Untargeted Lipidomics Analysis of the Cyanobacterium Synechocystis sp. PCC 6803: Lipid Composition Variation in Response to Alternative Cultivation Setups and to Gene Deletion
Abstract
1. Introduction
2. Results and Discussion
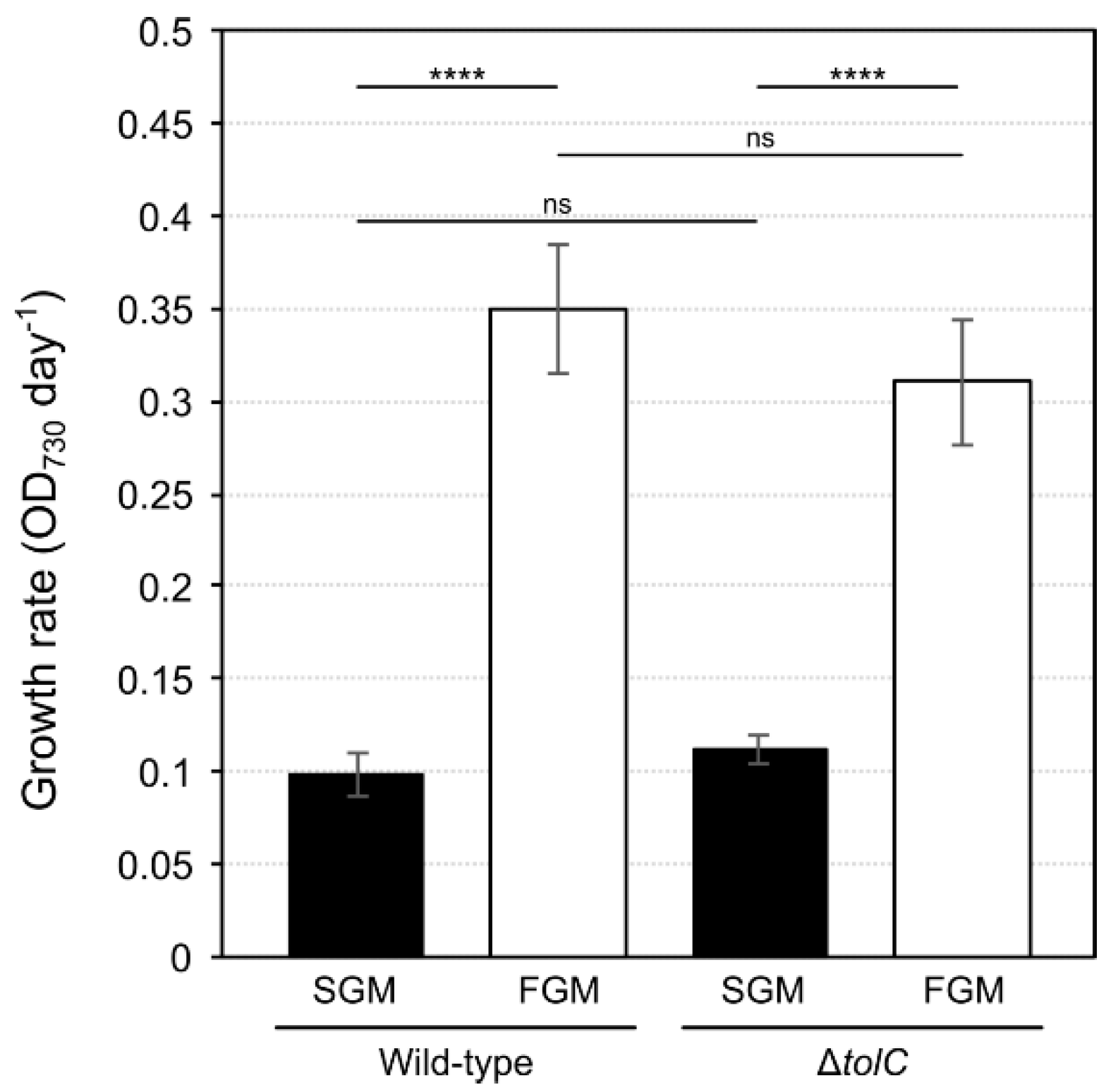
2.1. Different Photoautotrophic Cultivation Setups Result in Marked Differences in Cyanobacterial Growth Rates
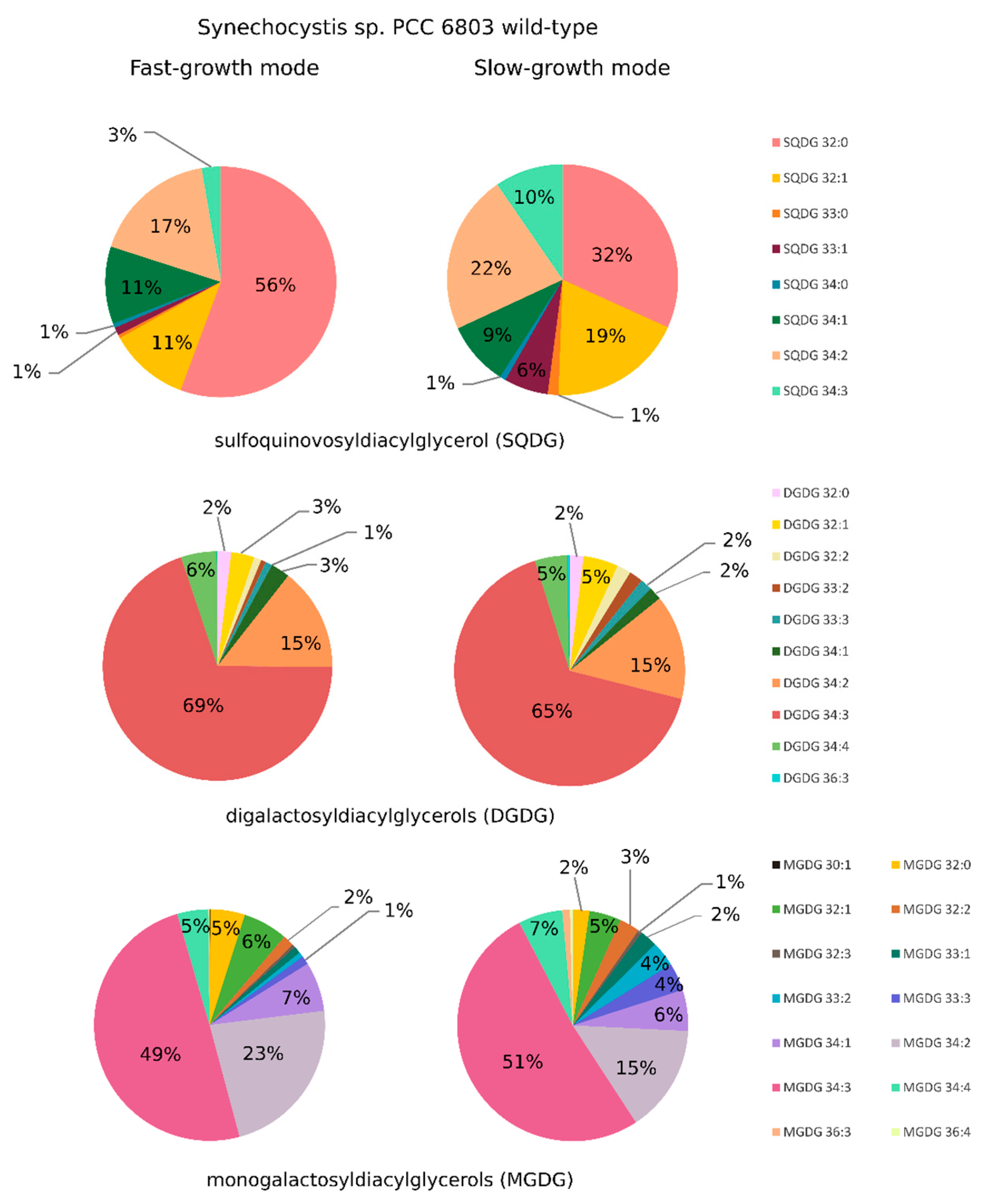
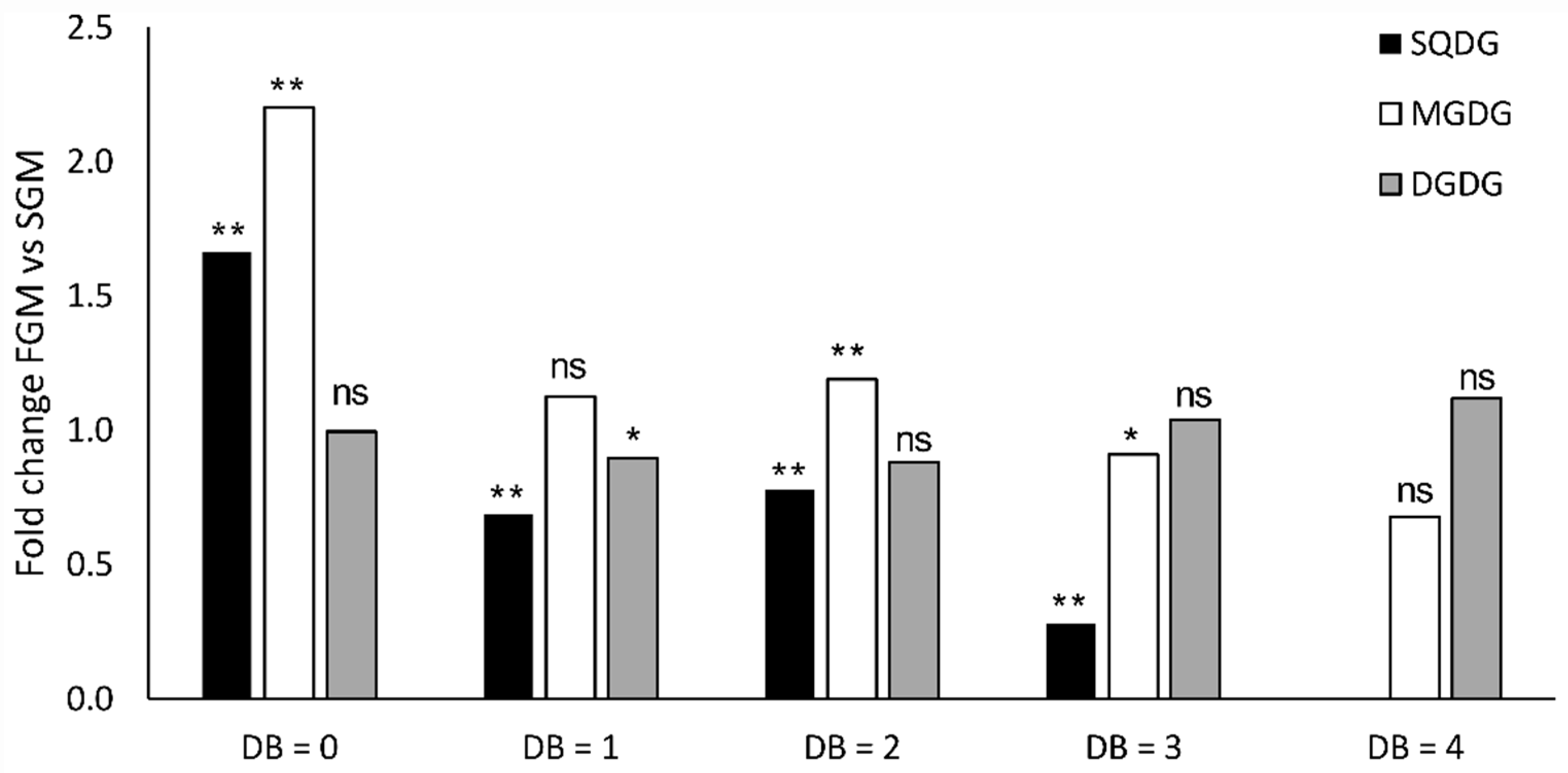
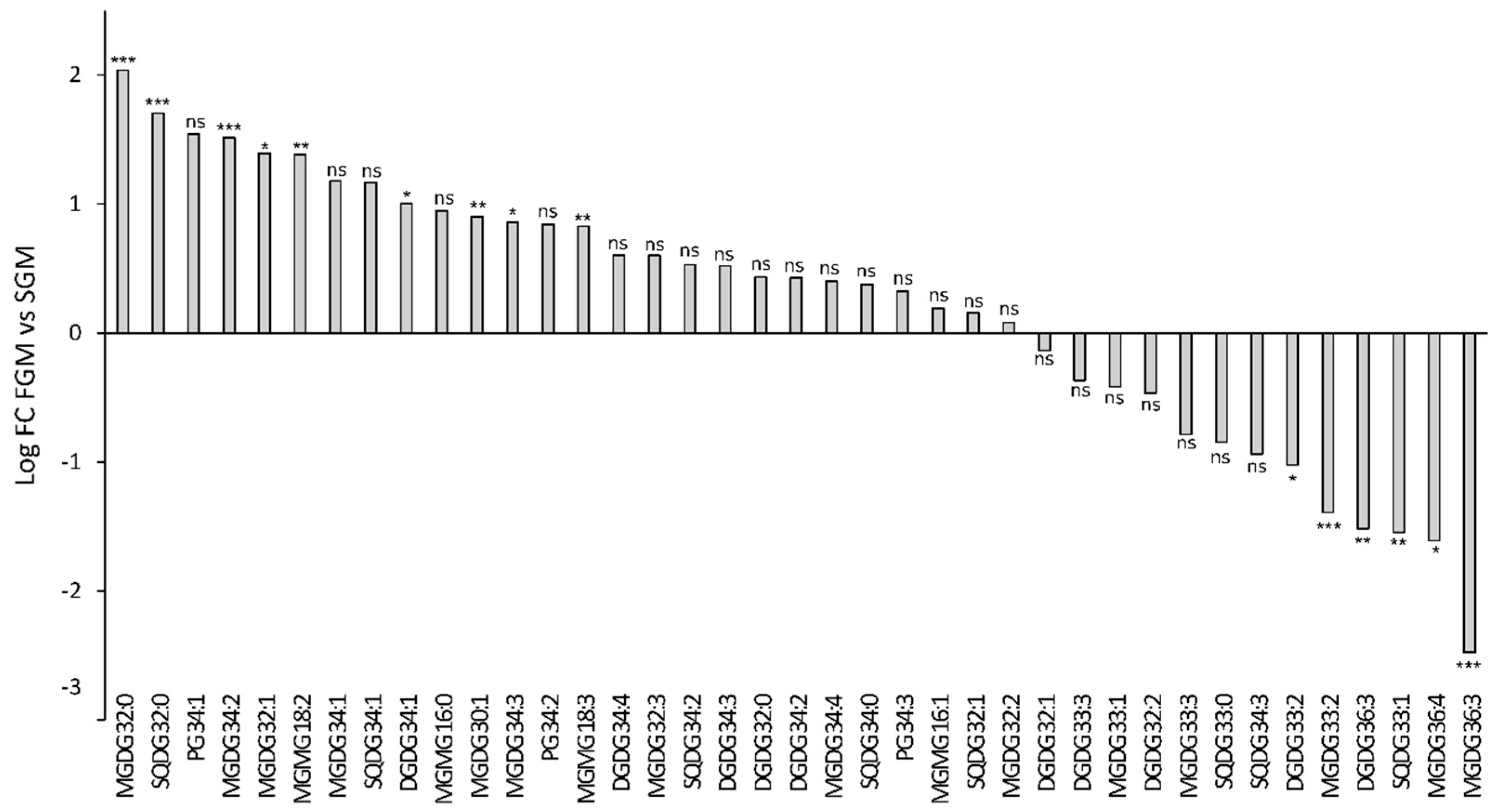
2.2. Synechocystis sp. PCC 6803 Lipidomic Variations in Cells Cultivated in SGM and in FGM
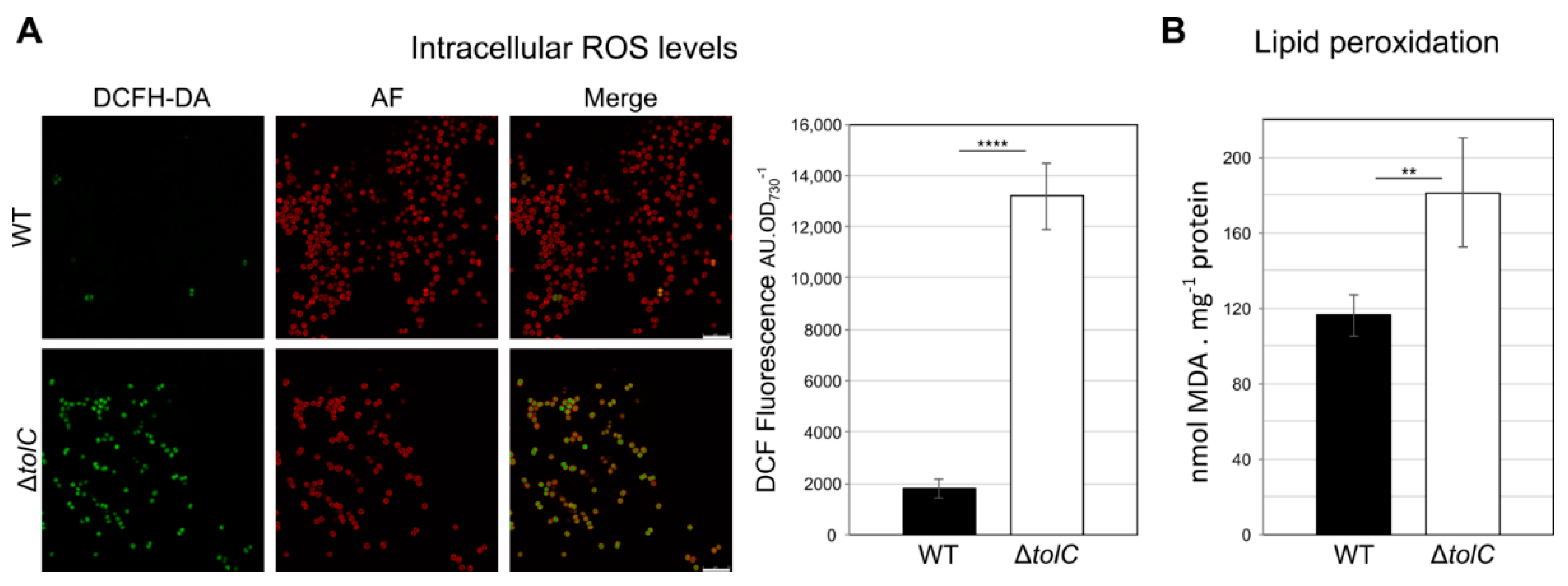
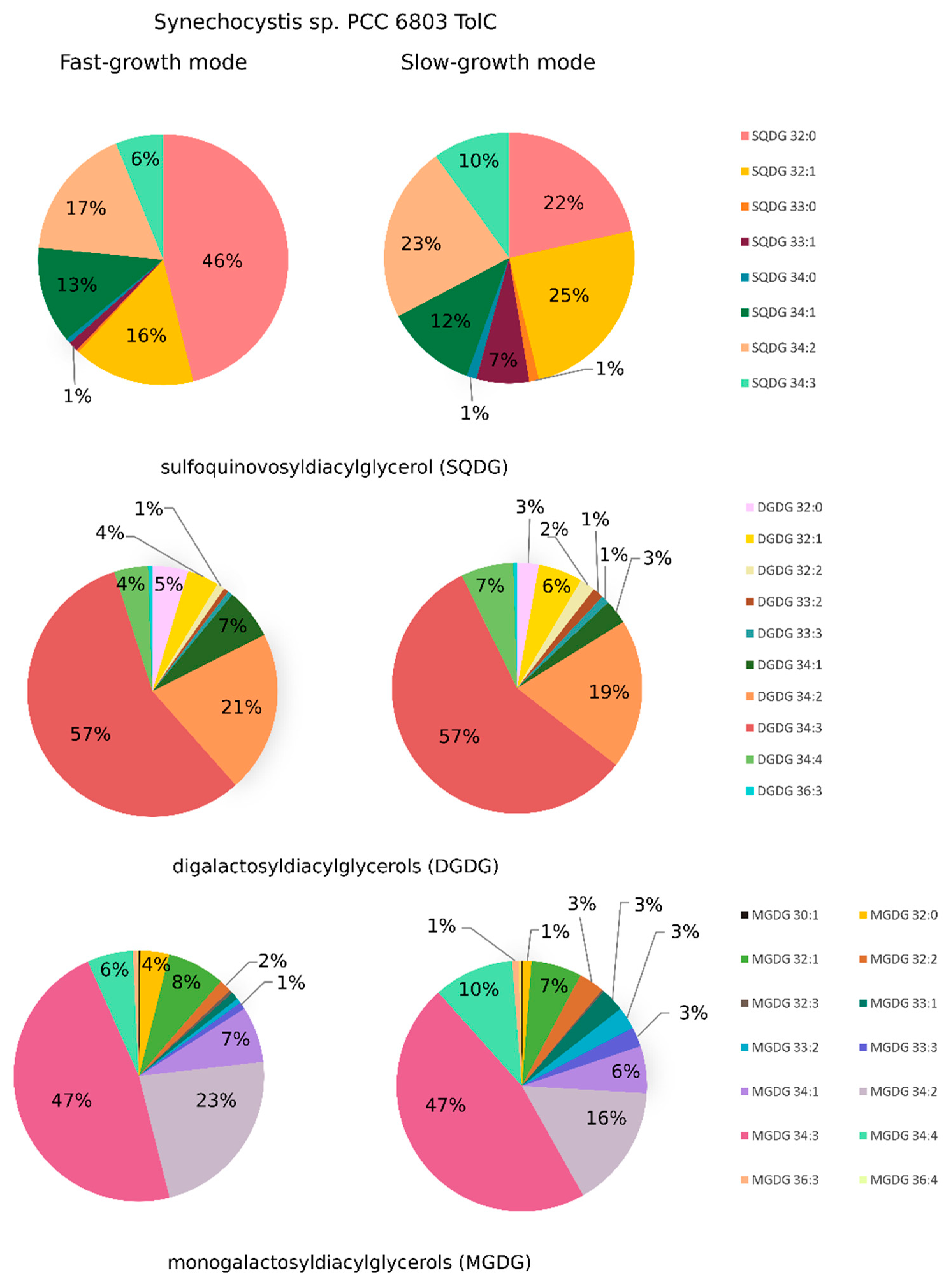
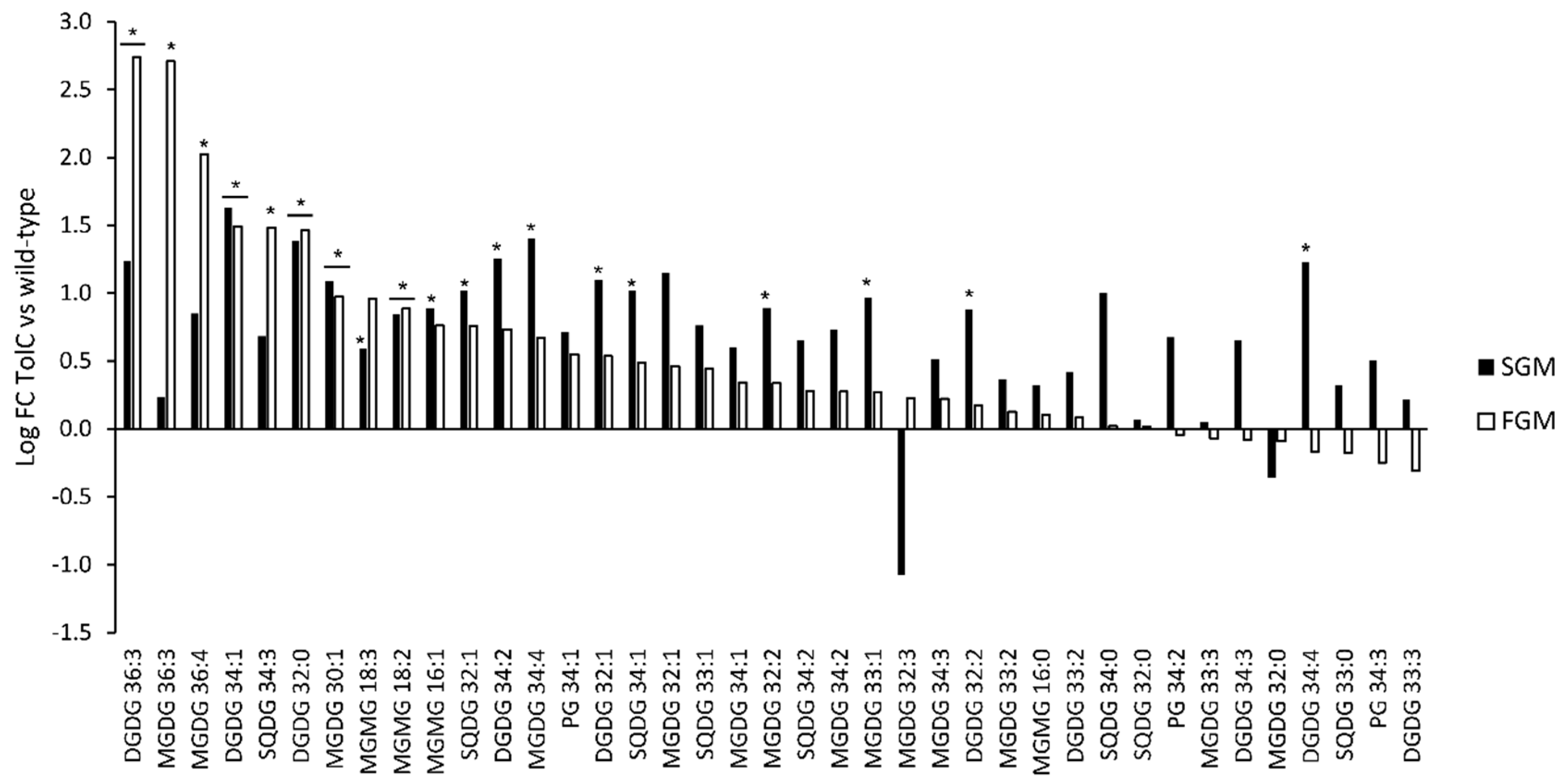
2.3. Synechocystis sp. PCC 6803 tolC-Mutant Cells are Under High Oxidative Stress, Show Elevated Levels of Lipid Peroxidation, and Present Lipidome Composition Variation in response to Different Cultivation Setups
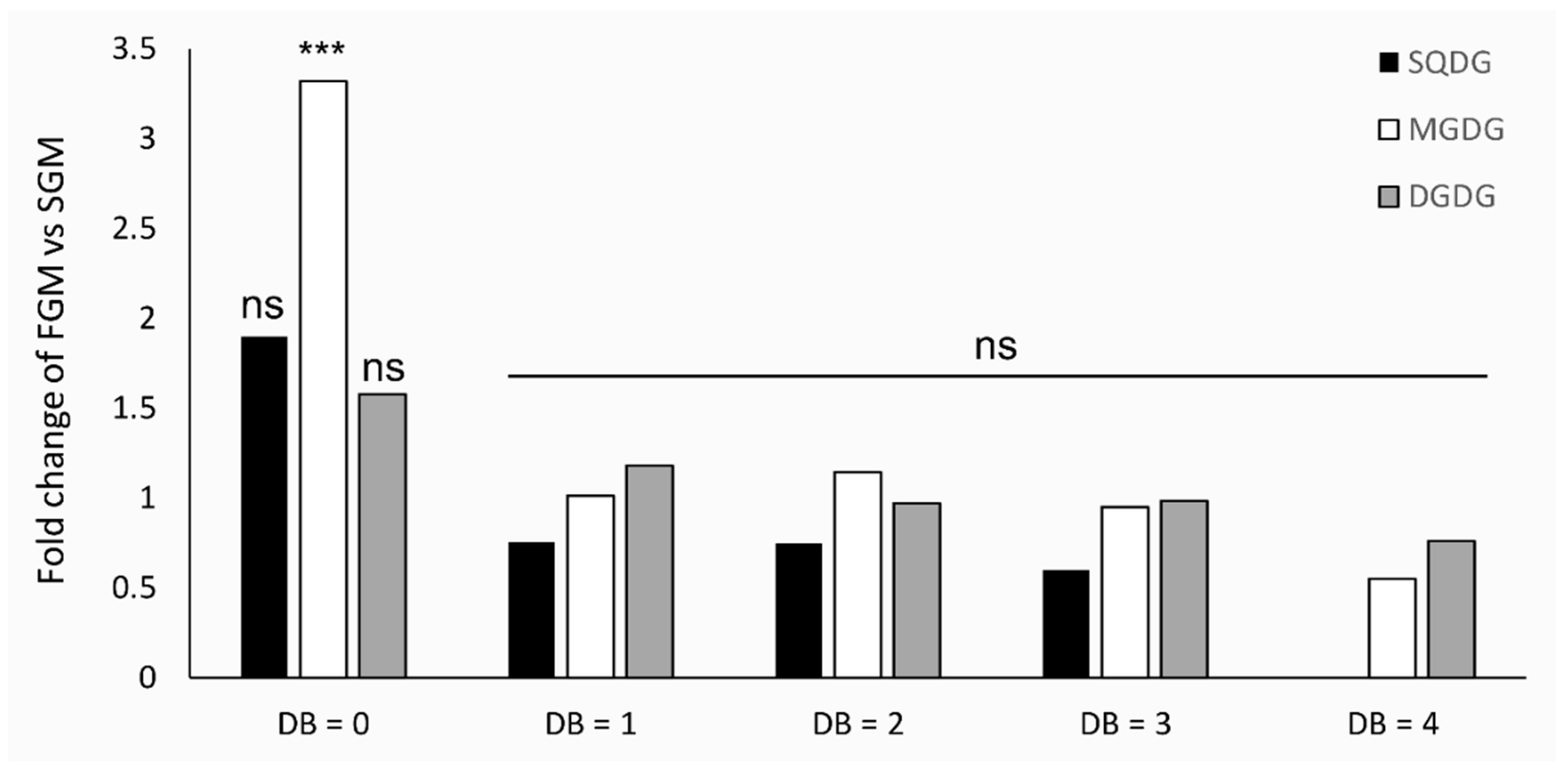
2.4. Lipidome Composition Variation is More Evident between Cells Cultivated in SGM and in FGM than in response to the Deletion of tolC
3. Materials and Methods
3.1. Strains and Growth Conditions
3.2. Sample Preparation
3.3. Reversed-Phase Liquid Chromatography–Quadrupole-Time of Flight Mass Spectrometry (RPLC-Q-TOF-MS) and Tandem Mass Spectrometry (MS/MS) Analysis of Lipid Extracts
3.4. Data Treatment and Analysis
3.5. Lipid Group Identification
3.6. Determination of Intracellular Reactive Oxygen Species (ROS) and Lipid Peroxidation Levels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DG | diacylglycerol |
| MGDG | monogalactosyldiacylglycerol |
| DGDG | digalactosyldiacylglycerol |
| SQDG | sulfoquinovosyldiacylglycerol |
| PG | Phosphatidylglycerol |
| LAHG | Light-activated heterotrophic growth |
| FGM | Fast-growth mode |
| SGM | Slow-growth mode |
| PS | Photosystem |
| OCFA | Odd-chain fatty acids |
| EVs | Extracellular vesicles |
| DCF | Dichlorodihydrofluorescein |
| ROS | Reactive oxygen species |
References
- Branco dos Santos, F.; Du, W.; Hellingwerf, K.J. Synechocystis: Not Just a Plug-Bug for CO2, but a Green E. coli. Front. Bioeng. Biotechnol. 2014, 2, 36. [Google Scholar] [CrossRef] [PubMed]
