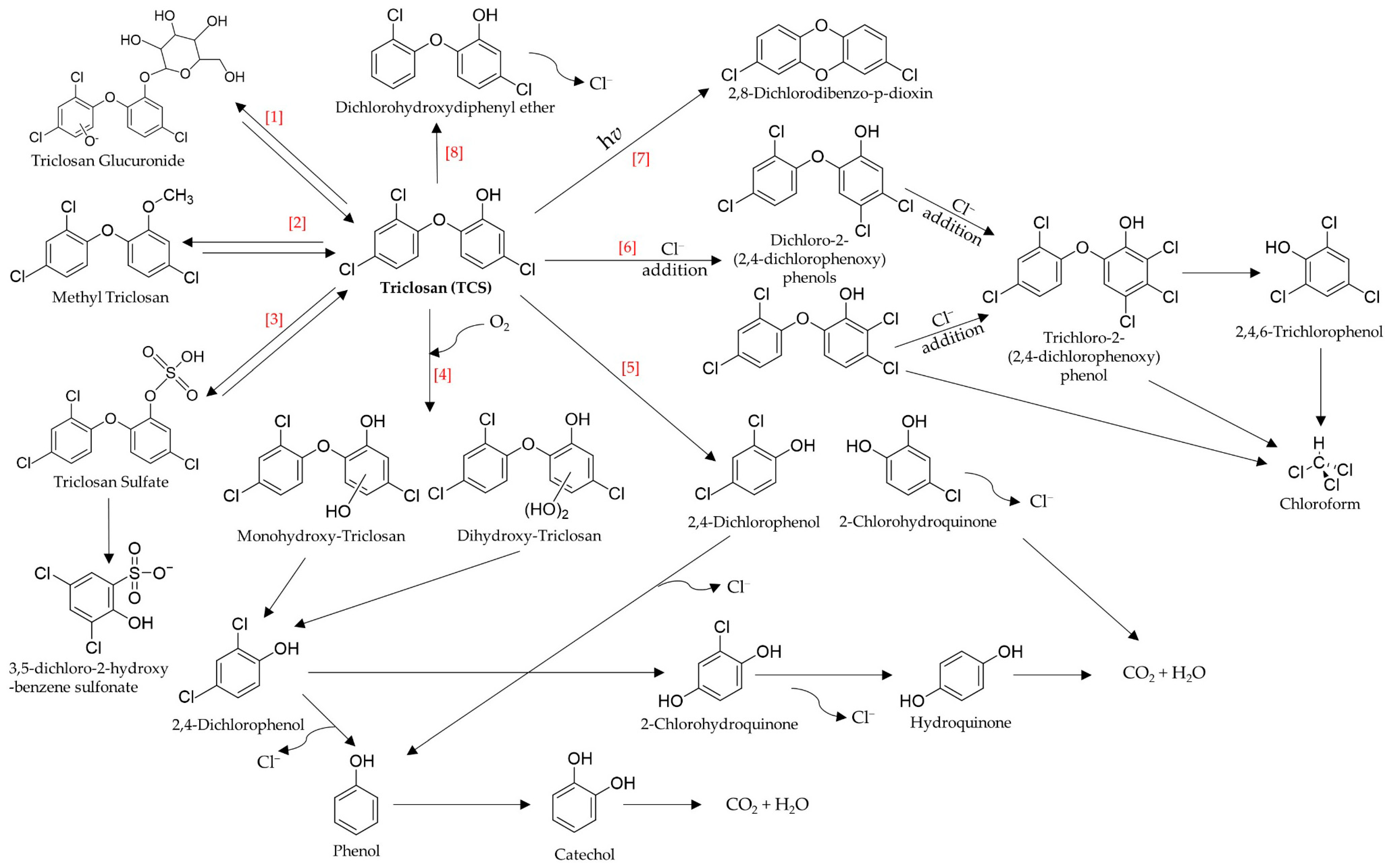
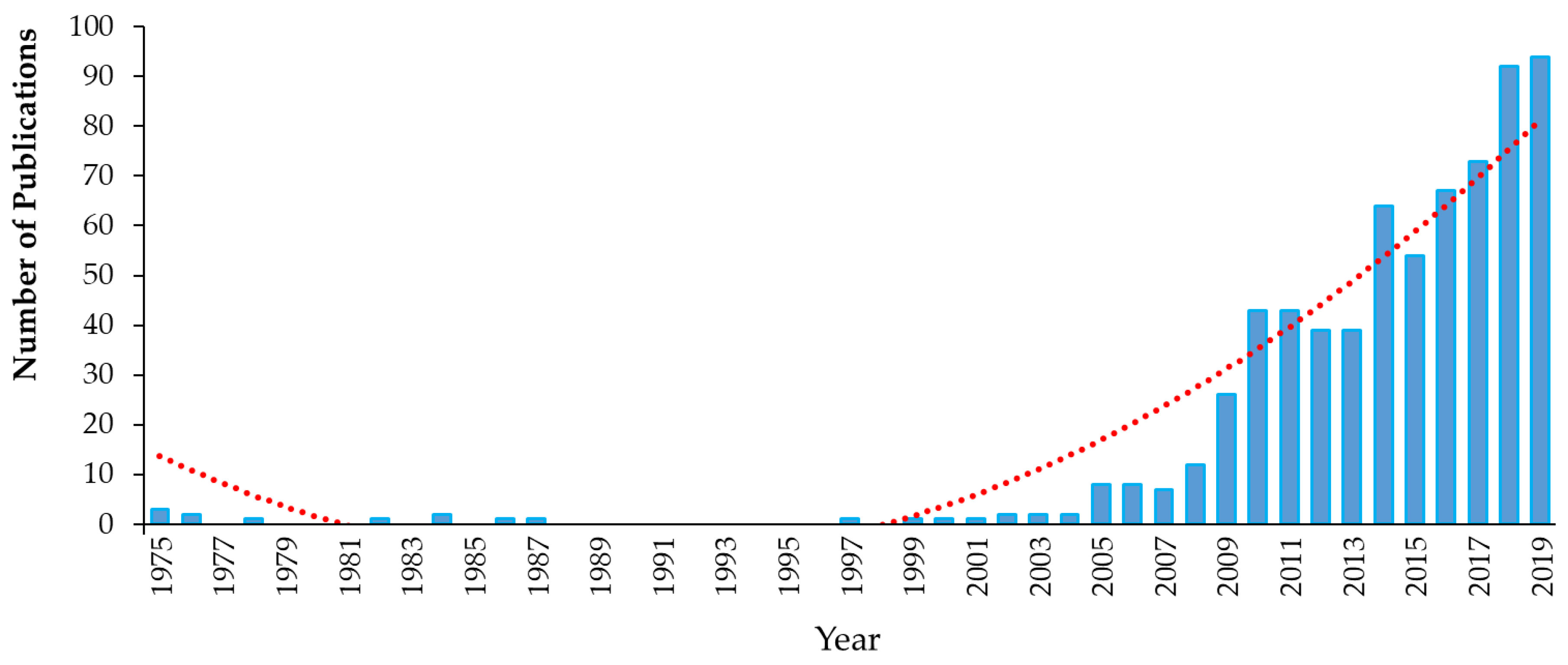
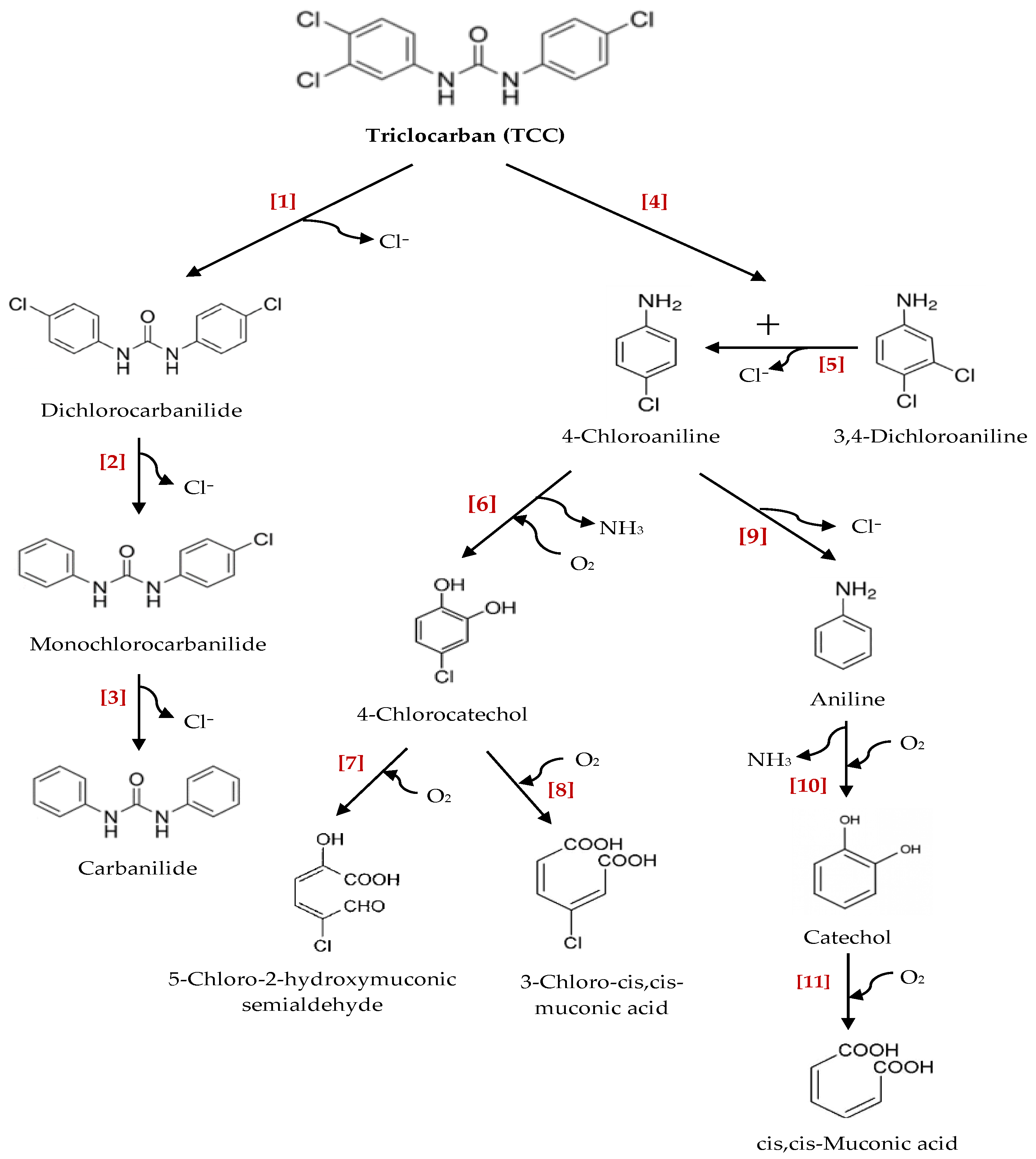

| Property | Triclosan (TCS) |
|---|---|
| Formula | C12H7Cl3O2 |
| CAS number | 3380-34-5 |
| Structure | 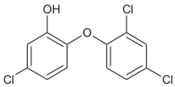 |
| Molecular weight (g/mole) | 289.5 g/mole |
| Log Kow | 4.76 † |
| Solubility in water (mg/L at 20 °C) | 10.0 ‡ |
| pKa | 7.9 † |
| Melting Point (°C) | 55–57 ‡ |
| Sludge Source | Treatment Conditions | TCS Levels in Raw Sludge | TCS Levels in Treated Sludge | Transformation Products/Metabolites Levels in Sludge | Reference |
|---|---|---|---|---|---|
| Three different WWTPs: WWTP (A): Primary treatment only (small facility <25,000 people). WWTP (B): Chemically enhanced primary treatment (large facility >1 million people). WWTP (C): Primary + secondary BNR treatment (medium facility >500,000 people). | WWTP (A): Aerobic digestion. WWTP (B): None. WWTP (C): MH anaerobic digestion. | WWTP (A): 2247 ± 185 ng/g dw WWTP (B): 447 ± 21 ng/g dw WWTP (C): 196 ± 14 ng/g dw (primary) and 174 ± 12 ng/g dw in secondary sludges | WWTP (A): 2299 ng/g dw WWTP (B): 393 ng/g dw WWTP (C): 310 ng/g dw | Study also examined TCS transformation products/metabolites: • 111 ng/g dw 2,4-DCP found in digested sludge from WWTP (B) (< LOD of 1.0 ng/g dw in WWTPs (A) and (B)) • MeTCS was 262 ng/g dw in digested sludge from WWTP (A); < LOD of 1.0 ng/g dw in WWTP (B) and 26.2 ng/g dw in WWTP (C) • DCDD was < LOD (3 ng/g dw) in all three facilities | [126] |
| Very large east-coast U.S. municipal WWTP (daily flow 1.25 million m3/day). Facility uses primary sedimentation, activated sludge and tertiary treatment (including nitrification–denitrification). | Cambi™ thermal hydrolysis followed by anaerobic digestion (TH-AD). Anaerobic digester operated at 37 °C with a 22-day SRT. | Mixed sludge before TH-AD treatment: TCS: 6884 to 7489 ng/g dw; MeTCS: 248 to 280 ng/g dw; 2,4-DCP: 204 to 283 ng/g dw. | 10,872 to 12,345 ng/g dw | Study also examined TCS transformation products/metabolites. Effluent from TH-AD contained: • MeTCS: 326 to 340 ng/g dw • 2,4-DCP: 396–661 ng/g dw • TCS-SO4 was not detected in samples collected within the TH-AD process | [127] |
