Determination of Methemoglobin in Hemoglobin Submicron Particles Using NMR Relaxometry
Abstract
1. Introduction
2. Results and Discussion
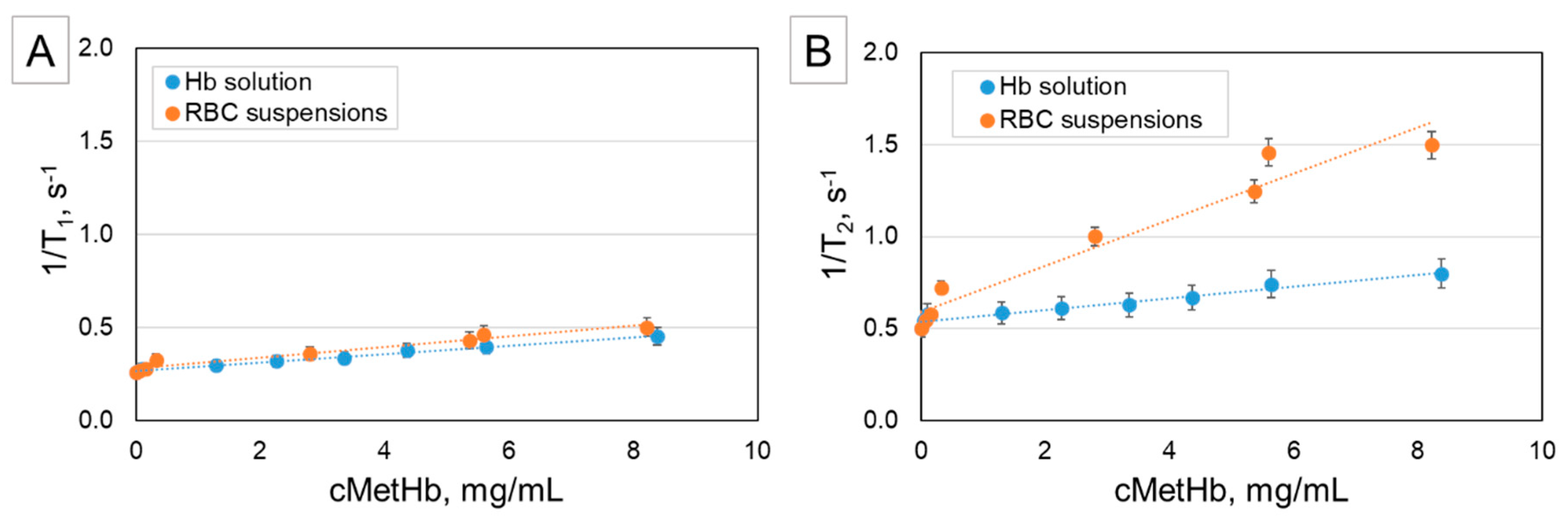
2.1. NMR of Human Hb/MetHb Solutions and RBCs/MetHb-RBC Suspensions
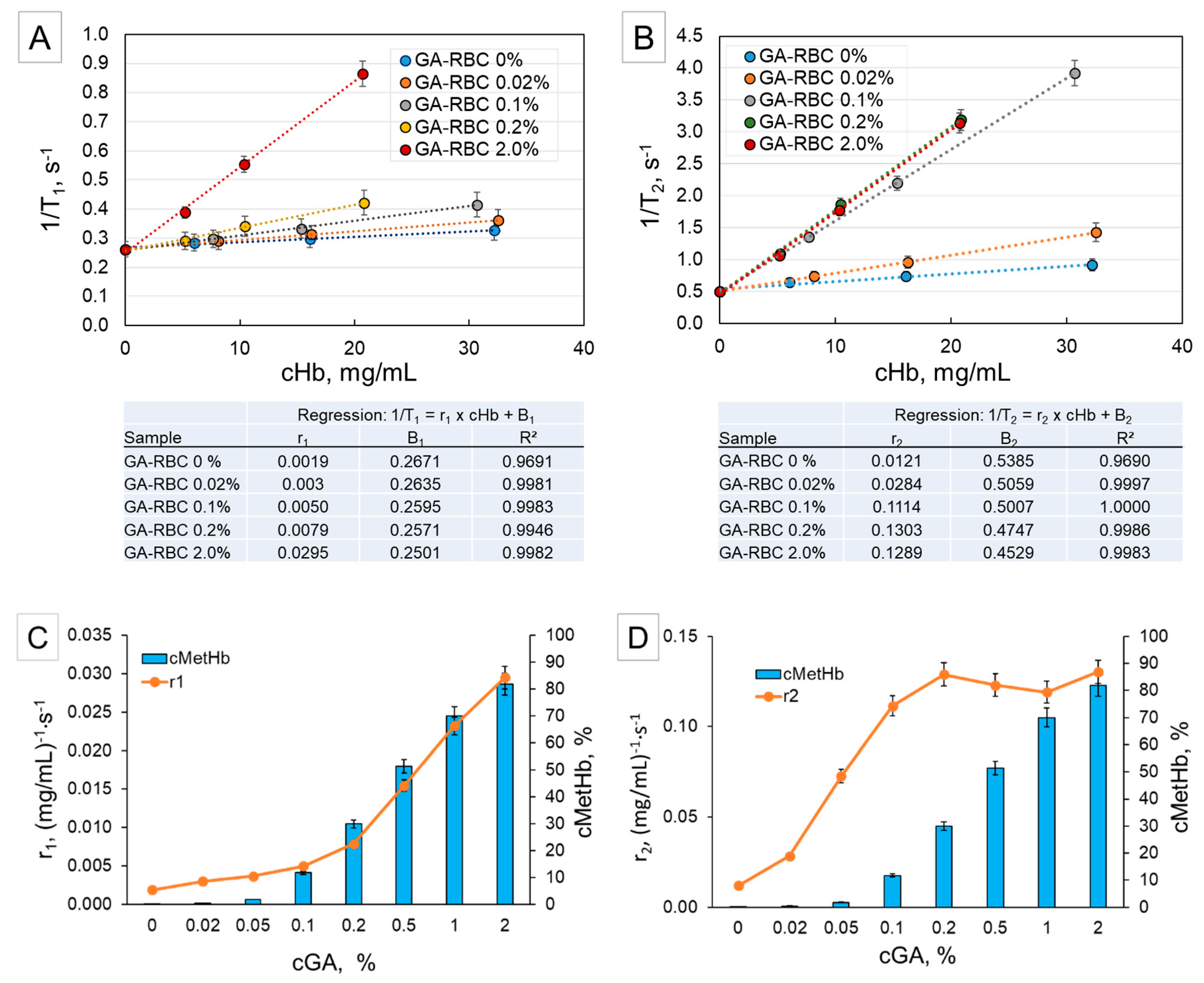
2.2. NMR of RBCs Treated with GA (Influence of Crosslinking)
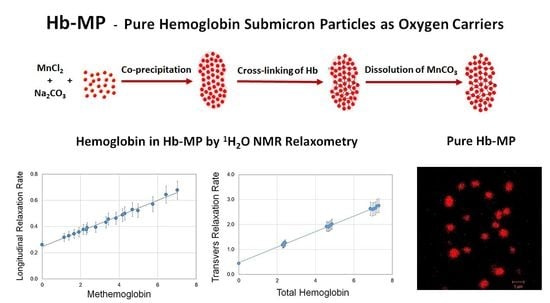
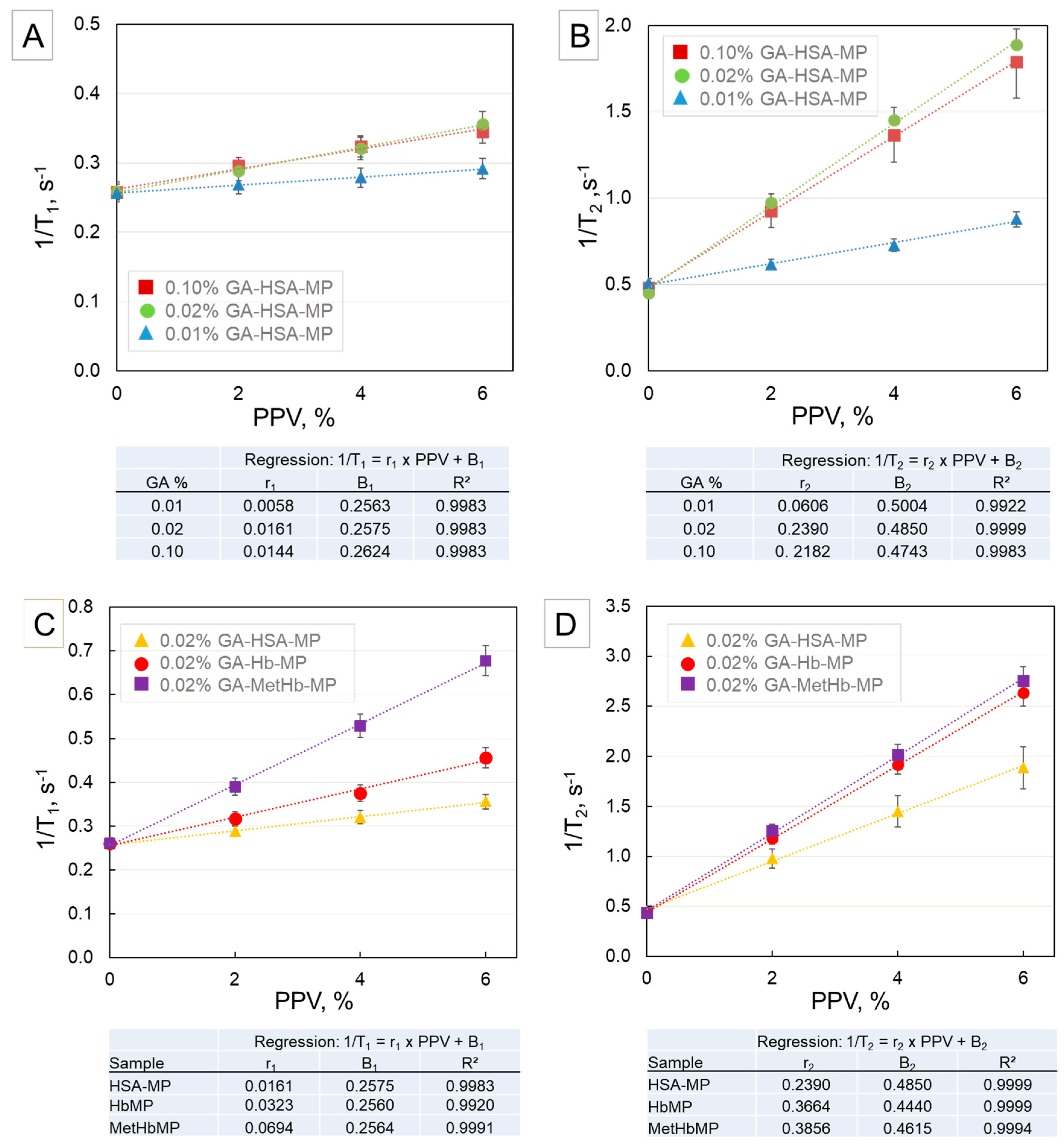
2.3. NMR of HSA-MP, Hb-MP, and MetHb-MP
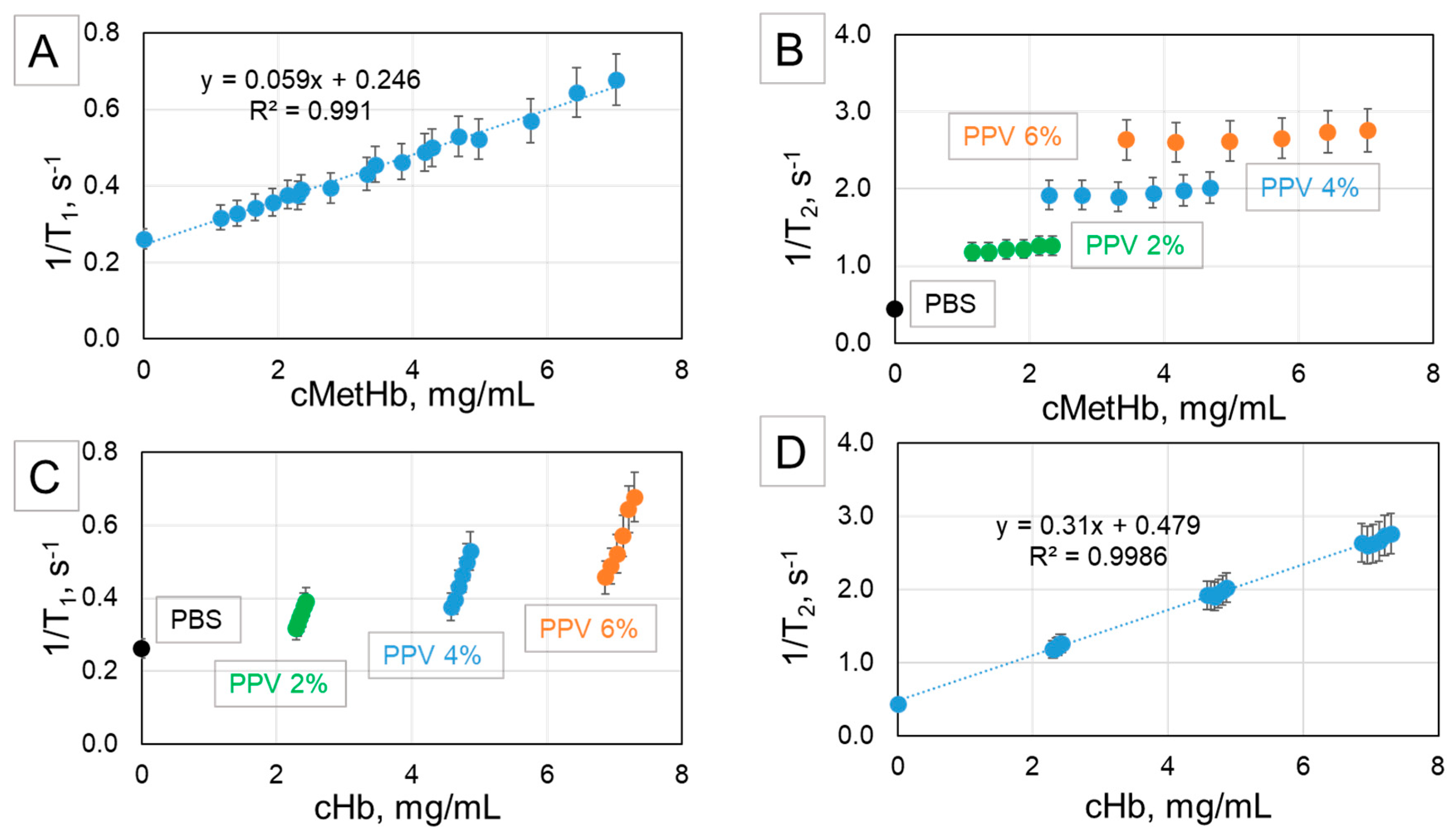
2.4. NMR as a Tool for Determination of Total Hb and MetHb in Hb-MP
3. Materials and Methods
3.1. Materials
3.2. Preparation of Vital RBC, GA-Treated RBC (GA-RBC), and Sodium Nitrite Treated RBC (MetHb-RBC) Suspensions
3.3. Preparation of Hb and MetHb Solutions
3.4. Determination of Oxyhemoglobin in RBC Suspensions and Hb Solutions
3.5. Preparation and Characterization of Hemoglobin Microparticles (Hb-MP), Human Serum Albumin Microparticles (HSA-MP), and Methemoglobin Particles (MetHb-MP)
3.6. NMR Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Antonini, E.; Brunori, M. Hemoglobin. Annu. Rev. Biochem. 1970, 39, 977–1042. [Google Scholar] [CrossRef]
- Riggs, A. Functional properties of hemoglobins. Physiol. Rev. 1965, 45, 619–673. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, A. Methemoglobinemia. Am. J. Med. Sci. 1985, 289, 200–209. [Google Scholar] [CrossRef] [PubMed]
- White, J.C.; Beaver, G.H. A review of the varieties of human haemoglobin in health and disease. J. Clin. Pathol. 1954, 7, 175–200. [Google Scholar] [CrossRef] [PubMed]