- Eungrasamee, K.; Incharoensakdi, A.; Lindblad, P.; Jantaro, S. Synechocystis sp. PCC 6803 overexpressing genes involved in CBB cycle and free fatty acid cycling enhances the significant levels of intracellular lipids and secreted free fatty acids. Sci. Rep. 2020, 10, 4515. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sheng, J.; Curtiss, R., III. Fatty acid production in genetically modified cyanobacteria. Proc. Natl. Acad. Sci. USA 2011, 108, 6899–6904. [Google Scholar] [CrossRef] [PubMed]
- Eungrasamee, K.; Miao, R.; Incharoensakdi, A.; Lindblad, P.; Jantaro, S. Improved lipid production via fatty acid biosynthesis and free fatty acid recycling in engineered Synechocystis sp. PCC 6803. Biotechnol. Biofuels 2019, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Neag, E.; Török, A.I.; Cadar, O.; Băbălău-Fuss, V.; Roman, C. Enhancing lipid production of Synechocystis PCC 6803 for biofuels production, through environmental stress exposure. Renew. Energy 2019, 143, 243–251. [Google Scholar] [CrossRef]
- Wang, X.; Xiong, X.; Sa, N.; Roje, S.; Chen, S. Metabolic engineering of enhanced glycerol-3-phosphate synthesis to increase lipid production in Synechocystis sp. PCC 6803. Appl. Microbiol. Biotechnol. 2016, 100, 6091–6101. [Google Scholar] [CrossRef] [PubMed]
- Gombos, Z.; Wada, H.; Murata, N. Unsaturation of fatty acids in membrane lipids enhances tolerance of the cyanobacterium Synechocystis PCC6803 to low-temperature photoinhibition. Proc. Natl. Acad. Sci. USA 1992, 89, 9959–9963. [Google Scholar] [CrossRef]
- Wada, H.; Murata, N. Temperature-Induced Changes in the Fatty Acid Composition of the Cyanobacterium, Synechocystis PCC6803. Plant Physiol. 1990, 92, 1062–1069. [Google Scholar] [CrossRef]
- Kern, J.; Zouni, A.; Guskov, A.; Krauss, N. Lipids in the Structure of Photosystem I, Photosystem II and the Cytochrome b6f Complex. In Lipids in Photosynthesis: Essential and Regulatory Functions. Advances in Photosynthesis and Respiration; Wada, H., Murata, N., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 203–241. [Google Scholar]
- Mamedov, M.; Hayashi, H.; Murata, N. Effects of glycinebetaine and unsaturation of membrane lipids on heat stability of photosynthetic electron-transport and phosphorylation reactions in Synechocystis PCC6803. Biochim. Biophys. Acta (BBA)-Bioenergy 1993, 1142, 1–5. [Google Scholar] [CrossRef]
