3D Spheroids Derived from Human Lipedema ASCs Demonstrated Similar Adipogenic Differentiation Potential and ECM Remodeling to Non-Lipedema ASCs In Vitro
Abstract
:1. Introduction
2. Results
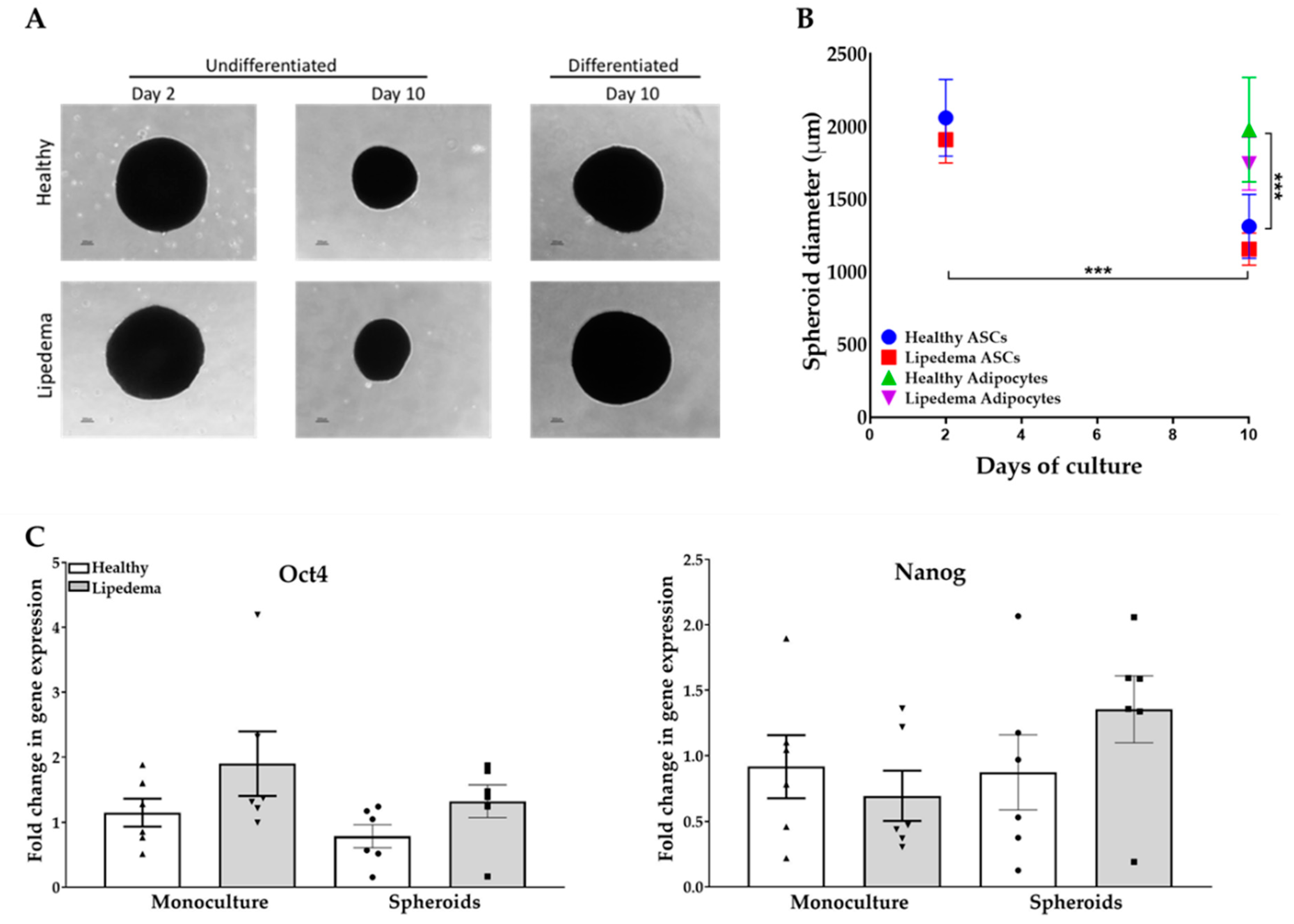
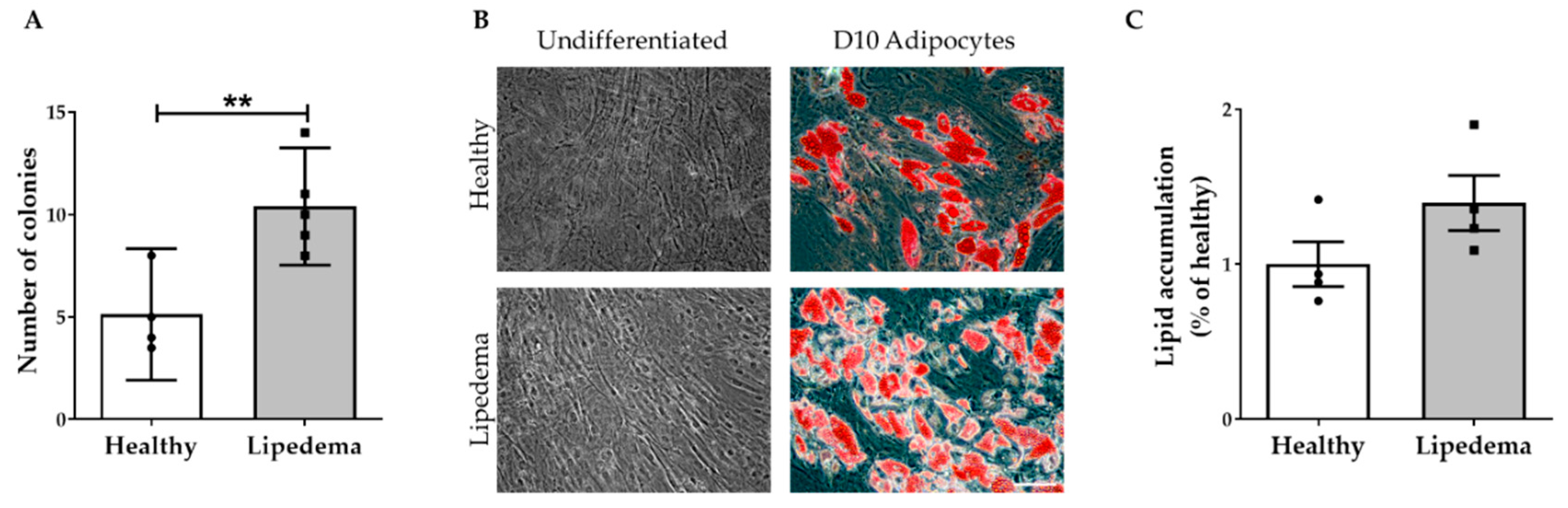
2.1. ASC Spheroid Formation and Stemness Characterization
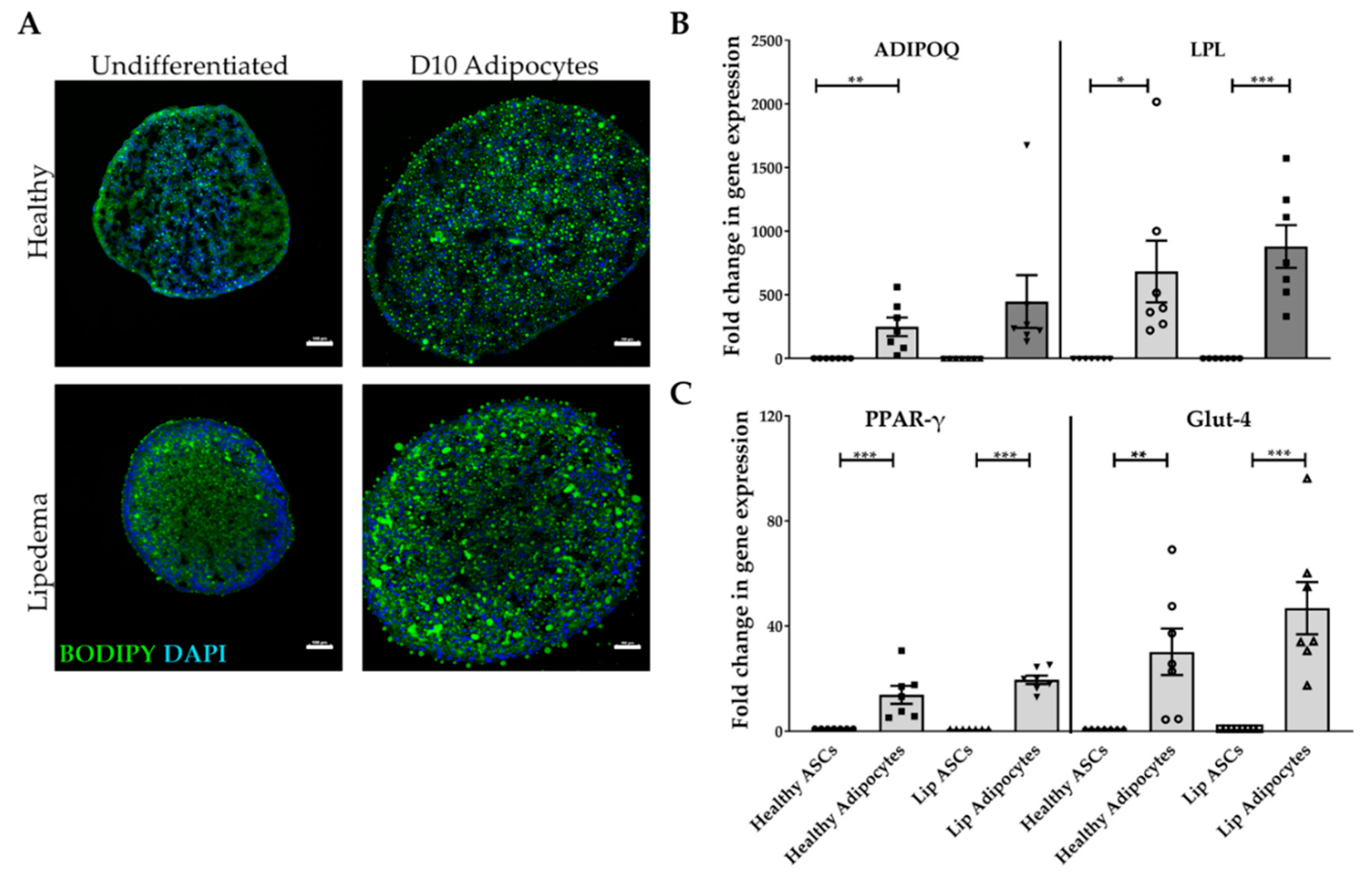
2.2. Adipogenic Differentiation of ASC-Derived Spheroids
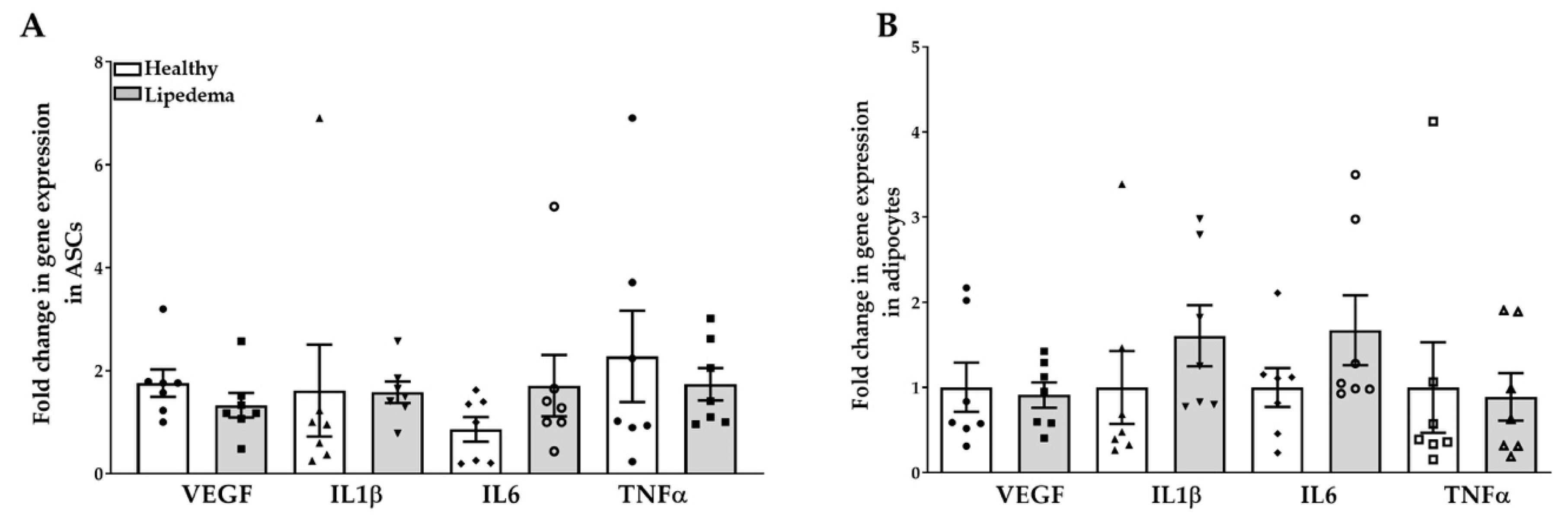
2.3. Levels of Inflammatory Gene Expression in ASC Spheroids
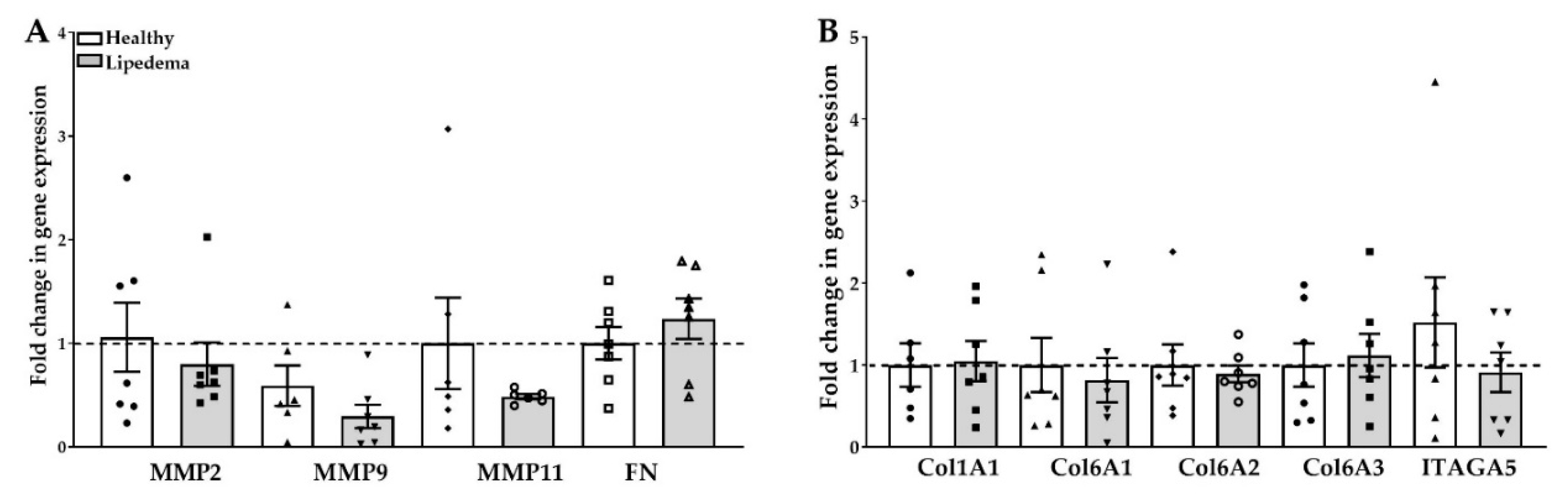
2.4. Fibrosis in ASC Spheroids
2.5. Expression of ECM Components in 3D Differentiated Spheroids
2.6. Retention of Stemness Properties in ASC-Derived from 3D Spheroids
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Formation of ASC Spheroids
4.3. Adipogenic Differentiation of ASC Spheroids
4.4. RNA Isolation and Quantitative Real-Time PCR (qRT-PCR)
4.5. Preparation of Frozen Sections and Immunofluorescence
4.6. Characterizing ASCs Derived from 3D Spheroids
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ASCs | Adipose tissue-derived stem cells |
| AT | Adipose tissue |
| ECM | Extracellular matrix |
| MMP | Matrix metalloproteinase |
| LN | Laminin |
| FN | Fibronectin |
| SVF | Stromal vascular fraction |
| CD | Cluster of differentiation |
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| ADIPOQ | Adiponectin |
| LPL | Lipoprotein lipase |
| PPAR-γ | Peroxisome proliferator-activated receptor gamma |
| Glut4 | Glucose transporter type 4 |
| Lip | Lipedema |
| IL | Interleukin |
| VEGF | Vascular endothelial growth factor |
| TNFα | Tumor necrosis factor |
| Col | Collagen |
| CFU-F | Colony-forming unit fibroblast |
References
- Al-Ghadban, S.; Bunnell, B.A. Adipose Tissue-Derived Stem Cells: Immunomodulatory Effects and Therapeutic Potential. Physiology 2020, 35, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-Derived Stem Cells for Regenerative Medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Ajay, A.K. Fat on sale: Role of adipose-derived stem cells as anti-fibrosis agent in regenerative medicine. Stem Cell Res. Ther. 2015, 6, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strong, A.L.; Neumeister, M.W.; Levi, B. Stem Cells and Tissue Engineering: Regeneration of the Skin and Its Contents. Clin. Plast. Surg. 2017, 44, 635–650. [Google Scholar] [CrossRef] [PubMed]
