A Novel Method for Visualizing Melanosome and Melanin Distribution in Human Skin Tissues
Abstract
:1. Introduction
2. Results
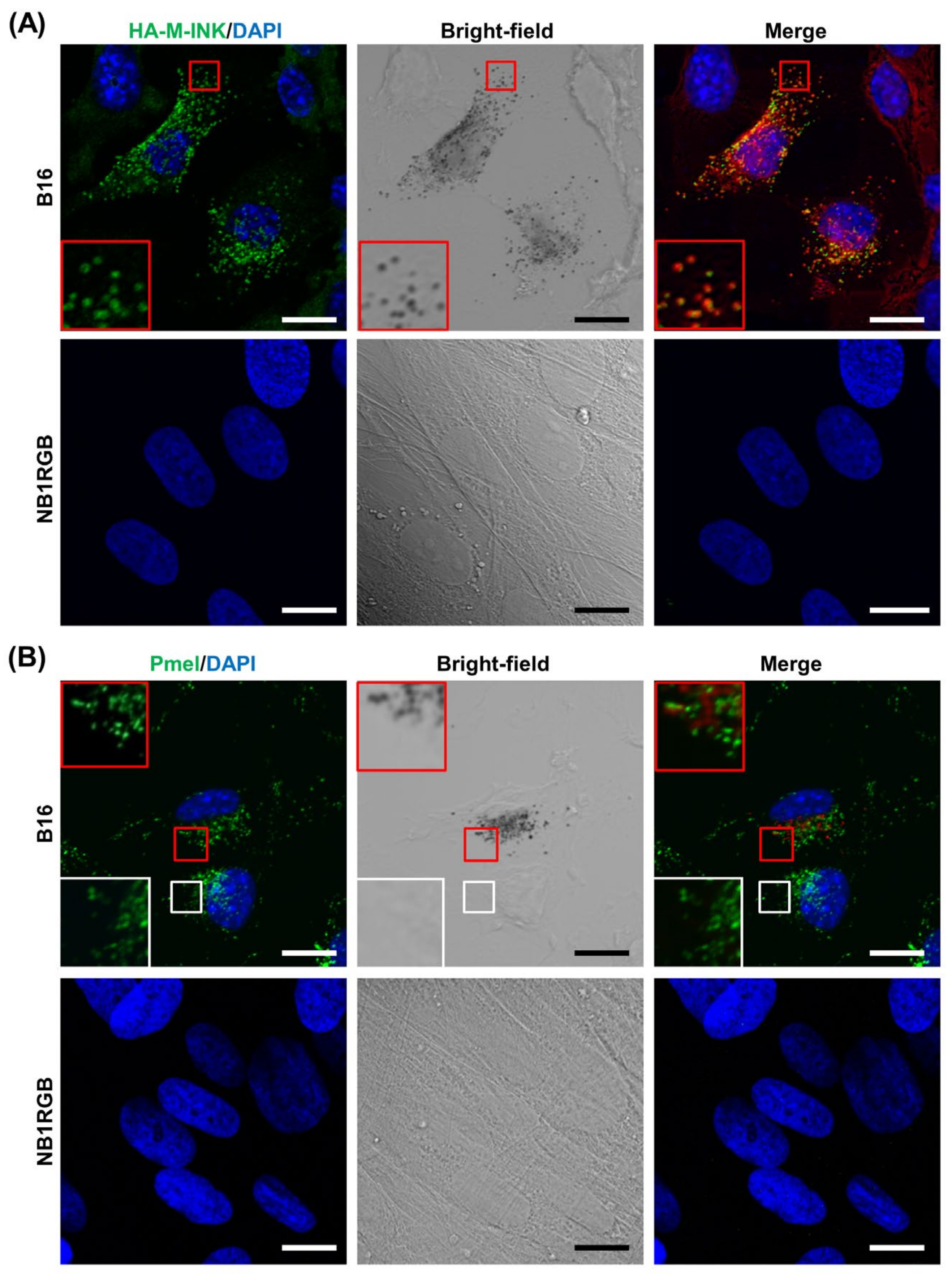
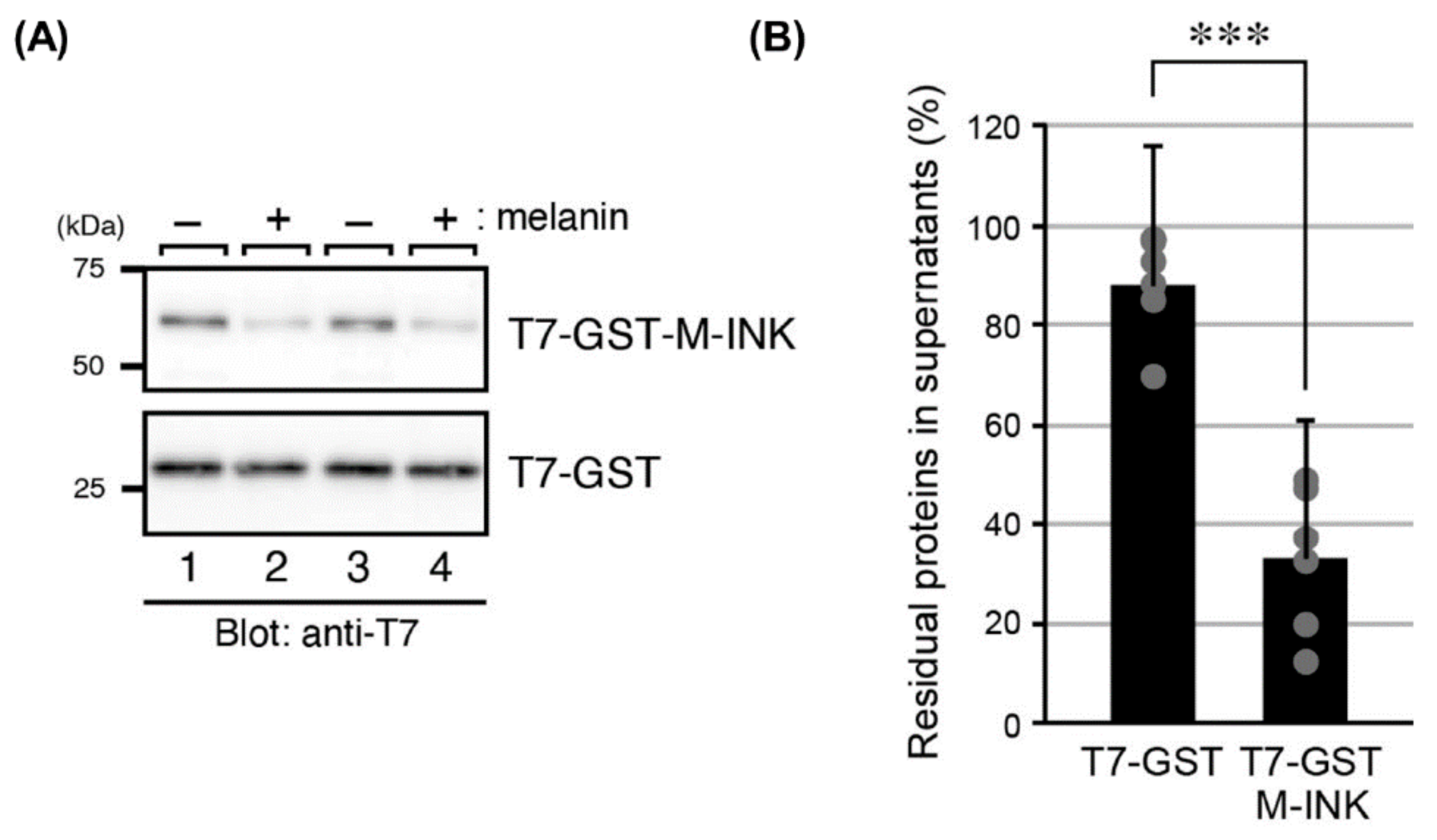
2.1. M-INK Recognizes Both Mature Melanosomes and Purified Melanin
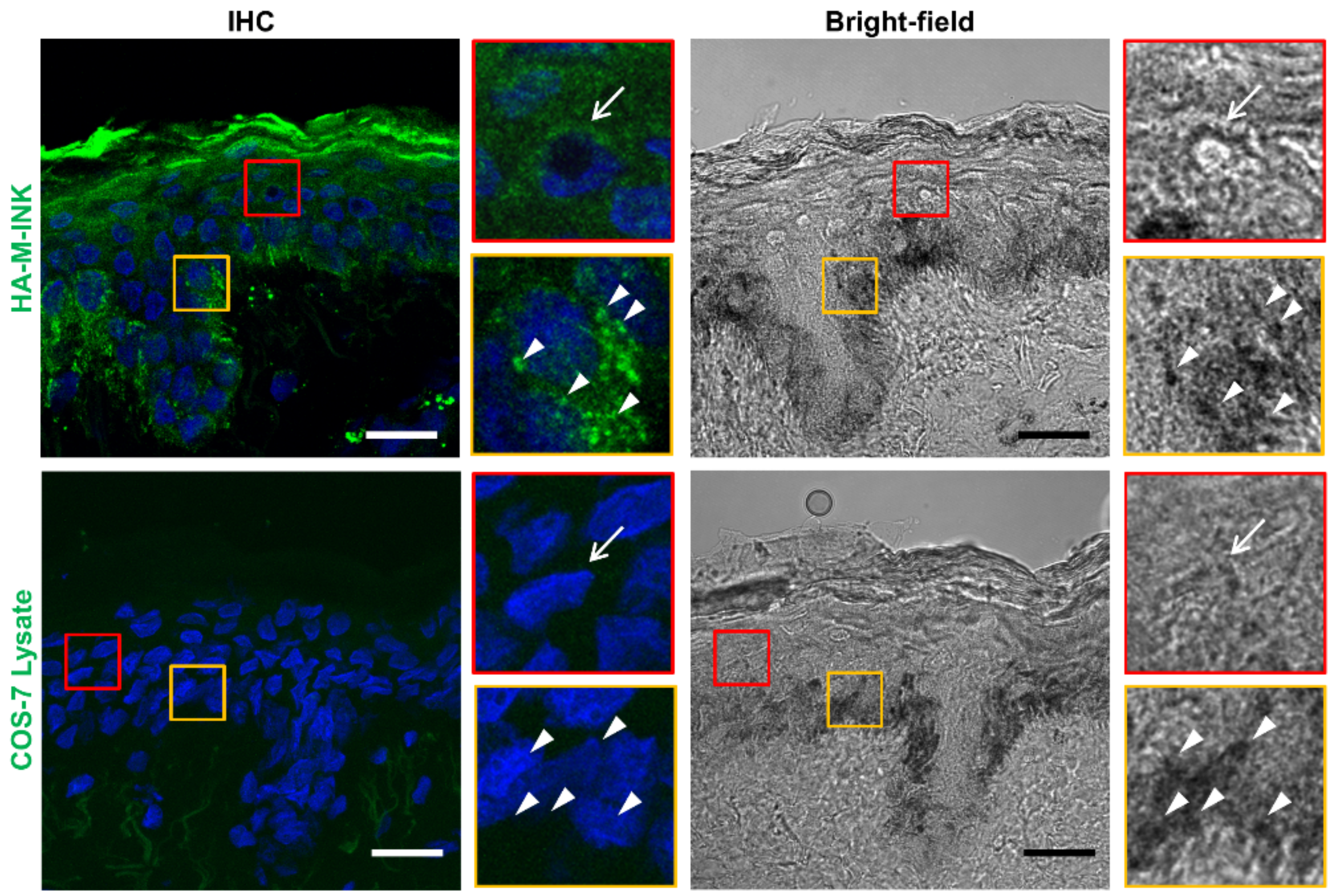
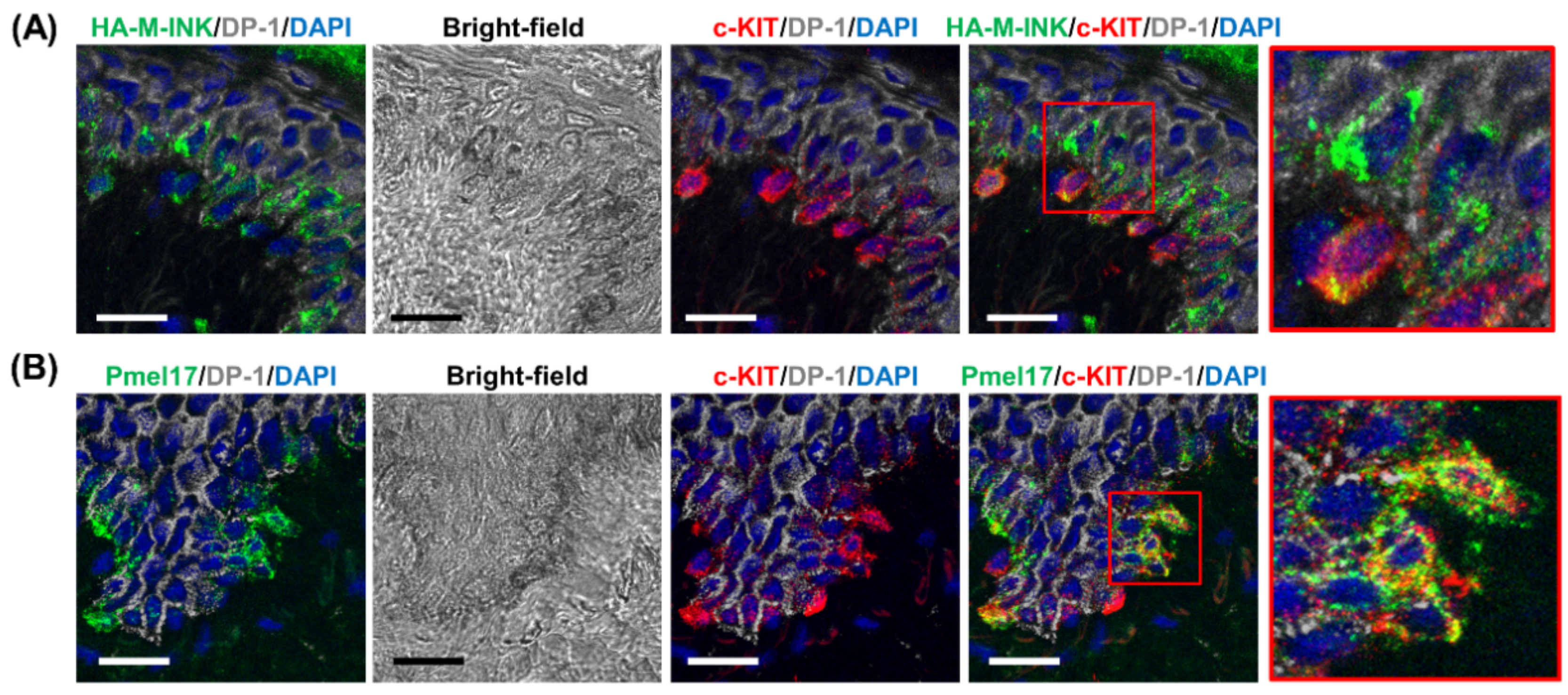
2.2. HA-M-INK Recognizes Melanin or Black Mature Melanosomes in Human Skin Tissue
2.3. Correlation between Skin Phototypes and Fluorescence Signals by HA-M-INK
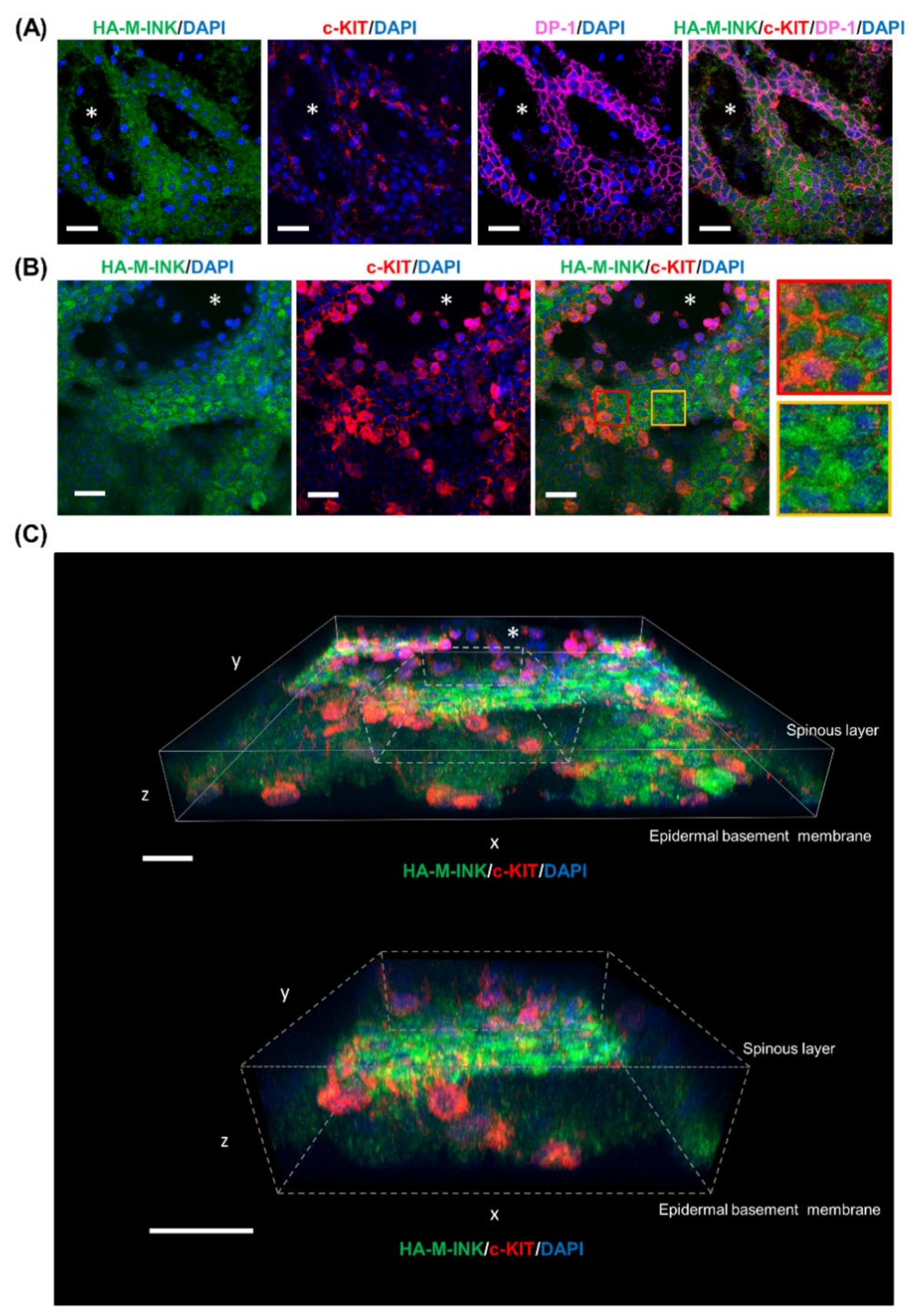
2.4. Three-Dimensional (3D) Reconstruction of Epidermal Tissue Structure
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Plasmid Construction
4.3. Preparation of HA-M-INK
4.4. Cell Cultures
4.5. Interaction of M-INK with Purified Melanin In Vitro
4.6. Tissues
4.7. Skin Color Measurements
4.8. Preparation of Epidermal Sheets and Skin Specimens
4.9. Immunohistochemistry
4.10. Confocal Microscopy
4.11. Measurement of Fluorescence Intensity
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D | Two-Dimensional |