- Tasaka, Y.; Gombos, Z.; Nishiyama, Y.; Mohanty, P.; Ohba, T.; Ohki, K.; Murata, N. Targeted mutagenesis of acyl-lipid desaturases in Synechocystis: Evidence for the important roles of polyunsaturated membrane lipids in growth, respiration and photosynthesis. EMBO J. 1996, 15, 6416–6425. [Google Scholar] [CrossRef]
- Cuellar-Bermudez, S.P.; Romero-Ogawa, M.A.; Vannela, R.; Lai, Y.S.; Rittmann, B.E.; Parra-Saldivar, R. Effects of light intensity and carbon dioxide on lipids and fatty acids produced by Synechocystis sp. PCC6803 during continuous flow. Algal Res. 2015, 12, 10–16. [Google Scholar] [CrossRef]
- Mavroudakis, L.; Valsami, E.-A.; Grafanaki, S.; Andreadaki, T.-P.; Ghanotakis, D.F.; Pergantis, S.A. The effect of nitrogen starvation on membrane lipids of Synechocystis sp. PCC 6803 investigated by using easy ambient sonic-spray ionization mass spectrometry. Biochim. Biophys. Acta (BBA)-Biomembr. 2019, 1861, 183027. [Google Scholar] [CrossRef]
- Kovacs, T.; Szalontai, B.; Kłodawska, K.; Vladkova, R.; Malec, P.; Gombos, Z.; Laczko-Dobos, H. Photosystem I oligomerization affects lipid composition in Synechocystis sp. PCC 6803. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2019, 1864, 1384–1395. [Google Scholar] [CrossRef] [PubMed]
- Mizusawa, N.; Sakurai, I.; Sato, N.; Wada, H. Lack of digalactosyldiacylglycerol increases the sensitivity of Synechocystis sp. PCC 6803 to high light stress. FEBS Lett. 2009, 583, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Vannela, R.; Rittmann, B.E. Evaluation of methods to extract and quantify lipids from Synechocystis PCC 6803. Bioresour. Technol. 2011, 102, 1697–1703. [Google Scholar] [CrossRef] [PubMed]
- Gombos, Z.; Wada, H.; Varkonyi, Z.; Los, D.A.; Murata, N. Characterization of the Fad12 mutant of Synechocystis that is defective in Δ12 acyl-lipid desaturase activity. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1996, 1299, 117–123. [Google Scholar] [CrossRef]
- Moreau, P.; Bessoule, J.J.; Mongrand, S.; Testet, E.; Vincent, P.; Cassagne, C. Lipid trafficking in plant cells. Prog. Lipid Res. 1998, 37, 371–391. [Google Scholar] [CrossRef]
