Abstract
Herein, the generation of decarboxylated derivatives of gomphrenin pigments exhibiting potential health-promoting properties and the kinetics of their extraction during tea brewing from the purple flowers of Gomphrena globosa L. in aqueous and aqueous citric acid solutions were investigated. Time-dependent concentration monitoring of natural gomphrenins and their tentative identification was carried out by LC-DAD-ESI-MS/MS. The high content of acylated gomphrenins and their principal decarboxylation products, 2-, 15-, 17-decarboxy-gomphrenins, along with minor levels of their bidecarboxylated derivatives, were reported in the infusions. The identification was supported by the determination of molecular formulas of the extracted pigments by liquid chromatography coupled with high-resolution mass spectrometry (LCMS-IT-TOF). The influence of plant matrix on gomphrenins’ stability and generation of their derivatives, including the extraction kinetics, was determined by studying the concentration profiles in the primary and diluted infusions. Isolated and purified acylated gomphrenins from the same plant material were used for the preliminary determination of their decarboxylated derivatives. The acylated gomphrenins were found to be more stable than nonacylated ones. Citric acid addition had a degradative influence on natural gomphrenins mainly during the longer tea brewing process (above 15 min); however, the presence of plant matrix significantly increased the stability for betacyanins’ identification.
1. Introduction
Gomphrena globosa L. commonly known as globe amaranth is an annual herbaceous, edible plant belonging to the family of Amaranthaceae. It is widely cultivated in China and the tropical regions of Central America. G. globosa inflorescences occur basically in three varieties: white, red, and violet. They are utilized in traditional Chinese medicine in the preparation of cough syrups to treat respiratory system diseases. Dried gomphrena blossoms are characterized by unique fragrance and exhibit numerous pro-health properties. They are commonly used for the preparation of tea infusions. Furthermore, globe amaranth flowers are sold on a commercial scale as tisanes and used as a popular ingredient in blooming teas which are commonly known as flowering tea [1]. Nowadays, the number of people suffering from civilization diseases such as diabetes, hypertension, and cancer, is increasing. Therefore, natural products comprising biologically active compounds attract much attention [2]. These products implemented into a daily diet may play an important role in the prevention and treatment of many health problems [3]. The violet G. globosa inflorescences are a precious source of many antioxidants involving betalains. These compounds are proven to exhibit numerous pro-health properties, owing to the high content of hydroxyl groups that participate in free radical scavenging [3,4,5,6,7]. Their antihemorrhage, antimicrobial, antiinflammatory activity, and analgesic effects have been reported [8,9,10,11,12,13]. Betalains are vacuolar, natural plant pigments. They are present in plants belonging to the Caryophyllales order [14,15]. It is worth noting that betacyanins acylated by hydroxycinnamic acid derivatives are present in gomphrena flowers at relatively high levels. To simplify, they are frequently denominated as gomphrenins. Accordingly, nonacylated gomphrenin I is a common name for betanidin-6-O-β-glucoside, whereas gomphrenin II and III are betanidin-6-O-(6′-O-trans-4-coumaroyl)-β-glucoside and betanidin-6-O-(6′-O-trans-feruloyl)-β- -glucoside, respectively [16,17,18]. Their chemical structures have been completely elucidated by nuclear magnetic resonance and mass spectrometry [19,20]. The structure of gomphrenin IV has been tentatively elucidated as a betanidin-6-O-(6′-O-sinapoyl)-β-glucoside because of its coelution with gomphrenin III. Betanidin 6-O-glucosides are very rare compounds and hitherto, they have been identified only in G. globosa inflorescences, B. alba L. fruits and its variety B. rubra L. (the main source of nonacylated gomphrenin I) as well as in B. glabra bracts [19,20,21,22,23,24,25,26]. Previous reports indicated that decarboxylated betacyanin derivatives are formed in preparations subjected to thermal processing as a result of betalains’ sensitivity to elevated temperatures [21,24,27,28,29,30,31,32]. Thermal decarboxylation may occur at different degrees. In general, 2-decarboxy- and 17-decarboxy-betacyanins, which exist in two diastereomeric forms, are formed in the greatest amounts. Furthermore, 15-decarboxy-betacyanins with the lost chiral center at the C-15 carbon are observed, therefore, there are no additional diastereomers of these derivatives. Frequently, after prolonged heating, bidecarboxy- and even tridecarboxy-betacyanins can be formed as well. Decarboxylated betacyanins are less polar than the initial forms of betacyanins, therefore, their retention on reversed-phase in HPLC is greater [21,24]. Despite a few phytochemical studies concerning the presence of phenolic compounds in G. globosa flowers, there is a lack of reports referring to the comprehensive extraction of these relevant compounds during thermal processing close to tea brewing conditions [7,28]. For that reason, our study aimed to evaluate the kinetics of betacyanins’ extraction in tea infusion prepared from G. globosa flowers and to determine betacyanin degradation products. Research on the influence of citric acid addition and matrix presence on the stability of the compounds during the preparation of G. globosa infusions as well as the comparative experiments with single purified gomphrenins derived from G. globosa flowers was also performed.
2. Results and Discussion
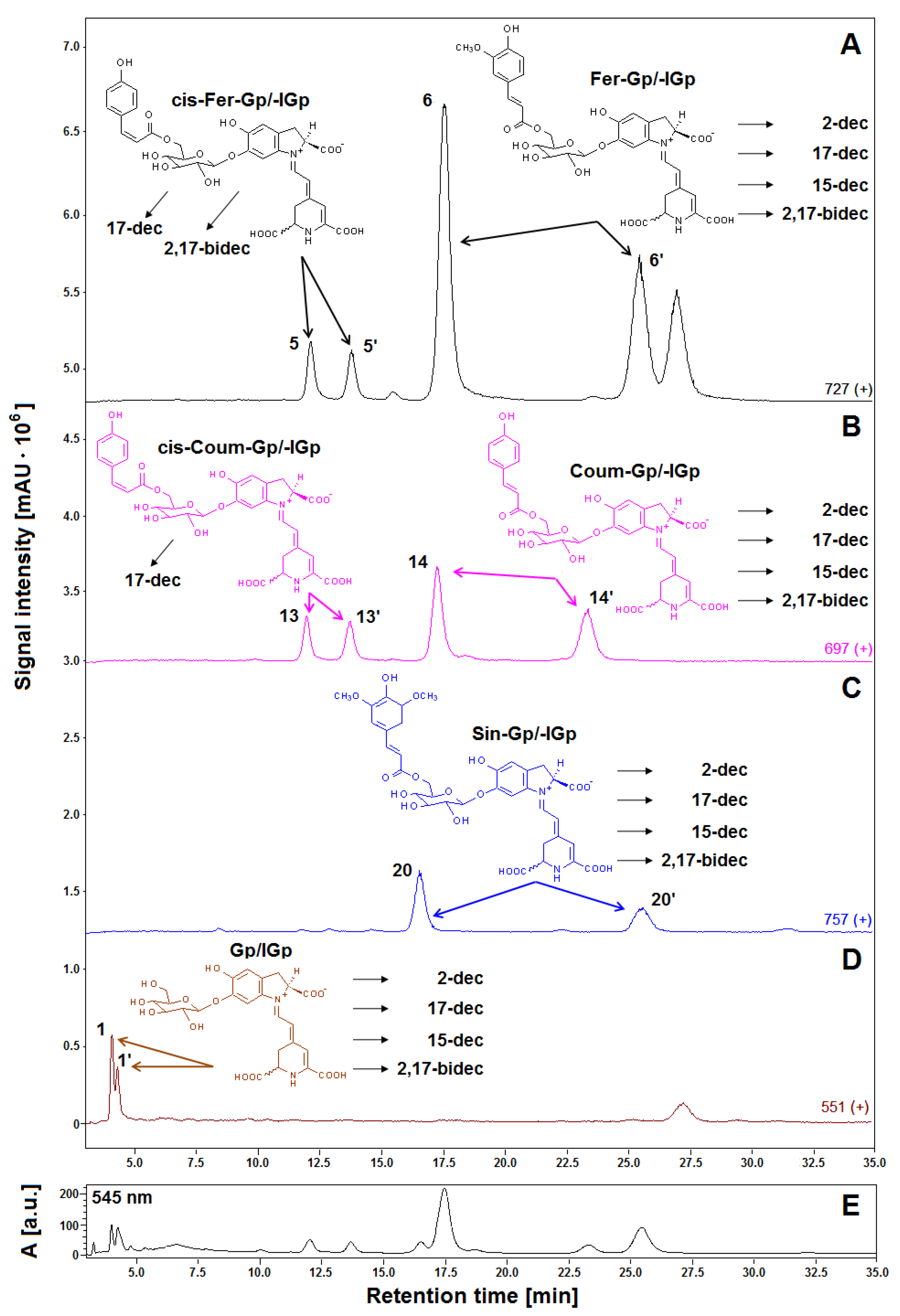
Previous studies revealed a typical primary betacyanin profile in the extract of violet G. globosa flowers [12,22]. Chromatograms recorded in selected ion monitoring mode (SIM), and DAD chromatogram registered at 545 nm, obtained for a lyophilized sample of G. globosa extract not subjected to heating are depicted in Figure 1. Additionally, chemical patterns of natural gomphrenins and abbreviation names of the observed decarboxylated derivatives are presented. The presence of sinapoyled diastereomers 20/20′ has never been confirmed in natural extracts of G. globose, except for low-resolution mass spectrometry (Table 1) [12,22]; therefore, additional high-resolution measurements and confirmation of the molecular formulas were performed by LCMS-IT-TOF (Figure 2, Table 2).
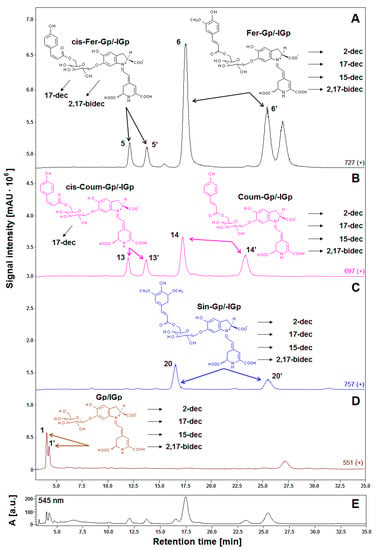
Figure 1.
LCMS gomphrenin profiles (A–D) recorded in selected ion monitoring (SIM) mode of the freeze-dried representative extract obtained from purple G. globosa flowers and chemical structures of natural gomphrenins present in the extract which undergo decarboxylation during the tea brewing process leading to obtaining products which had their abbreviations placed. The LC-DAD fingerprint (λ 545 nm) of the betacyanin extract is also depicted for an indication of the pigments (E).

Table 1.
Chromatographic, spectrophotometric, and mass spectrometric data of the analysed natural gomphrenins and their derivatives in G. globosa floral extracts after tea brewing at 90 °C.