| Heat-treated biosolid pellets from a full-scale WWTP in the United Kingdom. | n/a | n/a | 11,220 to 28,220 ng/g dw | Biosolid pellets had 35 to 69 ng/g dw of MeTCS | [128] |
| Five American WWTPs, including two activated sludge (treating wastwater from ~400,000 and 27,000 people) and three tricking filter (all treating wastewater from <3100 people). | Full-scale aerobic digestion and anaerobic digestion of mixed sludge. Process specifics not provided. | • Activated sludge facilities: - 8750 to 14,700 ng/g dw TCS in primary; - 900 to 4,200 ng/g dw TCS in secondary sludges. • Tricking filter facilities: - 7500 to 12,200 ng/g dw TCS in primary; - 7300 ng/g dw TCS in secondary sludges. | • Digested sludge values not reported in activated sludge facilities • 530 to 15,600 ng/g dw in tricking filter facilities | Study also examined MeTCS and higher-chlorinated TCS compounds: • MeTCS in activated sludge facilities: - 50 to 260 ng/g dw in primary - 310 to 1030 ng/g dw in secondary • MeTCS in tricking filter facilities: - 140 to 200 ng/g dw in primary sludge - 450 ng/g dw in secondary sludge • Low levels of higher-chlorinated TCS in digested tricking filter sludge: - < LOD to 110 ng/g dw of 4,5-dichloro-2-(2,4-dichloro-phenoxy)-phenol - < LOD to 100 ng/g dw of 5,6-dichloro-2-(2,4-dichloro-phenoxy)-phenol - <LOD and 80 ng/g dw of 4,5,6-trichloro-2- (2,4-dichloro-phenoxy)-phenol | [41] |
| Mixed fermented primary and secondary sludge (33:67% by weight) from a medium-sized Canadian BNR WWTP (>40,000 people). | • Bench-scale anaerobic digester (35 °C) and cycling aerobic/anoxic (22 °C) digesters operated at SRTs between 5 and 20 days • Sequential AD (35 °C) and cycling aerobic/anoxic (22 and 35 °C, respectively) digesters operated at various first: second stage SRTs (total SRT