- Joyard, J.; Teyssier, E.; Miège, C.; Berny-Seigneurin, D.; Maréchal, E.; Block, M.A.; Dorne, A.-J.; Rolland, N.; Ajlani, G.; Douce, R. The Biochemical Machinery of Plastid Envelope Membranes. Plant Physiol. 1998, 118, 715–723. [Google Scholar] [CrossRef]
- Plohnke, N.; Seidel, T.; Kahmann, U.; Rögner, M.; Schneider, D.; Rexroth, S. The Proteome and Lipidome of Synechocystis sp. PCC 6803 Cells Grown under Light-Activated Heterotrophic Conditions. Mol. Cell. Proteom. 2015, 14, 572–584. [Google Scholar] [CrossRef]
- Hewelt-Belka, W.; Nakonieczna, J.; Belka, M.; Bączek, T.; Namieśnik, J.; Kot-Wasik, A. Untargeted Lipidomics Reveals Differences in the Lipid Pattern among Clinical Isolates of Staphylococcus aureus Resistant and Sensitive to Antibiotics. J. Proteome Res. 2016, 15, 914–922. [Google Scholar] [CrossRef]
- Hines, K.M.; Xu, L. Lipidomic consequences of phospholipid synthesis defects in Escherichia coli revealed by HILIC-ion mobility-mass spectrometry. Chem. Phys. Lipids 2019, 219, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Hameed, S.; Kumar, P.; Singh, S.; Fatima, Z. Comparative lipidomics of drug sensitive and resistant Mycobacterium tuberculosis reveals altered lipid imprints. 3 Biotech 2017, 7, 325. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.; Martins, N.M.; Santos, M.; Pinto, F.; Büttel, Z.; Couto, N.A.S.; Wright, P.C.; Tamagnini, P. The versatile TolC-like Slr1270 in the cyanobacterium Synechocystis sp. PCC 6803. Environ. Microbiol. 2016, 18, 486–502. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.F.; Pacheco, C.C.; Tamagnini, P.; Oliveira, P. Identification of inner membrane translocase components of TolC-mediated secretion in the cyanobacterium Synechocystis sp. PCC 6803. Environ. Microbiol. 2018, 20, 2354–2369. [Google Scholar] [CrossRef]
- Agarwal, R.; Whitelegge, J.P.; Saini, S.; Shrivastav, A.P. The S-layer biogenesis system of Synechocystis 6803: Role of Sll1180 and Sll1181 (E. coli HlyB and HlyD analogs) as type-I secretion components for Sll1951 export. Biochim. Biophys. Acta (BBA)-Biomembr. 2018, 1860, 1436–1446. [Google Scholar] [CrossRef]
- Bellefleur, M.P.A.; Wanda, S.-Y.; Curtiss, R. Characterizing active transportation mechanisms for free fatty acids and antibiotics in Synechocystis sp. PCC 6803. BMC Biotechnol. 2019, 19, 5. [Google Scholar] [CrossRef]
