Bone Fracture Enhanced Blood-Brain Barrier Breakdown in the Hippocampus and White Matter Damage of Stroke Mice
Abstract
1. Introduction
2. Results
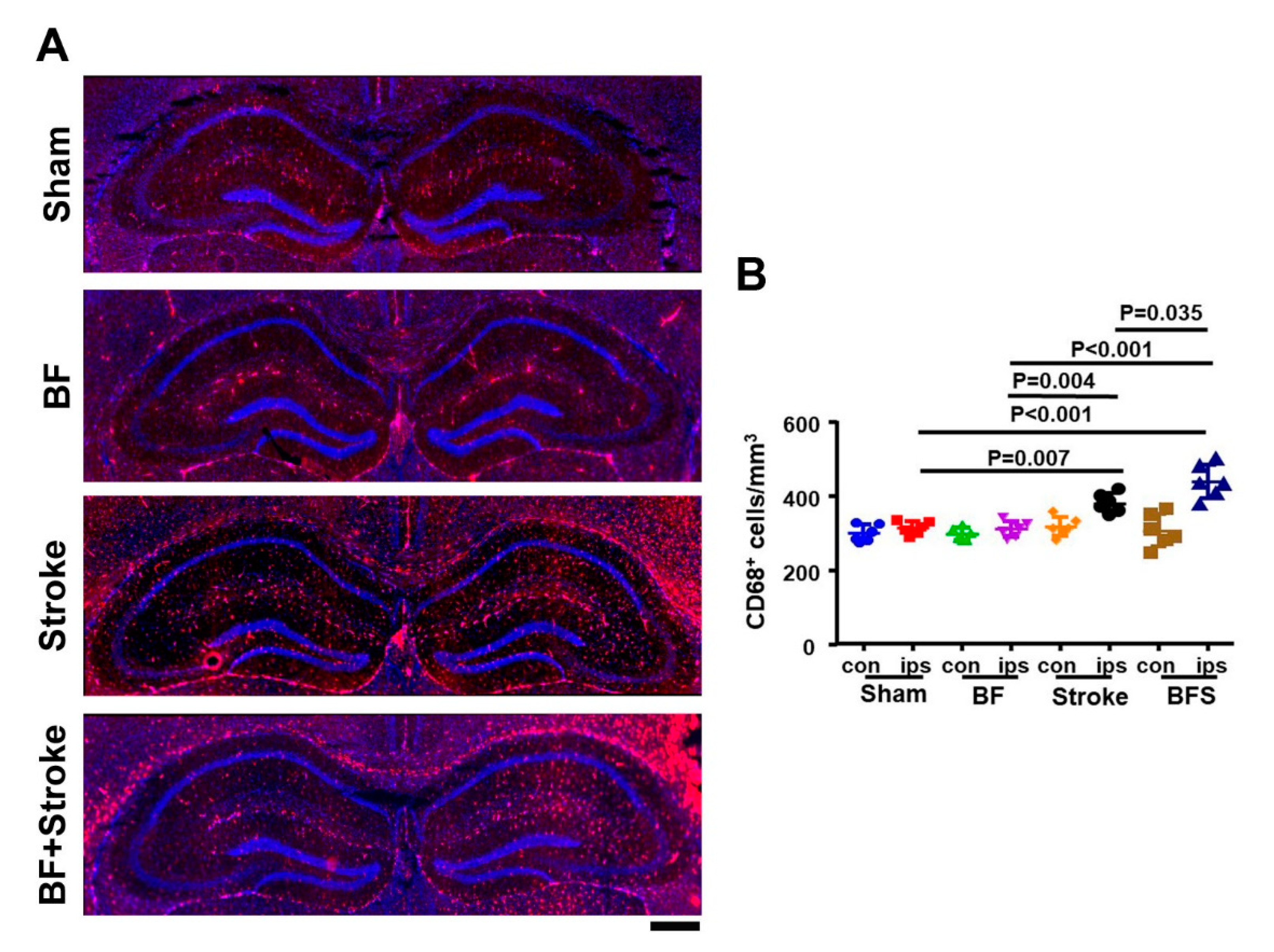
2.1. Stroke and BF+Stroke Mice Have Increased Number of CD68+ Cells in the Hippocampal CA1 Region Ipsilateral to the Stroke Injury
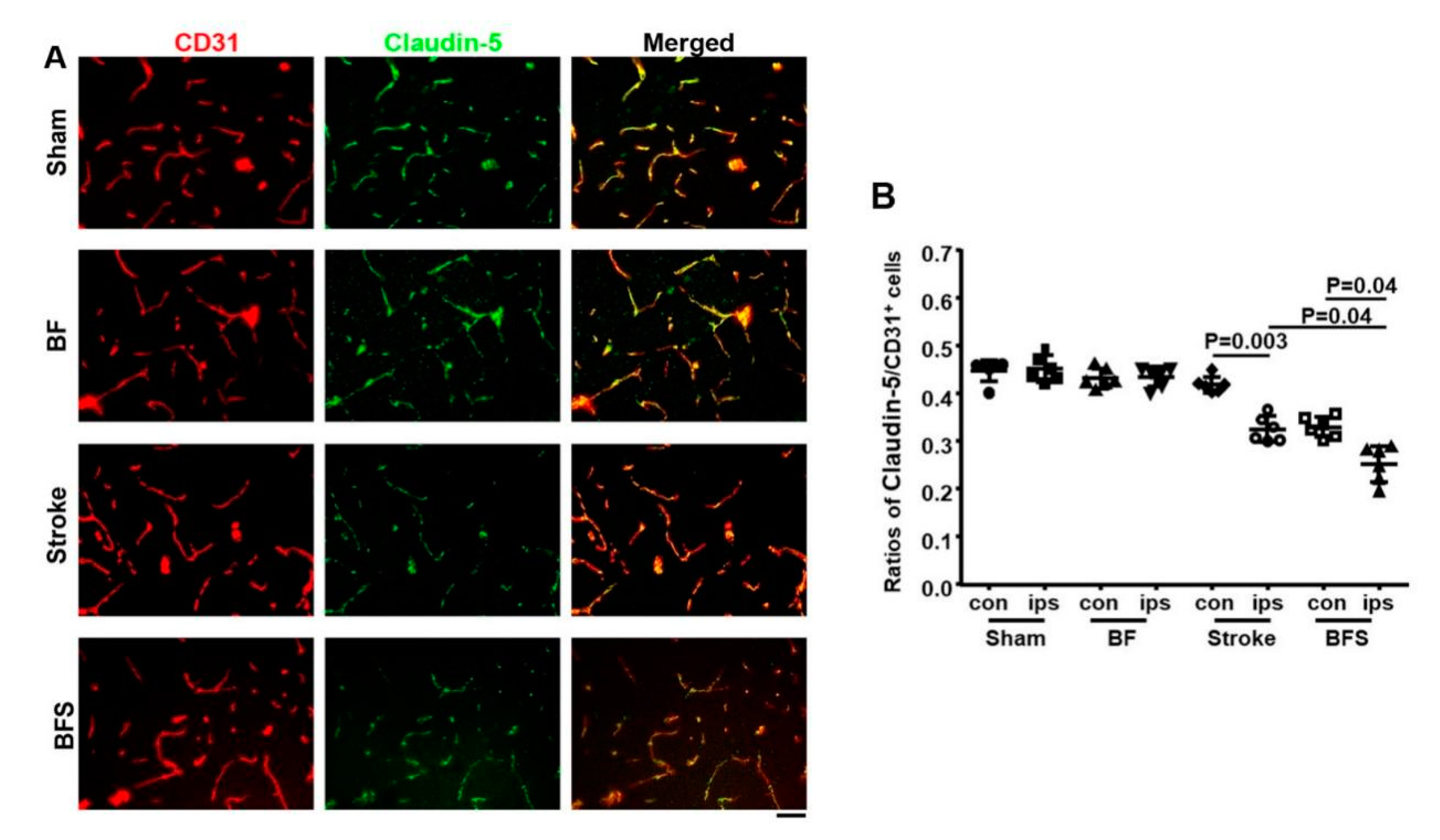
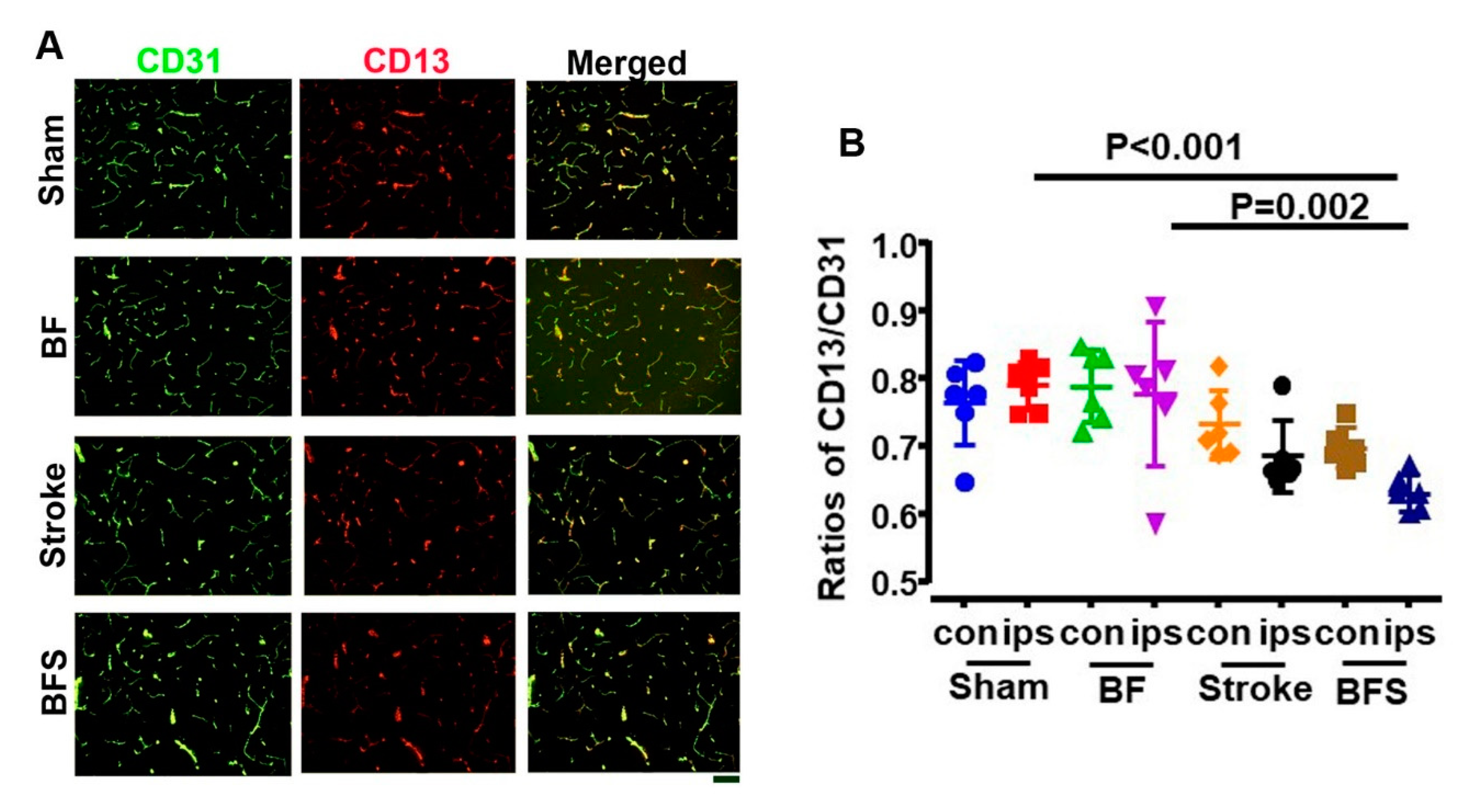
2.2. BF Exacerbates BBB Breakdown in the Hippocampus
2.3. BF Exacerbated WM Damage in the Basal Ganglia of Stroke Brain
3. Discussion
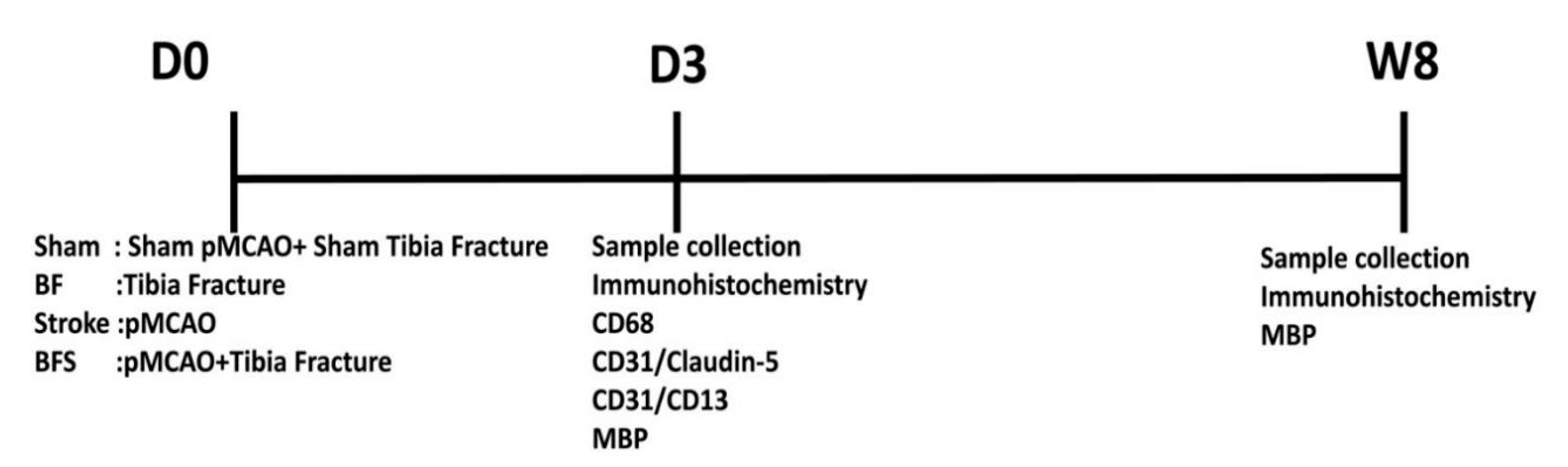
4. Materials and Methods
4.1. Animals
4.2. BF Surgery
4.3. pMCAO Procedure
4.4. Immunohistochemistry
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BF | tibia fracture |
| MCA | middle cerebral artery |
| pMCAO | permanent occlusion of distal middle cerebral artery |
| BBB | blood brain barrier |
| WM | white matter |
| MBP | myelin basic protein |
References
- Jauch, E.C.; Saver, J.L.; Adams, H.P., Jr.; Bruno, A.; Connors, J.J.; Demaerschalk, B.M.; Khatri, P.; McMullan, P.W., Jr.; Qureshi, A.I.; Rosenfield, K.; et al. Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 870–947. [Google Scholar] [CrossRef]
- Vidal, S.M.; Chaudhry, F.S.; Schneck, M. Management of acute ischemic stroke. Hosp Pract 2013, 41, 108–122. [Google Scholar] [CrossRef]
- Kapral, M.K.; Fang, J.; Alibhai, S.M.; Cram, P.; Cheung, A.M.; Casaubon, L.K.; Prager, M.; Stamplecoski, M.; Rashkovan, B.; Austin, P.C. Risk of fractures after stroke: Results from the Ontario Stroke Registry. Neurology 2017, 88, 57–64. [Google Scholar] [CrossRef]
- Fisher, A.; Srikusalanukul, W.; Davis, M.; Smith, P. Poststroke hip fracture: Prevalence, clinical characteristics, mineral-bone metabolism, outcomes, and gaps in prevention. Stroke Res. Treat. 2013, 2013, 641943. [Google Scholar] [CrossRef]
