Circulating Tumour Cell Expression of Immune Markers as Prognostic and Therapeutic Biomarkers in Head and Neck Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Results
2.1. Included Studies and Patient Clinical Demographic Data
2.2. CTC Detection and Disease Stage
2.3. Immune Marker Expression and Disease Stage
2.4. HPV Status
2.5. CTC Immune-Marker Expression as Tumour Therapeutic and Treatment Response Biomarkers
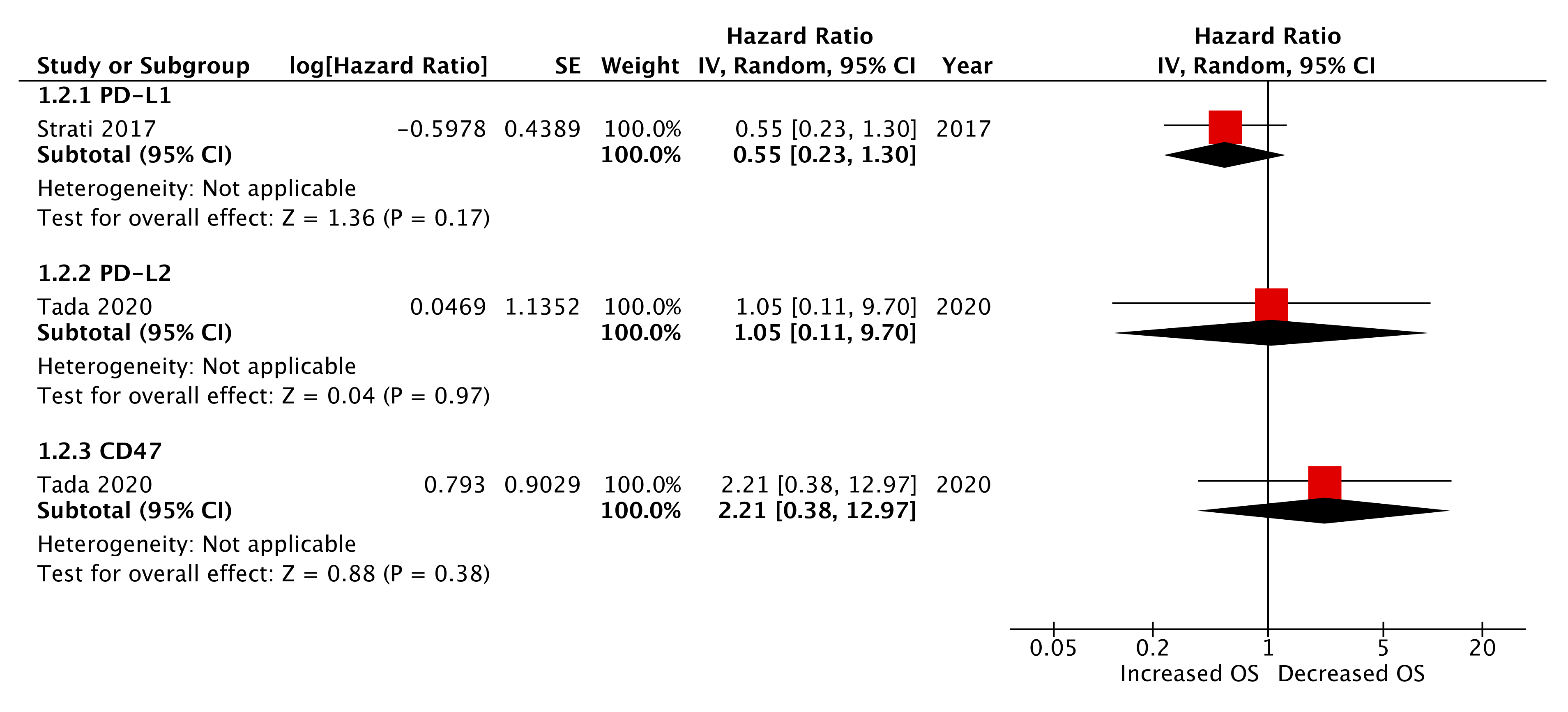
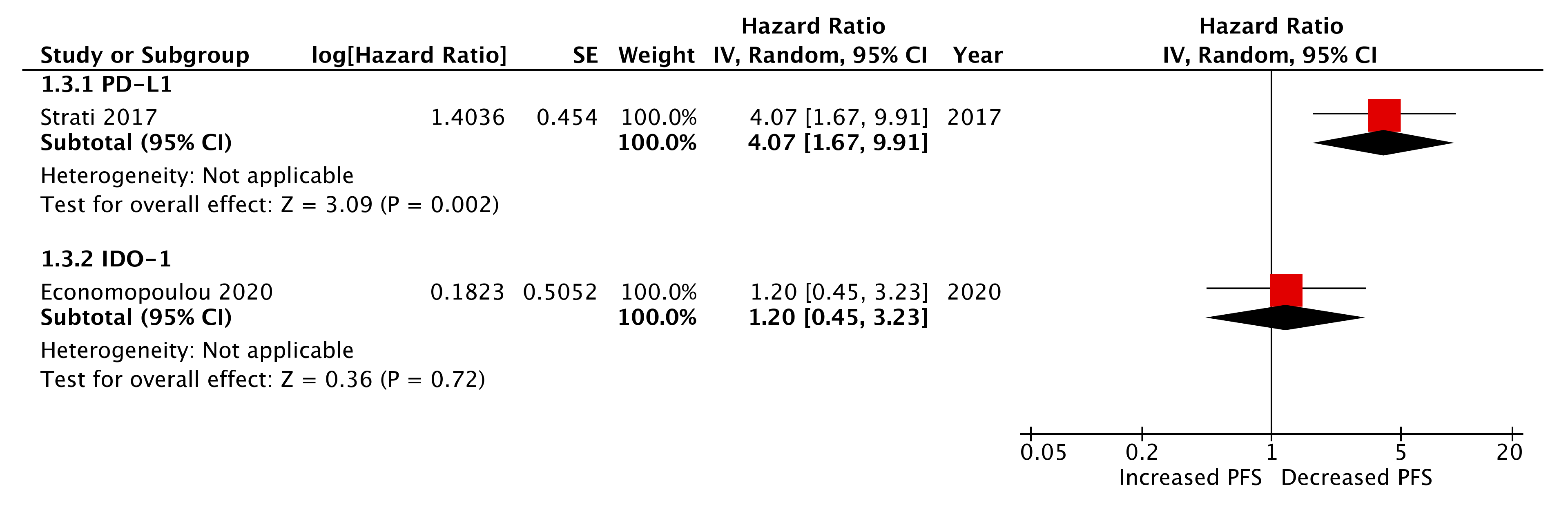
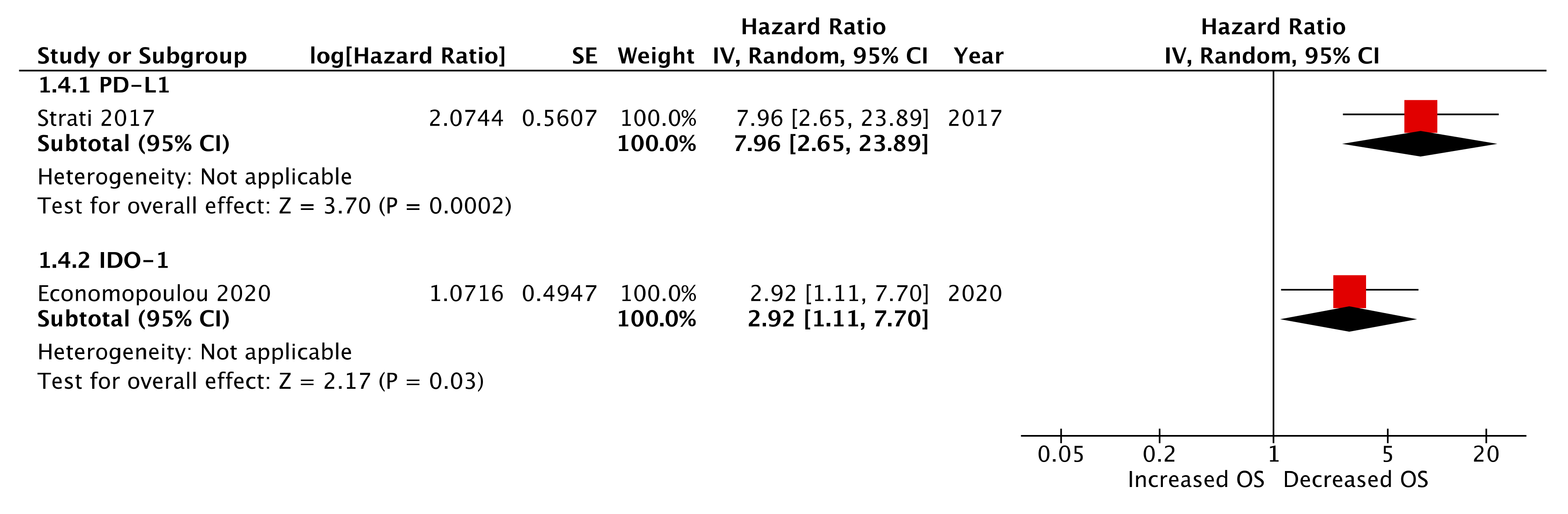
2.6. Pre-Treatment (Baseline) CTC Immune Marker Expression as a Prognostic Biomarker
2.7. Post-Treatment CTC Immune Marker Expression
2.8. Study Quality
3. Discussion
4. Methods
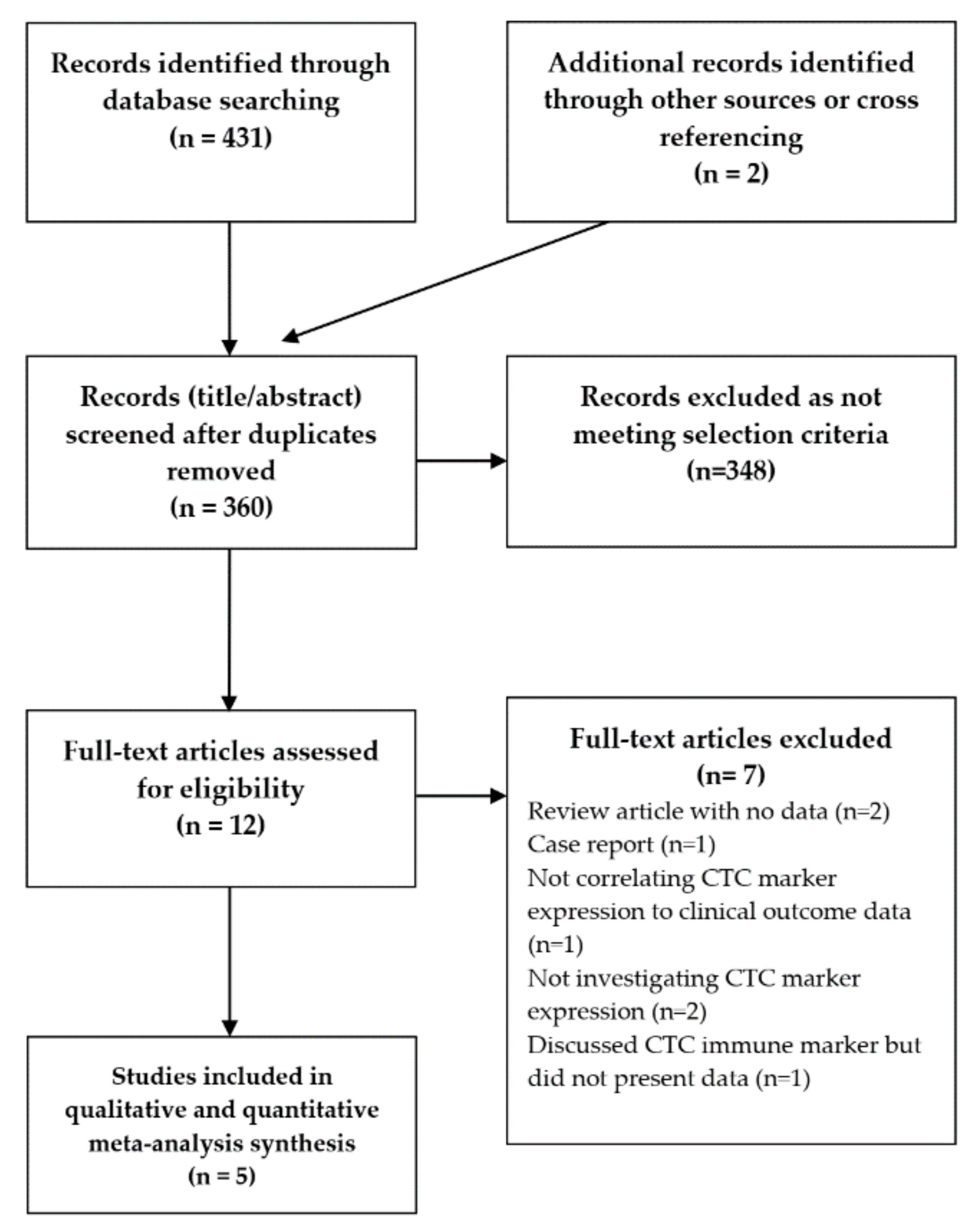
4.1. Search Strategy and Inclusion Criteria
4.2. Study Selection and Data Extraction
4.3. Data Analysis
5. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Sacco, A.G.; Cohen, E.E. Current treatment options for recurrent or metastatic head and neck squamous cell carcinoma. J. Clin. Oncol. 2015, 33, 3305–3315. [Google Scholar] [CrossRef] [PubMed]
- Vermorken, J.B.; Specenier, P. Optimal treatment for recurrent/metastatic head and neck cancer. Ann. Oncol. 2010, 21, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Vermorken, J.B.; Mesia, R.; Rivera, F.; Remenar, E.; Kawecki, A.; Rottey, S.; Erfan, J.; Zabolotnyy, D.; Kienzer, H.R.; Cupissol, D.; et al. Platinum-Based Chemotherapy plus Cetuximab in Head and Neck Cancer. N. Engl. J. Med. 2008, 359, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Schwaederle, M.; Zhao, M.; Lee, J.; Eggermont, A.; Schilsky, R.; Mendelsohn, J.; Lazar, V.; Kurzrock, R. Impact of Precision Medicine in Diverse Cancers: A Meta-Analysis of Phase II Clinical Trials. J. Clin. Oncol. 2015, 33. [Google Scholar] [CrossRef] [PubMed]
