Effects of Estrogen Receptor and Wnt Signaling Activation on Mechanically Induced Bone Formation in a Mouse Model of Postmenopausal Bone Loss
Abstract
1. Introduction
2. Results
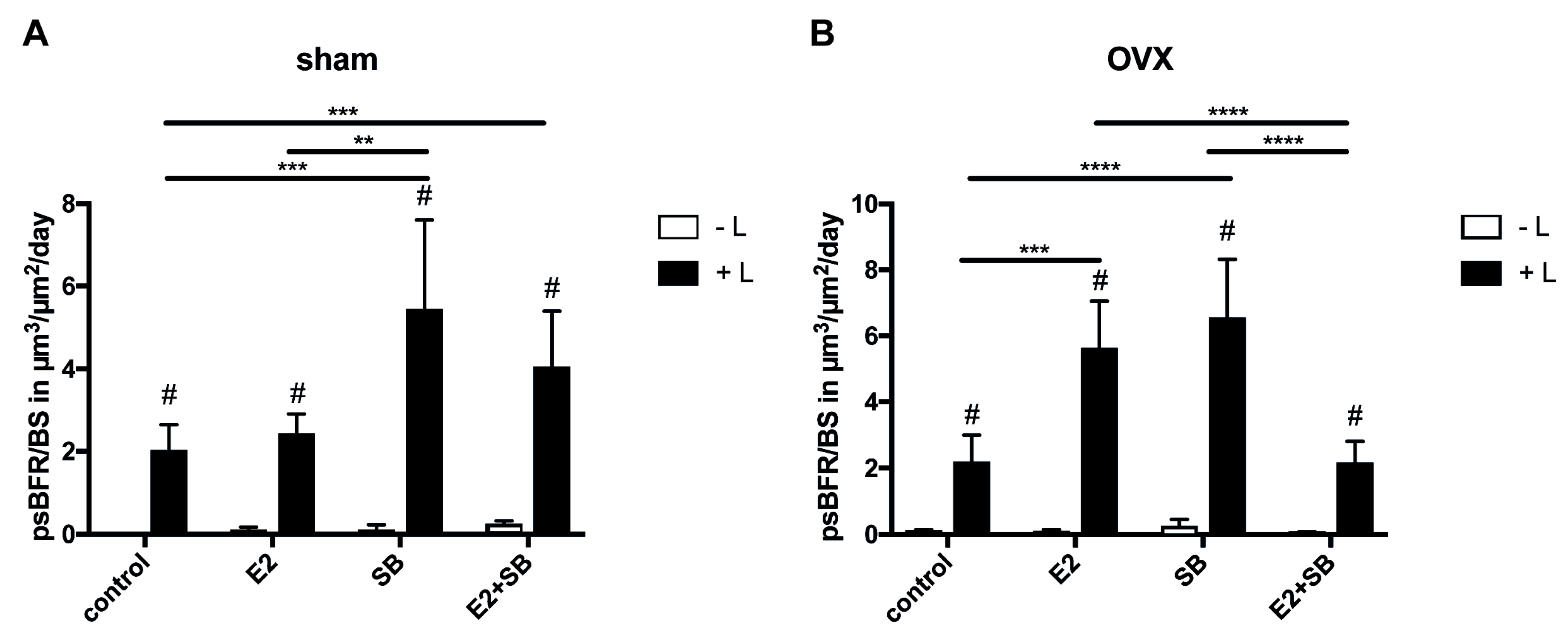
2.1. Effects of Ovariectomy (OVX) and Estradiol Treatment
2.2. Mechanical Loading Increases Cortical Bone Formation and Activates ER or β-Catenin Signaling in Ovariectomized Mice
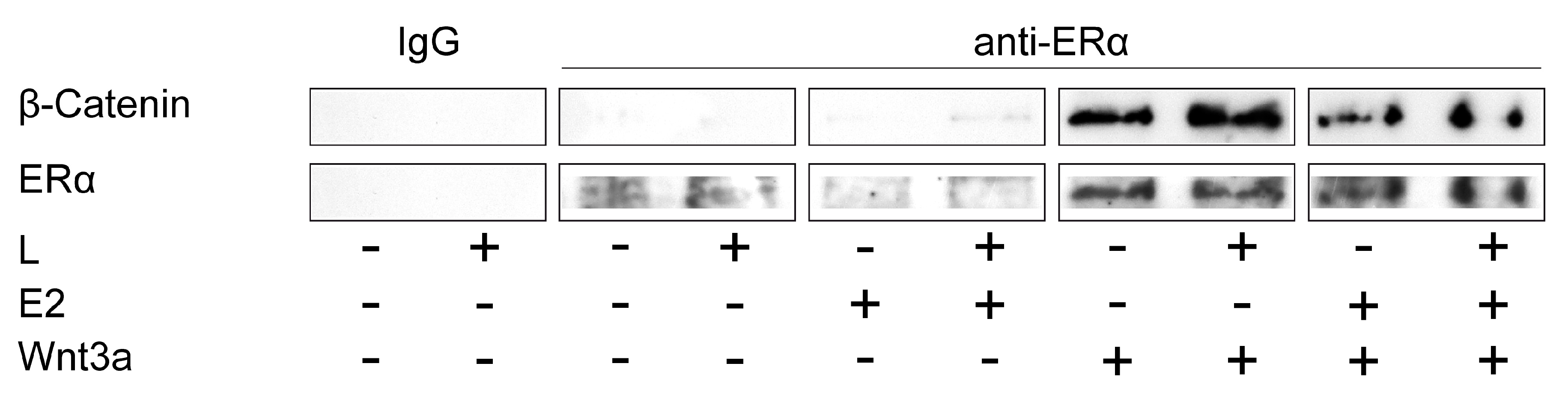
2.3. ERα and Wnt/β-Catenin Signaling Interact in the Mechanically Induced Response of Preosteoblastic Cells
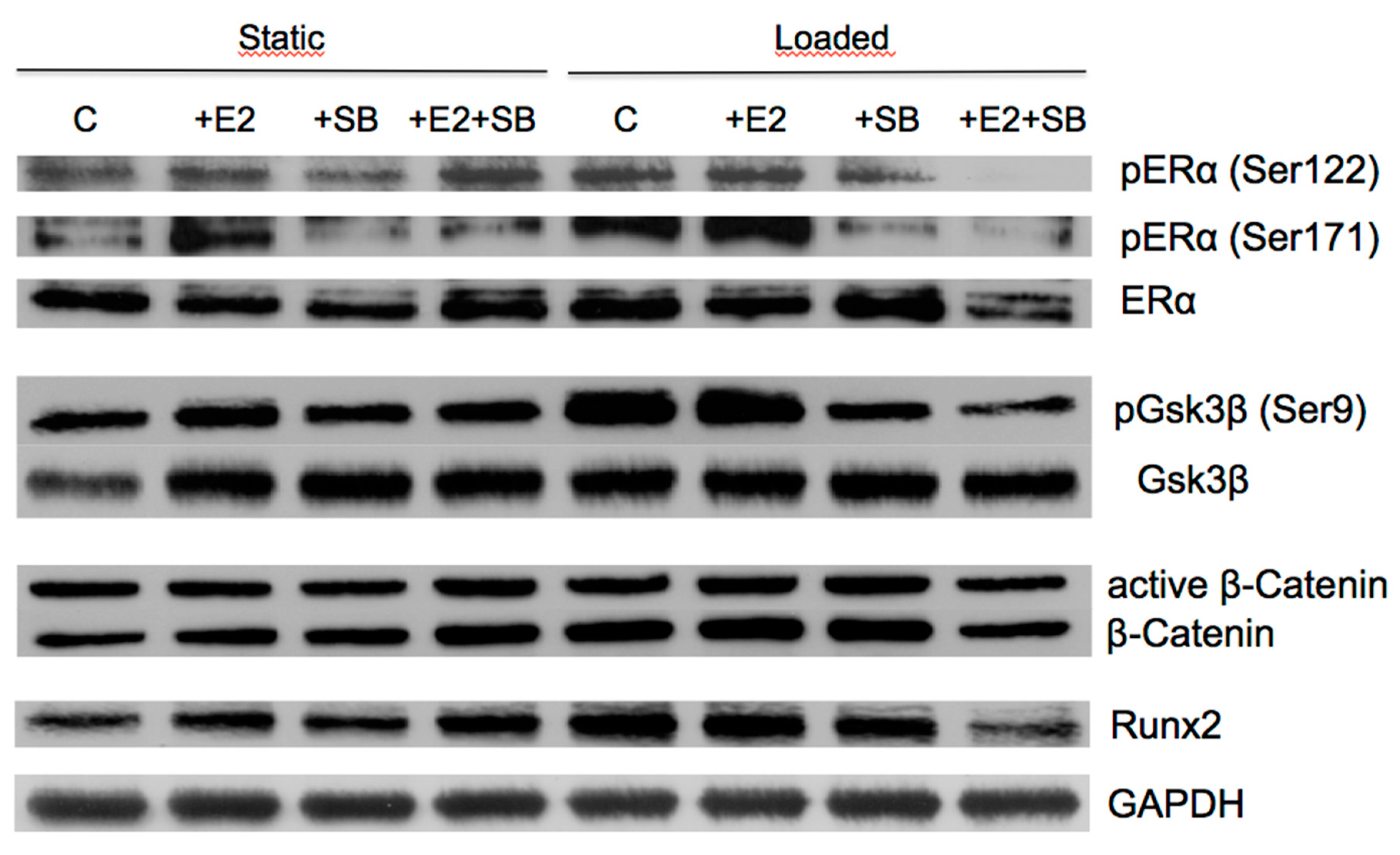
2.4. Mechanical Loading Increases Wnt and ERα Target Protein Expression and Activates ERα or β-Catenin Signaling in Preosteoblastic Cells
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Ex Vivo Calibration of the Ulna Loading Regimen and Mechanical Loading In Vivo
4.3. Ovariectomy and Estradiol and Gsk-3β Inhibitor Treatments
4.4. Histomorphometry and Bone Microstructure Measurements
4.5. Mechanical Loading In Vitro
4.6. Co-Immunoprecipitation
4.7. Western Blot Analysis
4.8. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harada, S.; Rodan, G.A. Control of osteoblast function and regulation of bone mass. Nature 2003, 423, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Frost, H.M. A 2003 update of bone physiology and Wolff’s Law for clinicians. Angle Orthod. 2004, 74, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Hadjidakis, D.J.; Androulakis, I.I. Bone remodeling. Ann. N. Y. Acad. Sci. 2006, 1092, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Riggs, B.L.; Khosla, S.; Melton, L.J., III. Sex steroids and the construction and conservation of the adult skeleton. Endocr. Rev. 2002, 23, 279–302. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Jessop, H.; Suswillo, R.; Zaman, G.; Lanyon, L. Endocrinology: Bone adaptation requires oestrogen receptor-alpha. Nature 2003, 424, 389. [Google Scholar] [CrossRef]
- Manolagas, S.C.; O’Brien, C.A.; Almeida, M. The role of estrogen and androgen receptors in bone health and disease. Nat. Rev. Endocrinol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Heldring, N.; Pike, A.; Andersson, S.; Matthews, J.; Cheng, G.; Hartman, J.; Tujague, M.; Strom, A.; Treuter, E.; Warner, M.; et al. Estrogen receptors: How do they signal and what are their targets. Physiol. Rev. 2007, 87, 905–931. [Google Scholar] [CrossRef] [PubMed]