- Huo, K.; Hashim, S.I.; Yong, K.L.; Su, H.; Qu, Q.M. Impact and risk factors of post-stroke bone fracture. World J. Exp. Med. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Coutinho, E.S.; Fletcher, A.; Bloch, K.V.; Rodrigues, L.C. Risk factors for falls with severe fracture in elderly people living in a middle-income country: A case control study. BMC Geriatr. 2008, 8, 21. [Google Scholar] [CrossRef]
- Myint, P.K.; Poole, K.E.; Warburton, E.A. Hip fractures after stroke and their prevention. QJM 2007, 100, 539–545. [Google Scholar] [CrossRef]
- Sennerby, U.; Melhus, H.; Gedeborg, R.; Byberg, L.; Garmo, H.; Ahlbom, A.; Pedersen, N.L.; Michaelsson, K. Cardiovascular diseases and risk of hip fracture. JAMA 2009, 302, 1666–1673. [Google Scholar] [CrossRef]
- Kang, J.H.; Chung, S.D.; Xirasagar, S.; Jaw, F.S.; Lin, H.C. Increased risk of stroke in the year after a hip fracture: A population-based follow-up study. Stroke 2011, 42, 336–341. [Google Scholar] [CrossRef]
- Lakshminarayan, K.; Schissel, C.; Anderson, D.C.; Vazquez, G.; Jacobs, D.R., Jr.; Ezzeddine, M.; Luepker, R.V.; Virnig, B.A. Five-year rehospitalization outcomes in a cohort of patients with acute ischemic stroke: Medicare linkage study. Stroke 2011, 42, 1556–1562. [Google Scholar] [CrossRef]
- Hacke, W.; Bousser, M.G.; Ford, G.A.; Bath, P.M.; Brainin, M.; Caso, V.; Cervera, V.; Chamorro, A.; Cordnnier, C. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc. Dis. 2008, 25, 457–507. [Google Scholar] [CrossRef]
- Frost, S.A.; Nguyen, N.D.; Black, D.A.; Eisman, J.A.; Nguyen, T.V. Risk factors for in-hospital post-hip fracture mortality. Bone 2011, 49, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Degos, V.; Maze, M.; Vacas, S.; Hirsch, J.; Guo, Y.; Shen, F.; Jun, K.; van Rooijen, N.; Gressens, P.; Young, W.L.; et al. Bone fracture exacerbates murine ischemic cerebral injury. Anesthesiology 2013, 118, 1362–1372. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Li, L.; Wang, L.; Degos, V.; Maze, M.; Su, H. Alpha-7 nicotinic acetylcholine receptor agonist treatment reduces neuroinflammation, oxidative stress, and brain injury in mice with ischemic stroke and bone fracture. J. Neurochem. 2014, 131, 498–508. [Google Scholar] [CrossRef]