- Beeson, W.; Woods, E.; Agha, R. Tissue Engineering, Regenerative Medicine, and Rejuvenation in 2010: The Role of Adipose-Derived Stem Cells. Facial Plast. Surg. 2011, 27, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Conese, M.; Annacontini, L.; Carbone, A.; Beccia, E.; Cecchino, L.R.; Parisi, D.; Di Gioia, S.; Lembo, F.; Angiolillo, A.; Mastrangelo, F.; et al. The Role of Adipose-Derived Stem Cells, Dermal Regenerative Templates, and Platelet-Rich Plasma in Tissue Engineering-Based Treatments of Chronic Skin Wounds. Stem Cells Int. 2020, 2020, 1–17. [Google Scholar] [CrossRef]
- Alicka, M.; Major, P.; Wysocki, M.; Marycz, K. Adipose-Derived Mesenchymal Stem Cells Isolated from Patients with Type 2 Diabetes Show Reduced “Stemness” through an Altered Secretome Profile, Impaired Anti-Oxidative Protection, and Mitochondrial Dynamics Deterioration. J. Clin. Med. 2019, 8, 765. [Google Scholar] [CrossRef] [Green Version]
- Serena, C.; Keiran, N.; Ceperuelo-Mallafré, V.; Ejarque, M.; Fradera, R.; Roche, K.; Nuñez-Roa, C.; Vendrell, J.; Fernández-Veledo, S. Obesity and Type 2 Diabetes Alters the Immune Properties of Human Adipose Derived Stem Cells. Stem Cells 2016, 34, 2559–2573. [Google Scholar] [CrossRef]
- Oñate, B.; Vilahur, G.; Camino-López, S.; Díez-Caballero, A.; Ballesta-López, C.; Ybarra, J.; Badimon, L. Stem cells isolated from adipose tissue of obese patients show changes in their transcriptomic profile that indicate loss in stemcellness and increased commitment to an adipocyte-like phenotype. BMC Genom. 2013, 14, 625. [Google Scholar] [CrossRef] [Green Version]
- Bunnell, B.A.; Flaat, M.; Gagliardi, C.; Patel, B.; Ripoll, C. Adipose-derived stem cells: Isolation, expansion and differentiation☆. Methods 2008, 45, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Gimble, J.M.; Bunnell, B.A. Adipose-Derived Stem Cells Methods and Protocols; Humana Press: New York, NY, USA, 2011. [Google Scholar]
- Mineda, K.; Feng, J.; Ishimine, H.; Takada, H.; Doi, K.; Kuno, S.; Kinoshita, K.; Kanayama, K.; Kato, H.; Mashiko, T.; et al. Therapeutic Potential of Human Adipose-Derived Stem/Stromal Cell Microspheroids Prepared by Three-Dimensional Culture in Non-Cross-Linked Hyaluronic Acid Gel. Stem Cells Transl. Med. 2015, 4, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- Mueller-Klieser, W. Three-dimensional cell cultures: From molecular mechanisms to clinical applications. Am. J. Physiol. Physiol. 1997, 273, C1109–C1123. [Google Scholar] [CrossRef]