| 3D | Three-Dimensional |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DP-1 | Desmoplakin 1 |
| FBS | Fetal Bovine Serum |
| GST | Glutathione S-transferase |
| HA-M-INK | Hemagglutinin-Tagged M-INK |
| IHC | Immunohistochemical |
| L-Dopa | 3,4-Dihydroxyphenylalanine |
| M-INK | Melanocore-Interacting Kif1c-Tail |
| PAGE | Polyacrylamide Gel Electrophoresis |
| PBS | Phosphate-Buffered Saline |
| T7-GST-M-INK | T7-GST-Tagged M-INK |
| Tyrp1 | Tyrosinase-Related Protein 1 |
References
- Boissy, R.E. Melanosome transfer to and translocation in the keratinocyte. Exp. Dermatol. 2003, 12, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Byers, H. Melanosome processing in keratinocytes. In The Pigmentary System: Physiology and Pathophysiology, 2nd ed.; Nordlund, J.J., Boissy, R.E., Hearing, V.J., King, R.A., Oetting, W.S., Ortonne, J.P., Eds.; Wiley: Hoboken, NJ, USA, 2007; pp. 181–190. [Google Scholar]
- Yamashita, T.; Kuwahara, T.; Gonzalez, S.; Takahashi, M. Non-invasive visualization of melanin and melanocytes by reflectance-mode confocal microscopy. J. Investig. Dermatol. 