- Wang, L.; Kang, S.; Zou, D.; Zhan, L.; Li, Z.; Zhu, W.; Su, H. Bone Fracture Pre-Ischemic Stroke Exacerbates Ischemic Cerebral Injury in Mice. PLoS ONE 2016, 11, e0153835. [Google Scholar] [CrossRef]
- Zou, D.; Luo, M.; Han, Z.; Zhan, L.; Zhu, W.; Kang, S.; Bao, C.; Li, Z.; Nelson, J.; Zhang, R.; et al. Activation of Alpha-7 Nicotinic Acetylcholine Receptor Reduces Brain Edema in Mice with Ischemic Stroke and Bone Fracture. Mol. Neurobiol. 2017, 54, 8278–8286. [Google Scholar] [CrossRef]
- Doyle, K.P.; Quach, L.N.; Sole, M.; Axtell, R.C.; Nguyen, T.V.; Soler-Llavina, G.J.; Jurado, S.; Han, J.; Steinman, L.; Longo, F.M.; et al. B-lymphocyte-mediated delayed cognitive impairment following stroke. J. Neurosci. 2015, 35, 2133–2145. [Google Scholar] [CrossRef]
- Seitz, D.P.; Adunuri, N.; Gill, S.S.; Rochon, P.A. Prevalence of dementia and cognitive impairment among older adults with hip fractures. J. Am. Med. Dir. Assoc. 2011, 12, 556–564. [Google Scholar] [CrossRef]
- Tatemichi, T.K.; Paik, M.; Bagiella, E.; Desmond, D.W.; Pirro, M.; Hanzawa, L.K. Dementia after stroke is a predictor of long-term survival. Stroke 1994, 25, 1915–1919. [Google Scholar] [CrossRef]
- Marengoni, A.; Corrao, S.; Nobili, A.; Tettamanti, M.; Pasina, L.; Salerno, F.; Iorio, A.; Marcucci, M.; Bonometti, F.; Mannucci, P.M. In-hospital death according to dementia diagnosis in acutely ill elderly patients: The REPOSI study. Int. J. Geriatr. Psychiatry 2011, 26, 930–936. [Google Scholar] [CrossRef]
- Marengoni, A.; Nobili, A.; Romano, V.; Tettamanti, M.; Pasina, L.; Djade, S.; Corrao, S.; Salerno, F.; Iorio, A.; Marcucci, M.; et al. Adverse clinical events and mortality during hospitalization and 3 months after discharge in cognitively impaired elderly patients. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Lo Coco, D.; Lopez, G.; Corrao, S. Cognitive impairment and stroke in elderly patients. Vasc. Health Risk Manag. 