Early Postnatal Exposure to a Low Dose of Nanoparticulate Silver Induces Alterations in Glutamate Transporters in Brain of Immature Rats
Abstract
1. Introduction
2. Results
2.1. Temporal Profile of Silver Concentrations in Serum and Brains of Exposed Rats
2.2. Ultrastructural Alterations in Neurons but Not in Astrocytes under Exposure to AgNPs
2.3. Exposure to AgNPs/Ag+ Affects the Expression and Functional Activity of Neuronal Excitatory Amino Acid Carrier 1 (EAAC1)
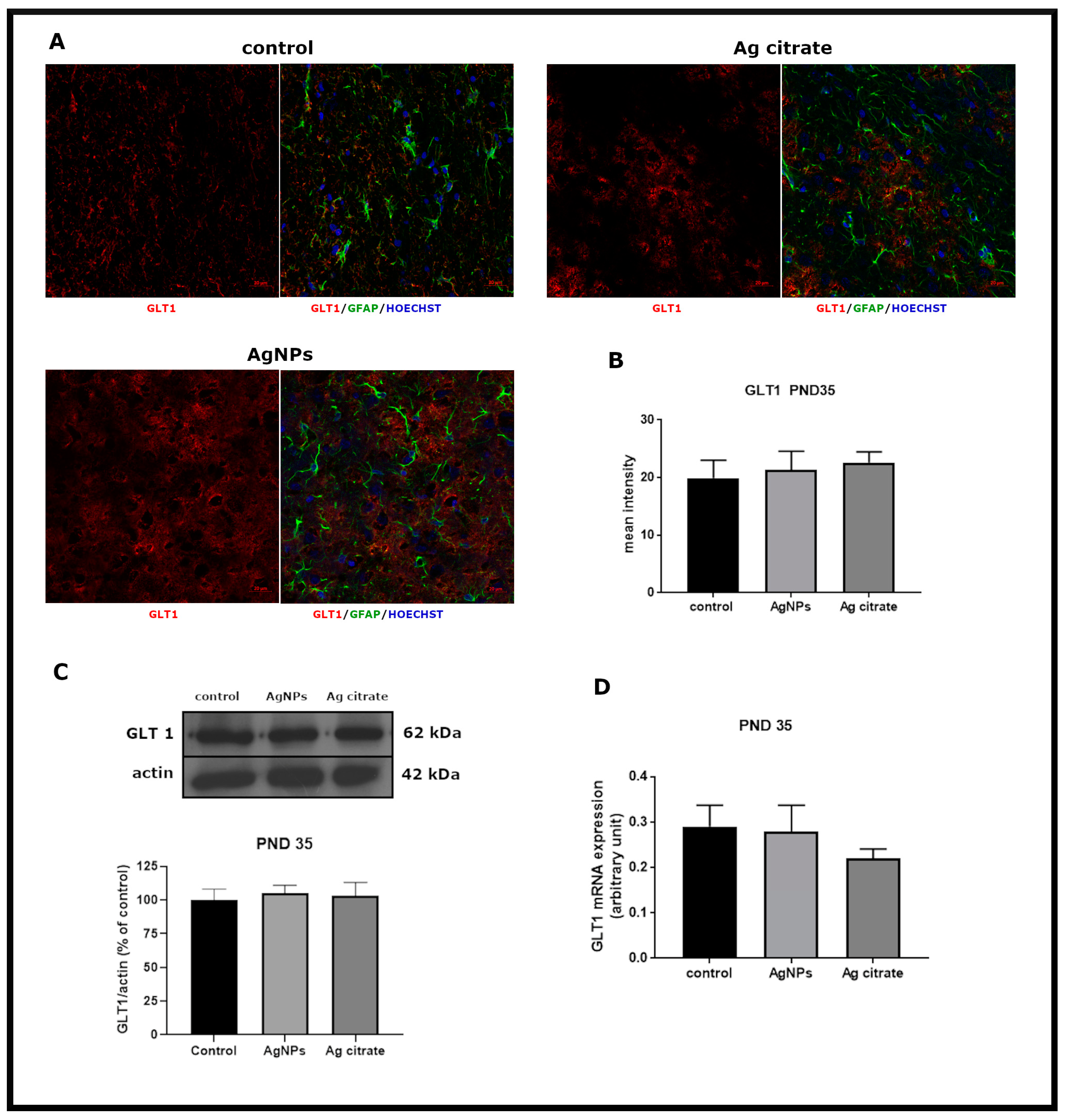
2.4. Exposure to AgNPs/Ag+ Affects the Function but Not the Expression of Glial Glutamate Transporters GLT-1 and GLAST
3. Discussion
3.1. Exposure to AgNPs/Ag+ during the Developmental Period Results in Stress-Like Ultrastructural Alterations in Neuronal ER and Downregulation of Neuronal Excitatory Amino Acid Carrier 1 (EAAC1)
3.2. Influence of AgNPs on Glial Glutamate Transporters GLT-1 and GLAST
4. Materials and Methods
4.1. Silver Nanoparticles (AgNPs)
4.2. Animal Model of Developmental Exposure to a Low Dose of AgNPs
4.3. Determination of Silver Concentration with Inductively-Coupled Plasma Mass Spectrometry (ICP-MS)
4.4. Analysis of Gene Expression by qPCR
4.5. Western Blot Analysis
4.6. Preparation of Brain Fractions
4.7. [3H] Glutamate Transport Assay
4.8. Immunohistochemical Procedure and Microscopic Analysis
4.9. Ultrastructural Analysis by TEM
4.10. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Benn, T.; Cavanagh, B.; Hristovski, K.; Posner, J.D.; Westerhoff, P. The release of nanosilver from consumer products used in the home. J. Environ. Qual. 2010, 39, 1875–1882. [Google Scholar] [CrossRef]
- Tulve, N.S.; Stefaniak, A.B.; Vance, M.E.; Rogers, K.; Mwilu, S.; LeBouf, R.F.; Schwegler-Berry, D.; Willis, R.; Thomas, T.A.; Marr, L.C. Characterization of silver nanoparticles in selected consumer products and its relevance for predicting children’s potential exposures. Int. J. Hyg. Environ. Health 2015, 218, 345–357. [Google Scholar] [CrossRef]
- Quadros, M.E.; Pierson, R., 4th; Tulve, N.S.; Willis, R.; Rogers, K.; Thomas, T.A.; Marr, L.C. Release of silver from nanotechnology-based consumer products for children. Environ. Sci. Technol. 2013, 47, 8894–8901. [Google Scholar] [CrossRef]
- Hubal, E.A.C.; Sheldon, L.S.; Burke, J.M.; McCurdy, T.R.; Berry, M.R.; Rigas, M.L.; Zartarian, V.G.; Freeman, N.C.G. Children’s exposure assessment: A review of factors influencing children’s exposure, and the data available to characterize and assess that exposure. Environ. Health Perspect. 2000, 108, 475. [Google Scholar] [CrossRef]
