Phoenixin: More than Reproductive Peptide
Abstract
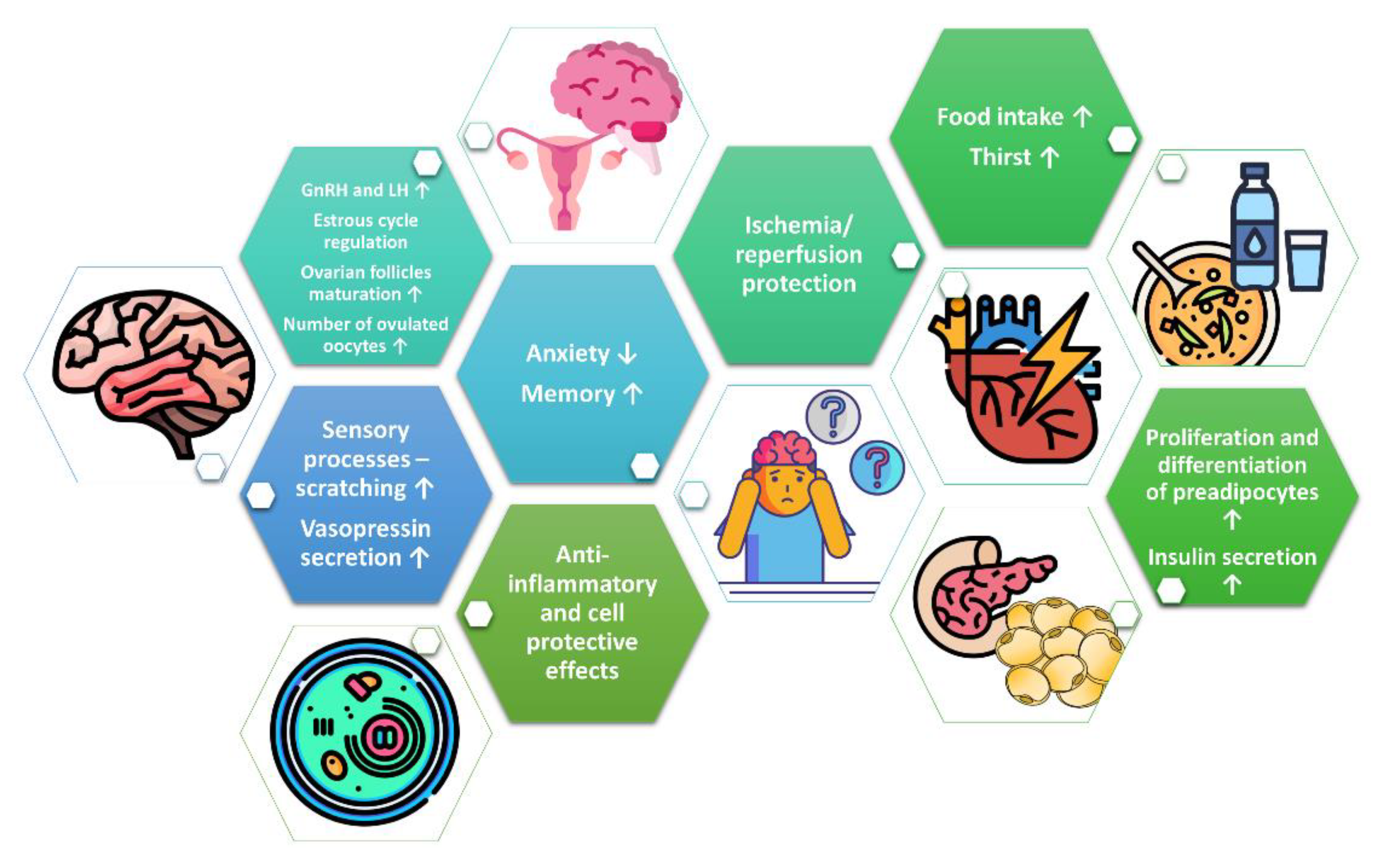
:1. Introduction
2. Characterisation of Phoenixin and GPR173 Receptor
3. Phoenixin in Reproduction System
4. Phoenixin in the Regulation of Food Intake and Thirst
5. Phoenixin in Memory and Anxiety
6. Other Effects of Phoenixin in Central Nervous System
7. Phoenixin as Modulator of Lipid and Glucose Metabolism
8. Cell-Protective and Anti-Inflammatory Effects of Phoenixin
9. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| Arc | Arcuate nucleus |
| Aβ | Amyloid β |
| COX1 | Cytochrome C oxidase subunit 1 |
| CREB | cAMP response element-binding protein |
| DHA | Dioxoheptanoic acid |
| ERK1/2 | Extracellular signal-regulated kinases 1/2 |
| FSH | Follicle-stimulating hormone |
| GnRH | Gonadotropin-releasing hormone |
| GnRHR | Gonadotropin-releasing hormone receptor |
| GPR173 | G protein-coupled receptor 173 |
| KP-13 | Kisspeptin-13 |
| LDH | Lactate dehydrogenase |
| LH | Luteinizing hormone |
| NTS | Nucleus of the solitary tract |
| NRF1 | Nuclear respiratory factor 1 |
| PCOS | Polycystic ovary syndrome |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PNX | Phoenixin |
| Smim20 | Small integral membrane protein 20 |
| SREB | Super Conserved Receptor Expressed in Brain |
| TFAM | Mitochondrial transcription factor A |
| VMH | Ventromedial nucleus of the hypothalamus |
References
- Corbiere, A.; Vaudry, H.; Chan, P.; Walet-Balieu, M.L.; Lecroq, T.; Lefebvre, A.; Pineau, C.; Vaudry, D. Strategies for the Identification of Bioactive Neuropeptides in Vertebrates. Front. Neurosci. 2019, 13, 948. [Google Scholar] [CrossRef]
- Samson, W.K.; Zhang, J.V.; Avsian-Kretchmer, O.; Cui, K.; Yosten, G.L.; Klein, C.; Lyu, R.M.; Wang, Y.X.; Chen, X.Q.; Yang, J.; et al. Neuronostatin encoded by the somatostatin gene regulates neuronal, cardiovascular, and metabolic functions. J. Biol. Chem. 2008, 283, 31949–31959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yosten, G.L.; Lyu, R.M.; Hsueh, A.J.; Avsian-Kretchmer, O.; Chang, J.K.; Tullock, C.W.; Dun, S.L.; Dun, N.; Samson, W.K. A novel reproductive peptide, phoenixin. J. Neuroendocrinol. 2013, 25, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Stein, L.M.; Tullock, C.W.; Mathews, S.K.; Garcia-Galiano, D.; Elias, C.F.; Samson, W.K.; Yosten, G.L. Hypothalamic action of phoenixin to control reproductive hormone secretion in females: Importance of the orphan G protein-coupled receptor Gpr173. