Lipid Rafts and Dopamine Receptor Signaling
Abstract
1. Introduction
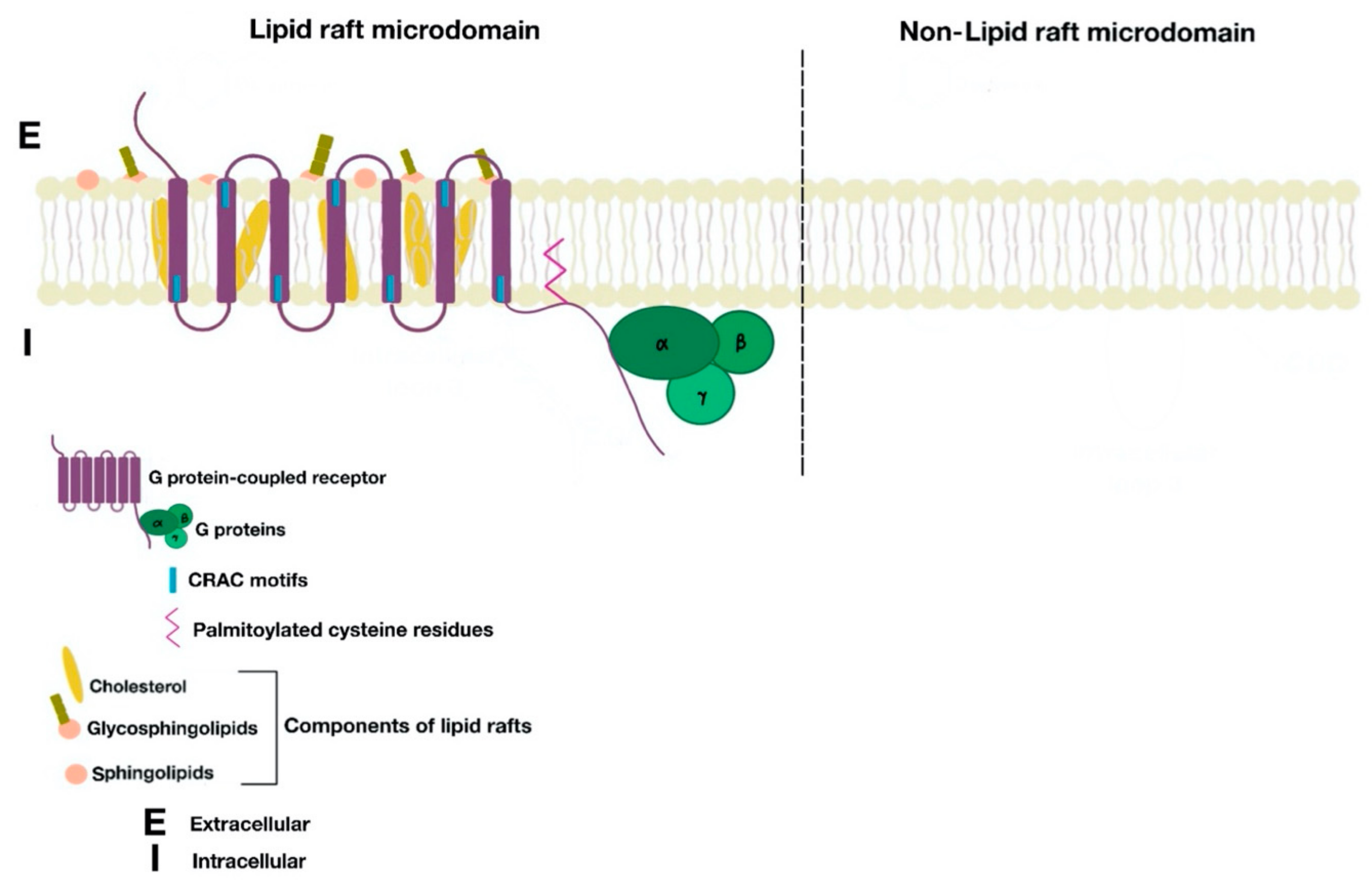
2. Definition of Lipid Rafts, Structure, Function, and Associated Components
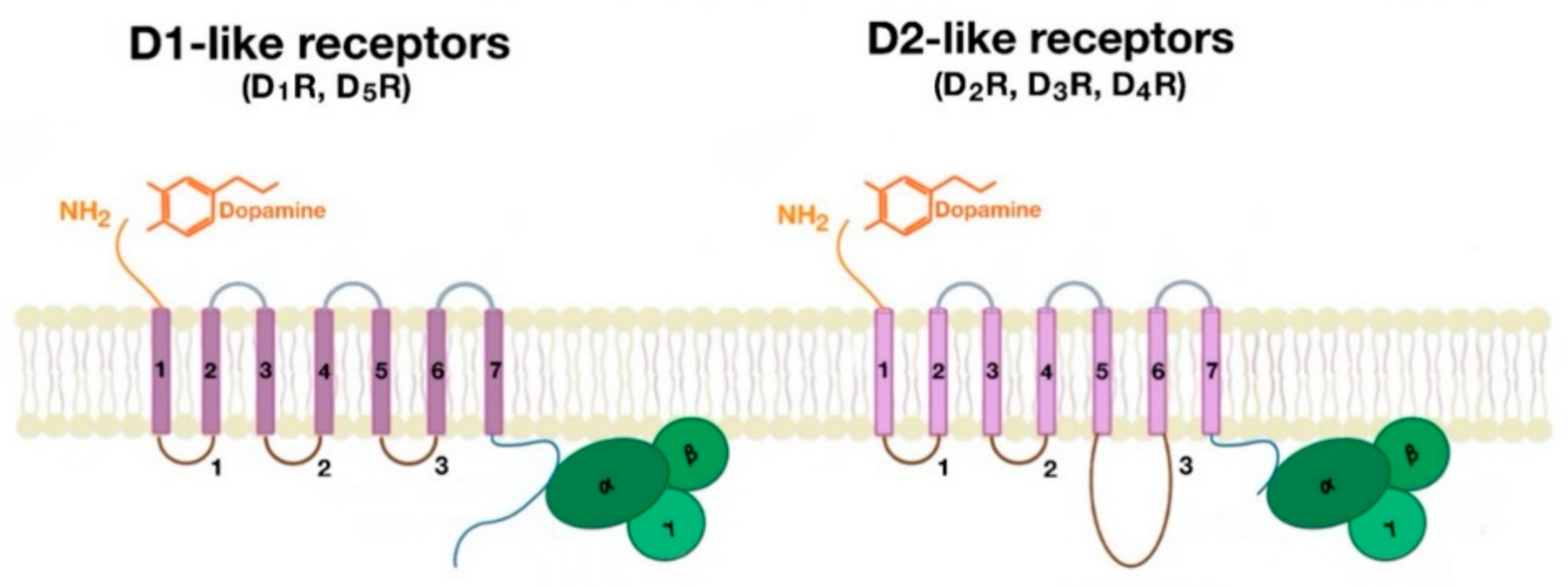
3. Definition of Dopamine and Classification of Dopamine Receptors
4. Function of Renal D1-Like and D2-Like Receptors
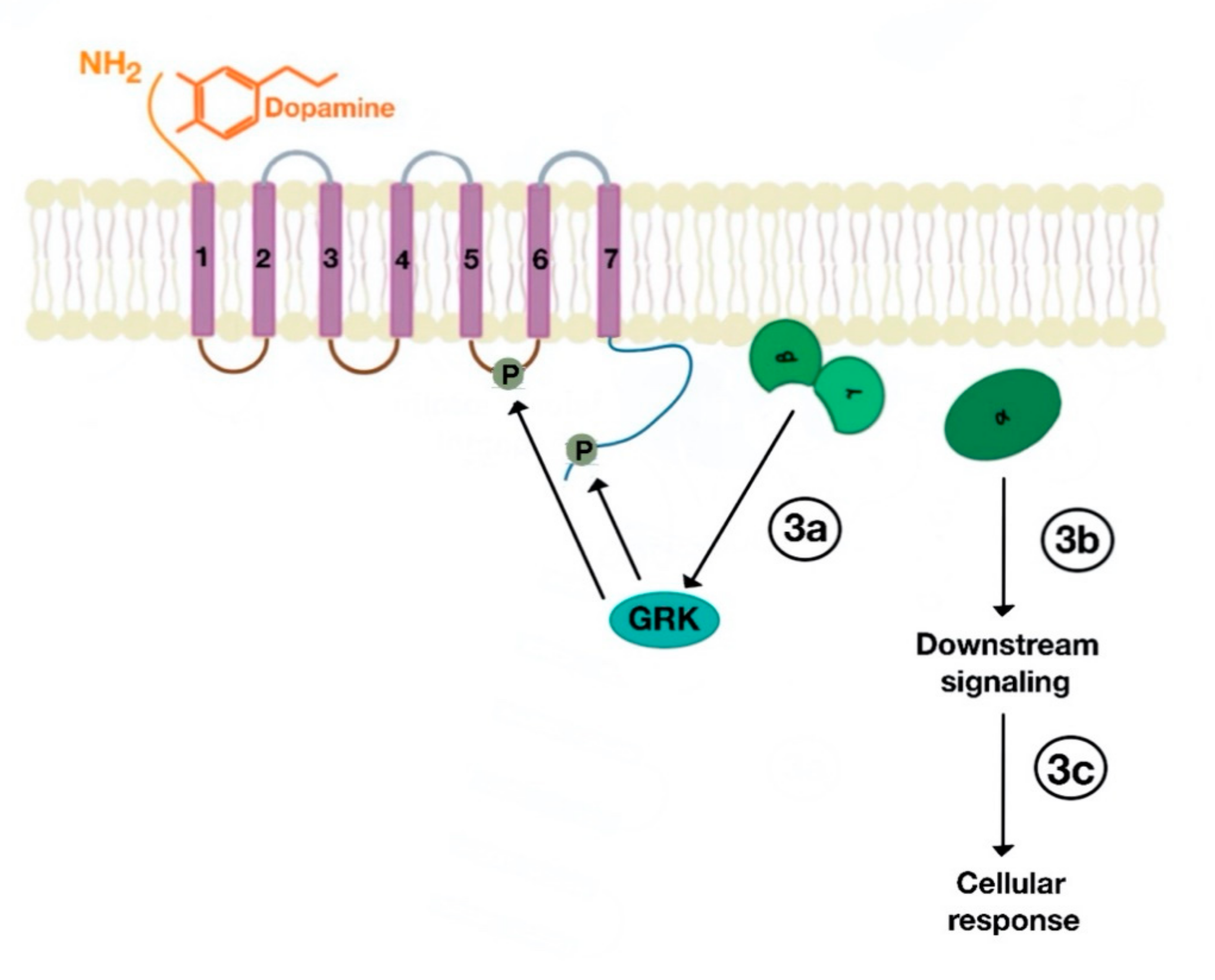
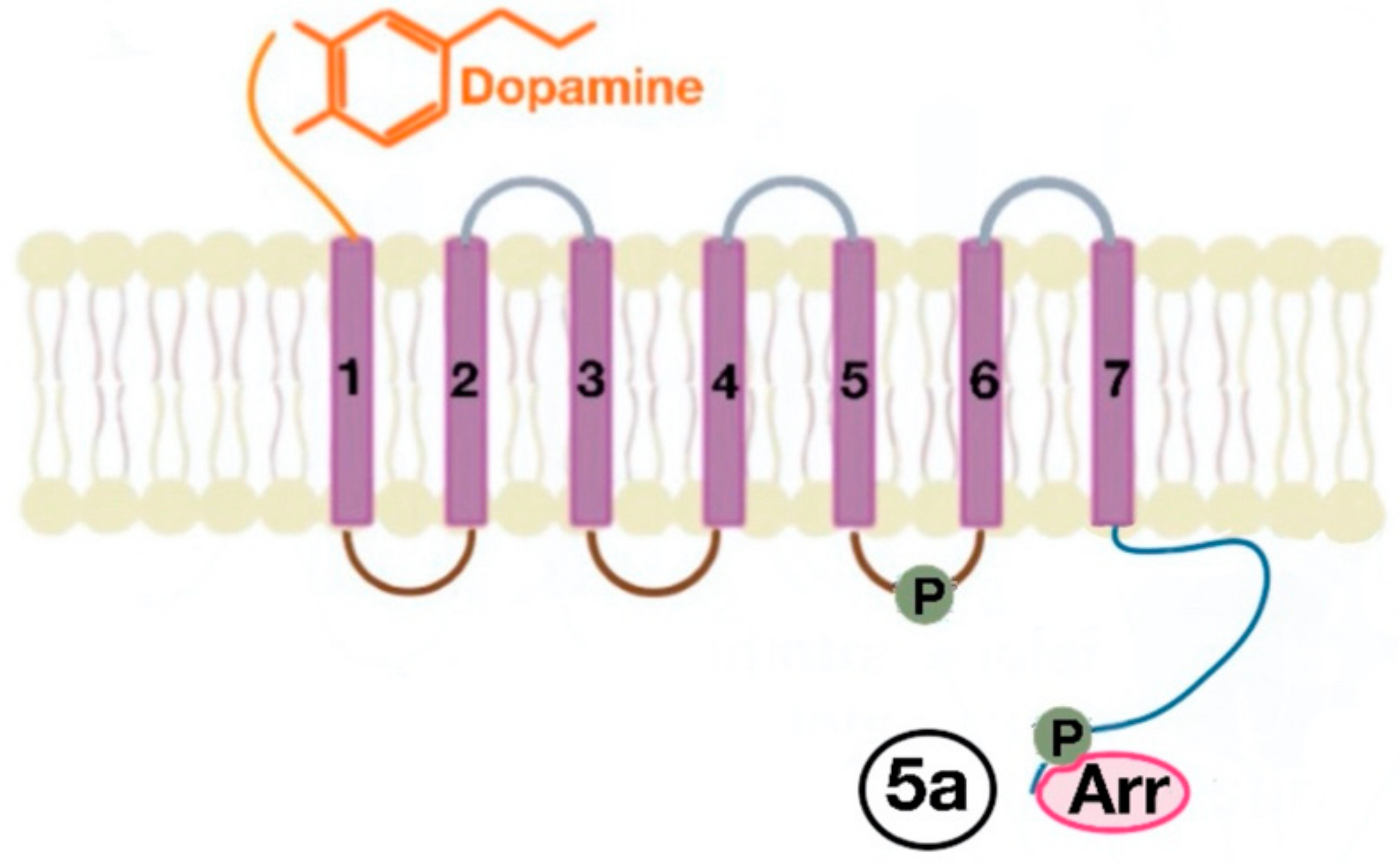
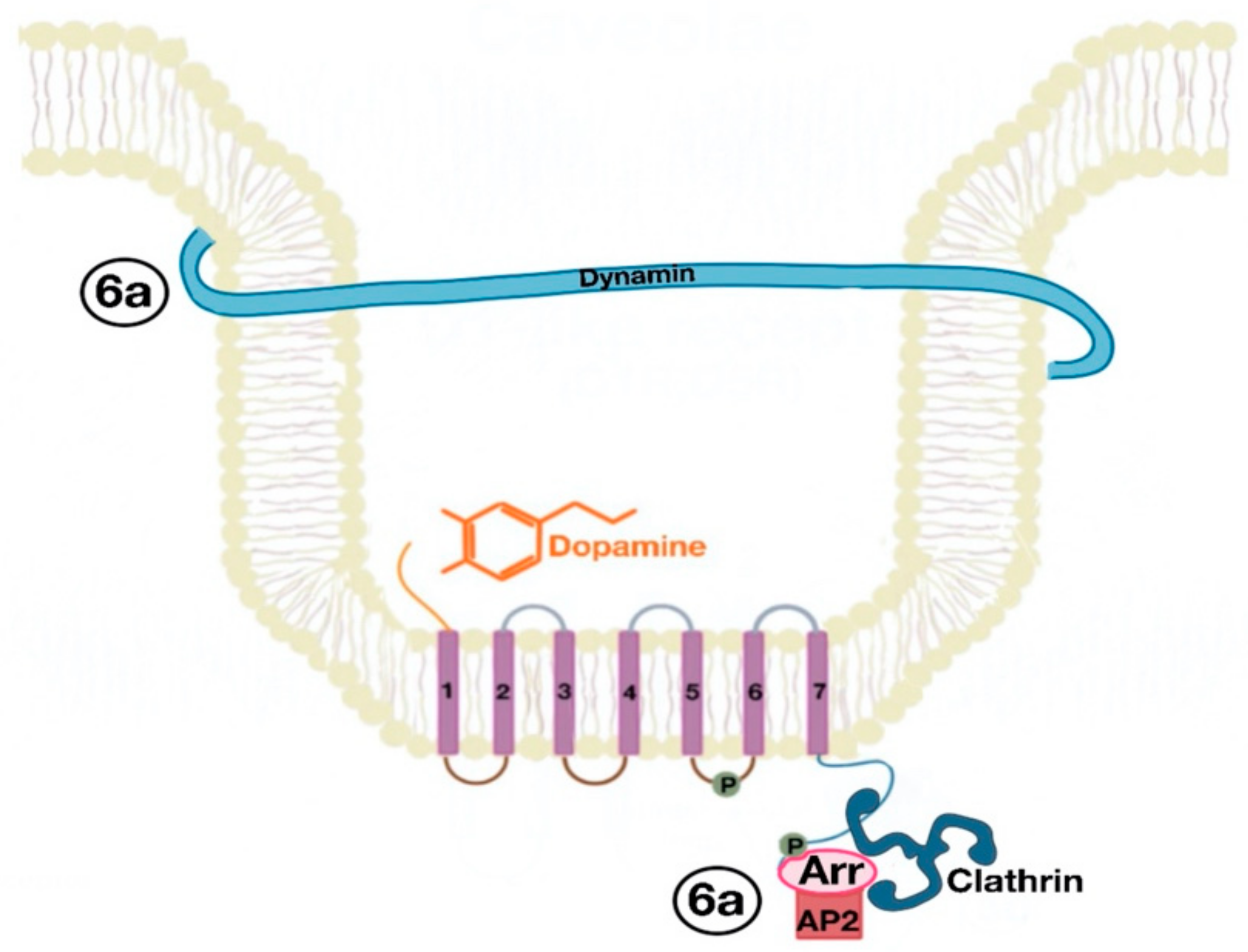
5. Signaling Pathway of Renal Dopamine Receptors
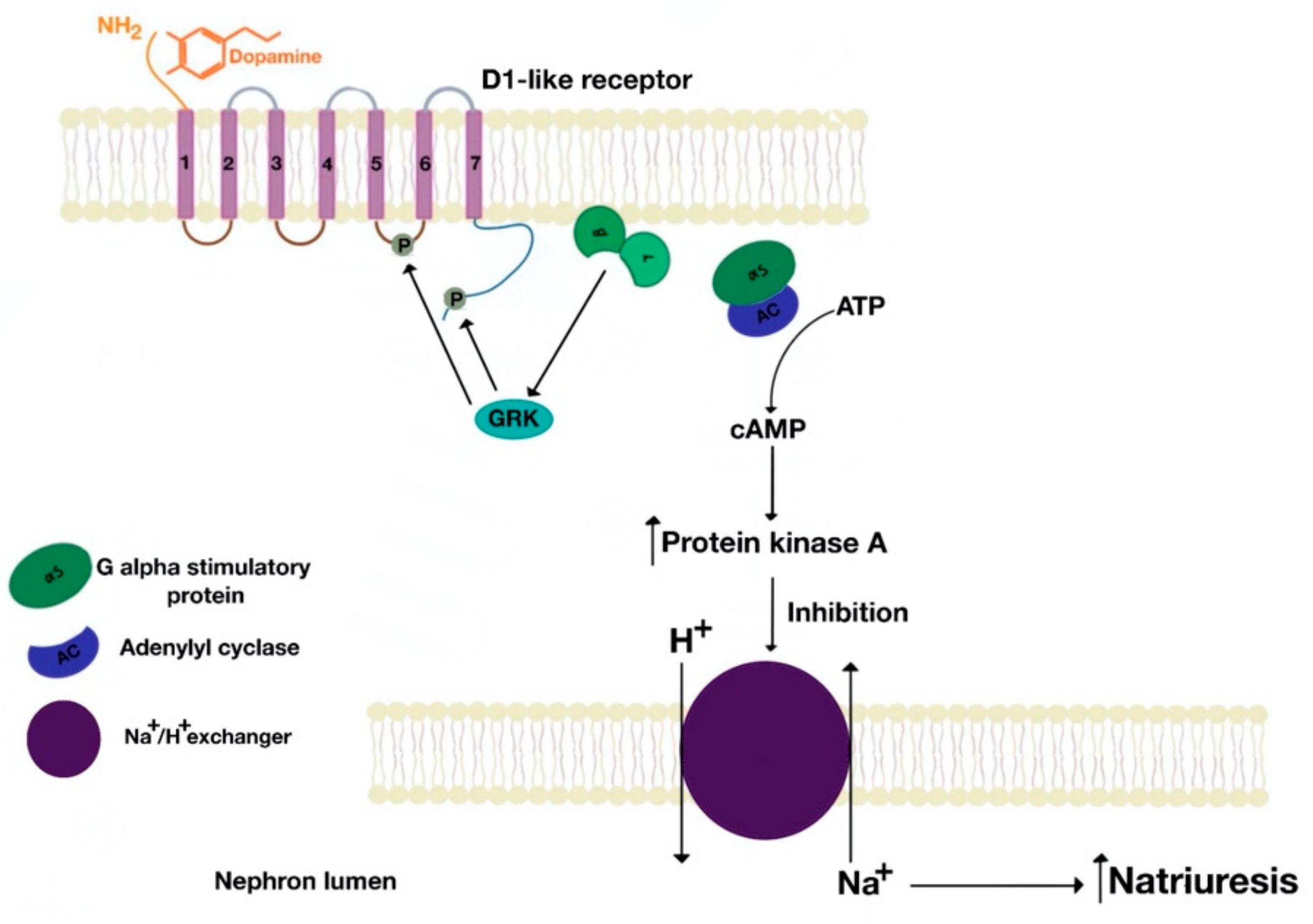
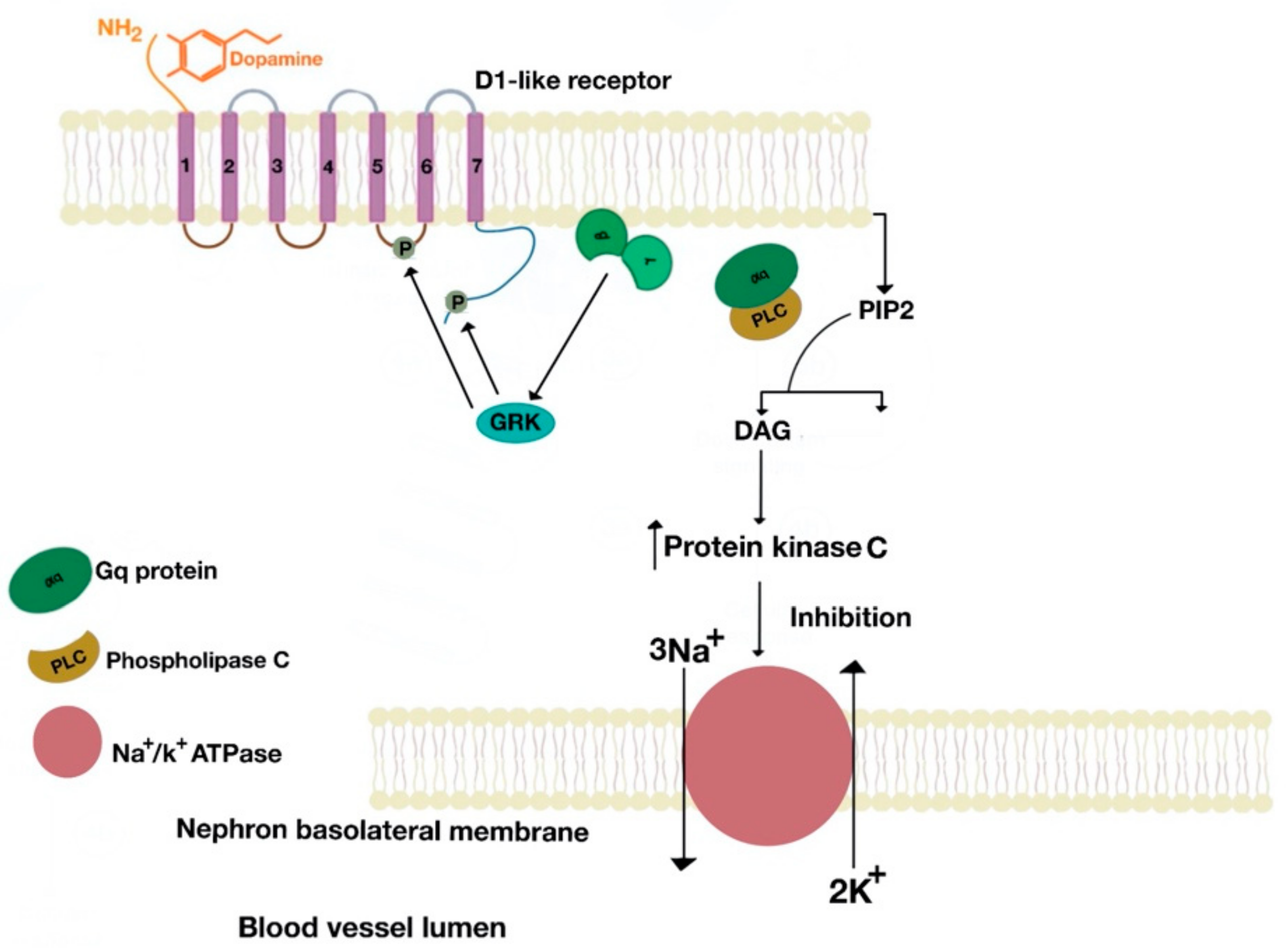
5.1. D1-Like Receptor Signaling Pathway
5.2. D2-Like Receptor Signaling Pathway
6. Role of the D1-Like and D2-Like Receptors in Natriuresis and Antinatriuresis
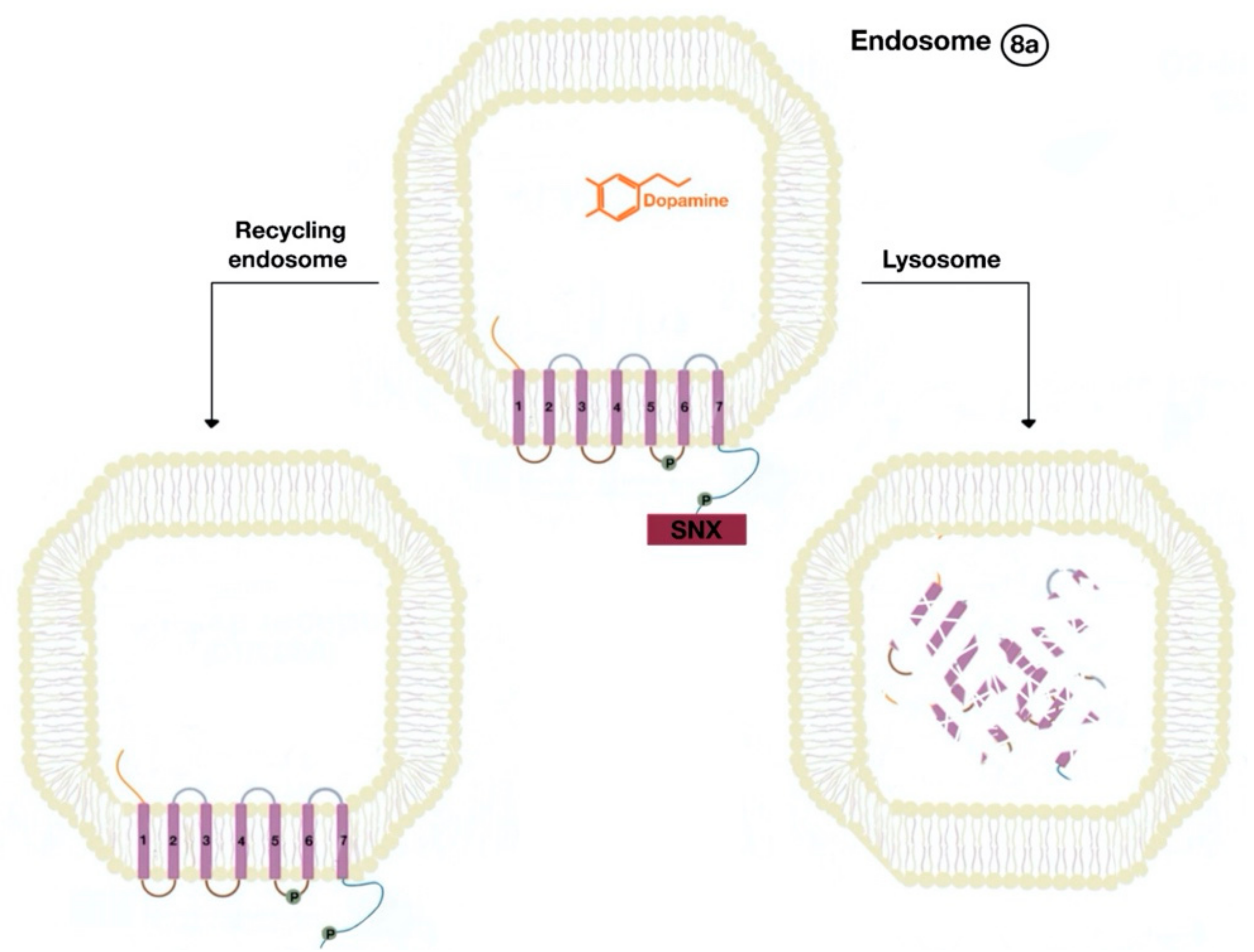
7. Renal D1-Like Receptors and Lipid Rafts
8. Renal D2-Like Receptors and Lipid Rafts
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Simons, K.; Ikonen, E. How cells handle cholesterol. Science 2000, 290, 1721–1726. [Google Scholar] [CrossRef]
- Villar, V.A.; Cuevas, S.; Zheng, X.; Jose, P.A. Localization and signaling of GPCRs in lipid rafts. Methods Cell Biol. 2016, 132, 3–23. [Google Scholar] [CrossRef]
- Armando, I.; Villar, V.A.; Jose, P.A. Dopamine and renal function and blood pressure regulation. Compr. Physiol. 2011, 1, 1075–1117. [Google Scholar] [CrossRef]
- Zeng, C.; Felder, R.A.; Jose, P.A. A new approach for treatment of hypertension: modifying D1 dopamine receptor function. Cardiovasc. Hematol. Agents Med. Chem. 2006, 4, 369–377. [Google Scholar] [CrossRef]
- Wang, X.; Villar, V.A.; Armando, I.; Eisner, G.M.; Felder, R.A.; Jose, P.A. Dopamine, kidney, and hypertension: Studies in dopamine receptor knockout mice. Pediatr. Nephrol. 2008, 23, 2131–2146. [Google Scholar] [CrossRef]
- Lingwood, D.; Simons, K. Lipid rafts as a membrane-organizing principle. Science 2010, 327, 46–50. [Google Scholar] [CrossRef]
- Busija, A.R.; Patel, H.H.; Insel, P.A. Caveolins and cavins in the trafficking, maturation, and degradation of caveolae: Implications for cell physiology. Am. J. Physiol. Cell Physiol. 2017, 312, C459–C477. [Google Scholar] [CrossRef]
- Guo, Y.; Jose, P.A. C-terminal di-leucine motif of dopamine D1 receptor plays an important role in its plasma membrane trafficking. PLoS ONE 2011, 6, e29204. [Google Scholar] [CrossRef]
- Wootten, D.; Christopoulos, A.; Marti-Solano, M.; Babu, M.M.; Sexton, P.M. Mechanisms of signalling and biased agonism in G protein-coupled receptors. Nat. Rev. Mol. Cell Biol 2018, 19, 638–653. [Google Scholar] [CrossRef]
- Rosenbaum, D.M.; Rasmussen, S.G.; Kobilka, B.K. The structure and function of G-protein-coupled receptors. Nature 2009, 459, 356–363. [Google Scholar] [CrossRef]
- Drake, M.T.; Shenoy, S.K.; Lefkowitz, R.J. Trafficking of G protein-coupled receptors. Circ. Res. 2006, 99, 570–582. [Google Scholar] [CrossRef]
- Jafurulla, M.; Tiwari, S.; Chattopadhyay, A. Identification of cholesterol recognition amino acid consensus (CRAC) motif in G-protein coupled receptors. Biochem. Biophys. Res. Commun. 2011, 404, 569–573. [Google Scholar] [CrossRef]
- Jean-Charles, P.Y.; Kaur, S.; Shenoy, S.K. G Protein-Coupled Receptor Signaling Through β-Arrestin-Dependent Mechanisms. J. Cardiovasc. Pharmacol. 2017, 70, 142–158. [Google Scholar] [CrossRef]
- Lefkowitz, R.J. G protein-coupled receptors. III. New roles for receptor kinases and beta-arrestins in receptor signaling and desensitization. J. Biol. Chem. 1998, 273, 18677–18680. [Google Scholar] [CrossRef]