- Tang, S.; Wang, M.; Germ, K.E.; Du, H.-M.; Sun, W.-J.; Gao, W.-M.; Mayer, G.D. Health implications of engineered nanoparticles in infants and children. World J. Pediatr. 2015, 11, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Mureşanu, D.F.; Patnaik, R.; Sharma, H.S. Size- and age-dependent neurotoxicity of engineered metal nanoparticles in rats. Mol. Neurobiol. 2013, 48, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Piett, C.; Farkas, S.; Qazzaz, M.; Syed, N.I. Silver nanoparticles (AgNPs) cause degeneration of cytoskeleton and disrupt synaptic machinery of cultured cortical neurons. Mol. Brain 2013, 6, 29. [Google Scholar] [CrossRef]
- Tang, J.; Xiong, L.; Wang, S.; Wang, J.; Liu, L.; Li, J.; Wan, Z.; Xi, T. Influence of silver nanoparticles on neurons and blood-brain barrier via subcutaneous injection in rats. Appl. Surf. Sci. 2008, 255, 502–504. [Google Scholar] [CrossRef]
- Skalska, J.; Frontczak-Baniewicz, M.; Strużyńska, L. Synaptic degeneration in rat brain after prolonged oral exposure to silver nanoparticles. Neurotoxicology 2015, 46, 145–154. [Google Scholar] [CrossRef]
- Luther, E.M.; Schmidt, M.M.; Diendorf, J.; Epple, M.; Dringen, R. Upregulation of metallothioneins after exposure of cultured primary astrocytes to silver nanoparticles. Neurochem. Res. 2012, 37, 1639–1648. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.S.; Ali, S.F.; Hussain, S.M.; Schlager, J.J.; Sharma, A. Influence of engineered nanoparticles from metals on the blood-brain barrier permeability, cerebral blood flow, brain edema and neurotoxicity. an experimental study in the rat and mice using biochemical and morphological approaches. J. Nanosci. Nanotechnol. 2009, 9, 5055–5072. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska-Bouta, B.; Sulkowski, G.; Frontczak-Baniewicz, M.; Skalska, J.; Sałek, M.; Orzelska-Górka, J.; Strużyńska, L. Ultrastructural and biochemical features of cerebral microvessels of adult rat subjected to a low dose of silver nanoparticles. Toxicology 2018, 408, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Danbolt, N.C. Glutamate uptake. Prog. Neurobiol. 2001, 65, 1–105. [Google Scholar] [CrossRef]
- Dong, X.-X.; Wang, Y.; Qin, Z.-H. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol. Sin. 2009, 30, 379–387. [Google Scholar] [CrossRef]
- Saunders, N.R.; Liddelow, S.A.; Dziegielewska, K.M. Barrier mechanisms in the developing brain. Front. Pharmacol. 2012, 3, 46. [Google Scholar] [CrossRef]
- Hull, M.S.; Bowman, D.M. Nanomaterials ecotoxicology: A case study with nanosilver. In Nanotechnology Environmental Health and Safety: Risks, Regulation and Management, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 117–142. [Google Scholar]
- Barnes, J.R.; Mukherjee, B.; Rogers, B.C.; Nafar, F.; Gosse, M.; Parsons, M.P. The Relationship Between Glutamate Dynamics and Activity-Dependent Synaptic Plasticity. J. Neurosci. 2020, 40, 2793–2807. [Google Scholar] [CrossRef]