2016, 12, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Barba, R.; Martinez-Espinosa, S.; Rodriguez-Garcia, E.; Pondal, M.; Vivancos, J.; Del Ser, T. Poststroke dementia: Clinical features and risk factors. Stroke 2000, 31, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Leys, D.; Henon, H.; Mackowiak-Cordoliani, M.A.; Pasquier, F. Poststroke dementia. Lancet Neurol. 2005, 4, 752–759. [Google Scholar] [CrossRef]
- Bejot, Y.; Aboa-Eboule, C.; Durier, J.; Rouaud, O.; Jacquin, A.; Ponavoy, E.; Richard, D.; Moreau, T.; Giroud, M. Prevalence of early dementia after first-ever stroke: A 24-year population-based study. Stroke 2011, 42, 607–612. [Google Scholar] [CrossRef]
- Savva, G.M.; Stephan, B.C. Epidemiological studies of the effect of stroke on incident dementia: A systematic review. Stroke 2010, 41, e41–e46. [Google Scholar] [CrossRef] [PubMed]
- Cibelli, M.; Fidalgo, A.R.; Terrando, N.; Ma, D.; Monaco, C.; Feldmann, M.; Takata, M.; Lever, I.J.; Nanchahal, J.; Fanselow, M.S.; et al. Role of interleukin-1beta in postoperative cognitive dysfunction. Ann. Neurol. 2010, 68, 360–368. [Google Scholar] [CrossRef]
- Moller, J.T.; Cluitmans, P.; Rasmussen, L.S.; Houx, P.; Rasmussen, H.; Canet, J.; Rabbitt, P.; Jolles, J.; Larsen, K.; Hanning, C.D.; et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet 1998, 351, 857–861. [Google Scholar] [CrossRef]
- Salazar, F.; Donate, M.; Boget, T.; Bogdanovich, A.; Basora, M.; Torres, F.; Fabregas, N. Intraoperative warming and post-operative cognitive dysfunction after total knee replacement. Acta Anaesthesiol. Scand. 2011, 55, 216–222. [Google Scholar] [CrossRef]
- Gruber-Baldini, A.L.; Hosseini, M.; Orwig, D.; Grattan, L.; Chiles Shaffer, N.; Hochberg, M.; Magaziner, J. Cognitive Differences between Men and Women who Fracture their Hip and Impact on Six-Month Survival. J. Am. Geriatr. Soc. 2017, 65, e64–e69. [Google Scholar] [CrossRef]
- Terrando, N.; Monaco, C.; Ma, D.; Foxwell, B.M.; Feldmann, M.; Maze, M. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc. Natl. Acad. Sci. USA 2010, 107, 20518–20522. [Google Scholar] [CrossRef]