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R489–R496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, J.H.; He, Z.; Peng, Y.L.; Jin, W.D.; Wang, Z.; Mu, L.Y.; Chang, M.; Wang, R. Phoenixin-14 enhances memory and mitigates memory impairment induced by Abeta1-42 and scopolamine in mice. Brain Res. 2015, 1629, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.H.; He, Z.; Peng, Y.L.; Jin, W.D.; Mu, J.; Xue, H.X.; Wang, Z.; Chang, M.; Wang, R. Effects of Phoenixin-14 on anxiolytic-like behavior in mice. Behav. Brain Res. 2015, 286, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Billert, M.; Wojciechowicz, T.; Jasaszwili, M.; Szczepankiewicz, D.; Wasko, J.; Kazmierczak, S.; Strowski, M.Z.; Nowak, K.W.; Skrzypski, M. Phoenixin-14 stimulates differentiation of 3T3-L1 preadipocytes via cAMP/Epac-dependent mechanism. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Billert, M.; Kolodziejski, P.A.; Strowski, M.Z.; Nowak, K.W.; Skrzypski, M. Phoenixin-14 stimulates proliferation and insulin secretion in insulin producing INS-1E cells. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 118533. [Google Scholar] [CrossRef] [PubMed]
- Soya, S.; Sakurai, T. Evolution of Orexin Neuropeptide System: Structure and Function. Front. Neurosci. 2020, 14, 691. [Google Scholar] [CrossRef]
- Kim, S.; Nam, Y.; Shin, S.J.; Park, Y.H.; Jeon, S.G.; Kim, J.I.; Kim, M.J.; Moon, M. The Potential Roles of Ghrelin in Metabolic Syndrome and Secondary Symptoms of Alzheimer’s Disease. Front. Neurosci. 2020, 14, 583097. [Google Scholar] [CrossRef]
- Navarro, V.M. Metabolic regulation of kisspeptin—The link between energy balance and reproduction. Nat. Rev. Endocrinol. 2020, 16, 407–420. [Google Scholar] [CrossRef]
- Palasz, A.; Rojczyk, E.; Bogus, K.; Worthington, J.J.; Wiaderkiewicz, R. The novel neuropeptide phoenixin is highly co-expressed with nesfatin-1 in the rat hypothalamus, an immunohistochemical study. Neurosci. Lett. 2015, 592, 17–21. [Google Scholar] [CrossRef]
- Lyu, R.M.; Huang, X.F.; Zhang, Y.; Dun, S.L.; Luo, J.J.; Chang, J.K.; Dun, N.J. Phoenixin: A novel peptide in rodent sensory ganglia. Neuroscience 2013, 250, 622–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dennerlein, S.; Oeljeklaus, S.; Jans, D.; Hellwig, C.; Bareth, B.; Jakobs, S.; Deckers, M.; Warscheid, B.; Rehling, P. MITRAC7 Acts as a COX1-Specific Chaperone and Reveals a Checkpoint during Cytochrome c Oxidase Assembly. Cell Rep. 2015, 12, 1644–1655. [Google Scholar] [CrossRef] [Green Version]
- Cowan, A.; Lyu, R.M.; Chen, Y.H.; Dun, S.L.; Chang, J.K.; Dun, N.J. Phoenixin: A candidate pruritogen in the mouse. Neuroscience 2015, 310, 541–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elphick, M.R.; Mirabeau, O.; Larhammar, D. Evolution of neuropeptide signalling systems. J. Exp. Biol. 2018, 221. [Google Scholar] [CrossRef] [Green Version]
- Rocca, C.; Scavello, F.; Granieri, M.C.; Pasqua, T.; Amodio, N.; Imbrogno, S.; Gattuso, A.; Mazza, R.; Cerra, M.C.; Angelone, T. Phoenixin-14: Detection and novel physiological implications in cardiac modulation and cardioprotection. Cell. Mol. Life Sci. 2018, 75, 743–756. [Google Scholar] [CrossRef]
