Fragmentation Study, Dual Anti-Bactericidal and Anti-Viral Effects and Molecular Docking of Cobalt(III) Complexes
Abstract
1. Introduction
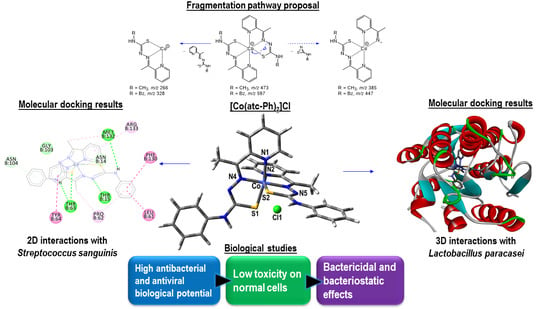
2. Results and Discussion
2.1. Structural Characterization
2.2. Stability Studies
2.3. Biological Activity Studies
2.3.1. Antibacterial Activity
2.3.2. Antiviral Activity
2.4. Molecular Docking
3. Experiment
3.1. Materials
3.2. Preparation of the Complexes
3.3. Instruments
3.4. Antibacterial Assays
3.4.1. Determination of MICs and MBC
3.4.2. Time–Kill Curves
3.5. Antiviral Assays
3.5.1. Cell Culture
3.5.2. In Vitro Cytotoxic Activity Evaluation by MTT Assays
3.5.3. Assessment of CHIKV Replication
3.6. Statistical Analysis
3.7. Molecular Docking Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MIC | Minimum inhibitory concentration |
| CBM | Minimum bactericidal concentration |
| PDB | Protein Data Bank |
| CCDC | Cambridge Crystallographic Data Centre |
| AD4 | Autodock4.2 |
| RMS | Root mean square |
| ESI | Electrospray Ionization |
| TMS | Tetamethylsilane |
References
- Konkankit, C.C.; Marker, S.C.; Knopf, K.M.; Wilson, J.J. Anticancer activity of complexes of the third row transition metals, rhenium, osmium, and iridium. Dalton Trans. 2018, 47, 9934–9974. [Google Scholar] [CrossRef]
- Budimir, A. Metal ions, Alzheimer’s disease and chelation therapy. Acta Pharm. 2011, 61, 1–14. [Google Scholar] [CrossRef]
- Oliveira, C.G.; Romero-Canelón, I.; Silva, M.M.; Coverdale, J.P.C.; Maia, P.I.S.; Batista, A.A.; Castelli, S.; Desideri, A.; Sadler, P.J.; Deflon, V.M. Palladium(II) complexes with thiosemicarbazones derived from pyrene as topoisomerase IB inhibitors. Dalton Trans. 2019, 48, 16509–16517. [Google Scholar] [CrossRef]
- Kostelidou, A.; Kalogiannis, S.; Begou, O.-A.; Perdih, F.; Turel, I.; Psomas, G. Synthesis, structure and biological activity of copper(II) complexes with gatifloxacin. Polyhedron 2016, 119, 359–370. [Google Scholar] [CrossRef]
- Carneiro, Z.A.; Lima, J.C.; Lopes, C.D.; Gaspari, A.P.S.; de Albuquerque, S.; Dinelli, L.R.; Veloso-Silva, L.L.W.; Paganelli, M.O.; Libardi, S.H.; Oliveira, C.G.; et al. Heterobimetallic nickel(II) and palladium(II) complexes derived from S-benzyl-N- (ferrocenyl)methylenedithiocarbazate: Trypanocidal activity and interaction with Trypanosoma cruzi Old Yellow Enzyme (TcOYE). Eur. J. Med. Chem. 2019, 180, 213–223. [Google Scholar] [CrossRef]
- Alghamdi, N.J.; Balaraman, L.; Emhoff, K.A.; Salem, A.M.H.; Wei, R.; Zhou, A.; Boyd, W.C. Cobalt(II) Diphenylazodioxide Complexes Induce Apoptosis in SK-HEP-1 Cells. ACS Omega 2019, 4, 14503–14510. [Google Scholar] [CrossRef]
- Oliveira, C.G.; Romero-Canelón, I.; Coverdale, J.P.C.; Maia, P.I.S.; Clarkson, G.J.; Deflon, V.M.; Sadler, P.J. Novel tetranuclear PdII and PtII anticancer complexes derived from pyrene thiosemicarbazones. Dalton Trans. 2020, 49, 9595–9604. [Google Scholar] [CrossRef]
- Lopes, E.O.; Oliveira, C.G.; Silva, P.B.; Eismann, C.E.; Suárez, C.A.; Menegário, A.A.; Leite, C.Q.F.; Deflon, V.M.; Pavan, F.R. Novel Zinc(II) Complexes [Zn(atc-Et)2] and [Zn(atc-Ph)2]: In Vitro and in Vivo Antiproliferative Studies. Int. J. Mol. Sci. 2016, 17, 781. [Google Scholar] [CrossRef]