- Prossnitz, E.R.; Barton, M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat. Rev. Endocrinol. 2011, 7, 715–726. [Google Scholar] [CrossRef]
- Khosla, S.; Oursler, M.J.; Monroe, D.G. Estrogen and the skeleton. Trends Endocrinol. Metab. 2012, 23, 576–581. [Google Scholar] [CrossRef]
- Saxon, L.K.; Galea, G.; Meakin, L.; Price, J.; Lanyon, L.E. Estrogen receptors alpha and beta have different gender-dependent effects on the adaptive responses to load bearing in cancellous and cortical bone. Endocrinology 2012, 153, 2254–2266. [Google Scholar] [CrossRef]
- Windahl, S.H.; Saxon, L.; Borjesson, A.E.; Lagerquist, M.K.; Frenkel, B.; Henning, P.; Lerner, U.H.; Galea, G.L.; Meakin, L.B.; Engdahl, C.; et al. Estrogen receptor-alpha is required for the osteogenic response to mechanical loading in a ligand-independent manner involving its activation function 1 but not 2. J. Bone Min. Res. 2013, 28, 291–301. [Google Scholar] [CrossRef]
- Little, R.D.; Carulli, J.P.; Del Mastro, R.G.; Dupuis, J.; Osborne, M.; Folz, C.; Manning, S.P.; Swain, P.M.; Zhao, S.C.; Eustace, B.; et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am. J. Hum. Genet. 2002, 70, 11–19. [Google Scholar] [CrossRef]
- Gong, Y.; Slee, R.B.; Fukai, N.; Rawadi, G.; Roman-Roman, S.; Reginato, A.M.; Wang, H.; Cundy, T.; Glorieux, F.H.; Lev, D.; et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 2001, 107, 513–523. [Google Scholar] [CrossRef]
- Regard, J.B.; Zhong, Z.; Williams, B.O.; Yang, Y. Wnt signaling in bone development and disease: Making stronger bone with Wnts. Cold Spring Harb. Perspect. Biol. 2012, 4. [Google Scholar] [CrossRef]
- Baron, R.; Kneissel, M. WNT signaling in bone homeostasis and disease: From human mutations to treatments. Nat. Med. 2013, 19, 179–192. [Google Scholar] [CrossRef]
- Zhao, L.; Shim, J.W.; Dodge, T.R.; Robling, A.G.; Yokota, H. Inactivation of Lrp5 in osteocytes reduces young’s modulus and responsiveness to the mechanical loading. Bone 2013, 54, 35–43. [Google Scholar] [CrossRef]
- Javaheri, B.; Stern, A.; Lara, N.; Dallas, M.; Zhao, H.; Liu, Y.; Bonewald, L.F.; Johnson, M.L. Deletion of a single beta-catenin allele in osteocytes abolishes the bone anabolic response to loading. J. Bone Min. Res. 2013. [Google Scholar] [CrossRef]