- Bodansky, O. Methemoglobinemia and methemoglobin-producing compounds. Pharmacol. Rev. 1951, 3, 144–191. [Google Scholar]
- Benz, E.J.; Ebert, B.L. Hemoglobin Variants Associated With Hemolytic Anemia, Altered Oxygen Affinity, and Methemoglobinemias. In Hematology: Basic Principles and Practice; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 608–615. ISBN 9781455740413. [Google Scholar]
- Brunelle, J.A.; Degtiarov, A.M.; Moran, R.F.; Race, L.A. Simultaneous measurement of total hemoglobin and its derivatives in blood using CO-oximeters: Analytical principles; Their application in selecting analytical wavelengths and reference methods; A comparison of the results of the choices made. Scand. J. Clin. Lab. Investig. 1996, 56, 47–69. [Google Scholar] [CrossRef]
- Whitehead, R.D.; Mei, Z.; Mapango, C.; Jefferds, M.E.D. Methods and analyzers for hemoglobin measurement in clinical laboratories and field settings. Ann. N. Y. Acad. Sci. 2019, 1450, 147. [Google Scholar] [CrossRef]
- Haymond, S.; Cariappa, R.; Eby, C.S.; Scott, M.G. Laboratory Assessment of Oxygenation in Methemoglobinemia. Clin. Chem. 2005, 51, 434–444. [Google Scholar] [CrossRef]
- Cortazzo, J.A.; Lichtman, A.D. Methemoglobinemia: A review and recommendations for management. J. Cardiothorac. Vasc. Anesth. 2014, 28, 1043–1047. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, Z.Z.; Georgieva, R.; Smuda, K.; Steffen, A.; Sendeski, M.; Voigt, A.; Patzak, A.; Bäumler, H. Nonvasoconstrictive Hemoglobin Particles as Oxygen Carriers. ACS Nano 2013, 7, 7454–7461. [Google Scholar] [CrossRef]