2005, 124, 235–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Den Bossche, K.; Naeyaert, J.M.; Lambert, J. The quest for the mechanism of melanin transfer. Traffic 2006, 7, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Lillie, R.D. Metal reduction reactions of the melanins: Histochemical studies. J. Histochem. Cytochem. 1957, 5, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Yonei, N.; Kaminaka, C.; Kimura, A.; Furukawa, F.; Yamamoto, Y. Two patterns of solar lentigines: A histopathological analysis of 40 Japanese women. J. Dermatol. 2012, 39, 829–832. [Google Scholar] [CrossRef]
- Noblesse, E.; Nizard, C.; Cario-Andre, M.; Lepreux, S.; Pain, C.; Schnebert, S.; Taieb, A.; Kurfurst, R. Skin ultrastructure in senile lentigo. Skin Pharmacol. Physiol. 2006, 19, 95–100. [Google Scholar] [CrossRef]
- Hurbain, I.; Romao, M.; Sextius, P.; Bourreau, E.; Marchal, C.; Bernerd, F.; Duval, C.; Raposo, G. Melanosome distribution in keratinocytes in different skin types: Melanosome clusters are not degradative organelles. J. Investig. Dermatol. 2018, 138, 647–656. [Google Scholar] [CrossRef] [Green Version]
