Adipokines in the Skin and in Dermatological Diseases
Abstract
1. Introduction
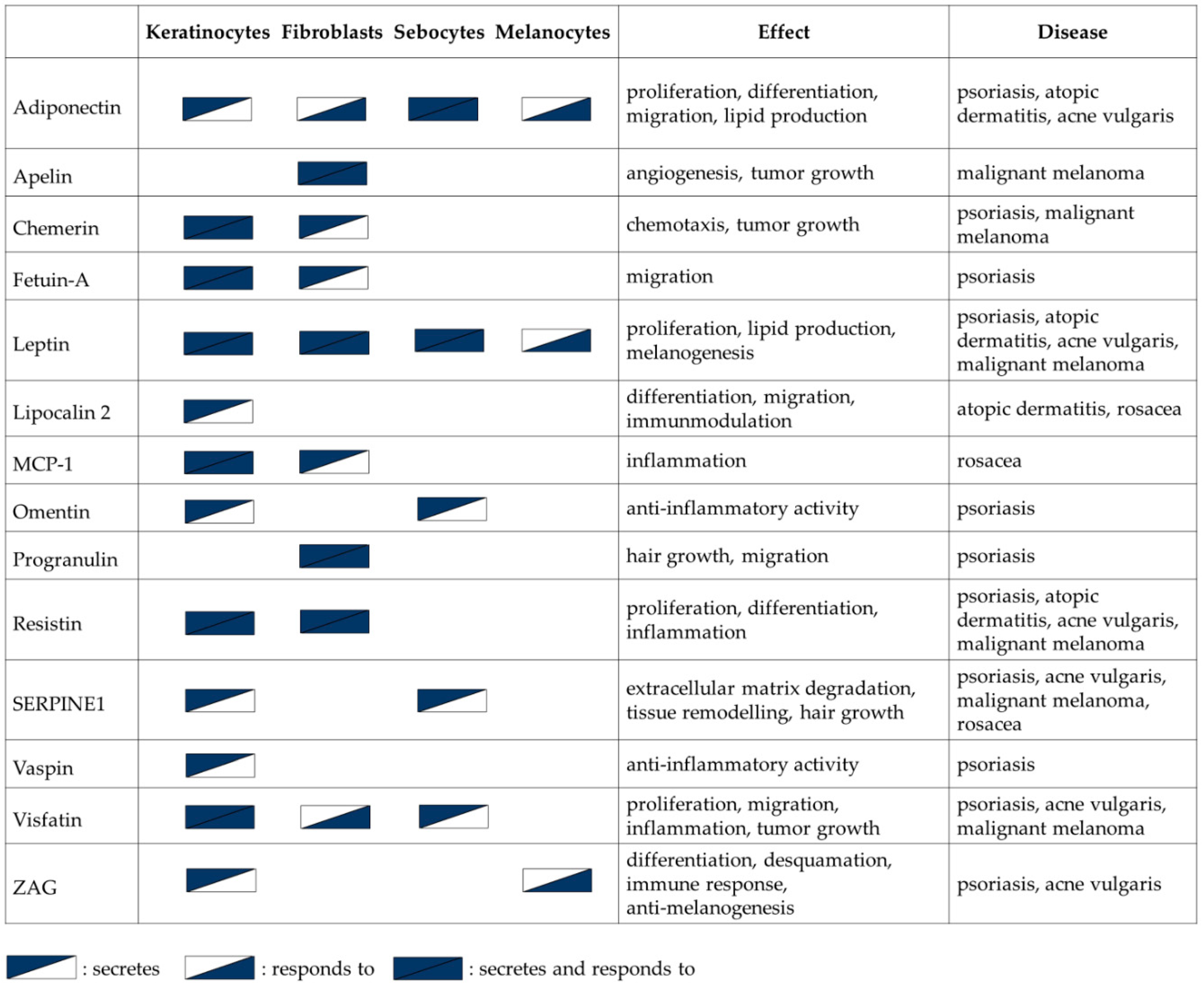
2. Expression of Adipokines in Skin Cells
2.1. Keratinocytes
2.2. Fibroblasts
2.3. Sebocytes
3. Adipokines in Physiological Processes in the Skin
3.1. Melanogenesis
3.2. Hair Growth
3.3. Wound Healing
4. Adipokines in Skin Diseases
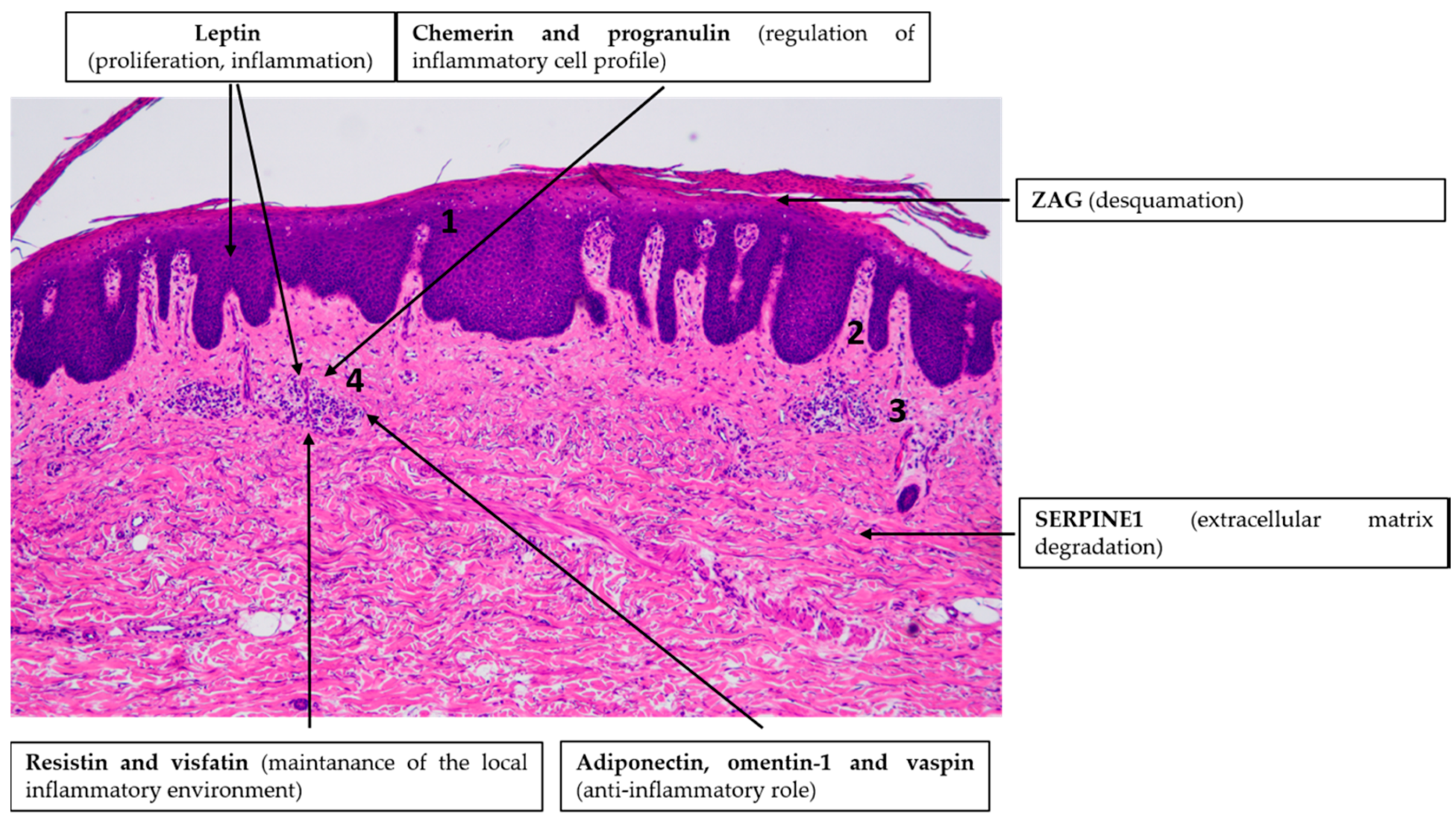
4.1. Psoriasis
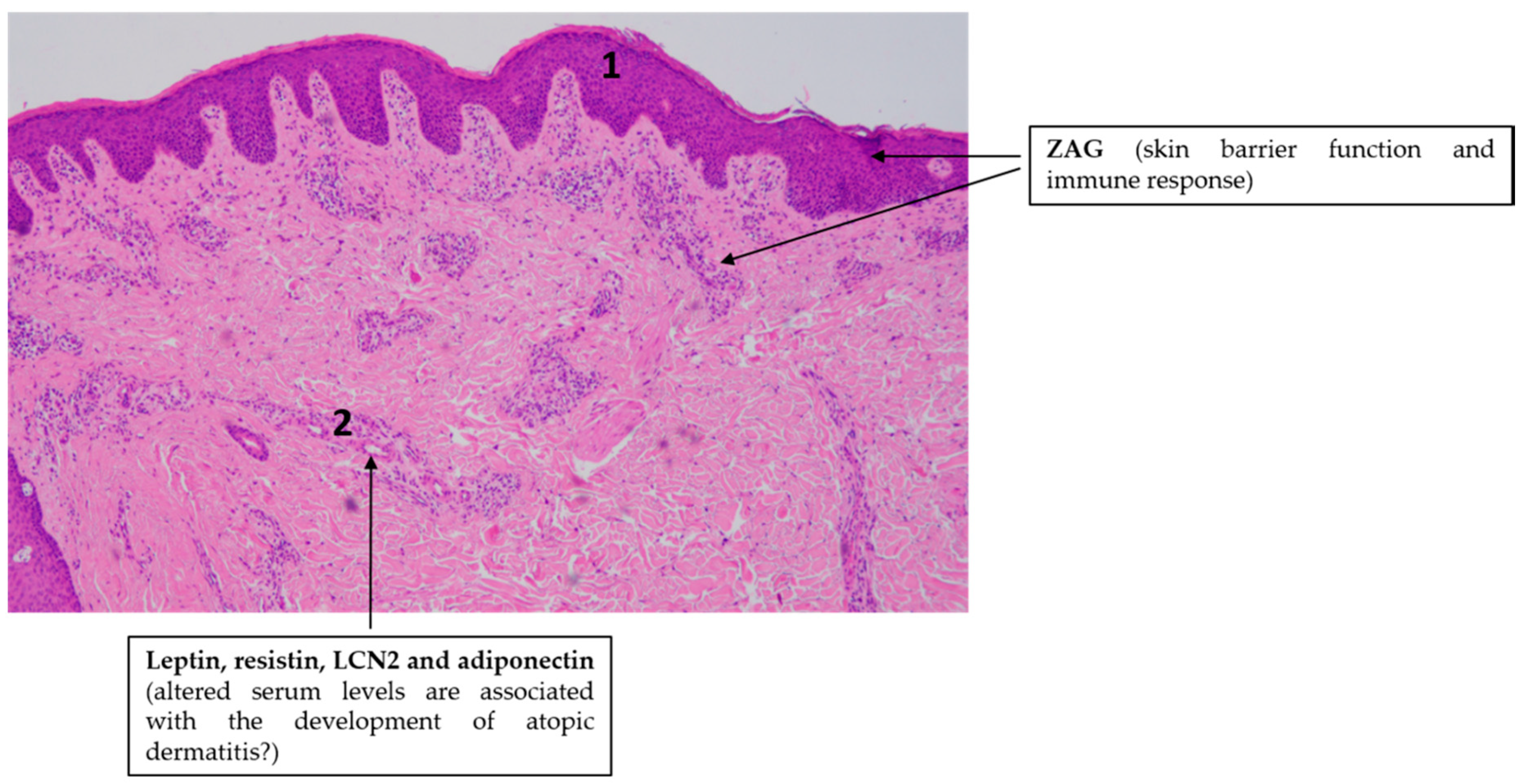
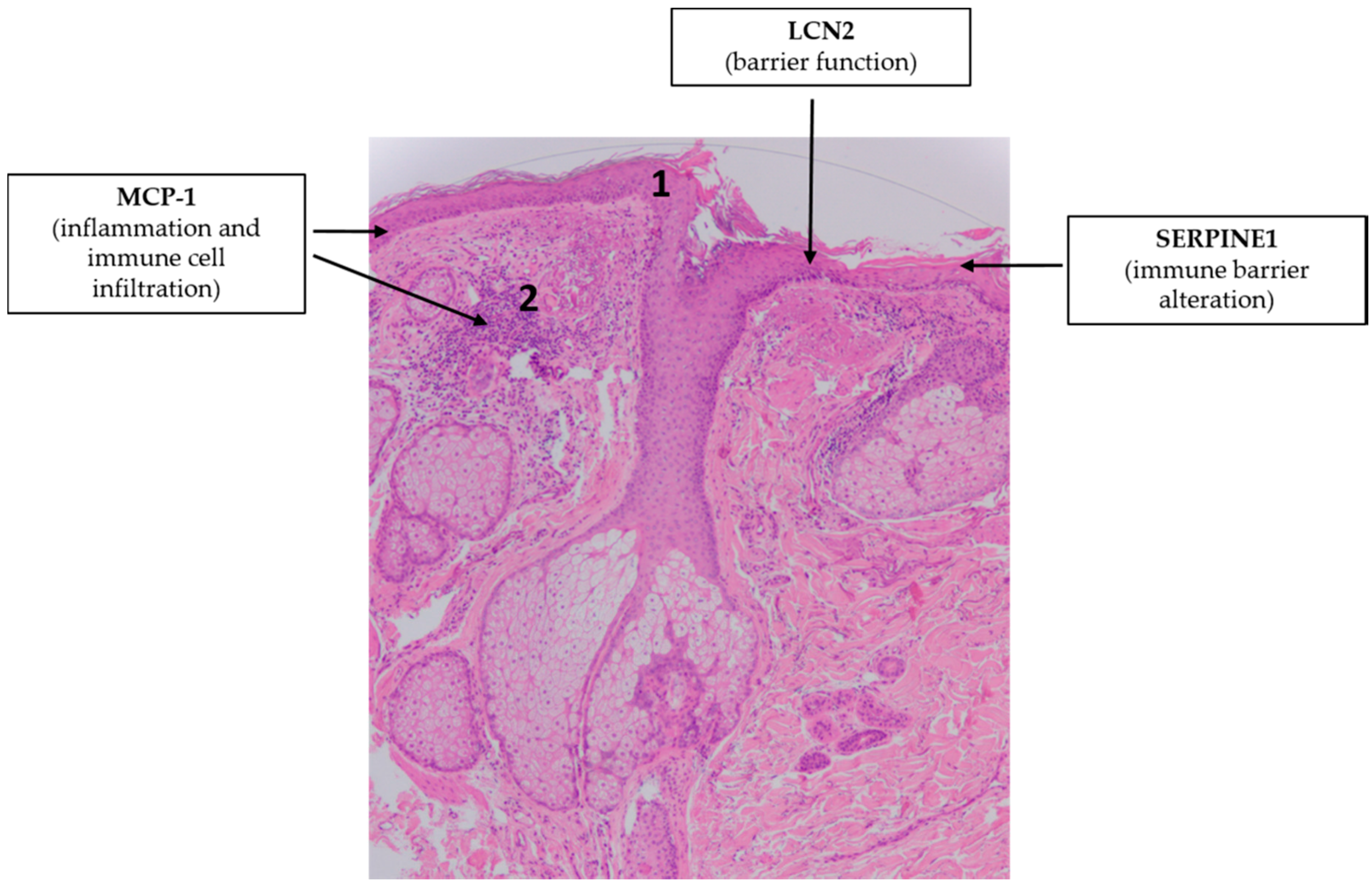
4.2. Atopic Dermatitis (AD)
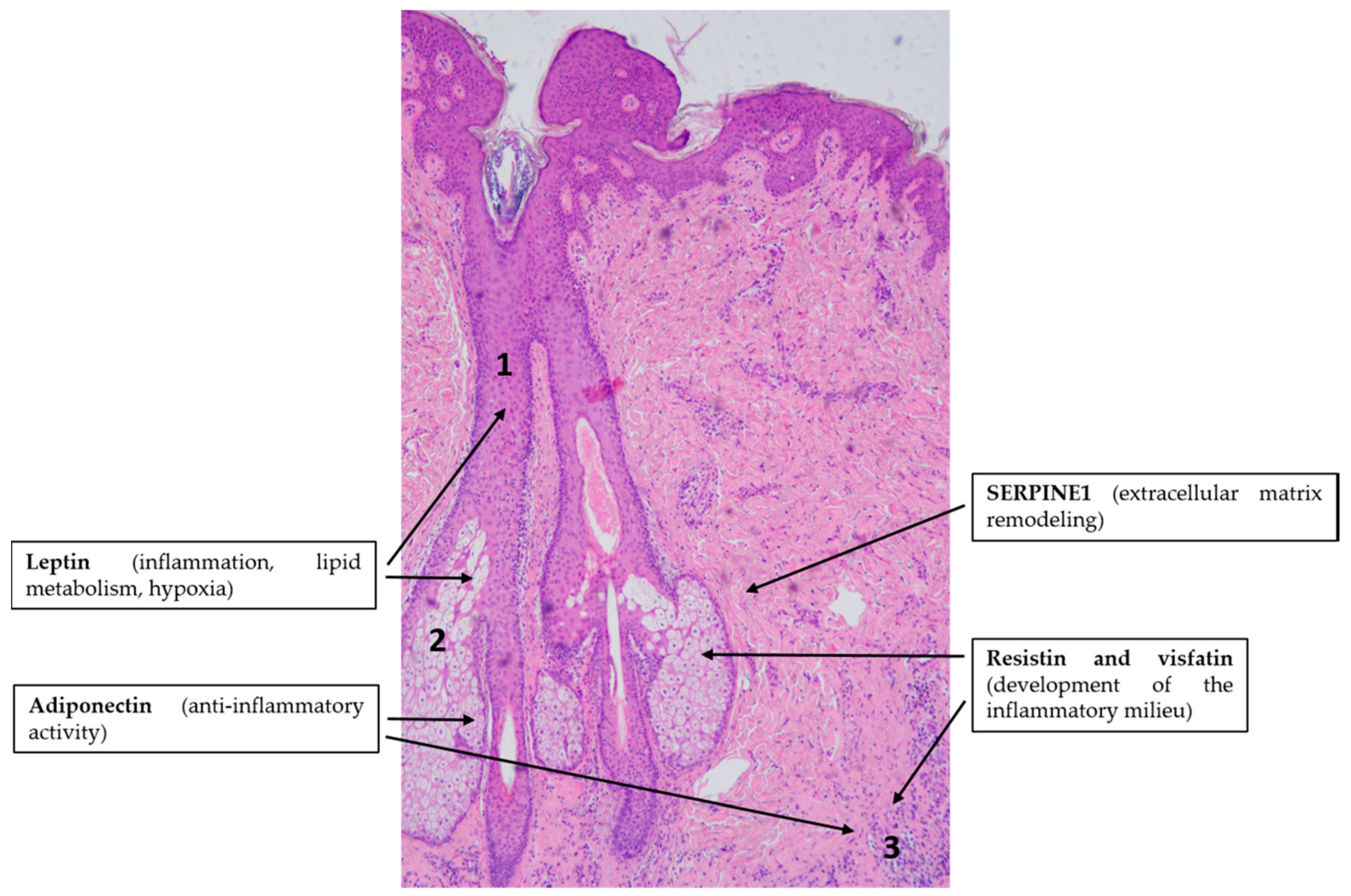
4.3. Acne Vulgaris
4.4. Rosacea
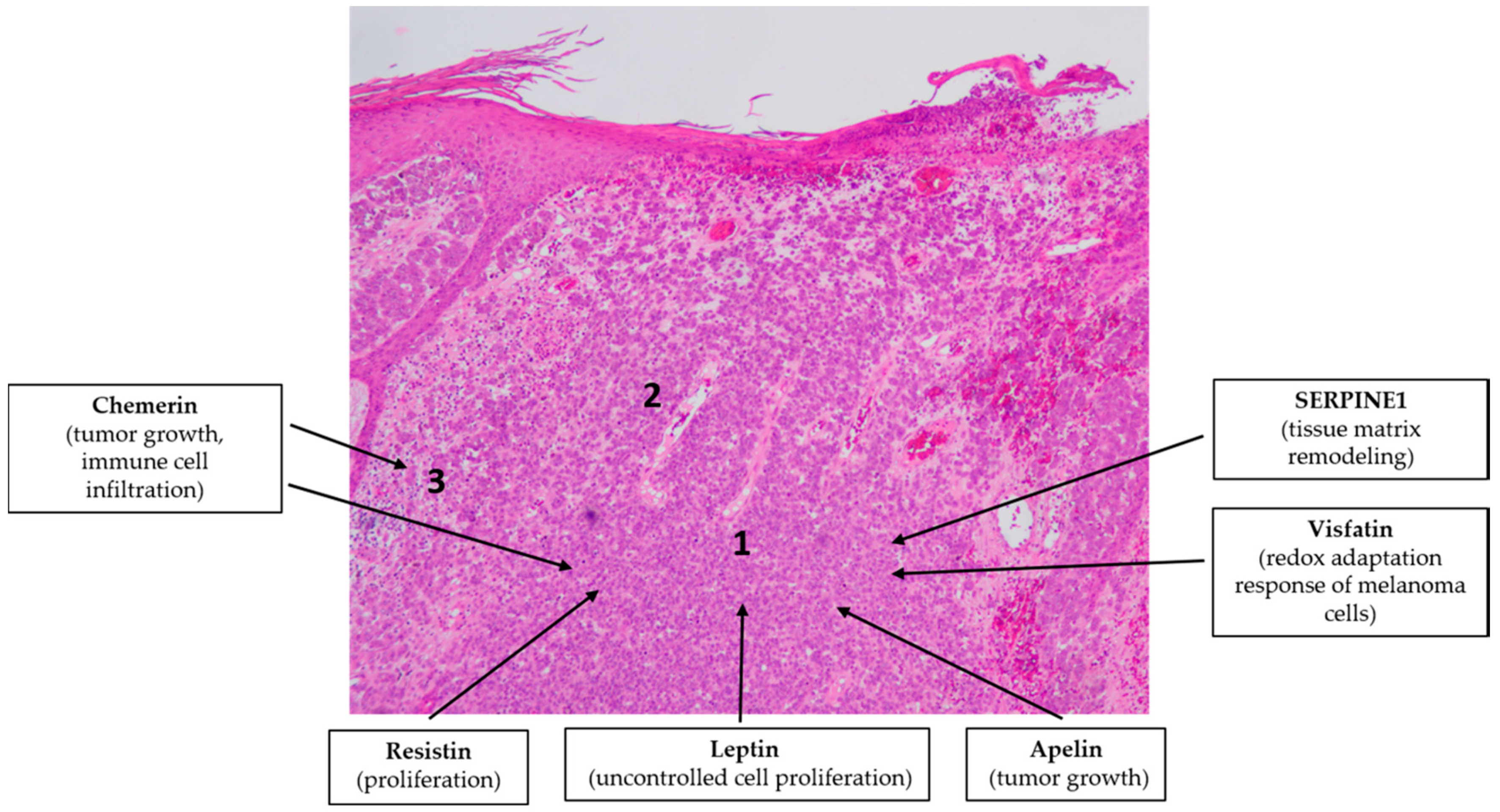
4.5. Malignant Melanoma
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-LOX | 5-lipoxygenase |
| AA | Alopecia areata |
| ACRP/AdipoQ | Adiponectin |
| AD | Atopic dermatitis |
| ADIPOR | Adiponectin receptor |
| AGA | Androgenetic alopecia |
| Akt | Protein kinase B |
| AMPK | AMP-activated protein kinase |
| APJ | Apelin receptor |
| BMI | Body mass index |
| BRAF | B-Raf proto-oncogene serine/threonine kinase |
| C/EBP | CCAAT/enhancer-binding protein |
| CCL2 | C-C motif ligand 2 |
| CMKLR1 | Chemerin chemokine-like receptor 1 |
| COX-2 | Cyclooxygenase-2 |
| CRTCs | CREB-regulated transcription co-activators |
| CXCL | (C-X-C motif) ligand |
| DC | Dendritic cell |
| DGAT | Diglyceride acyltransferase |
| DP | Dermal papilla |
| DMBPA/TPA | Dimethylbenz[a]anthracene/12-O tetradecanoylphorbol-13-acetate |
| DWAT | Dermal white adipose tissue |
| EGF | Epidermal growth factor |
| EGFR | Epidermal growth factor receptor |
| ERK | Extracellular signal-regulated kinases |
| GPR1 | G protein-coupled receptor 1 |
| hBD-2 | Human β-defensin-2 |
| HF | Hair follicle |
| HIF-1α | Hypoxia-inducible factor 1-α |
| HS | Hidradenitis suppurativa |
| IFN-γ | Interferon-γ |
| IL | Interleukin |
| JAK2 | Janus kinase 2 |
| JNK | c-Jun N-terminal kinase |
| LCN2 | Lipocalin2 |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinase |
| MC1R | Melanocortin 1 receptor |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MEK | Mitogen-activated protein kinase kinase |
| MMP | Matrix metalloproteinase |
| MSH | Melanocyte stimulating hormone |
| mTORC1 | Mammalian target of rapamycin complex 1 |
| NAMPT | Nicotinamide phosphoribosyltransferase |
| NF-κB | Nuclear factor kappa-β |
| NGAL | Neutrophil gelatinase–associated lipocalin |
| NHK | Normal human keratinocyte |
| NK cell | Natural killer cell |
| Ob-Rb | Leptin receptor |
| PAI-1 | Plasminogen activator inhibitor type-1 |
| PASI | Psoriasis area and severity index |
| PBEF | Pre-B-cell colony-enhancing factor |
| PI3K | Phosphatidylinositol 3-kinas |
| RARRES2 | Retinoic acid receptor responder 2 |
| RBP | Retinol-binding protein |
| ROS | Reactive oxygen species |
| SCORAD | SCORing Atopic Dermatitis |
| SERPIN | Serine protease inhibitor |
| SG | Sebaceous gland |
| SNP | Single nucleotide polymorphism |
| SOCS-3 | Suppressor of cytokine signaling 3 |
| SREBP1 | Sterol regulatory element binding protein 1 |
| STAT3 | Signal transducer and activator of transcription factor 3 |
| STRA6 | Signaling receptor and transporter of retinol STRA6 |
| SZ95 | Human sebaceous gland cell line |
| Tcf | Transcription factor |
| TGFβ | Transforming growth factor β |
| Th | T helper cell |
| TIG2 | Tazarotene-induced gene 2 protein |
| TLR | Toll-like receptor |
| TNF-α | Tumor necrosis factor-α |
| UVB | Ultraviolet B |
| VEGF | Vascular endothelial growth factor; |
| WAT | White adipose tissue |
| ZAG | Zinc α2-Glycoprotein |
References
- Stolarczyk, E. Adipose tissue inflammation in obesity: A metabolic or immune response? Curr. Opin. Pharmacol. 2017, 37, 35–40. [Google Scholar] [CrossRef]
- Mittal, B. Subcutaneous adipose tissue & visceral adipose tissue. Indian J. Med. Res. 2019, 149, 571–573. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2010, 11, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Fasshauer, M.; Bluher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Reilly, S.M.; Saltiel, A.R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 2017, 13, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Atawia, R.T.; Bunch, K.L.; Toque, H.A.; Caldwell, R.B.; Caldwell, R.W. Mechanisms of obesity-induced metabolic and vascular dysfunctions. Front. Biosci. (Landmark Ed.) 2019, 24, 890–934. [Google Scholar]
- Jialal, I.; Devaraj, S. Subcutaneous adipose tissue biology in metabolic syndrome. Horm. Mol. Biol. Clin. Investig. 2018, 33. [Google Scholar] [CrossRef]
- Burhans, M.S.; Hagman, D.K.; Kuzma, J.N.; Schmidt, K.A.; Kratz, M. Contribution of Adipose Tissue Inflammation to the Development of Type 2 Diabetes Mellitus. Compr. Physiol. 2018, 9, 1–58. [Google Scholar] [CrossRef]
- Giralt, M.; Cereijo, R.; Villarroya, F. Adipokines and the Endocrine Role of Adipose Tissues. Handb. Exp. Pharmacol. 2016, 233, 265–282. [Google Scholar] [CrossRef]
- Raucci, R.; Rusolo, F.; Sharma, A.; Colonna, G.; Castello, G.; Costantini, S. Functional and structural features of adipokine family. Cytokine 2013, 61, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, N.; Falcão-Pires, I.; Leite-Moreira, A.F. Adipokines and their receptors: Potential new targets in cardiovascular diseases. Future Med. Chem. 2015, 7, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Gorska, E.; Popko, K.; Stelmaszczyk-Emmel, A.; Ciepiela, O.; Kucharska, A.; Wasik, M. Leptin receptors. Eur. J. Med. Res. 2010, 15 (Suppl. 2), 50–54. [Google Scholar] [CrossRef] [PubMed]
- Banas, M.; Zegar, A.; Kwitniewski, M.; Zabieglo, K.; Marczynska, J.; Kapinska-Mrowiecka, M.; LaJevic, M.; Zabel, B.A.; Cichy, J. The expression and regulation of chemerin in the epidermis. PLoS ONE 2015, 10, e0117830. [Google Scholar] [CrossRef] [PubMed]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Wysocka, M.B.; Pietraszek-Gremplewicz, K.; Nowak, D. The Role of Apelin in Cardiovascular Diseases, Obesity and Cancer. Front. Physiol. 2018, 9, 557. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Kwak, M.K.; Kim, H.J.; Ahima, R.S. Linking resistin, inflammation, and cardiometabolic diseases. Korean J. Intern. Med. 2017, 32, 239–247. [Google Scholar] [CrossRef]
- Romacho, T.; Valencia, I.; Ramos-González, M.; Vallejo, S.; López-Esteban, M.; Lorenzo, O.; Cannata, P.; Romero, A.; San Hipólito-Luengo, A.; Gómez-Cerezo, J.F.; et al. Visfatin/eNampt induces endothelial dysfunction in vivo: A role for Toll-Like Receptor 4 and NLRP3 inflammasome. Sci. Rep. 2020, 10, 5386. [Google Scholar] [CrossRef]
- Trepanowski, J.F.; Mey, J.; Varady, K.A. Fetuin-A: A novel link between obesity and related complications. Int. J. Obes. 2015, 39, 734–741. [Google Scholar] [CrossRef]
- Tanabe, H.; Fujii, Y.; Okada-Iwabu, M.; Iwabu, M.; Nakamura, Y.; Hosaka, T.; Motoyama, K.; Ikeda, M.; Wakiyama, M.; Terada, T.; et al. Crystal structures of the human adiponectin receptors. Nature 2015, 520, 312–316. [Google Scholar] [CrossRef]
- Ceperuelo-Mallafre, V.; Ejarque, M.; Duran, X.; Pachon, G.; Vazquez-Carballo, A.; Roche, K.; Nunez-Roa, C.; Garrido-Sanchez, L.; Tinahones, F.J.; Vendrell, J.; et al. Zinc-alpha2-Glycoprotein Modulates AKT-Dependent Insulin Signaling in Human Adipocytes by Activation of the PP2A Phosphatase. PLoS ONE 2015, 10, e0129644. [Google Scholar] [CrossRef] [PubMed]
- Rahman, F.A.; Krause, M.P. PAI-1, the Plasminogen System, and Skeletal Muscle. Int. J. Mol. Sci. 2020, 21, 7066. [Google Scholar] [CrossRef] [PubMed]