- Wu, S.M.; Shih, L.H.; Lee, J.Y.; Shen, Y.J.; Lee, H.H. Estrogen enhances activity of Wnt signaling during osteogenesis by inducing Fhl1 expression. J. Cell. Biochem. 2015, 116, 1419–1430. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, V.J.; Muzylak, M.; Sunters, A.; Zaman, G.; Saxon, L.K.; Price, J.S.; Lanyon, L.E. Wnt/beta-catenin signaling is a component of osteoblastic bone cell early responses to load-bearing and requires estrogen receptor alpha. J. Biol. Chem. 2007, 282, 20715–20727. [Google Scholar] [CrossRef]
- Lau, K.H.; Kapur, S.; Kesavan, C.; Baylink, D.J. Up-regulation of the Wnt, estrogen receptor, insulin-like growth factor-I, and bone morphogenetic protein pathways in C57BL/6J osteoblasts as opposed to C3H/HeJ osteoblasts in part contributes to the differential anabolic response to fluid shear. J. Biol. Chem. 2006, 281, 9576–9588. [Google Scholar] [CrossRef]
- Liedert, A.; Wagner, L.; Seefried, L.; Ebert, R.; Jakob, F.; Ignatius, A. Estrogen receptor and Wnt signaling interact to regulate early gene expression in response to mechanical strain in osteoblastic cells. Biochem. Biophys. Res. Commun. 2010, 394, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Lannigan, D.A. Estrogen receptor phosphorylation. Steroids 2003, 68, 1–9. [Google Scholar] [CrossRef]
- Li, C.Y.; Schaffler, M.B.; Wolde-Semait, H.T.; Hernandez, C.J.; Jepsen, K.J. Genetic background influences cortical bone response to ovariectomy. J. Bone Min. Res. 2005, 20, 2150–2158. [Google Scholar] [CrossRef]
- Sugiyama, T.; Galea, G.L.; Lanyon, L.E.; Price, J.S. Mechanical loading-related bone gain is enhanced by tamoxifen but unaffected by fulvestrant in female mice. Endocrinology 2010, 151, 5582–5590. [Google Scholar] [CrossRef] [PubMed]
- Hagino, H.; Raab, D.M.; Kimmel, D.B.; Akhter, M.P.; Recker, R.R. Effect of ovariectomy on bone response to in vivo external loading. J. Bone Min. Res. 1993, 8, 347–357. [Google Scholar] [CrossRef]
- Cheng, M.Z.; Zaman, G.; Rawlinson, S.C.; Suswillo, R.F.; Lanyon, L.E. Mechanical loading and sex hormone interactions in organ cultures of rat ulna. J. Bone Min. Res. 1996, 11, 502–511. [Google Scholar] [CrossRef]