- Prinz, P.; Scharner, S.; Friedrich, T.; Schalla, M.; Goebel-Stengel, M.; Rose, M.; Stengel, A. Central and peripheral expression sites of phoenixin-14 immunoreactivity in rats. Biochem. Biophys. Res. Commun. 2017, 493, 195–201. [Google Scholar] [CrossRef]
- Kalamon, N.; Blaszczyk, K.; Szlaga, A.; Billert, M.; Skrzypski, M.; Pawlicki, P.; Gorowska-Wojtowicz, E.; Kotula-Balak, M.; Blasiak, A.; Rak, A. Levels of the neuropeptide phoenixin-14 and its receptor GRP173 in the hypothalamus, ovary and periovarian adipose tissue in rat model of polycystic ovary syndrome. Biochem. Biophys. Res. Commun. 2020, 528, 628–635. [Google Scholar] [CrossRef]
- Nguyen, X.P.; Nakamura, T.; Osuka, S.; Bayasula, B.; Nakanishi, N.; Kasahara, Y.; Muraoka, A.; Hayashi, S.; Nagai, T.; Murase, T.; et al. Effect of the neuropeptide phoenixin and its receptor GPR173 during folliculogenesis. Reproduction 2019, 158, 25–34. [Google Scholar] [CrossRef]
- Wang, M.; Deng, S.P.; Chen, H.P.; Jiang, D.N.; Tian, C.X.; Yang, W.; Wu, T.L.; Zhu, C.H.; Zhang, Y.; Li, G.L. Phoenixin participated in regulation of food intake and growth in spotted scat, Scatophagus argus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2018, 226, 36–44. [Google Scholar] [CrossRef]
- McIlwraith, E.K.; Loganathan, N.; Belsham, D.D. Regulation of Gpr173 expression, a putative phoenixin receptor, by saturated fatty acid palmitate and endocrine-disrupting chemical bisphenol A through a p38-mediated mechanism in immortalized hypothalamic neurons. Mol. Cell. Endocrinol. 2019, 485, 54–60. [Google Scholar] [CrossRef]
- Rajeswari, J.J.; Unniappan, S. Phoenixin-20 Stimulates mRNAs Encoding Hypothalamo-Pituitary-Gonadal Hormones, is Pro-Vitellogenic, and Promotes Oocyte Maturation in Zebrafish. Sci. Rep. 2020, 10, 6264. [Google Scholar] [CrossRef] [Green Version]
- Suszka-Switek, A.; Palasz, A.; Filipczyk, L.; Menezes, I.C.; Mordecka-Chamera, K.; Angelone, T.; Bogus, K.; Bacopoulou, F.; Worthington, J.J.; Wiaderkiewicz, R. The GnRH analogues affect novel neuropeptide SMIM20/phoenixin and GPR173 receptor expressions in the female rat hypothalamic-pituitary-gonadal (HPG) axis. Clin. Exp. Pharmacol. Physiol. 2019, 46, 350–359. [Google Scholar] [CrossRef]
- Haddock, C.J.; Almeida-Pereira, G.; Stein, L.M.; Yosten, G.L.C.; Samson, W.K. A novel regulator of thirst behavior: Phoenixin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R1027–R1035. [Google Scholar] [CrossRef]
- Friedrich, T.; Schalla, M.A.; Lommel, R.; Goebel-Stengel, M.; Kobelt, P.; Rose, M.; Stengel, A. Restraint stress increases the expression of phoenixin immunoreactivity in rat brain nuclei. Brain Res. 2020, 1743, 146904. [Google Scholar] [CrossRef]