- Anderson, S.L.; McIntosh, L. Light-activated heterotrophic growth of the cyanobacterium Synechocystis sp. strain PCC 6803: A blue-light-requiring process. J. Bacteriol. 1991, 173, 2761–2767. [Google Scholar] [CrossRef]
- Flores, C.; Santos, M.; Pereira, S.B.; Mota, R.; Rossi, F.; De Philippis, R.; Couto, N.; Karunakaran, E.; Wright, P.C.; Oliveira, P.; et al. The alternative sigma factor SigF is a key player in the control of secretion mechanisms in Synechocystis sp. PCC 6803. Environ. Microbiol. 2019, 21, 343–359. [Google Scholar] [CrossRef]
- Huang, Q.; Jiang, F.; Wang, L.; Yang, C. Design of Photobioreactors for Mass Cultivation of Photosynthetic Organisms. Engineering 2017, 3, 318–329. [Google Scholar] [CrossRef]
- Posten, C. Design principles of photo-bioreactors for cultivation of microalgae. Eng. Life Sci. 2009, 9, 165–177. [Google Scholar] [CrossRef]
- Eichenberger, W. Lipids of Chlamydomonas reinhardi under different growth conditions. Phytochemistry 1976, 15, 459–463. [Google Scholar] [CrossRef]
- Khotimchenko, S.V.; Yakovleva, I.M. Lipid composition of the red alga Tichocarpus crinitus exposed to different levels of photon irradiance. Phytochemistry 2005, 66, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Minnikin, D.E.; Abdolrahimzadeh, H.; Baddiley, J. Variation of polar lipid composition of Bacillus subtilis (Marburg) with different growth conditions. FEBS Lett. 1972, 27, 16–18. [Google Scholar] [CrossRef]
- Yeh, K.-L.; Chang, J.-S. Effects of cultivation conditions and media composition on cell growth and lipid productivity of indigenous microalga Chlorella vulgaris ESP-31. Bioresour. Technol. 2012, 105, 120–127. [Google Scholar] [CrossRef]
- Hoiczyk, E.; Hansel, A. Cyanobacterial Cell Walls: News from an Unusual Prokaryotic Envelope. J. Bacteriol. 2000, 182, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Hewelt-Belka, W.; Nakonieczna, J.; Belka, M.; Bączek, T.; Namieśnik, J.; Kot-Wasik, A. Comprehensive methodology for Staphylococcus aureus lipidomics by liquid chromatography and quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2014, 1362, 62–74. [Google Scholar] [CrossRef]