- Kloypan, C.; Prapan, A.; Suwannasom, N.; Chaiwaree, S.; Kaewprayoon, W.; Steffen, A.; Xiong, Y.; Baisaeng, N.; Georgieva, R.; Bäumler, H. Improved oxygen storage capacity of haemoglobin submicron particles by one-pot formulation. Artif. Cells Nanomed. Biotechnol. 2018, 46, S964–S972. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Georgieva, R.; Steffen, A.; Smuda, K.; Bäumler, H. Structure and properties of hybrid biopolymer particles fabricated by co-precipitation cross-linking dissolution procedure. J. Colloid Interface Sci. 2018, 514, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Bäumler, H.; Xiong, Y.; Liu, Z.Z.; Patzak, A.; Georgieva, R. Novel Hemoglobin Particles-Promising New-Generation Hemoglobin-Based Oxygen Carriers. Artif. Organs 2014, 38, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Eike, J.H.; Palmer, A.F. Effect of glutaraldehyde concentration on the physical properties of polymerized hemoglobin-based oxygen carriers. Biotechnol. Prog. 2004, 20, 1225–1232. [Google Scholar] [CrossRef]
- Prapan, A.; Suwannasom, N.; Kloypan, C.; Chaiwaree, S.; Steffen, A.; Xiong, Y.; Kao, I.; Pruß, A.; Georgieva, R.; Bäumler, H. Surface Modification of Hemoglobin-Based Oxygen Carriers Reduces Recognition by Haptoglobin, Immunoglobulin, and Hemoglobin Antibodies. Coatings 2019, 9, 454. [Google Scholar] [CrossRef]
- Gomori, J.M.; Grossman, R.I.; Yu-Ip, C.; Asakura, T. NMR relaxation times of blood: Dependence on field strength, oxidation state, and cell integrity. J. Comput. Assist. Tomogr. 1987, 11, 684–690. [Google Scholar] [CrossRef]
