Intrathecal Inflammation in Progressive Multiple Sclerosis
Abstract
1. Introduction
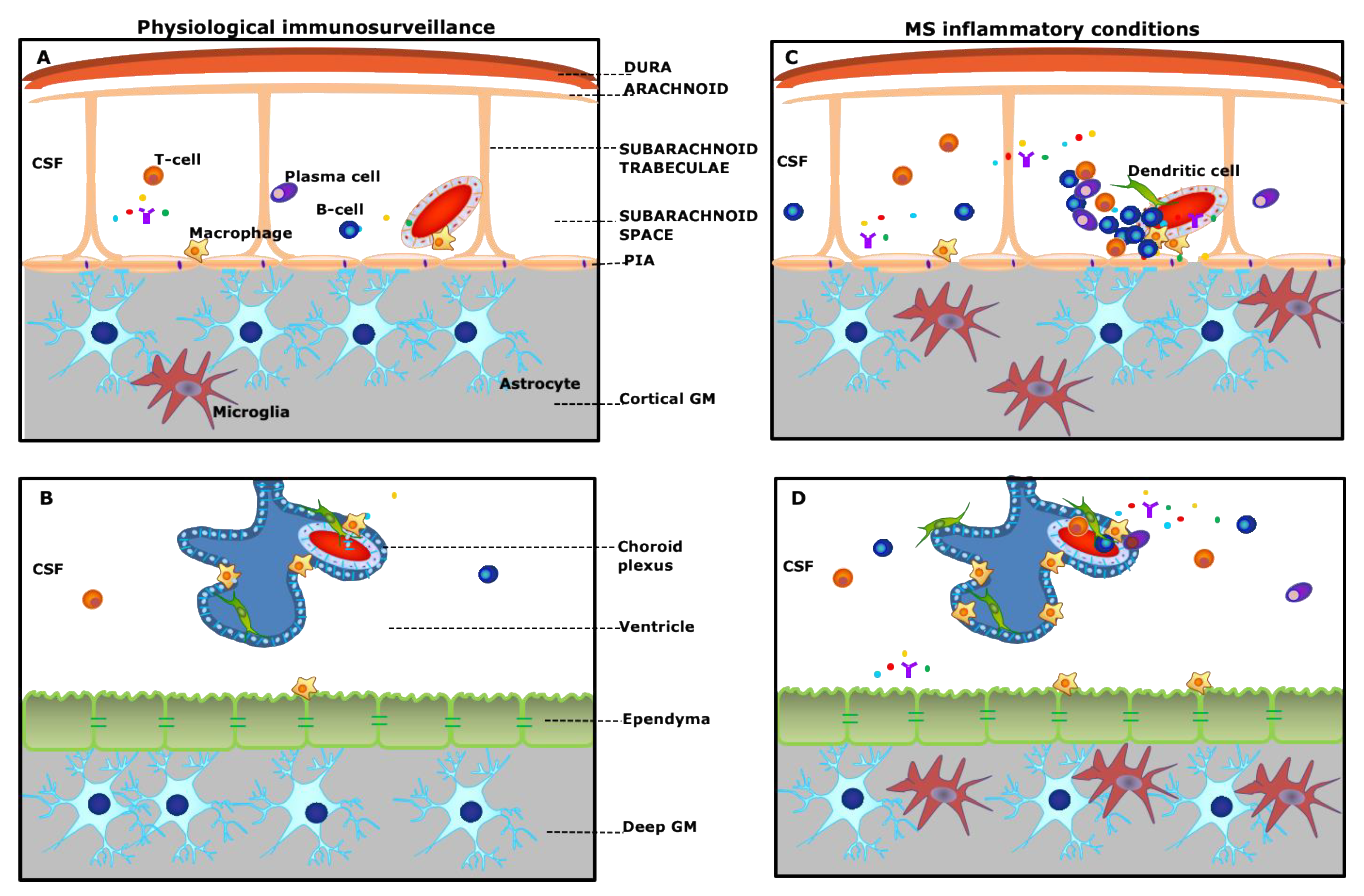
2. Meningeal Inflammation
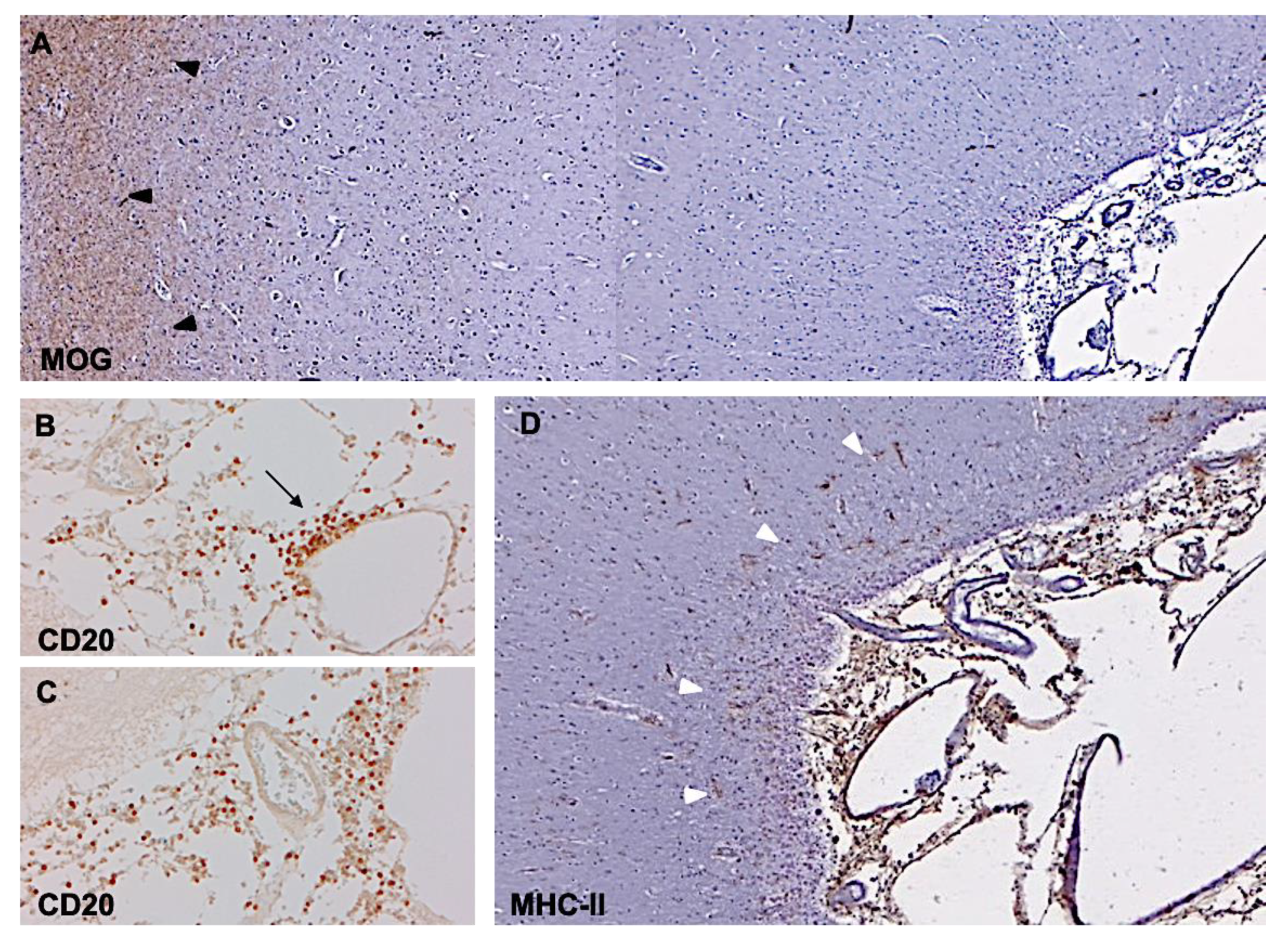
3. Subpial Lesions
4. CSF Inflammation
- Diagnostic, including antibodies against aquaporin 4 and MOG, vascular cell adhesion molecules, glial fibrillary acidic protein (GFAP), complement components, cytokines (IL-6), and chemokines CXCL13);
- Prognostic, including chitinase 3- like1 (CHI3L1), CXCL13, GFAP;
- Monitoring of therapy response and side effects, including CXCL13, IL-6, IL-8CHI3L1, sCD21, sCD27 [40].