- Almeida, M.; Iyer, S.; Martin-Millan, M.; Bartell, S.M.; Han, L.; Ambrogini, E.; Onal, M.; Xiong, J.; Weinstein, R.S.; Jilka, R.L.; et al. Estrogen receptor-alpha signaling in osteoblast progenitors stimulates cortical bone accrual. J. Clin. Investig. 2013, 123, 394–404. [Google Scholar] [CrossRef]
- Haffner-Luntzer, M.; Kovtun, A.; Lackner, I.; Modinger, Y.; Hacker, S.; Liedert, A.; Tuckermann, J.; Ignatius, A. Estrogen receptor alpha- (ERalpha), but not ERbeta-signaling, is crucially involved in mechanostimulation of bone fracture healing by whole-body vibration. Bone 2018, 110, 11–20. [Google Scholar] [CrossRef]
- Wehrle, E.; Liedert, A.; Heilmann, A.; Wehner, T.; Bindl, R.; Fischer, L.; Haffner-Luntzer, M.; Jakob, F.; Schinke, T.; Amling, M.; et al. The impact of low-magnitude high-frequency vibration on fracture healing is profoundly influenced by the oestrogen status in mice. Dis. Models Mech. 2015, 8, 93–104. [Google Scholar] [CrossRef]
- Haffner-Luntzer, M.; Lackner, I.; Liedert, A.; Fischer, V.; Ignatius, A. Effects of low-magnitude high-frequency vibration on osteoblasts are dependent on estrogen receptor alpha signaling and cytoskeletal remodeling. Biochem. Biophys. Res. Commun. 2018, 503, 2678–2684. [Google Scholar] [CrossRef] [PubMed]
- Zaman, G.; Cheng, M.Z.; Jessop, H.L.; White, R.; Lanyon, L.E. Mechanical strain activates estrogen response elements in bone cells. Bone 2000, 27, 233–239. [Google Scholar] [CrossRef]
- Jessop, H.L.; Sjoberg, M.; Cheng, M.Z.; Zaman, G.; Wheeler-Jones, C.P.; Lanyon, L.E. Mechanical strain and estrogen activate estrogen receptor alpha in bone cells. J. Bone Min. Res. 2001, 16, 1045–1055. [Google Scholar] [CrossRef]
- Zaman, G.; Jessop, H.L.; Muzylak, M.; De Souza, R.L.; Pitsillides, A.A.; Price, J.S.; Lanyon, L.L. Osteocytes use estrogen receptor alpha to respond to strain but their ERalpha content is regulated by estrogen. J. Bone Min. Res. 2006, 21, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Lanyon, L.; Skerry, T. Postmenopausal osteoporosis as a failure of bone’s adaptation to functional loading: A hypothesis. J. Bone Min. Res. 2001, 16, 1937–1947. [Google Scholar] [CrossRef]