- Vitorino, H.A.; Mantovanelli, L.; Zanotto, F.P.; Espósito, B.P. Iron Metallodrugs: Stability, Redox Activity and Toxicity against Artemia salina. PLoS ONE 2015, 10, e0121997. [Google Scholar] [CrossRef]
- Rodríguez, M.R.; Plá, J.D.; Balsa, L.M.; León, I.E.; Piro, O.E.; Echeverría, G.A.; García-Tojal, J.; Pis-Diez, R.; Parajón-Costa, B.S.; González-Baró, A.C. Cu(II) and Zn(II) complexes with a poly-functional ligand derived from o-vanillin and thiophene. Crystal structure, physicochemical properties, theoretical studies and cytotoxicity assays against human breast cancer cells. New J. Chem. 2019, 43, 7120–7129. [Google Scholar] [CrossRef]
- Slator, C.; Molphy, Z.; McKee, V.; Long, C.; Brown, T.; Kellett, A. Di-copper metallodrugs promote NCI-60 chemotherapy via singlet oxygen and superoxide production with tandem TA/TA and AT/AT oligonucleotide discrimination. Nucleic Acids Res. 2018, 46, 2733–2750. [Google Scholar] [CrossRef]
- Ambika, S.; Manojkumar, Y.; Arunachalam, S.; Gowdhami, B.; Meenakshi Sundaram, K.K.; Solomon, R.V.; Venuvanalingam, P.; Akbarsha, M.A.; Sundararaman, M. Biomolecular Interaction, Anti-Cancer and Anti-Angiogenic Properties of Cobalt(III) Schiff Base Complexes. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Oliveira, C.G.; da S Maia, P.I.; Souza, P.C.; Pavan, F.R.; Leite, C.Q.F.; Viana, R.B.; Batista, A.A.; Nascimento, O.R.; Deflon, V.M. Manganese(II) complexes with thiosemicarbazones as potential anti-Mycobacterium tuberculosis agents. J. Inorg. Biochem. 2014, 132, 21–29. [Google Scholar] [CrossRef]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The essential metals for humans: A brief overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef]
- Zhang, Y.; Rodionov, D.A.; Gelfand, M.S.; Gladyshev, V.N. Comparative genomic analyses of nickel, cobalt and vitamin B12 utilization. BMC Genomics 2009, 10, 1–26. [Google Scholar] [CrossRef]
- Chang, E.L.; Simmers, C.; Knight, D.A. Cobalt Complexes as Antiviral and Antibacterial Agents. Pharmaceuticals 2010, 3, 1711–1728. [Google Scholar] [CrossRef]
- Czarnek, K.; Terpiłowska, S.; Siwicki, A.K. Selected aspects of the action of cobalt ions in the human body. Cent. Eur. J. Immunol. 2015, 40, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Heffern, M.C.; Yamamoto, N.; Holbrook, R.J.; Eckermann, A.L.; Meade, T.J. Cobalt Derivatives as Promising Therapeutic Agents. Curr. Opin. Chem. Biol. 2013, 17, 189–196. [Google Scholar] [CrossRef]
- Hall, M.D.; Failes, T.W.; Yamamoto, N.; Hambley, T.W. Bioreductive activation and drug chaperoning in cobalt pharmaceuticals. Dalton Trans. 2007, 36, 3983–3990. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.A.; Lium, E.K.; Silverstein, S.J. Herpes simplex virus type 1 entry is inhibited by the cobalt chelate complex CTC-96. J. Virol. 2001, 75, 4117–4128. [Google Scholar] [CrossRef]
- Pelosi, G.; Bisceglie, F.; Bignami, F.; Ronzi, P.; Schiavone, P.; Re, M.C.; Casoli, C.; Pilotti, E. Antiretroviral Activity of Thiosemicarbazone Metal Complexes. J. Med. Chem. 2010, 53, 8765–8769. [Google Scholar] [CrossRef]
