Al-Tolerant Barley Mutant hvatr.g Shows the ATR-Regulated DNA Damage Response to Maleic Acid Hydrazide
Abstract
1. Introduction
2. Results
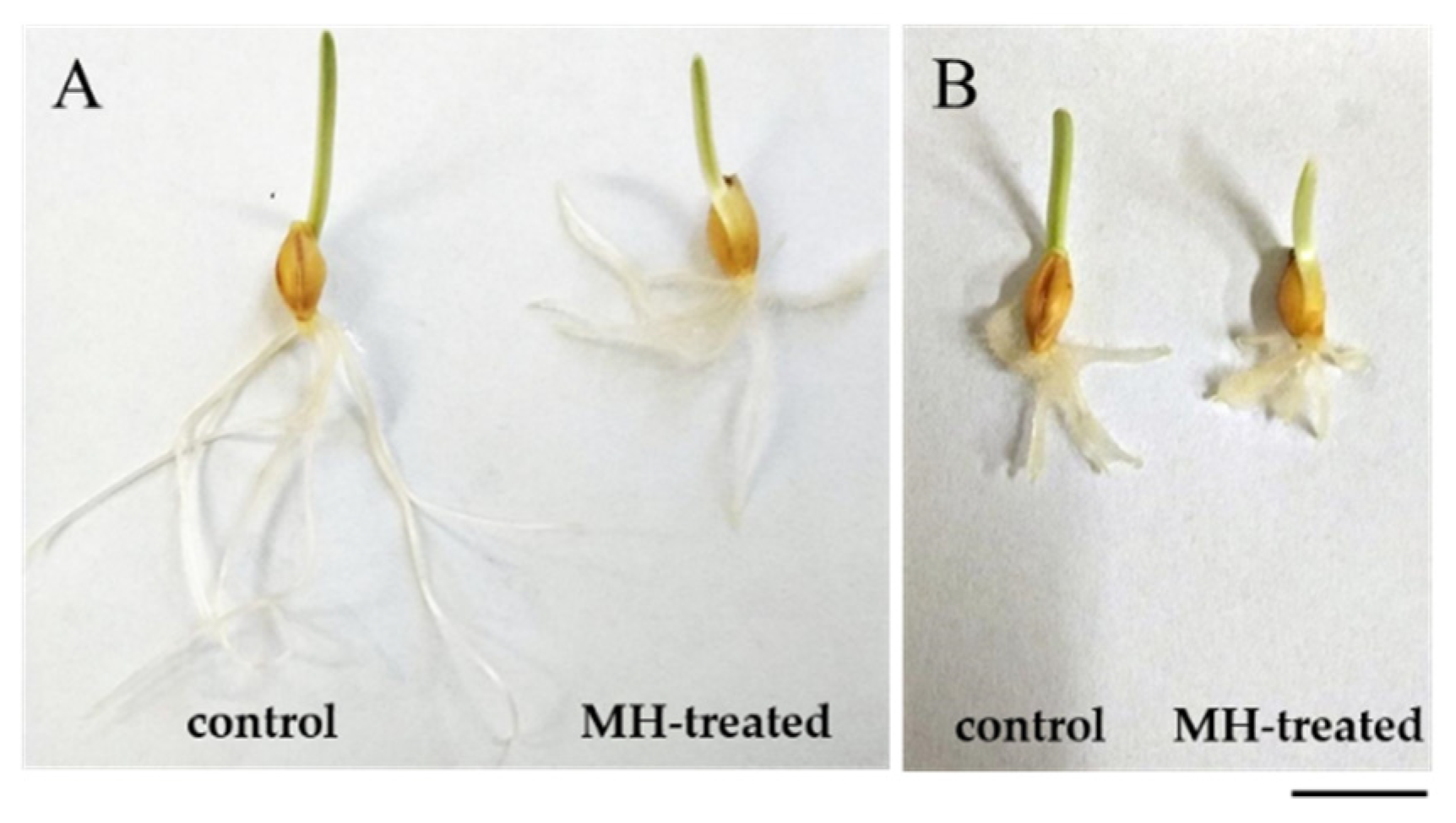
2.1. Response of hvatr.g Mutant and Its Parental Line to MH Treatment
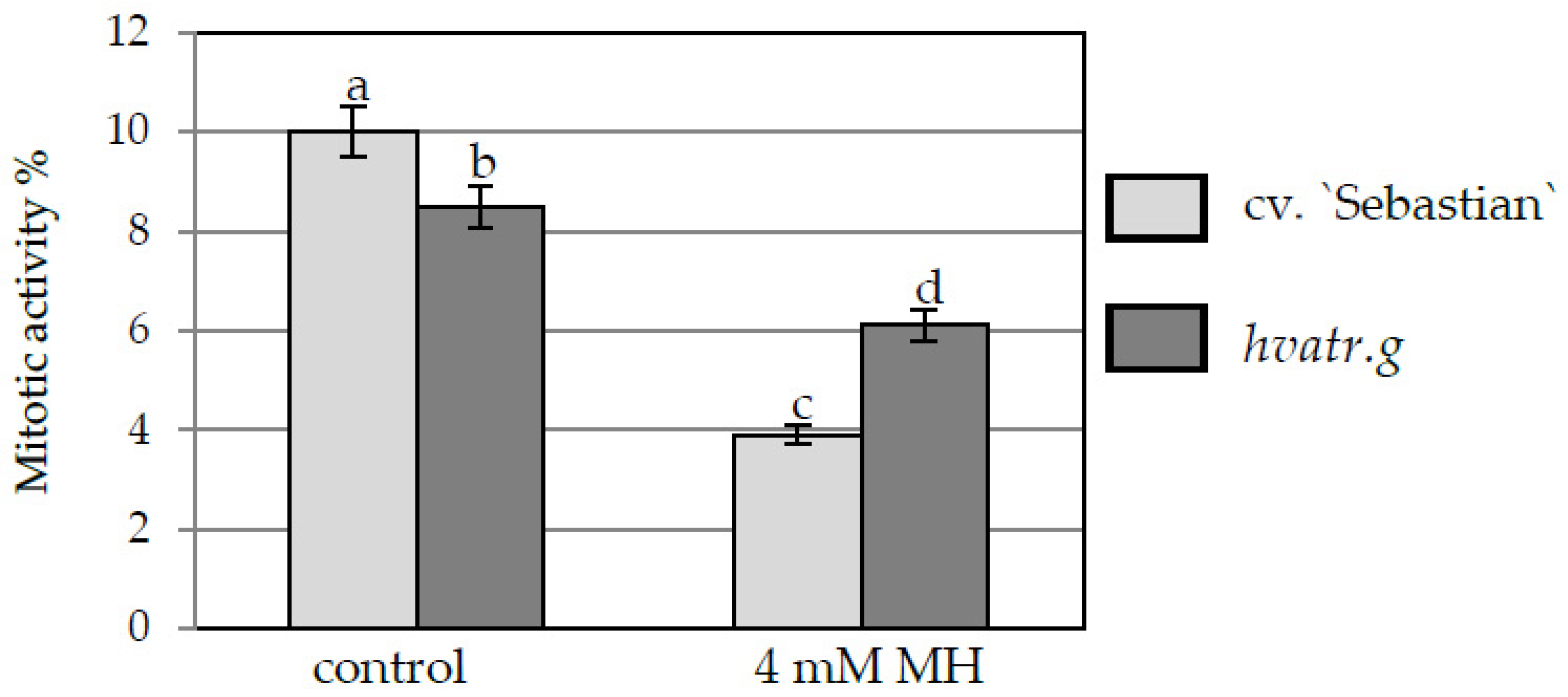
2.1.1. Mitotic Activity
2.1.2. DNA Damage
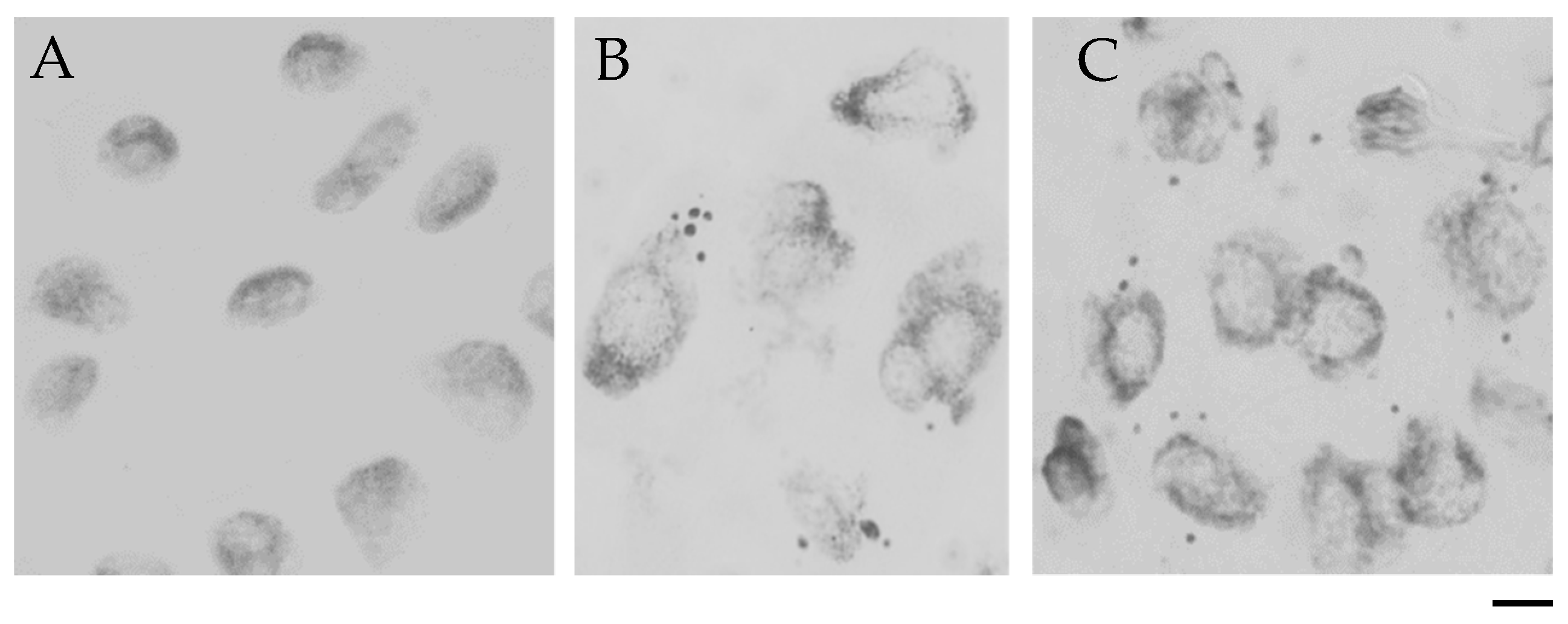
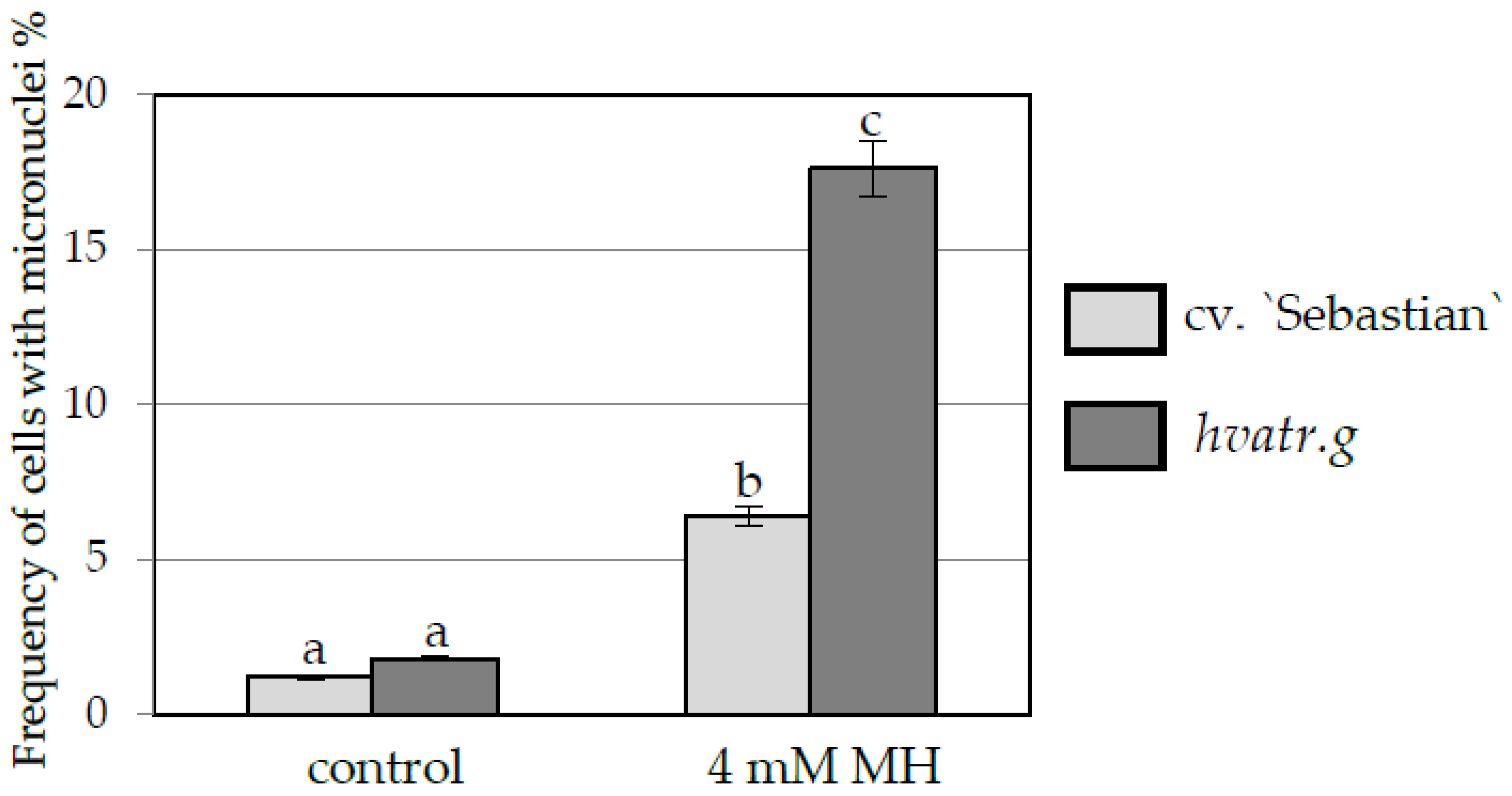
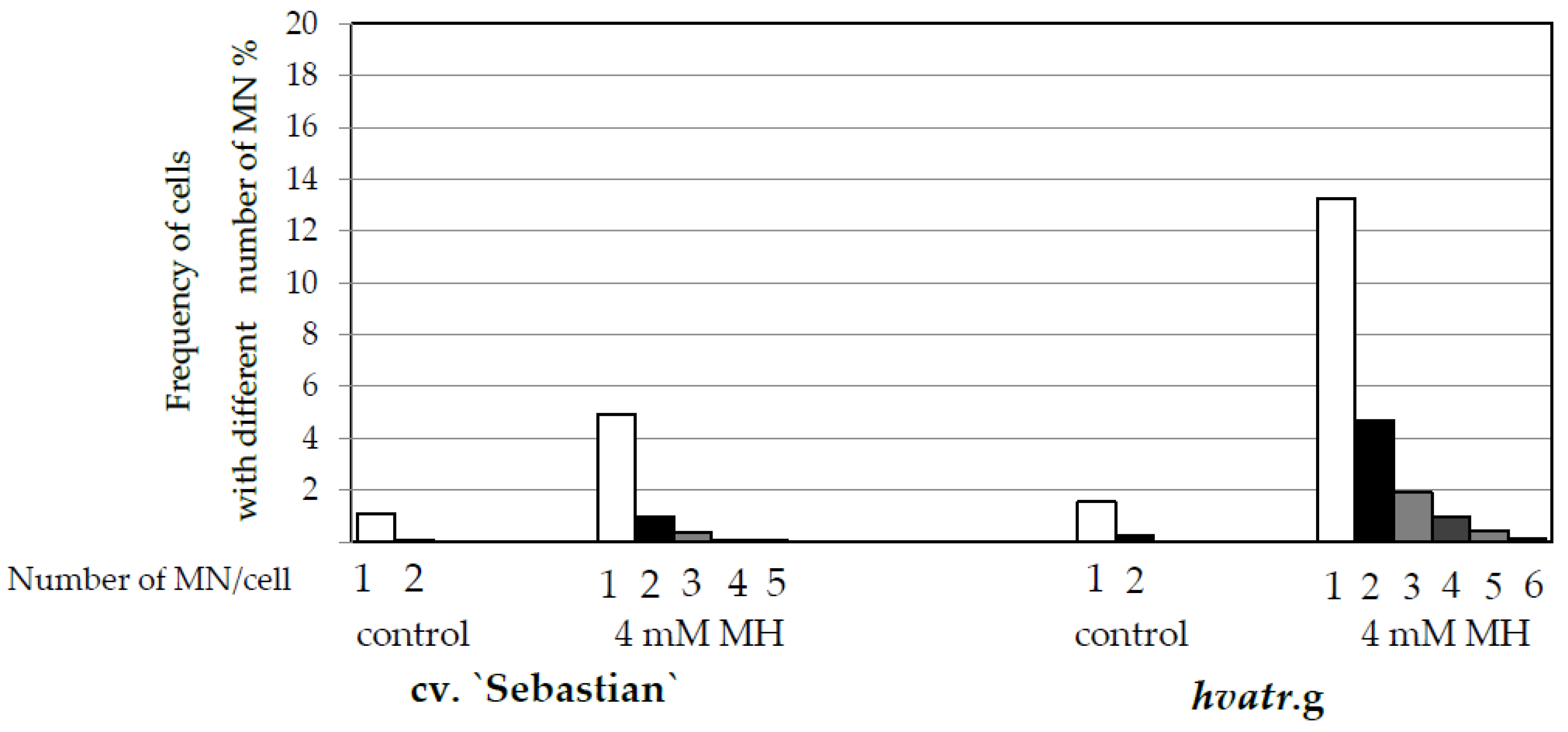
Frequency of Cells with Micronuclei
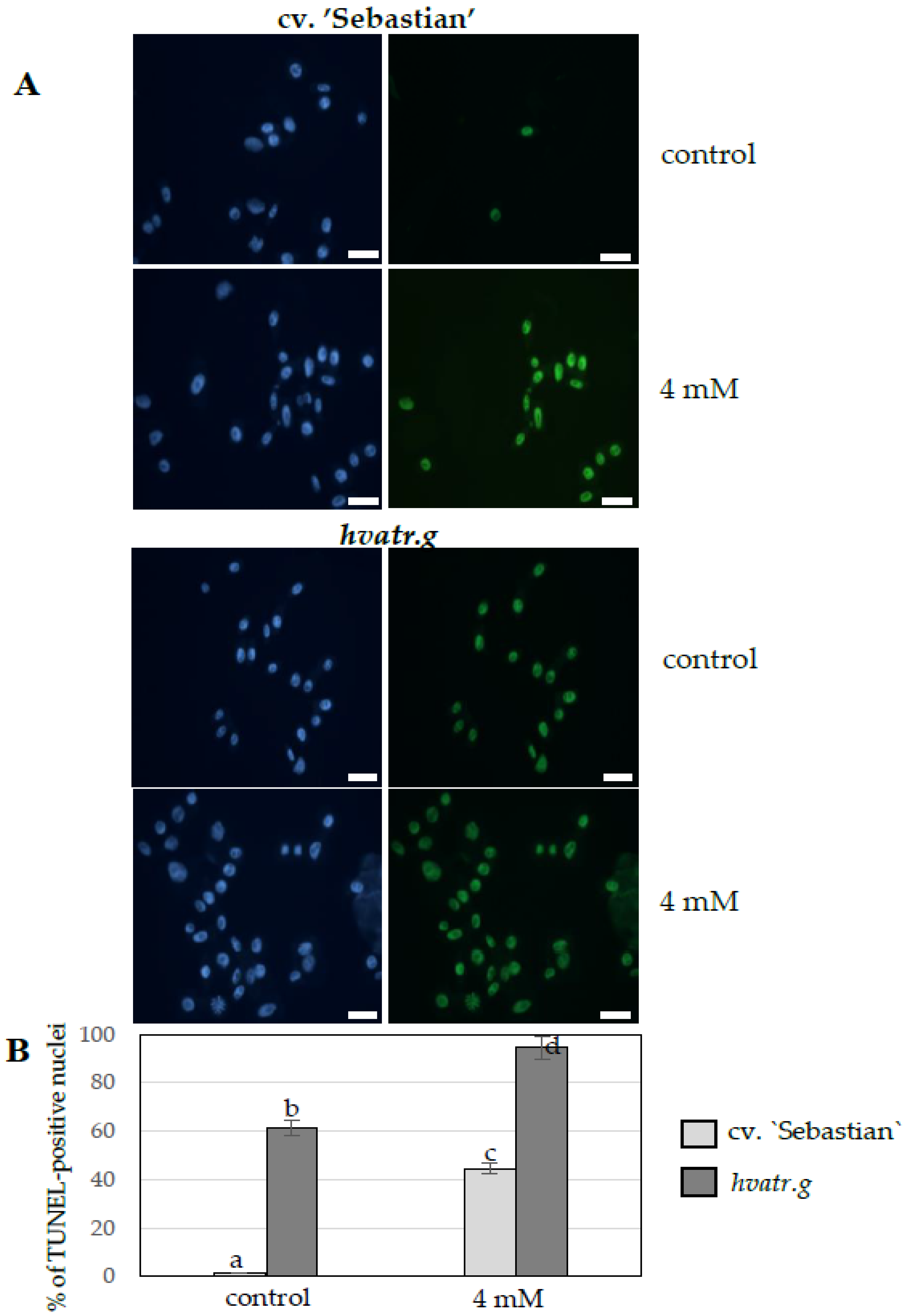
TUNEL Test
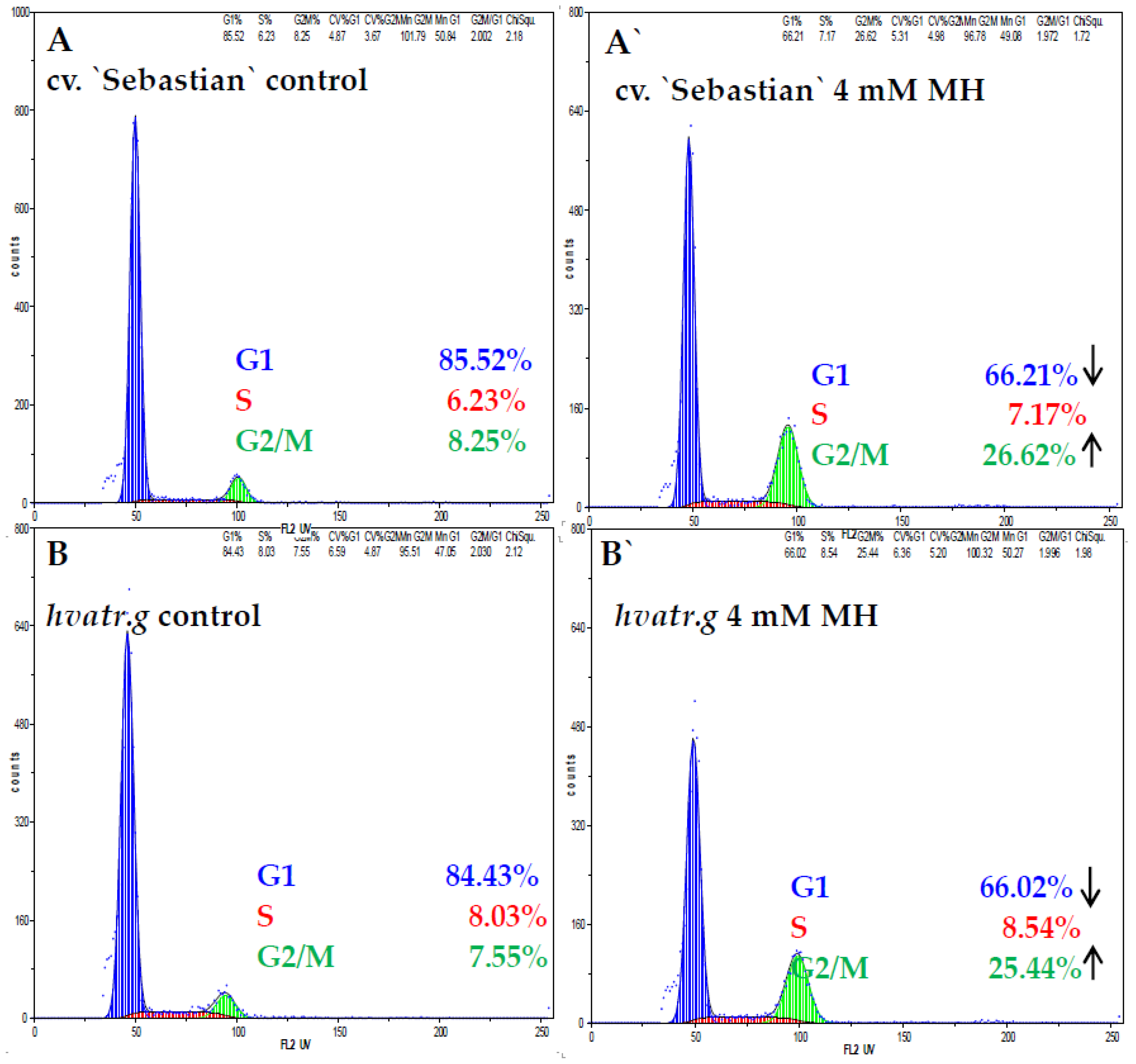
2.1.3. Cell Cycle Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. MH Treatment
4.3. Analysis of Mitotic Activity, the Frequency of Micronuclei and Damaged Nuclei
4.4. TUNEL Test
4.5. Cell Cycle Analysis using Flow Cytometry
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hu, Z.; Cools, T.; De Veylder, L. Mechanisms Used by Plants to Cope with DNA Damage. Annu. Rev. Plant Biol. 2016, 67, 439–462. [Google Scholar] [CrossRef] [PubMed]
- Lanier, C.; Bernard, F.; Dumez, S.; Leclercq-Dransart, J.; Lemière, S.; Vandenbulcke, F.; Nesslany, F.; Platel, A.; Devred, I.; Hayet, A.; et al. Combined toxic effects and DNA damage to two plant species exposed to binary metal mixtures (Cd/Pb). Ecotoxicol. Environ. Saf. 2019, 167, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, K.; Roy, S. An insight into the mechanism of DNA damage response in plants—Role of SUPPRESSOR OF GAMMA RESPONSE 1: An overview. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2020, 819, 111689. [Google Scholar] [CrossRef] [PubMed]
- Delhaize, E.; Ryan, P.R. Aluminum Toxicity and Tolerance in Plants. Plant Physiol. 1995, 107, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Kochian, L.; Piñeros, M.; Hoekenga, O. The Physiology, Genetics and Molecular Biology of Plant Aluminum Resistance and Toxicity. Plant Soil 2005, 274, 175–195. [Google Scholar] [CrossRef]
- Kochian, L.; Piñeros, M.; Liu, J.; Magalhaes, J. Plant Adaptation to Acid Soils: The Molecular Basis for Crop Aluminum Resistance. Annu. Rev. Plant Biol. 2015, 66, 571–598. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, J.; Chen, M.; Li, L.; Wang, L.; Huang, C.-F. The Cell Cycle Checkpoint Regulator ATR Is Required for Internal Aluminum Toxicity Mediated Root Growth Inhibition in Arabidopsis. Front. Plant Sci. 2018, 9, 118. [Google Scholar] [CrossRef]
- Chen, P.; Sjorgen, C.A.; Larsen, P.B.; Schnittger, A. A multi-level response to DNA damage induced by aluminium. Plant J. 2019, 98, 479–491. [Google Scholar] [CrossRef]
- Jaskowiak, J.; Kwasniewska, J.; Milewska-Hendel, A.; Kurczynska, E.U.; Szurman-Zubrzycka, M.; Szarejko, I. Aluminum Alters the Histology and Pectin Cell Wall Composition of Barley Roots. Int. J. Mol. Sci. 2019, 20, 3039. [Google Scholar] [CrossRef]
- Riaz, M.; Yan, L.; Wu, X.; Hussain, S.; Aziz, O.; Jiang, C. Mechanisms of organic acids and boron induced tolerance of aluminum toxicity: A review. Ecotoxicol. Environ. Saf. 2018, 165, 25–35. [Google Scholar] [CrossRef]
- Eekhout, T.; Larsen, P.; De Veylder, L. Modification of DNA Checkpoints to Confer Aluminum Tolerance. Trends Plant Sci. 2016, 22, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Rounds, M.; Larsen, P. Aluminum-Dependent Root-Growth Inhibition in Arabidopsis Results from AtATR-Regulated Cell-Cycle Arrest. Curr. Biol. 2008, 18, 1495–1500. [Google Scholar] [CrossRef] [PubMed]
- Culligan, K.M.; Robertson, C.E.; Foreman, J.; Doerner, P.; Britt, A.B. ATR and ATM play both distinct and additive roles in response to ionizing radiation. Plant J. 2006, 48, 947–961. [Google Scholar] [CrossRef] [PubMed]
- Nisa, M.U.; Huang, Y.; Benhamed, M.; Raynaud, C. The Plant DNA Damage Response: Signaling Pathways Leading to Growth Inhibition and Putative Role in Response to Stress Conditions. Front. Plant Sci. 2019, 10, 653. [Google Scholar] [CrossRef] [PubMed]