of 20 days). | Mixed sludge contained: • 2323 to 6127 ng/g dw TCS; • 111 to 214 ng/g dw TCS-SO4. | • Anaerobic digester effluent with 4832 to 7080 ng/g dw at different SRTs • Aerobic/anoxic digester effluent with 1183 and 4532 ng/g dw at different SRTs • Sequential digester effluent with 2430 to 6875 ng/g dw | Study also examined TCS transformation products/metabolites: • Low levels of 2,4-DCP were found in anaerobic digester effluent but were below LOQ. Anaerobic digesters were also effective in removing TCS-SO4, which had levels between 91.8 and 194.1 ng/g dw in effluent • 2,4-DCP below LOD in aerobic/anoxic digester effluent, near-complete removal of TCS-SO4 (15.0 to 40.1 ng/g dw in effluent) • Near complete removal of TCS-SO4 in sequential anaerobic/aerobic/anoxic digesters (11.3 and 137.6 ng/g dw) | [80,129] |
| Mixed fermented primary and secondary sludge (33:67% by volume) from a medium-sized Canadian BNR WWTP (>40,000 people). | Bench-scale anaerobic digesters operated at SRTs of 20, 12 and 6 days under MH and TH temperatures. | TCS in mixed primary and secondary BNR sludge: • 6760 to 8240 ng/g dw during cold months; • 1700 to 3530 ng/g dw during warmer months • Mean conc.: 4450 ± 2750 ng/g dw | • 8775 ng/g to 10,990 ng/g dw for TH digestates at different SRTs • 9120 to 12,790 ng/g dw for MH digestates at different SRTs | Study examined TCS-gluc, TCS-SO4, 5,6- dichloro-2-(2,4-dichloro-phenoxy)-phenol and 4,5,6-trichloro-2-(2,4-dichloro-phenoxy)-phenol and 2,3,4-TCP, but were below the LOD (1.44, 0.12, 0.58, 0.09 and 0.26 ppb, respectively) | [124] |
| 19 full-scale Spanish WWTPs located in a mix of rural, industrial and urban areas. | n/a | n/a | 54 to 2987 ng/g dw with a mean concentration of 1194 ng/g dw | 4 to 311 ng/g dw for MeTCS, with a mean concentration of 54 ng/g dw | [130] |
| Large WWTP in the Mid-Atlantic region of the U.S. employing primary treatment and secondary treatment (activated sludge with added nitrification–denitrification). | Dewatered and treated with lime (~15% by weight). | n/a | 19,100 ng/g dw | 1000 ng/g dw MeTCS | [131,132] |