- Worby, C.A.; Dixon, J.E. Sorting out the cellular functions of sorting nexins. Nat. Rev. Mol. Cell Biol. 2002, 3, 919–931. [Google Scholar] [CrossRef]
- Yang, J.; Villar, V.A.M.; Rozyyev, S.; Jose, P.A.; Zeng, C. The emerging role of sorting nexins in cardiovascular diseases. Clin. Sci. 2019, 133, 723–737. [Google Scholar] [CrossRef]
- Von Zastrow, M. Mechanisms regulating membrane trafficking of G protein-coupled receptors in the endocytic pathway. Life Sci. 2003, 74, 217–224. [Google Scholar] [CrossRef]
- Banday, A.A.; Lokhandwala, M.F. Dopamine receptors and hypertension. Curr. Hypertens. Rep. 2008, 10, 268–275. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Harris, R.C. Antihypertensive mechanisms of intra-renal dopamine. Curr. Opin. Nephrol. Hypertens. 2015, 24, 117–122. [Google Scholar] [CrossRef][Green Version]
- Missale, C.; Nash, S.R.; Robinson, S.W.; Jaber, M.; Caron, M.G. Dopamine receptors: From structure to function. Physiol. Rev. 1998, 78, 189–225. [Google Scholar] [CrossRef]
- Beaulieu, J.M.; Gainetdinov, R.R. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 2011, 63, 182–217. [Google Scholar] [CrossRef]
- Jose, P.A.; Eisner, G.M.; Felder, R.A. Role of dopamine receptors in the kidney in the regulation of blood pressure. Curr. Opin. Nephrol. Hypertens. 2002, 11, 87–92. [Google Scholar] [CrossRef]
- Jose, P.A.; Eisner, G.M.; Felder, R.A. Role of dopamine in the pathogenesis of hypertension. Clin. Exp. Pharmacol. Physiol. Suppl. 1999, 26, S10–S13. [Google Scholar]
- Preto, A.J.; Barreto, C.A.V.; Baptista, S.J.; Almeida, J.G.; Lemos, A.; Melo, A.; Cordeiro, M.N.D.S.; Kurkcuoglu, Z.; Melo, R.; Moreira, I.S. Understanding the Binding Specificity of G-Protein Coupled Receptors toward G-Proteins and Arrestins: Application to the Dopamine Receptor Family. J. Chem. Inf. Model. 2020, 60, 3969–3984. [Google Scholar] [CrossRef]
- Hussain, T.; Lokhandwala, M.F. Renal dopamine receptors and hypertension. Exp. Biol. Med. 2003, 228, 134–142. [Google Scholar] [CrossRef]
- Albrecht, F.E.; Xu, J.; Moe, O.W.; Hopfer, U.; Simonds, W.F.; Orlowski, J.; Jose, P.A. Regulation of NHE3 activity by G protein subunits in renal brush-border membranes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 278, R1064–R1073. [Google Scholar] [CrossRef]
- Kimura, K.; White, B.H.; Sidhu, A. Coupling of human D-1 dopamine receptors to different guanine nucleotide binding proteins. Evidence that D-1 dopamine receptors can couple to both Gs and G(o). J. Biol. Chem. 1995, 270, 14672–14678. [Google Scholar] [CrossRef]
- Sidhu, A.; Kimura, K.; Uh, M.; White, B.H.; Patel, S. Multiple coupling of human D5 dopamine receptors to guanine nucleotide binding proteins Gs and Gz. J. Neurochem. 1998, 70, 2459–2467. [Google Scholar] [CrossRef]
- Zheng, S.; Yu, P.; Zeng, C.; Wang, Z.; Yang, Z.; Andrews, P.M.; Felder, R.A.; Jose, P.A. Galpha12- and Galpha13-protein subunit linkage of D5 dopamine receptors in the nephron. Hypertension 2003, 41, 604–610. [Google Scholar] [CrossRef]
- Jin, L.Q.; Wang, H.Y.; Friedman, E. Stimulated D(1) dopamine receptors couple to multiple Galpha proteins in different brain regions. J. Neurochem. 2001, 78, 981–990. [Google Scholar] [CrossRef]
- Roosterman, D. Agonist-dependent and -independent dopamine-1-like receptor signalling differentially regulates downstream effectors. FEBS J. 2014, 281, 4792–4804. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.X.; Monsma, F.J.; Sibley, D.R.; Chiodo, L.A. D2L, D2S, and D3 dopamine receptors stably transfected into NG108-15 cells couple to a voltage-dependent potassium current via distinct G protein mechanisms. Synapse 1996, 24, 156–164. [Google Scholar] [CrossRef]
