The Role of Ionic Liquids in the Pharmaceutical Field: An Overview of Relevant Applications
Abstract
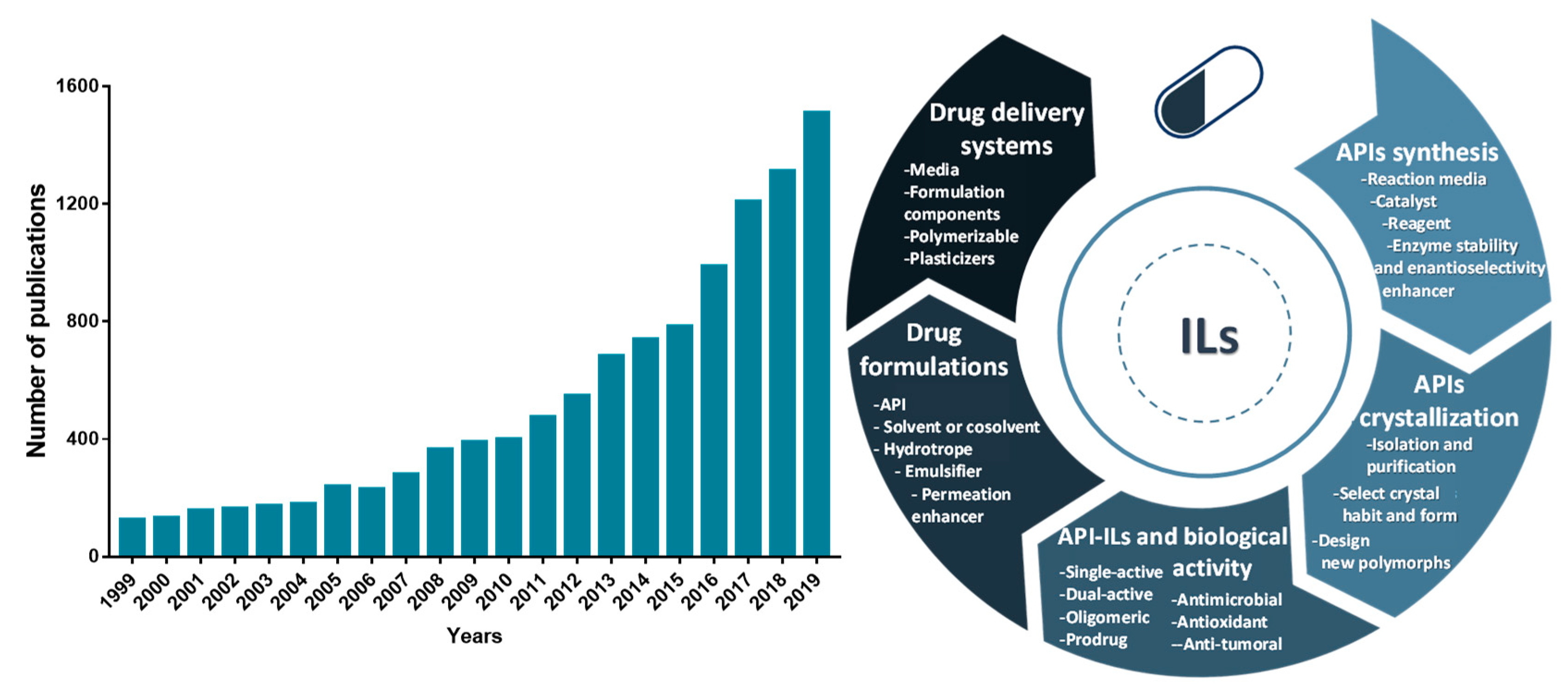
:1. Introduction
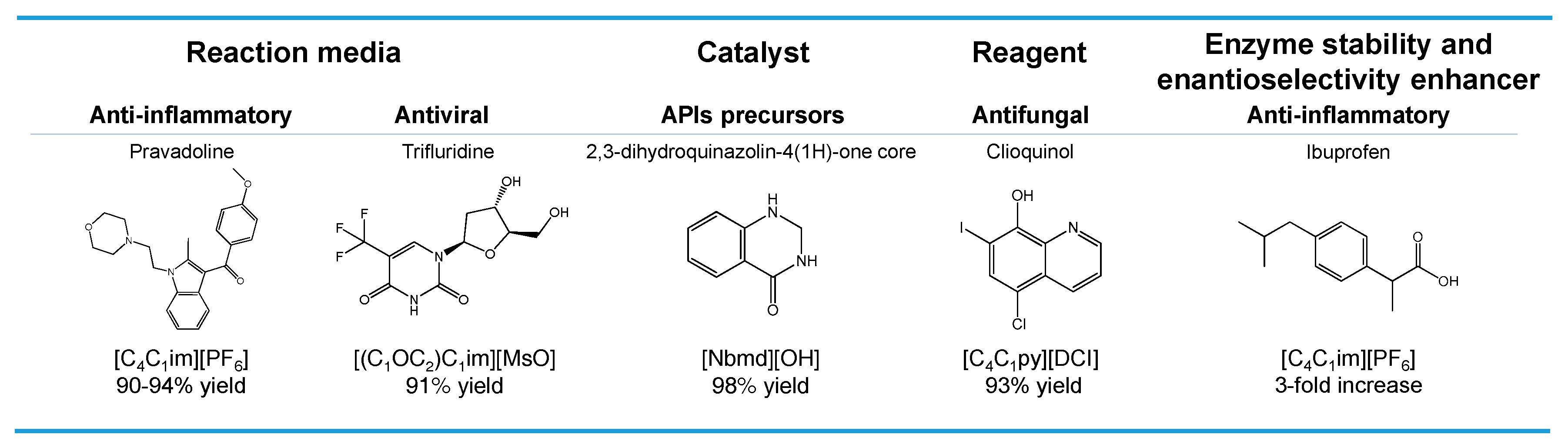
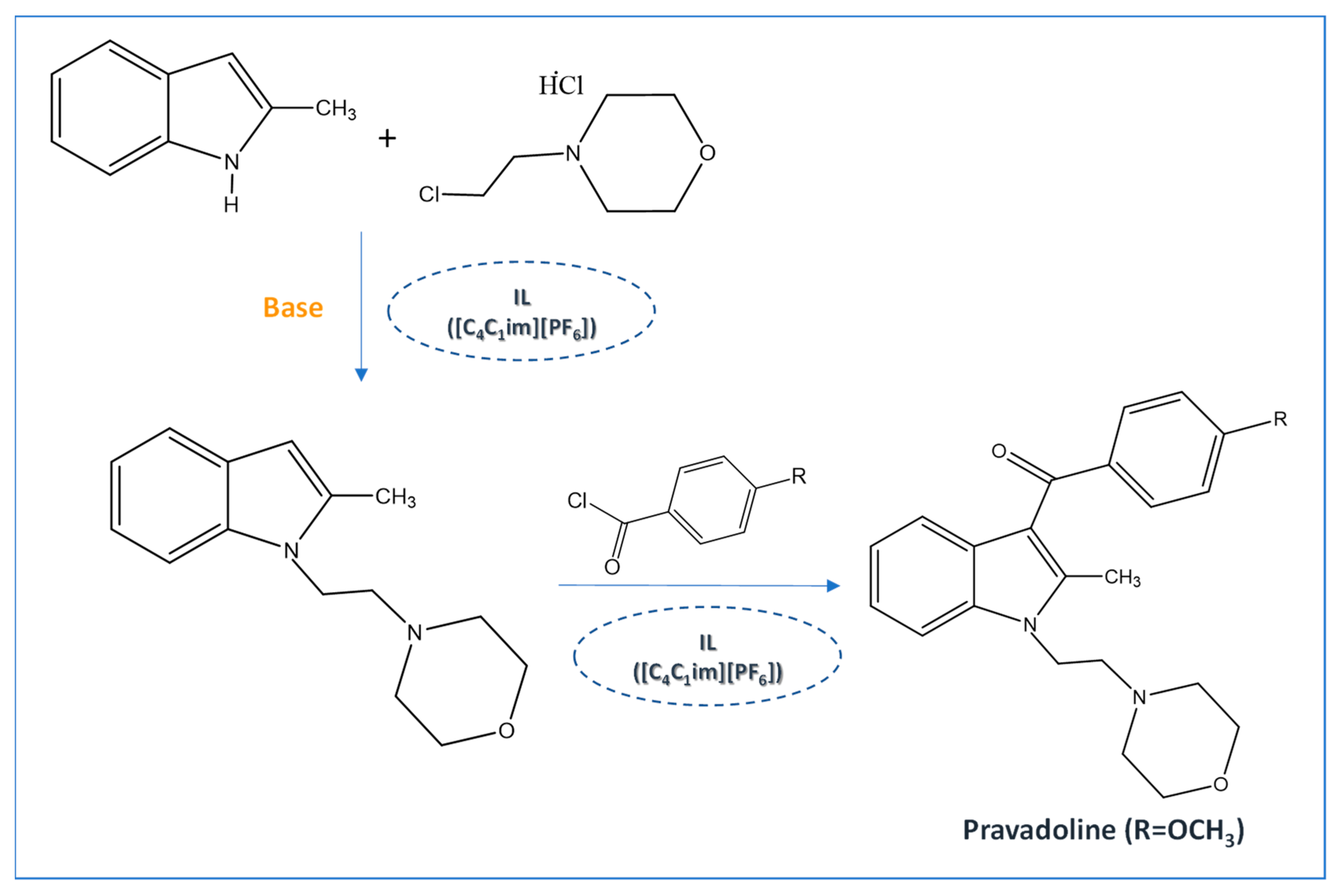
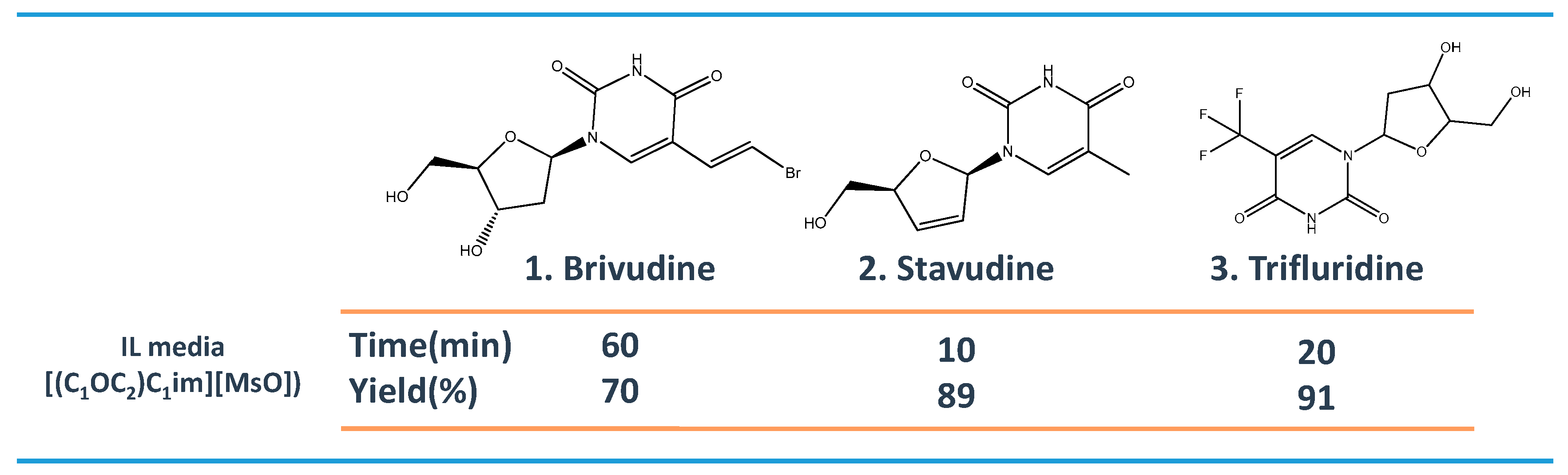
2. ILs in the Synthesis of Pharmaceutical Compounds
3. ILs as Solvents, Co-Solvents or Emulsifiers for APIs Solubilization
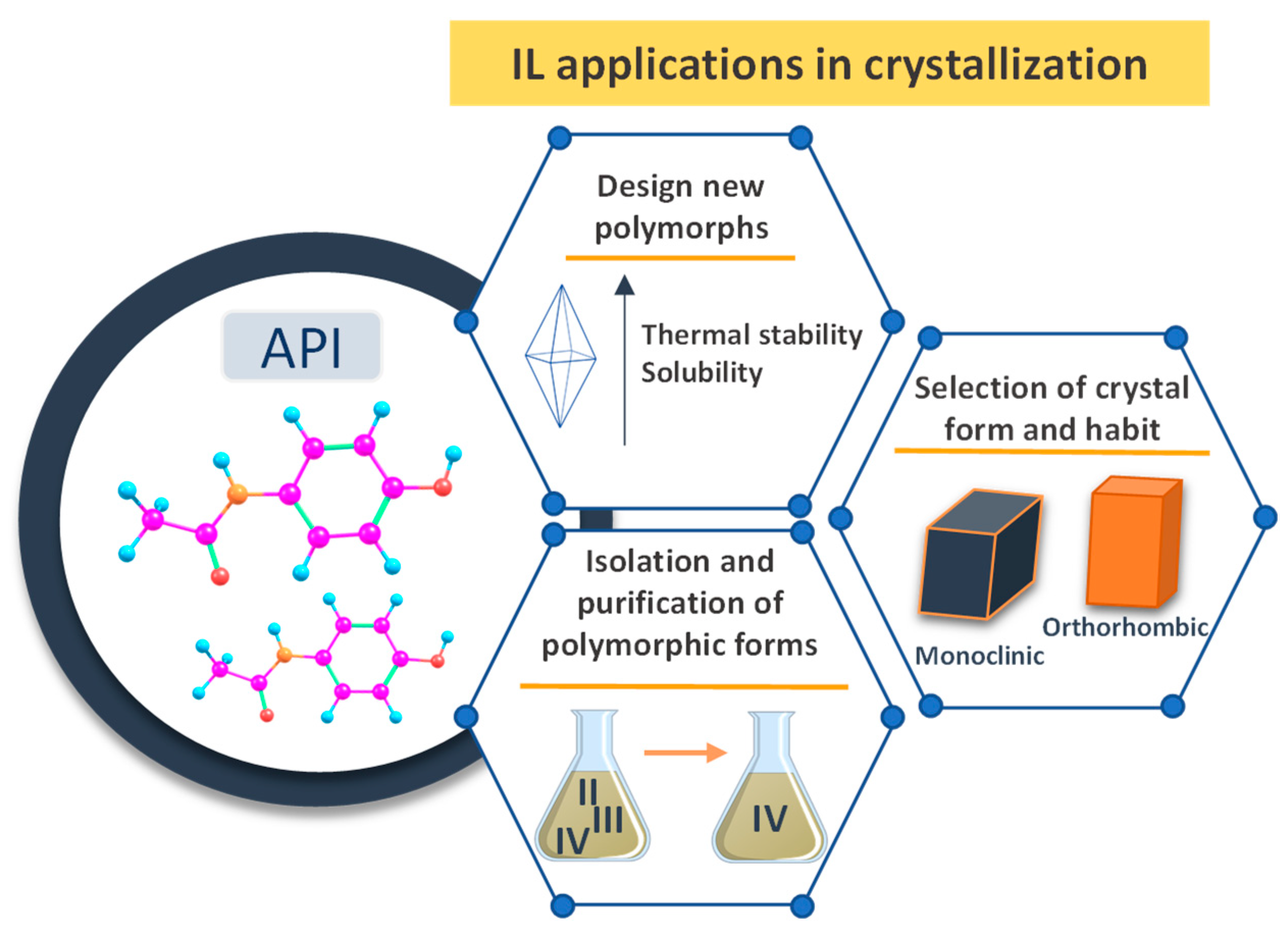
4. ILs in APIs Crystallization
5. ILs with Biological Activity
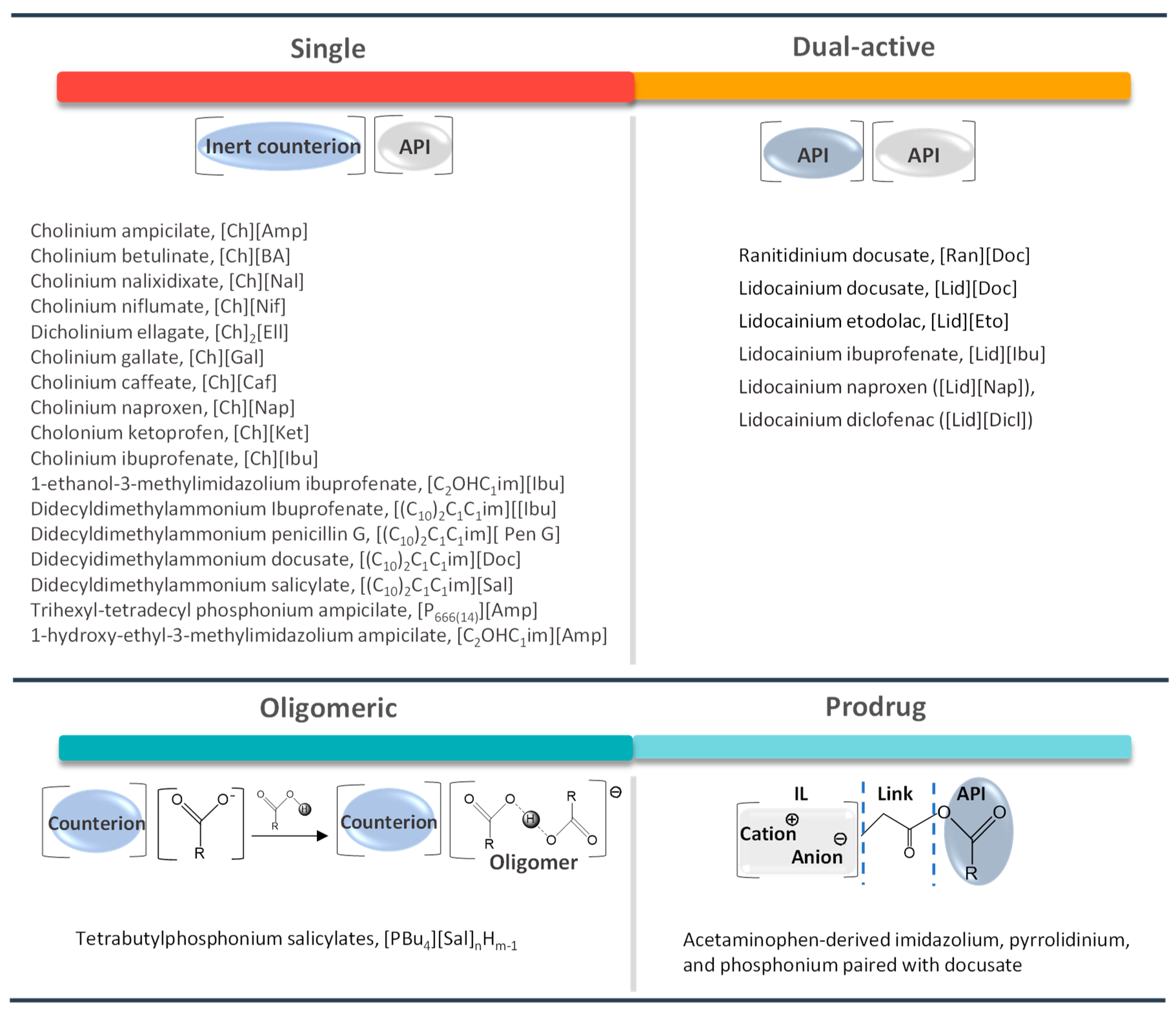
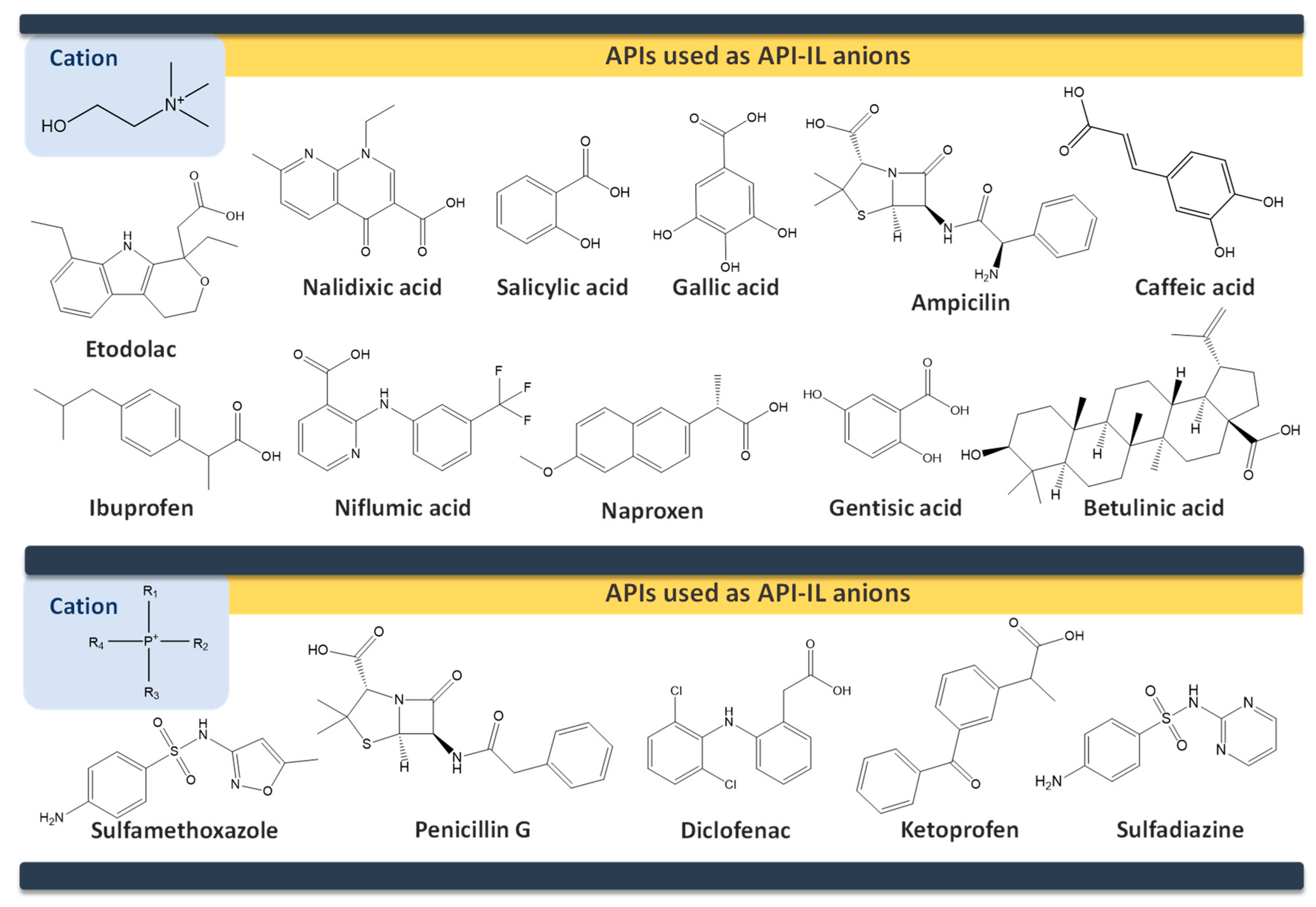
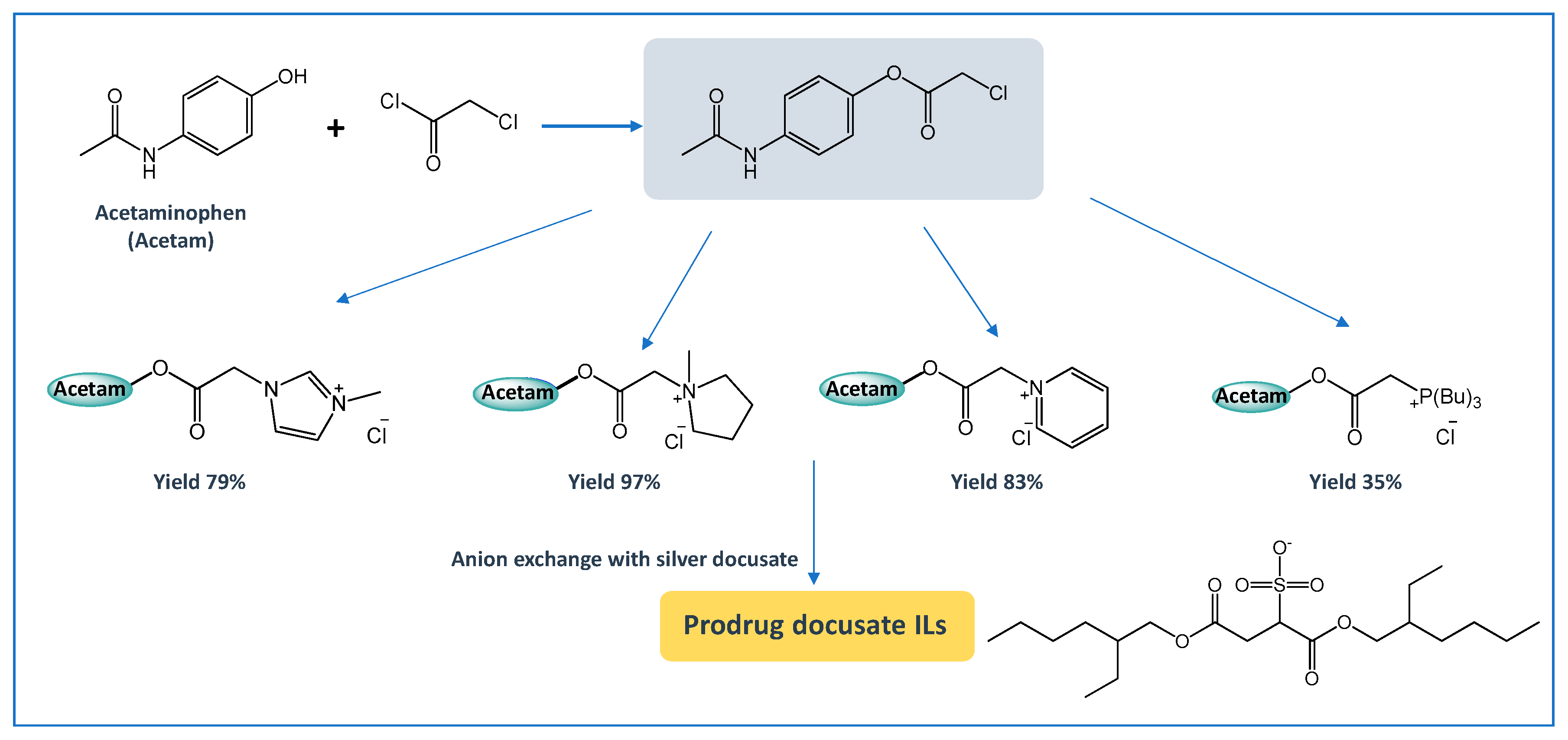
6. API-ILs as Liquid Forms of APIs
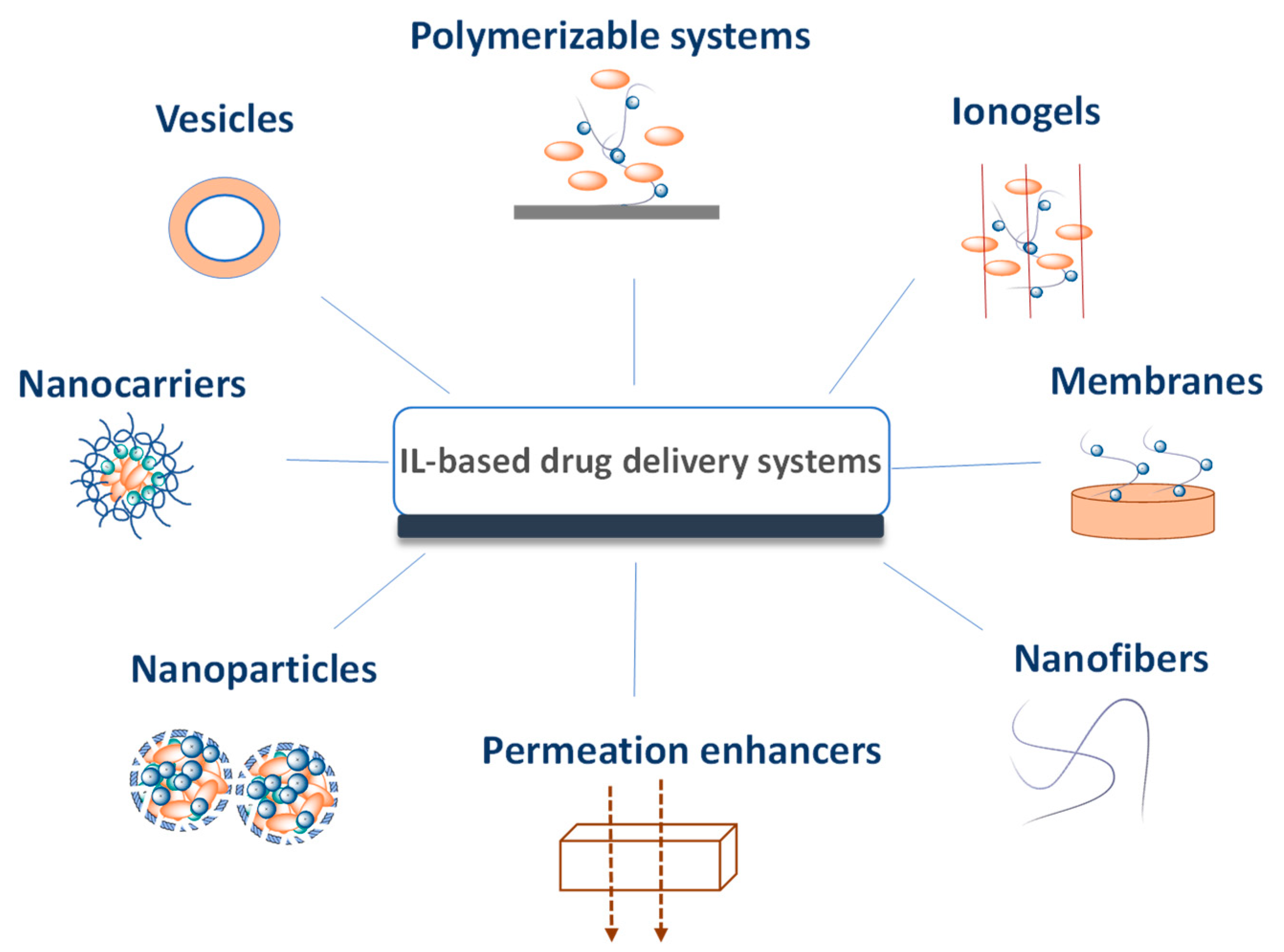
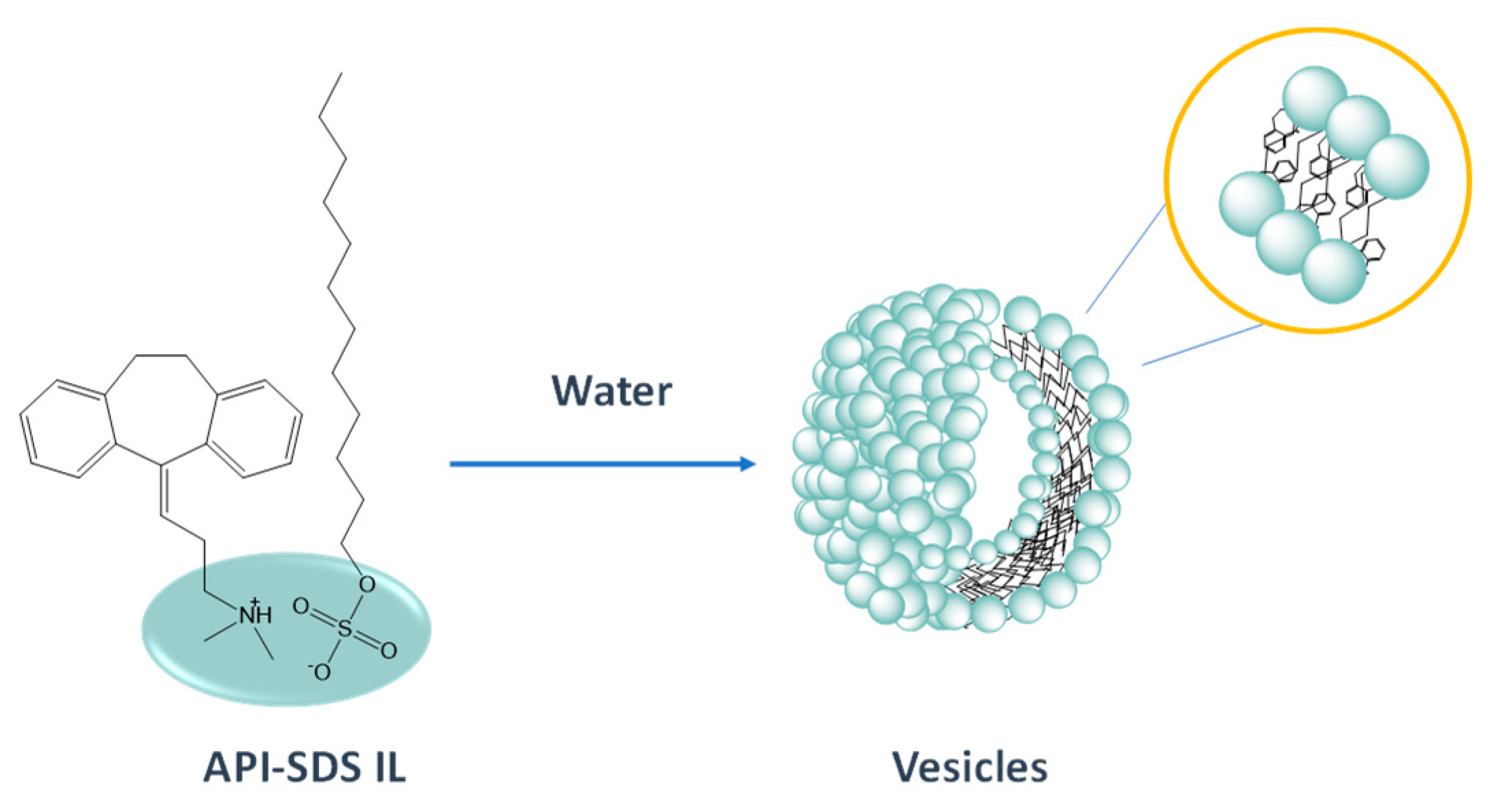
7. IL-Based Drug Delivery Systems
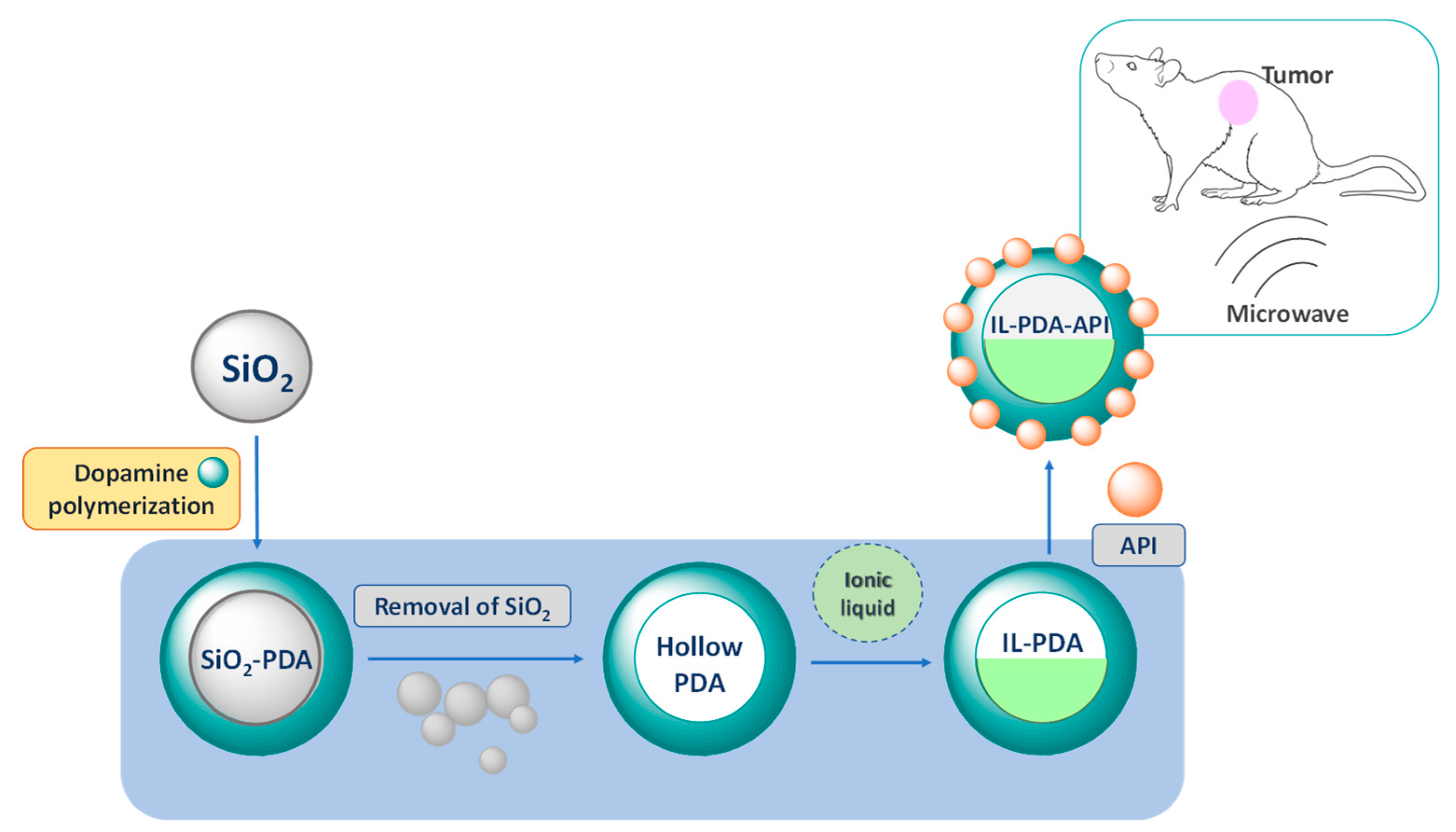
7.1. Intravenous Drug Delivery
7.2. Oral Drug Delivery
7.3. Topical and Transdermal Drug Delivery
8. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| General | |
| 3-CPP | 3-chloro-1-phenyl-1-propanone |
| AGB-ILs | Ionic liquids analogues of glycine-betaine |
| APIs | Active pharmaceutical ingredients |
| API-ILs | Ionic liquid comprising active pharmaceutical ingredients |
| BC | Bacterial cellulose |
| Cellulose-g-PLLA | Cellulose-graft-poly (L-lactide) |
| CrEL | Ethoxylated castor oil |
| DHQ | 2,3-Dihydroquinazolin-4(1H)-one |
| DMAP | 4-Dimethylaminopyridine |
| DMF | Dimethylformamide |
| DMSO | Dimethyl sulfoxide |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| FTIR–ATR | Fourier transform infrared spectroscopy—attenuated total reflectance |
| HSal | Salicylic acid (neutral form) |
| IC50 | Half maximal inhibitory concentration |
| IL | Ionic liquid |
| IL/O | IL-in-oil |
| IL/w | IL-in-water |
| LCOS | Linoleic acid-grafted chitosan oligosaccharide |
| LFER | Linear free energy relationship |
| MIC | Minimal inhibitory concentration |
| MBC | Minimal biocidal concentration |
| NSAID | Non-steroidal anti-inflammatory |
| [Na][Sal] | Sodium salicylate |
| O/IL | Oil-in-IL |
| PC12 | Pheochromocytoma cells |
| PLGA | Poly (lactic-co-glycolic acid) |
| QSAR | Quantitative structure-activity relationship |
| SAIL | Surface-active ionic liquid |
| (S)-CPPO | (S)-3-chloro-1-phenyl-1-propanol |
| SEDDs | Self-emulsifying drug delivery systems |
| TTAB | Tetradecyltrimethylammonium bromide |
| W/IL | Water-in-IL |
| Ionic Liquids | |
| Ammonium | |
| [C4C1im][BF4] | 1-butyl-3-methylimidazolium tetrafluoroborate |
| [(C4C1C1m][BF4] | 1-butyl-2,3dimethylimidazolium tetrafluoroborate |
| [C6C1im][BF4] | 1-hexyl-3-methylimidazolium tetrafluoroborate |
| [C8C1im][BF4] | 1-octyl-3-methylimidazolium tetrafluoroborate |
| [(CH2CH=C2)C2m][BF4] | 1-allyl-3-ethylimidazolium tetrafluoroborate |