- Bartosh, T.J.; Ylöstalo, J.H.; Mohammadipoor, A.; Bazhanov, N.; Coble, K.; Claypool, K.; Lee, R.H.; Choi, H.; Prockop, D.J. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc. Natl. Acad. Sci. USA 2010, 107, 13724–13729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrenko, Y.; Syková, E.; Šárka, K. The therapeutic potential of three-dimensional multipotent mesenchymal stromal cell spheroids. Stem Cell Res. Ther. 2017, 8, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, I.S.; Rhie, J.-W.; Kim, S.-H. A novel three-dimensional adipose-derived stem cell cluster for vascular regeneration in ischemic tissue. Cytotherapy 2014, 16, 508–522. [Google Scholar] [CrossRef]
- Cheng, N.-C.; Chen, S.-Y.; Li, J.-R.; Young, T.-H. Short-Term Spheroid Formation Enhances the Regenerative Capacity of Adipose-Derived Stem Cells by Promoting Stemness, Angiogenesis, and Chemotaxis. Stem Cells Transl. Med. 2013, 2, 584–594. [Google Scholar] [CrossRef]
- Frith, J.E.; Thomson, B.; Genever, P.G. Dynamic Three-Dimensional Culture Methods Enhance Mesenchymal Stem Cell Properties and Increase Therapeutic Potential. Tissue Eng. Part C Methods 2010, 16, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Chaicharoenaudomrung, N.; Kunhorm, P.; Noisa, P. Three-dimensional cell culture systems as an in vitro platform for cancer and stem cell modeling. World J. Stem Cells 2019, 11, 1065–1083. [Google Scholar] [CrossRef]
- O’Donnell, B.T.; Al-Ghadban, S.; Ives, C.J.; L’Ecuyer, M.P.; Monjure, T.A.; Romero-Lopez, M.; Bunnell, B.A. Adipose Tissue-Derived Stem Cells Retain Their Adipocyte Differentiation Potential in Three-Dimensional Hydrogels and Bioreactors (†). Biomolecules 2020, 10, 1070. [Google Scholar] [CrossRef]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. ASSAY Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Xie, J.; Yao, Y.; Cun, X.; Lin, S.; Tian, T.; Zhu, B.; Lin, Y. Angiogenesis in a 3D model containing adipose tissue stem cells and endothelial cells is mediated by canonical Wnt signaling. Bone Res. 2017, 5, 17048. [Google Scholar] [CrossRef]
- Morrison, R.J.; Nasser, H.B.; Kashlan, K.N.; Zopf, D.A.; Milner, D.J.; Flanangan, C.L.; Wheeler, M.B.; Green, G.E.; Hollister, S. Co-culture of adipose-derived stem cells and chondrocytes on three-dimensionally printed bioscaffolds for craniofacial cartilage engineering. Laryngoscope 2018, 128, E251–E257. [Google Scholar] [CrossRef]