- McMullen, C.; Chung, C.; Hernandez-Prera, J. Evolving Role of Human Papillomavirus as a Clinically Significant Biomarker in Head and Neck Squamous Cell Carcinoma. Expert Rev. Mol. Diagn. 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- Eze, N.; Lo, Y.-C.; Burtness, B. Biomarker driven treatment of head and neck squamous cell cancer. Cancers Head Neck 2017, 2, 6. [Google Scholar] [CrossRef]
- Seiwert, T.Y.; Burtness, B.; Mehra, R.; Weiss, J.; Berger, R.; Eder, J.P.; Heath, K.; McClanahan, T.; Lunceford, J.; Gause, C.; et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): An open-label, multicentre, phase 1b trial. Lancet Oncol. 2016, 17, 956–965. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Forster, M.D.; Devlin, M.-J. Immune Checkpoint Inhibition in Head and Neck Cancer. Front. Oncol. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.E.W.; Bell, R.B.; Bifulco, C.B.; Burtness, B.; Gillison, M.L.; Harrington, K.J.; Le, Q.-T.; Lee, N.Y.; Leidner, R.; Lewis, R.L.; et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J. Immunother. Cancer 2019, 7, 184. [Google Scholar] [CrossRef] [PubMed]
- Havel, J.J.; Chowell, D.; Chan, T.A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer 2019, 19, 133–150. [Google Scholar] [CrossRef]
- Ayers, M.; Ribas, A.; Mcclanahan, T.K.; Ayers, M.; Lunceford, J.; Nebozhyn, M.; Murphy, E.; Loboda, A.; Kaufman, D.R.; Albright, A.; et al. IFN- g – related mRNA profile predicts clinical response to PD-1 blockade Find the latest version: IFN- γ – related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Investig. 2017, 127, 2930–2940. [Google Scholar] [CrossRef]
- Gibney, G.T.; Weiner, L.M.; Atkins, M.B. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016, 17, e542–e551. [Google Scholar] [CrossRef]
- Kloten, V.; Lampignano, R.; Krahn, T.; Schlange, T. Circulating Tumor Cell PD-L1 Expression as Biomarker for Therapeutic Efficacy of Immune Checkpoint Inhibition in NSCLC. Cells 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Chambers, A.; Groon, A.; MacDonald, I. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. 2002, 2, 563–572. [Google Scholar] [CrossRef]