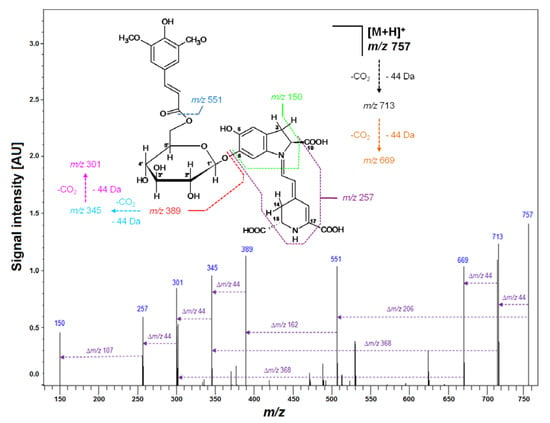
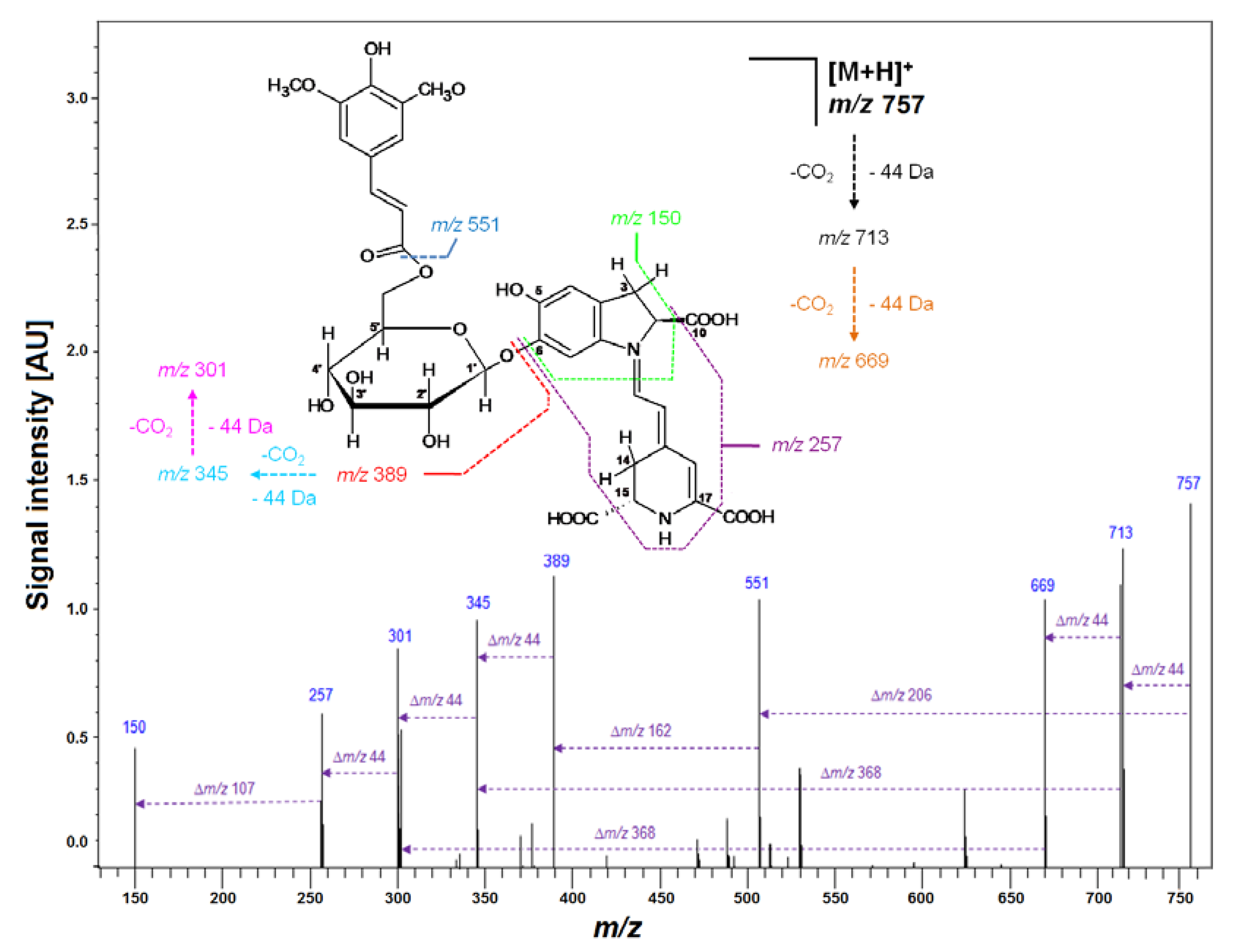
Figure 2.
Fragmentation pattern obtained by LCMS-IT-TOF for purified Sin-Gp 20 isolated from purple G. globosa flowers.

Table 2.
High-resolution mass spectrometric data obtained by LCMS-IT-TOF measurements of purified acylated gomphrenins isolated from G. globosa flowers and their derivatives generated after heating of acylated gomphrenins at 90 °C in aqueous citric acid solutions.
The dominant presence of the feruloyl-gomphrenin (Fer-Gp) 6, coumaroyl-gomphrenin (Coum-Gp) 14 and their diastereomers (Fer-IGp) 6′, (Coum-IGp) 14′ with significantly lower quantities of sinapoyl-gomphrenin/-isogomphrenin (Sin-Gp/-IGp) 20/20′ and nonacylated gomphrenin I/isogomphrenin I (Gp/IGp) 1/1′ was confirmed. For the aim of simplification, we propose to refer to the acylated pigments as respective “hydroxycinnamoyled gomphrenins” as well as to their abbreviations (Table 1) instead of selected “gomphrenins II, III, and IV”. Accordingly, nonacylated “gomphrenin I” will be referred to as “gomphrenin”. In the previous study [12], additional cis-isomers of coumaroyl-gomphrenin (cis-Coum-Gp/-IGp) 13/13′ and feruloyl-gomphrenin (cis-Fer-Gp/-IGp) 5/5′ were tentatively detected.
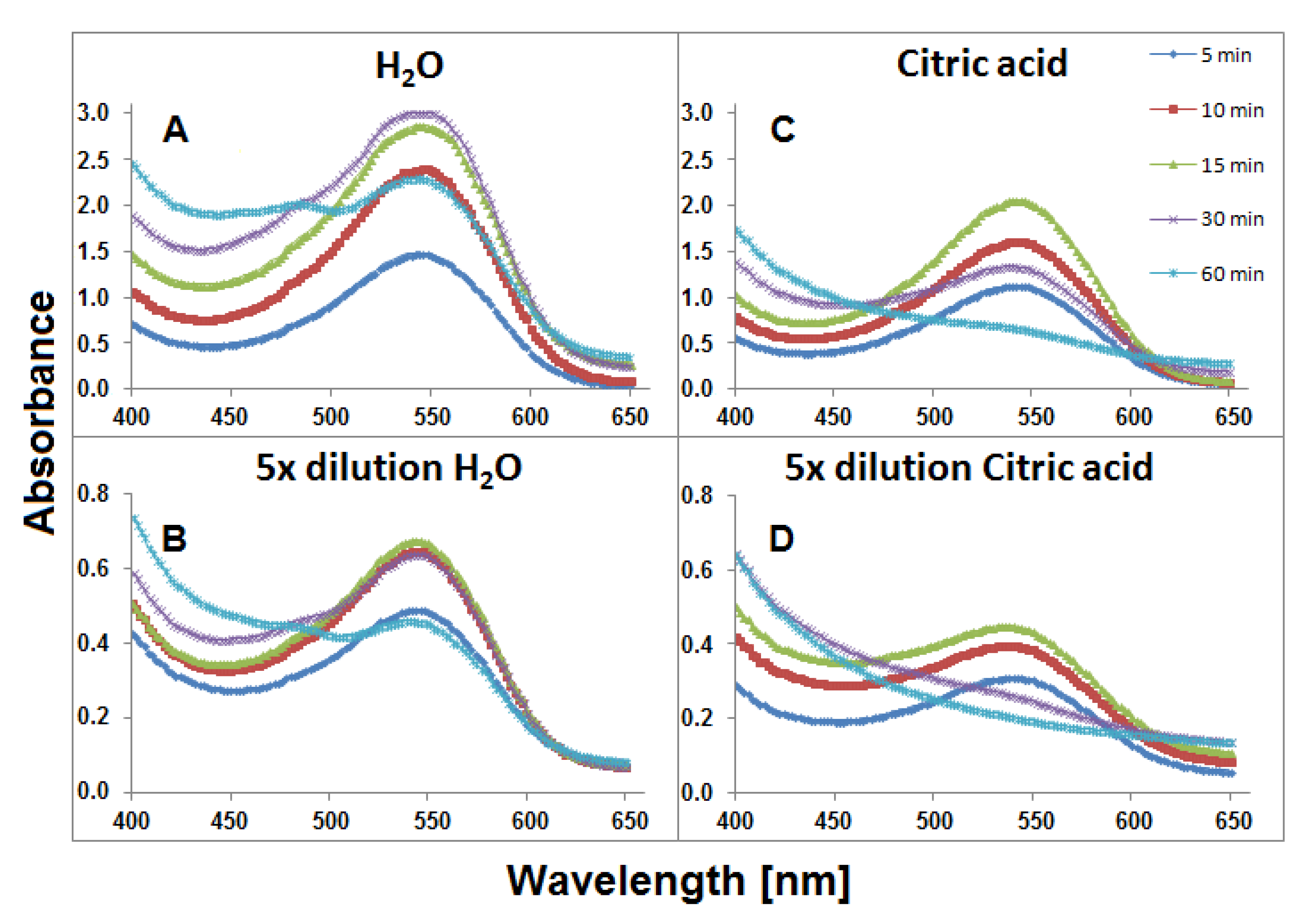
For 0.94 g or 0.19 g weighted portions of G. globosa flowers which were brewing in 6 mL of water for 60 min, two series of solutions were obtained, nondiluted, and fivefold diluted, respectively. The temperature (90 °C) of the tea brewing process was high enough for monitoring changes in the compositions of the resulting mixtures and close to home tea brewing conditions. The spectra of the visible range of the obtained extract samples collected during heat processing are shown in Figure 3. Higher absorbance values were observed for the samples which were extracted in aqueous solutions (Figure 3A,B) in comparison to aqueous citric acid solutions. It indicates mild extraction conditions of gomphrenins in water without citric acid addition. A stronger influence of citric acid addition on the stability of these compounds can be observed in Figure 3C,D. After 60 min, for undiluted samples, the absorption maximum was not clearly observed and there was no absorption band for diluted samples after 30 min.
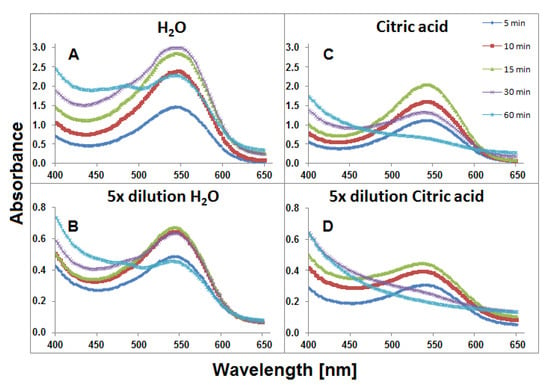
Figure 3.