- Pahontu, E.; Julea, F.; Rosu, T.; Purcarea, V.; Chumakov, Y.; Petrenco, P.; Gulea, A. Antibacterial, antifungal and in vitro antileukaemia activity of metal complexes with thiosemicarbazones. J. Cell Mol. Med. 2015, 19, 865–878. [Google Scholar] [CrossRef]
- Gonçalves, A.C.R.; Carneiro, Z.A.; Oliveira, C.G.; Danuello, A.; Guerra, W.; Oliveira, R.J.; Ferreira, F.B.; Veloso-Silva, L.L.W.; Batista, F.A.H.; Borges, J.C.; et al. PtII, PdII and AuIII complexes with a thiosemicarbazone derived from diacethylmonooxime: Structural analysis, trypanocidal activity, cytotoxicity and first insight into the antiparasitic mechanism of action. Eur. J. Med. Chem. 2017, 141, 615–631. [Google Scholar] [CrossRef]
- Andres, S.A.; Bajaj, K.; Vishnosky, N.S.; Peterson, M.A.; Mashuta, M.S.; Buchanan, R.M.; Bates, P.J.; Grapperhaus, C.A. Synthesis, Characterization, and Biological Activity of Hybrid Thiosemicarbazone–Alkylthiocarbamate Metal Complexes. Inorg. Chem. 2020, 59, 4924–4935. [Google Scholar] [CrossRef]
- Pavan, F.R.; da S Maia, P.I.; Leite, S.R.A.; Deflon, V.M.; Batista, A.A.; Sato, D.N.; Franzblau, S.G.; Leite, C.Q.F. Thiosemicarbazones, semicarbazones, dithiocarbazates and hydrazide/hydrazones: Anti—Mycobacterium tuberculosis activity and cytotoxicity. Eur. J. Med. Chem. 2010, 45, 1898–1905. [Google Scholar] [CrossRef]
- Rosu, T.; Gulea, A.; Nicolae, A.; Georgescu, R. Complexes of 3dn Metal Ions with Thiosemicarbazones: Synthesis and Antimicrobial Activity. Molecules 2007, 12, 782–796. [Google Scholar] [CrossRef]
- Oliveira, C.G.; da S Maia, P.I.; Miyata, M.; Pavan, F.R.; Leite, C.Q.F.; de Almeida, E.T.; Deflon, V.M. Cobalt(III) complexes with thiosemicarbazones as potential anti-Mycobacterium tuberculosis agents. J. Braz. Chem. Soc. 2014, 25, 1848–1856. [Google Scholar] [CrossRef]
- Di, L.; Kerns, E.H. Lipophilicity Methods. In Drug-Like Properties, 2nd ed.; Di, L., Kerns, E.H., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 299–306. ISBN 978-0-12-801076-1. [Google Scholar]
- Zhang, R.; Qin, X.; Kong, F.; Chen, P.; Pan, G. Improving cellular uptake of therapeutic entities through interaction with components of cell membrane. Drug Deliv. 2019, 26, 328–342. [Google Scholar] [CrossRef]
- Echeverría, J.; Opazo, J.; Mendoza, L.; Urzúa, A.; Wilkens, M. Structure-Activity and Lipophilicity Relationships of Selected Antibacterial Natural Flavones and Flavanones of Chilean Flora. Molecules 2017, 22, 608. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.M. A Bioinorganic Approach to Fragment-Based Drug Discovery Targeting Metalloenzymes. Acc. Chem. Res. 2017, 50, 1–21. [Google Scholar] [CrossRef]
- Meija, J.; Coplen, T.B.; Berglund, M.; Brand, W.A.; Bièvre, P.D.; Gröning, M.; Holden, N.E.; Irrgeher, J.; Loss, R.D.; Walczyk, T.; et al. Atomic weights of the elements 2013 (IUPAC Technical Report). Pure Appl. Chem. 2016, 88, 265–291. [Google Scholar] [CrossRef]
- Mjos, K.D.; Orvig, C. Metallodrugs in Medicinal Inorganic Chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef]