| Large WWTP in the Mid-Atlantic region of the U.S. employing primary treatment and secondary treatment (activated sludge with added nitrification–denitrification). | Dewatered and treated with lime (~15% by weight). | • 19,200 ng/g dw TCS • 80 ng/g dw MeTCS | 16,200 ng/g dw TCS | 160 ± 30 ng/g dw MeTCS | [42] |
| Property | Triclocarban (TCC) |
|---|---|
| Formula | C13H9Cl3N2O |
| CAS number | 101-20-2 |
| Structure | 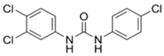 |
| Molecular weight (g/mole) | 315.578 |
| Log Kow (at 25 °C, pH 7) | 4.9 † |
| Solubility in water (mg/L at 25 °C) | 0.65–1.55 † |
| pKa | 12.7 ‡ |
| Vapour pressure (mm Hg at 25 °C) | 3.61 × 10−9 § |
| Melting point (°C) | 255–256 ‡ |
| Sludge Source | Treatment Conditions | TCC Levels in Raw Sludge | TCC Levels in Treated Sludge | Transformation Products/Metabolites Levels in Sludge | Reference |
|---|---|---|---|---|---|
| Primary sludge samples from various WWTPs located in five different U.S. states | n/a | 7500 to 25,900 ng/g dw for primary sludge, with a mean concentration of 19,300 ± 7100 ng/g dw | n/a | • 3′-Cl-TCC: 80 to 500 ng/g dw for primary sludge, with a mean concentration of 275 ± 150 ng/g dw • DCC: 7 to 30 ng/g dw for primary sludge, with a mean concentration of 16 ± 9 ng/g dw | [213] |
| 100 sewage sludge samples from 25 full-scale WWTPs in 18 U.S. states | n/a | n/a | •21,600 to 43,200 ng/g dw for undigested sludge† (median conc.: 34,900 ± 7200 ng/g dw) •4700 to 63,000 ng/g dw for anaerobically digested biosolids (median conc.: 27,600 ± 9600 ng/g dw) •16,400 to 19,600 ng/g dw for aerobically digested biosolids (median conc.: 18,000 ng/g dw) | • 3′-Cl-TCC: - 1700 to 4300 ng/g dw for undigested sludge - 40 to 5000 ng/g dw for anaerobically digested biosolids - 2400 to 2700 ng/g dw for aerobically digested biosolids • DCC: - 250 to 590 ng/g dw for undigested sludge - 30 to 14,900 ng/g dw for anaerobically digested biosolids - 210 to 740 ng/g dw for aerobically digested biosolids | [201] |