Visible spectra of aqueous (A and B) and citric acid (C and D) infusions of G. globosa flowers obtained within 60 min of tea brewing at 90 °C.
This resulted from the lower impact of the plant matrix which presumably supported the stability of the pigments. The slight absorption maximum at approximately 480 nm is observed in Figure 3A after 60 min. It indicates the presence of other conversion products, presumably oxidized, of natural gomphrenins. Decarboxylated degradation products of natural gomphrenins formed during the tea brewing process in aqueous and citric acid solutions are listed in Table 1. All the detected degradation products of the pigments were less polar than their corresponding precursors. For the most prominent degradation products of acylated gomphrenins, additional confirmation was obtained by LCMS-IT-TOF analyses (Table 2). A series of selected chromatograms obtained for the processed flowers in the experiments as well as for selected purified and heated pigments are depicted in Figure 4.
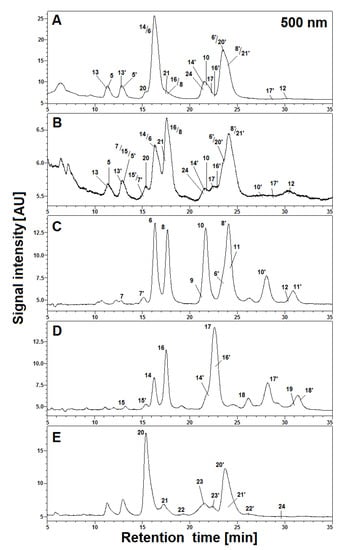
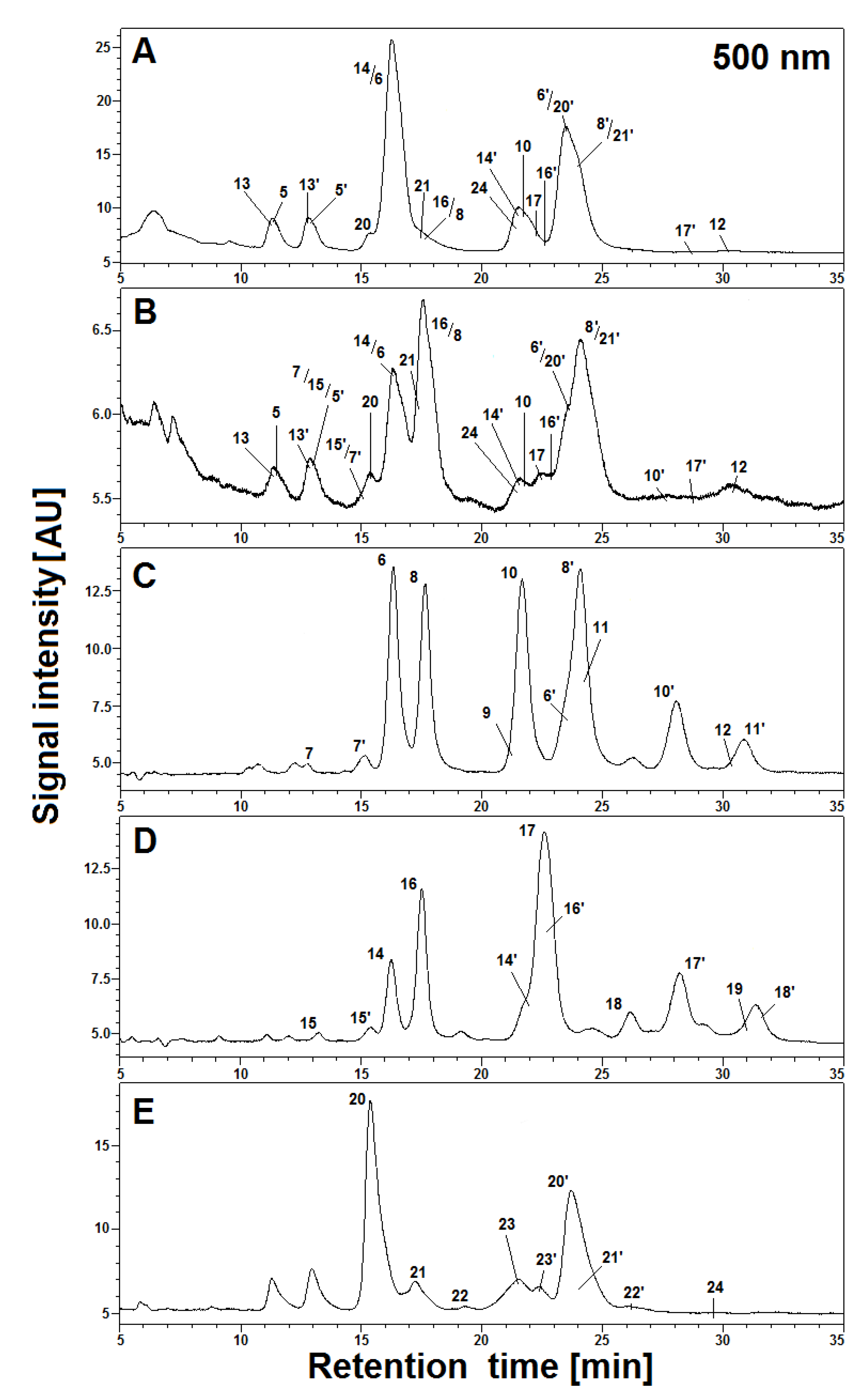
Figure 4.
HPLC profiles (λ 500 nm) of acylated gomphrenin and gomphrenin-based derivatives (A and B) in 5× diluted tea infusions of purple G. globosa flowers obtained in aqueous (A) and citric acid solutions (B) after 30 min of brewing at 90 °C, as well as referential HPLC profiles of purified feruloylated (C), coumaroylated (D), and sinapoylated (E) gomphrenin derivatives obtained after 20 min (C and D) and 10 min (E) of heating at 90 °C in citric acid solutions.
2.1. Extraction of Gomphrenins during Tea Brewing of G. globosa in Aqueous Solutions
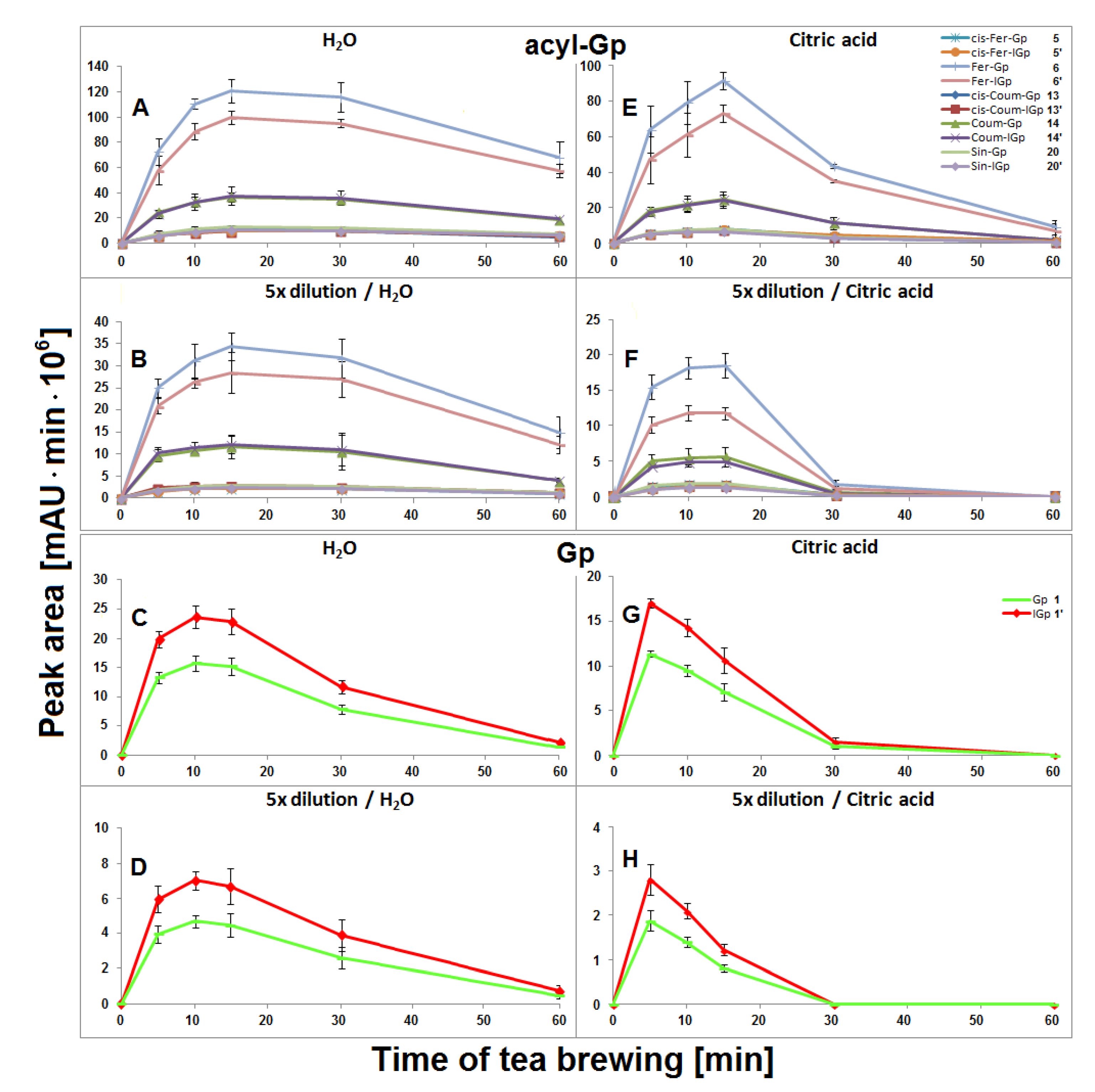
The highest concentration of all acylated gomphrenins in undiluted and fivefold diluted samples were obtained after 15 min of extraction, but the degradation of substrates was faster for diluted samples, presumably as a result of the diminished stabilizing effect of the matrix in diluted solutions (Figure 5). The matrix effect in aqueous solutions was not clearly observed for Gp/-IGp 1/1′, but these betacyanins were, in general, less stable during tea brewing than the acylated ones. The greatest signal intensity for Gp/-IGp 1/1′ was observed after 10 min of thermal treatment.