- Kwon-Chung, K.J.; Hill, W.B.; Bennett, J.E. New, special stain for histopathological diagnosis of cryptococcosis. J. Clin. Microbiol. 1981, 13, 383–387. [Google Scholar] [CrossRef] [Green Version]
- Watt, B.; van Niel, G.; Raposo, G.; Marks, M.S. PMEL: A pigment cell-specific model for functional amyloid formation. Pigment. Cell Melanoma Res. 2013, 26, 300–315. [Google Scholar] [CrossRef] [Green Version]
- Delevoye, C.; Giordano, F.; van Niel, G.; Raposo, G. Biogenesis of melanosomes—The chessboard of pigmentation. Med. Sci. (Paris) 2011, 27, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Marubashi, S.; Fukuda, M. Rab7B/42 is functionally involved in protein degradation on melanosomes in keratinocytes. Cell Struct. Funct. 2020, 45, 45–55. [Google Scholar] [CrossRef] [Green Version]
- Ishida, M.; Marubashi, S.; Fukuda, M. M-INK, a novel tool for visualizing melanosomes and melanocores. J. Biochem. 2017, 161, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Andreassi, L.; Flori, L. Practical applications of cutaneous colorimetry. Clin. Dermatol. 1995, 13, 369–373. [Google Scholar] [CrossRef]