- Payne, K.; Brooks, J.; Spruce, R.; Batis, N.; Taylor, G.; Nankivell, P.; Mehanna, H. Circulating Tumour Cell Biomarkers in Head and Neck Cancer: Current Progress and Future Prospects. Cancers (Basel) 2019, 11, 1115. [Google Scholar] [CrossRef]
- Economopoulou, P.; Koutsodontis, G.; Strati, A.; Kirodimos, E.; Giotakis, E.; Maragoudakis, P.; Prikas, C.; Papadimitriou, N.; Perisanidis, C.; Gagari, E.; et al. Surrogates of immunologic cell death (ICD) and chemoradiotherapy outcomes in head and neck squamous cell carcinoma (HNSCC). Oral Oncol. 2019, 94, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Strati, A.; Koutsodontis, G.; Papaxoinis, G.; Angelidis, I.; Zavridou, M.; Economopoulou, P.; Kotsantis, I.; Avgeris, M.; Mazel, M.; Perisanidis, C.; et al. Prognostic significance of PD-L1 expression on circulating tumor cells in patients with head and neck squamous cell carcinoma. Ann. Oncol. 2017, 28, 1923–1933. [Google Scholar] [CrossRef]
- Kulasinghe, A.; Kapeleris, J.; Kimberley, R.; Mattarollo, S.R.; Thompson, E.W.; Thiery, J.-P.; Kenny, L.; O’byrne, K.; Punyadeera, C. The prognostic significance of circulating tumor cells in head and neck and non-small-cell lung cancer. Cancer Med. 2018, 7, 5910–5919. [Google Scholar] [CrossRef]
- Chikamatsu, K.; Tada, H.; Takahashi, H.; Kuwabara-Yokobori, Y.; Ishii, H.; Ida, S.; Shino, M. Expression of immune-regulatory molecules in circulating tumor cells derived from patients with head and neck squamous cell carcinoma. Oral Oncol. 2019, 89, 34–39. [Google Scholar] [CrossRef]
- Tada, H.; Takahashi, H.; Kuwabara-Yokobori, Y.; Shino, M.; Chikamatsu, K. Molecular profiling of circulating tumor cells predicts clinical outcome in head and neck squamous cell carcinoma. Oral Oncol. 2020, 102, 104558. [Google Scholar] [CrossRef]
- Economopoulou, P.; Kladi-Skandali, A.; Strati, A.; Koytsodontis, G.; Kirodimos, E.; Giotakis, E.; Maragoudakis, P.; Gagari, E.; Maratou, E.; Dimitriadis, G.; et al. Prognostic impact of indoleamine 2,3-dioxygenase 1 (IDO1) mRNA expression on circulating tumour cells of patients with head and neck squamous cell carcinoma. ESMO Open 2020, 5, e000646. [Google Scholar] [CrossRef]