- Lepiarczyk, E.; Bossowska, A.; Majewska, M.; Skowronska, A.; Kaleczyc, J.; Majewski, M. Distribution and chemical coding of phoenixin-immunoreactive nerve structures in the spinal cord of the pig. Ann. Anat. 2020, 232, 151559. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Rotllant, G.E.; Cummins, S.F.; Elizur, A.; Ventura, T. Insights Into Sexual Maturation and Reproduction in the Norway Lobster (Nephrops norvegicus) via in silico Prediction and Characterization of Neuropeptides and G Protein-coupled Receptors. Front. Endocrinol. 2018, 9, 430. [Google Scholar] [CrossRef] [Green Version]
- Treen, A.K.; Luo, V.; Belsham, D.D. Phoenixin Activates Immortalized GnRH and Kisspeptin Neurons Through the Novel Receptor GPR173. Mol. Endocrinol. 2016, 30, 872–888. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Su, D.; Wang, Q.; Chong, Z.; Zhu, Q.; He, W.; Wang, W. Phoenixin 14 inhibits ischemia/reperfusion-induced cytotoxicity in microglia. Arch. Biochem. Biophys. 2020, 689, 108411. [Google Scholar] [CrossRef]
- Matsumoto, M.; Saito, T.; Takasaki, J.; Kamohara, M.; Sugimoto, T.; Kobayashi, M.; Tadokoro, M.; Matsumoto, S.; Ohishi, T.; Furuichi, K. An evolutionarily conserved G-protein coupled receptor family, SREB, expressed in the central nervous system. Biochem. Biophys. Res. Commun. 2000, 272, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Guvenc, G.; Altinbas, B.; Kasikci, E.; Ozyurt, E.; Bas, A.; Udum, D.; Niaz, N.; Yalcin, M. Contingent role of phoenixin and nesfatin-1 on secretions of the male reproductive hormones. Andrologia 2019, 51, e13410. [Google Scholar] [CrossRef]
- Schalla, M.A.; Stengel, A. Current Understanding of the Role of Nesfatin-1. J. Endocr. Soc. 2018, 2, 1188–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedrich, T.; Schalla, M.A.; Scharner, S.; Kuhne, S.G.; Goebel-Stengel, M.; Kobelt, P.; Rose, M.; Stengel, A. Intracerebroventricular injection of phoenixin alters feeding behavior and activates nesfatin-1 immunoreactive neurons in rats. Brain Res. 2019, 1715, 188–195. [Google Scholar] [CrossRef]
- Kim, J.; Yang, H. Nesfatin-1 as a new potent regulator in reproductive system. Dev. Reprod. 2012, 16, 253–264. [Google Scholar] [CrossRef]
- Wang, M.; Chen, H.P.; Zhai, Y.; Jiang, D.N.; Liu, J.Y.; Tian, C.X.; Wu, T.L.; Zhu, C.H.; Deng, S.P.; Li, G.L. Phoenixin: Expression at different ovarian development stages and effects on genes ralated to reproduction in spotted scat, Scatophagus argus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2019, 228, 17–25. [Google Scholar] [CrossRef]
- Ullah, K.; Ur Rahman, T.; Wu, D.D.; Lin, X.H.; Liu, Y.; Guo, X.Y.; Leung, P.C.K.; Zhang, R.J.; Huang, H.F.; Sheng, J.Z. Phoenixin-14 concentrations are increased in association with luteinizing hormone and nesfatin-1 concentrations in women with polycystic ovary syndrome. Clin. Chim. Acta 2017, 471, 243–247. [Google Scholar] [CrossRef]