- Langelueddecke, C.; Roussa, E.; Fenton, R.A.; Thévenod, F. Expression and function of the lipocalin-2 (24p3/NGAL) receptor in rodent and human intestinal epithelia. PLoS ONE 2013, 8, e71586. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, B.; Hao, C.; Huang, X.; Li, X.; Huang, Y.; Luo, Z. Omentin-A Novel Adipokine in Respiratory Diseases. Int. J. Mol. Sci. 2017, 19, 73. [Google Scholar] [CrossRef]
- Zhang, Y.; Chua, S., Jr. Leptin Function and Regulation. Compr. Physiol. 2017, 8, 351–369. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, P. The role of adipokines in chronic inflammation. Immunotargets Ther. 2016, 5, 47–56. [Google Scholar] [CrossRef]
- Francisco, V.; Pino, J.; Gonzalez-Gay, M.A.; Mera, A.; Lago, F.; Gómez, R.; Mobasheri, A.; Gualillo, O. Adipokines and inflammation: Is it a question of weight? Br. J. Pharmacol. 2018, 175, 1569–1579. [Google Scholar] [CrossRef]
- Wolk, K.; Sabat, R. Adipokines in psoriasis: An important link between skin inflammation and metabolic alterations. Rev. Endocr. Metab. Disord. 2016, 17, 305–317. [Google Scholar] [CrossRef]
- Lynch, M.; Ahern, T.; Sweeney, C.M.; Malara, A.; Tobin, A.M.; O’Shea, D.; Kirby, B. Adipokines, psoriasis, systemic inflammation, and endothelial dysfunction. Int. J. Dermatol. 2017, 56, 1103–1118. [Google Scholar] [CrossRef]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef]
- Pan, W.W.; Myers, M.G., Jr. Leptin and the maintenance of elevated body weight. Nat. Rev. Neurosci. 2018, 19, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllou, G.A.; Paschou, S.A.; Mantzoros, C.S. Leptin and Hormones: Energy Homeostasis. Endocrinol. Metab. Clin. 2016, 45, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Tong, K.M.; Shieh, D.C.; Chen, C.P.; Tzeng, C.Y.; Wang, S.P.; Huang, K.C.; Chiu, Y.C.; Fong, Y.C.; Tang, C.H. Leptin induces IL-8 expression via leptin receptor, IRS-1, PI3K, Akt cascade and promotion of NF-kappaB/p300 binding in human synovial fibroblasts. Cell. Signal. 2008, 20, 1478–1488. [Google Scholar] [CrossRef]
- Palhinha, L.; Liechocki, S.; Hottz, E.D.; Pereira, J.; de Almeida, C.J.; Moraes-Vieira, P.M.M.; Bozza, P.T.; Maya-Monteiro, C.M. Leptin Induces Proadipogenic and Proinflammatory Signaling in Adipocytes. Front. Endocrinol. 2019, 10, 841. [Google Scholar] [CrossRef] [PubMed]
- Poeggeler, B.; Schulz, C.; Pappolla, M.A.; Bodó, E.; Tiede, S.; Lehnert, H.; Paus, R. Leptin and the skin: A new frontier. Exp. Dermatol. 2010, 19, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Lee, E.; Jin, S.H.; Ahn, S.; Kim, S.O.; Kim, J.; Choi, D.; Lim, K.M.; Lee, S.T.; Noh, M. Leptin regulates the pro-inflammatory response in human epidermal keratinocytes. Arch. Dermatol. Res. 2018, 310, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Kanda, N.; Watanabe, S. Leptin enhances human beta-defensin-2 production in human keratinocytes. Endocrinology 2008, 149, 5189–5198. [Google Scholar] [CrossRef]
- Rico, L.; Del Rio, M.; Bravo, A.; Ramirez, A.; Jorcano, J.L.; Page, M.A.; Larcher, F. Targeted overexpression of leptin to keratinocytes in transgenic mice results in lack of skin phenotype but induction of early leptin resistance. Endocrinology 2005, 146, 4167–4176. [Google Scholar] [CrossRef][Green Version]
- Samal, B.; Sun, Y.; Stearns, G.; Xie, C.; Suggs, S.; McNiece, I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol. Cell Biol. 1994, 14, 1431–1437. [Google Scholar] [CrossRef]
- Lee, B.C.; Song, J.; Lee, A.; Cho, D.; Kim, T.S. Visfatin Promotes Wound Healing through the Activation of ERK1/2 and JNK1/2 Pathway. Int. J. Mol. Sci. 2018, 19, 3642. [Google Scholar] [CrossRef]
- Kanda, N.; Hau, C.S.; Tada, Y.; Tatsuta, A.; Sato, S.; Watanabe, S. Visfatin enhances CXCL8, CXCL10, and CCL20 production in human keratinocytes. Endocrinology 2011, 152, 3155–3164. [Google Scholar] [CrossRef] [PubMed]
- Hau, C.S.; Kanda, N.; Noda, S.; Tatsuta, A.; Kamata, M.; Shibata, S.; Asano, Y.; Sato, S.; Watanabe, S.; Tada, Y. Visfatin enhances the production of cathelicidin antimicrobial peptide, human beta-defensin-2, human beta-defensin-3, and S100A7 in human keratinocytes and their orthologs in murine imiquimod-induced psoriatic skin. Am. J. Pathol. 2013, 182, 1705–1717. [Google Scholar] [CrossRef] [PubMed]
- Managò, A.; Audrito, V.; Mazzola, F.; Sorci, L. Extracellular nicotinate phosphoribosyltransferase binds Toll like receptor 4 and mediates inflammation. Nat. Commun. 2019, 10, 4116. [Google Scholar] [CrossRef] [PubMed]
- Travelli, C.; Colombo, G.; Mola, S.; Genazzani, A.A.; Porta, C. NAMPT: A pleiotropic modulator of monocytes and macrophages. Pharm. Res. 2018, 135, 25–36. [Google Scholar] [CrossRef] [PubMed]
- La Manna, G.; Ghinatti, G.; Tazzari, P.L.; Alviano, F.; Ricci, F.; Capelli, I.; Cuna, V.; Todeschini, P.; Brunocilla, E.; Pagliaro, P.; et al. Neutrophil gelatinase-associated lipocalin increases HLA-G(+)/FoxP3(+) T-regulatory cell population in an in vitro model of PBMC. PLoS ONE 2014, 9, e89497. [Google Scholar] [CrossRef]
- Mallbris, L.; O’Brien, K.P.; Hulthén, A.; Sandstedt, B.; Cowland, J.B.; Borregaard, N.; Ståhle-Bäckdahl, M. Neutrophil gelatinase-associated lipocalin is a marker for dysregulated keratinocyte differentiation in human skin. Exp. Dermatol. 2002, 11, 584–591. [Google Scholar] [CrossRef]
- Wolk, K.; Wenzel, J.; Tsaousi, A.; Witte-Händel, E.; Babel, N.; Zelenak, C.; Volk, H.D.; Sterry, W.; Schneider-Burrus, S.; Sabat, R. Lipocalin-2 is expressed by activated granulocytes and keratinocytes in affected skin and reflects disease activity in acne inversa/hidradenitis suppurativa. Br. J. Dermatol. 2017, 177, 1385–1393. [Google Scholar] [CrossRef]
- Shao, S.; Cao, T.; Jin, L.; Li, B.; Fang, H.; Zhang, J.; Zhang, Y.; Hu, J.; Wang, G. Increased Lipocalin-2 Contributes to the Pathogenesis of Psoriasis by Modulating Neutrophil Chemotaxis and Cytokine Secretion. J. Investig. Dermatol. 2016, 136, 1418–1428. [Google Scholar] [CrossRef]
- Béke, G.; Dajnoki, Z.; Kapitány, A.; Gáspár, K.; Medgyesi, B.; Póliska, S.; Hendrik, Z.; Péter, Z.; Törőcsik, D.; Bíró, T.; et al. Immunotopographical Differences of Human Skin. Front. Immunol. 2018, 9, 424. [Google Scholar] [CrossRef]
- Flevaris, P.; Vaughan, D. The Role of Plasminogen Activator Inhibitor Type-1 in Fibrosis. Semin. Thromb. Hemost. 2017, 43, 169–177. [Google Scholar] [CrossRef]
- Rømer, J.; Lund, L.R.; Eriksen, J.; Ralfkiaer, E.; Zeheb, R.; Gelehrter, T.D.; Danø, K.; Kristensen, P. Differential expression of urokinase-type plasminogen activator and its type-1 inhibitor during healing of mouse skin wounds. J. Investig. Dermatol. 1991, 97, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Lund, L.R.; Eriksen, J.; Ralfkiaer, E.; Rømer, J. Differential expression of urokinase-type plasminogen activator, its receptor, and inhibitors in mouse skin after exposure to a tumor-promoting phorbol ester. J. Investig. Dermatol. 1996, 106, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Jacenik, D.; Fichna, J. Chemerin in immune response and gastrointestinal pathophysiology. Clin. Chim. Acta Int. J. Clin. Chem. 2020, 504, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huo, J.; Zhang, D.; Hu, G.; Zhang, Y. Chemerin/ChemR23 axis triggers an inflammatory response in keratinocytes through ROS-sirt1-NF-kappaB signaling. J. Cell. Biochem. 2019, 120, 6459–6470. [Google Scholar] [CrossRef] [PubMed]