| 100 sewage sludge samples from 86 domestic and 16 industrial WWTPs in China | n/a | <3 to 43,300 ng/g dw with 95% DF | n/a | • 3′-Cl-TCC: 22 to 580 ng/g dw with 100% DF • DCC: <2 to 23,890 ng/g dw with 94% DF • MCC: <2 to 120 ng/g dw with 92% DF • NCC: 3 to 1340 ng/g dw with 90% DF • 2′−OH-TCC: <1 to 2340 ng/g dw with 94% DF • 3′−OH-TCC: <1 to 1250 ng/g dw with 91% DF | [206] |
| Un-pretreated and MW-pretreated mixed sludge samples (FPS:TWAS = 33:67% by volume) | Bench-scale anaerobic digesters operated at SRTs of 20, 12 and 6 days under MH and TH temperatures | 300 to 1800 ng/g dw for mixed sludge with a mean concentration of 1030 ± 470 ng/g dw | • 620 to 1900 ng/g dw for TH digestates at different SRTs • 940 to 1900 ng/g dw for MH digestates at different SRTs | • NCC: 250 to 1020 ng/g dw for TH digestates and 130 to 675 ng/g dw for MH digestates at different SRTs • 4-Cl-TCC, monochloroanilines and 4-chlorocatechol: below the LOD (0.70, 0.12 and 0.87 ppb, respectively) • MCC and DCC: below the LOQ for digestates (0.25 and 0.25 ppb, respectively) | [44] |
| Property | Benzalkonium Compounds | ||
|---|---|---|---|
| BAC-12 | BAC-14 | BAC-16 | |
| Formula | C21H38ClN | C23H42ClN | C25H46ClN |
| CAS number | 139-07-1 | 139-08-2 | 122-18-9 |
| Structure | 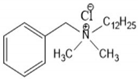 | 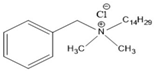 | 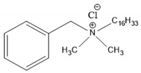 |
| Molecular weight (g/mole) | 339.99 g/mole | 368.04 g/mole | 396.09 g/mole |
| Log Kow | 2.93 † | 3.91 † | 4.89 ‡ |
| Solubility in water (mg/L at 25 °C) | 1230 § | 100 § | 8.5 § |
| pKa | NA | NA | NA |
| Melting point (°C) | 42–44 * | 47–52 * | 54–56 * |
| Sludge Source | Treatment Conditions | BAC Levels in Raw Sludge | BAC Levels in Treated Sludge | Transformation Products/Metabolites in Sludge | Reference |
|---|---|---|---|---|---|
