Chitosan Oligosaccharides Suppress Nuclear Factor-Kappa B Activation and Ameliorate Experimental Autoimmune Uveoretinitis in Mice
Abstract
:1. Introduction
2. Results
2.1. COS Significantly Decreased EAU in Mice
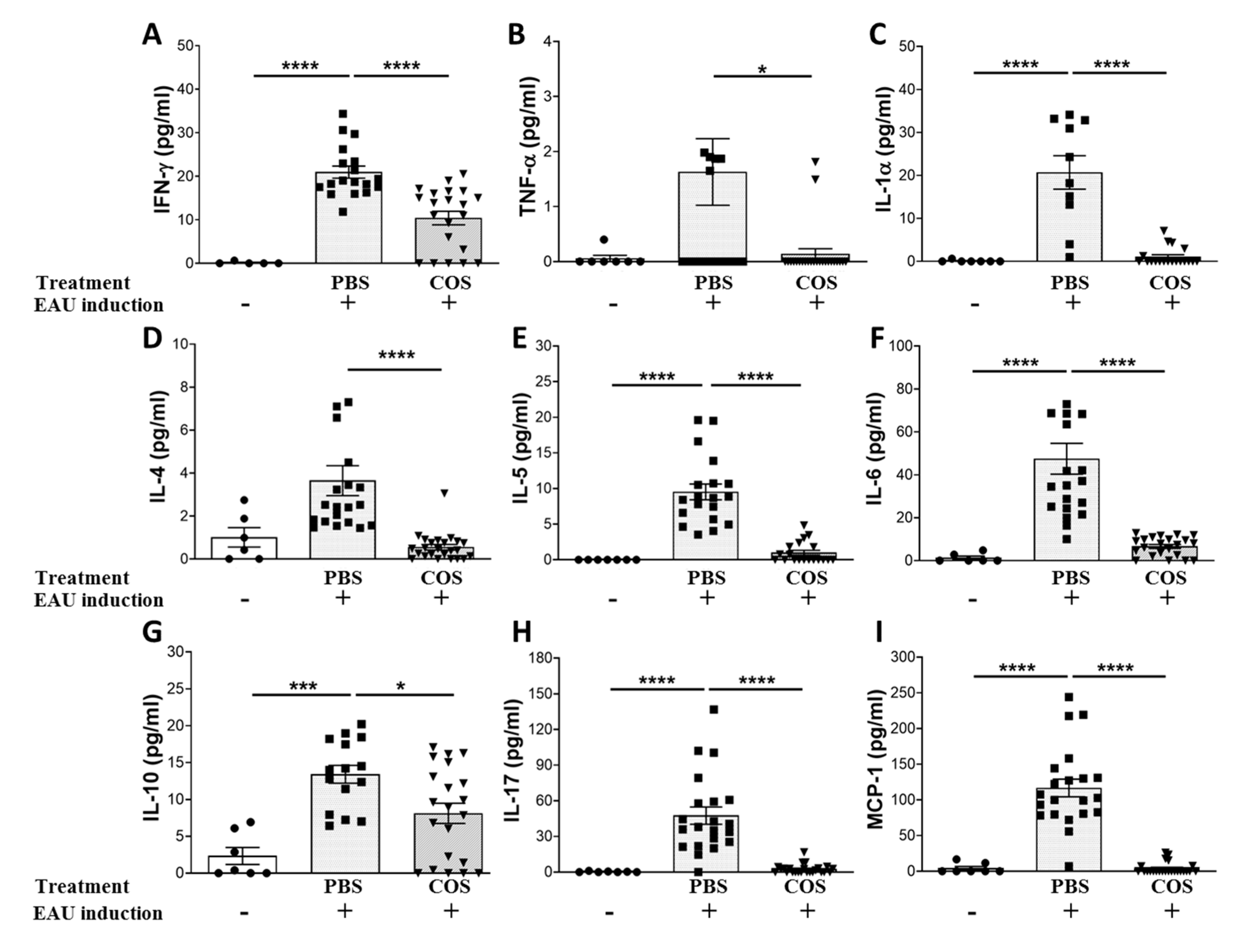
2.2. The Influence of COS Treatment on Inflammatory Mediators in the Retinas of EAU Mice
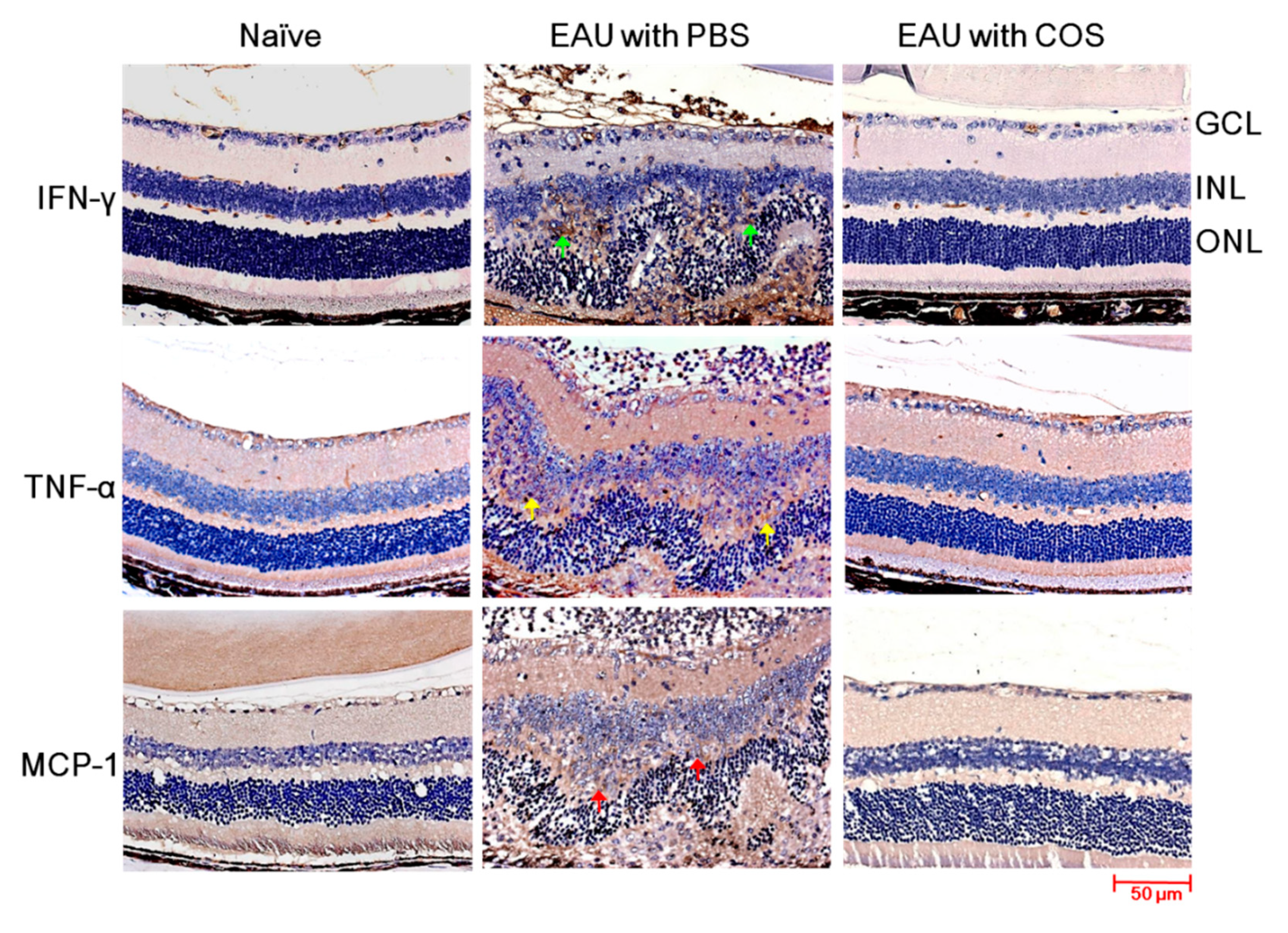
2.3. Decreased Expression of IFN-γ, TNF-α and MCP-1 in the Retinas of COS-Treated EAU Mice
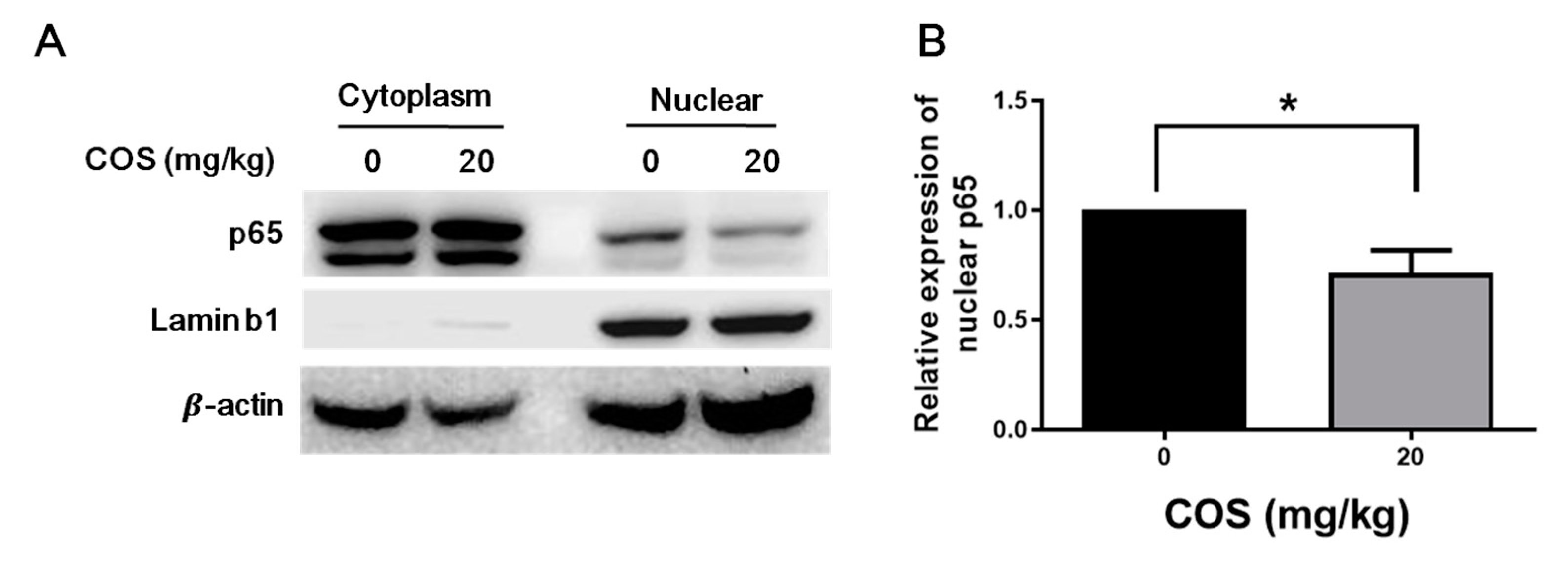
2.4. COS Suppresses NF-κB Activation in Retinas of Mice with EAU Induction
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Induction and Treatment of EAU
4.3. Histopathological Evaluation
4.4. Immunohistochemical and Immunofluorescence Staining of Inflammatory Mediators and p65 in Mouse Retinas
4.5. Luminex Assay
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| COS | Chitosan oligosaccharides |
| PBS | Phosphate-buffered saline |
| ROS | Reactive oxygen species |
| EAU | Experimental autoimmune uveoretinitis |
| IRBP | Interphotoreceptor retinoid-binding protein |
| IL | Interleukin |
| IFN | Interferon |
| TNF | Tumor necrosis factor |
| MCP | Monocyte chemoattractant protein |
References
- Tsirouki, T.; Dastiridou, A.; Symeonidis, C.; Tounakaki, O.; Brazitikou, I.; Kalogeropoulos, C.; Androudi, S. A focus on the epidemiology of uveitis. Ocul. Immunol. Inflamm. 2018, 26, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Miserocchi, E.; Fogliato, G.; Modorati, G.; Bandello, F. Review on the worldwide epidemiology of uveitis. Eur. J. Ophthalmol. 2013, 23, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.T.; Yao, T.C.; Huang, J.L.; Yeh, K.W.; Hwang, Y.S. Clinical manifestations in uveitis patients with and without rheumatic disease in a Chinese population in Taiwan. J. Microbiol. Immunol. Infect. 2017, 50, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Chuang, C.T.; Chu, M.Y.; Sheu, S.J. Patterns and etiologies of uveitis at a tertiary referral center in Taiwan. Ocul. Immunol. Inflamm. 2017, 25, S31–S38. [Google Scholar] [CrossRef]