- Campiani, G.; Fattorusso, C.; Angelis, M.; Catalanotti, B.; Butini, S.; Fattorusso, R.; Fiorini, I.; Nacci, V.; Novellino, E. Neuronal high-affinity sodium-dependent glutamate transporters (EAATs): Targets for the development of novel therapeutics against neurodegenerative diseases. Curr. Pharm. Des. 2003, 9, 599–625. [Google Scholar] [CrossRef]
- Gegelashvili, G.; Robinson, M.B.; Trotti, D.; Rauen, T. Regulation of glutamate transporters in health and disease. Prog. Brain Res. 2001, 132, 267–286. [Google Scholar] [CrossRef]
- Cheville, N.F. Ultrastructural Pathology the Comparative Cellular Basis of Disease, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2009. [Google Scholar]
- Rothstein, J.D.; Martin, L.; Levey, A.I.; Dykes-Hoberg, M.; Jin, L.; Wu, D.; Nash, N.; Kuncl, R.W. Localization of neuronal and glial glutamate transporters. Neuron 1994, 13, 713–725. [Google Scholar] [CrossRef]
- Bianchi, M.G.; Bardelli, D.; Chiu, M.; Bussolati, O. Changes in the expression of the glutamate transporter EAAT3/EAAC1 in health and disease. Cell. Mol. Life Sci. 2014, 71, 2001–2015. [Google Scholar] [CrossRef]
- Malik, A.R.; Willnow, T.E. Excitatory amino acid transporters in physiology and disorders of the central nervous system. Int. J. Mol. Sci. 2019, 20, 5671. [Google Scholar] [CrossRef] [PubMed]
- McGivan, J.D.; Nicholson, B. Regulation of high-affinity glutamate transport by amino acid deprivation and hyperosmotic stress. Am. J. Physiol. 1999, 277, F498–F500. [Google Scholar] [CrossRef] [PubMed]
- Cameron, S.J.; Hosseinian, F.; Willmor, W.G. A Current Overview of the Biological and Cellular Effects of Nanosilver. Int. J. Mol. Sci. 2018, 19, 2030. [Google Scholar] [CrossRef] [PubMed]
- Wasąg, P.; Lenartowski, R. Nuclear matrix—structure, function and pathogenesis. Postepy Hig. Med. Dosw. 2016, 70, 1206–1219. [Google Scholar]
- Scimemi, A.; Tian, H.; Diamond, J.S. Neuronal transporters regulate glutamate clearance, nmda receptor activation, and synaptic plasticity in the hippocampus. J. Neurosci. 2009, 29, 14581–14595. [Google Scholar] [CrossRef]
- Greish, K.; Alqahtani, A.A.; Alotaibi, A.F.; Abdulla, A.M.; Bukelly, A.T.; Alsobyani, F.M.; Alharbi, G.H.; Alkiyumi, I.S.; Aldawish, M.M.; Alshahrani, T.F.; et al. The Effect of Silver Nanoparticles on Learning, Memory and Social Interaction in BALB/C Mice. Int. J. Environ. Res. Public Health 2019, 16, 148. [Google Scholar] [CrossRef]
- Chen, Y.; Swanson, R.A. The glutamate transporters EAAT2 and EAAT3 mediate cysteine uptake in cortical neuron cultures. J. Neurochem. 2003, 84, 1332–1339. [Google Scholar] [CrossRef]
- Himi, T.; Ikeda, M.; Yasuhara, T.; Nishida, M.; Morita, I. Role of neuronal glutamate transporter in the cysteine uptake and intracellular glutathione levels in cultured cortical neurons. J. Neural Transm. 2003, 110, 1337–1348. [Google Scholar] [CrossRef]