- Del Bino, S.; Bernerd, F. Variations in skin colour and the biological consequences of ultraviolet radiation exposure. Br. J. Dermatol. 2013, 169, 33–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarafder, A.K.; Bolasco, G.; Correia, M.S.; Pereira, F.J.C.; Iannone, L.; Hume, A.N.; Kirkpatrick, N.; Picardo, M.; Torrisi, M.R.; Rodrigues, I.P.; et al. Rab11b mediates melanin transfer between donor melanocytes and acceptor keratinocytes via coupled exo/endocytosis. J. Investig. Dermatol. 2014, 134, 1056–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raposo, G.; Tenza, D.; Murphy, D.M.; Berson, J.F.; Marks, M.S. Distinct protein sorting and localization to premelanosomes, melanosomes, and lysosomes in pigmented melanocytic cells. J. Cell Biol. 2001, 152, 809–824. [Google Scholar] [CrossRef] [Green Version]
- Staricco, R.J.; Pinkus, H. Quantitative and qualitative data on the pigment cells of adult human epidermis. J. Investig. Dermatol. 1957, 28, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Fitzpatrick, T.B.; Breathnach, A.S. The epidermal melanin unit system. Dermatol. Wochenschr. 1963, 147, 481–489. [Google Scholar]
- Yoshida-Amano, Y.; Hachiya, A.; Ohuchi, A.; Kobinger, G.P.; Kitahara, T.; Takema, Y.; Fukuda, M. Essential role of RAB27A in determining constitutive human skin color. PLoS ONE 2012, 7, e41160. [Google Scholar] [CrossRef] [Green Version]
- Correia, M.S.; Moreiras, H.; Pereira, F.J.C.; Neto, M.V.; Festas, T.C.; Tarafder, A.K.; Ramalho, J.S.; Seabra, M.C.; Barral, D.C. Melanin transferred to keratinocytes resides in nondegradative endocytic compartments. J. Investig. Dermatol. 2018, 138, 637–646. [Google Scholar] [CrossRef] [Green Version]
- Ebanks, J.P.; Koshoffer, A.; Wickett, R.R.; Schwemberger, S.; Babcock, G.; Hakozaki, T.; Boissy, R.E. Epidermal keratinocytes from light vs. dark skin exhibit differential degradation of melanosomes. J. Investig. Dermatol. 2011, 131, 1226–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murase, D.; Hachiya, A.; Takano, K.; Hicks, R.; Visscher, M.O.; Kitahara, T.; Hase, T.; Takema, Y.; Yoshimori, T. Autophagy has a significant role in determining skin color by regulating melanosome degradation in keratinocytes. J. Investig. Dermatol. 