- Jabs, D.A.; Rosenbaum, J.T.; Foster, C.S.; Holland, G.N.; Jaffe, G.J.; Louie, J.S.; Nussenblatt, R.B.; Stiehm, E.R.; Tessler, H.; Van Gelder, R.N.; et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: Recommendations of an expert panel. Am. J. Ophthalmol. 2000, 130, 492–513. [Google Scholar] [CrossRef]
- Gasparin, F.; Takahashi, B.S.; Scolari, M.R.; Gasparin, F.; Pedral, L.S.; Damico, F.M. Experimental models of autoimmune inflammatory ocular diseases. Arq. Bras. Oftalmol. 2012, 75, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Horai, R.; Caspi, R.R. Cytokines in autoimmune uveitis. J. Interferon Cytokine Res. 2011, 31, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Kitamei, H.; Iwabuchi, K.; Namba, K.; Yoshida, K.; Yanagawa, Y.; Kitaichi, N.; Kitamura, M.; Ohno, S.; Onoe, K. Amelioration of experimental autoimmune uveoretinitis (EAU) with an inhibitor of nuclear factor-kappaB (NF-kappaB), pyrrolidine dithiocarbamate. J. Leukoc. Biol. 2006, 79, 1193–1201. [Google Scholar] [CrossRef]
- Iwata, D.; Kitaichi, N.; Miyazaki, A.; Iwabuchi, K.; Yoshida, K.; Namba, K.; Ozaki, M.; Ohno, S.; Umezawa, K.; Yamashita, K.; et al. Amelioration of experimental autoimmune uveoretinitis with nuclear factor-{kappa}B Inhibitor dehydroxy methyl epoxyquinomicin in mice. Invest. Ophthalmol. Vis. Sci 2010, 51, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.E.; Pepple, K.L. Cytokines in uveitis. Curr. Opin. Ophthalmol. 2018, 29, 267–274. [Google Scholar] [CrossRef]
- Hsu, S.M.; Yang, C.H.; Shen, F.H.; Chen, S.H.; Lin, C.J.; Shieh, C.C. Proteasome inhibitor bortezomib suppresses nuclear factor-kappa B activation and ameliorates eye inflammation in experimental autoimmune uveitis. Mediat. Inflamm. 2015, 2015, 847373. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.M.; Yang, C.H.; Teng, Y.T.; Tsai, H.Y.; Lin, C.Y.; Lin, C.J.; Shieh, C.C.; Chen, S.H. Suppression of the reactive oxygen response alleviates experimental autoimmune uveitis in mice. Int. J. Mol. Sci. 2020, 21, 3261. [Google Scholar] [CrossRef] [PubMed]