- Li, Z.; Wei, M.; Lyu, H.; Huo, K.; Wang, L.; Zhang, M.; Su, H. Fracture shortly before stroke in mice leads to hippocampus inflammation and long-lasting memory dysfunction. J. Cereb. Blood Flow Metab. 2020, 40, 446–455. [Google Scholar] [CrossRef]
- Blanchette, M.; Daneman, R. Formation and maintenance of the BBB. Mech. Dev. 2015, 138 Pt 1, 8–16. [Google Scholar] [CrossRef]
- Brzica, H.; Abdullahi, W.; Ibbotson, K.; Ronaldson, P.T. Role of Transporters in Central Nervous System Drug Delivery and Blood-Brain Barrier Protection: Relevance to Treatment of Stroke. J. Cent. Nerv. Syst. Dis. 2017, 9, 1179573517693802. [Google Scholar] [CrossRef]
- Abdullahi, W.; Tripathi, D.; Ronaldson, P.T. Blood-brain barrier dysfunction in ischemic stroke: Targeting tight junctions and transporters for vascular protection. Am. J. Physiol. Cell Physiol. 2018, 315, C343–C356. [Google Scholar] [CrossRef]
- Terrando, N.; Eriksson, L.I.; Ryu, J.K.; Yang, T.; Monaco, C.; Feldmann, M.; Jonsson Fagerlund, M.; Charo, I.F.; Akassoglou, K.; Maze, M. Resolving postoperative neuroinflammation and cognitive decline. Ann. Neurol. 2011, 70, 986–995. [Google Scholar] [CrossRef]
- Terrando, N.; Brzezinski, M.; Degos, V.; Eriksson, L.I.; Kramer, J.H.; Leung, J.M.; Miller, B.L.; Seeley, W.W.; Vacas, S.; Weiner, M.W.; et al. Perioperative cognitive decline in the aging population. Mayo Clin. Proc. 2011, 86, 885–893. [Google Scholar] [CrossRef]
- Hayakawa, K.; Qiu, J.; Lo, E.H. Biphasic actions of HMGB1 signaling in inflammation and recovery after stroke. Ann. N. Y. Acad. Sci. 2010, 1207, 50–57. [Google Scholar] [CrossRef]
- Chamorro, A.; Hallenbeck, J. The harms and benefits of inflammatory and immune responses in vascular disease. Stroke 2006, 37, 291–293. [Google Scholar] [CrossRef]
- Lo, E.H.; Rosenberg, G.A. The neurovascular unit in health and disease: Introduction. Stroke 2009, 40, S2–S3. [Google Scholar] [CrossRef]