- Shan, Y.; Liu, Y.; Yang, L.; Nie, H.; Shen, S.; Dong, C.; Bai, Y.; Sun, Q.; Zhao, J.; Liu, H. Lipid profiling of cyanobacteria Synechococcus sp. PCC 7002 using two-dimensional liquid chromatography with quadrupole time-of-flight mass spectrometry. J. Sep. Sci. 2016, 39, 3745–3753. [Google Scholar] [CrossRef]
- Wada, H.; Murata, N. Membrane Lipids in Cyanobacteria. In Lipids in Photosynthesis: Structure, Function and Genetics. Advances in Photosynthesis and Respiration; Siegenthaler, P.-A., Murata, N., Eds.; Springer: Dordrecht, The Netherlands, 1998; pp. 65–81. [Google Scholar] [CrossRef]
- Los, D.A.; Murata, N. Structure and expression of fatty acid desaturases. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1998, 1394, 3–15. [Google Scholar] [CrossRef]
- Los, D.A.; Murata, N. Membrane fluidity and its roles in the perception of environmental signals. Biochim. Biophys. Acta (BBA)-Biomembr. 2004, 1666, 142–157. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Los, D.A.; Murata, N. Regulatory Roles in Photosynthesis of Unsaturated Fatty Acids in Membrane Lipids. In Lipids in Photosynthesis: Essential and Regulatory Functions. Advances in Photosynthesis and Respiration; Wada, H., Murata, N., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 373–388. [Google Scholar]
- Páli, T.; Garab, G.; Horváth, L.I.; Kóta, Z. Functional significance of the lipid-protein interface in photosynthetic membranes. Cell. Mol. Life Sci. CMLS 2003, 60, 1591–1606. [Google Scholar] [CrossRef]
- Hoyo, J.; Guaus, E.; Torrent-Burgués, J. Monogalactosyldiacylglycerol and digalactosyldiacylglycerol role, physical states, applications and biomimetic monolayer films. Eur. Phys. J. E 2016, 39, 39. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, W.; Xu, X.; Zhang, H.; Wang, J.; Xian, M. Production of free monounsaturated fatty acids by metabolically engineered Escherichia coli. Biotechnol. Biofuels 2014, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Peter, D.; Hölzl, G. The Role of Glycolipids in Photosynthesis. In Lipids in Photosynthesis: Essential and Regulatory Functions. Advances in Photosynthesis and Respiration; Wada, H., Murata, N., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 265–282. [Google Scholar]
- Jones, M.R. Lipids in photosynthetic reaction centres: Structural roles and functional holes. Prog. Lipid Res. 2007, 46, 56–87. [Google Scholar] [CrossRef] [PubMed]