- Oliveira-Costa, J.P.; de Carvalho, A.F.; da Silveira, G.G.; Amaya, P.; Wu, Y.; Park, K.-J.J.; Gigliola, M.P.; Lustberg, M.; Buim, M.E.C.; Ferreira, E.N.; et al. Gene expression patterns through oral squamous cell carcinoma development: PD-L1 expression in primary tumor and circulating tumor cells. Oncotarget 2015, 6, 20902–20920. [Google Scholar] [CrossRef]
- Kulasinghe, A.; Perry, C.; Kenny, L.; Warkiani, M.E.; Nelson, C.; Punyadeera, C. PD-L1 expressing circulating tumour cells in head and neck cancers. BMC Cancer 2017, 17, 333. [Google Scholar] [CrossRef]
- Yang, W.; Wong, M.C.M.; Thomson, P.J.; Li, K.-Y.; Su, Y. The prognostic role of PD-L1 expression for survival in head and neck squamous cell carcinoma: A systematic review and meta-analysis. Oral Oncol. 2018, 86, 81–90. [Google Scholar] [CrossRef]
- Khattak, M.A.; Reid, A.; Freeman, J.; Pereira, M.; McEvoy, A.; Lo, J.; Frank, M.H.; Meniawy, T.; Didan, A.; Spencer, I.; et al. PD-L1 Expression on Circulating Tumor Cells May Be Predictive of Response to Pembrolizumab in Advanced Melanoma: Results from a Pilot Study. Oncologist 2020, 25, e520–e527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, X.; Liu, Y.; Zhang, T.; Wang, Z.; Gu, M.; Li, Y.; Wang, D.D.; Li, W.; Lin, P.P. PD-L1+ aneuploid circulating tumor endothelial cells (CTECs) exhibit resistance to the checkpoint blockade immunotherapy in advanced NSCLC patients. Cancer Lett. 2020, 469, 355–366. [Google Scholar] [CrossRef]
- Zhai, L.; Ladomersky, E.; Lenzen, A.; Nguyen, B.; Patel, R.; Lauing, K.L.; Wu, M.; Wainwright, D.A. IDO1 in cancer: A Gemini of immune checkpoints. Cell. Mol. Immunol. 2018, 15, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, Q.; Liu, Q.; Wang, C.; Yao, R.; Wang, Y. CTC immune escape mediated by PD-L1. Med. Hypotheses 2016, 93, 138–139. [Google Scholar] [CrossRef]