| [C4C1im]Br | 1-butyl-3-methylimidazolium bromide |
| [C14C1im]Br | 1-methyl-3-tetradecylimidazolium bromide |
| [C8im]Cl | N-octylimidazolium chloride |
| [C1C8im]Cl | 1-dodecyl-3-methylimidazolium chloride |
| [C1C1C8im]Cl | 1-methyl-3-tetradecylimidazolium chloride |
| [C6C1im]Cl | 1-hexyl-3-methylimidazolium chloride |
| [C8C1im]Cl | 3-methyl-1-octylimidazolium chloride |
| [C12C1im]Cl | 1-hexadecyl-3-methylimidazolium chloride |
| [C14C1im]Cl | 1-octyl-3-methyl imidazolium chloride |
| [(CH2CH=C2)]Cl | 1-allyl-3-methylimidazolium chloride |
| [C2C1im][CH3COO] | 1-ethyl-3-methylimidazolium acetate |
| [C2C1im][CH3OHPO2] | 1-ethyl-3-methylimidazolium methylphosphonate |
| [C1C1im][(CH3O)2PO2] | Dimethylimidazolium dimethylphosphate |
| [C4C1im][C8OSO3] | 1-butyl-3-methylimidazolium octylsulfate |
| [C1C1im][C12SO3] | Dimethylimidazolium dodecanesulfate |
| [C2C1im][EtSO4] | 1-ethyl-3-methylimidazolium ethylsulfate |
| [C4C1im][N(CN)2] | 1-butyl-3-methylimidazolium dicyanamide |
| [C2][NTf2] | N-ethyl-2-hydroxy-N,N-dimethylethanammonium bis(trifluoromethylsulfonyl)amide) |
| [C2C1im][NTf2] | 1-ethyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide |
| [C4C1im][NTf2] | 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide |
| [C6C1im][NTf2] | 1-hexyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide |
| [C10C1im][NTf2] | 1-decyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide |
| [C2C1im][CF3O3S] | 1-ethyl-3-methylimidazolium trifluoromethanesulfonate |
| [C4C1im][CF3O3S] | 1-butyl-3-methylimidazolium trifluoromethanesulfonate |
| [C10C1im][CF3O3S] | 1-decyl-3-methylimidazolium trifluoromethanesulfonate |
| [C4C1im][PF6] | 1-butyl-3-methylimidazolium hexafluorophosphate |
| [C6C1im][PF6] | 1-hexyl-3-methylimidazolium hexafluorophosphate |
| [C8C1im][PF6] | 1-octyl-3-methylimidazolium hexafluorophosphate |
| [C4C1im][SCN]) | 1-butyl-3-methylimidazolium thiocyanate |
| [(C₂)₃NC₂]Br | Triethyl[2-ethoxy-2-oxoethyl]ammonium bromide |
| [C4NH3][CH3COO] | N-butylammonium acetate |
| [C6NH3][CH3COO] | N-hexylammonium acetate |
| [C8NH3][CH3COO] | N-octylammonium acetate |
| [C4NH3][oleate] | N-butylammonium oleate |
| [C6NH3][oleate] | N-hexylammonium oleate |
| [C8NH3][oleate] | N-octylammonium oleate |
| [(C1OC2)C1im][MsO] | 1-methoxyethyl-3-methylimidazolium methanesulfonate |
| [C2OHC1im]Cl | 1-(2-hydroxyethyl)-3-methylimidazolium chloride |
| [DDA][NO3] | Didecyldimethylammonium nitrate |
| [N4,1,1,1][NTf2] | N-trimethyl-N-butylammonium bis(trifluoromethanesulfonyl)imide |
| Cholinium | |
| [Ch][Ala] | Cholinium alaninate |
| [Ch][Ile] | Cholinium isoleucine |
| [Ch][geranate2(H)] | Cholinium geranate |
| [Ch][Glu] | Cholinium L-glutaminate |
| [Ch][Gly] | Cholinium glycinate |