- Werner, P.; Hussy, N.; Buell, G.; Jones, K.A.; North, R.A. D2, D3, and D4 dopamine receptors couple to G protein-regulated potassium channels in Xenopus oocytes. Mol. Pharmacol. 1996, 49, 656–661. [Google Scholar] [PubMed]
- Momiyama, T.; Koga, E. Dopamine D(2)-like receptors selectively block N-type Ca(2+) channels to reduce GABA release onto rat striatal cholinergic interneurones. J. Physiol. 2001, 533, 479–492. [Google Scholar] [CrossRef]
- Gao, D.Q.; Canessa, L.M.; Mouradian, M.M.; Jose, P.A. Expression of the D2 subfamily of dopamine receptor genes in kidney. Am. J. Physiol. 1994, 266, F646–F650. [Google Scholar] [CrossRef]
- Senogles, S.E. D2s dopamine receptor mediates phospholipase D and antiproliferation. Mol. Cell. Endocrinol. 2003, 209, 61–69. [Google Scholar] [CrossRef]
- Obadiah, J.; Avidor-Reiss, T.; Fishburn, C.S.; Carmon, S.; Bayewitch, M.; Vogel, Z.; Fuchs, S.; Levavi-Sivan, B. Adenylyl cyclase interaction with the D2 dopamine receptor family; differential coupling to Gi, Gz, and Gs. Cell. Mol. Neurobiol. 1999, 19, 653–664. [Google Scholar] [CrossRef]
- Newman-Tancredi, A.; Cussac, D.; Audinot, V.; Pasteau, V.; Gavaudan, S.; Millan, M.J. G protein activation by human dopamine D3 receptors in high-expressing Chinese hamster ovary cells: A guanosine-5’-O-(3-[35S]thio)- triphosphate binding and antibody study. Mol. Pharmacol. 1999, 55, 564–574. [Google Scholar]
- Du, Z.; Yan, Q.; Wan, L.; Weinbaum, S.; Weinstein, A.M.; Wang, T. Regulation of glomerulotubular balance. I. Impact of dopamine on flow-dependent transport. Am. J. Physiol. Ren. Physiol. 2012, 303, F386–F395. [Google Scholar] [CrossRef]
- Agnoli, G.C.; Cacciari, M.; Garutti, C.; Ikonomu, E.; Lenzi, P.; Marchetti, G. Effects of extracellular fluid volume changes on renal response to low-dose dopamine infusion in normal women. Clin. Physiol. 1987, 7, 465–479. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Yao, B.; Wang, S.; Fan, X.; Wu, G.; Yang, H.; Yin, H.; Yang, S.; Harris, R.C. Intrarenal dopamine deficiency leads to hypertension and decreased longevity in mice. J. Clin. Investig. 2011, 121, 2845–2854. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Jose, P.A. Dopamine receptors: Important antihypertensive counterbalance against hypertensive factors. Hypertension 2011, 57, 11–17. [Google Scholar] [CrossRef]
- Carey, R.M. Theodore Cooper Lecture: Renal dopamine system: Paracrine regulator of sodium homeostasis and blood pressure. Hypertension 2001, 38, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.; Ward, A.S.; Foltin, R.W.; Fischman, M.W. Effects of ecopipam, a selective dopamine D1 antagonist, on smoked cocaine self-administration by humans. Psychopharmacology 2001, 155, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Henderson, D.C.; Daley, T.B.; Kunkel, L.; Rodrigues-Scott, M.; Koul, P.; Hayden, D. Clozapine and hypertension: A chart review of 82 patients. J. Clin. Psychiatry 2004, 65, 686–689. [Google Scholar] [CrossRef] [PubMed]
- Armando, I.; Konkalmatt, P.; Felder, R.A.; Jose, P.A. The renal dopaminergic system: Novel diagnostic and therapeutic approaches in hypertension and kidney disease. Transl. Res. 2015, 165, 505–511. [Google Scholar] [CrossRef][Green Version]