- Fang, I.M.; Yang, C.H.; Yang, C.M. Chitosan oligosaccharides attenuate ocular inflammation in rats with experimental autoimmune anterior uveitis. Mediat. Inflamm. 2014, 2014, 827847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muanprasat, C.; Chatsudthipong, V. Chitosan oligosaccharide: Biological activities and potential therapeutic applications. Pharmacol. Ther. 2017, 170, 80–97. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.K. Neuroprotective properties of chitosan and its derivatives. Mar. Drugs 2010, 8, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Du, Y.; Xiao, L.; Li, Z.; Gao, X. Enzymic preparation of water-soluble chitosan and their antitumor activity. Int. J. Biol. Macromol. 2002, 31, 111–117. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, S.Q.; Du, Y.M.; Peng, H.; Sun, L.P. Carboxymethyl-chitosan protects rabbit chondrocytes from interleukin-1beta-induced apoptosis. Eur. J. Pharmacol. 2006, 541, 1–8. [Google Scholar] [CrossRef]
- Joodi, G.; Ansari, N.; Khodagholi, F. Chitooligosaccharide-mediated neuroprotection is associated with modulation of Hsps expression and reduction of MAPK phosphorylation. Int. J. Biol. Macromol. 2011, 48, 726–735. [Google Scholar] [CrossRef]
- Fang, I.M.; Yang, C.H.; Yang, C.M.; Chen, M.S. Chitosan oligosaccharides attenuates oxidative-stress related retinal degeneration in rats. PLoS ONE 2013, 8, e77323. [Google Scholar] [CrossRef] [PubMed]
- Fang, I.M.; Yang, C.M.; Yang, C.H. Chitosan oligosaccharides prevented retinal ischemia and reperfusion injury via reduced oxidative stress and inflammation in rats. Exp. Eye Res. 2015, 130, 38–50. [Google Scholar] [CrossRef]
- Zhu, L.; Li, R.; Jiao, S.; Wei, J.; Yan, Y.; Wang, Z.A.; Li, J.; Du, Y. Blood-brain barrier permeable chitosan oligosaccharides interfere with beta-amyloid aggregation and alleviate beta-amyloid protein mediated neurotoxicity and neuroinflammation in a dose- and degree of polymerization-dependent manner. Mar. Drugs 2020, 18, 488. [Google Scholar] [CrossRef]