- Skalska, J.; Dąbrowska-Bouta, B.; Strużyńska, L. Oxidative stress in rat brain but not in liver following oral administration of a low dose of nanoparticulate silver. Food Chem. Toxicol. 2016, 97, 307–315. [Google Scholar] [CrossRef]
- Aoyama, K.; Nakaki, T. Neuroprotective properties of the excitatory amino acid carrier 1 (EAAC1). Amino Acids 2013, 45, 133–142. [Google Scholar] [CrossRef]
- Plaitakis, A.; Shashidharan, P. Glutamate transport and metabolism in dopaminergic neurons of substantia nigra: Implications for the pathogenesis of Parkinson’s disease. J. Neurol. 2000, 247, II25–II35. [Google Scholar] [CrossRef] [PubMed]
- Nafia, I.; Re, D.B.; Masméjean, F.; Melon, C.; Kachidian, P.; Kerkerian-Le-Goff, L.; Nieoullon, A.; Had-Aissouni, L. Preferential vulnerability of mesencephalic dopamine neurons to glutamate transporter dysfunction. J. Neurochem. 2008, 105, 484–496. [Google Scholar] [CrossRef]
- Aoyama, K.; Suh, S.W.; Hamby, A.M.; Liu, J.; Chan, W.Y.; Chen, Y.; Swanson, R.A. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat. Neurosci. 2006, 9, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.; Correale, D.M.; Robinson, M.B. Substrate-induced up-regulation of Na(+)-dependent glutamate transport activity. Neurochem. Int. 2000, 37, 147–162. [Google Scholar] [CrossRef]
- Gegelashvili, G.; Schousboe, A. High affinity glutamate transporters: Regulation of expression and activity. Mol. Pharmacol. 1997, 52, 6–15. [Google Scholar] [CrossRef]
- Poitry-Yamate, C.L.; Vutskits, L.; Rauen, T. Neuronal-induced and glutamate-dependent activation of glial glutamate transporter function. J. Neurochem. 2002, 82, 987–997. [Google Scholar] [CrossRef]
- Rose, E.M.; Koo, J.C.P.; Antflick, J.E.; Ahmed, S.M.; Angers, S.; Hampson, D.R. Glutamate Transporter Coupling to Na,K-ATPase. J. Neurosci. 2009, 29, 8143–8155. [Google Scholar] [CrossRef]
- Hussain, S.; Meneghini, E.; Moosmayer, M.; Lacotte, D.; Anner, B.M. Potent and reversible interaction of silver with pure Na,K-ATPase and Na,K-ATPase-liposomes. Biochim. Biophys. Acta Biomembr. 1994, 1190, 402–408. [Google Scholar] [CrossRef]
- Lima, T.; Bernfur, K.; Vilanova, M.; Cedervall, T. Understanding the lipid and protein corona formation on different sized polymeric nanoparticles. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Therien, A.G.; Blostein, R. Mechanisms of sodium pump regulation. Am. J. Physiol. Cell Physiol. 2000, 279, C541–C566. [Google Scholar] [CrossRef]
- Kimelberg, H.K.; Papahadjopoulos, D. Phospholipid requirements for (Na1,K1)-ATPase activity: Head group specificity and fatty acid fluidity. Biochim. Biophys. Acta Biomembr. 1972, 282, 277–292. [Google Scholar] [CrossRef]