- Gildea, J.J.; Xu, P.; Carlson, J.M.; Gaglione, R.T.; Bigler Wang, D.; Kemp, B.A.; Reyes, C.M.; McGrath, H.E.; Carey, R.M.; Jose, P.A.; et al. The sodium-bicarbonate cotransporter NBCe2 (slc4a5) expressed in human renal proximal tubules shows increased apical expression under high-salt conditions. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R1447–R1459. [Google Scholar] [CrossRef]
- Kunimi, M.; Seki, G.; Hara, C.; Taniguchi, S.; Uwatoko, S.; Goto, A.; Kimura, S.; Fujita, T. Dopamine inhibits renal Na+:HCO3- cotransporter in rabbits and normotensive rats but not in spontaneously hypertensive rats. Kidney Int. 2000, 57, 534–543. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Felder, R.A.; Carey, R.M. Selective inhibition of the renal dopamine subtype D1A receptor induces antinatriuresis in conscious rats. Hypertension 1999, 33, 504–510. [Google Scholar] [CrossRef]
- Albrecht, F.E.; Drago, J.; Felder, R.A.; Printz, M.P.; Eisner, G.M.; Robillard, J.E.; Sibley, D.R.; Westphal, H.J.; Jose, P.A. Role of the D1A dopamine receptor in the pathogenesis of genetic hypertension. J. Clin. Investig. 1996, 97, 2283–2288. [Google Scholar] [CrossRef]
- Bacic, D.; Kaissling, B.; McLeroy, P.; Zou, L.; Baum, M.; Moe, O.W. Dopamine acutely decreases apical membrane Na/H exchanger NHE3 protein in mouse renal proximal tubule. Kidney Int. 2003, 64, 2133–2141. [Google Scholar] [CrossRef] [PubMed]
- Felder, C.C.; Albrecht, F.E.; Campbell, T.; Eisner, G.M.; Jose, P.A. cAMP-independent, G protein-linked inhibition of Na+/H+ exchange in renal brush border by D1 dopamine agonists. Am. J. Physiol. 1993, 264, F1032–F1037. [Google Scholar] [CrossRef] [PubMed]
- Gesek, F.A.; Schoolwerth, A.C. Hormonal interactions with the proximal Na(+)-H+ exchanger. Am. J. Physiol. 1990, 258, F514–F521. [Google Scholar] [CrossRef]
- Gesek, F.A.; Schoolwerth, A.C. Hormone responses of proximal Na(+)-H+ exchanger in spontaneously hypertensive rats. Am. J. Physiol. 1991, 261, F526–F536. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.C.; Fan, L.; Crowder, L.A.; Karim-Jimenez, Z.; Murer, H.; Moe, O.W. Dopamine acutely stimulates Na+/H+ exchanger (NHE3) endocytosis via clathrin-coated vesicles: Dependence on protein kinase A-mediated NHE3 phosphorylation. J. Biol. Chem. 2001, 276, 26906–26915. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, W.; Du, P.; Yu, K.Q.; Fu, W. Molecular insights into the D1R agonist and D2R/D3R antagonist effects of the natural product (-)-stepholidine: Molecular modeling and dynamics simulations. J. Phys. Chem. B 2012, 116, 8121–8130. [Google Scholar] [CrossRef] [PubMed]
- Mohr, P.; Decker, M.; Enzensperger, C.; Lehmann, J. Dopamine/serotonin receptor ligands. 12(1): SAR studies on hexahydro-dibenz[d,g]azecines lead to 4-chloro-7-methyl-5,6,7,8,9,14-hexahydrodibenz[d,g]azecin-3-ol, the first picomolar D5-selective dopamine-receptor antagonist. J. Med. Chem. 2006, 49, 2110–2116. [Google Scholar] [CrossRef]
- Gildea, J.J.; Shah, I.T.; Van Sciver, R.E.; Israel, J.A.; Enzensperger, C.; McGrath, H.E.; Jose, P.A.; Felder, R.A. The cooperative roles of the dopamine receptors, D1R and D5R, on the regulation of renal sodium transport. Kidney Int. 2014, 86, 118–126. [Google Scholar] [CrossRef]
- Narkar, V.; Hussain, T.; Lokhandwala, M. Role of tyrosine kinase and p44/42 MAPK in D(2)-like receptor-mediated stimulation of Na(+), K(+)-ATPase in kidney. Am. J. Physiol. Ren. Physiol. 2002, 282, F697–F702. [Google Scholar] [CrossRef]