- Khan, R.A.; Tăbăcaru, A.; Ali, F.; Koo, B.H. Anticancer and Antimicrobial Properties of Inorganic Compounds/Nanomaterials. Bioinorg. Chem. Appl. 2019, 2019, 1–2. [Google Scholar] [CrossRef]
- van Rijt, S.H.; Sadler, P.J. Current applications and future potential for bioinorganic chemistry in the development of anticancer drugs. Drug Discov. Today 2009, 14, 1089–1097. [Google Scholar] [CrossRef]
- Zhang, P.; Sadler, P.J. Redox-Active Metal Complexes for Anticancer Therapy. Eur. J. Inorg. Chem. 2017, 2017, 1541–1548. [Google Scholar] [CrossRef]
- Maia, P.I.S.; Pavan, F.R.; Leite, C.Q.F.; Abram, U.; Lang, E.S.; Batista, A.A.; Deflon, V.M. Thiosemicarbazone complexes of group 10 metals. Preparation, structural analysis and anti-Mycobacterium tuberculosis activity. In Metal Ions in Biology and Medicine; Pele, L., Powell, J.J., Kinrade, S., Jugdaohsingh, R., Collery, P., Maymard, I., Badawi, A., Eds.; John Libbey Eurotext: Paris, France, 2011; Volume 11, pp. 164–171. [Google Scholar]
- O’Shea, R.; Moser, H.E. Physicochemical Properties of Antibacterial Compounds: Implications for Drug Discovery. J. Med. Chem. 2008, 51, 2871–2878. [Google Scholar] [CrossRef] [PubMed]
- da S Maia, P.I.; Graminha, A.; Pavan, F.R.; Leite, C.Q.F.; Batista, A.A.; Back, D.F.; Lang, E.S.; Ellena, J.; Lemos, S.D.S.; Salistre-de-Araujo, H.S.; et al. Palladium(II) complexes with thiosemicarbazones: Syntheses, characterization and cytotoxicity against breast cancer cells and Anti-Mycobacterium tuberculosis activity. J. Braz. Chem. Soc. 2010, 21, 1177–1186. [Google Scholar] [CrossRef]
- Pohjala, L.; Utt, A.; Varjak, M.; Lulla, A.; Merits, A.; Ahola, T.; Tammela, P. Inhibitors of Alphavirus Entry and Replication Identified with a Stable Chikungunya Replicon Cell Line and Virus-Based Assays. PLoS ONE 2011, 6, e28923. [Google Scholar] [CrossRef]
- Pahonțu, E.; Proks, M.; Shova, S.; Lupașcu, G.; Ilieș, D.-C.; Bărbuceanu, Ș.-F.; Socea, L.-I.; Badea, M.; Păunescu, V.; Istrati, D.; et al. Synthesis, characterization, molecular docking studies and in vitro screening of new metal complexes with Schiff base as antimicrobial and antiproliferative agents. Appl. Organomet. Chem. 2019, 33, e5185. [Google Scholar] [CrossRef]
- Gorgulu, G.; Cicek, M.B.; Dede, B. Novel Aminoketooxime Ligand and Its Cu(II) and Mn(II) Complexes: Synthesis, Characterization and Molecular Docking Studies. Acta Phys. Pol. A 2018, 133, 250–255. [Google Scholar] [CrossRef]
- Richardson, D.R.; Kalinowski, D.S.; Richardson, V.; Sharpe, P.C.; Lovejoy, D.B.; Islam, M.; Bernhardt, P.V. 2-Acetylpyridine thiosemicarbazones are potent iron chelators and antiproliferative agents: Redox activity, iron complexation and characterization of their antitumor activity. J. Med. Chem. 2009, 52, 1459–1470. [Google Scholar] [CrossRef]
- Santiago, P.H.O.; Santiago, M.B.; Martins, C.H.G.; Gatto, C.C. Copper(II) and zinc(II) complexes with Hydrazone: Synthesis, crystal structure, Hirshfeld surface and antibacterial activity. Inorg. Chim. Acta 2020, 508, 119632. [Google Scholar] [CrossRef]