- Han, Z.; Shen, F.; He, Y.; Degos, V.; Camus, M.; Maze, M.; Young, W.L.; Su, H. Activation of alpha-7 nicotinic acetylcholine receptor reduces ischemic stroke injury through reduction of pro-inflammatory macrophages and oxidative stress. PLoS ONE 2014, 9, e105711. [Google Scholar] [CrossRef]
- Mathew, R.O.; Hsu, W.H.; Young, Y. Effect of comorbidity on functional recovery after hip fracture in the elderly. Am. J. Phys. Med. Rehabil. 2013, 92, 686–696. [Google Scholar] [CrossRef]
- Wang, C.; Yue, H.; Hu, Z.; Shen, Y.; Ma, J.; Li, J.; Wang, X.D.; Wang, L.; Sun, B.; Shi, P.; et al. Microglia mediate forgetting via complement-dependent synaptic elimination. Science 2020, 367, 688–694. [Google Scholar] [CrossRef]
- Palencia, G.; Garcia, E.; Osorio-Rico, L.; Trejo-Solis, C.; Escamilla-Ramirez, A.; Sotelo, J. Neuroprotective effect of thalidomide on MPTP-induced toxicity. Neurotoxicology 2015, 47, 82–87. [Google Scholar] [CrossRef]
- Ballabh, P.; Braun, A.; Nedergaard, M. The blood-brain barrier: An overview: Structure, regulation, and clinical implications. Neurobiol. Dis. 2004, 16, 1–13. [Google Scholar] [CrossRef]
- Edwards, D.N.; Bix, G.J. Roles of blood-brain barrier integrins and extracellular matrix in stroke. Am. J. Physiol. Cell Physiol. 2019, 316, C252–C263. [Google Scholar] [CrossRef]
- Fields, R.D. Neuroscience. Change in the brain’s white matter. Science 2010, 330, 768–769. [Google Scholar] [CrossRef]
- Fu, J.H.; Lu, C.Z.; Hong, Z.; Dong, Q.; Luo, Y.; Wong, K.S. Extent of white matter lesions is related to acute subcortical infarcts and predicts further stroke risk in patients with first ever ischaemic stroke. J. Neurol. Neurosurg. Psychiatry 2005, 76, 793–796. [Google Scholar] [CrossRef]
- Li, L.; Simoni, M.; Kuker, W.; Schulz, U.G.; Christie, S.; Wilcock, G.K.; Rothwell, P.M. Population-based case-control study of white matter changes on brain imaging in transient ischemic attack and ischemic stroke. Stroke 2013, 44, 3063–3070. [Google Scholar] [CrossRef]