- Rogan, H.; Ilagan, F.; Tong, X.; Chu, C.R.; Yang, F. Microribbon-hydrogel composite scaffold accelerates cartilage regeneration in vivo with enhanced mechanical properties using mixed stem cells and chondrocytes. Biomaterials 2020, 228, 119579. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ma, J.; Gao, Y.; Dong, M.; Zheng, Z.; Li, Y.; Tan, R.; She, Z.; Yang, L. Epithelial differentiation of human adipose-derived stem cells (hASCs) undergoing three-dimensional (3D) cultivation with collagen sponge scaffold (CSS) via an indirect co-culture strategy. Stem Cell Res. Ther. 2020, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Resch, A.; Wolf, S.; Mann, A.; Weiss, T.; Stetco, A.-L.; Radtke, C. Co-Culturing Human Adipose Derived Stem Cells and Schwann Cells on Spider Silk—A New Approach as Prerequisite for Enhanced Nerve Regeneration. Int. J. Mol. Sci. 2018, 20, 71. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.; Zhang, Y.; Jansen, J.A.; Beucken, J.J.V.D. Effect of monocytes/macrophages on the osteogenic differentiation of adipose-derived mesenchymal stromal cells in 3D co-culture spheroids. Tissue Cell 2017, 49, 461–469. [Google Scholar] [CrossRef]
- Buso, G.; Depairon, M.; Tomson, D.; Raffoul, W.; Vettor, R.; Mazzolai, L. Lipedema: A Call to Action! Obesity 2019, 27, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghadban, S.; Herbst, K.L.; Bunnell, B.A. Lipedema: A Painful Adipose Tissue Disorder. In Adipose Tissue—An Update; Szablewski, L., Ed.; IntechOpen: London, UK, 2019. [Google Scholar]
- Herbst, K.L. Subcutaneous Adipose Tissue Diseases: Dercum Disease, Lipedema, Familial Multiple Lipomatosis, and Madelung Disease; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dungan, K., Grossman, A., Hershman, J.M., Hofland, H.J., Kaltsas, G., et al., Eds.; Endotext: South Dartmouth, MA, USA, 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK552156/ (accessed on 1 September 2020).
- Suga, H.; Araki, J.; Aoi, N.; Kato, H.; Higashino, T.; Yoshimura, K. Adipose tissue remodeling in lipedema: Adipocyte death and concurrent regeneration. J. Cutan. Pathol. 2009, 36, 1293–1298. [Google Scholar] [CrossRef]