- Yamaguchi, I.; Walk, S.F.; Jose, P.A.; Felder, R.A. Dopamine D2L receptors stimulate Na+/K(+)-ATPase activity in murine LTK- cells. Mol. Pharmacol. 1996, 49, 373–378. [Google Scholar]
- Zhang, Y.; Fu, C.; Asico, L.D.; Villar, V.A.; Ren, H.; He, D.; Wang, Z.; Yang, J.; Jose, P.A.; Zeng, C. Role of Gα(12)- and Gα(13)-protein subunit linkage of D(3) dopamine receptors in the natriuretic effect of D(3) dopamine receptor in kidney. Hypertens. Res. 2011, 34, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, H.; Lu, X.; He, D.; Han, Y.; Wang, H.; Zeng, C.; Shi, W. Inhibition of D4 Dopamine Receptors on Insulin Receptor Expression and Effect in Renal Proximal Tubule Cells. J. Am. Heart Assoc. 2016, 5, e002448. [Google Scholar] [CrossRef] [PubMed]
- Smit, A.J.; Meijer, S.; Wesseling, H.; Donker, A.J.; Reitsma, W.D. Dissociation of renal vasodilator and natriuretic effects of dopamine during sulpiride infusion in normal man. Eur. J. Clin. Pharmacol. 1990, 39, 221–226. [Google Scholar] [CrossRef]
- Luippold, G.; Zimmermann, C.; Mai, M.; Kloor, D.; Starck, D.; Gross, G.; Mühlbauer, B. Dopamine D(3) receptors and salt-dependent hypertension. J. Am. Soc. Nephrol. 2001, 12, 2272–2279. [Google Scholar] [PubMed]
- Fantini, J.; Di Scala, C.; Baier, C.J.; Barrantes, F.J. Molecular mechanisms of protein-cholesterol interactions in plasma membranes: Functional distinction between topological (tilted) and consensus (CARC/CRAC) domains. Chem. Phys. Lipids 2016, 199, 52–60. [Google Scholar] [CrossRef]
- Chidlow, J.H.; Sessa, W.C. Caveolae, caveolins, and cavins: Complex control of cellular signalling and inflammation. Cardiovasc. Res. 2010, 86, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Sun, M.; Villar, V.A.; Zhang, Y.; Weinman, E.J.; Felder, R.A.; Jose, P.A. Differential dopamine receptor subtype regulation of adenylyl cyclases in lipid rafts in human embryonic kidney and renal proximal tubule cells. Cell. Signal. 2014, 26, 2521–2529. [Google Scholar] [CrossRef]
- Han, W.; Li, H.; Villar, V.A.; Pascua, A.M.; Dajani, M.I.; Wang, X.; Natarajan, A.; Quinn, M.T.; Felder, R.A.; Jose, P.A.; et al. Lipid rafts keep NADPH oxidase in the inactive state in human renal proximal tubule cells. Hypertension 2008, 51, 481–487. [Google Scholar] [CrossRef]
- Villar, V.A.; Armando, I.; Sanada, H.; Frazer, L.C.; Russo, C.M.; Notario, P.M.; Lee, H.; Comisky, L.; Russell, H.A.; Yang, Y.; et al. Novel role of sorting nexin 5 in renal D(1) dopamine receptor trafficking and function: Implications for hypertension. FASEB J. 2013, 27, 1808–1819. [Google Scholar] [CrossRef]
- Tiu, A.C.; Yang, J.; Asico, L.D.; Konkalmatt, P.; Zheng, X.; Cuevas, S.; Wang, X.; Lee, H.; Mazhar, M.; Felder, R.A.; et al. Lipid rafts are required for effective renal D1 dopamine receptor function. FASEB J. 2020, 34, 6999–7017. [Google Scholar] [CrossRef]
- Gildea, J.J.; Israel, J.A.; Johnson, A.K.; Zhang, J.; Jose, P.A.; Felder, R.A. Caveolin-1 and dopamine-mediated internalization of NaKATPase in human renal proximal tubule cells. Hypertension 2009, 54, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Gildea, J.J.; Kemp, B.A.; Howell, N.L.; Van Sciver, R.E.; Carey, R.M.; Felder, R.A. Inhibition of renal caveolin-1 reduces natriuresis and produces hypertension in sodium-loaded rats. Am. J. Physiol. Ren. Physiol. 2011, 300, F914–F920. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.M.; Hasbi, A.; Mattocks, M.; Fan, T.; O’Dowd, B.F.; George, S.R. Regulation of D1 dopamine receptor trafficking and signaling by caveolin-1. Mol. Pharmacol. 2007, 72, 1157–1170. [Google Scholar] [CrossRef] [PubMed]