- Ojeda-Hernandez, D.D.; Canales-Aguirre, A.A.; Matias-Guiu, J.; Gomez-Pinedo, U.; Mateos-Diaz, J.C. Potential of chitosan and its derivatives for biomedical applications in the central nervous system. Front. Bioeng. Biotechnol. 2020, 8, 389. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. NF-kappaB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal. Transduct. Target. Ther. 2017, 2. [Google Scholar] [CrossRef] [Green Version]
- Levy-Clarke, G.; Jabs, D.A.; Read, R.W.; Rosenbaum, J.T.; Vitale, A.; Van Gelder, R.N. Expert panel recommendations for the use of anti-tumor necrosis factor biologic agents in patients with ocular inflammatory disorders. Ophthalmology 2014, 121, 785–796.e3. [Google Scholar] [CrossRef]
- Sanchez-Cano, D.; Callejas-Rubio, J.L.; Ruiz-Villaverde, R.; Rios-Fernandez, R.; Ortego-Centeno, N. Off-label uses of anti-TNF therapy in three frequent disorders: Behcet’s disease, sarcoidosis, and noninfectious uveitis. Mediat. Inflamm. 2013, 2013, 286857. [Google Scholar] [CrossRef]
- Kohen, R.; Nyska, A. Oxidation of biological systems: Oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. 2002, 30, 620–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gracy, R.W.; Talent, J.M.; Kong, Y.; Conrad, C.C. Reactive oxygen species: The unavoidable environmental insult? Mutat. Res. 1999, 428, 17–22. [Google Scholar] [CrossRef]
- Luo, T.; Xia, Z. A small dose of hydrogen peroxide enhances tumor necrosis factor-alpha toxicity in inducing human vascular endothelial cell apoptosis: Reversal with propofol. Anesth. Analg. 2006, 103, 110–116, table of contents. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Bai, X.F.; Du, Y.G. Chitosan oligosaccharides protect mice from LPS challenge by attenuation of inflammation and oxidative stress. Int. Immunopharmacol. 2011, 11, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.J.; Moon, M.E.; Park, H.S.; Kim, H.W.; Im, S.Y.; Lee, J.H.; Kim, Y.H. Effects of chitosan oligosaccharide (COS) on the glycerol-induced acute renal failure in vitro and in vivo. Food Chem. Toxicol. 2008, 46, 710–716. [Google Scholar] [CrossRef]