- Dubois-Vedrenne, I.; De Henau, O.; Robert, V.; Langa, F.; Javary, J.; Al Delbany, D.; Vosters, O.; Angelats-Canals, E.; Vernimmen, M.; Luangsay, S.; et al. Expression of Bioactive Chemerin by Keratinocytes Inhibits Late Stages of Tumor Development in a Chemical Model of Skin Carcinogenesis. Front. Oncol. 2019, 9, 1253. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Skiba, D.S.; Touyz, R.M.; Harrison, D.G. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc. Res. 2017, 113, 1009–1023. [Google Scholar] [CrossRef] [PubMed]
- Mai, W.; Lu, D.; Liu, X.; Chen, L. MCP-1 produced by keratinocytes is associated with leucocyte recruitment during elicitation of nickel-induced occupational allergic contact dermatitis. Toxicol. Ind. Health 2018, 34, 36–43. [Google Scholar] [CrossRef]
- Li, J.; Farthing, P.M.; Thornhill, M.H. Oral and skin keratinocytes are stimulated to secrete monocyte chemoattractant protein-1 by tumour necrosis factor-alpha and interferon-gamma. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2000, 29, 438–444. [Google Scholar] [CrossRef]
- Giustizieri, M.L.; Mascia, F.; Frezzolini, A.; De Pità, O.; Chinni, L.M.; Giannetti, A.; Girolomoni, G.; Pastore, S. Keratinocytes from patients with atopic dermatitis and psoriasis show a distinct chemokine production profile in response to T cell-derived cytokines. J. Allergy Clin. Immunol. 2001, 107, 871–877. [Google Scholar] [CrossRef]
- Lee, W.J.; Jo, S.Y.; Lee, M.H.; Won, C.H.; Lee, M.W.; Choi, J.H.; Chang, S.E. The Effect of MCP-1/CCR2 on the Proliferation and Senescence of Epidermal Constituent Cells in Solar Lentigo. Int. J. Mol. Sci. 2016, 17, 948. [Google Scholar] [CrossRef]
- Yamashiro, S.; Takeya, M.; Kuratsu, J.; Ushio, Y.; Takahashi, K.; Yoshimura, T. Intradermal injection of monocyte chemoattractant protein-1 induces emigration and differentiation of blood monocytes in rat skin. Int. Arch. Allergy Immunol. 1998, 115, 15–23. [Google Scholar] [CrossRef]
- Nakamura, K.; Williams, I.R.; Kupper, T.S. Keratinocyte-derived monocyte chemoattractant protein 1 (MCP-1): Analysis in a transgenic model demonstrates MCP-1 can recruit dendritic and Langerhans cells to skin. J. Investig. Dermatol. 1995, 105, 635–643. [Google Scholar] [CrossRef]
- Huang, X.; Yang, Z. Resistin’s, obesity and insulin resistance: The continuing disconnect between rodents and humans. J. Endocrinol. Investig. 2016, 39, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Harrison, W.J.; Bull, J.J.; Seltmann, H.; Zouboulis, C.C.; Philpott, M.P. Expression of lipogenic factors galectin-12, resistin, SREBP-1, and SCD in human sebaceous glands and cultured sebocytes. J. Investig. Dermatol. 2007, 127, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Lee, B.; Puan, K.J.; Lee, W.; Luis, B.S.; Yusof, N.; Andiappan, A.K.; Del Rosario, R. Resistin expression in human monocytes is controlled by two linked promoter SNPs mediating NFKB p50/p50 binding and C-methylation. Sci. Rep. 2019, 9, 15245. [Google Scholar] [CrossRef]
- Kelly, M.; Widjaja-Adhi, M.A.; Palczewski, G.; von Lintig, J. Transport of vitamin A across blood-tissue barriers is facilitated by STRA6. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2016, 30, 2985–2995. [Google Scholar] [CrossRef]
- Skazik, C.; Amann, P.M.; Heise, R.; Marquardt, Y.; Czaja, K.; Kim, A.; Rühl, R.; Kurschat, P.; Merk, H.F.; Bickers, D.R.; et al. Downregulation of STRA6 expression in epidermal keratinocytes leads to hyperproliferation-associated differentiation in both in vitro and in vivo skin models. J. Investig. Dermatol. 2014, 134, 1579–1588. [Google Scholar] [CrossRef]
- Kidoya, H.; Naito, H.; Takakura, N. Apelin induces enlarged and nonleaky blood vessels for functional recovery from ischemia. Blood 2010, 115, 3166–3174. [Google Scholar] [CrossRef]
- Lv, S.Y.; Cui, B.; Chen, W.D.; Wang, Y.D. Apelin/APJ system: A key therapeutic target for liver disease. Oncotarget 2017, 8, 112145–112151. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, H.J.; Lim, Y.; Yang, S.J. Involvement of resveratrol in crosstalk between adipokine adiponectin and hepatokine fetuin-A in vivo and in vitro. J. Nutr. Biochem. 2015, 26, 1254–1260. [Google Scholar] [CrossRef]
- Wang, X.Q.; Hung, B.S.; Kempf, M.; Liu, P.Y.; Dalley, A.J.; Saunders, N.A.; Kimble, R.M. Fetuin-A promotes primary keratinocyte migration: Independent of epidermal growth factor receptor signalling. Exp. Dermatol. 2010, 19, e289–e292. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.H.; Wang, Y.D.; Qi, X.Y.; Wang, Y.Y.; Li, J.Y.; Li, H.; Zhang, P.Y.; Liao, H.L.; Li, M.H.; Liao, Z.Z.; et al. Zinc alpha2 glycoprotein protects against obesity-induced hepatic steatosis. Int. J. Obes. 2018, 42, 1418–1430. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Arany, I.; Apisarnthanarax, N.; Rajaraman, S.; Tyring, S.K.; Horikoshi, T.; Brysk, H.; Brysk, M.M. Response of keratinocytes from normal and psoriatic epidermis to interferon-gamma differs in the expression of zinc-alpha(2)-glycoprotein and cathepsin D. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2000, 14, 565–571. [Google Scholar] [CrossRef]
- Wang, Z.V.; Scherer, P.E. Adiponectin, the past two decades. J. Mol. Cell Biol. 2016, 8, 93–100. [Google Scholar] [CrossRef]
- Bjursell, M.; Ahnmark, A.; Bohlooly, Y.M.; William-Olsson, L.; Rhedin, M.; Peng, X.R.; Ploj, K.; Gerdin, A.K.; Arnerup, G.; Elmgren, A.; et al. Opposing effects of adiponectin receptors 1 and 2 on energy metabolism. Diabetes 2007, 56, 583–593. [Google Scholar] [CrossRef]
- Ouchi, N.; Kihara, S.; Arita, Y.; Nishida, M.; Matsuyama, A.; Okamoto, Y.; Ishigami, M.; Kuriyama, H.; Kishida, K.; Nishizawa, H.; et al. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation 2001, 103, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Argueta, J.G.; Masuhiro, Y.; Kagishita, M.; Nonaka, K.; Saito, T.; Hanazawa, S.; Yamashita, Y. Adiponectin inhibits Toll-like receptor family-induced signaling. FEBS Lett. 2005, 579, 6821–6826. [Google Scholar] [CrossRef]
- Wolf, A.M.; Wolf, D.; Rumpold, H.; Enrich, B.; Tilg, H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem. Biophys. Res. Commun. 2004, 323, 630–635. [Google Scholar] [CrossRef]
- Won, C.H.; Yoo, H.G.; Park, K.Y.; Shin, S.H.; Park, W.S.; Park, P.J.; Chung, J.H.; Kwon, O.S.; Kim, K.H. Hair growth-promoting effects of adiponectin in vitro. J. Investig. Dermatol. 2012, 132, 2849–2851. [Google Scholar] [CrossRef]
- Shibata, S.; Tada, Y.; Asano, Y.; Hau, C.S.; Kato, T.; Saeki, H.; Yamauchi, T.; Kubota, N.; Kadowaki, T.; Sato, S. Adiponectin regulates cutaneous wound healing by promoting keratinocyte proliferation and migration via the ERK signaling pathway. J. Immunol. 2012, 189, 3231–3241. [Google Scholar] [CrossRef]
- Hong, S.P.; Seo, H.S.; Shin, K.O.; Park, K.; Park, B.C.; Kim, M.H.; Park, M.; Kim, C.D.; Seo, S.J. Adiponectin Enhances Human Keratinocyte Lipid Synthesis via SIRT1 and Nuclear Hormone Receptor Signaling. J. Investig. Dermatol. 2019, 139, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Kageyama, A.; Tsumano, T.; Nishimoto, S.; Fukuda, K.; Yokoyama, S.; Oguma, T.; Fujita, K.; Yoshimoto, S.; Yanai, A.; et al. Effects of adiponectin on growth and differentiation of human keratinocytes--implication of impaired wound healing in diabetes. Biochem. Biophys. Res. Commun. 2008, 374, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Park, K.Y.; Lee, M.K.; Jin, T.; Seo, S.J. Adiponectin Suppresses UVB-Induced Premature Senescence and hBD2 Overexpression in Human Keratinocytes. PLoS ONE 2016, 11, e0161247. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Park, K.Y.; Seo, S.J. Adiponectin Upregulates Filaggrin Expression via SIRT1-Mediated Signaling in Human Normal Keratinocytes. Ann. Dermatol. 2017, 29, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhu, K.J.; Liu, J.L.; Xu, G.X.; Liu, W.; Jiang, F.X.; Zheng, H.F.; Quan, C. Omentin-1 plasma levels and omentin-1 expression are decreased in psoriatic lesions of psoriasis patients. Arch. Dermatol. Res. 2015, 307, 455–459. [Google Scholar] [CrossRef]