- Nezames, C.; Sjogren, C.; Barajas, J.; Larsen, P. The Arabidopsis Cell Cycle Checkpoint Regulators TANMEI/ALT2 and ATR Mediate the Active Process of Aluminum-Dependent Root Growth Inhibition. Plant Cell 2012, 24, 608–621. [Google Scholar] [CrossRef]
- Jaskowiak, J.; Tkaczyk, O.; Slota, M.; Kwasniewska, J.; Szarejko, I. Analysis of aluminium toxicity in Hordeum vulgare roots with an emphasis on DNA integrity and cell cycle. PLoS ONE 2018, 13, e0193156. [Google Scholar] [CrossRef]
- Sjogren, C.; Bolaris, S.; Larsen, P. Aluminum-Dependent Terminal Differentiation of the Arabidopsis Root Tip Is Mediated through an ATR-, ALT2-, and SOG1-Regulated Transcriptional Response. Plant Cell 2015, 27, 2501–2515. [Google Scholar] [CrossRef]
- Sjogren, C.; Larsen, P. SUV2, which encodes an ATR-related cell cycle checkpoint and putative plant ATRIP, is required for aluminium-dependent root growth inhibition in Arabidopsis. Plant Cell Environ. 2017, 40, 1849–1860. [Google Scholar] [CrossRef]
- Culligan, K.; Tissier, A.; Britt, A. ATR Regulates a G2-Phase Cell-Cycle Checkpoint in Arabidopsis thaliana. Plant Cell 2004, 16, 1091–1104. [Google Scholar] [CrossRef]
- Szurman-Zubrzycka, M.; Nawrot, M.; Jelonek, J.; Dziekanowski, M.; Kwasniewska, J.; Szarejko, I. ATR, a DNA Damage Signaling Kinase, Is Involved in Aluminum Response in Barley. Front. Plant Sci. 2019, 10, 1299. [Google Scholar] [CrossRef]