- Mortimer, R.H.; Lev-Gur, M.; Freeman, R.; Fleischer, N. Pituitary response to bolus and continuous intravenous infusion of luteinizing hormone-releasing factor in normal women and women with polycystic ovarian syndrome. Am. J. Obstet. Gynecol. 1978, 130, 630–634. [Google Scholar] [CrossRef]
- Jeffcoate, S.L.; Brooks, R.V.; London, D.R.; Prunty, F.T.; Rhodes, P. Secretion of C19-steroids and oestrogens in the polycystic ovary syndrome. Ovarian studies in vivo and in vitro (including studies in vitro on a coincidental granulosa cell tumour). J. Endocrinol. 1968, 42, 229–243. [Google Scholar] [CrossRef]
- Schalla, M.; Prinz, P.; Friedrich, T.; Scharner, S.; Kobelt, P.; Goebel-Stengel, M.; Rose, M.; Stengel, A. Phoenixin-14 injected intracerebroventricularly but not intraperitoneally stimulates food intake in rats. Peptides 2017, 96, 53–60. [Google Scholar] [CrossRef]
- Stengel, A.; Goebel, M.; Tache, Y. Nesfatin-1: A novel inhibitory regulator of food intake and body weight. Obes. Rev. 2011, 12, 261–271. [Google Scholar] [CrossRef] [Green Version]
- Rajeswari, J.J.; Blanco, A.M.; Unniappan, S. Phoenixin-20 suppresses food intake, modulates glucoregulatory enzymes, and enhances glycolysis in zebrafish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R917–R928. [Google Scholar] [CrossRef]
- Palasz, A.; Tyszkiewicz-Nwafor, M.; Suszka-Switek, A.; Bacopoulou, F.; Dmitrzak-Weglarz, M.; Dutkiewicz, A.; Slopien, A.; Janas-Kozik, M.; Wilczynski, K.M.; Filipczyk, L.; et al. Longitudinal study on novel neuropeptides phoenixin, spexin and kisspeptin in adolescent inpatients with anorexia nervosa—Association with psychiatric symptoms. Nutr. Neurosci. 2019, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Misra, M.; Miller, K.K.; Kuo, K.; Griffin, K.; Stewart, V.; Hunter, E.; Herzog, D.B.; Klibanski, A. Secretory dynamics of ghrelin in adolescent girls with anorexia nervosa and healthy adolescents. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E347–E356. [Google Scholar] [CrossRef] [Green Version]
- Sedlackova, D.; Kopeckova, J.; Papezova, H.; Vybiral, S.; Kvasnickova, H.; Hill, M.; Nedvidkova, J. Changes of plasma obestatin, ghrelin and NPY in anorexia and bulimia nervosa patients before and after a high-carbohydrate breakfast. Physiol. Res. 2011, 60, 165–173. [Google Scholar] [CrossRef]
- McIlwraith, E.K.; Loganathan, N.; Belsham, D.D. Phoenixin Expression Is Regulated by the Fatty Acids Palmitate, Docosahexaenoic Acid and Oleate, and the Endocrine Disrupting Chemical Bisphenol A in Immortalized Hypothalamic Neurons. Front. Neurosci. 2018, 12, 838. [Google Scholar] [CrossRef]
- Lopez-Rodriguez, D.; Franssen, D.; Sevrin, E.; Gerard, A.; Balsat, C.; Blacher, S.; Noel, A.; Parent, A.S. Persistent vs Transient Alteration of Folliculogenesis and Estrous Cycle After Neonatal vs Adult Exposure to Bisphenol A. Endocrinology 2019, 160, 2558–2572. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.P.; Xie, Y.; Meng, X.Y.; Kang, J.S. History and progress of hypotheses and clinical trials for Alzheimer’s disease. Signal Transduct. Target. Ther. 2019, 4, 29. [Google Scholar] [CrossRef]