- Saalbach, A.; Vester, K.; Rall, K.; Tremel, J.; Anderegg, U.; Beck-Sickinger, A.G.; Blüher, M.; Simon, J.C. Vaspin--a link of obesity and psoriasis? Exp. Dermatol. 2012, 21, 309–312. [Google Scholar] [CrossRef]
- Saalbach, A.; Tremel, J.; Herbert, D.; Schwede, K.; Wandel, E.; Schirmer, C.; Anderegg, U.; Beck-Sickinger, A.G.; Heiker, J.T.; Schultz, S.; et al. Anti-Inflammatory Action of Keratinocyte-Derived Vaspin: Relevance for the Pathogenesis of Psoriasis. Am. J. Pathol. 2016, 186, 639–651. [Google Scholar] [CrossRef]
- Di Carlo, S.E.; Peduto, L. The perivascular origin of pathological fibroblasts. J. Clin. Investig. 2018, 128, 54–63. [Google Scholar] [CrossRef]
- Chen, J.H.; Goh, K.J.; Rocha, N.; Groeneveld, M.P. Evaluation of human dermal fibroblasts directly reprogrammed to adipocyte-like cells as a metabolic disease model. Dis. Models Mech. 2017, 10, 1411–1420. [Google Scholar] [CrossRef]
- Glasow, A.; Kiess, W.; Anderegg, U.; Berthold, A.; Bottner, A.; Kratzsch, J. Expression of leptin (Ob) and leptin receptor (Ob-R) in human fibroblasts: Regulation of leptin secretion by insulin. J. Clin. Endocrinol. Metab. 2001, 86, 4472–4479. [Google Scholar] [CrossRef]
- Ambrosini, G.; Nath, A.K.; Sierra-Honigmann, M.R.; Flores-Riveros, J. Transcriptional activation of the human leptin gene in response to hypoxia. Involvement of hypoxia-inducible factor 1. J. Biol. Chem. 2002, 277, 34601–34609. [Google Scholar] [CrossRef] [PubMed]
- Ezure, T.; Amano, S. Adiponectin and leptin up-regulate extracellular matrix production by dermal fibroblasts. Biofactors 2007, 31, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Li, J.; Liu, H.; Jian, X.; Zou, Q.; Zhao, Q.; Le, Q.; Chen, H.; Gao, X.; He, C. Adiponectin Is Involved in Connective Tissue Growth Factor-Induced Proliferation, Migration and Overproduction of the Extracellular Matrix in Keloid Fibroblasts. Int. J. Mol. Sci. 2017, 18, 1044. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.L.; Huang, L.H.; Tsai, H.Y.; Chang, H.I. Dermal Lipogenesis Inhibits Adiponectin Production in Human Dermal Fibroblasts while Exogenous Adiponectin Administration Prevents against UVA-Induced Dermal Matrix Degradation in Human Skin. Int. J. Mol. Sci. 2016, 17, 1129. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Liu, L.; Yang, Y.; Tamaki, Z.; Wei, J.; Marangoni, R.G.; Bhattacharyya, S.; Summer, R.S.; Ye, B.; Varga, J. The adipokine adiponectin has potent anti-fibrotic effects mediated via adenosine monophosphate-activated protein kinase: Novel target for fibrosis therapy. Arthritis Res. Ther. 2012, 14, R229. [Google Scholar] [CrossRef] [PubMed]
- Farsam, V.; Basu, A.; Gatzka, M.; Treiber, N.; Schneider, L.A.; Mulaw, M.A.; Lucas, T.; Kochanek, S.; Dummer, R.; Levesque, M.P.; et al. Senescent fibroblast-derived Chemerin promotes squamous cell carcinoma migration. Oncotarget 2016, 7, 83554–83569. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Sekiguchi, A.; Fujiwara, C.; Uchiyama, A.; Uehara, A.; Ogino, S.; Torii, R.; Ishikawa, O.; Motegi, S.I. Inhibitory Regulation of Skin Fibrosis in Systemic Sclerosis by Apelin/APJ Signaling. Arthritis Rheumatol. 2018, 70, 1661–1672. [Google Scholar] [CrossRef]
- He, Z.; Ong, C.H.; Halper, J.; Bateman, A. Progranulin is a mediator of the wound response. Nat. Med. 2003, 9, 225–229. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Picardo, M.; Ju, Q.; Kurokawa, I.; Törőcsik, D.; Bíró, T.; Schneider, M.R. Beyond acne: Current aspects of sebaceous gland biology and function. Rev. Endocr. Metab. Disord. 2016, 17, 319–334. [Google Scholar] [CrossRef]
- Nagy, I.; Pivarcsi, A.; Kis, K.; Koreck, A.; Bodai, L.; McDowell, A.; Seltmann, H.; Patrick, S.; Zouboulis, C.C.; Kemény, L. Propionibacterium acnes and lipopolysaccharide induce the expression of antimicrobial peptides and proinflammatory cytokines/chemokines in human sebocytes. Microbes Infect. 2006, 8, 2195–2205. [Google Scholar] [CrossRef]
- Kovács, D.; Lovászi, M.; Póliska, S.; Oláh, A. Sebocytes differentially express and secrete adipokines. Exp. Dermatol. 2016, 25, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Törőcsik, D.; Kovács, D.; Camera, E.; Lovászi, M.; Cseri, K.; Nagy, G.G.; Molinaro, R.; Rühl, R.; Tax, G.; Szabó, K.; et al. Leptin promotes a proinflammatory lipid profile and induces inflammatory pathways in human SZ95 sebocytes. Br. J. Dermatol. 2014, 171, 1326–1335. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Smith, S.J.; Tow, B.; Elias, P.M.; Farese, R.V., Jr. Leptin modulates the effects of acyl CoA:diacylglycerol acyltransferase deficiency on murine fur and sebaceous glands. J. Clin. Investig. 2002, 109, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C. Is sebocyte-derived leptin the missing link between hyperseborrhea, ductal hypoxia, inflammation and comedogenesis in acne vulgaris? Exp. Dermatol. 2016, 25, 181–182. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.R.; Lee, J.H.; Sohn, K.C.; Lee, Y.; Seo, Y.J.; Kim, C.D.; Lee, J.H.; Hong, S.P.; Seo, S.J.; Kim, S.J.; et al. Adiponectin Signaling Regulates Lipid Production in Human Sebocytes. PLoS ONE 2017, 12, e0169824. [Google Scholar] [CrossRef]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef]
- Morpurgo, G.; Fioretti, B.; Catacuzzeno, L. The increased incidence of malignant melanoma in obese individuals is due to impaired melanogenesis and melanocyte DNA repair. Med. Hypotheses 2012, 78, 533–535. [Google Scholar] [CrossRef]
- Bang, S.; Won, K.H.; Moon, H.R.; Yoo, H.; Hong, A.; Song, Y. Novel regulation of melanogenesis by adiponectin via the AMPK/CRTC pathway. Pigment Cell Melanoma Res. 2017, 30, 553–557. [Google Scholar] [CrossRef]
- Chung, B.Y.; Noh, T.K.; Yang, S.H.; Kim, I.H.; Lee, M.W.; Yoon, T.J.; Chang, S.E. Gene expression profiling in melasma in Korean women. Dermatology 2014, 229, 333–342. [Google Scholar] [CrossRef]
- Hale, L.P. Zinc alpha-2-glycoprotein regulates melanin production by normal and malignant melanocytes. J. Investig. Dermatol. 2002, 119, 464–470. [Google Scholar] [CrossRef]
- Bagherani, N. The Newest Hypothesis about Vitiligo: Most of the Suggested Pathogeneses of Vitiligo Can Be Attributed to Lack of One Factor, Zinc-alpha2-Glycoprotein. ISRN Dermatol. 2012, 2012, 405268. [Google Scholar] [CrossRef] [PubMed]
- El-Rifaie, A.; Gohary, Y.M.; Abd-El Aziz, G.M.; Owies, F.O. Zinc-alpha2-Glycoprotein (ZAG): A New Deficiency in Vitiligo Patients. Skinmed 2019, 17, 248–253. [Google Scholar] [PubMed]
- Saxena, N.; Mok, K.W.; Rendl, M. An updated classification of hair follicle morphogenesis. Exp. Dermatol. 2019, 28, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.W.; Kloepper, J.; Langan, E.A.; Kim, Y.; Yeo, J.; Kim, M.J.; Hsi, T.C.; Rose, C.; Yoon, G.S.; Lee, S.J.; et al. A Guide to Studying Human Hair Follicle Cycling In Vivo. J. Investig. Dermatol. 2016, 136, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Paus, R.; Langan, E.A.; Vidali, S.; Ramot, Y.; Andersen, B. Neuroendocrinology of the hair follicle: Principles and clinical perspectives. Trends Mol. Med. 2014, 20, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Grymowicz, M.; Rudnicka, E. Hormonal Effects on Hair Follicles. Int. J. Mol. Sci. 2020, 21, 5342. [Google Scholar] [CrossRef] [PubMed]