- Loll, B.; Kern, J.; Saenger, W.; Zouni, A.; Biesiadka, J. Towards complete cofactor arrangement in the 3.0 Å resolution structure of photosystem II. Nature 2005, 438, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Endo, K.; Kobayashi, K.; Wada, H. Sulfoquinovosyldiacylglycerol has an Essential Role in Thermosynechococcus elongatus BP-1 under Phosphate-Deficient Conditions. Plant Cell Physiol. 2016, 57, 2461–2471. [Google Scholar] [CrossRef]
- Aoki, M.; Sato, N.; Meguro, A.; Tsuzuki, M. Differing involvement of sulfoquinovosyl diacylglycerol in photosystem II in two species of unicellular cyanobacteria. Eur. J. Biochem. 2004, 271, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Beld, J.; Abbriano, R.; Finzel, K.; Hildebrand, M.; Burkart, M.D. Probing fatty acid metabolism in bacteria, cyanobacteria, green microalgae and diatoms with natural and unnatural fatty acids. Mol. Biosyst. 2016, 12, 1299–1312. [Google Scholar] [CrossRef]
- Horning, M.G.; Martin, D.B.; Karmen, A.; Vagelos, P.R. Fatty Acid Synthesis in Adipose Tissue: II. Enzymatic synthesis of branched chain and odd-numbered fatty acids. J. Biol. Chem. 1961, 236, 669–672. [Google Scholar]
- Sen, N.; Schlenk, H. The structure of polyenoic odd- and even-numbered fatty acids of mullet (Mugil cephalus). J. Am. Oil Chem. Soc. 1964, 41, 241–247. [Google Scholar] [CrossRef]
- Borges, K.; Sonnewald, U. Triheptanoin—A medium chain triglyceride with odd chain fatty acids: A new anaplerotic anticonvulsant treatment? Epilepsy Res. 2012, 100, 239–244. [Google Scholar] [CrossRef][Green Version]
- Jenkins, B.; West, J.A.; Koulman, A. A review of odd-chain fatty acid metabolism and the role of pentadecanoic Acid (C15:0) and heptadecanoic Acid (C17:0) in health and disease. Molecules 2015, 20, 2425–2444. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-K.; Ledesma-Amaro, R.; Nicaud, J.-M. De novo Biosynthesis of Odd-Chain Fatty Acids in Yarrowia lipolytica Enabled by Modular Pathway Engineering. Front. Bioeng. Biotechnol. 2020, 7, 484. [Google Scholar] [CrossRef] [PubMed]
- Kulp, A.; Kuehn, M.J. Biological Functions and Biogenesis of Secreted Bacterial Outer Membrane Vesicles. Annu. Rev. Microbiol. 2010, 64, 163–184. [Google Scholar] [CrossRef] [PubMed]