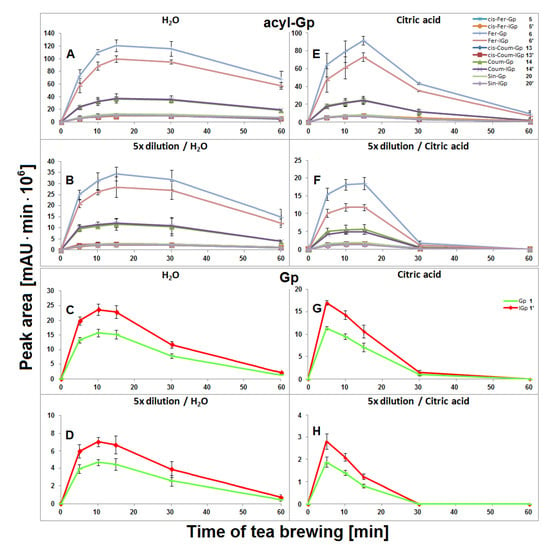
Figure 5.
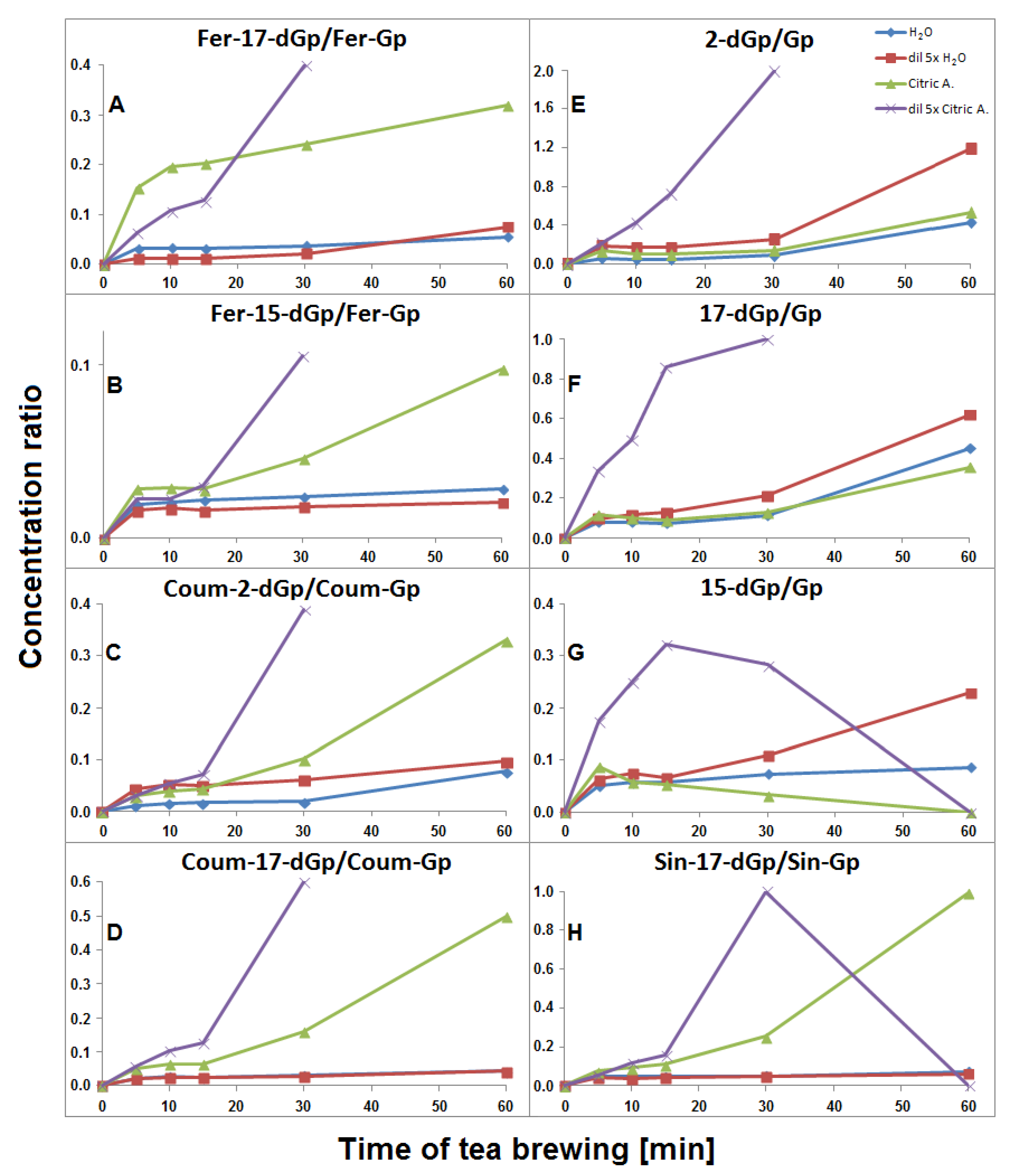
Time-dependent concentration profiles of gomphrenins and acylated gomphrenins obtained after brewing purple G. globosa flowers at 90 °C in undiluted (A,C,E,G) and 5× diluted (B,D,F,H) aqueous and citric acid solutions. Standard deviation values (SD) of nonacylated pigments 1/1′ in citric acid solutions were multiplied by a factor of 3 and 2 for undiluted (G) and diluted samples (H), respectively.
2.2. Generation and Identification of 17-Decarboxylated Derivatives of Gomphrenins in Aqueous Solutions
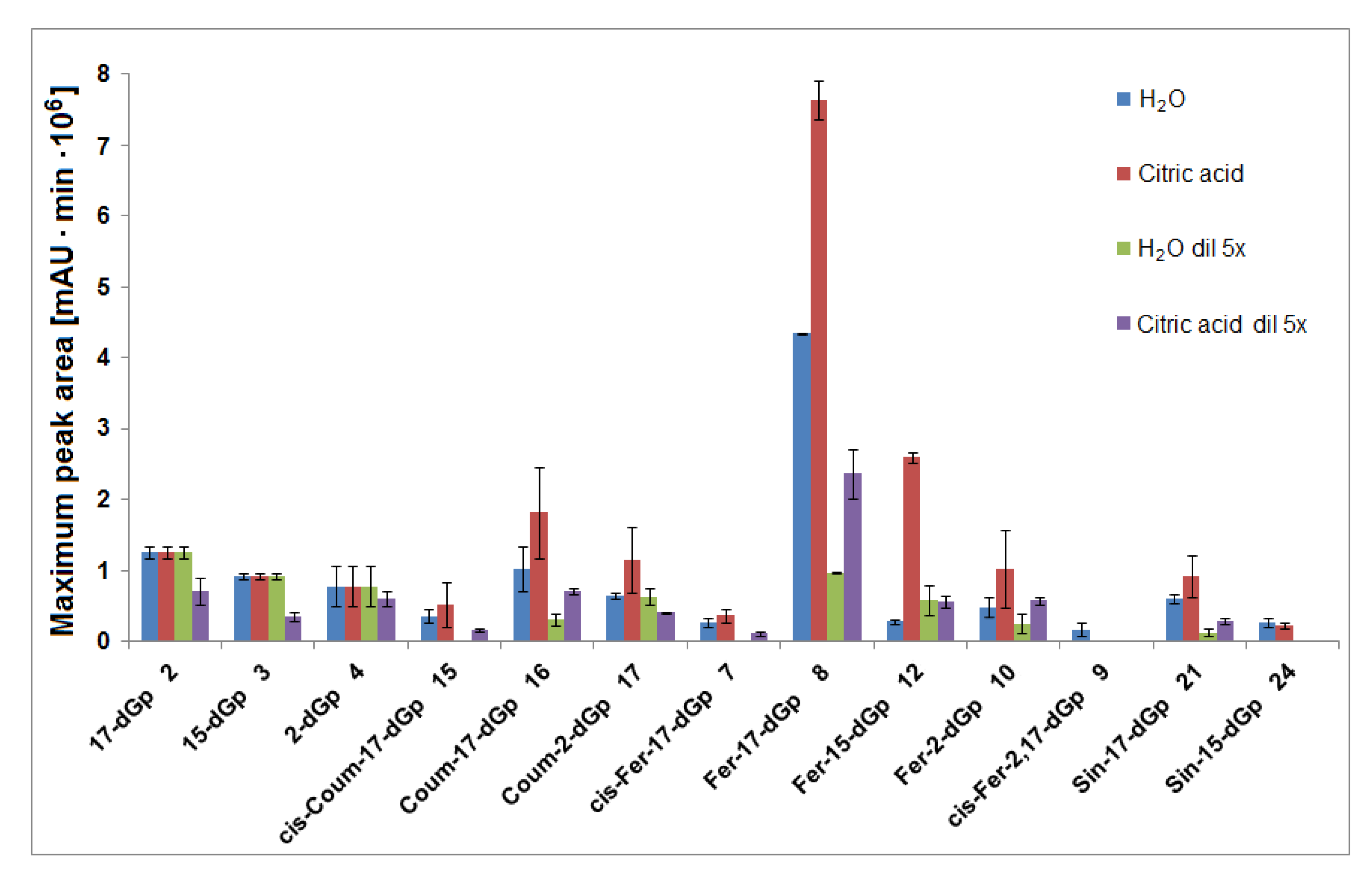
Interpretation of the LC-DAD and LC-MS spectra obtained in the HPLC gradient System 5 revealed that the main products appeared to be mono-decarboxylated derivatives for all gomphrenins due to the loss of CO2 from the corresponding precursors (Figure 4 and Figure 6). Based on previous studies [24], a group of distinct chromatographic peaks with characteristic absorption maxima at λmax 507, 515, 512, and 517 nm, influenced by the bathochromic effect of the acyl substituents, was attributed to 17-decarboxylated derivatives of nonacylated gomphrenins (17-dGp/-dIGp) 2/2′ as well as feruloylated (Fer-17-dGp/-dIGp) 8/8′, coumaroylated (Coum-17-dGp/-dIGp) 14/14′, and sinapoylated gomphrenins (Sin-17-dGp/-dIGp) 21/21′, respectively. Similarly, cis-feruloyl-17-decarboxy-gomphrenin/-isogomphrenin (cis-Fer-17-dGp/-dIGp) 7/7′ and cis-coumaroyl-17-decarboxy-gomphrenin/-isogomphrenin (cis-Coum-17-dGp/-dIGp) 15/15′ were tentatively detected (Table 1) only in undiluted samples due to very low signal intensities (Figure 6).
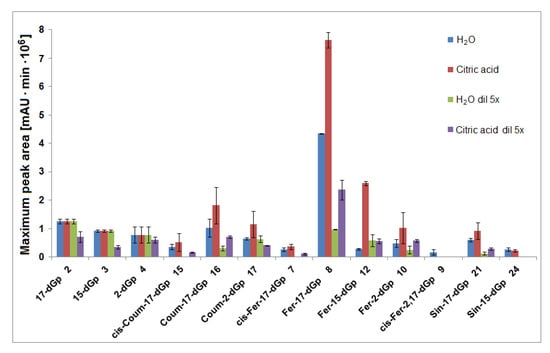
Figure 6.
Maxima of time-dependent concentration profiles of decarboxylated gomphrenin derivatives generated in the course of 60 min tea brewing experiments in aqueous and citric acid solutions at 90 °C.