- Tóth, K.F.; Ádám, D. Cannabinoid Signaling in the Skin: Therapeutic Potential of the “C(ut)annabinoid” System. Molecules 2019, 24, 918. [Google Scholar] [CrossRef]
- Iguchi, M.; Aiba, S.; Yoshino, Y.; Tagami, H. Human follicular papilla cells carry out nonadipose tissue production of leptin. J. Investig. Dermatol. 2001, 117, 1349–1356. [Google Scholar] [CrossRef]
- Sumikawa, Y.; Inui, S.; Nakajima, T.; Itami, S. Hair cycle control by leptin as a new anagen inducer. Exp. Dermatol. 2014, 23, 27–32. [Google Scholar] [CrossRef]
- Tiede, S.; Kloepper, J.E.; Ernst, N.; Poeggeler, B.; Kruse, C.; Paus, R. Nestin in human skin: Exclusive expression in intramesenchymal skin compartments and regulation by leptin. J. Investig. Dermatol. 2009, 129, 2711–2720. [Google Scholar] [CrossRef]
- Yang, C.C.; Sheu, H.M.; Chung, P.L.; Chang, C.H.; Tsai, Y.S.; Hughes, M.W.; Tuan, T.L.; Huang, L.L. Leptin of dermal adipose tissue is differentially expressed during the hair cycle and contributes to adipocyte-mediated growth inhibition of anagen-phase vibrissa hair. Exp. Dermatol. 2015, 24, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Foster, A.R.; Nicu, C.; Schneider, M.R.; Hinde, E.; Paus, R. Dermal white adipose tissue undergoes major morphological changes during the spontaneous and induced murine hair follicle cycling: A reappraisal. Arch. Dermatol. Res. 2018, 310, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.B. Direct and indirect effects of leptin on adipocyte metabolism. Biochim. Biophys. Acta 2014, 1842, 414–423. [Google Scholar] [CrossRef]
- Yang, C.C.; Chung, P.L.; Lin, L.Y.; Hughes, M.W.; Tsai, Y.S. Higher plasma leptin is associated with higher risk of androgenetic alopecia in men. Exp. Dermatol. 2017, 26, 524–526. [Google Scholar] [CrossRef] [PubMed]
- Lyons-Giordano, B.; Lazarus, G.S. Skin abnormalities in mice transgenic for plasminogen activator inhibitor 1: Implications for the regulation of desquamation and follicular neogenesis by plasminogen activator enzymes. Dev. Biol. 1995, 170, 289–298. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kato, M.; Hasunuma, N.; Nakayama, R.; Takeda, J.; Itami, S.; Taira, M.; Manabe, M.; Osada, S. Progranulin, a secreted tumorigenesis and dementia-related factor, regulates mouse hair growth. J. Dermatol. Sci. 2009, 53, 234–236. [Google Scholar] [CrossRef]
- Incel-Uysal, P.; Akdogan, N.; Alli, N.; Oktem, A.; Candar, T.; Topcuoglu, C.; Turhan, T. Assessment of Metabolic Profile and Ischemia-modified Albumin Level in Patients with Alopecia Areata: A Case-Control Study. Indian J. Dermatol. 2019, 64, 12–18. [Google Scholar] [CrossRef]
- Narla, S.; Azzam, M.; Townsend, S.; Vellaichamy, G.; Marzano, A.V. Identifying key components and therapeutic targets of the immune system in hidradenitis suppurativa with an emphasis on neutrophils. Br. J. Dermatol. 2020. [Google Scholar] [CrossRef]
- Stallmeyer, B.; Kämpfer, H.; Podda, M.; Kaufmann, R.; Pfeilschifter, J.; Frank, S. A novel keratinocyte mitogen: Regulation of leptin and its functional receptor in skin repair. J. Investig. Dermatol. 2001, 117, 98–105. [Google Scholar] [CrossRef]
- Tadokoro, S.; Ide, S.; Tokuyama, R.; Umeki, H.; Tatehara, S.; Kataoka, S.; Satomura, K. Leptin promotes wound healing in the skin. PLoS ONE 2015, 10, e0121242. [Google Scholar] [CrossRef]
- Frank, S.; Stallmeyer, B.; Kämpfer, H.; Kolb, N.; Pfeilschifter, J. Leptin enhances wound re-epithelialization and constitutes a direct function of leptin in skin repair. J. Clin. Investig. 2000, 106, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Murad, A.; Nath, A.K.; Cha, S.T.; Demir, E.; Flores-Riveros, J.; Sierra-Honigmann, M.R. Leptin is an autocrine/paracrine regulator of wound healing. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2003, 17, 1895–1897. [Google Scholar] [CrossRef] [PubMed]
- Lerman, O.Z.; Galiano, R.D.; Armour, M.; Levine, J.P.; Gurtner, G.C. Cellular dysfunction in the diabetic fibroblast: Impairment in migration, vascular endothelial growth factor production, and response to hypoxia. Am. J. Pathol. 2003, 162, 303–312. [Google Scholar] [CrossRef]
- Miao, Q.; Ku, A.T.; Nishino, Y.; Howard, J.M.; Rao, A.S.; Shaver, T.M.; Garcia, G.E.; Le, D.N.; Karlin, K.L.; Westbrook, T.F.; et al. Tcf3 promotes cell migration and wound repair through regulation of lipocalin 2. Nat. Commun. 2014, 5, 4088. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, Y.; Cheng, H.; Liu, Y.; Zou, X.; Zhan, N.; Xiao, S.; Xia, Y. Transcription factor 7-like 1 dysregulates keratinocyte differentiation through upregulating lipocalin 2. Cell Death Discov. 2016, 2, 16028. [Google Scholar] [CrossRef]
- Fitsialos, G.; Chassot, A.A.; Turchi, L.; Dayem, M.A.; LeBrigand, K.; Moreilhon, C.; Meneguzzi, G.; Buscà, R.; Mari, B.; Barbry, P.; et al. Transcriptional signature of epidermal keratinocytes subjected to in vitro scratch wounding reveals selective roles for ERK1/2, p38, and phosphatidylinositol 3-kinase signaling pathways. J. Biol. Chem. 2007, 282, 15090–15102. [Google Scholar] [CrossRef]
- Providence, K.M.; Higgins, P.J. PAI-1 expression is required for epithelial cell migration in two distinct phases of in vitro wound repair. J. Cell. Physiol. 2004, 200, 297–308. [Google Scholar] [CrossRef]
- Dipietro, L.A.; Reintjes, M.G.; Low, Q.E.; Levi, B.; Gamelli, R.L. Modulation of macrophage recruitment into wounds by monocyte chemoattractant protein-1. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2001, 9, 28–33. [Google Scholar] [CrossRef]
- Akazawa, Y.; Sayo, T.; Sugiyama, Y.; Sato, T.; Akimoto, N.; Ito, A.; Inoue, S. Adiponectin resides in mouse skin and upregulates hyaluronan synthesis in dermal fibroblasts. Connect. Tissue Res. 2011, 52, 322–328. [Google Scholar] [CrossRef]
- Herédi, E.; Csordás, A.; Clemens, M.; Adám, B.; Gáspár, K.; Törőcsik, D.; Nagy, G.; Adány, R.; Gaál, J.; Remenyik, E.; et al. The prevalence of obesity is increased in patients with late compared with early onset psoriasis. Ann. Epidemiol. 2013, 23, 688–692. [Google Scholar] [CrossRef]
- Wong, Y.; Nakamizo, S.; Tan, K.J.; Kabashima, K. An Update on the Role of Adipose Tissues in Psoriasis. Front. Immunol. 2019, 10, 1507. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Zhang, S.; Wu, R.; Su, X.; Peng, D.; Zhao, M.; Su, Y. New insights into different adipokines in linking the pathophysiology of obesity and psoriasis. Lipids Health Dis. 2019, 18, 171. [Google Scholar] [CrossRef] [PubMed]
- Cerman, A.A.; Bozkurt, S.; Sav, A.; Tulunay, A.; Elbaşi, M.O.; Ergun, T. Serum leptin levels, skin leptin and leptin receptor expression in psoriasis. Br. J. Dermatol. 2008, 159, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Aly, D.G.; Abdallah, I.Y.; Hanafy, N.S.; Elsaie, M.L.; Hafiz, N.A. Elevated serum leptin levels in nonobese patients with psoriasis. J. Drugs Dermatol. JDD 2013, 12, e25-9. [Google Scholar]
- Kyriakou, A.; Patsatsi, A.; Sotiriadis, D.; Goulis, D.G. Serum Leptin, Resistin, and Adiponectin Concentrations in Psoriasis: A Meta-Analysis of Observational Studies. Dermatology 2017, 233, 378–389. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Zhao, Y.; Geng, L.; Song, F.; Chen, H.D. Psoriasis is associated with increased levels of serum leptin. Br. J. Dermatol. 2008, 158, 1134–1135. [Google Scholar] [CrossRef]