| Treated, untreated and digested sludge samples from 11 Swedish WWTPs | • Full-scale biological process in different treatment combinations • 10 WWTPs used aerobic sludge digestion while only one WWTP did not have sludge digestion | n/a | • BAC-10: 24 to 210 ng/g dw for digested sludge (median conc.: 100 ng/g dw) • BAC-12: 8800 to 89,000 ng/g dw for digested sludge (median conc.: 29,000 ng/g dw) • BAC-14: 3200 to 60,000 ng/g dw for digested sludge (median conc.: 17,000 ng/g dw) • BAC-16: 990 to 4900 ng/g dw for digested sludge (median conc.: 2200 ng/g dw) | n/a | [261] |
| Spiked sludge used to check adsorption by wastewater sludge | Bench-scale unit that consisted of a Pyrex glass tube was used for thermocatalytic low temperature conversion of sludge | • BAC-12: 0.1 mg/g dw spiked dewatered sludge • BAC-14: 0.1 mg/g dw spiked dewatered sludge | • BAC-12: 0.1 mg/g spiked dried sludge, n = 9 • BAC-14: 0.1 mg/g spiked dried sludge, n = 3 | n/a | [300] |
| Digested sludge samples from three different Swedish WWTPs | Full-scale conventional biological process of different sizes and treatment configurations • Plant 1: Secondary clarifier sludge treated by anaerobic digesters at temperature of 34–37 °C and SRT of 15 days • Plant 2: Primary clarifier sludge of 1.7-h retention time introduced to the digester with SRT of 20 days at 37 °C Plant 3: Primary sludge introduced to the digesters, treated with SRT of 25–30 days at 37 °C | • BAC-12: The total influent mass flow percentage of BAC-12 for primary sludge in the three WWTPs: 62, 55 and 25%, respectively • BAC-14: The total influent mass flow percentage of BAC-14 for primary sludge in the three WWTPs: 74, 54 and 41%, respectively | • BAC-12: Total influent mass flow percentage of BAC-12 for digested sludge in the three WWTPs: 67, 43 and 42%, respectively • BAC-14: The influent mass flow percentage of BAC-14 for digested sludge at the three WWTPS: 96, 65 and 74%, respectively | n/a | [257] |