| [Ch][Leu] | Cholinium leucinate |
| [Ch][Phe] | Cholinium phenylalanine |
| [Ch][Pro] | Cholinium prolinate |
| [Ch][Se] | Cholinium serinate |
| [Ch][Try] | Cholinium tryptophan |
| Morpholinium | |
| [Nbmd][OH] | 4,4′-(butane-1,4-diyl)bis(4-dodecyl-morpholin-4-ium)hydroxide |
| Phosphonium | |
| [P444(14)]Cl | Tributyltetradecylphosphonium chloride |
| [P6,6,6,14]Cl | Trihexyltetradecylphosphonium chloride |
| [P6,6,6,14][NTf2] | Trihexyltetradecylphosphonium bis(trifluoromethylsulfonyl)imide |
| Pyridinium | |
| [C4C1py][DCI] | 1-butyl-3-methylpyridinium dichloroiodate |
| [C6C6OCOpy][N(CN)2] | 1-hexyl-3-hexyloxycarbonylpyridinium dicyanamide |
| [C6C6OCOpy][NTf2] | 1-hexyl-3-hexyloxycarbonylpyridinium bis(trifluoromethylsulfonyl)imide |
| Pyrrolidinium | |
| [Pyrr4,1][NTf2] | Butylmethylpyrrolidinium bis(trifluorosulfonyl)amide |
| API-ILs | |
| [C4C1im][Ibu] | 1-butyl-3-methylimidazolium ibuprofenate |
| [(C10)2C1C1im][Doc] | Didecyidimethylammonium docusate |
| [(C10)2C1C1im][[Ibu] | Didecyldimethylammonium ibuprofenate |
| [(C10)2C1C1im][Pen G] | Didecyldimethylammonium penicillin G |
| [(C10)2C1C1im][Sal] | Didecyldimethylammonium salicylate |
| [Ch][Amp] | Cholinium ampicilate |
| [Ch][BA] | Cholinium betulinate |
| [Ch][B3] | Cholinium nicotinate |
| [Ch][B5] | Cholinium pantothenate |
| [Ch][B6] | Cholinium pyridoxylate |
| [Ch][Caf] | Cholinium caffeate |
| [Ch][Gal] | Cholinium gallate |
| [Ch][Ibu] | Cholinium ibuprofenate |
| [Ch][Ket] | Cholonium ketoprofen |
| [Ch][Nal] | Cholinium nalixidixate |
| [Ch][Nap] | Cholinium naproxen |
| [Ch][Nif] | Cholinium niflumate |
| [Ch][Sal] | Cholinium salicylate |
| [Ch]2[Ell] | Dicholinium ellagate |
| [C2OHC1im][Amp] | 1-hydroxy-ethyl-3-methylimidazolium ampicilate |
| [C2OHC1im][Ibu] | 1-ethanol-3-methylimidazolium ibuprofenate |
| [C1Pyrr][Sal] | 1-methylpyrrolidinium salicylate |
| [HN444][Sal] | Tributylammonium salicylate |
| [Lid][Asp] | Lidocainium acetylsalicylate |
| [Lid][Dicl] | Lidocainium diclofenac |
| [Lid][Doc] | Lidocainium docusate |
| [Lid][Eto] | Lidocainium etodolac |
| [Lid][Ibu] | Lidocainium ibuprofenate |
| [Lid][Nap] | Lidocainium naproxenum |
| [Ran][Doc] | Ranitidine docusate |
| [mPEG3N444][Sal] | Triethylene glycol monomethyl ether tributylammonium salicylate |
| [P4444][Ibu] | Tetrabutylphosphonium ibuprofenate |
| [P666(14)][Amp] | Trihexyl-tetradecyl phosphonium ampicilate |
| [PBu4][Sal]nHm-1 | Tetrabutylphosphonium salicylates |
| [ProOEt][Ibu] | Ethylester ibuprofenate |
References
- World Health Organization Medicines Strategy-Contries at the Core. Available online: https://apps.who.int/iris/bitstream/handle/10665/84307/WHO_EDM_2004.5_eng.pdf;jsessionid=0F733DD987692B73A234E9FB8C10D40B?sequence=1 (accessed on 20 May 2020).