- Vacas, S.; Degos, V.; Tracey, K.J.; Maze, M. High-mobility group box 1 protein initiates postoperative cognitive decline by engaging bone marrow-derived macrophages. Anesthesiology 2014, 120, 1160–1167. [Google Scholar] [CrossRef]
- Andersson, U.; Wang, H.; Palmblad, K.; Aveberger, A.C.; Bloom, O.; Erlandsson-Harris, H.; Janson, A.; Kokkola, R.; Zhang, M.; Yang, H.; et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J. Exp. Med. 2000, 192, 565–570. [Google Scholar] [CrossRef]
- Merlini, M.; Rafalski, V.A.; Rios Coronado, P.E.; Gill, T.M.; Ellisman, M.; Muthukumar, G.; Subramanian, K.S.; Ryu, J.K.; Syme, C.A.; Davalos, D.; et al. Fibrinogen Induces Microglia-Mediated Spine Elimination and Cognitive Impairment in an Alzheimer’s Disease Model. Neuron 2019, 101, 1099–1108.e6. [Google Scholar] [CrossRef]
- Cacheaux, L.P.; Ivens, S.; David, Y.; Lakhter, A.J.; Bar-Klein, G.; Shapira, M.; Heinemann, U.; Friedman, A.; Kaufer, D. Transcriptome profiling reveals TGF-beta signaling involvement in epileptogenesis. J. Neurosci. 2009, 29, 8927–8935. [Google Scholar] [CrossRef]
- Ivens, S.; Kaufer, D.; Flores, L.P.; Bechmann, I.; Zumsteg, D.; Tomkins, O.; Seiffert, E.; Heinemann, U.; Friedman, A. TGF-beta receptor-mediated albumin uptake into astrocytes is involved in neocortical epileptogenesis. Brain 2007, 130, 535–547. [Google Scholar] [CrossRef]
- Weissberg, I.; Wood, L.; Kamintsky, L.; Vazquez, O.; Milikovsky, D.Z.; Alexander, A.; Oppenheim, H.; Ardizzone, C.; Becker, A.; Frigerio, F.; et al. Albumin induces excitatory synaptogenesis through astrocytic TGF-beta/ALK5 signaling in a model of acquired epilepsy following blood-brain barrier dysfunction. Neurobiol. Dis. 2015, 78, 115–125. [Google Scholar] [CrossRef]
- Festoff, B.W.; Sajja, R.K.; van Dreden, P.; Cucullo, L. HMGB1 and thrombin mediate the blood-brain barrier dysfunction acting as biomarkers of neuroinflammation and progression to neurodegeneration in Alzheimer’s disease. J. Neuroinflamm. 2016, 13, 194. [Google Scholar] [CrossRef]