- Aime, S.; Dastrú, W.; Fasano, M.; Arnelli, A.; Castagnola, M.; Giardina, B.; Ascenzi, P. Quantitative determination of methemoglobin by measuring the solvent-water proton-nuclear magnetic resonance relaxation rate. Clin. Chem. 1992, 38, 2401–2404. [Google Scholar] [CrossRef]
- Tentori, L.; Salvati, A.M. Hemoglobinometry in Human Blood. Methods Enzymol. 1981, 76, 707–715. [Google Scholar] [CrossRef]
- Bass, J.; Sostman, H.D.; Boyko, O.; Koepke, J.A. Effects of cell membrane disruption on the relaxation rates of blood and clot with various methemoglobin concentrations. Investig. Radiol. 1990, 25, 1232–1237. [Google Scholar] [CrossRef]
- Thulborn, K.R.; Waterton, J.C.; Matthews, P.M.; Radda, G.K. Oxygenation dependence of the transverse relaxation time of water protons in whole blood at high field. BBA Gen. Subj. 1982, 714, 265–270. [Google Scholar] [CrossRef]
- Păltineanu, B.; Voinea, S. Spin-spin proton transverse relaxation times studies of red blood cell membrene in rabbits with experimental atherosclerosis. Anim. Sci. 2017, 60, 174–182. [Google Scholar]
- Stefanovic, B.; Pike, G.B. Human whole-blood relaxometry at 1.5T: Assessment of diffusion and exchange models. Magn. Reson. Med. 2004, 52, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Kumar, P. Assessment of the histological state of the healing wound. Plast. Aesthetic Res. 2015, 2, 239. [Google Scholar] [CrossRef]