- Oishi, K.; Zheng, B.; Kuo, J.F. Inhibition of Na,K-ATPase and sodium pump by protein kinase C regulators sphingosine, lysophosphatidylcholine, and oleic acid. J. Biol. Chem. 1990, 265, 70–75. [Google Scholar] [PubMed]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Booth, R.F.G.; Clark, J.B. A rapid method for the preparation of relatively pure metabolically competent synaptosomes from rat brain. Biochem. J. 1978, 176, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Daniels, K.K.; Vickroy, T.W. Simultaneous isolation of glial and neuronal fractions from rat brain homogenates: Comparison of high-affinity L-glutamate transport properties. Neurochem. Res. 1998, 23, 103–113. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Divac, I.; Fonnum, F.; Storm-Mathisen, J. High affinity uptake of glutamate in terminals of corticostriatal axons. Nat. Cell Biol. 1977, 266, 377–378. [Google Scholar] [CrossRef]
- Peng, L.; Hertz, L.; Huang, R.; Sonnewald, U.; Petersen, S.B.; Westergaard, N.; Larsson, O.; Schousboe, A. Utilization of glutamine and of TCA cycle constituents as precursors for transmitter glutamate and GABA. Dev. Neurosci. 1993, 15, 367–377. [Google Scholar] [CrossRef]








| Group | Silver Concentration | |||
|---|---|---|---|---|
| Serum (µg/L) | Brain (mg/kg w.w.) | |||
| Short-Term (PND 35) | Long-Term (PND 90) | Short-Term (PND 35) | Long-Term (PND 90) | |
| Control | 1.10 ± 0.79 | <DL1 | <DL2 | <DL2 |
| AgNPs | 22.57 ± 5.57 **** | 0.42 ± 0.06 #### | 0.15 ± 0.01 *** | 0.20 ± 0.03 ** |
| Ag citrate | 23.23 ± 4.83 **** | 0.40 ± 0.14 #### | 0.23 ± 0.03 ***,+++ | 0.53 ± 0.11 ****,+++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dąbrowska-Bouta, B.; Sulkowski, G.; Sałek, M.; Gewartowska, M.; Sidoryk-Węgrzynowicz, M.; Strużyńska, L. Early Postnatal Exposure to a Low Dose of Nanoparticulate Silver Induces Alterations in Glutamate Transporters in Brain of Immature Rats. Int. J. Mol. Sci. 2020, 21, 8977. https://doi.org/10.3390/ijms21238977
Dąbrowska-Bouta B, Sulkowski G, Sałek M, Gewartowska M, Sidoryk-Węgrzynowicz M, Strużyńska L. Early Postnatal Exposure to a Low Dose of Nanoparticulate Silver Induces Alterations in Glutamate Transporters in Brain of Immature Rats. International Journal of Molecular Sciences. 2020; 21(23):8977. https://doi.org/10.3390/ijms21238977
Chicago/Turabian StyleDąbrowska-Bouta, Beata, Grzegorz Sulkowski, Mikołaj Sałek, Magdalena Gewartowska, Marta Sidoryk-Węgrzynowicz, and Lidia Strużyńska. 2020. "Early Postnatal Exposure to a Low Dose of Nanoparticulate Silver Induces Alterations in Glutamate Transporters in Brain of Immature Rats" International Journal of Molecular Sciences 21, no. 23: 8977. https://doi.org/10.3390/ijms21238977
APA StyleDąbrowska-Bouta, B., Sulkowski, G., Sałek, M., Gewartowska, M., Sidoryk-Węgrzynowicz, M., & Strużyńska, L. (2020). Early Postnatal Exposure to a Low Dose of Nanoparticulate Silver Induces Alterations in Glutamate Transporters in Brain of Immature Rats. International Journal of Molecular Sciences, 21(23), 8977. https://doi.org/10.3390/ijms21238977