2013, 133, 2416–2424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuda, M. Synaptotagmin-like protein (Slp) homology domain 1 of Slac2-a/melanophilin is a critical determinant of GTP-dependent specific binding to Rab27A. J. Biol. Chem. 2002, 277, 40118–40124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzpatrick, T.B. Ultraviolet-induced pigmentary changes: Benefits and hazards. Curr. Probl. Dermatol. 1986, 15, 25–38. [Google Scholar]
- Seitz, J.C.; Whitmore, C.G. Measurement of erythema and tanning responses in human skin using a tri-stimulus colorimeter. Dermatologica 1988, 177, 70–75. [Google Scholar] [CrossRef]
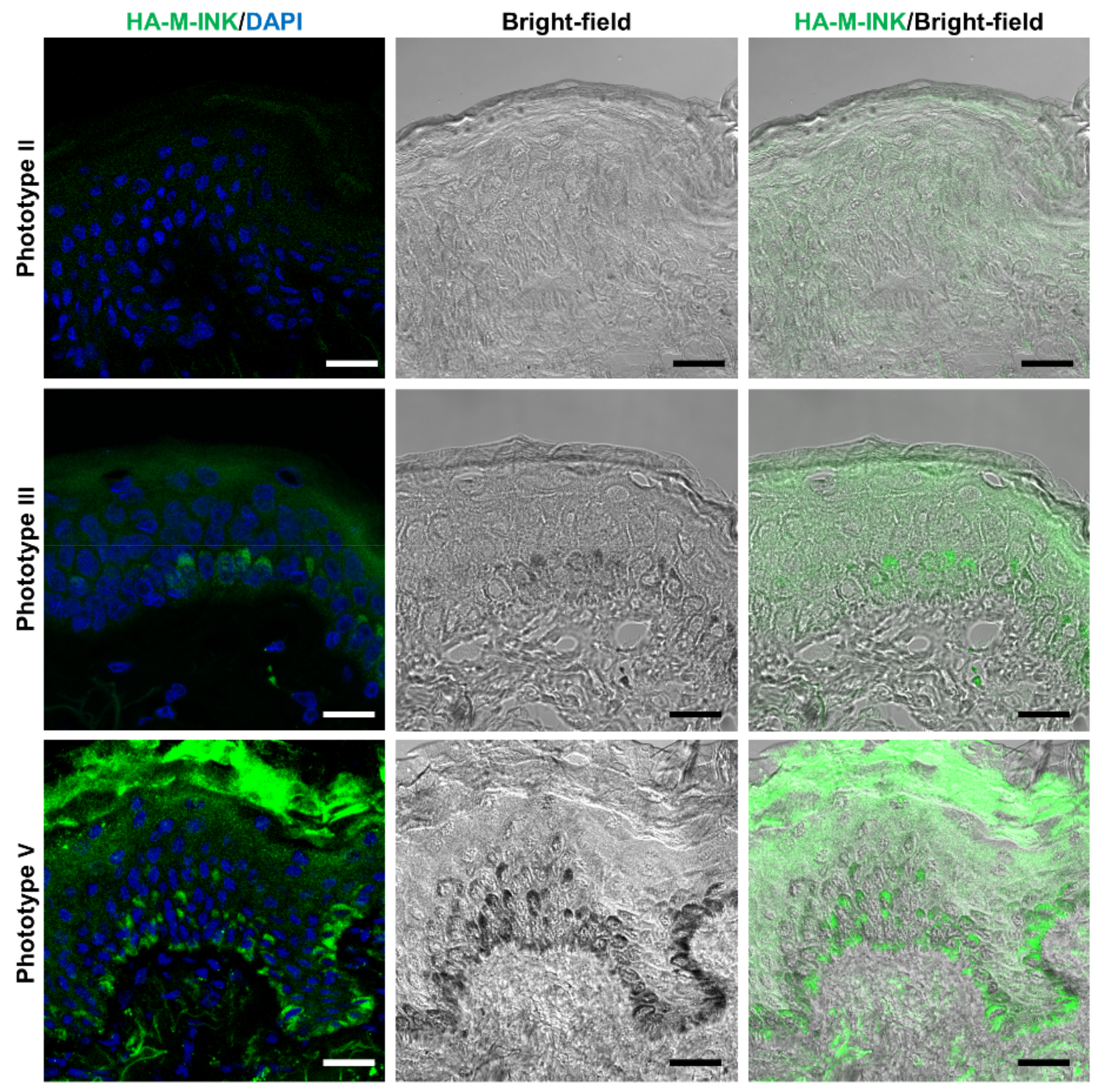
| No. | Phototype | Age | Sex | Source | L* Value | Fluorescence Intensity of HA-M-INK |
|---|---|---|---|---|---|---|
| 1 | II | 54 | Female | Abdomen | 77.86 | 3.69 |
| 2 | III | 50 | Female | Breast | 73.78 | 9.41 |