- Koenig, S.H.; Brown, R.D.; Lindstrom, T.R. Interactions of solvent with the heme region of methemoglobin and fluoro-methemoglobin. Biophys. J. 1981, 34, 397–408. [Google Scholar] [CrossRef]
- La Mar, G.N.; Chatfield, M.J.; Peyton, D.H.; de Ropp, J.S.; Smith, W.S.; Krishnamoorthi, R.; Satterlee, J.D.; Erman, J.E. Solvent isotope effects on NMR spectral parameters in high-spin ferric hemoproteins: An indirect probe for distal hydrogen bonding. Biochim. Biophys. Acta (BBA)/Protein Struct. Mol. 1988, 956, 267–276. [Google Scholar] [CrossRef]
- Brooks, R.A.; Di Chiro, G.; Patronas, N. MR imaging of cerebral hematomas at different field strengths: Theory and applications. J. Comput. Assist. Tomogr. 1989, 13, 194–208. [Google Scholar] [CrossRef]
- Bäumler, H.; Halbhuber, K.J.; Stibenz, D.; Lerche, D. Topo-optical investigations of human erythrocyte glycocalyx conformational changes induced by dextran. BBA Gen. Subj. 1987, 923, 22–28. [Google Scholar] [CrossRef]
- Morel, F.M.M.; Baker, R.F.; Wayland, H.; Knust-Graichen, P.V. Quantitation of human red blood cell fixation by glutaraldehyde. J. Cell Biol. 1971, 48, 91–100. [Google Scholar] [CrossRef]
- Folland, R.; Steven, J.H.; Charlesby, A. Proton spin relaxation in liquid polydimethylsiloxane: Molecular motion and network formation. J. Polym. Sci. Polym. Phys. Ed. 1978, 16, 1041–1057. [Google Scholar] [CrossRef]
- Chen, E.-L.; Kim, R.J. Magnetic Resonance Water Proton Relaxation in Protein Solutions and Tissue: T1ρ Dispersion Characterization. PLoS ONE 2010, 5, e8565. [Google Scholar] [CrossRef]