- Staub, E.; Fiziev, P.; Rosenthal, A.; Hinzmann, B. Insights into the evolution of the nucleolus by an analysis of its protein domain repertoire. BioEssays 2004, 26, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Szurman-Zubrzycka, M.; Zbieszczyk, J.; Marzec, M.; Jelonek, J.; Chmielewska, B.; Kurowska, M.; Krok, M.; Daszkowska-Golec, A.; Guzy-Wróbelska, J.; Gruszka, D.; et al. HorTILLUS—A Rich and Renewable Source of Induced Mutations for Forward/Reverse Genetics and Pre-breeding Programs in Barley (Hordeum vulgare L.). Front. Plant Sci. 2018, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Rank, J.; Lopez, L.C.; Nielsen, M.; Moretton, J. Genotoxicity of maleic hydrazide, acridine and DEHP in Allium cepa root cells performed by two different laboratories. Hereditas 2002, 136, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Juchimiuk, J.; Hering, B.; Maluszynska, J. Multicolour FISH in an analysis of chromosome aberrations induced by N-nitroso-N-methylurea and maleic hydrazide in barley cells. J. Appl. Genet. 2007, 48, 99–106. [Google Scholar] [CrossRef]
- Kwasniewska, J.; Grabowska, M.; Kwasniewski, M.; Kolano, B. Comet-FISH using rDNA probes in analysis of mutagen-induced DNA damage in plant cells. Environ. Mol. Mutagen. 2012, 53, 369–375. [Google Scholar] [CrossRef]