- Yuruyen, M.; Gultekin, G.; Batun, G.C.; Yavuzer, H.; Akcan, F.E.; Doventas, A.; Emul, M. Does plasma phoenixin level associate with cognition? Comparison between subjective memory complaint, mild cognitive impairment, and mild Alzheimer’s disease. Int. Psychogeriatr. 2017, 29, 1–8. [Google Scholar] [CrossRef]
- Hofmann, T.; Weibert, E.; Ahnis, A.; Elbelt, U.; Rose, M.; Klapp, B.F.; Stengel, A. Phoenixin is negatively associated with anxiety in obese men. Peptides 2017, 88, 32–36. [Google Scholar] [CrossRef]
- Neumann, I.D.; Landgraf, R. Balance of brain oxytocin and vasopressin: Implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012, 35, 649–659. [Google Scholar] [CrossRef]
- Csabafi, K.; Jaszberenyi, M.; Bagosi, Z.; Liptak, N.; Telegdy, G. Effects of kisspeptin-13 on the hypothalamic-pituitary-adrenal axis, thermoregulation, anxiety and locomotor activity in rats. Behav. Brain Res. 2013, 241, 56–61. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; He, Z.; Peng, Y.; Jin, W.; Wang, Z.; Han, R.; Chang, M.; Wang, R. Kisspeptin-13 enhances memory and mitigates memory impairment induced by Aβ1–42 in mice novel object and object location recognition tasks. Neurobiol. Learn. Mem. 2015, 123, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Pałasz, A.; Janas-Kozik, M.; Borrow, A.; Arias-Carrión, O.; Worthington, J.J. The potential role of the novel hypothalamic neuropeptides nesfatin-1, phoenixin, spexin and kisspeptin in the pathogenesis of anxiety and anorexia nervosa. Neurochem. Int. 2018, 113, 120–136. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Xian, D.; Yang, L.; Xiong, X.; Lai, R.; Zhong, J. Pruritus: Progress toward Pathogenesis and Treatment. BioMed Res. Int. 2018, 2018, 9625936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grover, H.M.; Smith, P.M.; Ferguson, A.V. Phoenixin influences the excitability of nucleus of the solitary tract neurones, effects which are modified by environmental and glucocorticoid stress. J. Neuroendocrinol. 2020, 32, e12855. [Google Scholar] [CrossRef]
- Schalla, M.A.; Goebel-Stengel, M.; Friedrich, T.; Kuhne, S.G.; Kobelt, P.; Rose, M.; Stengel, A. Restraint stress affects circulating NUCB2/nesfatin-1 and phoenixin levels in male rats. Psychoneuroendocrinology 2020, 122, 104906. [Google Scholar] [CrossRef]
- Brown, C.H. Magnocellular Neurons and Posterior Pituitary Function. Compr. Physiol. 2016, 6, 1701–1741. [Google Scholar] [CrossRef]
- Gasparini, S.; Stein, L.M.; Loewen, S.P.; Haddock, C.J.; Soo, J.; Ferguson, A.V.; Kolar, G.R.; Yosten, G.L.C.; Samson, W.K. Novel regulator of vasopressin secretion: Phoenixin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R623–R628. [Google Scholar] [CrossRef] [PubMed]