| Sewage sludge samples from 52 municipalities in China | Full-scale conventional biological treatment process of various combinations for municipal WWTPs | n/a | • BAC-8 to BAC-18 homologues: 0.09 to 190 µg/g dw for freshly digested freeze-dried sludge (median conc.: 1.09 µg/g dw) | n/a | [256] |
| Anaerobic digestion of waste activated sludge collected from a local WWTP in Harbin, China | Full-scale conventional activated sludge process for treating municipal wastewater | • BACs: The base level of BACs in raw sludge was 48 ± 7 µg/g TSS | • BAC: Waste-activated sludge was amended with BACs at 2, 8, 15, 25 and 50 mg/g of TSS to investigate performance and microbial community responses of anaerobic digestion | n/a | [301] |
| Exposure Route | LD50 Concentration |
|---|---|
| Intravenous | 14 mg/kg body weight |
| Oral | 240 mg/kg body weight |
| Intraperitoneal | 15 mg/kg body weight |
| Subcutaneous | 400 mg/kg body weight |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbott, T.; Kor-Bicakci, G.; Islam, M.S.; Eskicioglu, C. A Review on the Fate of Legacy and Alternative Antimicrobials and Their Metabolites during Wastewater and Sludge Treatment. Int. J. Mol. Sci. 2020, 21, 9241. https://doi.org/10.3390/ijms21239241
Abbott T, Kor-Bicakci G, Islam MS, Eskicioglu C. A Review on the Fate of Legacy and Alternative Antimicrobials and Their Metabolites during Wastewater and Sludge Treatment. International Journal of Molecular Sciences. 2020; 21(23):9241. https://doi.org/10.3390/ijms21239241
Chicago/Turabian StyleAbbott, Timothy, Gokce Kor-Bicakci, Mohammad S. Islam, and Cigdem Eskicioglu. 2020. "A Review on the Fate of Legacy and Alternative Antimicrobials and Their Metabolites during Wastewater and Sludge Treatment" International Journal of Molecular Sciences 21, no. 23: 9241. https://doi.org/10.3390/ijms21239241
APA StyleAbbott, T., Kor-Bicakci, G., Islam, M. S., & Eskicioglu, C. (2020). A Review on the Fate of Legacy and Alternative Antimicrobials and Their Metabolites during Wastewater and Sludge Treatment. International Journal of Molecular Sciences, 21(23), 9241. https://doi.org/10.3390/ijms21239241