- Lima, S.; Matinha-Cardoso, J.; Tamagnini, P.; Oliveira, P. Extracellular Vesicles: An Overlooked Secretion System in Cyanobacteria. Life 2020, 10, 129. [Google Scholar] [CrossRef]
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [CrossRef]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Chong, J.; Xia, J. MetaboAnalystR: An R package for flexible and reproducible analysis of metabolomics data. Bioinformatics 2018, 34, 4313–4314. [Google Scholar] [CrossRef]
- Sartain, M.J.; Dick, D.L.; Rithner, C.D.; Crick, D.C.; Belisle, J.T. Lipidomic analyses of Mycobacterium tuberculosis based on accurate mass measurements and the novel “Mtb LipidDB”. J. Lipid Res. 2011, 52, 861–872. [Google Scholar] [CrossRef]
- Esterbauer, H.; Cheeseman, K.H. [42] Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1990; Volume 186, pp. 407–421. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hewelt-Belka, W.; Kot-Wasik, Á.; Tamagnini, P.; Oliveira, P. Untargeted Lipidomics Analysis of the Cyanobacterium Synechocystis sp. PCC 6803: Lipid Composition Variation in Response to Alternative Cultivation Setups and to Gene Deletion. Int. J. Mol. Sci. 2020, 21, 8883. https://doi.org/10.3390/ijms21238883
Hewelt-Belka W, Kot-Wasik Á, Tamagnini P, Oliveira P. Untargeted Lipidomics Analysis of the Cyanobacterium Synechocystis sp. PCC 6803: Lipid Composition Variation in Response to Alternative Cultivation Setups and to Gene Deletion. International Journal of Molecular Sciences. 2020; 21(23):8883. https://doi.org/10.3390/ijms21238883
Chicago/Turabian StyleHewelt-Belka, Weronika, Ágata Kot-Wasik, Paula Tamagnini, and Paulo Oliveira. 2020. "Untargeted Lipidomics Analysis of the Cyanobacterium Synechocystis sp. PCC 6803: Lipid Composition Variation in Response to Alternative Cultivation Setups and to Gene Deletion" International Journal of Molecular Sciences 21, no. 23: 8883. https://doi.org/10.3390/ijms21238883
APA StyleHewelt-Belka, W., Kot-Wasik, Á., Tamagnini, P., & Oliveira, P. (2020). Untargeted Lipidomics Analysis of the Cyanobacterium Synechocystis sp. PCC 6803: Lipid Composition Variation in Response to Alternative Cultivation Setups and to Gene Deletion. International Journal of Molecular Sciences, 21(23), 8883. https://doi.org/10.3390/ijms21238883