- Rodda, S.J.; McMahon, A.P. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development 2006, 133, 3231–3244. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Lee, B.H.; Song, K.B.; Park, R.W.; Kim, I.S.; Sohn, K.Y.; Jo, J.S.; Ryoo, H.M. Expression patterns of bone-related proteins during osteoblastic differentiation in MC3T3-E1 cells. J. Cell Biochem. 1996, 61, 609–618. [Google Scholar] [CrossRef]
- Karsenty, G.; Ducy, P.; Starbuck, M.; Priemel, M.; Shen, J.; Geoffroy, V.; Amling, M. Cbfa1 as a regulator of osteoblast differentiation and function. Bone 1999, 25, 107–108. [Google Scholar] [CrossRef]
- Ziros, P.G.; Gil, A.P.; Georgakopoulos, T.; Habeos, I.; Kletsas, D.; Basdra, E.K.; Papavassiliou, A.G. The bone-specific transcriptional regulator Cbfa1 is a target of mechanical signals in osteoblastic cells. J. Biol. Chem. 2002, 277, 23934–23941. [Google Scholar] [CrossRef]
- Varea, O.; Garrido, J.J.; Dopazo, A.; Mendez, P.; Garcia-Segura, L.M.; Wandosell, F. Estradiol activates beta-catenin dependent transcription in neurons. PLoS ONE 2009, 4, e5153. [Google Scholar] [CrossRef]
- Mendez, P.; Garcia-Segura, L.M. Phosphatidylinositol 3-kinase and glycogen synthase kinase 3 regulate estrogen receptor-mediated transcription in neuronal cells. Endocrinology 2006, 147, 3027–3039. [Google Scholar] [CrossRef][Green Version]
- Lee, K.C.; Maxwell, A.; Lanyon, L.E. Validation of a technique for studying functional adaptation of the mouse ulna in response to mechanical loading. Bone 2002, 31, 407–412. [Google Scholar] [CrossRef]
- Rubin, C.T.; Lanyon, L.E. Dynamic strain similarity in vertebrates; an alternative to allometric limb bone scaling. J. Theor. Biol. 1984, 107, 321–327. [Google Scholar] [CrossRef]
- Parfitt, A.M.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R. Bone histomorphometry: Standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 1987, 2, 595–610. [Google Scholar] [CrossRef]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, A.M. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2013, 28, 2–17. [Google Scholar] [CrossRef]
- Haffner-Luntzer, M.; Heilmann, A.; Rapp, A.E.; Roessler, R.; Schinke, T.; Amling, M.; Ignatius, A.; Liedert, A. Antagonizing Midkine Accelerates Fracture Healing in Mice by Enhanced Bone Formation in the Fracture Callus. Br. J. Pharm. 2016, 173, 2237–2249. [Google Scholar] [CrossRef]