- Baran, A.; Flisiak, I.; Jaroszewicz, J.; Świderska, M. Serum adiponectin and leptin levels in psoriatic patients according to topical treatment. J. Dermatol. Treat. 2015, 26, 134–138. [Google Scholar] [CrossRef]
- Johnston, A.; Arnadottir, S.; Gudjonsson, J.E.; Aphale, A.; Sigmarsdottir, A.A.; Gunnarsson, S.I.; Steinsson, J.T.; Elder, J.T.; Valdimarsson, H. Obesity in psoriasis: Leptin and resistin as mediators of cutaneous inflammation. Br. J. Dermatol. 2008, 159, 342–350. [Google Scholar] [CrossRef]
- Mitsuyama, S.; Abe, F.; Kimura, M.; Yoshida, M.; Higuchi, T. Association between leptin gene expression in subcutaneous adipose tissue and circulating leptin levels in obese patients with psoriasis. Arch. Dermatol. Res. 2015, 307, 539–544. [Google Scholar] [CrossRef]
- Takahashi, H.; Tsuji, H.; Honma, M.; Ishida-Yamamoto, A.; Iizuka, H. Increased plasma resistin and decreased omentin levels in Japanese patients with psoriasis. Arch. Dermatol. Res. 2013, 305, 113–116. [Google Scholar] [CrossRef]
- Huang, K.; Chen, A.; Zhang, X.; Song, Z.; Xu, H.; Cao, J.; Yin, Y. Progranulin is preferentially expressed in patients with psoriasis vulgaris and protects mice from psoriasis-like skin inflammation. Immunology 2015, 145, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Rubina, K.A.; Sysoeva, V.Y.; Zagorujko, E.I.; Tsokolaeva, Z.I.; Kurdina, M.I.; Parfyonova, Y.V.; Tkachuk, V.A. Increased expression of uPA, uPAR, and PAI-1 in psoriatic skin and in basal cell carcinomas. Arch. Dermatol. Res. 2017, 309, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.A.; Mohamed, S.A. Serum levels of visfatin and omentin-1 in patients with psoriasis and their relation to disease severity. Br. J. Dermatol. 2012, 167, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Sereflican, B.; Goksugur, N.; Bugdayci, G.; Polat, M.; Haydar Parlak, A. Serum Visfatin, adiponectin, and tumor necrosis factor alpha (TNF-alpha) levels in patients with psoriasis and their correlation with disease severity. Acta Dermatovenerol. Croat. 2016, 24, 13–19. [Google Scholar]
- Albanesi, C.; Scarponi, C.; Pallotta, S.; Daniele, R.; Bosisio, D.; Madonna, S.; Fortugno, P.; Gonzalvo-Feo, S.; Franssen, J.D.; Parmentier, M.; et al. Chemerin expression marks early psoriatic skin lesions and correlates with plasmacytoid dendritic cell recruitment. J. Exp. Med. 2009, 206, 249–258. [Google Scholar] [CrossRef]
- Rollman, O.; Vahlquist, A. Psoriasis and vitamin A. Plasma transport and skin content of retinol, dehydroretinol and carotenoids in adult patients versus healthy controls. Arch. Dermatol. Res. 1985, 278, 17–24. [Google Scholar] [CrossRef]
- Uysal, S.; Yılmaz, F.M.; Karatoprak, K.; Artüz, F.; Cumbul, N.U. The levels of serum pentraxin3, CRP, fetuin-A, and insulin in patients with psoriasis. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 3453–3458. [Google Scholar]
- Gerdes, S.; Osadtschy, S.; Buhles, N.; Baurecht, H.; Mrowietz, U. Cardiovascular biomarkers in patients with psoriasis. Exp. Dermatol. 2014, 23, 322–325. [Google Scholar] [CrossRef]
- Uyar, B.; Akyildiz, M.; Solak, A.; Genc, B.; Saklamaz, A. Relationship Between Serum Fetuin-A Levels and Carotid Intima-media Thickness in Turkish Patients with Mild to Moderate Psoriasis. A Case-control Study. Acta Dermatovenerol. Croat. 2015, 23, 171–177. [Google Scholar]
- Li, R.C.; Krishnamoorthy, P.; DerOhannessian, S.; Doveikis, J.; Wilcox, M.; Thomas, P.; Rader, D.J.; Reilly, M.P.; Van Voorhees, A.; Gelfand, J.M.; et al. Psoriasis is associated with decreased plasma adiponectin levels independently of cardiometabolic risk factors. Clin. Exp. Dermatol. 2014, 39, 19–24. [Google Scholar] [CrossRef]
- Coimbra, S.; Oliveira, H.; Reis, F.; Belo, L.; Rocha, S.; Quintanilha, A.; Figueiredo, A.; Teixeira, F.; Castro, E.; Rocha-Pereira, P.; et al. Circulating adipokine levels in Portuguese patients with psoriasis vulgaris according to body mass index, severity and therapy. J. Eur. Acad. Dermatol. Venereol 2010, 24, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Saeki, H.; Tada, Y.; Karakawa, M.; Komine, M.; Tamaki, K. Serum high molecular weight adiponectin levels are decreased in psoriasis patients. J. Dermatol. Sci. 2009, 55, 62–63. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, S.; Pinter, A.; Biermann, M.; Papavassilis, C.; Reinhardt, M. Adiponectin levels in a large pooled plaque psoriasis study population. J. Dermatol. Treat. 2020, 31, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Turan, H.; Yaykasli, K.O.; Soguktas, H.; Yaykasli, E.; Aliagaoglu, C.; Erdem, T.; Karkucak, M.; Kaya, E.; Ucgun, T.; Bahadir, A. Omentin serum levels and omentin gene Val109Asp polymorphism in patients with psoriasis. Int. J. Dermatol. 2014, 53, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Tada, Y.; Hau, C.S.; Mitsui, A.; Kamata, M.; Asano, Y.; Sugaya, M.; Kadono, T.; Masamoto, Y.; Kurokawa, M.; et al. Adiponectin regulates psoriasiform skin inflammation by suppressing IL-17 production from gammadelta-T cells. Nat. Commun. 2015, 6, 7687. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Tada, Y.; Hau, C.; Tatsuta, A.; Yamamoto, M.; Kamata, M.; Karakawa, M.; Asano, Y.; Mitsui, H.; Sugaya, M.; et al. Adiponectin as an anti-inflammatory factor in the pathogenesis of psoriasis: Induction of elevated serum adiponectin levels following therapy. Br. J. Dermatol. 2011, 164, 667–670. [Google Scholar] [CrossRef] [PubMed]
- Ataseven, A.; Kesli, R. Novel inflammatory markers in psoriasis vulgaris: Vaspin, vascular adhesion protein-1 (VAP-1), and YKL-40. G. Ital. Dermatol. Venereol. Organo Uff. Soc. Ital. Dermatol. Sifilogr. 2016, 151, 244–250. [Google Scholar]
- Rittié, L.; Tejasvi, T.; Harms, P.W.; Xing, X.; Nair, R.P.; Gudjonsson, J.E.; Swindell, W.R.; Elder, J.T. Sebaceous Gland Atrophy in Psoriasis: An Explanation for Psoriatic Alopecia? J. Investig. Dermatol. 2016, 136, 1792–1800. [Google Scholar] [CrossRef]
- Karpouzis, A.; Tripsianis, G.; Gatzidou, E. Assessment of Leptin Gene Polymorphism rs2060713 in Psoriasis Vulgaris. Int. Sch. Res. Not. 2014, 2014, 845272. [Google Scholar] [CrossRef]
- Abdel Hay, R.M.; Rashed, L.A. Association between the leptin gene 2548G/A polymorphism, the plasma leptin and the metabolic syndrome with psoriasis. Exp. Dermatol. 2011, 20, 715–719. [Google Scholar] [CrossRef]
- Torres, T.; Bettencourt, N.; Ferreira, J.; Carvalho, C.; Mendonça, D.; Vasconcelos, C.; Selores, M.; Silva, B. Lack of association between leptin, leptin receptor, adiponectin gene polymorphisms and epicardial adipose tissue, abdominal visceral fat volume and atherosclerotic burden in psoriasis patients. Arch. Physiol. Biochem. 2015, 121, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Werfel, T.; Allam, J.P.; Biedermann, T.; Eyerich, K.; Gilles, S.; Guttman-Yassky, E.; Hoetzenecker, W.; Knol, E.; Simon, H.U.; Wollenberg, A.; et al. Cellular and molecular immunologic mechanisms in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2016, 138, 336–349. [Google Scholar] [CrossRef] [PubMed]
- Kimata, H. Elevated serum leptin in AEDS. Allergy 2002, 57, 179. [Google Scholar] [CrossRef]
- Jaworek, A.K.; Szepietowski, J.C. Adipokines as biomarkers of atopic dermatitis in adults. J. Clin. Med. 2020, 9, 2858. [Google Scholar] [CrossRef]