- Somkuwar, S.S.; Fannon, M.J.; Head, B.P.; Mandyam, C.D. Methamphetamine reduces expression of caveolin-1 in the dorsal striatum: Implication for dysregulation of neuronal function. Neuroscience 2016, 328, 147–156. [Google Scholar] [CrossRef][Green Version]
- Wang, C.; Niu, M.; Zhou, Z.; Zheng, X.; Zhang, L.; Tian, Y.; Yu, X.; Bu, G.; Xu, H.; Ma, Q.; et al. VPS35 regulates cell surface recycling and signaling of dopamine receptor D1. Neurobiol. Aging 2016, 46, 22–31. [Google Scholar] [CrossRef]
- Yu, P.; Yang, Z.; Jones, J.E.; Wang, Z.; Owens, S.A.; Mueller, S.C.; Felder, R.A.; Jose, P.A. D1 dopamine receptor signaling involves caveolin-2 in HEK-293 cells. Kidney Int. 2004, 66, 2167–2180. [Google Scholar] [CrossRef]
- Liu, C.; Xi, B. Pooled analyses of the associations of polymorphisms in the GRK4 and EMILIN1 genes with hypertension risk. Int. J. Med. Sci. 2012, 9, 274–279. [Google Scholar] [CrossRef]
- Yang, J.; Villar, V.A.; Jones, J.E.; Jose, P.A.; Zeng, C. G protein-coupled receptor kinase 4: Role in hypertension. Hypertension 2015, 65, 1148–1155. [Google Scholar] [CrossRef]
- Felder, R.A.; Sanada, H.; Xu, J.; Yu, P.Y.; Wang, Z.; Watanabe, H.; Asico, L.D.; Wang, W.; Zheng, S.; Yamaguchi, I.; et al. G protein-coupled receptor kinase 4 gene variants in human essential hypertension. Proc. Natl. Acad. Sci. USA 2002, 99, 3872–3877. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, C.; Villar, V.A.; Chen, S.Y.; Konkalmatt, P.; Wang, X.; Asico, L.D.; Jones, J.E.; Yang, Y.; Sanada, H.; et al. Human GRK4γ142V Variant Promotes Angiotensin II Type I Receptor-Mediated Hypertension via Renal Histone Deacetylase Type 1 Inhibition. Hypertension 2016, 67, 325–334. [Google Scholar] [CrossRef]
- Allen, S.J.; Parthasarathy, G.; Darke, P.L.; Diehl, R.E.; Ford, R.E.; Hall, D.L.; Johnson, S.A.; Reid, J.C.; Rickert, K.W.; Shipman, J.M.; et al. Structure and Function of the Hypertension Variant A486V of G Protein-coupled Receptor Kinase 4. J. Biol. Chem. 2015, 290, 20360–20373. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Villar, V.A.; Armando, I.; Jose, P.A.; Zeng, C. G Protein-Coupled Receptor Kinases: Crucial Regulators of Blood Pressure. J. Am. Heart Assoc. 2016, 5, e003519. [Google Scholar] [CrossRef] [PubMed]
- Rayner, B.; Ramesar, R. The importance of G protein-coupled receptor kinase 4 (GRK4) in pathogenesis of salt sensitivity, salt sensitive hypertension and response to antihypertensive treatment. Int. J. Mol. Sci. 2015, 16, 5741–5749. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Xu, J.; Bengra, C.; Jose, P.A.; Felder, R.A. Desensitization of human renal D1 dopamine receptors by G protein-coupled receptor kinase 4. Kidney Int. 2002, 62, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Sanada, H.; Yoneda, M.; Yatabe, J.; Williams, S.M.; Bartlett, J.; White, M.J.; Gordon, L.N.; Felder, R.A.; Eisner, G.M.; Armando, I.; et al. Common variants of the G protein-coupled receptor type 4 are associated with human essential hypertension and predict the blood pressure response to angiotensin receptor blockade. Pharm. J. 2016, 16, 3–9. [Google Scholar] [CrossRef]
- Kimura, L.; Angeli, C.B.; Auricchio, M.T.; Fernandes, G.R.; Pereira, A.C.; Vicente, J.P.; Pereira, T.V.; Mingroni-Netto, R.C. Multilocus family-based association analysis of seven candidate polymorphisms with essential hypertension in an African-derived semi-isolated Brazilian population. Int. J. Hypertens. 2012, 2012, 859219. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Han, W.; Villar, V.A.; Keever, L.B.; Lu, Q.; Hopfer, U.; Quinn, M.T.; Felder, R.A.; Jose, P.A.; Yu, P. D1-like receptors regulate NADPH oxidase activity and subunit expression in lipid raft microdomains of renal proximal tubule cells. Hypertension 2009, 53, 1054–1061. [Google Scholar] [CrossRef]