- Ende, M.T.; Ende, D.J. Chemical Engineering in the Pharmaceutical Industry: Drug Product Design, Development and Modeling, 2nd ed.; Wiley: Hoboken, NJ, USA, 2019. [Google Scholar]
- Shamshina, J.L.; Rogers, R.D. Overcoming the problems of solid state drug formulations with ionic liquids. Ther. Deliv. 2014, 5, 489–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrn, S.; Pfeiffer, R.; Ganey, M.; Hoiber, C.; Poochikian, G. Pharmaceutical Solids: A Strategic Approach to Regulatory Considerations. Pharm. Res. 1995, 12, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Kalepu, S.; Nekkanti, V. Insoluble drug delivery strategies: Review of recent advances and business prospects. Acta Pharm. Sin. B 2015, 5, 442–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharm. 2012, 2012, 195727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brittain, H.G.; Grant, D.J.R. Effects of Polymorphism and Solid-State Solvation on Solubility and Dissolution Rate. In Polymorphism in Pharmaceutical Solids; Taylor and Francis: Abingdon, UK, 2009; pp. 436–480. [Google Scholar]
- Censi, R.; Di Martino, P. Polymorph Impact on the Bioavailability and Stability of Poorly Soluble Drugs. Molecules 2015, 20, 18759–18776. [Google Scholar] [CrossRef] [Green Version]
- Bauer, J.; Spanton, S.; Henry, R.; Quick, J.; Dziki, W.; Porter, W.; Morris, J. Ritonavir: An Extraordinary Case of Conformational Polymorphism. Pharm. Res. 2001, 18, 859–866. [Google Scholar] [CrossRef]
- Hulme, A.T.; Price, S.L.; Tocher, D.A. A New Polymorph of 5-Fluorouracil Found Following Computational Crystal Structure Predictions. J. Am. Chem. Soc. 2005, 127, 1116–1117. [Google Scholar] [CrossRef]
- Florence, A.T.; Attwood, D. Physicochemical Principles of Pharmacy, 4th ed.; Pharmaceutical Press: London, UK, 2006. [Google Scholar]
- Cue, B.W.; Zhang, J. Green process chemistry in the pharmaceutical industry. Green Chem. Lett. Rev. 2009, 2, 193–211. [Google Scholar] [CrossRef]
- El-Yafi, A.K.E.Z.; El-Zein, H. Technical crystallization for application in pharmaceutical material engineering: Review article. Asian J. Pharm. Sci. 2015, 10, 283–291. [Google Scholar] [CrossRef] [Green Version]
- Jain, N.; Kumar, A.; Chauhan, S.; Chauhan, S.M.S. Chemical and biochemical transformations in ionic liquids. Tetrahedron 2005, 61, 1015–1060. [Google Scholar] [CrossRef]
- Olivier-bourbigou, H.; Magna, L. Ionic liquids: Perspectives for organic and catalytic reactions. J. Mol. Catal. A Chem. 2002, 183, 419–437. [Google Scholar] [CrossRef]
- Cave, G.W.V.; Raston, L.; Scott, J.L. Recent advances in solventless organic reactions: Towards benign synthesis with remarkable versatility. Chem. Commun. 2001, 2001, 2159–2169. [Google Scholar] [CrossRef]
- Ehrenström-Reiz, G.; Reiz, S.; Stockman, O. Topical Anaesthesia with EMLA, a New Lidocaine-Prilocaine Cream and the Cusum Technique for Detection of Minimal Application Time. Acta Anaesthesiol. Scand. 1983, 27, 510–512. [Google Scholar] [CrossRef] [PubMed]
- Shamshina, J.L.; Kelley, S.P.; Gurau, G.; Rogers, R.D. Develop ionic liquid drugs. Nature 2015, 528, 188–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freire, M.G.; Cláudio, A.F.M.; Araújo, J.M.M.; Coutinho, J.A.P.; Marrucho, I.M.; Canongia Lopes, J.N.; Rebelo, L.P.N. Aqueous biphasic systems: A boost brought about by using ionic liquids. Chem. Soc. Rev. 2012, 41, 4966–4995. [Google Scholar] [CrossRef]
- Earle, M.J.; Esperança, J.M.S.S.; Gile, M.; Lopes, J.N.C.; Rebelo, L.P.N.; Magee, J.W.; Seddon, K.R.; Widegren, J.A. The distillation and volatility of ionic liquids. Nature 2005, 439, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Cláudio, A.F.M.; Neves, M.C.; Shimizu, K.; Canongia Lopes, J.N.; Freire, M.G.; Coutinho, J.A.P. The magic of aqueous solutions of ionic liquids: Ionic liquids as a powerful class of catanionic hydrotropes. Green Chem. 2015, 17, 3948–3963. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Barber, P.S.; Rogers, R.D. Ionic liquids in drug delivery. Expert Opin. Drug Deliv. 2013, 10, 1367–1381. [Google Scholar] [CrossRef]
- Castro, L.; Pereira, P.; Freire, M.; Pedro, A. Progress in the Development of Aqueous Two-Phase Systems Comprising Ionic Liquids for the Downstream Processing of Protein- Based Biopharmaceuticals. Am. Pharm. Rev. 2019, 1–6. [Google Scholar]
- Ventura, P.M.; Silva, F.A.; Quental, M.V.; Mondal, D.; Freire, M.G. Ionic-Liquid-Mediated Extraction and Separation Processes for Bioactive Compounds: Past, Present, and Future Trends. Chem. Rev. 2017, 117, 6984–7052. [Google Scholar] [CrossRef]
- McQueen, L.; Lai, D. Ionic liquid aqueous two-phase systems from a pharmaceutical perspective. Front. Chem. 2019, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egorova, K.S.; Ananikov, V.P. Biological Activity of Ionic Liquids Involving Ionic and Covalent Binding: Tunable Drug Development Platform. In Encyclopedia of Ionic Liquids; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–8. ISBN 9789811067396. [Google Scholar]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef] [PubMed]
- Tanner, E.E.L.; Curreri, A.M.; Balkaran, J.P.R.; Selig-wober, N.C.; Yang, A.B.; Kendig, C.; Fluhr, M.P.; Kim, N.; Mitragotri, S. Design Principles of Ionic Liquids for Transdermal Drug Delivery. Adv. Mater. 2019, 31, 1901103. [Google Scholar] [CrossRef] [PubMed]
- Dobler, D.; Schmidts, T.; Klingenhöfer, I.; Runkel, F. Ionic liquids as ingredients in topical drug delivery systems. Int. J. Pharm. 2013, 441, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Adawiyah, N.; Moniruzzaman, M.; Hawatulaila, S.; Goto, M. Ionic liquids as a potential tool for drug delivery systems. Med. Chem. Commun. 2016, 7, 1881–1897. [Google Scholar] [CrossRef]
- Marrucho, I.M.; Branco, L.C.; Rebelo, L.P.N. Ionic Liquids in Pharmaceutical Applications. Annu. Rev. Chem. Biomol. Eng. 2014, 5, 527–546. [Google Scholar] [CrossRef]
- Huang, W.; Wu, X.; Qi, J.; Zhu, Q.; Wu, W.; Lu, Y.; Chen, Z. Ionic liquids: Green and tailor-made solvents in drug delivery. Drug Discov. Today 2020, 25, 901–908. [Google Scholar] [CrossRef]
- Frizzo, C.P.; Gindri, I.M.; Tier, A.Z.; Buriol, L.; Moreira, D.N.; Martins, M.A.P. Pharmaceutical Salts: Solids to Liquids by Using Ionic Liquid Design. In Ionic Liquids—New Aspects for the Future; IntechOpen: London, UK, 2013; pp. 557–579. [Google Scholar]
- Moniruzzaman, M.; Mahmood, H.; Goto, M. Chapter 15: Ionic Liquid Based Nanocarriers for Topical and Transdermal Drug Delivery. In Ionic Liquid Devices; Royal Society of Chemistry: London, UK, 2017; pp. 390–403. [Google Scholar]
- Sheldon, R.A. The E factor 25 years on: The rise of green chemistry and sustainability. Green Chem. 2017, 19, 18–43. [Google Scholar] [CrossRef]
- Subhedar, D.D.; Shaikh, M.H.; Nawale, L.; Yeware, A.; Sarkar, D.; Kalam, F.A.; Sangshetti, J.N.; Shingate, B.B. Novel tetrazoloquinoline—Rhodanine conjugates: Highly efficient synthesis and biological evaluation. Bioorg. Med. Chem. Lett. 2016, 26, 2278–2283. [Google Scholar] [CrossRef]
- Subhedar, D.D.; Shaikh, M.H.; Khan, F.A.K.; Sangshetti, J.N.; Khedkar, V.M.; Shingate, B.B. Facile synthesis of new N-sulfonamidyl-4- thiazolidinone derivatives and their biological evaluation. New J. Chem. 2016, 40, 3047–3058. [Google Scholar] [CrossRef]
- Tao, Y.; Dong, R.; Pavlidis, I.V.; Chen, B.; Tan, T. Using imidazolium-based ionic liquids as dual solvent-catalysts for sustainable synthesis of vitamin esters: Inspiration from bio- and organo-catalysis. Green Chem. 2016, 18, 1240–1248. [Google Scholar] [CrossRef] [Green Version]
- Siódmiak, T.; Marsza, M.P.; Proszowska, A. Ionic Liquids: A New Strategy in Pharmaceutical Synthesis. Mini Rev. Org. Chem. 2012, 9, 203–208. [Google Scholar] [CrossRef] [Green Version]
- Hubbard, C.D.; Illner, P.; van Eldik, R. Understanding chemical reaction mechanisms in ionic liquids: Successes and challenges. Chem. Soc. Rev. 2011, 20, 272–290. [Google Scholar] [CrossRef] [PubMed]
- Orrling, K.M.; Wu, X.; Russo, F. Fast, Acid-Free, and Selective Lactamization of Lactones in Ionic Liquids. J. Org. Chem. 2008, 8627–8630. [Google Scholar] [CrossRef] [PubMed]
- Zang, H.; Su, Q.; Mo, Y.; Cheng, B. Ionic liquid under ultrasonic irradiation towards a facile synthesis of pyrazolone derivatives. Ultrason. Sonochem. 2011, 18, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.R.; Srivastava, A.; Shamim, S.; Srivastava, A.; Waseem, M.A. An Efficient One-Pot Regioselective Approach Towards the Synthesis of Thiopyrano [2,3-d] thiazole-2-thiones Catalyzed by Basic Ionic Liquid under Microwave Irradiation. J. Heterocycl. Chem. 2016, 53, 849–858. [Google Scholar] [CrossRef]
- Yeung, K.; Farkas, M.E.; Qiu, Z.; Yang, Z. Friedel—Crafts acylation of indoles in acidic imidazolium chloroaluminate ionic liquid at room temperature. Tetrahedron Lett. 2002, 43, 5793–5795. [Google Scholar] [CrossRef]
- Güzel, Ö.; Salman, A.; Güzel, Ö.; Salman, A. Synthesis and biological evaluation of new 4- thiazolidinone derivatives. J. Enzyme Inhib. Med. Chem. 2009, 24, 1015–1023. [Google Scholar] [CrossRef]
- Kowsari, E.; Mallakmohammadi, M. Ultrasound promoted synthesis of quinolines using basic ionic liquids in aqueous media as a green procedure. Ultrason. Sonochem. 2011, 18, 447–454. [Google Scholar] [CrossRef]
- Shi, F.; Gu, Y.; Zhang, Q.; Deng, Y. Development of ionic liquids as green reaction media and catalysts. Catal. Surv. Asia 2004, 8, 179–186. [Google Scholar] [CrossRef]
- Bhatt, J.; Chudasama, C.; Patel, K.D. Microwave Assisted Synthesis of Pyrimidines in Ionic Liquid and Their Potency as Non-Classical Malarial Antifolates: Pyrimidines as Non-Classical Malarial Antifolates. Arch. Pharm. Chem. Life Sci. 2016, 349, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, H.; Vaghefi, S. One-Pot Wittig and Knoevenagel Reactions in Ionic Liquid as Convenient Methods for the Synthesis of Coumarin Derivatives One-Pot Wittig and Knoevenagel Reactions in Synthesis of Coumarin Derivatives. Synth. Commun. 2009, 39, 1666–1678. [Google Scholar] [CrossRef]
- Rao, V.A.; Tiwari, R.; Chhikara, B.S.; Shirazi, A.N.; Parang, K.; Kumar, A. Copper triflate-mediated synthesis of 1,3,5- triarylpyrazoles in [bmim][PF6] ionic liquid and evaluation of their anticancer activities. RSC Adv. 2013, 35, 15396–15403. [Google Scholar] [CrossRef] [PubMed]
- Earle, M.J.; Mccormac, B.; Seddon, K.R. The first high yield green route to a pharmaceutical in a room temperature ionic liquid. Green Chem. 2000, 2, 261–262. [Google Scholar] [CrossRef]
- Lai, R.; Wu, X.; Lv, S.; Zhang, C.; He, M.; Chen, Y.; Wang, Q.; Hai, L.; Wu, Y. Synthesis of indoles and quinazolines via additive- controlled selective C–H activation/annulation of N-arylamidines and sulfoxonium ylides. Chem. Commun. 2019, 55, 4039–4042. [Google Scholar] [CrossRef]
- Mazzoni, O.; Diurno, M.V.; Bosco, A.M.; Novellino, E.; Grieco, P.; Esposito, G.; Calignano, A.; Russo, R.; Università, M.; Li, F.; et al. Synthesis and Pharmacological Evaluation of Analogs of Indole-Based Cannabimimetic Agents. Chem. Biol. Drug Des. 2010, 75, 106–114. [Google Scholar] [CrossRef]
- Martins, M.A.P.; Frizzo, C.P.; Moreira, D.N.; Zanatta, N.; Bonacorso, H.G. Ionic Liquids in Heterocyclic Synthesis. Chem. Rev. 2008, 108, 2015–2050. [Google Scholar] [CrossRef]
- Liu, B.K.; Wang, N.; Chen, Z.C.; Wu, Q.; Lin, X.F. Markedly enhancing lipase-catalyzed synthesis of nucleoside drugs ester by using a mixture system containing organic solvents and ionic liquid. Bioorg. Med. Chem. Lett. 2006, 16, 3769–3771. [Google Scholar] [CrossRef]
- Kumar, V.; Malhotra, S.V. Synthesis of nucleoside-based antiviral drugs in ionic liquids. Bioorg. Med. Chem. Lett. 2008, 18, 5640–5642. [Google Scholar] [CrossRef]
- Deshmukh, A.; Gore, B.; Thulasiram, H.V.; Swamy, V.P. Recyclable ionic liquid iodinating reagent for solvent free, regioselective iodination of activated aromatic and heteroaromatic amines. RSC Adv. 2015, 5, 88311–88315. [Google Scholar] [CrossRef]
- Harrison, I.T.; Lewis, B.; Nelson, P.; Rooks, W.; Roszkowski, A.; Tomolonis, A.; Fried, J.H. Nonsteroidal Antiinflammatory Agents. I. 6- Substituted 2-Naphthylacetic Acids. J. Med. Chem. 1970, 13, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Wan, K.T.; Davis, M.E. Asymmetric synthesis of naproxen by a new heterogeneous catalyst. J. Catal. 1995, 152, 25–30. [Google Scholar] [CrossRef]
- Mena, S.; Santiago, S.; Gallardo, I.; Guirado, G. Sustainable and efficient electrosynthesis of naproxen using carbon dioxide and ionic liquids. Chemosphere 2020, 245, 125557. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Damarla, K.; Kumar, A.; Saikia, P.J.; Sarma, D. Gemini basic ionic liquid as bi-functional catalyst for the synthesis of 2,3-dihydroquinazolin-4(1 H)-ones at room temperature. Tetrahedron Lett. 2020, 61, 151587. [Google Scholar] [CrossRef]
- Kim, K.; Song, B.; Choi, M.; Kim, M. Biocatalysis in Ionic Liquids: Markedly Enhanced Enantioselectivity of Lipase. Org. Lett. 2001, 3, 1507–1509. [Google Scholar] [CrossRef]
- Gamenara, D.; Domínguez, P.; María, D. Candida spp. redox machineries: An ample biocatalytic platform for practical applications and academic insights. Biotechnol. Adv. 2009, 27, 278–285. [Google Scholar] [CrossRef]
- Fronza, G.; Fuganti, C.; Grasselli, P.; Mele, A. On the Mode of Bakers’ Yeast Transformation of 3-Chloropropiophenone and Related Ketones. Synthesis of (2S)-[2-2H]Propiophenone, (R)-Fluoxetine, and (R)- and (S)-Fenfluramine. J. Org. Chem. 1991, 56, 6019–6023. [Google Scholar] [CrossRef]
- Jeong, H.; Uhm, K.; Kim, H. Production of chiral compound using recombinant Escherichia coli cells co-expressing reductase and glucose dehydrogenase in an ionic liquid/water two phase system. J. Mol. Catal. B Enzym. 2011, 70, 114–118. [Google Scholar] [CrossRef]
- Pleuvry, B.J. Factors affecting drug absorption and distribution. Pharmacology 2005, 6, 135–138. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Dissolution Testing and Acceptance Criteria for Immediate-Release Solid Oral Dosage Form. Drug Products Containing High. Solubility Drug Substances (Guidance for Industry). Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/dissolution-testing-and-acceptance-criteria-immediate-release-solid-oral-dosage-form-drug-products (accessed on 26 May 2020).