- Yu, P.; Venkat, P.; Chopp, M.; Zacharek, A.; Shen, Y.; Liang, L.; Landschoot-Ward, J.; Liu, Z.; Jiang, R.; Chen, J. Deficiency of tPA Exacerbates White Matter Damage, Neuroinflammation, Glymphatic Dysfunction and Cognitive Dysfunction in Aging Mice. Aging Dis. 2019, 10, 770–783. [Google Scholar] [CrossRef]
- Feng, X.; Valdearcos, M.; Uchida, Y.; Lutrin, D.; Maze, M.; Koliwad, S.K. Microglia mediate postoperative hippocampal inflammation and cognitive decline in mice. JCI Insight 2017, 2, e91229. [Google Scholar] [CrossRef]
- Degos, V.; Westbroek, E.M.; Lawton, M.T.; Hemphill, J.C., 3rd; Del Zoppo, G.J.; Young, W.L. Perioperative management of coagulation in nontraumatic intracerebral hemorrhage. Anesthesiology 2013, 119, 218–227. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Lyu, H.; Huo, K.; Do Prado, L.B.; Tang, C.; Wang, Z.; Li, Q.; Wong, J.; Su, H. Bone Fracture Enhanced Blood-Brain Barrier Breakdown in the Hippocampus and White Matter Damage of Stroke Mice. Int. J. Mol. Sci. 2020, 21, 8481. https://doi.org/10.3390/ijms21228481
Huang J, Lyu H, Huo K, Do Prado LB, Tang C, Wang Z, Li Q, Wong J, Su H. Bone Fracture Enhanced Blood-Brain Barrier Breakdown in the Hippocampus and White Matter Damage of Stroke Mice. International Journal of Molecular Sciences. 2020; 21(22):8481. https://doi.org/10.3390/ijms21228481
Chicago/Turabian StyleHuang, Jinhao, Haiyan Lyu, Kang Huo, Leandro B. Do Prado, Chaoliang Tang, Zhanqiang Wang, Qifeng Li, Julia Wong, and Hua Su. 2020. "Bone Fracture Enhanced Blood-Brain Barrier Breakdown in the Hippocampus and White Matter Damage of Stroke Mice" International Journal of Molecular Sciences 21, no. 22: 8481. https://doi.org/10.3390/ijms21228481
APA StyleHuang, J., Lyu, H., Huo, K., Do Prado, L. B., Tang, C., Wang, Z., Li, Q., Wong, J., & Su, H. (2020). Bone Fracture Enhanced Blood-Brain Barrier Breakdown in the Hippocampus and White Matter Damage of Stroke Mice. International Journal of Molecular Sciences, 21(22), 8481. https://doi.org/10.3390/ijms21228481