- Al-Ghadban, S.; Cromer, W.; Allen, M.; Ussery, C.; Badowski, M.; Harris, D.; Herbst, K.L. Dilated Blood and Lymphatic Microvessels, Angiogenesis, Increased Macrophages, and Adipocyte Hypertrophy in Lipedema Thigh Skin and Fat Tissue. J. Obes. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Felmerer, G.; Stylianaki, A.; Hägerling, R.; Wang, A.; Ströbel, P.; Hollmén, M.; Lindenblatt, N.; Gousopoulos, E. Adipose Tissue Hypertrophy, An Aberrant Biochemical Profile and Distinct Gene Expression in Lipedema. J. Surg. Res. 2020, 253, 294–303. [Google Scholar] [CrossRef]
- Felmerer, G.; Stylianaki, A.; Hollmén, M.; Ströbel, P.; Stepniewski, A.; Wang, A.; Frueh, F.S.; Kim, B.-S.; Giovanoli, P.; Lindenblatt, N.; et al. Increased levels of VEGF-C and macrophage infiltration in lipedema patients without changes in lymphatic vascular morphology. Sci. Rep. 2020, 10, 10947. [Google Scholar] [CrossRef]
- Priglinger, E.; Wurzer, C.; Steffenhagen, C.; Maier, J.; Hofer, V.; Peterbauer, A.; Nuernberger, S.; Redl, H.; Wolbank, S.; Sandhofer, M. The adipose tissue–derived stromal vascular fraction cells from lipedema patients: Are they different? Cytotherapy 2017, 19, 849–860. [Google Scholar] [CrossRef]
- Al-Ghadban, S.; Diaz, Z.T.; Singer, H.J.; Mert, K.B.; Bunnell, B.A. Increase in Leptin and PPAR-γ Gene Expression in Lipedema Adipocytes Differentiated in vitro from Adipose-Derived Stem Cells. Cells 2020, 9, 430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siems, W.; Grune, T.; Voss, P.; Brenke, R. Anti-fibrosclerotic effects of shock wave therapy in lipedema and cellulite. BioFactors 2005, 24, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, S.; Zhang, X.; Pei, M. Significance of Cellular Cross-Talk in Stromal Vascular Fraction of Adipose Tissue in Neovascularization. Arter. Thromb. Vasc. Biol. 2019, 39, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Kubo, Y.; Kaidzu, S.; Nakajima, I.; Takenouchi, K.; Nakamura, F. Organization of extracellular matrix components during differentiation of adipocytes in long-term culture. Vitro Cell Dev. Biol. Anim. 2000, 36, 38–44. [Google Scholar] [CrossRef]
- Mariman, E.C.M.; Wang, P. Adipocyte extracellular matrix composition, dynamics and role in obesity. Cell. Mol. Life Sci. 2010, 67, 1277–1292. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, I.; Aso, H.; Yamaguchi, T.; Ozutsumi, K. Adipose tissue extracellular matrix: Newly organized by adipocytes during differentiation. Differentiation 1998, 63, 193–200. [Google Scholar] [CrossRef]