| 3 | V | 27 | Female | Abdomen | 43.37 | 12.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshikawa-Murakami, C.; Mizutani, Y.; Ryu, A.; Naru, E.; Teramura, T.; Homma, Y.; Fukuda, M. A Novel Method for Visualizing Melanosome and Melanin Distribution in Human Skin Tissues. Int. J. Mol. Sci. 2020, 21, 8514. https://doi.org/10.3390/ijms21228514
Yoshikawa-Murakami C, Mizutani Y, Ryu A, Naru E, Teramura T, Homma Y, Fukuda M. A Novel Method for Visualizing Melanosome and Melanin Distribution in Human Skin Tissues. International Journal of Molecular Sciences. 2020; 21(22):8514. https://doi.org/10.3390/ijms21228514
Chicago/Turabian StyleYoshikawa-Murakami, Chikako, Yuki Mizutani, Akemi Ryu, Eiji Naru, Takashi Teramura, Yuta Homma, and Mitsunori Fukuda. 2020. "A Novel Method for Visualizing Melanosome and Melanin Distribution in Human Skin Tissues" International Journal of Molecular Sciences 21, no. 22: 8514. https://doi.org/10.3390/ijms21228514
APA StyleYoshikawa-Murakami, C., Mizutani, Y., Ryu, A., Naru, E., Teramura, T., Homma, Y., & Fukuda, M. (2020). A Novel Method for Visualizing Melanosome and Melanin Distribution in Human Skin Tissues. International Journal of Molecular Sciences, 21(22), 8514. https://doi.org/10.3390/ijms21228514