- Kwasniewska, J.; Kwasniewski, M. Comet-FISH for the evaluation of plant DNA damage after mutagenic treatments. J. Appl. Genet. 2013, 54, 407–415. [Google Scholar] [CrossRef]
- Michaelis, A.; Rieger, R. On the distribution between chromosomes of chemically induced chromatid aberrations: Studies with a new karyotype of Vicia faba. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1968, 6, 81–92. [Google Scholar] [CrossRef]
- Nicoloff, H.; Rieger, R.; Michaelis, A. Deletion clustering in specific chromosome segments of Hordeum vulgare and Vicia faba. Biol. Zentralbl. 1979, 98, 527–535. [Google Scholar]
- Maluszynska, J.; Maluszynski, M. The influence of MNUA and MH on the cell cycle and DNA contents in meristematic cells of barley. Acta Biol. 1983, 11, 227–237. [Google Scholar]
- Marcano, L.; Carruyo, I.; Del Campo, A.; Montiel, X. Cytotoxicity and mode of action of maleic hydrazide in root tips of Allium cepa L. Environ. Res. 2004, 94, 221–226. [Google Scholar] [CrossRef]
- Jabee, F.; Ansari, M.Y.; Shahab, D. Studies on the Effect of Maleic Hydrazide on Root Tip Cells and Pollen Fertility in Trigonella foenum-graceum L. Turk. J. Bot. 2008, 32, 332–344. [Google Scholar]