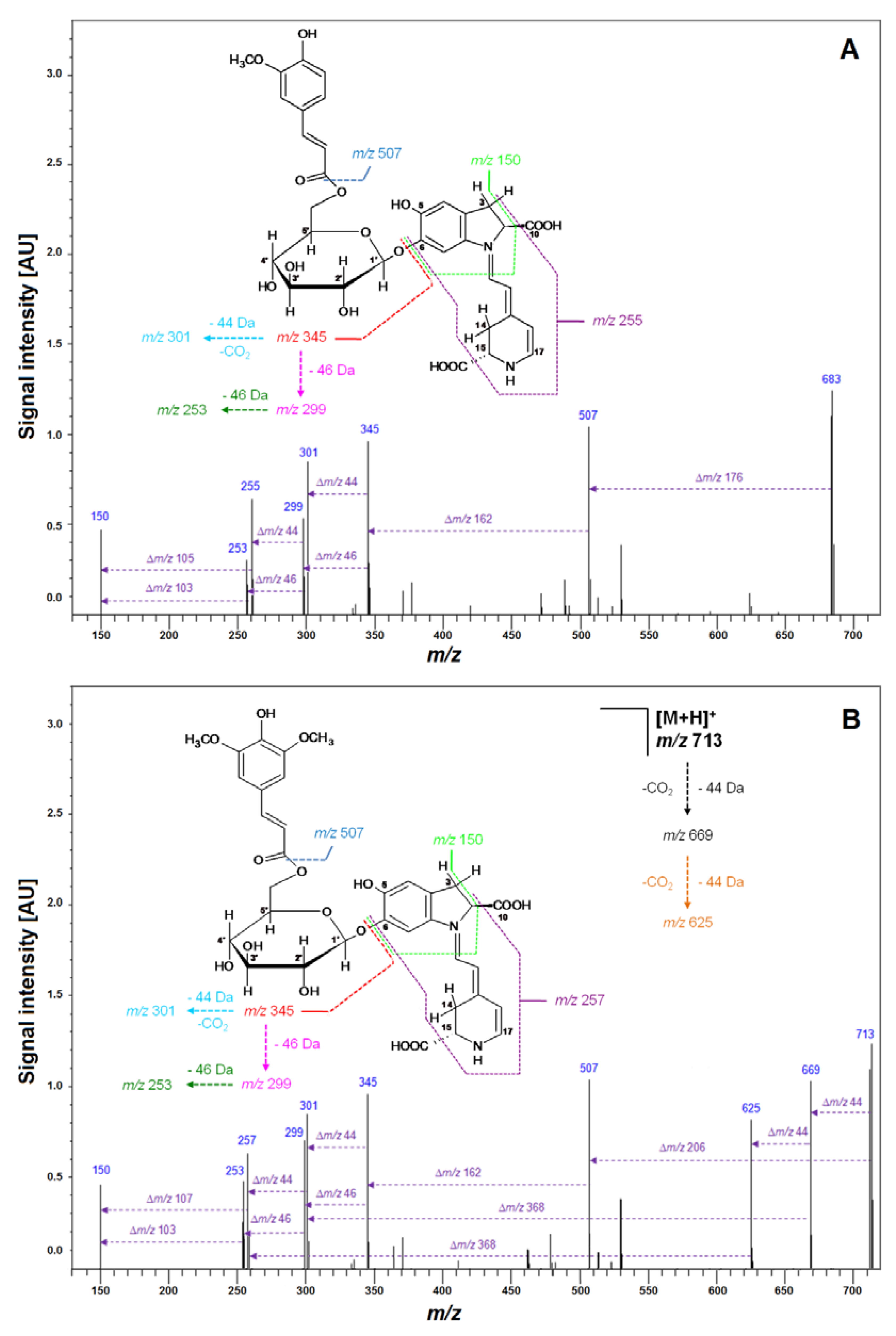
Subsequently, LCMS-IT-TOF analyses yielding m/z 683.2081 (C33H34N2O14, calculated m/z: 683.2083) confirmed the molecular formula of Fer-17-dGp 8 (Table 2). The observed fragmentation pathway in the MS2 mode afforded a signal at m/z 507 (Table 2), indicating detachment of a feruloyl moiety at the glucosyl ring of mono-decarboxylated gomphrenin (683 − 507 = 176 Da). Other daughter ions of Fer-17-dGp 8 detected at m/z 345 and 301 were assigned to mono- and bi-decarboxylated betanidin as a result of further deglycosylation (507 − 345 = 162 Da) and decarboxylation at carbon C-2 or C-15 (345 − 301 = 44 Da), respectively. The full fragmentation pattern is depicted in Figure 7. Likewise, HRMS determination of the molecular formula of C32H32N2O13 for the precursor ion of Coum-17-dGp 16 at m/z 653 supported the presence of a decarboxylated coumaroylated gomphrenin (determined m/z 653.1990; calculated m/z 653.1977). The fragmentation pattern of Coum-17-dGp 16 was analogous to that of Fer-17-dGp 8 and allowed for detection of the detachment of coumaroyl moiety (653 − 507 = 146 Da) from the glucosyl ring of mono-decarboxylated gomphrenin as well as the fragmentation ions at m/z 345 and 301. Fragmentation ions at m/z 507 and 345 were observed for cis-Fer- and cis-Coum-17-dGp (7 and 15). Sin-17-dGp 21 exhibited a precursor ion [M + H] + at m/z 713 and its molecular formula, C34H36N2O15, was obtained in HRMS analyses (determined m/z 713.2205; calculated m/z 713.2188). Its fragmentation pathway was analogous to the previously described Fer-17-dGp 8 as well as Coum-17-dGp 16, and it was presented in Figure 4. Detection of a fragmentation ion at m/z 507 supported the presence of Sin-17-dGp 21 (713 − 507 = 206 Da).
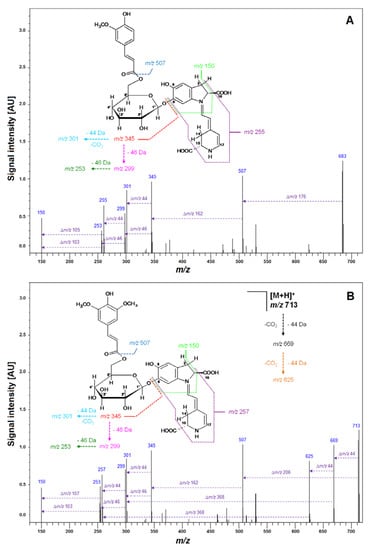
Figure 7.
Fragmentation patterns obtained by LCMS-IT-TOF for Fer-17-dGp 8 (A) and Sin-17-dGp 21 (B) formed in the course of heating at 90 °C for 20 min of isolated Fer-Gp 6 and Sin-Gp 20 from G. globosa flowers.
2.3. Concentration Profiles of 17-Decarboxylated-Gomphrenins in Aqueous Solutions
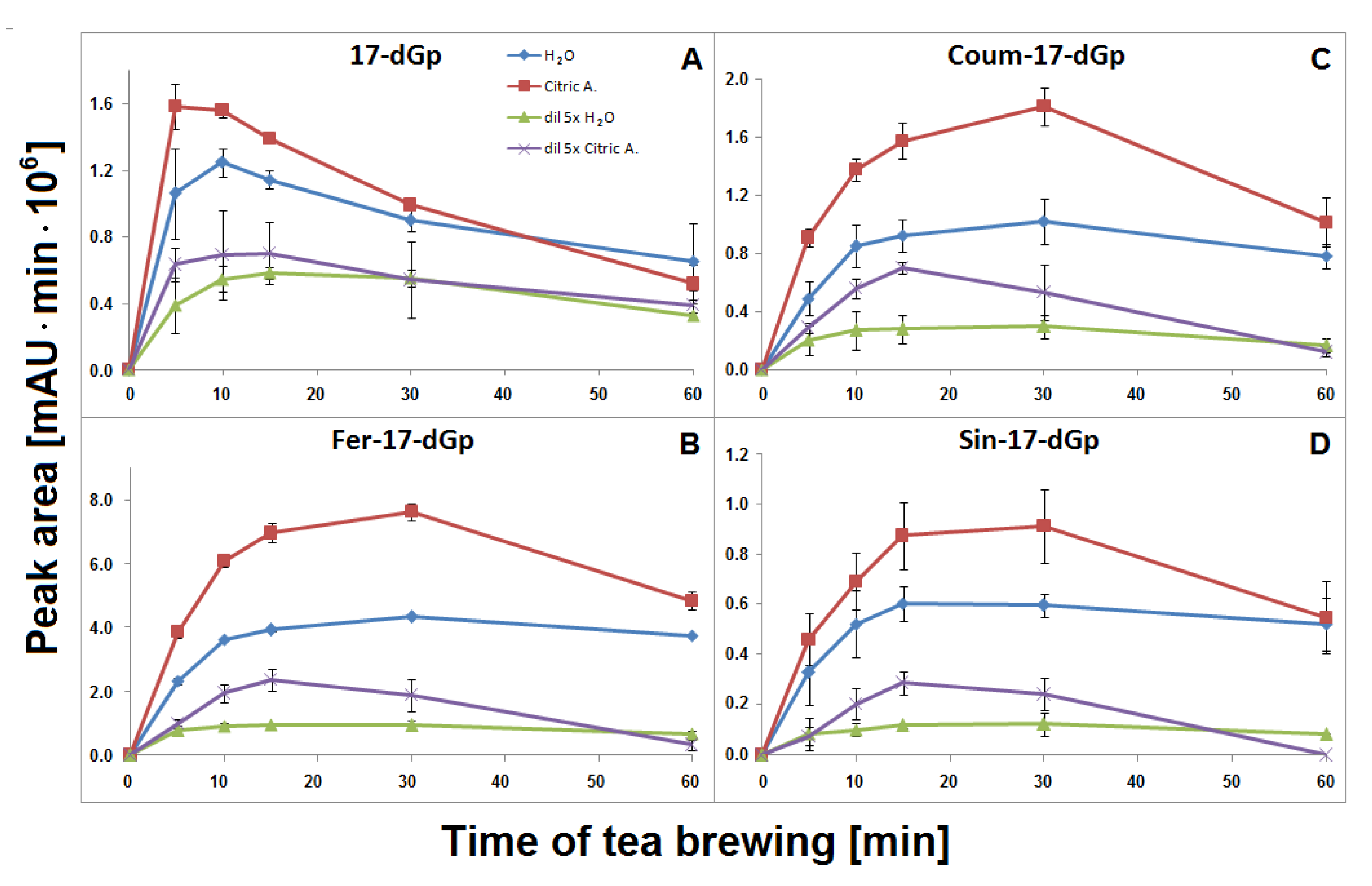
The time-dependent concentration profiles for 17-dGp 2 as well as acylated Fer-17-dGp 8, Coum-17-dGp 16, and Sin-17-dGp 21 in all the samples during tea brewing are shown in Figure 8A–D. The maximal concentrations for acylated derivatives 8, 16, and 21 were evidently shifted to 30 min in comparison to their parent substrates 6, 14, and 20 (Figure 5), for which the maxima were observed at ca. 15 min of the brewing experiment. This indicates that a continuous decarboxylation effect took place in the substrates Fer-Gp 6 and Coum-Gp 14 as a result of their heating in the presence of relatively high quantities of the inflorescence matrix. This effect is less demonstrated for the diluted samples (Figure 8B,C) presumably as a result of a lower concentration of the pigments and a lower stabilizing effect of the matrix, therefore, faster degradation of the generated pigments was observed. For the Sin-17-dGp 21, these differences were less pronounced (Figure 8D), most probably because of the much lower concentration of the parent substrate Sin-Gp 20 in the flowers. Concentration ratios (CR) of the resulting Fer-17-dGp 8, Coum-17-dGp 16, and Sin-17-dGp 21 to their respective natural gomphrenins slowly increased in the course of heating both in concentrated and diluted samples (Figure 9A,D,H, respectively). For 17-dGp 2, the concentration maximum was observed at 10–15 min of brewing (Figure 8A). This presumably results from a low concentration of Gp 1 in the flowers, but also from its much lower stability (evidenced in Figure 5C,D). The CR value of 17-dGp 2 to nonacylated gomphrenin 1 (Figure 9F) increased definitely faster than CR for acylated decarboxylated gomphrenins presumably due to lower stability of the initial pigment. For cis-Fer-17-dGp 7 and cis-Coum-17-dGp 15, the concentration profiles (data not shown) were virtually the same as for Fer-17-dGp 8 and Coum-17-dGp 16.