- Nicolazzo, C.; Raimondi, C.; Mancini, M.; Caponnetto, S.; Gradilone, A.; Gandini, O.; Mastromartino, M.; del Bene, G.; Prete, A.; Longo, F.; et al. Monitoring PD-L1 positive circulating tumor cells in non-small cell lung cancer patients treated with the PD-1 inhibitor Nivolumab. Sci. Rep. 2016, 6, 31726. [Google Scholar] [CrossRef]
- Yue, C.; Jiang, Y.; Li, P.; Wang, Y.; Xue, J.; Li, N.; Li, D.; Wang, R.; Dang, Y.; Hu, Z.; et al. Dynamic change of PD-L1 expression on circulating tumor cells in advanced solid tumor patients undergoing PD-1 blockade therapy. Oncoimmunology 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Guibert, N.; Delaunay, M.; Lusque, A.; Boubekeur, N.; Rouquette, I.; Clermont, E.; Mourlanette, J.; Gouin, S.; Dormoy, I.; Favre, G.; et al. PD-L1 Expression in Circulating Tumor Cells of Advanced Non-Small Cell Lung Cancer Patients Treated With Nivolumab. Lung Cancer 2018, 120. [Google Scholar] [CrossRef] [PubMed]
- Janning, M.; Kobus, F.; Babayan, A.; Wikman, H.; Velthaus, J.-L.; Bergmann, S.; Schatz, S.; Falk, M.; Berger, L.-A.; Böttcher, L.-M.; et al. Determination of PD-L1 Expression in Circulating Tumor Cells of NSCLC Patients and Correlation with Response to PD-1/PD-L1 Inhibitors. Cancers (Basel) 2019, 11. [Google Scholar] [CrossRef]
- Chow, L.Q.M.; Haddad, R.; Gupta, S.; Mahipal, A.; Mehra, R.; Tahara, M.; Berger, R.; Eder, J.P.; Burtness, B.; Lee, S.-H.; et al. Antitumor Activity of Pembrolizumab in Biomarker-Unselected Patients With Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma: Results From the Phase Ib KEYNOTE-012 Expansion Cohort. J. Clin. Oncol. 2016, 34, 3838–3845. [Google Scholar] [CrossRef]
- Gettinger, S.; Horn, L.; Jackman, D.; Spigel, D.; Antonia, S.; Hellmann, M.; Powderly, J.; Heist, R.; Sequist, L.; Smith, D.; et al. Five-Year Follow-Up of Nivolumab in Previously Treated Advanced Non-Small-Cell Lung Cancer: Results From the CA209-003 Study. J. Clin. Oncol. 2018, 36. [Google Scholar] [CrossRef]