- Könczöl, K.; Pintér, O.; Ferenczi, S.; Varga, J.; Kovács, K.; Palkovits, M.; Zelena, D.; Toth, Z. Nesfatin-1 exerts long-term effect on food intake and body temperature. Int. J. Obes. 2012, 36, 1514–1521. [Google Scholar] [CrossRef] [Green Version]
- Muir, L.A.; Neeley, C.K.; Meyer, K.A.; Baker, N.A.; Brosius, A.M.; Washabaugh, A.R.; Varban, O.A.; Finks, J.F.; Zamarron, B.F.; Flesher, C.G.; et al. Adipose tissue fibrosis, hypertrophy, and hyperplasia: Correlations with diabetes in human obesity. Obesity 2016, 24, 597–605. [Google Scholar] [CrossRef]
- Ojha, A.; Ojha, U.; Mohammed, R.; Chandrashekar, A.; Ojha, H. Current perspective on the role of insulin and glucagon in the pathogenesis and treatment of type 2 diabetes mellitus. Clin. Pharmacol. 2019, 11, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Del Prato, S.; Marchetti, P. Beta- and alpha-cell dysfunction in type 2 diabetes. Horm. Metab. Res. 2004, 36, 775–781. [Google Scholar] [CrossRef]
- Oram, R.A.; Sims, E.K.; Evans-Molina, C. Beta cells in type 1 diabetes: Mass and function; sleeping or dead? Diabetologia 2019, 62, 567–577. [Google Scholar] [CrossRef] [Green Version]
- Yosten, G.L.C. Alpha cell dysfunction in type 1 diabetes. Peptides 2018, 100, 54–60. [Google Scholar] [CrossRef]
- Yang, Y.; Lv, Y.; Liu, J.; Zhang, S.; Li, Y.; Shi, Y. Phoenixin 20 promotes neuronal mitochondrial biogenesis via CREB-PGC-1alpha pathway. J. Mol. Histol. 2020, 51, 173–181. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, B.; Yang, S.; Tang, X.; Wang, J.; Wei, D. The protective effects of phoenixin-14 against lipopolysaccharide-induced inflammation and inflammasome activation in astrocytes. Inflamm. Res. 2020, 69, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Li, Y.; Ma, S.; Tang, Y.; Li, H. Phoenixin-20 Ameliorates Lipopolysaccharide-Induced Activation of Microglial NLRP3 Inflammasome. Neurotox. Res. 2020, 38, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Mai, N.; Prifti, V.; Kim, M.; Halterman, M.W. Characterization of neutrophil-neuronal co-cultures to investigate mechanisms of post-ischemic immune-mediated neurotoxicity. J. Neurosci. Methods 2020, 108782. [Google Scholar] [CrossRef]
- Zhang, B.; Li, J. Phoenixin-14 protects human brain vascular endothelial cells against oxygen-glucose deprivation/reoxygenation (OGD/R)-induced inflammation and permeability. Arch. Biochem. Biophys. 2020, 682, 108275. [Google Scholar] [CrossRef]
- Singh, V.; Roth, S.; Veltkamp, R.; Liesz, A. HMGB1 as a Key Mediator of Immune Mechanisms in Ischemic Stroke. Antioxid. Redox Signal. 2016, 24, 635–651. [Google Scholar] [CrossRef]
- Yang, F.; Huang, P.; Shi, L.; Liu, F.; Tang, A.; Xu, S. Phoenixin 14 Inhibits High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease in Experimental Mice. Drug Des. Dev. Ther. 2020, 14, 3865–3874. [Google Scholar] [CrossRef]