- Nagel, G.; Koenig, W.; Rapp, K.; Wabitsch, M.; Zoellner, I.; Weiland, S.K. Associations of adipokines with asthma, rhinoconjunctivitis, and eczema in German schoolchildren. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2009, 20, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Bostanci, I.; Atli, O.; Celebi, N.; Taşar, A.; Alpkarakoç, E.; Dallar, Y. Serum leptin level in children with atopic dermatitis-treated topical steroids. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2004, 15, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Balato, N.; Nino, M.; Patruno, C.; Matarese, G.; Ayala, F. “Eczemas” and leptin. Dermatitis 2011, 22, 320–323. [Google Scholar] [CrossRef]
- Machura, E.; Szczepanska, M.; Ziora, K.; Ziora, D.; Swietochowska, E.; Barc-Czarnecka, M.; Kasperska-Zajac, A. Evaluation of adipokines: Apelin, visfatin, and resistin in children with atopic dermatitis. Mediat. Inflamm. 2013, 2013, 760691. [Google Scholar] [CrossRef]
- Farag, A.G.A.; Hammam, M.A.; Khaled, H.N.; Soliman, S.; Tayel, N.R.; El-Shamendy, A.A.; Shehata, W.A. Resistin adipokin in atopic dermatitis patients: A clinical, biochemical, and genetic study. J. Cosmet. Dermatol. 2020. [Google Scholar] [CrossRef]
- Aizawa, N.; Ishiuji, Y.; Tominaga, M.; Sakata, S.; Takahashi, N.; Yanaba, K. Relationship between the Degrees of Itch and Serum Lipocalin-2 Levels in Patients with Psoriasis. J. Immunol. Res. 2019, 2019, 8171373. [Google Scholar] [CrossRef]
- Kamata, M.; Tada, Y.; Tatsuta, A.; Kawashima, T.; Shibata, S.; Mitsui, H.; Asano, Y.; Sugaya, M.; Kadono, T.; Kanda, N.; et al. Serum lipocalin-2 levels are increased in patients with psoriasis. Clin. Exp. Dermatol. 2012, 37, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.Y.; Shin, J.U.; Kim, J.H.; Kim, S.H.; Kim, B.M.; Kim, Y.H.; Park, S.; Kim, T.G.; Shin, K.O.; Park, K.; et al. ZAG Regulates the Skin Barrier and Immunity in Atopic Dermatitis. J. Investig. Dermatol. 2019, 139, 1648–1657.e1647. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Wu, W.H.; Bae, J.M.; Son, S.J.; Lee, J.H.; Han, T.Y. Serum leptin and adiponectin levels in atopic dermatitis (AD) and their relation to disease severity. J. Am. Acad. Dermatol. 2016, 75, 629–631. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Banihani, S.A.; Elmadhoun, R.A.; Khabour, O.F.; Alzoubi, K.H. The rs2167270 polymorphism of leptin gene is associated with atopic dermatitis. Derm. Endocrinol. 2018, 10, e1454191. [Google Scholar] [CrossRef]
- Banihani, S.A.; Abu-Alia, K.F.; Khabour, O.F. Association between resistin gene polymorphisms and atopic dermatitis. Biomolecules 2018, 8, 17. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Jourdan, E.; Picardo, M. Acne is an inflammatory disease and alterations of sebum composition initiate acne lesions. J. Eur. Acad. Dermatol. Venereol 2014, 28, 527–532. [Google Scholar] [CrossRef]
- Pérez-Pérez, A.; Vilariño-García, T.; Fernández-Riejos, P.; Martín-González, J.; Segura-Egea, J.J.; Sánchez-Margalet, V. Role of leptin as a link between metabolism and the immune system. Cytokine Growth Factor Rev. 2017, 35, 71–84. [Google Scholar] [CrossRef]
- Ingham, E.; Eady, E.A.; Goodwin, C.E.; Cove, J.H.; Cunliffe, W.J. Pro-inflammatory levels of interleukin-1 alpha-like bioactivity are present in the majority of open comedones in acne vulgaris. J. Investig. Dermatol. 1992, 98, 895–901. [Google Scholar] [CrossRef]
- Reis, B.S.; Lee, K.; Fanok, M.H.; Mascaraque, C.; Amoury, M.; Cohn, L.B.; Rogoz, A.; Dallner, O.S.; Moraes-Vieira, P.M.; Domingos, A.I.; et al. Leptin receptor signaling in T cells is required for Th17 differentiation. J. Immunol. 2015, 194, 5253–5260. [Google Scholar] [CrossRef]
- Kelhälä, H.L.; Palatsi, R.; Fyhrquist, N.; Lehtimäki, S.; Väyrynen, J.P.; Kallioinen, M.; Kubin, M.E.; Greco, D.; Tasanen, K.; Alenius, H.; et al. IL-17/Th17 pathway is activated in acne lesions. PLoS ONE 2014, 9, e105238. [Google Scholar] [CrossRef]
- Danby, F.W. Ductal hypoxia in acne: Is it the missing link between comedogenesis and inflammation? J. Am. Acad. Dermatol. 2014, 70, 948–949. [Google Scholar] [CrossRef] [PubMed]
- Dodd, K.M.; Yang, J.; Shen, M.H.; Sampson, J.R.; Tee, A.R. mTORC1 drives HIF-1alpha and VEGF-A signalling via multiple mechanisms involving 4E-BP1, S6K1 and STAT3. Oncogene 2015, 34, 2239–2250. [Google Scholar] [CrossRef] [PubMed]
- Kaymak, Y.; Adisen, E.; Ilter, N.; Bideci, A.; Gurler, D.; Celik, B. Dietary glycemic index and glucose, insulin, insulin-like growth factor-I, insulin-like growth factor binding protein 3, and leptin levels in patients with acne. J. Am. Acad. Dermatol. 2007, 57, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.C.; Lin, M.H.; Huang, Y.C. Association between circulating adipokines and acne vulgaris: A systematic review and meta-analysis. Australas J. Dermatol. 2019, 60, e361–e364. [Google Scholar] [CrossRef] [PubMed]
- Çerman, A.A.; Aktaş, E.; Altunay, İ.K.; Arıcı, J.E.; Tulunay, A.; Ozturk, F.Y. Dietary glycemic factors, insulin resistance, and adiponectin levels in acne vulgaris. J. Am. Acad. Dermatol. 2016, 75, 155–162. [Google Scholar] [CrossRef]
- Aydin, K.; Çetinözman, F.; Elcin, G.; Aksoy, D.Y.; Ucar, F.; Yildiz, B.O. Suppressed Adiponectin Levels and Increased Adiponectin Response to Oral Glucose Load in Lean Women with Severe Acne Normalizes after Isotretinoin Treatment. Dermatology 2017, 233, 314–319. [Google Scholar] [CrossRef]
- Karadag, A.S.; Ertugrul, D.T.; Takci, Z.; Bilgili, S.G.; Namuslu, M.; Ata, N.; Sekeroglu, R. The effect of isotretinoin on retinol-binding protein 4, leptin, adiponectin and insulin resistance in acne vulgaris patients. Dermatology 2015, 230, 70–74. [Google Scholar] [CrossRef]
- Hussain, S.; Faraz, A.; Iqbal, T. The RETN gene rs1862513 polymorphism as a novel predisposing marker for familial Acne vulgaris in a Pakistani population. Iran. J. Basic Med. Sci. 2015, 18, 526–528. [Google Scholar]
- Younis, S.; Blumenberg, M.; Javed, Q. Resistin gene polymorphisms are associated with acne and serum lipid levels, providing a potential nexus between lipid metabolism and inflammation. Arch. Dermatol. Res. 2016, 308, 229–237. [Google Scholar] [CrossRef]
- Soguktas, H.; Yaykasli, K.O.; Turan, H.; Kaya, E.; Yaykasli, E. Omentin Val/Val genotype increases predisposition to acne vulgaris without changing omentin serum level. Cell. Mol. Biol. 2018, 64, 81–86. [Google Scholar] [CrossRef]
- Ahn, C.S.; Huang, W.W. Rosacea Pathogenesis. Dermatol. Clin. 2018, 36, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Gerber, P.A.; Buhren, B.A.; Steinhoff, M.; Homey, B. Rosacea: The cytokine and chemokine network. J. Investig. Dermatology. Symp. Proc. 2011, 15, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Rainer, B.M.; Kang, S.; Chien, A.L. Rosacea: Epidemiology, pathogenesis, and treatment. Derm. Endocrinol. 2017, 9, e1361574. [Google Scholar] [CrossRef] [PubMed]
- Amir Ali, A.; Vender, R.; Vender, R. The Role of IL-17 in Papulopustular Rosacea and Future Directions. J. Cutan. Med. Surg. 2019, 23, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zeng, Q.; Chen, X.; Wang, G.; Zhang, H.; Yu, A.; Wang, H.; Hu, Y. The therapeutic effect of artesunate on rosacea through the inhibition of the JAK/STAT signaling pathway. Mol. Med. Rep. 2018, 17, 8385–8390. [Google Scholar] [CrossRef]
- Thibaut de Ménonville, S.; Rosignoli, C.; Soares, E.; Roquet, M.; Bertino, B.; Chappuis, J.P.; Defoin-Platel/Chaussade, C.; Piwnica, D. Topical Treatment of Rosacea with Ivermectin Inhibits Gene Expression of Cathelicidin Innate Immune Mediators, LL-37 and KLK5, in Reconstructed and Ex Vivo Skin Models. Dermatol. Ther. 2017, 7, 213–225. [Google Scholar] [CrossRef]