- Hirsch, F.R.; McElhinny, A.; Stanforth, D.; Ranger-Moore, J.; Jansson, M.; Kulangara, K.; Richardson, W.; Towne, P.; Hanks, D.; Vennapusa, B.; et al. PD-L1 Immunohistochemistry Assays for Lung Cancer: Results from Phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J. Thorac. Oncol. 2017, 12, 208–222. [Google Scholar] [CrossRef]
- McLaughlin, J.; Han, G.; Schalper, K.A.; Carvajal-Hausdorf, D.; Pelekanou, V.; Rehman, J.; Velcheti, V.; Herbst, R.; LoRusso, P.; Rimm, D.L. Quantitative Assessment of the Heterogeneity of PD-L1 Expression in Non-Small-Cell Lung Cancer. JAMA Oncol. 2016, 2, 46–54. [Google Scholar] [CrossRef]
- Szczerba, B.M.; Castro-Giner, F.; Vetter, M.; Krol, I.; Gkountela, S.; Landin, J.; Scheidmann, M.C.; Donato, C.; Scherrer, R.; Singer, J.; et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 2019, 566, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, G.; Sun, Q. PD-L1 expression on circulating tumor cells and prognosis of breast cancer patients. J. Clin. Oncol. 2019, 37, e14028. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- McShane, L.M.; Altman, D.G.; Sauerbrei, W.; Taube, S.E.; Gion, M.; Clark, G.M.; Statistics Subcommittee of the NCI-EORTC Working Group on Cancer Diagnostics. REporting recommendations for tumour MARKer prognostic studies (REMARK). Br. J. Cancer 2005, 93, 387–391. [Google Scholar] [CrossRef]
- Gurung, P.M.S.; Barnett, A.R.; Wilson, J.S.; Hudson, J.; Ward, D.G.; Messing, E.M.; Bryan, R.T. Prognostic DNA Methylation Biomarkers in High-risk Non-muscle-invasive Bladder Cancer: A Systematic Review to Identify Loci for Prospective Validation. Eur. Urol. Focus 2020, 6, 683–697. [Google Scholar] [CrossRef]

| Author | Year | Tumour Site/Stage | No. of Patients in Cohort | Cohort Gender | Age (Years) | Tumour Site | CTC Enrichment Technique | Marker Detection Methodology | Immune Marker(s) | Timepoint of CTC Analysis | Outcome Measure (Median) and (Range) F/U |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Strati et al. [18] | 2017 | Locally advanced HNSCC | 113 (pre-treatment = 94, post-treatment = 54) | Pre-treatment: M = 75, F = 19, post-treatment: M = 37, F = 17 | Pre-treatment: ≥65 = 40, <65 = 54, post-treatment: ≥65 = 25, <65 = 29 | Pre-treatment: Oropharynx = 21, Other = 73, Post-treatment: Oropharynx = 13, Other = 41 | Negative depletion (red cell lysis and CD45 MACS depletion) and EpCAM MACS enrichment | Gene expression using RT-qPCR | PD-L1 | Pre-treatment and post-treatment | PFS and OS [18.9 months (0.2–54.9)] |
| Kulasinghe et al. [19] | 2018 | HNSCC stage I–IV | 23 | M = 17, F = 6 | <60 = 10, >60 = 23 | Oral cavity = 9, Oropharynx = 14 | Microfluidic (ClearCell FX CTChip marker-independent) enrichment | Surface marker assessment with IF antibody | PD-L1 | Pre-treatment | PFS [not stated] |
| Chikamatsu et al. [20] | 2019 | R/M HNSCC | 30 | M = 27, F = 3 | Median 70.5 (range 53–86) | Oral cavity = 3, Nasopharynx = 1, Oropharynx = 3, Hypopharynx = 12, Larynx = 4, Paranasal sinuses = 6, Parotid gland = 1 | Negative depletion (density centrifugation and CD45 MACS depletion) | Gene expression using RT-qPCR | PD-L1, PD-L2, CD47 | R/M post-treatment | Biomarker of therapeutic PD-L1 target on tumour |
| Tada et al. [21] | 2020 | HNSCC stage I–IV | 44 | M = 41, F = 3 | <66 = 21, ≥66 = 23 | Nasal cavity = 3, Oral cavity = 4, Nasopharynx = 2, Oropharynx = 17, Hypopharynx = 14, Larynx = 4 | CellSieve microfilter | Gene expression using RT-qPCR | PD-L1, PD-L2, CD47 | Pre-treatment | PFS and OS [not stated] |
| Economopoulou et al. [22] | 2020 | Locally advanced HNSCC | 113 (pre-treatment = 94, post-treatment = 54) | Pre-treatment: M = 75, F = 19, post-treatment: M = 37, F = 17 | Pre-treatment: ≥65 = 40, <65 = 54, post-treatment: ≥65 = 25, <65 = 29 | Pre-treatment: Oropharynx = 21, Other = 73, post-treatment: Oropharynx = 13, Other = 41 | Negative depletion (red cell lysis and CD45 MACS depletion) and EpCAM MACS enrichment | Gene expression using RT-qPCR | IDO1 | Pre-treatment and post-treatment | PFS and OS [27.16 months (2.3–69.3)] |
| Marker | PFS | OS | |||||
|---|---|---|---|---|---|---|---|
| Pre-Treatment | HR | 95% CI | p Value | HR | 95% CI | p Value | |