- Charlesby, A. The use of pulsed NMR techniques in the measurement of radiation effects in polymer. Radiat. Phys. Chem. 1979, 14, 919–930. [Google Scholar] [CrossRef]
- Xiong, Y.; Steffen, A.; Andreas, K.; Müller, S.; Sternberg, N.; Georgieva, R.; Bäumler, H. Hemoglobin-based oxygen carrier microparticles: Synthesis, properties, and in vitro and in vivo investigations. Biomacromolecules 2012, 13, 3292–3300. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.A.; Ali, S.N.; Mahmood, R. Sodium nitrite-induced oxidative stress causes membrane damage, protein oxidation, lipid peroxidation and alters major metabolic pathways in human erythrocytes. Toxicol. Vitr. 2015, 29, 1878–1886. [Google Scholar] [CrossRef]
- Keszler, A.; Piknova, B.; Schechter, A.N.; Hogg, N. The reaction between nitrite and oxyhemoglobin: A mechanistic study. J. Biol. Chem. 2008, 283, 9615–9622. [Google Scholar] [CrossRef] [PubMed]
- Haldane, J. The ferricyanide method of determining the oxygen capacity of blood. J. Physiol. 1900, 25, 295–302. [Google Scholar] [CrossRef]
- Cook, S.F. The action of potassium cyanide and potassium ferricyanide on certain respiratory pigments. J. Gen. Physiol. 1928, 11, 339–348. [Google Scholar] [CrossRef] [PubMed]

| Values for Particle Suspensions with 20% PPV | ||||||
|---|---|---|---|---|---|---|
| Type of MP | cGA | Size | cHb (by AHD) | cFe (ICP-OES) | cOxyHb (O2 Release) | cMetHb (cHb—cOxyHb) |
| % | nm | mg/mL | mg/L | mg/mL | mg/mL | |
| Hb-MP | 0.02 | 804.5 ± 15.6 | 22.9 ± 3.3 | 77.4 ± 2.9 | 11.4 ± 0.5 | 11.5 ± 3.4 |