- Grodowska, K.; Parczewski, A. Organic solvents in the pharmaceutical industry. Acta Pol. Pharm. Drug Res. 2010, 67, 3–12. [Google Scholar]
- Mizuuchi, H.; Jaitely, V.; Murdan, S.; Florence, A.T. Room temperature ionic liquids and their mixtures: Potential pharmaceutical solvents. Eur. J. Pharm. Sci. 2008, 33, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Millard, J.W.; Alvarez-Núñez, F.A.; Yalkowsky, S.H. Solubilization by cosolvents: Establishing useful constants for the log-linear model. Int. J. Pharm. 2002, 245, 153–166. [Google Scholar] [CrossRef]
- Chaudhari, S.P.; Dugar, R.P. Application of surfactants in solid dispersion technology for improving solubility of poorly water soluble drugs. J. Drug Deliv. Sci. Technol. 2017, 41, 68–77. [Google Scholar] [CrossRef]
- Faria, R.A.; da Ponte, M.N.; Bogel-Łukasik, E. Solubility studies on the system of trihexyl(tetradecyl)phosphonium bis[(trifluoromethyl)sulfonyl]amide) ionic liquid and pharmaceutical and bioactive compounds. Fluid Phase Equilib. 2015, 385, 1–9. [Google Scholar] [CrossRef]
- dos Santos, A.D.; Morais, A.R.C.; Melo, C.; Bogel-Łukasik, R.; Bogel-Łukasik, E. Solubility of pharmaceutical compounds in ionic liquids. Fluid Phase Equilib. 2013, 356, 18–29. [Google Scholar] [CrossRef]
- Goindi, S.; Arora, P.; Kumar, N.; Puri, A. Development of novel ionic liquid-based microemulsion formulation for dermal delivery of 5-fluorouracil. AAPS PharmSciTech 2014, 15, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.B.; Bridson, R.H.; Leeke, G.A. Solubilities of pharmaceutical compounds in ionic liquids. J. Chem. Eng. Data 2011, 56, 2039–2043. [Google Scholar] [CrossRef]
- McCrary, P.D.; Beasley, P.A.; Gurau, G.; Narita, A.; Barber, P.S.; Cojocaru, O.A.; Rogers, R.D. Drug specific, tuning of an ionic liquid’s hydrophilic-lipophilic balance to improve water solubility of poorly soluble active pharmaceutical ingredients. New J. Chem. 2013, 37, 2196–2202. [Google Scholar] [CrossRef]
- Williams, H.D.; Sahbaz, Y.; Ford, L.; Nguyen, T.H.; Scammells, P.J.; Porter, C.J.H. Ionic liquids provide unique opportunities for oral drug delivery: Structure optimization and in vivo evidence of utility. Chem. Commun. 2014, 50, 1688–1690. [Google Scholar] [CrossRef]
- Manic, M.S.; Najdanovic-Visak, V. Solubility of erythromycin in ionic liquids. J. Chem. Thermodyn. 2012, 44, 102–106. [Google Scholar] [CrossRef]
- Goindi, S.; Kaur, R.; Kaur, R. An ionic liquid-in-water microemulsion as a potential carrier for topical delivery of poorly water soluble drug: Development, ex-vivo and in-vivo evaluation. Int. J. Pharm. 2015, 495, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Alawi, M.A.; Hamdan, I.I.; Sallam, A.A.; Heshmeh, N.A. Solubility enhancement of glibenclamide in choline-tryptophan ionic liquid: Preparation, characterization and mechanism of solubilization. J. Mol. Liq. 2015, 212, 629–634. [Google Scholar] [CrossRef]
- Melo, C.I.; Bogel-Łukasik, R.; Nunes da Ponte, M.; Bogel-Łukasik, E. Ammonium ionic liquids as green solvents for drugs. Fluid Phase Equilib. 2013, 338, 209–216. [Google Scholar] [CrossRef] [Green Version]
- Forte, A.; Melo, C.I.; Bogel-łukasik, R.; Bogel-łukasik, E. A favourable solubility of isoniazid, an antitubercular antibiotic drug, in alternative solvents. Fluid Phase Equilib. 2012, 318, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, M.R.; Moshikur, R.M.; Wakabayashi, R.; Tahara, Y.; Kamiya, N.; Moniruzzaman, M.; Goto, M. Ionic-Liquid-Based Paclitaxel Preparation: A New Potential Formulation for Cancer Treatment. Mol. Pharm. 2018, 15, 2484–2488. [Google Scholar] [CrossRef]
- Lourenço, C.; Melo, C.I.; Bogel-Łukasik, R.; Bogel-Łukasik, E. Solubility advantage of pyrazine-2-carboxamide: Application of alternative solvents on the way to the future pharmaceutical development. J. Chem. Eng. Data 2012, 57, 1525–1533. [Google Scholar] [CrossRef]
- Jaitely, V.; Mizuuchi, H.; Florence, A.T. Current-stimulated release of solutes solubilized in water-immiscible room temperature ionic liquids (RTILs). J. Drug Target. 2010, 18, 787–793. [Google Scholar] [CrossRef]
- Sintra, T.E.; Shimizu, K.; Ventura, S.P.M.; Shimizu, S.; Canongia Lopes, J.N.; Coutinho, J.A.P. Enhanced dissolution of ibuprofen using ionic liquids as catanionic hydrotropes. Phys. Chem. Chem. Phys. 2018, 20, 2094–2103. [Google Scholar] [CrossRef]
- Sanan, R.; Kaur, R.; Mahajan, R.K. Micellar Transitions in Catanionic Ionic liquid—Ibuprofen Aqueous Mixtures, Effects of Composition and Dilution. RSC Adv. 2014, 4, 64877–64889. [Google Scholar] [CrossRef]
- De Faria, E.L.P.; Shabudin, S.V.; Claúdio, A.F.M.; Válega, M.; Domingues, F.M.J.; Freire, C.S.R.; Silvestre, A.J.D.; Freire, M.G. Aqueous Solutions of Surface-Active Ionic Liquids: Remarkable Alternative Solvents to Improve the Solubility of Triterpenic Acids and Their Extraction from Biomass. ACS Sustain. Chem. Eng. 2017, 5, 7344–7351. [Google Scholar] [CrossRef]
- Cláudio, A.F.M.; Cognigni, A.; de Faria, E.L.P.; Silvestre, A.J.D.; Zirbs, R.; Freire, M.G.; Bica, K. Valorization of olive tree leaves: Extraction of oleanolic acid using aqueous solutions of surface-active ionic liquids. Sep. Purif. Technol. 2018, 204, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Hornedo, N.; Murphy, D. Significance of controlling crystallization mechanisms and kinetics in pharmaceutical systems. J. Pharm. Sci. 1999, 88, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.Q.; Chew, J.W.; Chow, P.S.; Tan, R.B.H. Recent advances in crystallization control: An industrial perspective. Chem. Eng. Res. Des. 2007, 85, 893–905. [Google Scholar] [CrossRef]
- An, J.H.; Kim, J.M.; Chang, S.M.; Kim, W.S. Application of ionic liquid to polymorphic design of pharmaceutical ingredients. Cryst. Growth Des. 2010, 10, 3044–3050. [Google Scholar] [CrossRef]
- Desiraju, G.R. Crystal engineering: A holistic view. Angew. Chem. Int. Ed. 2007, 46, 8342–8356. [Google Scholar] [CrossRef]
- Weber, C.C.; Kunov-Kruse, A.J.; Rogers, R.D.; Myerson, A.S. Manipulation of ionic liquid anion-solute-antisolvent interactions for the purification of acetaminophen. Chem. Commun. 2015, 51, 4294–4297. [Google Scholar] [CrossRef]
- An, J.H.; Jin, F.; Kim, H.S.; Ryu, H.C.; Kim, J.S.; Kim, H.M.; Kim, K.H.; Kiyonga, A.N.; Jung, K. Investigation of the Polymorphic Transformation of the Active Pharmaceutical Ingredient Clopidogrel Bisulfate Using the Ionic Liquid AEImBF4. Cryst. Growth Des. 2016, 16, 1829–1836. [Google Scholar] [CrossRef]
- An, J.H.; Jin, F.; Kim, H.S.; Ryu, H.C.; Kim, J.S.; Kim, H.M.; Kiyonga, A.N.; Min, D.S.; Youn, W.; Kim, K.H.; et al. Application of ionic liquid to polymorphic transformation of anti-viral/HIV drug adefovir dipivoxil. Arch. Pharm. Res. 2016, 39, 646–659. [Google Scholar] [CrossRef]
- An, J.H.; Kim, W.S. Antisolvent crystallization using ionic liquids as solvent and antisolvent for polymorphic design of active pharmaceutical ingredient. Cryst. Growth Des. 2013, 13, 31–39. [Google Scholar] [CrossRef]
- Weber, C.C.; Kulkarni, S.A.; Kunov-Kruse, A.J.; Rogers, R.D.; Myerson, A.S. The use of cooling crystallization in an ionic liquid system for the purification of pharmaceuticals. Cryst. Growth Des. 2015, 15, 4946–4951. [Google Scholar] [CrossRef]
- Berry, D.A.; Dye, S.R.; Ng, K.M. Synthesis of Drowning-Out Crystallization-Based Separations. AIChE J. 1997, 43, 91–103. [Google Scholar] [CrossRef]
- Viçosa, A.; Letourneau, J.J.; Espitalier, F.; Inês Ré, M. An innovative antisolvent precipitation process as a promising technique to prepare ultrafine rifampicin particles. J. Cryst. Growth 2012, 342, 80–87. [Google Scholar] [CrossRef] [Green Version]
- Martins, I.C.B.; Gomes, J.R.B.; Duarte, M.T.; Mafra, L. Understanding polymorphic control of pharmaceuticals using Imidazolium-based ionic liquid mixtures as crystallization directing agents. Cryst. Growth Des. 2017, 17, 428–432. [Google Scholar] [CrossRef]
- Reece, H.A.; Levendis, D.C. Polymorphs of gabapentin. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2008, 64, 105–108. [Google Scholar] [CrossRef]
- Dempah, K.E.; Barich, D.H.; Kaushal, A.M.; Zong, Z.; Desai, S.D.; Suryanarayanan, R.; Kirsch, L.; Munson, E.J. Investigating gabapentin polymorphism using solid-state NMR spectroscopy. AAPS PharmSciTech 2013, 14, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.B.; Bridson, R.H.; Leeke, G.A. Crystallisation control of paracetamol from ionic liquids. CrystEngComm 2014, 16, 10797–10803. [Google Scholar] [CrossRef]
- Mirmehrabi, M.; Rohani, S. An approach to solvent screening for crystallization of polymorphic pharmaceutical and fine chemicals. J. Pharm. Sci. 2005, 94, 1560–1576. [Google Scholar] [CrossRef]
- Hough, W.L.; Rogers, R.D. Ionic Liquids Then and Now: From Solvents to Materials to Active Pharmaceutical Ingredients. Bull. Chem. Soc. Jpn. 2007, 80, 2262–2269. [Google Scholar] [CrossRef]
- Carson, L.; Chau, P.K.W.; Earle, M.J.; Gilea, M.A.; Gilmore, B.F.; Gorman, S.P.; Mccann, T.; Seddon, K.R. Antibiofilm activities of 1-alkyl-3-methylimidazolium chloride ionic liquids. Green Chem. 2009, 44, 492–497. [Google Scholar] [CrossRef]
- Anvari, S.; Hajfarajollah, H.; Mokhtarani, B.; Enayati, M. Antibacterial and anti-adhesive properties of ionic liquids with various cationic and anionic heads toward pathogenic bacteria. J. Mol. Liq. 2016, 221, 685–690. [Google Scholar] [CrossRef]
- Taylor, P.; Nancharaiah, Y.V.; Reddy, G.K.K.; Lalithamanasa, P.; Venugopalan, V.P. The ionic liquid 1-alkyl-3-methylimidazolium demonstrates comparable antimicrobial and antibiofilm behavior to a cationic surfactant. Biofouling J. Bioadhes. Biofilm 2012, 28, 1141–1149. [Google Scholar] [CrossRef]
- Docherty, K.M.; Kulpa, C.F. Toxicity and antimicrobial activity of imidazolium and pyridinium ionic liquids. Green Chem. 2005, 7, 185–189. [Google Scholar] [CrossRef]
- Cornellas, A.; Perez, L.; Comelles, F.; Ribosa, I.; Manresa, A.; Garcia, M.T. Self-aggregation and antimicrobial activity of imidazolium and pyridinium based ionic liquids in aqueous solution. J. Colloid Interface Sci. 2011, 355, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Benedetto, A.; Bingham, R.J.; Ballone, P. Structure and dynamics of POPC bilayers in water solutions of room temperature ionic liquids. J. Chem. Phys. 2015, 142, 03B622_1. [Google Scholar] [CrossRef] [PubMed]
- Doria, O.F.; Castro, R.; Gutierrez, M.; Valenzuela, D.G.; Santos, L.; Ramirez, D.; Guzman, L. Novel Alkylimidazolium Ionic Liquids as an Antibacterial Alternative to Pathogens of the Skin and Soft Tissue Infections. Molecules 2018, 23, 2354. [Google Scholar] [CrossRef] [Green Version]