- D’Arrigo, M.; Ginestra, G.; Mandalari, G.; Furneri, P.M.; Bisignano, G. Synergism and postantibiotic effect of tobramycin and Melaleuca alternifolia (tea tree) oil against Staphylococcus aureus and Escherichia coli. Phytomedicine 2010, 17, 317–322. [Google Scholar] [CrossRef]
- Davis, J.L.; Hodge, H.M.; Campbell, W.E. Growth of Chikungunya Virus in Baby Hamster Kidney Cell (BHK-21-Clone 13) Suspension Cultures. Appl. Microbiol. 1971, 21, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Mf, S. Python: A programming language for software integration and development. J. Mol. Graph. Model 1999, 17, 57–61. [Google Scholar]







| Bacterial Strains | Hatc-Me | Hatc-Ph | [Co(atc-Me)2]+ | [Co(atc-Ph)2]+ | CoCl2·6H2O | CHD * | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Streptococcus mutans (ATCC 251755) | 12.5 | 12.5 | 100 | 100 | 400 | 400 | 0.78 | 0.78 | 100 | 100 | 0.46 | 0.46 |
| Streptococcus mitis (ATCC 49456) | 12.5 | 12.5 | 100 | 100 | 400 | 400 | 1.56 | 1.56 | 100 | 100 | 1.84 | 1.84 |
| Streptococcus sanguinis (ATCC 10556) | 6.25 | 6.25 | 100 | 100 | 400 | 400 | 0.39 | 0.39 | 100 | 100 | 0.92 | 0.92 |
| Streptococcus sobrinus (ATCC 33478) | 25 | 25 | 400 | 400 | 400 | 400 | 12.5 | 25 | >400 | >400 | 0.46 | 0.46 |
| Lactobacillus paracasei (ATCC 11578) | 50 | 50 | 400 | 400 | 400 | 400 | 0.78 | 0.78 | >400 | >400 | 0.92 | 0.92 |
| Streptococcus salivarius (ATCC 25975) | 25 | 25 | 100 | 100 | 400 | 400 | 3.12 | 3.12 | 100 | 100 | 0.92 | 0.92 |
| Enterococcus faecalis (ATCC 4082) | >400 | >400 | >400 | >400 | 400 | 400 | 6.25 | 6.25 | >400 | >400 | 3.68 | 3.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, L.d.P.; Silva, J.M.B.; Martins, D.O.S.; Santiago, M.B.; Martins, C.H.G.; Jardim, A.C.G.; Oliveira, G.S.; Pivatto, M.; Souza, R.A.C.; Franca, E.d.F.; et al. Fragmentation Study, Dual Anti-Bactericidal and Anti-Viral Effects and Molecular Docking of Cobalt(III) Complexes. Int. J. Mol. Sci. 2020, 21, 8355. https://doi.org/10.3390/ijms21218355
Fernandes LdP, Silva JMB, Martins DOS, Santiago MB, Martins CHG, Jardim ACG, Oliveira GS, Pivatto M, Souza RAC, Franca EdF, et al. Fragmentation Study, Dual Anti-Bactericidal and Anti-Viral Effects and Molecular Docking of Cobalt(III) Complexes. International Journal of Molecular Sciences. 2020; 21(21):8355. https://doi.org/10.3390/ijms21218355
Chicago/Turabian StyleFernandes, Laísa de P., Júlia M. B. Silva, Daniel O. S. Martins, Mariana B. Santiago, Carlos H. G. Martins, Ana C. G. Jardim, Guedmiller S. Oliveira, Marcos Pivatto, Rafael A. C. Souza, Eduardo de F. Franca, and et al. 2020. "Fragmentation Study, Dual Anti-Bactericidal and Anti-Viral Effects and Molecular Docking of Cobalt(III) Complexes" International Journal of Molecular Sciences 21, no. 21: 8355. https://doi.org/10.3390/ijms21218355
APA StyleFernandes, L. d. P., Silva, J. M. B., Martins, D. O. S., Santiago, M. B., Martins, C. H. G., Jardim, A. C. G., Oliveira, G. S., Pivatto, M., Souza, R. A. C., Franca, E. d. F., Deflon, V. M., Machado, A. E. H., & Oliveira, C. G. (2020). Fragmentation Study, Dual Anti-Bactericidal and Anti-Viral Effects and Molecular Docking of Cobalt(III) Complexes. International Journal of Molecular Sciences, 21(21), 8355. https://doi.org/10.3390/ijms21218355