| MetHb-MP | 0.02 | 809.2 ± 15.4 | 24.3 ± 3.1 | 82.5 ± 4.7 | 0.9 ± 0.3 | 23.4 ± 5.0 |
| HSA-MP | 0.01 | 803.6 ± 65.5 | - | - | - | - |
| HSA-MP | 0.02 | 776.2 ± 47.0 | - | - | - | - |
| HSA-MP | 0.10 | 823.6 ± 28.9 | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaewprayoon, W.; Suwannasom, N.; Kloypan, C.; Steffen, A.; Xiong, Y.; Schellenberger, E.; Pruß, A.; Georgieva, R.; Bäumler, H. Determination of Methemoglobin in Hemoglobin Submicron Particles Using NMR Relaxometry. Int. J. Mol. Sci. 2020, 21, 8978. https://doi.org/10.3390/ijms21238978
Kaewprayoon W, Suwannasom N, Kloypan C, Steffen A, Xiong Y, Schellenberger E, Pruß A, Georgieva R, Bäumler H. Determination of Methemoglobin in Hemoglobin Submicron Particles Using NMR Relaxometry. International Journal of Molecular Sciences. 2020; 21(23):8978. https://doi.org/10.3390/ijms21238978
Chicago/Turabian StyleKaewprayoon, Waraporn, Nittiya Suwannasom, Chiraphat Kloypan, Axel Steffen, Yu Xiong, Eyk Schellenberger, Axel Pruß, Radostina Georgieva, and Hans Bäumler. 2020. "Determination of Methemoglobin in Hemoglobin Submicron Particles Using NMR Relaxometry" International Journal of Molecular Sciences 21, no. 23: 8978. https://doi.org/10.3390/ijms21238978
APA StyleKaewprayoon, W., Suwannasom, N., Kloypan, C., Steffen, A., Xiong, Y., Schellenberger, E., Pruß, A., Georgieva, R., & Bäumler, H. (2020). Determination of Methemoglobin in Hemoglobin Submicron Particles Using NMR Relaxometry. International Journal of Molecular Sciences, 21(23), 8978. https://doi.org/10.3390/ijms21238978