- Cho, C.; Stolte, S.; Yun, Y. Comprehensive approach for predicting toxicological effects of ionic liquids on several biological systems using unified descriptors. Sci. Rep. 2016, 33403, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hodyna, D.; Kovalishyn, V.; Semenyuta, I.; Blagodatnyi, V.; Rogalsky, S.; Metelytsia, L. Imidazolium ionic liquids as effective antiseptics and disinfectants against drug resistant S. aureus: In silico and in vitro studies. Comput. Biol. Chem. 2018, 73, 127–138. [Google Scholar] [CrossRef]
- Zheng, W.; Huang, W.; Song, Z.; Tang, Z.; Sun, W. Insight into Structure-Antibacterial Activity of Amino Cation- based and Acetate Anion-based Ionic Liquids from the Computational Interaction with POPC Phospholipid Bilayer. Phys. Chem. Chem. Phys. 2020, 22, 15573–15581. [Google Scholar] [CrossRef]
- Fister, S.; Mester, P.; Sommer, J.; Witte, A.K.; Kalb, R.; Wagner, M.; Rossmanith, P. Virucidal Influence of Ionic Liquids on Phages P100 and MS2. Front. Microbiol. 2017, 8, 1608. [Google Scholar] [CrossRef]
- Bergamo, V.Z.; Donato, R.K.; Lana, D.F.D.; Donato, K.J.Z.; Ortega, G.G.; Schrekker, H.S. Imidazolium salts as antifungal agents: Strong antibiofilm activity against multidrug-resistant Candida tropicalis isolates. Lett. Appl. Microbiol. 2014, 60, 66–71. [Google Scholar] [CrossRef]
- Hough-troutman, W.L.; Smiglak, M.; Griffin, S.; Reichert, W.M.; Mirska, I.; Jodynis-liebert, J.; Adamska, T.; Nawrot, J.; Stasiewicz, M.; Rogers, D.; et al. Ionic liquids with dual biological function: Sweet and anti-microbial, hydrophobic quaternary ammonium-based salts. New J. Chem. 2009, 33, 26–33. [Google Scholar] [CrossRef]
- Petkovic, M.; Ferguson, J.; Bohn, A.; Trindade, J.; Martins, I.; Carvalho, M.B.; Leit, M.C.; Rodrigues, C.; Garcia, H.; Ferreira, R.; et al. Exploring fungal activity in the presence of ionic liquids. Green Chem. 2009, 11, 889–894. [Google Scholar] [CrossRef]
- Suchodolski, J.; Feder-kubis, J.; Krasowska, A. Antifungal activity of ionic liquids based on (−)-menthol: A mechanism study. Microbiol. Res. 2017, 197, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Sintra, T.E.; Luís, A.; Rocha, S.N.; Ferreira, A.I.M.C.L.; Gonçalves, F.; Santos, L.M.N.B.F.; Neves, B.M.; Freire, M.G.; Ventura, S.P.M.; Coutinho, J.A.P. Enhancing the antioxidant characteristics of phenolic acids by their conversion into cholinium salts. ACS Sustain. Chem. Eng. 2015, 3, 2558–2565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czerniak, K.; Walkiewicz, F. Synthesis and antioxidant properties of dicationic ionic liquids. New J. Chem. 2017, 41, 530–539. [Google Scholar] [CrossRef]
- Ahmad, N.A.; Jumbri, K.; Ramli, A.; Ghani, N.A.; Ahmad, H.; Kassim, M.A. Synthesis, characterisation and antioxidant properties of ferulate-based protic ionic liquids: Experimental and modelling approaches. J. Mol. Liq. 2019, 278, 309–319. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Morais, E.S.; Leite, A.C.; Mohamadou, A.; Holmbom, B.; Holmbom, T.; Neves, B.M.; Silvestre, A.J. Enhanced Extraction and Biological Activity of 7- hydroxymatairesinol obtained from Norway Spruce knots using Aqueous Solutions of Ionic Liquids. Green Chem. 2017, 19, 2626–2635. [Google Scholar] [CrossRef]
- Malhotra, S.V.; Kumar, V. A profile of the in vitro anti-tumor activity of imidazolium-based ionic liquids. Bioorg. Med. Chem. Lett. 2010, 20, 581–585. [Google Scholar] [CrossRef]
- Kumar, V.; Malhotra, S.V. Study on the potential anti-cancer activity of phosphonium and ammonium-based ionic liquids. Bioorg. Med. Chem. Lett. 2009, 19, 4643–4646. [Google Scholar] [CrossRef]
- Li, X.; Jing, C.; Lei, W.; Li, J.; Wang, J. Apoptosis caused by imidazolium-based ionic liquids in PC12 cells. Ecotoxicol. Environ. Saf. 2012, 83, 102–107. [Google Scholar] [CrossRef]
- Hough, W.L.; Smiglak, M.; Rodríguez, H.; Swatloski, R.P.; Spear, S.K.; Daly, D.T.; Pernak, J.; Grisel, J.E.; Carliss, R.D.; Soutullo, M.D.; et al. The third evolution of ionic liquids: Active pharmaceutical ingredients. New J. Chem. 2007, 31, 1429. [Google Scholar] [CrossRef]
- Dean, P.M.; Turanjanin, J.; Yoshizawa-fujita, M.; Macfarlane, D.R.; Scott, J.L. Exploring an Anti-Crystal Engineering Approach to the Preparation of Pharmaceutically Active Ionic Liquids. Cryst. Growth Des. 2009, 9, 1137–1145. [Google Scholar] [CrossRef]
- Sastry, N.V.; Singh, D.K. Surfactant and Gelation Properties of Acetylsalicylate Based Room Temperature Ionic Liquid in Aqueous Media. Langmuir 2016, 32, 10000–10016. [Google Scholar] [CrossRef] [PubMed]
- Suresh, C.; Zhao, H.; Gumbs, A.; Chetty, C.S.; Bose, H.S. New ionic derivatives of betulinic acid as highly potent anti-cancer agents. Bioorg. Med. Chem. Lett. 2012, 22, 1734–1738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherukuvada, S.; Nangia, A. Polymorphism in an API ionic liquid: Ethambutol dibenzoate trimorphs. CrystEngComm 2012, 14, 7840–7843. [Google Scholar] [CrossRef]
- Tourne, C.; Judeinstein, P.; In, M.; Viau, L. Surfactant properties of ionic liquids containing short alkyl chain imidazolium cations and ibuprofenate anions. Phys. Chem. Chem. Phys. 2011, 13, 15523–15529. [Google Scholar] [CrossRef]
- Ferraz, R.; Branco, L.C.; Prudêncio, C.; Noronha, J.P.; Petrovski, Ž. Ionic liquids as active pharmaceutical ingredients. ChemMedChem 2011, 6, 975–985. [Google Scholar] [CrossRef]
- Bica, K.; Rijksen, C.; Nieuwenhuyzen, M.; Rogers, R.D. In search of pure liquid salt forms of aspirin: Ionic liquid approaches with acetylsalicylic acid and salicylic acid. Phys. Chem. Chem. Phys. 2010, 12, 2011–2017. [Google Scholar] [CrossRef]
- Rogers, R.D.; Daly, D.T.; Swatloski, R.P.; Hough-Troutman, W.L.; Hough-Troutman, J.J.H.L.; Marcin, S.; Juliusz, P.; Spear, S.K. Multifunctional Ionic Liquid Compositions for Overcoming Polymorphism and Imparting Improved Properties for Active Pharmaceutical, Biological, Nutritional and Energetic Ingredients. U.S. Patent No. 8,232,265; PCT/US2006/039454; WO 2007/044693 A2 (2007), 18 October 2012. [Google Scholar]
- Frizzo, C.P.; Wust, K.; Tier, A.Z.; Vaucher, R.A.; Bolzan, L.P.; Terra, S.; Martins, M.A.P. Novel ibuprofenate- and docusate-based ionic liquids: Emergence of antimicrobial activity. RSC Adv. 2016, 6, 100476–100486. [Google Scholar] [CrossRef]
- Fernandez-Stefanuto, V.; Tojo, E. New Active Pharmaceutical Ingredient-Ionic Liquids (API-ILs) Derived from Indomethacin and Mebendazole. Proceedings 2018, 9, 48. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Holmes, S.S.; Baker, G.A.; Challa, S.; Bose, H.S.; Song, Z. Ionic derivatives of betulinic acid as novel HIV-1 protease inhibitors. J. Enzyme Inhib. Med. Chem. 2012, 27, 715–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Florindo, C.; Pereiro, A.B.; Vieira, N.S.M.; Matias, A.A.; Duarte, C.M.M.; Rebelo, P.N.; Marrucho, I.M. Cholinium-based ionic liquids with pharmaceutically active anions. RSC Adv. 2014, 28126–28132. [Google Scholar] [CrossRef]
- Balk, A.; Wiest, J.; Widmer, T.; Galli, B.; Holzgrabe, U.; Meinel, L. Transformation of acidic poorly water soluble drugs into ionic liquids. Eur. J. Pharm. Biopharm. 2015, 94, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Branco, L.C.; Jorda, N. Dipolar motions and ionic conduction in an ibuprofen derived ionic liquid. Phys. Chem. Chem. Phys. 2015, 17, 24108–24120. [Google Scholar] [CrossRef] [Green Version]
- Ferraz, R.; Branco, L.C.; Marrucho, I.M.; Araújo, J.M.; Rebelo, L.P.N.; da Ponte, M.N.; Petrovski, Ž. Development of novel ionic liquids based on ampicillin. Med. Chem. Commun. 2012, 3, 494–497. [Google Scholar] [CrossRef] [Green Version]
- Florindo, C.; Araújo, J.M.; Alves, F.; Matos, C.; Ferraz, R.; Prudêncio, C.; Marrucho, I.M. Evaluation of solubility and partition properties of ampicillin-based ionic liquids. Int. J. Pharm. 2013, 456, 553–559. [Google Scholar] [CrossRef] [Green Version]
- Ferraz, R.; Noronha, P. Advances Antibacterial activity of Ionic Liquids based on ampicillin against resistant bacteria. RSC Adv. 2014, 4, 4301–4307. [Google Scholar] [CrossRef] [Green Version]
- Ferraz, R.; Costa-Rodrigues, J.; Fernandes, M.H.; Santos, M.M.; Marrucho, I.M.; Rebelo, L.P.N.; Prudêncio, C.; Noronha, J.P.; Petrovski, Ž.; Branco, L.C. Antitumor Activity of Ionic Liquids Based on Ampicillin. ChemMedChem 2015, 10, 1480–1483. [Google Scholar] [CrossRef] [Green Version]
- Ferraz, R.; Noronha, J.; Murtinheira, F.; Nogueira, F.; Machado, M.; Prudêncio, M.; Parapini, S.; D’Alessandro, S.; Teixeira, C.; Gomes, A.; et al. Primaquine-based ionic liquids as a novel class of antimalarial hits. RSC Adv. 2016, 6, 56134–56138. [Google Scholar] [CrossRef]
- Ferraz, R.; Pinheiro, M.; Gomes, A.; Teixeira, C.; Prudêncio, C.; Reis, S.; Gomes, P. Effects of novel triple-stage antimalarial ionic liquids on lipid membrane models. Bioorg. Med. Chem. Lett. 2017, 27, 4190–4193. [Google Scholar] [CrossRef]
- Miwa, Y.; Hamamoto, H.; Ishida, T. Lidocaine self-sacrificially improves the skin permeation of the acidic and poorly water-soluble drug etodolac via its transformation into an ionic liquid. Eur. J. Pharm. Biopharm. 2016, 102, 92–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berton, P.; Di Bona, K.R.; Yancey, D.; Rizvi, S.A.A.; Gray, M.; Gurau, G.; Shamshina, J.L.; Rasco, J.F.; Rogers, R.D. Transdermal Bioavailability in Rats of Lidocaine in the Forms of Ionic Liquids, Salts, and Deep Eutectic. ACS Med. Chem. Lett. 2017, 8, 498–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansson, K.M.; Izgorodina, E.I.; Forsyth, M.; Macfarlane, D.R.; Seddon, K.R. Protic ionic liquids based on the dimeric and oligomeric anions: [(AcO)xHx-1]-. Phys. Chem. Chem. Phys. 2008, 10, 2972–2978. [Google Scholar] [CrossRef] [PubMed]
- Bica, K.; Rogers, R.D. Confused ionic liquid ions—A ‘“liquification”’ and dosage strategy for pharmaceutically active salts. Chem. Commun. 2010, 46, 1215–1217. [Google Scholar] [CrossRef]
- Stoimenovski, J.; Dean, P.M.; Izgorodina, E.I.; Macfarlane, D.R. Protic pharmaceutical ionic liquids and solids: Aspects of protonics. Faraday Discuss. 2012, 154, 335–352. [Google Scholar] [CrossRef]
- Hajnal, K.; Gabriel, H.; Aura, R.; Erzsébet, V.; Blanka, S.S. Prodrug Strategy in Drug Development. Acta Med. Marisiensis 2016, 62, 356–362. [Google Scholar] [CrossRef] [Green Version]
- N’Da, D.D. Prodrug Strategies for Enhancing the Percutaneous Absorption of Drugs. Molecules 2014, 19, 20780–20807. [Google Scholar] [CrossRef] [Green Version]
- Aguiar, A.J.; Krc, J.; Kinkel, A.W.; Samyn, J.C. Effect of polymorphism on the absorption of chloramphenicol from chloramphenicol palmitate. J. Pharm. Sci. 1967, 56, 847–853. [Google Scholar] [CrossRef]
- Cojocaru, O.A.; Bica, K.; Gurau, G.; Narita, A.; Mccrary, P.D.; Shamshina, J.L.; Barber, S.; Rogers, R.D. Prodrug ionic liquids: Functionalizing neutral active ionic liquid form. Med. Chem. Commun. 2013, 4, 559–563. [Google Scholar] [CrossRef]