- Hailstones, D.; Sleer, L.S.; Parton, R.G.; Stanley, K.K. Regulation of caveolin and caveolae by cholesterol in MDCK cells. J. Lipid Res. 1998, 39, 369–379. [Google Scholar]
- Wang, J.; Fedoseienko, A.; Chen, B.; Burstein, E.; Jia, D.; Billadeau, D.D. Endosomal receptor trafficking: Retromer and beyond. Traffic 2018, 19, 578–590. [Google Scholar] [CrossRef]
- Gallon, M.; Cullen, P.J. Retromer and sorting nexins in endosomal sorting. Biochem. Soc. Trans. 2015, 43, 33–47. [Google Scholar] [CrossRef]
- Teasdale, R.D.; Collins, B.M. Insights into the PX (phox-homology) domain and SNX (sorting nexin) protein families: Structures, functions and roles in disease. Biochem. J. 2012, 441, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Bek, M.J.; Zheng, S.; Xu, J.; Yamaguchi, I.; Asico, L.D.; Sun, X.G.; Jose, P.A. Differential expression of adenylyl cyclases in the rat nephron. Kidney Int. 2001, 60, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yang, J.; Jones, J.E.; Villar, V.A.; Yu, P.; Armando, I.; Felder, R.A.; Jose, P.A. Sorting nexin 5 and dopamine d1 receptor regulate the expression of the insulin receptor in human renal proximal tubule cells. Endocrinology 2015, 156, 2211–2221. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, J.; Villar, V.; Yang, J.A.; Jones, J.E.; Guo, Y.; Asico, L.D.; Armando, I.; Weinman, E.J.; Jose, P.A. Sorting nexin 19: A novel regulator of renal dopamine D1 receptor. Hypertension 2014, 64, A296. [Google Scholar]
- Heydorn, A.; Søndergaard, B.P.; Hadrup, N.; Holst, B.; Haft, C.R.; Schwartz, T.W. Distinct in vitro interaction pattern of dopamine receptor subtypes with adaptor proteins involved in post-endocytotic receptor targeting. FEBS Lett. 2004, 556, 276–280. [Google Scholar] [CrossRef]
- Villar, V.A.; Jones, J.E.; Armando, I.; Asico, L.D.; Escano, C.S.; Lee, H.; Wang, X.; Yang, Y.; Pascua-Crusan, A.M.; Palmes-Saloma, C.P.; et al. Sorting nexin 1 loss results in D5 dopamine receptor dysfunction in human renal proximal tubule cells and hypertension in mice. J. Biol. Chem. 2013, 288, 152–163. [Google Scholar] [CrossRef]
- Yang, J.; Asico, L.D.; Beitelshees, A.L.; Feranil, J.B.; Wang, X.; Jones, J.E.; Armando, I.; Cuevas, S.G.; Schwartz, G.L.; Gums, J.G.; et al. Sorting nexin 1 loss results in increased oxidative stress and hypertension. FASEB J. 2020, 34, 7941–7957. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Chen, C.; Guo, L.; Xu, Z.; Peng, X.; Wang, X.; Wang, J.; Wang, N.; Li, C.; Luo, X.; et al. Exposure to Maternal Diabetes Mellitus Causes Renal Dopamine D1 Receptor Dysfunction and Hypertension in Adult Rat Offspring. Hypertension 2018, 72, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.R.; Kouyoumdzian, N.M.; Rukavina Mikusic, N.L.; Kravetz, M.C.; Rosón, M.I.; Rodríguez Fermepin, M.; Fernández, B.E. Renal dopaminergic system: Pathophysiological implications and clinical perspectives. World J. Nephrol. 2015, 4, 196–212. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Celver, J.; Octeau, J.C.; Kovoor, A. Plasma membrane compartmentalization of D2 dopamine receptors. J. Biol. Chem. 2013, 288, 12554–12568. [Google Scholar] [CrossRef]