- Kwasniewska, J.; Kus, A.; Swoboda, M.; Braszewska-Zalewska, A. DNA replication after mutagenic treatment in Hordeum vulgare. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2016, 812, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ryu, T.; Lee, S.; Chung, B. Ionizing radiation manifesting DNA damage response in plants: An overview of DNA damage signaling and repair mechanisms in plants. Plant Sci. 2019, 278, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Kwasniewska, J.; Zubrzycka, K.; Kus, A. Impact of Mutagens on DNA Replication in Barley Chromosomes. Int. J. Mol. Sci. 2018, 19, 1070. [Google Scholar] [CrossRef] [PubMed]
- Seaton, D.D.; Krishnan, J. Model-Based Analysis of Cell Cycle Responses to Dynamically Changing Environments. PLoS Comput. Biol. 2016, 12, e1004604. [Google Scholar] [CrossRef]
- Szarejko, I.; Szurman-Zubrzycka, M.; Nawrot, M.; Marzec, M.; Gruszka, D.; Kurowska, M.; Chmielewska, B.; Zbieszczyk, J.; Jelonek, J.; Maluszynski, M. Creation of a TILLING in Barley After Chemical Mutagenesis with Sodium Azide and MNU. In Biotechnologies for Plant Mutation Breeding; Springer: Cham, Switzerland, 2017; pp. 91–111. [Google Scholar]
- Szurman-Zubrzycka, M.; Chmielewska, B.; Gajewska, P.; Szarejko, I. Mutation Detection by Analysis of DNA Heteroduplexes in TILLING Populations of Diploid Species. In Biotechnologies for Plant Mutation Breeding; Springer: Cham, Switzerland, 2017; pp. 281–303. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaskowiak, J.; Kwasniewska, J.; Szurman-Zubrzycka, M.; Rojek-Jelonek, M.; Larsen, P.B.; Szarejko, I. Al-Tolerant Barley Mutant hvatr.g Shows the ATR-Regulated DNA Damage Response to Maleic Acid Hydrazide. Int. J. Mol. Sci. 2020, 21, 8500. https://doi.org/10.3390/ijms21228500
Jaskowiak J, Kwasniewska J, Szurman-Zubrzycka M, Rojek-Jelonek M, Larsen PB, Szarejko I. Al-Tolerant Barley Mutant hvatr.g Shows the ATR-Regulated DNA Damage Response to Maleic Acid Hydrazide. International Journal of Molecular Sciences. 2020; 21(22):8500. https://doi.org/10.3390/ijms21228500
Chicago/Turabian StyleJaskowiak, Joanna, Jolanta Kwasniewska, Miriam Szurman-Zubrzycka, Magdalena Rojek-Jelonek, Paul B. Larsen, and Iwona Szarejko. 2020. "Al-Tolerant Barley Mutant hvatr.g Shows the ATR-Regulated DNA Damage Response to Maleic Acid Hydrazide" International Journal of Molecular Sciences 21, no. 22: 8500. https://doi.org/10.3390/ijms21228500
APA StyleJaskowiak, J., Kwasniewska, J., Szurman-Zubrzycka, M., Rojek-Jelonek, M., Larsen, P. B., & Szarejko, I. (2020). Al-Tolerant Barley Mutant hvatr.g Shows the ATR-Regulated DNA Damage Response to Maleic Acid Hydrazide. International Journal of Molecular Sciences, 21(22), 8500. https://doi.org/10.3390/ijms21228500