- Yuan, X.; Li, J.; Li, Y.; Deng, Z.; Zhou, L.; Long, J.; Tang, Y.; Zuo, Z.; Zhang, Y.; Xie, H. Artemisinin, a potential option to inhibit inflammation and angiogenesis in rosacea. Biomed. Pharmacother. Biomed. Pharmacother. 2019, 117, 109181. [Google Scholar] [CrossRef]
- Topcu-Yilmaz, P.; Atakan, N.; Bozkurt, B.; Irkec, M.; Aban, D.; Mesci, L.; Tezcan, I. Determination of tear and serum inflammatory cytokines in patients with rosacea using multiplex bead technology. Ocul. Immunol. Inflamm. 2013, 21, 351–359. [Google Scholar] [CrossRef]
- Fischer, J.; Meyer-Hoffert, U. Regulation of kallikrein-related peptidases in the skin—From physiology to diseases to therapeutic options. Thromb. Haemost. 2013, 110, 442–449. [Google Scholar] [CrossRef]
- Di Paolo, C.T.; Diamandis, E.P.; Prassas, I. The role of kallikreins in inflammatory skin disorders and their potential as therapeutic targets. Crit. Rev. Clin. Lab. Sci. 2020, 1–16. [Google Scholar] [CrossRef]
- Meyer-Hoffert, U. Reddish, scaly, and itchy: How proteases and their inhibitors contribute to inflammatory skin diseases. Arch. Immunol. Ther. Exp. 2009, 57, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Medgyesi, B.; Dajnoki, Z.; Béke, G.; Gáspár, K.; Szabó, I.L.; Janka, E.A.; Póliska, S.; Hendrik, Z.; Méhes, G.; Törőcsik, D.; et al. Rosacea Is Characterized by a Profoundly Diminished Skin Barrier. J. Investig. Dermatol. 2020, 140, 1938–1950.e1935. [Google Scholar] [CrossRef] [PubMed]
- Tímár, J.; Vizkeleti, L.; Doma, V.; Barbai, T.; Rásó, E. Genetic progression of malignant melanoma. Cancer Metastasis Rev. 2016, 35, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Sergentanis, T.N.; Antoniadis, A.G.; Gogas, H.J.; Antonopoulos, C.N.; Adami, H.O.; Ekbom, A.; Petridou, E.T. Obesity and risk of malignant melanoma: A meta-analysis of cohort and case-control studies. Eur. J. Cancer 2013, 49, 642–657. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef]
- Præstegaard, C.; Kjær, S.K.; Christensen, J.; Tjønneland, A.; Halkjær, J.; Jensen, A. Obesity and risks for malignant melanoma and non-melanoma skin cancer: Results from a large Danish prospective cohort study. J. Investig. Dermatol. 2015, 135, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Malvi, P.; Chaube, B.; Singh, S.V.; Mohammad, N.; Vijayakumar, M.V.; Singh, S.; Chouhan, S.; Bhat, M.K. Elevated circulatory levels of leptin and resistin impair therapeutic efficacy of dacarbazine in melanoma under obese state. Cancer Metab. 2018, 6, 2. [Google Scholar] [CrossRef]
- Brandon, E.L.; Gu, J.W.; Cantwell, L.; He, Z.; Wallace, G.; Hall, J.E. Obesity promotes melanoma tumor growth: Role of leptin. Cancer Biol. Ther. 2009, 8, 1871–1879. [Google Scholar] [CrossRef]
- Malvi, P.; Chaube, B.; Pandey, V.; Vijayakumar, M.V.; Boreddy, P.R.; Mohammad, N.; Singh, S.V.; Bhat, M.K. Obesity induced rapid melanoma progression is reversed by orlistat treatment and dietary intervention: Role of adipokines. Mol. Oncol. 2015, 9, 689–703. [Google Scholar] [CrossRef]
- Ellerhorst, J.A.; Diwan, A.H.; Dang, S.M.; Uffort, D.G.; Johnson, M.K.; Cooke, C.P.; Grimm, E.A. Promotion of melanoma growth by the metabolic hormone leptin. Oncol. Rep. 2010, 23, 901–907. [Google Scholar] [CrossRef]
- Chi, M.; Chen, J.; Ye, Y.; Tseng, H.Y.; Lai, F.; Tay, K.H.; Jin, L.; Guo, S.T.; Jiang, C.C.; Zhang, X.D. Adipocytes contribute to resistance of human melanoma cells to chemotherapy and targeted therapy. Curr. Med. Chem. 2014, 21, 1255–1267. [Google Scholar] [CrossRef] [PubMed]
- Gogas, H.; Trakatelli, M.; Dessypris, N.; Terzidis, A.; Katsambas, A.; Chrousos, G.P.; Petridou, E.T. Melanoma risk in association with serum leptin levels and lifestyle parameters: A case-control study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2008, 19, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, H.; Fukushima, S.; Masuguchi, S.; Yamashita, J.; Miyashita, A.; Nakahara, S.; Aoi, J.; Inoue, Y.; Jinnin, M.; Ihn, H. Serum levels of leptin receptor in patients with malignant melanoma as a new tumor marker. Exp. Dermatol. 2013, 22, 748–749. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, F.; Mehdipoor, R.; Zarkesh-Esfahani, H.; Javanmard, S.H. Leptin serves as angiogenic/mitogenic factor in melanoma tumor growth. Adv. Biomed. Res. 2016, 5, 127. [Google Scholar] [CrossRef]
- Berta, J.; Hoda, M.A.; Laszlo, V.; Rozsas, A.; Garay, T.; Torok, S.; Grusch, M.; Berger, W.; Paku, S.; Renyi-Vamos, F.; et al. Apelin promotes lymphangiogenesis and lymph node metastasis. Oncotarget 2014, 5, 4426–4437. [Google Scholar] [CrossRef] [PubMed]
- Bułdak, R.J.; Bułdak, Ł.; Polaniak, R.; Kukla, M.; Birkner, E.; Kubina, R.; Kabała-Dzik, A.; Duława-Bułdak, A.; Żwirska-Korczala, K. Visfatin affects redox adaptative responses and proliferation in Me45 human malignant melanoma cells: An in vitro study. Oncol. Rep. 2013, 29, 771–778. [Google Scholar] [CrossRef]
- Grolla, A.A.; Torretta, S.; Gnemmi, I.; Amoruso, A.; Orsomando, G.; Gatti, M.; Caldarelli, A.; Lim, D.; Penengo, L.; Brunelleschi, S.; et al. Nicotinamide phosphoribosyltransferase (NAMPT/PBEF/visfatin) is a tumoural cytokine released from melanoma. Pigment. Cell Melanoma Res. 2015, 28, 718–729. [Google Scholar] [CrossRef]
- Audrito, V.; Managò, A.; Zamporlini, F.; Rulli, E.; Gaudino, F.; Madonna, G.; D’Atri, S.; Antonini Cappellini, G.C.; Ascierto, P.A.; Massi, D.; et al. Extracellular nicotinamide phosphoribosyltransferase (eNAMPT) is a novel marker for patients with BRAF-mutated metastatic melanoma. Oncotarget 2018, 9, 18997–19005. [Google Scholar] [CrossRef]
- Pachynski, R.K.; Zabel, B.A.; Kohrt, H.E.; Tejeda, N.M.; Monnier, J.; Swanson, C.D.; Holzer, A.K.; Gentles, A.J.; Sperinde, G.V.; Edalati, A.; et al. The chemoattractant chemerin suppresses melanoma by recruiting natural killer cell antitumor defenses. J. Exp. Med. 2012, 209, 1427–1435. [Google Scholar] [CrossRef]
- Song, Y.; Yin, W.; Dan, Y.; Sheng, J.; Zeng, Y.; He, R. Chemerin partly mediates tumor-inhibitory effect of all-trans retinoic acid via CMKLR1-dependent natural killer cell recruitment. Immunology 2019, 157, 248–256. [Google Scholar] [CrossRef]
- Klein, R.M.; Bernstein, D.; Higgins, S.P.; Higgins, C.E.; Higgins, P.J. SERPINE1 expression discriminates site-specific metastasis in human melanoma. Exp. Dermatol. 2012, 21, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Mantzoros, C.S.; Trakatelli, M.; Gogas, H.; Dessypris, N.; Stratigos, A.; Chrousos, G.P.; Petridou, E.T. Circulating adiponectin levels in relation to melanoma: A case-control study. Eur. J. Cancer 2007, 43, 1430–1436. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Adipokines—Removing road blocks to obesity and diabetes therapy. Mol. Metab. 2014, 3, 230–240. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovács, D.; Fazekas, F.; Oláh, A.; Törőcsik, D. Adipokines in the Skin and in Dermatological Diseases. Int. J. Mol. Sci. 2020, 21, 9048. https://doi.org/10.3390/ijms21239048
Kovács D, Fazekas F, Oláh A, Törőcsik D. Adipokines in the Skin and in Dermatological Diseases. International Journal of Molecular Sciences. 2020; 21(23):9048. https://doi.org/10.3390/ijms21239048
Chicago/Turabian StyleKovács, Dóra, Fruzsina Fazekas, Attila Oláh, and Dániel Törőcsik. 2020. "Adipokines in the Skin and in Dermatological Diseases" International Journal of Molecular Sciences 21, no. 23: 9048. https://doi.org/10.3390/ijms21239048
APA StyleKovács, D., Fazekas, F., Oláh, A., & Törőcsik, D. (2020). Adipokines in the Skin and in Dermatological Diseases. International Journal of Molecular Sciences, 21(23), 9048. https://doi.org/10.3390/ijms21239048