- Genedani, S.; Guidolin, D.; Leo, G.; Filaferro, M.; Torvinen, M.; Woods, A.S.; Fuxe, K.; Ferré, S.; Agnati, L.F. Computer-assisted image analysis of caveolin-1 involvement in the internalization process of adenosine A2A-dopamine D2 receptor heterodimers. J. Mol. Neurosci. 2005, 26, 177–184. [Google Scholar] [CrossRef]
- Voulalas, P.J.; Schetz, J.; Undieh, A.S. Differential subcellular distribution of rat brain dopamine receptors and subtype-specific redistribution induced by cocaine. Mol. Cell. Neurosci. 2011, 46, 645–654. [Google Scholar] [CrossRef]
- Lan, H.; Teeter, M.M.; Gurevich, V.V.; Neve, K.A. An intracellular loop 2 amino acid residue determines differential binding of arrestin to the dopamine D2 and D3 receptors. Mol. Pharmacol. 2009, 75, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Wandelmer, J.; Dávalos, A.; de la Peña, G.; Cano, S.; Giera, M.; Canfrán-Duque, A.; Bracher, F.; Martín-Hidalgo, A.; Fernández-Hernando, C.; Lasunción, M.A.; et al. Haloperidol disrupts lipid rafts and impairs insulin signaling in SH-SY5Y cells. Neuroscience 2010, 167, 143–153. [Google Scholar] [CrossRef]
- Zhang, X.; Kim, K.M. Palmitoylation of the carboxyl-terminal tail of dopamine D4 receptor is required for surface expression, endocytosis, and signaling. Biochem. Biophys. Res. Commun. 2016, 479, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Free, R.B.; Namkung, Y.; Hazelwood, L.A.; Sibley, D.R. Sorting nexin-25 interacts with D1 and D2 dopamine receptors to regulate receptor expression and signaling. FASEB J. 2010, 21, 568–569. [Google Scholar] [CrossRef]
- Free, R.B.; Hazelwood, L.A.; Spalding, H.N.; Cabrera, D.M.; Sibley, D.R. Sorting nexin-25, a novel member of the dopamine receptor signalplex, up-regulates D1 and D2 dopamine receptor expression in HEK293 cells. FASEB J. 2007, 21, A423. [Google Scholar] [CrossRef]
- Rifkin, R.A.; Huyghe, D.; Li, X.; Parakala, M.; Aisenberg, E.; Moss, S.J.; Slesinger, P.A. GIRK currents in VTA dopamine neurons control the sensitivity of mice to cocaine-induced locomotor sensitization. Proc. Natl. Acad. Sci. USA 2018, 115, E9479–E9488. [Google Scholar] [CrossRef]
- Mystek, P.; Dutka, P.; Tworzydło, M.; Dziedzicka-Wasylewska, M.; Polit, A. The role of cholesterol and sphingolipids in the dopamine D1 receptor and G protein distribution in the plasma membrane. Biochem. Biophys. Acta 2016, 1861, 1775–1786. [Google Scholar]




| D1R and D5R | D2R and D3R | D2R and D4R | |
|---|---|---|---|
| Amino acid sequence conservation within transmembrane domain | 80% | 75% | 53% |
| D1R and D5R | D2R | D3R | D4R | |
|---|---|---|---|---|
| Number of consensus N-glycosylation sites | 2 | 4 | 3 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez, V.J.; Asico, L.D.; Jose, P.A.; Tiu, A.C. Lipid Rafts and Dopamine Receptor Signaling. Int. J. Mol. Sci. 2020, 21, 8909. https://doi.org/10.3390/ijms21238909
Martinez VJ, Asico LD, Jose PA, Tiu AC. Lipid Rafts and Dopamine Receptor Signaling. International Journal of Molecular Sciences. 2020; 21(23):8909. https://doi.org/10.3390/ijms21238909
Chicago/Turabian StyleMartinez, Victor J., Laureano D. Asico, Pedro A. Jose, and Andrew C. Tiu. 2020. "Lipid Rafts and Dopamine Receptor Signaling" International Journal of Molecular Sciences 21, no. 23: 8909. https://doi.org/10.3390/ijms21238909
APA StyleMartinez, V. J., Asico, L. D., Jose, P. A., & Tiu, A. C. (2020). Lipid Rafts and Dopamine Receptor Signaling. International Journal of Molecular Sciences, 21(23), 8909. https://doi.org/10.3390/ijms21238909