- Datta, R.; Podolsky, M.J.; Atabai, K. Fat fibrosis: Friend or foe? JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Chavey, C.; Mari, B.; Monthouel, M.-N.; Bonnafous, S.; Anglard, P.; Van Obberghen, E.; Tartare-Deckert, S. Matrix Metalloproteinases Are Differentially Expressed in Adipose Tissue during Obesity and Modulate Adipocyte Differentiation. J. Biol. Chem. 2003, 278, 11888–11896. [Google Scholar] [CrossRef] [Green Version]
- Lin, D.; Chun, T.-H.; Kang, L. Adipose extracellular matrix remodelling in obesity and insulin resistance. Biochem. Pharmacol. 2016, 119, 8–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceccarelli, S.; Pontecorvi, P.; Anastasiadou, E.; Napoli, C.; Marchese, C. Immunomodulatory Effect of Adipose-Derived Stem Cells: The Cutting Edge of Clinical Application. Front. Cell Dev. Biol. 2020, 8, 236. [Google Scholar] [CrossRef]
- Seo, Y.; Shin, T.-H.; Kim, H.-S. Current Strategies to Enhance Adipose Stem Cell Function: An Update. Int. J. Mol. Sci. 2019, 20, 3827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Liu, P.; Chen, L.; Wang, Y.; Wang, Z.; Zhang, B. The effects of spheroid formation of adipose-derived stem cells in a microgravity bioreactor on stemness properties and therapeutic potential. Biomaterials 2015, 41, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Ylöstalo, J.H.; Bartosh, T.J.; Coble, K.; Prockop, D.J. Human Mesenchymal Stem/Stromal Cells Cultured as Spheroids are Self-activated to Produce Prostaglandin E2 that Directs Stimulated Macrophages into an Anti-inflammatory Phenotype. Stem Cells 2012, 30, 2283–2296. [Google Scholar] [CrossRef] [Green Version]
- Pennings, S.; Liu, K.J.; Qian, H. The Stem Cell Niche: Interactions between Stem Cells and Their Environment. Stem Cells Int. 2018, 2018, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Dorst, N.; Oberringer, M.; Grässer, U.; Pohlemann, T.; Metzger, W. Analysis of cellular composition of co-culture spheroids. Ann. Anat. Anat. Anz. 2014, 196, 303–311. [Google Scholar] [CrossRef]
- Metzger, W.; Sossong, D.; Bächle, A.; Pütz, N.; Wennemuth, G.; Pohlemann, T.; Oberringer, M. The liquid overlay technique is the key to formation of co-culture spheroids consisting of primary osteoblasts, fibroblasts and endothelial cells. Cytotherapy 2011, 13, 1000–1012. [Google Scholar] [CrossRef]