- Sun, G.; Ren, Q.; Bai, L.; Zhang, L. Phoenixin-20 suppresses lipopolysaccharide-induced inflammation in dental pulp cells. Chem. Biol. Interact. 2020, 318, 108971. [Google Scholar] [CrossRef]

| Area | PNX-li | Smim20 mRNA | GPR173 mRNA | Species | Publications |
|---|---|---|---|---|---|
| Central nervous system | |||||
| Hypothalamus (without nucleus division) | +++ | +++ | +++ | Sa, R, Zf | [17,19,21,22,23,24] |
| Periventricular Nucleus | +++ | +++ | R | [3,4] | |
| Paraventricular Nucleus | +++ | ++ | R | [3,4,12] | |
| Zona Incerta | ++ | R | [3] | ||
| Ventromedial Hypothalamus | ++ | +++ | R | [3,4,12] | |
| Supraoptic Nucleus | +++ | +++ | R | [3,4,18,25,26] | |
| Lateral Hypothalamus | ++ | ++ | R | [3,4,12] | |
| Substantia Nigra | ++ | R | [3] | ||
| Edinger–Westphal Nucleus | ++ | R | [3] | ||
| Nucleus Tractus Solitarius | ++ | R | [3,13,18] | ||
| Central Amygdaloid Nucleus | +++ | +++ | R | [4,18,26] | |
| Arcuate Nucleus | + | ++ | R | [4,18,26] | |
| Raphe Pallidus | + | R | [18,26] | ||
| Area Postrema | ++ | R | [18] | ||
| Median Eminence | ++ | R | [3] | ||
| Pituitary | ++ | Sa | [3,21] | ||
| Anterior Pituitary Lobe | + | R | [3] | ||
| Posterior Pituitary Lobe | + | R | [3] | ||
| Cerebrum | + | R | [3] | ||
| Pons | + | R | [3] | ||
| Spinal Cord | +++ | R, M, P | [13,18,26,27] | ||
| Dorsal Root Ganglion | +++ | R, M | [13,15] | ||
| Peripheral tissues | |||||
| Heart | +++ | ++ | R, Sa, Zf | [3,13,17,21,23] | |
| Thymus | ++ | R | [3] | ||
| Lung | ++ | R | [3] | ||
| Gill | + | + | Sa, Zf | [21,23] | |
| Oesophagus | ++ | R | [3] | ||
| Stomach | ++ | R, Sa | [3,21] | ||
| Duodenum | ++ | ++ | R, Zf | [3,18,23] | |
| Jejunum | +++ | R | [3,18] | ||
| Ileum | ++ | R | [3,18] | ||
| Colon | −/+ | R | [3,18] | ||
| Pancreas | ++ | R | [3,8,18] | ||
| Liver | ++ | Zf | [23] | ||
| Adipocytes | ++ | ++ | ++ | R, M | [7,19] |
| Kidney | ++ | R, Sa | [3,21] | ||
| Spleen | ++ | R, Sa | [3,21] | ||
| Ovary | ++ | ++ | ++ | H, Sa, R, Zf | [20,21,23,24] |
| Ovarian follicles | ++ | ++ | ++ | H, R | [19,20] |
| Testis | + | + | Sa, R, Zf | [17,21,23] | |
| Muscle | + | R, Sa | [21,28] | ||
| Skin | ++ | ++ | M, Zf | [15,23] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Billert, M.; Rak, A.; Nowak, K.W.; Skrzypski, M. Phoenixin: More than Reproductive Peptide. Int. J. Mol. Sci. 2020, 21, 8378. https://doi.org/10.3390/ijms21218378
Billert M, Rak A, Nowak KW, Skrzypski M. Phoenixin: More than Reproductive Peptide. International Journal of Molecular Sciences. 2020; 21(21):8378. https://doi.org/10.3390/ijms21218378
Chicago/Turabian StyleBillert, Maria, Agnieszka Rak, Krzysztof W. Nowak, and Marek Skrzypski. 2020. "Phoenixin: More than Reproductive Peptide" International Journal of Molecular Sciences 21, no. 21: 8378. https://doi.org/10.3390/ijms21218378