| PD-L1 | Strati et al. 2017 | 1.43 | 0.63–3.25 | 0.39 | 0.55 | 0.23–1.30 | 0.17 |
| Kulasinghe et al. 2018 | 5.16 | 1.01–26.33 | 0.049 | ||||
| Tada et al. 2020 | 0.29 | 0.10–0.86 | 0.035 | not stated | not stated | 0.038 | |
| PD-L2 | Tada et al. 2020 | 0.81 | 0.24–2.77 | 0.74 | 1.05 | 0.11–9.70 | 0.97 |
| CD47 | Tada et al. 2020 | 1.56 | 0.51–4.69 | 0.45 | 2.21 | 0.33–12.97 | 0.45 |
| IDO1 * | Economopoulou et al. 2020 | 0.23 | 0.02–0.50 | 0.018 | 0.57 | 0.19–1.36 | 0.21 |
| Post-treatment | |||||||
| IDO1 * | Economopoulou et al. 2020 | 1.20 | 0.25–3.23 | 0.75 | 2.92 | 1.11–7.70 | 0.011 |
| PD-L1 * | Strati et al. 2017 | 4.07 | 1.67–9.91 | 0.002 ** | 7.96 | 2.65–23.89 | 0.0002 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Payne, K.; Pugh, M.; Brooks, J.; Batis, N.; Taylor, G.; Nankivell, P.; Mehanna, H. Circulating Tumour Cell Expression of Immune Markers as Prognostic and Therapeutic Biomarkers in Head and Neck Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2020, 21, 8229. https://doi.org/10.3390/ijms21218229
Payne K, Pugh M, Brooks J, Batis N, Taylor G, Nankivell P, Mehanna H. Circulating Tumour Cell Expression of Immune Markers as Prognostic and Therapeutic Biomarkers in Head and Neck Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2020; 21(21):8229. https://doi.org/10.3390/ijms21218229
Chicago/Turabian StylePayne, Karl, Matthew Pugh, Jill Brooks, Nikolaos Batis, Graham Taylor, Paul Nankivell, and Hisham Mehanna. 2020. "Circulating Tumour Cell Expression of Immune Markers as Prognostic and Therapeutic Biomarkers in Head and Neck Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 21, no. 21: 8229. https://doi.org/10.3390/ijms21218229
APA StylePayne, K., Pugh, M., Brooks, J., Batis, N., Taylor, G., Nankivell, P., & Mehanna, H. (2020). Circulating Tumour Cell Expression of Immune Markers as Prognostic and Therapeutic Biomarkers in Head and Neck Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 21(21), 8229. https://doi.org/10.3390/ijms21218229