- Staben, L.R.; Koenig, S.G.; Lehar, S.M.; Vandlen, R.; Zhang, D.; Chuh, J.; Yu, S.; Ng, C.; Guo, J.; Liu, Y.; et al. Targeted drug delivery through the traceless release of tertiary and heteroaryl amines from antibody–drug conjugates. Nat. Chem. 2016, 8, 1112–1119. [Google Scholar] [CrossRef]
- Dias, A.M.A.; Cortez, A.R.; Barsan, M.M.; Santos, J.B.; Brett, C.M.A.; De Sousa, H.C. Development of greener multi-responsive chitosan biomaterials doped with biocompatible ammonium ionic liquids. ACS Sustain. Chem. Eng. 2013, 1, 1480–1492. [Google Scholar] [CrossRef]
- De Almeida, T.S.; Júlio, A.; Mota, J.P.; Rijo, P.; Reis, C.P. An emerging integration between ionic liquids and nanotechnology: General uses and future prospects in drug delivery. Ther. Deliv. 2017, 8, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Halayqa, M.; Zawadzki, M.; Domańska, U.; Plichta, A. Polymer—Ionic liquid—Pharmaceutical conjugates as drug delivery systems. J. Mol. Struct. 2019, 1180, 573–584. [Google Scholar] [CrossRef]
- Khan, A.B.; Ali, M.; Malik, N.A.; Ali, A.; Patel, R. Role of 1-methyl-3-octylimidazolium chloride in the micellization behavior of amphiphilic drug amitriptyline hydrochloride. Colloids Surf. B Biointerfaces 2013, 112, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Mehrdad, A.; Miri, A.H. Aqueous solubility of acetaminophen in the presence of 1-hexyl-3-methyl imidazolium bromide, ionic liquid as co-solvent. Fluid Phase Equilib. 2016, 425, 51–56. [Google Scholar] [CrossRef]
- Oh, D.X.; Shin, S.; Lim, C.; Hwang, D.S. Dopamine-mediated sclerotization of regenerated chitin in ionic liquid. Materials 2013, 6, 3826–3839. [Google Scholar] [CrossRef]
- Silva, S.S.; Popa, E.G.; Gomes, M.E.; Oliveira, M.B.; Nayak, S.; Subia, B.; Mano, J.F.; Kundu, S.C.; Reis, R.L. Silk hydrogels from non-mulberry and mulberry silkworm cocoons processed with ionic liquids. Acta Biomater. 2013, 9, 8972–8982. [Google Scholar] [CrossRef] [Green Version]
- Meng, Z.; Zheng, X.; Tang, K.; Liu, J.; Ma, Z.; Zhao, Q. Dissolution and regeneration of collagen fibers using ionic liquid. Int. J. Biol. Macromol. 2012, 51, 440–448. [Google Scholar] [CrossRef]
- De Carvalho, R.N.L.; Lourenço, N.M.T.; Gomes, P.M.V.; Da Fonseca, L.J.P. Swelling behavior of gelatin-ionic liquid functional polymers. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 817–825. [Google Scholar] [CrossRef]
- Pandey, P.K.; Rawat, K.; Aswal, V.K.; Kohlbrecher, J.; Bohidar, H.B. DNA ionogel: Structure and self-assembly. Phys. Chem. Chem. Phys. 2017, 19, 804–812. [Google Scholar] [CrossRef]
- Rawat, K.; Aswal, V.K.; Bohidar, H.B. DNA-gelatin complex coacervation, UCST and first-order phase transition of coacervate to anisotropic ion gel in 1-methyl-3-octylimidazolium chloride ionic liquid solutions. J. Phys. Chem. B 2012, 116, 14805–14816. [Google Scholar] [CrossRef]
- Barber, P.S.; Griggs, C.S.; Bonner, J.R.; Rogers, R.D. Electrospinning of chitin nanofibers directly from an ionic liquid extract of shrimp shells. Green Chem. 2013, 15, 601–607. [Google Scholar] [CrossRef]
- Fu, R.; Ji, X.; Ren, Y.; Wang, G.; Cheng, B. Antibacterial blend films of cellulose and chitosan prepared from binary ionic liquid system. Fibers Polym. 2017, 18, 852–858. [Google Scholar] [CrossRef]
- Murugesan, S.; Wiencek, J.M.; Ren, R.X.; Linhardt, R.J. Benzoate-based room temperature ionic liquids—Thermal properties and glycosaminoglycan dissolution. Carbohydr. Polym. 2006, 63, 268–271. [Google Scholar] [CrossRef]
- Trivedi, T.J.; Srivastava, D.N.; Rogers, R.D.; Kumar, A. Agarose processing in protic and mixed protic-aprotic ionic liquids: Dissolution, regeneration and high conductivity, high strength ionogels. Green Chem. 2012, 14, 2831–2839. [Google Scholar] [CrossRef] [Green Version]
- Viau, L.; Tourné-Péteilh, C.; Devoisselle, J.M.; Vioux, A. Ionogels as drug delivery system: One-step sol-gel synthesis using imidazolium ibuprofenate ionic liquid. Chem. Commun. 2010, 46, 228–230. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xie, F.; Li, X.; Chen, L. Ionic liquids for the preparation of biopolymer materials for drug/gene delivery: A review. Green Chem. 2018, 20, 4169–4200. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Xu, H. The optimal choice of medication administration route regarding intravenous, intramuscular, and subcutaneous injection. Patient Prefer. Adherence 2015, 15, 923–942. [Google Scholar] [CrossRef] [Green Version]
- Maddison, J.E.; Page, S.W.; Dyke, T.M. Chapter 2—Clinical Pharmacokinetics, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Bharmoria, P.; Singh, T.; Kumar, A. Complexation of chitosan with surfactant like ionic liquids: Molecular interactions and preparation of chitosan nanoparticles. J. Colloid Interface Sci. 2013, 407, 361–369. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; Boamah, P.O.; Cao, L.; Zhang, Q.; Lu, Z.; Li, H. Homogeneous synthesis of linoleic acid-grafted chitosan oligosaccharide in ionic liquid and its self-assembly performance in aqueous solution. J. Appl. Polym. Sci. 2015, 132, 1–8. [Google Scholar] [CrossRef]
- Tang, W.; Liu, B.; Wang, S.; Liu, T.; Fu, C.; Ren, X.; Tan, L.; Duan, W.; Meng, X. Doxorubicin-loaded Ionic Liquid-Polydopamine nanoparticles for combined chemotherapy and microwave thermal therapy of cancer. RSC Adv. 2016, 6, 32434–32440. [Google Scholar] [CrossRef]
- Dong, H.; Xu, Q.; Li, Y.; Mo, S.; Cai, S.; Liu, L. The synthesis of biodegradable graft copolymer cellulose-graft-poly(l-lactide) and the study of its controlled drug release. Colloids Surf. B Biointerfaces 2008, 66, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Tahara, Y.; Tamura, M.; Kamiya, N.; Goto, M. Ionic liquid-assisted transdermal delivery of sparingly soluble drugs. Chem. Commun. 2010, 46, 1452–1454. [Google Scholar] [CrossRef] [PubMed]
- Parsi, E.; Salabat, A. Comparison of O/W and IL/W Microemulsion Systems as Potential Carriers of Sparingly Soluble Celecoxib Drug. J. Solut. Chem. 2020, 49, 68–82. [Google Scholar] [CrossRef]
- Demirkurt, B.; Cakan-akdogan, G.; Akdogan, Y. Preparation of albumin nanoparticles in water-in-ionic liquid microemulsions. J. Mol. Liq. 2019, 295, 111713. [Google Scholar] [CrossRef]
- Esson, M.M.; Mecozzi, S.; Mecozzi, S. Preparation, Characterization, and Formulation Optimization of Ionic-Liquid-in-Water Nanoemulsions toward Systemic Delivery of Amphotericin B. Mol. Pharm. 2020, 17, 2221–2226. [Google Scholar] [CrossRef]
- Taylor, P.; Hosseinzadeh, F.; Mahkam, M. Synthesis and characterization of ionic liquid functionalized polymers for drug delivery of an anti-inflammatory drug. Des. Monomers Polym. 2012, 15, 379–388. [Google Scholar]
- Rasouli, S.; Davaran, S.; Rasouli, F.; Mahkam, M.; Salehi, S. Synthesis, characterization and pH-controllable methotrexate release from biocompatible polymer/silica nanocomposite for anticancer drug delivery. Drug Deliv. 2013, 1, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, A.; Ibsen, K.; Brown, T.; Chen, R.; Agatemor, C.; Mitragotri, S. Ionic liquids for oral insulin delivery. Proc. Natl. Acad. Sci. USA 2018, 115, 7296–7301. [Google Scholar] [CrossRef] [Green Version]
- Bica, K.; Rodríguez, H.; Gurau, G.; Cojocaru, O.A.; Riisager, A.; Fehrmann, R.; Rogers, R.D. Pharmaceutically active ionic liquids with solids handling, enhanced thermal stability, and fast release. Chem. Commun. 2012, 48, 5422–5424. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Tian, T.; Gao, Y.; Ji, X.; Li, Z. Pharmaceutically Active Ionic Liquid Self-Assembled Vesicles for the Application as an Efficient Drug Delivery System. ChemPhysChem 2013, 14, 3454–3457. [Google Scholar] [CrossRef] [PubMed]
- Sahbaz, Y.; Williams, H.D.; Nguyen, T.; Saunders, J.; Ford, L.; Charman, S.A.; Scammells, P.J.; Porter, C.J.H. Transformation of Poorly Water-Soluble Drugs into Lipophilic Ionic Liquids Enhances Oral Drug Exposure from Lipid Based Formulations. Mol. Pharm. 2015, 12, 1980–1991. [Google Scholar] [CrossRef] [PubMed]
- Benson, H.A.E.; Grice, J.E.; Mohammed, Y.; Namjoshi, S.; Roberts, M.S. Topical and Transdermal Drug Delivery: From Simple Potions to Smart Technologies. Curr. Drug Deliv. 2019, 16, 444–460. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]
- Caparica, R.; Júlio, A.; Rosadoand, C.; Santos, T. Applicability of Ionic Liquids in Topical Drug Delivery Systems: A Mini Review. J. Pharmacol. Clin. Res. 2018, 4, 555649–555655. [Google Scholar] [CrossRef]
- Mahajan, S.; Sharma, R.; Mahajan, R.K. An Investigation of Drug Binding Ability of a Surface Active Ionic Liquid: Micellization, Electrochemical, and Spectroscopic Studies. Langmuir 2012, 18, 17238–17246. [Google Scholar] [CrossRef]
- Pal, A.; Yadav, A. Binding interactions of anesthetic drug with surface active ionic liquid. J. Mol. Liq. 2016, 222, 471–479. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Tamura, M.; Tahara, Y.; Kamiya, N.; Goto, M. Ionic liquid-in-oil microemulsion as a potential carrier of sparingly soluble drug: Characterization and cytotoxicity evaluation. Int. J. Pharm. 2010, 400, 243–250. [Google Scholar] [CrossRef]
- Islam, R.; Chowdhury, R.; Wakabayashi, R.; Kamiya, N. Ionic Liquid-In-Oil Microemulsions Prepared with Biocompatible Choline Carboxylic Acids for Improving the Transdermal Delivery of a Sparingly Soluble Drug. Pharmaceutics 2020, 12, 392. [Google Scholar] [CrossRef]
- Yoshiura, H.; Tamura, M.; Aso, M.; Kamiya, N.; Goto, M. Ionic Liquid-in-Oil Microemulsions as Potential Carriers for the Transdermal Delivery of Methotrexate. J. Chem. Eng. Jpn. 2013, 46, 794–796. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, J.; Zhang, D.; Yang, Y.; Zheng, L.; Qu, Y.; Yang, X.; Cui, X. Ionic liquid—Microemulsions assisting in the transdermal delivery of Dencichine: Preparation, in-vitro and in-vivo evaluations, and investigation of the permeation mechanism. Int. J. Pharm. 2018, 535, 120–131. [Google Scholar] [CrossRef] [PubMed]
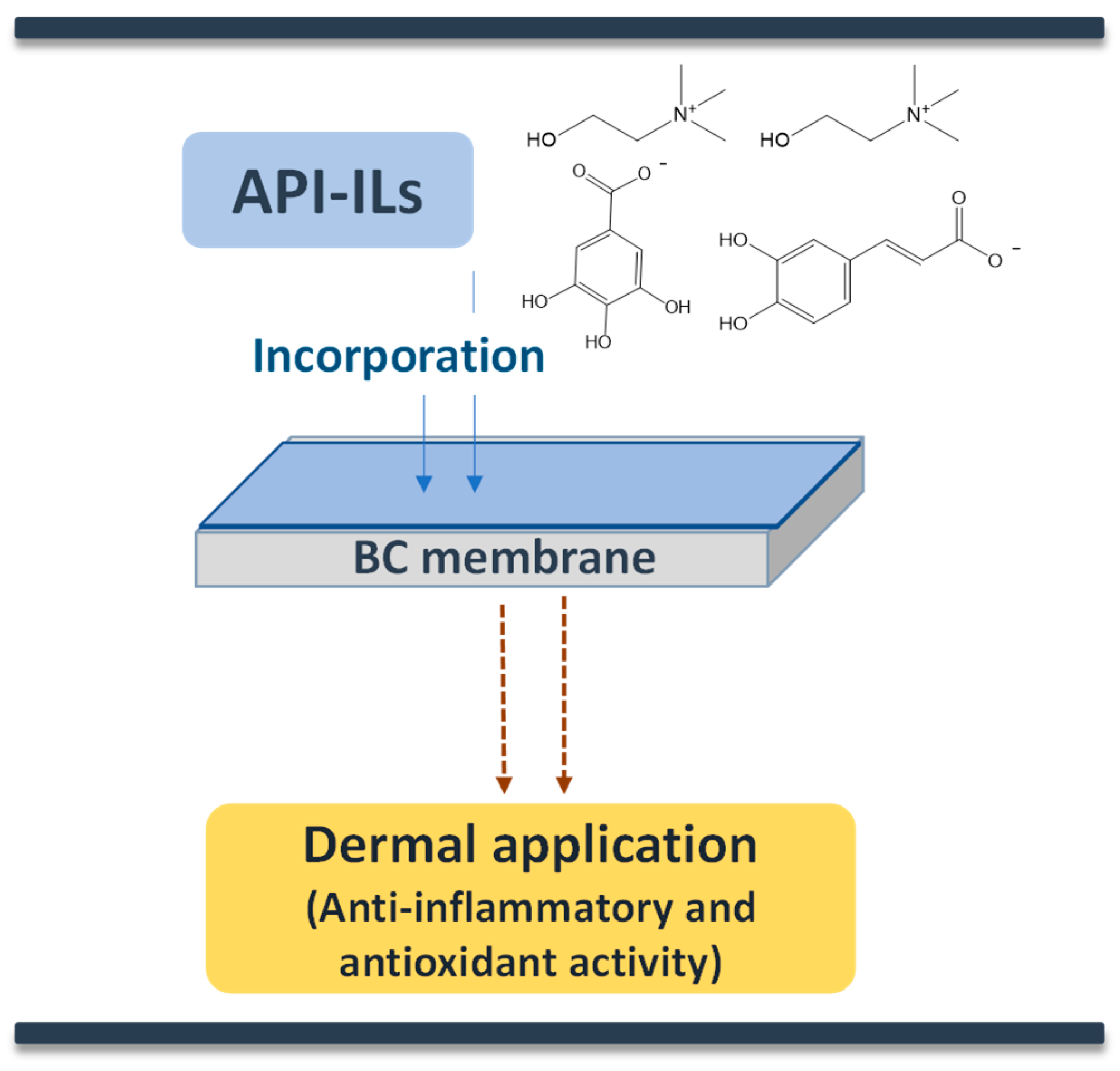
- Morais, E.S.; Silva, N.H.C.S.; Sintra, T.E.; Santos, S.A.O.; Neves, B.M.; Almeida, I.F.; Costa, P.C.; Correia-Sá, I.; Ventura, S.P.M.; Silvestre, A.J.D.; et al. Anti-inflammatory and antioxidant nanostructured cellulose membranes loaded with phenolic-based ionic liquids for cutaneous application. Carbohydr. Polym. 2019, 206, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Chantereau, G.; Sharma, M.; Abednejad, A.; Neves, B.M.; Se, G.; Freire, M.G.; Freire, C.S.R.; Silvestre, A.J.D. Design of Nonsteroidal Anti-Inflammatory Drug-Based Ionic Liquids with Improved Water Solubility and Drug Delivery. ACS Sustain. Chem. Eng. 2019, 7, 14126–14134. [Google Scholar] [CrossRef]