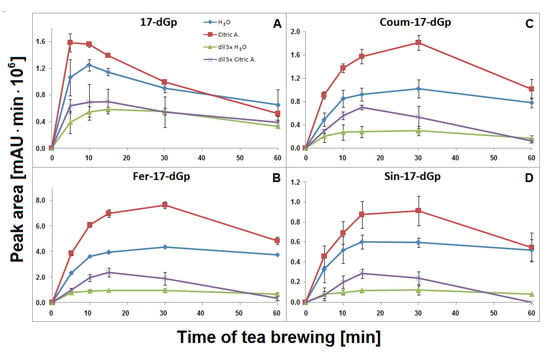
Figure 8.
Time-dependent concentration profiles of 17-decarbooxy-derivatives of nonacylated gomphrenin (A) and feruloylated, coumaroylated, and sinapoylated gomphrenins (B–D, respectively) generated during brewing of purple G. globosa flowers at 90 °C in undiluted and 5× diluted aqueous and citric acid solutions. These data represent time-dependent concentration of generated of 2-, 15-, and 2,17-decarboxy-gomphrenins. For clarity, calculated SD values for aqueous solutions were divided by a factor of 2 for 17-dGp (A) in undiluted aqueous solution of citric acid and diluted aqueous solutions and by a factor of 2 and 5 for Coum-17-dGp in undiluted aqueous and citric acid samples, respectively (C).
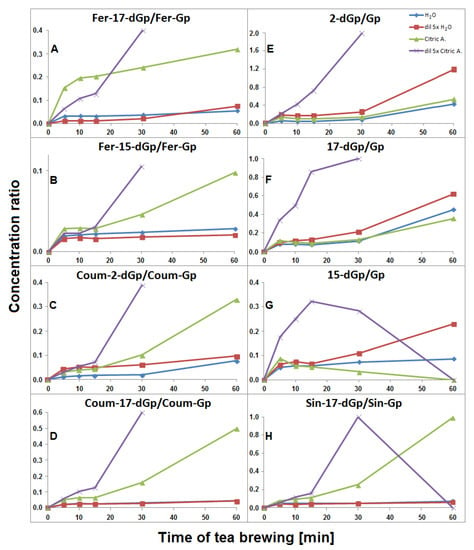
Figure 9.
Time-dependent concentration ratio CR of generated feruloylated (A and B), coumaroylated (C and D) and sinapoylated (H) as well as non-acylated (E–G) decarboxylated derivatives to their corresponding natural gomphrenins during G. globosa thermal processing at 90 °C. The ratios obtained for diluted citric acid samples after 60 min are not shown for most compounds due to a very high degradation rate of natural gomphrenins in citric acid solutions.
2.4. Generation and Identification of 2-Decarboxylated Derivatives of Gomphrenins in Aqueous Solutions
Other mono-decarboxylated compound 10 and the pair of 17/17′ were assigned to feruloyl-2-decarboxy-gomphrenin (Fer-2-dGp) and coumaroyl-2-decarboxy-gomphrenin/-isogomphrenin (Coum-2-dGp/-dIGp), respectively. These compounds displayed the characteristic absorption maxima at λmax 537 and 539 nm [24,28,29] and characteristic protonated molecular ions [M + H] + at m/z 683 and 653. For Fer-2-dGp 10, LCMS-IT-TOF analyses yielding m/z 683.2075 confirmed the molecular formula of C32H32N2O13. The same fragmentation ions as for Fer-17-dGp 8 were observed for Fer-2-dGp 10, besides the ion at m/z 301 that was not detected (Table 2).
2.5. Concentration Profiles of 2-Decarboxylated-Gomphrenins in Aqueous Solutions
Fer-2-dGp 10 was generated in the concentrated and diluted samples mainly within the first 10–15 min of tea brewing and most probably underwent following chemical changes (by decarboxylation) during the rest of the process, similarly to Fer-17-dGp 8 (Figure 8B). The diastereomer Fer-2-dIGp 10′ was not observed in the aqueous tea infusion samples. The time-dependent concentration profile of Coum-2-dGp 17 was very similar to the profile obtained for Coum-17-dGp 16 during thermal treatment (Figure 8C). CR values of Coum-2-dGp 17 slowly increased, similarly to Coum-17-dGp 16 (Figure 9C). For gomphrenin, one chromatographic peak, corresponding to 2-decarboxy-gomphrenin/-isogomphrenin 4/4′, was observed. This signal was characterized by absorption maximum at λmax 533 nm, which is coherent with the previous study [24]. Moreover, these data are close to the results obtained for betanin thermal degradation with 2-decarboxy-betanin/-isobetanin being the products present in heated betanin-rich red beet juice [21,25,26,27,30]. The concentration profile and the CR value of 2-dGp 4 was comparable to 17-dGp 2 (Figure 8A and Figure 9E).
2.6. Generation and Identification of 15-Decarboxylated Derivatives of Gomphrenins in Aqueous Solutions
The 15-decarboxy-derivatives, which were formed with a loss of the chiral center at carbon C-15 and exhibited only single chromatographic peaks, were tentatively identified for gomphrenin as well as feruloylated and sinapoylated gomphrenins. 15-decarboxy-gomphrenin (15-dGp) 3 and feruloyl-15-decarboxy-gomphrenin (Fer-15-dGp) 12 displayed the characteristic absorption maxima at λmax 530 nm. LC-MS and HRMS LCMS-IT-TOF spectra and the fragmentation ions were analogous to the data for 17-decarboxy-derivatives. The absorption maxima could not be observed for sinapoyl-15-decarboxy-gomphrenin (Sin-15-dGp) 24 due to low signal intensity.
2.7. Concentration Profiles of 15-Decarboxylated-Gomphrenins in Aqueous Solutions
The time-dependent concentration profiles of the acylated derivatives (Fer-15-dGp 12 and Sin-15-dGp 24) were close to the profiles of 17-decarboxylated derivative 8 (Figure 8B), while the nonacylated derivative 3 level decreased after 10 min, similarly to 17-dGp 2 (Figure 8A). Comparable concentration levels were observed for Fer-15-dGp 12 and Sin-15-dGp 24 in concentrated samples, whereas Sin-15-dGp 24 was not detected in diluted samples (Figure 6). The CR values of Fer-15-dGp 12 were comparable to Fer-17-dGp 8 during tea brewing, whereas the CR ratio of resulting 15-dGp 3 in dilute aqueous samples increased much faster (Figure 9B,G).
2.8. Generation of Bi-Decarboxylated Derivatives of Gomphrenins
In the course of tea brewing, one bi-decarboxylated derivative of the native acylated gomphrenin was tentatively detected as cis-feruloyl-2,17-bidecarboxy-gomphrenin (cis-Fer-2,17-dGp) 9. The concentration profile of cis-Fer-2,17-dGp 9 was analogous to Fer-17-dGp (Figure 8B). This compound was not completely detected in diluted aqueous samples due to extremely low signal intensity (Figure 6).
2.9. Extraction of Gomphrenins during Tea Brewing of G. globosa in Aqueous Citric Acid Solutions
In general, lower concentrations of extracted natural gomphrenins were determined during tea brewing in aqueous citric acid solutions in comparison to aqueous solutions. Exploration of the tea brewing data obtained for citric acid solutions revealed similar profiles of betacyanin degradation products to aqueous solutions. Cis-Fer-2,17-dGp 9 (m/z 639) previously observed in aqueous solutions was not detected in the citric acid solutions (Figure 6). The greatest concentration of acylated gomphrenins was obtained after 15 min of tea brewing for undiluted and fivefold diluted samples (Figure 5E,F), and their fast decline was observed within the next 15 min. In contrast to aqueous solutions, the degradation of substrates, cis-Fer-Gp/-IGp 5/5′, Fer-Gp/-IGp 6/6′, cis-Coum-Gp/-IGp 13/13′, Coum-Gp/-IGp 14/14′, and Sin-Gp/-IGp 20/20′ was much faster especially for diluted samples, with no substrates being retained after 60 min of the experiment (Figure 5F). The pair of Gp/-IGp 1/1′ was more labile than acylated gomphrenins, reaching the greatest concentration after 5 min and degrading very quickly during the next 25 min of tea brewing (Figure 5G,H).