- Kumar, M.N.; Muzzarelli, R.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef]
- Lee, D.J.; Taylor, A.W. Both MC5r and A2Ar are required for protective regulatory immunity in the spleen of post-experimental autoimmune uveitis in mice. J. Immunol. 2013, 191, 4103–4111. [Google Scholar] [CrossRef] [Green Version]
- Namba, K.; Kitaichi, N.; Nishida, T.; Taylor, A.W. Induction of regulatory T cells by the immunomodulating cytokines alpha-melanocyte-stimulating hormone and transforming growth factor-beta2. J. Leukoc. Biol. 2002, 72, 946–952. [Google Scholar]



| Treatment | Incidence of Mice with Clinical Score ≧ 2 | Mean Peak Disease Score |
|---|---|---|
| PBS | 32/41 | 2.30 ± 0.16 |
| COS (L) b | 13/30 | 1.20 ± 0.18 2 |
| COS (H) c | 0/27 1 | 0.10 ± 0.06 3,4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, S.-M.; Yang, C.-H.; Tsai, H.-Y.; Lin, C.-J.; Fang, Y.-H.; Shieh, C.-C.; Chen, S.-H. Chitosan Oligosaccharides Suppress Nuclear Factor-Kappa B Activation and Ameliorate Experimental Autoimmune Uveoretinitis in Mice. Int. J. Mol. Sci. 2020, 21, 8326. https://doi.org/10.3390/ijms21218326
Hsu S-M, Yang C-H, Tsai H-Y, Lin C-J, Fang Y-H, Shieh C-C, Chen S-H. Chitosan Oligosaccharides Suppress Nuclear Factor-Kappa B Activation and Ameliorate Experimental Autoimmune Uveoretinitis in Mice. International Journal of Molecular Sciences. 2020; 21(21):8326. https://doi.org/10.3390/ijms21218326
Chicago/Turabian StyleHsu, Sheng-Min, Chang-Hao Yang, Hsien-Yang Tsai, Chia-Jhen Lin, Yi-Hsuan Fang, Chi-Chang Shieh, and Shun-Hua Chen. 2020. "Chitosan Oligosaccharides Suppress Nuclear Factor-Kappa B Activation and Ameliorate Experimental Autoimmune Uveoretinitis in Mice" International Journal of Molecular Sciences 21, no. 21: 8326. https://doi.org/10.3390/ijms21218326