- Hoefner, C.; Muhr, C.; Horder, H.; Wiesner, M.; Wittmann, K.; Lukaszyk, D.; Radeloff, K.; Winnefeld, M.; Becker, M.; Blunk, T.; et al. Human Adipose-Derived Mesenchymal Stromal/Stem Cell Spheroids Possess High Adipogenic Capacity and Acquire an Adipose Tissue-like Extracellular Matrix Pattern. Tissue Eng. Part A 2020, 26, 915–926. [Google Scholar] [CrossRef]
- Nicolas, J.; Magli, S.; Rabbachin, L.; Sampaolesi, S.; Nicotra, F.; Russo, L. 3D Extracellular Matrix Mimics: Fundamental Concepts and Role of Materials Chemistry to Influence Stem Cell Fate. Biomacromolecules 2020, 21, 1968–1994. [Google Scholar] [CrossRef]
- Gattazzo, F.; Urciuolo, A.; Bonaldo, P. Extracellular matrix: A dynamic microenvironment for stem cell niche. Biochim. Biophys. Acta (BBA) Gen. Subj. 2014, 1840, 2506–2519. [Google Scholar] [CrossRef]
- Adapala, V.J.; Adedokun, S.A.; Considine, R.V.; Ajuwon, K.M. Acute inflammation plays a limited role in the regulation of adipose tissue COL1A1 protein abundance. J. Nutr. Biochem. 2012, 23, 567–572. [Google Scholar] [CrossRef]
- Gesta, S.; Guntur, K.; Majumdar, I.D.; Akella, S.; Vishnudas, V.K.; Sarangarajan, R.; Narain, N.R. Reduced expression of collagen VI alpha 3 (COL6A3) confers resistance to inflammation-induced MCP1 expression in adipocytes. Obesity 2016, 24, 1695–1703. [Google Scholar] [CrossRef]
- Arcidiacono, B.; Chiefari, E.; Laria, A.E.; Messineo, S.; Bilotta, F.L.; Britti, D.; Foti, D.P.; Foryst-Ludwig, A.; Kintscher, U.; Brunetti, A. Expression of matrix metalloproteinase-11 is increased under conditions of insulin resistance. World J. Diabetes 2017, 8, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Dali-Youcef, N.; Hnia, K.; Blaise, S.; Messaddeq, N.; Blanc, S.; Postic, C.; Rio, M.C. Matrix metalloproteinase 11 protects from diabesity and promotes metabolic switch. Sci. Rep. 2016, 6, 25140. [Google Scholar] [CrossRef]
- Kapur, S.K.; Wang, X.; Shang, H.; Yun, S.; Li, X.; Feng, G.; Khurgel, M.; Katz, A.J. Human adipose stem cells maintain proliferative, synthetic and multipotential properties when suspension cultured as self-assembling spheroids. Biofabrication 2012, 4, 025004. [Google Scholar] [CrossRef] [Green Version]

| Characteristics | Healthy | Lipedema |
|---|---|---|
| N | 8 | 8 |
| Sex | Female | Female |
| Age | 47.86 ± 2.28 | 44.57 ± 3.99 |
| BMI | 27.30 ± 1.042 | 30.43 ± 1.163 |
| Stage 1 | − | 12% |
| Stage 2 | − | 63% |