2.10. Generation of Decarboxylated Derivatives of Gomphrenins in Aqueous Citric Acid Solutions and Their Time-Dependent Concentration Profiles
Similarly to aqueous tea samples, the pairs of diastereomers of Fer-, Coum- and Sin-17-dGp/-IGp (8/8′, 16/16′, 21/21′, respectively) were detected in citric acid tea infusions. An increase of their concentrations was observed during the first 30 min in the undiluted samples (Figure 8B–D) with a subsequent slight decline, in contrast to their precursors (Figure 5E). A similar effect was observed for diluted samples, however, in that case, the decline was observed after the first 15 min of tea brewing (Figure 8B–D). Interestingly, cis-Fer-17-dGp 7 and cis-Coum-17-dG 15 were detected both in undiluted and diluted citric acid samples, whereas these compounds were not present in diluted aqueous samples (Figure 6). For Fer-17-dGp 8 (Figure 9A) and Coum-17-dGp 16 (Figure 9D), a very strong increase of their time-dependent CR values in all samples extracted in citric acid solutions was observed. An increase of the CR ratio for Sin-17-dGp 21 (Figure 9H) could be observed during the heating of citric acid undiluted samples, whereas in diluted samples, an increase of the ratio was noticed until 30 min, with a subsequent decrease at 60 min. These changes were not observed in aqueous solutions because natural gomphrenins were more stable which resulted in low and steady CR ratios. For 17-dGp 2 (Figure 9F), an increase of the CR ratio was observed during the heating of the diluted samples. Other detected mono-decarboxylated derivatives, 17 and 12, were assigned to Coum-2-dGp and Fer-15-dGp, respectively. Their concentration profiles were the same as for Coum-17-dGp 16 and Fer-17-dGp 8, respectively, for undiluted and diluted samples (Figure 8C). For Coum-2-dGp 17 (Figure 9C) and Fer-15-dGp 12 (Figure 9B), very similar signal CR ratios were observed to the CR for Coum-17-dGp 16 (Figure 9D) and Fer-17-dGp 8 (Figure 9F). Fer-2-dIGp 10′ (m/z 683) was detected (Table 1), while this compound was not observed in aqueous samples (data not shown). The maximum signal intensity for 15-dGp 12 was observed after 5 min and 15 min of heating in undiluted and diluted aqueous solutions of citric acid, respectively. The Signal of Sin-15-dGp 24 started diminishing after 30 min and 15 min for undiluted and diluted samples, respectively (Figure 8D). The CR ratios obtained for 17-dGp 2 and 2-dGp 4 (Figure 9F and Figure 9E, respectively) increased faster than the ratios for the acylated derivatives for all diluted samples. In contrast, 15-dGp 3 (Figure 9G) was the most labile and was not present in citric acid solutions after 60 min of heating.
2.11. Studies on Model Acylated Gomphrenins Isolated from G. globosa Extract
For the aim of selective generation of simplified profiles of acylated gomphrenin derivatives for clear referencing of complex mixtures obtained during the tea brewing study, other heating experiments were performed on purified diastereomers isolated from G. globosa extract. These selected pigments were heated for 10–20 min at 90 °C only in aqueous citric acid solutions which were sufficient for providing clear comprehensive profiles of the referential derivatives and enabled finding the absorption maxima for most of the relevant chromatographically studied compounds. In a few cases, some derivatives generated in the model experiments were not detected in the tea brewing products. Selected chromatograms of the reaction mixtures obtained by heating of purified acylated gomphrenins registered at λ 500 nm are depicted in Figure 4C–E.
2.12. Identification of Thermally Mono-Decarboxylated Derivatives of Isolated Gomphrenins
Similarly to the tea brewing experiments, heating of the isolated model gomphrenins resulted primarily in the generation of their corresponding 17-decarboxylated derivatives Fer-, Coum-, and Sin-17-dGp/-dIGp (8/8′, 16/16′, 21/21′). From the 2-decarboxylated derivatives, Fer-2-dGp/-dIGp 10/10′ and Coum-2-dGp/-dIGp 17/17′ were present in great concentrations (Table 1). The pair of 22/22′, previously not detected in the tea brewing products, was tentatively assigned in the heating products of Sin-Gp/-IGp (20/20′) to sinapoyl-2-decarboxy-gomphrenin/-isogomphrenin (Sin-2-dGp/-dIGp), based on the absorption λmax 536 nm and the results of HRMS analyses which confirmed the molecular formula (measured m/z 713.2156 vs. calculated 713.2188, Table 2). LCMS-IT-TOF fragmentation yielded three ions of the same molecular formula, as obtained for the fragmentation ions of the isomeric compound Sin-17-dGp 21. 15-decarboxylated derivatives of all the acylated gomphrenins (Fer-15-dGp 12, Coum-15-dGp 19, and Sin-15-dGp 24) were tentatively detected in the heating products as well (Table 1).
2.13. Identification of Thermally Bi-Decarboxylated Derivatives of Isolated Acylated Gomphrenins
LC-DAD-ESI-MS/MS analyses of heating products generated from purified acylated gomphrenins resulted in the detection of a wider variety of bi-decarboxylated betacyanins than during the tea brewing experiments. Chromatographic peaks corresponding to Fer-2,17-dGp/-dIGp 11/11′ were detected based on absorption maximum (λmax 520 nm) and the measured molecular formula of C32H34N2O12 (m/z 639.2174) by HRMS (Table 2), as well as the fragmentation patterns in the MS2 mode which afforded m/z signal at 463, indicating detachment of a feruloyl moiety within the glucosyl ring of bi-decarboxylated gomphrenin/isogomphrenin (639 − 463 = 176 Da). Other daughter ions of Fer-2,17-dGp/-dIGp 11/11′ detected at m/z 301 and 257 were assigned to bi-decarboxylated betanidin/isobetanidin as a result of further deglycosylation (463 − 301 = 162 Da) as well as to tri-decarboxylated betanidin/isobetanidin (301 − 257 = 44 Da) as a result of decarboxylation at C-15. The analogous pair of diastereomers 18/18′ with λmax 515 nm and m/z 609.2085 (Table 2) as well as similar fragmentation was assigned to coumaroyl-2,17-bidecarboxy- -gomphrenin/-isogomphrenin (Coum-2,17-dGp/-dIGp). A very weak signal for a pair of 23/23′ was tentatively assigned to sinapoyl-2,17-decarboxy-gomphrenin/-isogomphrenin (Sin-2,17-dGp/-dIGp) with m/z 669. This study revealed a complex variety of decarboxylated derivatives present in tea infusions of violet G. globosa dried flowers. In general, citric acid addition exhibited a degradative influence on natural gomphrenins’ stability, mainly during the longer tea brewing process (above 15 min), however, the presence of plant matrix significantly increased the stability of identified betacyanins. Moreover, the acylated gomphrenins exhibited greater stability than gomphrenins 1/1′.
3. Materials and Methods
3.1. Plant Material
Dried purple flowers of Gomphrena globosa L. were purchased from a China market.
3.2. Reagents
Formic acid, acetone, LC-MS grade methanol, and water were received from Sigma Chemical Co. (St. Louis, MO, USA). Citric acid was obtained from POCH (Gliwice, Poland).
3.3. Tea Brewing of Purple G. Globosa Flowers
To determine the influence of plant matrix on betacyanins’ stability, two (0.940 g) or two fifths (0.188 g) of flowers were brewing at 90 °C in 6 mL of solvent (demi-water or 1% aqueous citric acid). Heated samples were collected subsequently for LC-DAD-ESI-MS analyses after 5, 10, 15, 30, and 60 min, respectively. All of the experiments were performed in triplicate and standard deviation (SD) was calculated. Previously isolated single diastereomers of acylated gomphrenins were used for additional heating experiments in a citric acid solution to preliminarily determine degradation products.
3.4. Preparation of Plant Material for Semi-Preparative Chromatography
The extraction of 1 kg of plant material was performed in a 1% aqueous solution of formic acid (v/v). The obtained extract was pumped through the 3 cm layer of a silica gel in a Büchner funnel to separate the solid particles and the suspension. Separation and purification of the extract proceeded onto the ion exchange bed of a strong anion exchanger SepraTM ZT-SAX with a 30 μm pore size (Phenomenex, Torrance, CA, USA). After adsorption, pigments were eluted by a solution of 5% aqueous formic acid in 50% acetone (v/v). The collected eluate was concentrated in a rotary evaporator under reduced pressure at 25 °C and then purified by semi-preparative liquid chromatography.
3.5. Semi-Preparative Chromatography
For purification and isolation of gomphrenin pigments from G. globosa flowers, a semi-preparative HPLC system with a pump (Knauer HPLC PUMP 64, Knauer, Berlin, Germany), a UV−Vis detector (Knauer), and LP-Chrom operating software, (Lipopharm, Zblewo, Poland) equipped with a C18 (250 × 50 mm i.d., 40 μm) column (Interchim, Montlucon, France) and a 10 × 10 mm guard column (Phenomenex), was applied. Separations were carried out under the following gradient system (System 1) composed of 1% aqueous formic acid (A) and acetone (B) as follows: 0 min, 20% B; increasing linearly to 20 min, 25% B; increasing linearly to 30 min, 30% B; increasing linearly to 40 min, 35% B. The injection volume was 30 mL and the flow rate was 50 mL/min. For further purification of gomphrenin I, the following gradient system (System 2) consisting of 1% aqueous formic acid (A) and acetone (B) was applied: 0 min, 8% B; increasing linearly to 20 min, 10% B; increasing linearly to 30 min, 12% B; increasing linearly to 40 min, 14% B. Purified gomphrenin II and III were obtained using an isocratic preparative HPLC system (System 3) composed of 1% aqueous formic acid (A) and acetone (B) and it was as follows: 0 min, 25% B to 30 min. Finally, for gomphrenin IV purification, the following isocratic system (System 4) composed of 4% aqueous formic acid (A) and acetone (B) was as follows: 15% B for 30 min. Detection was performed at λ 505 nm with a PDA UV/Vis detector.
3.6. LC-DAD-ESI-MS/MS Analyses
For qualitative and quantitative analyses of tea infusion samples as well as fractions collected during semi-preparative chromatographic separation, a low resolution LC-MS-8030 mass spectrometric system (Shimadzu, Kyoto, Japan) coupled to LC-20ADXR HPLC pumps, an injector model SIL-20ACXR, and a PDA detector (photodiode array) model SPD-M20A, all controlled with LabSolutions software, version 5.60 SP1 (Shimadzu), was applied. The samples were eluted through a 150 × 4.6 mm i.d., 5.0 μm, Kinetex C18 chromatographic column preceded by a guard column of the same material (Phenomenex). The injection volume was 50 μL and the flow rate was 0.5 mL/min. The column was thermostated at 40 °C. Samples solutions were pumped through the column under the following elution gradient system (System 5) composed of 2% aqueous formic acid (A) and pure methanol (B) as follows: 0 min, 26% B; increasing linearly to 32 min, 90% B; increasing linearly to 35 min, 12% B; increasing linearly to 40 min, 14% B. The column was thermostated at 40 °C. The injection volume was 50 μL and the flow rate was 0.5 mL/min. The detection was performed in the full PDA range and at selected wavelengths (540, 505, 480, and 440 nm). The ionization electrospray source operated in positive mode (ESI+) at an electrospray voltage of 4.5 kV, capillary temperature 250 °C, and using N2 as a sheath gas. The LC-MS system was controlled by LabSolutions software, version 5.60 SP1 (Shimadzu), recording total ion chromatograms, mass spectra, and ion chromatograms in selected ion monitoring mode (SIM), as well as the fragmentation spectra. Argon was used as the collision gas for the collision-induced dissociation (CID) experiments. The relative collision energies for MS/MS analyses were set at −35 V.