- Liedert, A.; Mattausch, L.; Rontgen, V.; Blakytny, R.; Vogele, D.; Pahl, M.; Bindl, R.; Neunaber, C.; Schinke, T.; Harroch, S.; et al. Midkine-deficiency increases the anabolic response of cortical bone to mechanical loading. Bone 2011, 48, 945–951. [Google Scholar] [CrossRef]

| Parameter. | Sham | OVX | OVX+E2 |
|---|---|---|---|
| Estrogen Level (Serum) | |||
| Estrogen in pg/mL | 13.7 ± 4.1 | 9.9 ± 1.2 | 78.7 ± 13.6 bc |
| Uterus | |||
| Uterus weight in mg | 225.2 ± 80.2 | 66.7 ± 31.8 a | 283.7 ± 41.5 c |
| Trabecular Bone (L5) | |||
| BV/TV in % | 13.9 ± 1.1 | 11.6 ± 1.3 | 16.8 ± 2.4 bc |
| Tb.Th in mm | 0.059 ± 0.002 | 0.058 ± 0.002 | 0.062 ± 0.003 c |
| Tb.N in 1/mm | 2.4 ± 0.2 | 2.0 ± 0.2 | 2.7 ± 0.4 c |
| Tb.Sp in mm | 0.26 ± 0.02 | 0.30 ± 0.02 | 0.25 ± 0.02 c |
| Cortical Bone (Ulna) | |||
| Ct.Th in mm | 0.180 ± 0.007 | 0.168 ± 0.007 a | 0.172 ± 0.004 |
| Ma.Ar in mm2 | 0.028 ± 0.002 | 0.037 ± 0.001 a | 0.032 ± 0.002 bc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liedert, A.; Nemitz, C.; Haffner-Luntzer, M.; Schick, F.; Jakob, F.; Ignatius, A. Effects of Estrogen Receptor and Wnt Signaling Activation on Mechanically Induced Bone Formation in a Mouse Model of Postmenopausal Bone Loss. Int. J. Mol. Sci. 2020, 21, 8301. https://doi.org/10.3390/ijms21218301
Liedert A, Nemitz C, Haffner-Luntzer M, Schick F, Jakob F, Ignatius A. Effects of Estrogen Receptor and Wnt Signaling Activation on Mechanically Induced Bone Formation in a Mouse Model of Postmenopausal Bone Loss. International Journal of Molecular Sciences. 2020; 21(21):8301. https://doi.org/10.3390/ijms21218301
Chicago/Turabian StyleLiedert, Astrid, Claudia Nemitz, Melanie Haffner-Luntzer, Fabian Schick, Franz Jakob, and Anita Ignatius. 2020. "Effects of Estrogen Receptor and Wnt Signaling Activation on Mechanically Induced Bone Formation in a Mouse Model of Postmenopausal Bone Loss" International Journal of Molecular Sciences 21, no. 21: 8301. https://doi.org/10.3390/ijms21218301
APA StyleLiedert, A., Nemitz, C., Haffner-Luntzer, M., Schick, F., Jakob, F., & Ignatius, A. (2020). Effects of Estrogen Receptor and Wnt Signaling Activation on Mechanically Induced Bone Formation in a Mouse Model of Postmenopausal Bone Loss. International Journal of Molecular Sciences, 21(21), 8301. https://doi.org/10.3390/ijms21218301