| Stage 3 | − | 25% |
| Name | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| IL-6 | GTAGCCGCCCCACACAGACAGCC | GCCATCTTTGGAAGGTTC |
| LPL | GAGATTTCTCTGTATGGCACC | CTGCAAATGAGACACTTTCTC |
| IL-1 β | TCCCCAGCCCTTTTGTTGA | TTAGAACCA AATGTGGCCGTG |
| VEGF | AGGCCCACAGGGATTTTCTT | ATCAAACCTCACCAAGGCCA |
| Glut-4 | AGC AGC TCT CTG GCA TCA AT | CAA TGG AGA CGT AGC ACA TG |
| Leptin | GAAGACCACATCCACACACG | AGCTCAGCCAGACCCATCTA |
| PPAR-γ | AGGCGAGGGCGATCTTG | CCCATCATTAAGGAATTCATGTCATA |
| GAPDH | CGCTGAGTACGTCGTGGAGTC | GCAGGAGGCATTGCAGATGA |
| TNF-α | GAG CCA GCT CCC TCT ATT TA | GGG AAC AGC CTA TTG TTC AG |
| Oct4 | GACAGGGGGAGGGGAGGAGCTAGG | CTTCCCTCCAACCAGTTGCCCCAAAC |
| Nanog | AGTCCCAAAGGCAAACAACCCACTTC | TGCTGGAGGCTGAGGTATTTCTGTCTC |
| Col1 A1 | CATGTTCAGCTTTGTGGACCTC, | AGGTGATTGGTGGGATGTCTT |
| Col6 A1 | GACCTCGGACCTGTTGGGTAC | TACCCCATCTCCCCCTTCAC |
| Col6 A2 | CTGCGACAAGCCACAGCAG | GGGCACACGATCTGAGGGT |
| Col6 A3 | GAGCAGCTTGACAACATTGCC | GCCCAGAGCACTTGCAGG |
| ITAGA5 | GACAGGGAAGAGCGGGCACTATGG | GTCCCTTCCCGGCCGGTAAAACTC |
| MMP-2 | TTGACGGTAAGGACGGACTC | ACTTGCAGTACTCCCCATCG |
| MMP-9 | TTGACAGCGACAAGAAGTGG | GCCATTCACGTCGTCCTTAT |
| MMP-11 | ATTTGGTTCTTCCAAGGTGCTCAGT | CCTCGGAAGAAGTAGATCTTGTTCT |
| ADIPOQ | AACATGCCCATTCGCTTTAC | AGAGGCTGACCTTCACATCC |
| Fibronectin | TTCCTTGCTGGTATCATGGCA | TATTCGGTTCCCGGTTCCA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ghadban, S.; Pursell, I.A.; Diaz, Z.T.; Herbst, K.L.; Bunnell, B.A. 3D Spheroids Derived from Human Lipedema ASCs Demonstrated Similar Adipogenic Differentiation Potential and ECM Remodeling to Non-Lipedema ASCs In Vitro. Int. J. Mol. Sci. 2020, 21, 8350. https://doi.org/10.3390/ijms21218350
Al-Ghadban S, Pursell IA, Diaz ZT, Herbst KL, Bunnell BA. 3D Spheroids Derived from Human Lipedema ASCs Demonstrated Similar Adipogenic Differentiation Potential and ECM Remodeling to Non-Lipedema ASCs In Vitro. International Journal of Molecular Sciences. 2020; 21(21):8350. https://doi.org/10.3390/ijms21218350
Chicago/Turabian StyleAl-Ghadban, Sara, India A. Pursell, Zaidmara T. Diaz, Karen L. Herbst, and Bruce A. Bunnell. 2020. "3D Spheroids Derived from Human Lipedema ASCs Demonstrated Similar Adipogenic Differentiation Potential and ECM Remodeling to Non-Lipedema ASCs In Vitro" International Journal of Molecular Sciences 21, no. 21: 8350. https://doi.org/10.3390/ijms21218350