3.7. Chromatographic Analyses with Detection by Ion-Trap Time-of-Flight System (LCMS-IT-TOF)
The mass spectrometer (Shimadzu) with electrospray ionization mode (ESI) coupled to the HPLC Prominence (Shimadzu) was applied to record all mass spectra. The compounds were separated on a 50 × 2.1 mm i.d., 1.9 μm Shim Pack GISS C18 column (Shimadzu) thermostated at 40 °C. Samples were dosed in a volume of 2 μL and the flow rate was 0.2 mL/min. The separation of the analytes was performed in the same gradient systems as in the case of LC-DAD-ESI-MS/MS. The parameters of LCMS-IT-TOF spectrometer were set as follows: curved desolvation line (CDL) and heat block temperature was 230 °C, the nebulizing gas flow rate was 1.5 L/min, and capillary voltage was 4.5 kV. Positive ion mode with the mass range within 100–2000 Da was applied for recording all mass spectra. The collision energy was in the range of 12–50% independence on the structure of the compounds. The Formula Predictor within LCMS Solution software was used for the elaboration of results obtained in high-resolution mass spectrometry experiments (HRMS). Only an empirical formula with a mass error below 5 ppm was considered.
4. Conclusions
Herein, it is the first qualitative and quantitative report on gomphrenin derivatives formed during a tea brewing process in aqueous and aqueous citric acid solutions. The presence of gomphrenins in G. globosa flower extract may exhibit a positive impact on human health by regular drinking of its tea infusions. The rich profile of decarboxylated gomphrenins may contribute to their antioxidative and other pro-health activities; however, further research on the group of these biologically active compounds is still needed.
Author Contributions
Conceptualization, S.W.; methodology, S.W.; software, N.D.; validation, S.W. and N.D.; formal analysis, N.D. and S.W.; investigation, N.D. and S.W.; resources, E.D.; data curation, S.W. and N.D.; writing—original draft preparation, N.D. and S.W.; writing—review and editing, A.K.-J., J.O., T.Ś. and S.W.; visualization, K.S., A.S.-K., and M.A.; supervision, S.W., project administration, S.W.; funding acquisition, T.Ś. and J.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financed by Polish National Science Centre for years 2018–2021 (Project No. UMO-2017/27/B/NZ9/02831).
Acknowledgments
The authors thank Beata Wileńska and Bartłomiej Fedorczyk from Laboratory of Biologically Active Compounds (Warsaw University) for the excellent technical assistance with LCMS-IT-TOF experiments.
Conflicts of Interest
The authors declare no conflict of interests.
References
- Vieira, C.C.J.; Mercier, H.; Chu, E.P.; Figueiredo-Ribeiro, R.C.L. Gomphrena Species (Globe Amaranth). In Vitro Culture and Production of Secondary Metabolites; Springer: Berlin/Heidelberg, Germany, 1994; pp. 257–270. [Google Scholar]
- Roriz, C.L.; Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. HPLC-profiles of tocopherols, sugars, and organic acids in three medicinal plants consumed as infusions. Int. J. Food Sci. 2014, 2014, 241481. [Google Scholar] [CrossRef]
- Kumorkiewicz, A.; Sutor, K.; Nemzer, B.; Pietrzkowski, Z.; Wybraniec, S. Thermal decarboxylation of betacyanins in red beet betalain-rich extract. Polish J. Food Nutr. Sci. 2020, 70, 7–14. [Google Scholar] [CrossRef]
- Burda, S.; Oleszek, W. Antioxidant and antiradical activities of flavonoids. J. Agric. Food Chem. 2001, 49, 2774–2779. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Sun, M.; Xing, J.; Corke, H. Antioxidant phenolic constituents in roots of Rheum officinale and Rubia cordifolia: Structure-radical scavenginq activity relationships. J. Agric. Food Chem. 2004, 52, 7884–7890. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Sun, M.; Corke, H. Antioxidant activity of betalains from plants of the Amaranthaceae. J. Agric. Food Chem. 2003, 51, 2288–2294. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.R.; Valentão, P.; Faria, J.; Ferreres, F.; Sousa, C.; Gil-Izquierdo, A.; Pinho, B.R.; Andrade, P.B. Phytochemical investigations and biological potential screening with cellular and non-cellular models of globe amaranth (Gomphrena globosa L.) inflorescences. Food Chem. 2012, 135, 756–763. [Google Scholar] [CrossRef]
- Vulić, J.J.; Ćebović, T.N.; Čanadanović, V.M.; Ćetković, G.S.; Djilas, S.M.; Čanadanović-Brunet, J.M.; Velićanski, A.S.; Cvetković, D.D.; Tumbas, V.T. Antiradical, antimicrobial and cytotoxic activities of commercial beetroot pomace. Food Funct. 2013, 4, 713–721. [Google Scholar] [CrossRef]
- Kanner, J.; Harel, S.; Granit, R. Betalains—A new class of dietary cationized antioxidants. J. Agric. Food Chem. 2001, 49, 5178–5185. [Google Scholar] [CrossRef]
- Ben Haj Koubaier, H.; Snoussi, A.; Essaidi, I.; Chaabouni, M.M.; Thonart, P.; Bouzouita, N. Betalain and Phenolic Compositions, Antioxidant Activity of Tunisian Red Beet (Beta vulgaris L.conditiva) Roots and Stems Extracts. Int. J. Food Prop. 2014, 17, 1934–1945. [Google Scholar] [CrossRef]
- Allegra, M.; D’Acquisto, F.; Tesoriere, L.; Attanzio, A.; Livrea, M.A. Pro-oxidant activity of indicaxanthin from Opuntia ficus indica modulates arachidonate metabolism and prostaglandin synthesis through lipid peroxide production in LPS-stimulated RAW 264.7 macrophages. Redox Biol. 2014, 2, 892–900. [Google Scholar] [CrossRef][Green Version]
- Gandía-Herrero, F.; Escribano, J.; García-Carmona, F. Structural implications on color, fluorescence, and antiradical activity in betalains. Planta 2010, 232, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Wybraniec, S.; Starzak, K.; Pietrzkowski, Z. Chlorination of Betacyanins in Several Hypochlorous Acid Systems. J. Agric. Food Chem. 2016, 64, 2865–2874. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Vargas, F.; Jiménez, A.R.; Paredes-López, O.; Francis, F.J. Natural pigments: Carotenoids, anthocyanins, and betalains—Characteristics, biosynthesis, processing, and stability. Crit. Rev. Food Sci. Nutr. 2000, 40, 173–289. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Roriz, C.L.; Morales, P.; Barros, L.; Ferreira, I.C.F.R. Coloring attributes of betalains: A key emphasis on stability and future applications. Food Funct. 2017, 8, 1357–1372. [Google Scholar] [CrossRef]
- Kugler, F.; Stintzing, F.C.; Carle, R. Characterisation of betalain patterns of differently coloured inflorescences from Gomphrena globosa L. and Bougainvillea sp. by HPLC–DAD–ESI–MS n. Anal. Bioanal. Chem. 2007, 387, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Strack, D.; Vogt, T.; Schliemann, W. Recent advances in betalain research. Phytochemistry 2003, 62, 247–269. [Google Scholar] [CrossRef]
- Jerz, G.; Gebers, N.; Szot, D.; Szaleniec, M.; Winterhalter, P.; Wybraniec, S. Separation of amaranthine-type betacyanins by ion-pair high-speed countercurrent chromatography. J. Chromatogr. A 2014, 1344, 42–50. [Google Scholar] [CrossRef]
- Strack, D.; Vogt, T.; Schliemann, W. Recent Advances in Betalain Research. ChemInform 2003, 34. [Google Scholar] [CrossRef]
- Wybraniec, S.; Platzner, I.; Geresh, S.; Gottlieb, H.E.; Haimberg, M.; Mogilnitzki, M.; Mizrahi, Y. Betacyanins from vine cactus Hylocereus polyrhizus. Phytochemistry 2001, 58, 1209–1212. [Google Scholar] [CrossRef]
- Wybraniec, S. Formation of decarboxylated betacyanins in heated purified betacyanin fractions from red beet root (Beta vulgaris L.) monitored by LC-MS/MS. J. Agric. Food Chem. 2005, 53, 3483–3487. [Google Scholar] [CrossRef]
- Spórna-Kucab, A.; Jagodzińska, J.; Wybraniec, S. Separation of betacyanins from purple flowers of Gomphrena globosa L. by ion-pair high-speed counter-current chromatography. J. Chromatogr. A 2017, 1489, 51–57. [Google Scholar] [CrossRef]
- Tuwalska, D.; Starzak, K.; Szot, D.; Wybraniec, S.; Winterhalter, P.; Jerz, G. Semi-synthesis of red beet betacyanin ethyl-esters by esterification. Challenges Mod. Technol. 2014, 5, 27–31. [Google Scholar]
- Kumorkiewicz, A.; Wybraniec, S. Thermal Degradation of Major Gomphrenin Pigments in the Fruit Juice of Basella alba L. (Malabar Spinach). J. Agric. Food Chem. 2017, 65, 7500–7508. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Sun, M.; Corke, H. Identification and distribution of simple and acylated betacyanins in the Amaranthaceae. J. Agric. Food Chem. 2001, 49, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Heuer, S.; Richter, S.; Metzger, J.W.; Wray, V.; Nimtzt, M.; Strack, D. Betacyanins from bracts of Bougainvillea glabra. Phytochemistry 1994, 37, 761–767. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Xing, J.; Sun, M.; Corke, H. Rapid identification of betacyanins from Amaranthus tricolor, Gomphrena globosa, and Hylocereus polyrhizus by matrix-assisted laser desorption/ ionization quadrupole ion trap time-of-flight mass spectrometry (MALDI-QIT-TOF MS). J. Agric. Food Chem. 2006, 54, 6520–6526. [Google Scholar] [CrossRef]
- Ferreres, F.; Gil-Izquierdo, A.; Valentão, P.; Andrade, P.B. Structural characterization of phenolics and betacyanins in Gomphrena globosa by high-performance liquid chromatography-diode array detection/electrospray ionization multi-stage mass spectrometry. Rapid Commun. Mass Spectrom. 2011, 25, 3441–3446. [Google Scholar] [CrossRef]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Stability and color changes of thermally treated betanin, phyllocactin, and hylocerenin solutions. J. Agric. Food Chem. 2006, 54, 390–398. [Google Scholar] [CrossRef]
- Wybraniec, S.; Mizrahi, Y. Generation of decarboxylated and dehydrogenated betacyanins in thermally treated purified fruit extract from purple pitaya (Hylocereus polyrhizus) monitored by LC-MS/MS. J. Agric. Food Chem. 2005, 53, 6704–6712. [Google Scholar] [CrossRef]
- Gonçalves, L.C.P.; Di Genova, B.M.; Dörr, F.A.; Pinto, E.; Bastos, E.L. Effect of dielectric microwave heating on the color and antiradical capacity of betanin. J. Food Eng. 2013, 118, 49–55. [Google Scholar] [CrossRef]
- Tesoriere, L.; Fazzari, M.; Angileri, F.; Gentile, C.; Livrea, M.A. In vitro digestion of betalainic foods. Stability and bioaccessibility of betaxanthins and betacyanins and antioxidative potential of food digesta. J. Agric. Food Chem. 2008, 56, 10487–10492. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).