- Chantereau, G.; Sharma, M.; Abednejad, A.; Vilela, C.; Costa, E.M.; Veiga, M.; Antunes, F.; Pintado, M.M.; Sèbe, G.; Coma, V.; et al. Bacterial nanocellulose membranes loaded with vitamin B-based ionic liquids for dermal care applications. J. Mol. Liq. 2020, 302, 112547. [Google Scholar] [CrossRef]
- Miwa, Y.; Hamamoto, H.; Hikake, S.; Kuwabara, Y. A Phase I, Randomized, Open-Label, Cross-Over Study of the Pharmacokinetics, Dermal Tolerability, and Safety of MRX-7EAT Etodolac-Lidocaine Topical Patch in Healthy Volunteers. J. Pain 2013, 14, S72. [Google Scholar] [CrossRef]
- Abednejad, A.; Ghaee, A.; Morais, E.S.; Sharma, M.; Neves, B.M.; Freire, M.G.; Nourmohammadi, J.; Mehrizi, A.A. Polyvinylidene fluoride–Hyaluronic acid wound dressing comprised of ionic liquids for controlled drug delivery and dual therapeutic behavior. Acta Biomater. 2019, 100, 142–157. [Google Scholar] [CrossRef] [PubMed]
- Júlio, A.; Caparica, R.; Lima, S.A.C.; Fernandes, A.S.; Rosado, C.; Prazeres, D.M.F.; Reis, S.; Almeida, S. De Ionic Liquid-Polymer Nanoparticle Hybrid Systems as New Tools to Deliver Poorly Soluble Drugs. Nanomaterials 2019, 9, 1148. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Wang, H.; Cui, X.; Wang, C.; Zhang, D.; Wang, H.; Cui, X.; Wang, C. Evaluations of imidazolium ionic liquids as novel skin permeation enhancers for drug transdermal delivery. Pharm. Dev. Technol. ISSN 2016, 22, 511–520. [Google Scholar] [CrossRef]
- Furukawa, S.Y.; Hattori, G.; Sakai, S.; Kamiya, N. Highly Efficient and Low Toxic Skin Penetrants Composed of Amino Acid Ionic Liquids. RSC Adv. 2016, 6, 87753–87755. [Google Scholar] [CrossRef]
- Zavgorodnya, O.; Shamshina, J.L.; Mittenthal, M.; McCrary, P.D.; Rachiero, G.P.; Titi, H.M.; Rogers, R.D. Polyethylene Glycol Derivatization of the Non-active Ion in Active Pharmaceutical Ingredient Ionic Liquids Enhances Transdermal Delivery. New J. Chem. 2017, 41, 1499–1508. [Google Scholar] [CrossRef]
- Zakrewsky, M.; Lovejoy, K.S.; Kern, T.L.; Miller, T.E.; Le, V.; Nagy, A. Ionic liquids as a class of materials for transdermal delivery and pathogen neutralization. Proc. Natl. Acad. Sci. USA 2014, 111, 13313–13318. [Google Scholar] [CrossRef] [Green Version]
- Zech, O.; Thomaier, S.; Kolodziejski, A.; Touraud, D.; Grillo, I.; Kunz, W. Ionic Liquids in Microemulsions—A Concept To Extend the Conventional Thermal Stability Range of Microemulsions. Chem. Eur. J. 2010, 16, 783–786. [Google Scholar] [CrossRef] [Green Version]
- Simon, L.S.; Zhao, S.Z.; Arguelles, L.M.; Lefkowith, J.B. Economic and Gastrointestinal Safety Comparisons of Etodolac, Nabumetone, and Oxaprozin from Insurance Claims Data from Patients with Arthritis. Clin. Ther. 1998, 20, 1218–1235. [Google Scholar] [CrossRef]
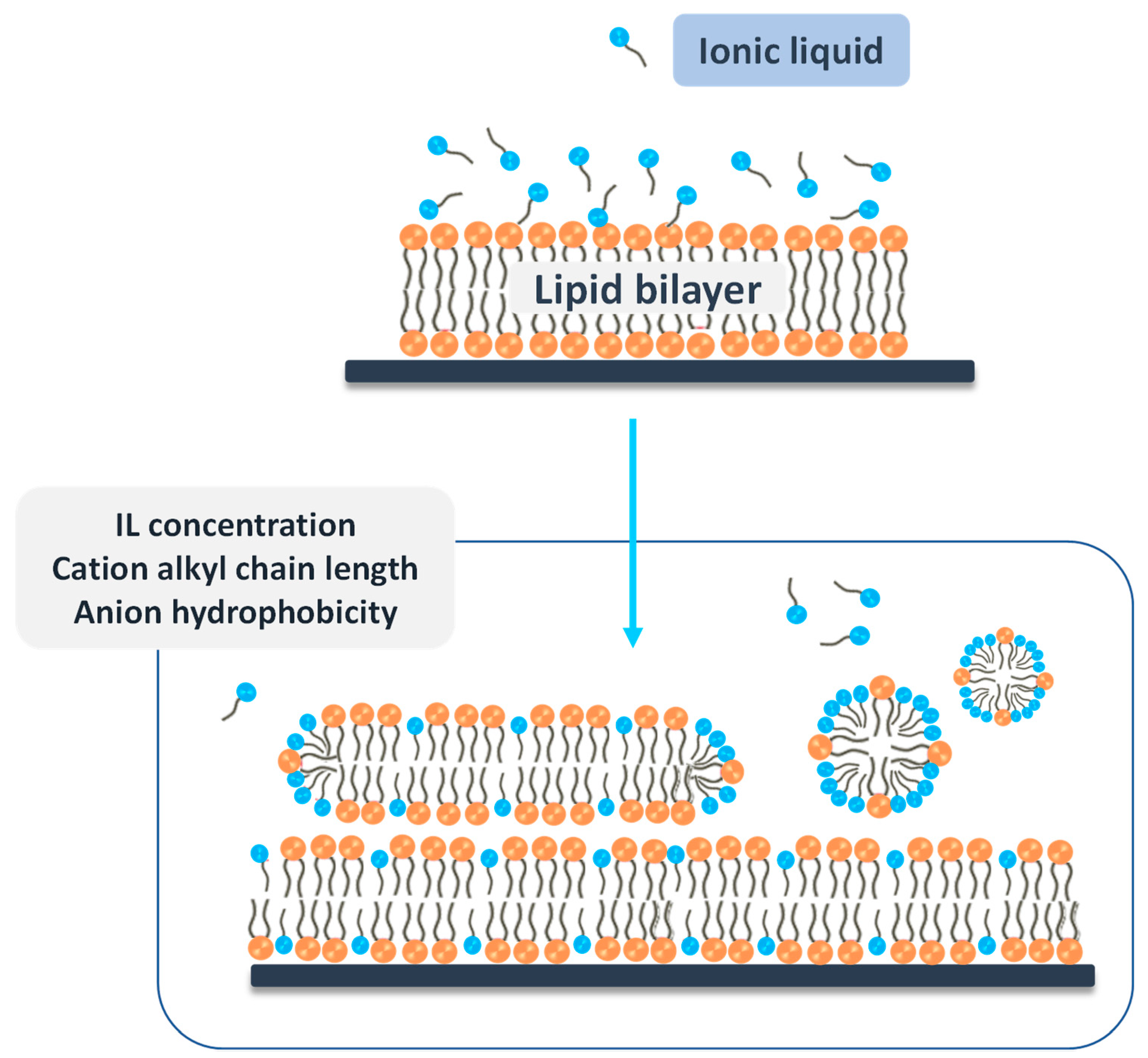
- Jing, B.; Lan, N.; Qiu, J.; Zhu, Y. Interaction of Ionic Liquids with Lipid Bilayer: A Biophysical Study of Ionic Liquid Cytotoxicity. J. Phys. Chem. B 2016, 120, 2781–2789. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, K.; Mitragotri, S. Mechanistic Analysis of Chemical Permeation Enhancers for Oral Drug Delivery. Pharm. Res. 2008, 25, 1412–1419. [Google Scholar] [CrossRef] [PubMed]
- Agatemor, C.; Ibsen, K.N.; Tanner, E.E.; Mitragotri, S. Ionic Liquids for Addressing Unmet Needs in Healthcare. Bioeng. Transl. Med. 2017, 3, 7–25. [Google Scholar]
- Kubota, K.; Shibata, A.; Yamaguchi, T. The molecular assembly of the ionic liquid/aliphatic carboxylic acid/aliphatic amine as effective and safety transdermal permeation enhancers. Eur. J. Pharm. Sci. 2016, 86, 75–83. [Google Scholar] [CrossRef]
- Janus, E.; Ossowicz, P.; Klebeko, J.; Nowak, A.; Duchnik, W.; Klimowicz, A. Enhancement of ibuprofen solubility and skin permeation by conjugation with L -valine alkyl esters. RSC Adv. 2020, 10, 7570–7584. [Google Scholar] [CrossRef] [Green Version]







| API | Structure | Water Solubility | IL | Solubility | Reference |
|---|---|---|---|---|---|
| 4-Hydroxycoumarin |  | - | [P666(14)][NTf2] | 0.0524 a * | [72] |
| [C2C1im][CF3O3S] | 0.1107 a * | ||||
| [C4C1im][CF3O3S] | 0.0907 a * | [73] | |||
| 5-Fluorouracil |  | 12.21 b * | [C4C1im]Br | 31.19 b * | [74] |
| Acetaminophen |  | 98.8 c19.16 b | [C4C1im][BF4] | >132 c | [69] |
| [C8C1im][BF4] | 126 c | ||||
| [C4C1im][PF6] | 52 c | ||||
| [C8C1im][PF6] | 10 c | ||||
| [C6C1im][PF6] | 13.21 b | [75] | |||
| Acetylcysteine |  | - | [C2C1im][CF3O3S] | 0.1711a * | [73] |
| [C4C1im][CF3O3S] | 0.1088 a * | ||||
| [C4C1im][NTf2] | 0.0866 a * | ||||
| [C6C1im][NTf2] | 0.0635 a | ||||
| [C10C1im][NTf2] | 0.0102 a * | ||||
| Albendazole |  | 0.0020 c | [C4C1im][BF4] | 1.49 c | [69] |
| [C6C1im][BF4] | 2.97 c | ||||
| [C8C1im] [BF4] | 7.2 c | ||||
| [C4C1im] [PF6] | 29 c | ||||
| [C6C1im] [PF6] | 53 c | ||||
| [C8C1im] [PF6] | >75 c | ||||
| Amphotericin B |  | 2.0 × 10−4 b | [C2C1im][CH3COO] | 85 b | [76] |
| [C4NH3][CH3COO] | 30 b | ||||
| [C6NH3][CH3COO] | 30 b | ||||
| [C8NH3][CH3COO] | 20 b | ||||
| [C4NH3][Oleate] | <5 b | ||||
| [C6NH3][Oleate] | <5 b | ||||
| [C8NH3][Oleate] | <5 b | ||||
| Danazol |  | 0.00030 c | [C4C1im] [BF4] | 18.9 c | [69] |
| [C8C1im] [BF4] | >59 c | ||||
| [C4C1im] [PF6] | 11.9 c | ||||
| [C8C1im] [PF6] | 35 c | ||||
| [C6C6OCOpy][N(CN)2 | >90 d | [77] | |||
| [C6C6OCOpy][NTf2] | 25 d | ||||
| Erythromycin |  | - | [C4C1im] [NTf2] | 0.037 a * | [78] |
| [C10C1im] [NTf2] | 0.072 a | ||||
| [P666(14)]Cl | 0.085 a | ||||
| [N4,1,1,1][NTf2] | 0.053 a | ||||
| [Pyrr4,1][NTf2] | 0.017 a | ||||
| Etodolac |  | Insoluble | [C4C1im] [PF6] | 374.33 b * | [79] |
| Fenofibrate |  | - | [C6C6OCOpy][N(CN)2] | >125 d | [77] |
| [C6C6OCOpy][NTf2] | >130 d | ||||
| Glibenclamide |  | 2.4 × 10−6 b * | [Ch][Try] | 9.89 b * | [80] |
| Ibuprofen |  | 0.124 | [C4C1im] [PF6] | 6.95 b | [75] |
| [C6C1im] [PF6] | 26.38 b | ||||
| [P666(14)][NTf2] | 0.0528 a | [72] | |||
| Isoniazid |  | - | [DDA][NO3] | 0.0452 a * | [81] |
| [C2][NTf2] | 0.0235 a | [82] | |||
| [C4C1im][NTf2] | 0.004 c | ||||
| [C6C1im][NTf2] | 0.003 c | ||||
| [P666(14)][NTf2] | 0.0651 c | [72] | |||
| Itraconazole |  | 1.0 × 10−6 b | [C2C1im][CH3COO] | <5 b | [76] |
| [C4NH3][CH3COO] | <5 b | ||||
| [C6NH3][CH3COO] | <5 b | ||||
| [C8NH3][CH3COO] | <5 b | ||||
| [C4NH3][Oleate] | <5 b | ||||
| [C6NH3][Oleate] | <5 b | ||||
| [C8NH3][Oleate] | <5 b | ||||
| [C6C6OCOpy][N(CN)2] | 40 d | [77] | |||
| - | [C6C6OCOpy][NTf2] | 8 d | |||
| Paclitaxel |  | <4.0 × 10−6 b | [Ch][Gly] | 22.34 b | [83] |
| [Ch][Ala] | 18.52 b | ||||
| [Ch][Pro] | 16.16 b | ||||
| [Ch][Phe] | 14.15 b | ||||
| [Ch][Ile] | 9.39 b | ||||
| [Ch][Ser] | 7.32 b | ||||
| [Ch][Leu] | 6.61 b | ||||
| Pyrazinecarboxamide |  | - | [C2C1im] [NTf2] | 0.0048 a | [84] |
| [C4C1im] [NTf2] | 0.0054 a* | ||||
| [C6C1im] [NTf2] | 0.0050 a * | ||||
| [C8C1im] [NTf2] | 0.0052 a | ||||
| [C10C1im] [NTf2] | 0.0046 a | ||||
| [C10C1im][CF3O3S] | 0.0116 a | ||||
| [C2][NTf2] | 0.0165 a | [81] | |||
| [P666(14)][NTf2] | 0.0125 a | [72] | |||
| Thymoquinone |  | - | [P666(14)][NTf2] | 0.1105 a | [72] |
| IL | DPPH Free Radical Scavenging (µM) | Reference Compound | DPPH Free Radical Scavenging (µM) | Reference |
|---|---|---|---|---|
| 2-(methylamino)ethanol ferulate | 17.40 | Ferulic acid | 21.40 | [124] |
| 2-(propylamino)ethanol ferulate | 16.61 | |||
| 2-(butylamino)ethanol ferulate | 16.34 | |||
| 3-dimethylamino-1-propanol ferulate | 12.93 | |||
| 3-diethylamino-1-propanol ferulate | 14.09 | |||
| Bis(ammonium) protocatechuate | 5.06–5.98 | Protocatechuic acid | 15.83 | [123] |
| Cholinium caffeate | 2.55 | Caffeic acid | 1.99 | [122] |
| Cholinium syringate | 2.44 | Syringic acid | 2.04 | [122] |
| Cholinium vanillate | 16.03 | Vanillic acid | 80.46 | [122] |
| Dicholinium ellagate | 1.22 | Ellagic acid | 0.79 | [122] |
| Strategy | IL | IL Role | API | Reference |
|---|---|---|---|---|
| Micellar system | [C14C1im]Br | Surfactant | Dopamine hydrochloride Acetylcholine chloride | [196] |
| Micellar system | [C12C1im]Cl | Surfactant | Ibuprofen | [87] |
| Micellar system | [C12C1im]Cl [C14C1im]Cl | Surfactant | Lidocaine hydrochloride | [197] |
| Microemulsion | [C6C1im]Cl [C4C1im][PF6] | Aqueous/Oil phase | Reichardt’s dye (drug model) | [29] |
| Microemulsion | [C4C1im][PF6] | Oil phase | Etodolac | [79] |
| Microemulsion | [C1C1im][(CH3O)2PO2] | Aqueous phase | Acyclovir | [198] |
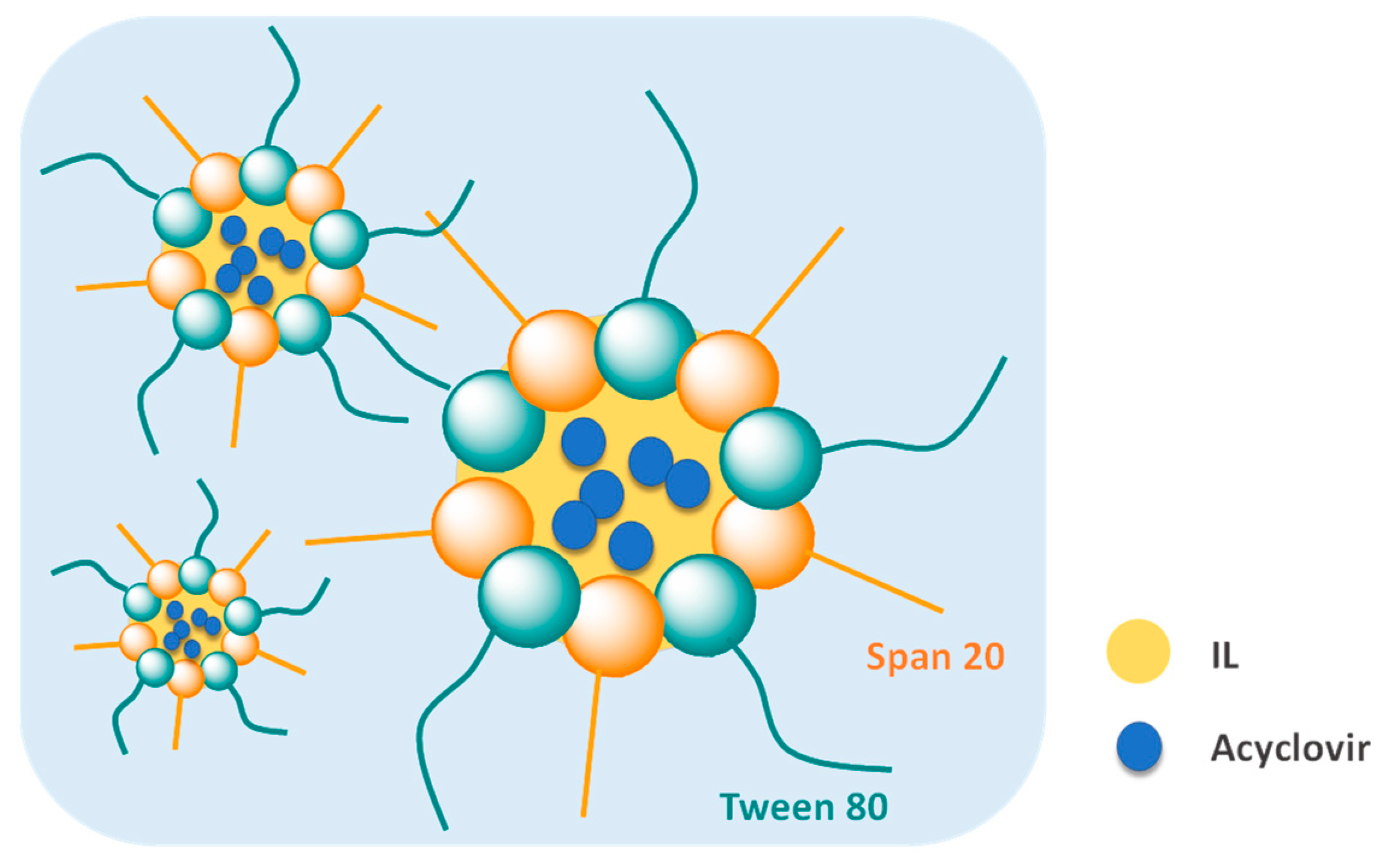
| Microemulsion | [Ch][formate] [Ch][lactate] [Ch][propionate] [Ch][oleate] | Non-aqueous phase; Surfactant in oil phase | Acyclovir | [199] |
| Microemulsion | [C1C1im][(CH3O)2PO2] | Aqueous phase | Methotrexate | [200] |
| Microemulsion | [C2OHC1]Cl [C1C1im][C12SO3] | Aqueous phase; Surfactant phase | Dencichine | [201] |
| Bacterial nanocellulose membranes | [Ch][Caf] [Ch][Gal] | API | Caffeic acid Gallic acid | [202] |
| Bacterial nanocellulose membranes | [Ch][Ibu] [Ch][Nap] [Ch][Ket] | API | Ibuprofen Naproxen Ketoprofen | [203] |
| Bacterial nanocellulose membranes | [Ch][B3] [Ch][B5] [Ch][B6] | API | Niacin Pantothenic acid Pyridoxine | [204] |
| Patch | [Lid][Eto] | Dual API | Lidocaine Etodolac | [205] |
| Polyvinvylidene fluoride membrane | [Lid][Nap] [Lid][Ibu] [Lid][Dicl] | Dual API | Naproxen Ibuprofen Diclofenac | [206] |
| PLGA nanoparticles | [Ch][Phe] [Ch][Glu] | API solubilization | Rutin | [207] |
| Permeation enhancers | [C8im]Cl [C1C8im]Cl [C1C1C8im]Cl | Membrane disruption | Testosterone | [208] |
| Permeation enhancers | [ProOEt][Ibu] | API | Ibuprofen | [209] |
| Permeation enhancers | [mPEG3N444][Sal] [HN444][Sal] [Ch][Sal] [C1Pyrr][Sal] | API | Salicylic acid | [210] |
| Permeation enhancers | [Ch][geranate2(H)] | API | Geranic acid | [211] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedro, S.N.; R. Freire, C.S.; Silvestre, A.J.D.; Freire, M.G. The Role of Ionic Liquids in the Pharmaceutical Field: An Overview of Relevant Applications. Int. J. Mol. Sci. 2020, 21, 8298. https://doi.org/10.3390/ijms21218298
Pedro SN, R. Freire CS, Silvestre AJD, Freire MG. The Role of Ionic Liquids in the Pharmaceutical Field: An Overview of Relevant Applications. International Journal of Molecular Sciences. 2020; 21(21):8298. https://doi.org/10.3390/ijms21218298
Chicago/Turabian StylePedro, Sónia N., Carmen S. R. Freire, Armando J. D. Silvestre, and Mara G. Freire. 2020. "The Role of Ionic Liquids in the Pharmaceutical Field: An Overview of Relevant Applications" International Journal of Molecular Sciences 21, no. 21: 8298. https://doi.org/10.3390/ijms21218298
APA StylePedro, S. N., R. Freire, C. S., Silvestre, A. J. D., & Freire, M. G. (2020). The Role of Ionic Liquids in the Pharmaceutical Field: An Overview of Relevant Applications. International Journal of Molecular Sciences, 21(21), 8298. https://doi.org/10.3390/ijms21218298