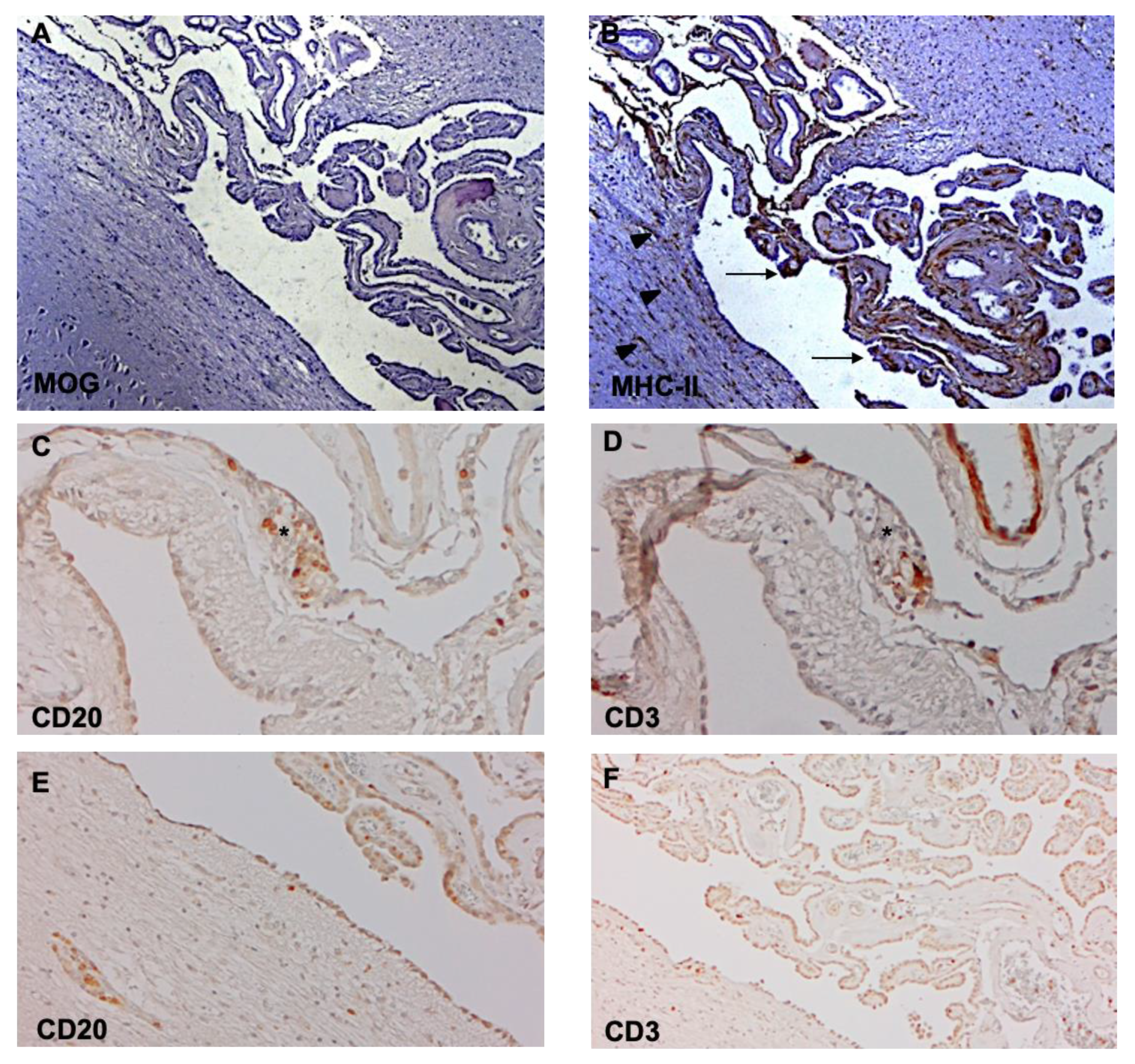
5. Choroid Plexus Inflammation
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Reich, D.S.; Lucchinetti, C.F.; Calabresi, P.A. Multiple sclerosis. N. Engl. J. Med. 2018, 378, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, W.J.; Hardy, T.A.; Fazekas, F.; Miller, D.H. Diagnosis of multiple sclerosis: Progress and challenges. Lancet 2017, 389, 1336–1346. [Google Scholar] [CrossRef]
- Absinta, M.; Lassmann, H.; Trapp, B.D. Mechanisms underlying progression in multiple sclerosis. Curr. Opin. Neurol. 2020, 33, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Baecher-Allan, C.; Kaskow, B.J.; Weiner, H.L. Multiple sclerosis: Mechanisms and immunotherapy. Neuron. 2018, 97, 742–768. [Google Scholar] [CrossRef] [PubMed]
- Plum, F. Ion Homeostasis in Cisternal and Lumbar Cerebrospinal Fluid. N. Engl. J. Med. 1975, 293, 1041–1042. [Google Scholar] [CrossRef]
- Brinker, T.; Stopa, E.; Morrison, J.; Klinge, P. A new look at cerebrospinal fluid circulation. Fluids Barriers CNS. 2014, 11, 10. [Google Scholar] [CrossRef]
- Nakada, T.; Kwee, I.L. Fluid dynamics inside the brain barrier: Current concept of interstitial flow, glymphatic flow, and cerebrospinal fluid circulation in the brain. Neuroscientist 2018, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kelley, K.W.; Shimada, A. Neuroinflammation and the blood-brain-interface: New findings in brain pathology. Clin. Exp. Neuroimmunol. 2020, 11, 16–20. [Google Scholar] [CrossRef]
- Magliozzi, R.; Howell, O.W.; Reeves, C.; Roncaroli, F.; Nicholas, R.; Serafini, B.; Aloisi, F.; Reynolds, R. A gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann. Neurol. 2010, 68, 477–493. [Google Scholar] [CrossRef]
- Magliozzi, R.; Hametner, S.; Facchiano, F.; Marastoni, D.; Rossi, S.; Castellaro, M.; Poli, A.; Lattanzi, F.; Visconti, A.; Nicholas, R.; et al. Iron homeostasis, complement, and coagulation cascade as CSF signature of cortical lesions in early multiple sclerosis. Ann. Clin. Transl. Neurol. 2019, 6, 2150–2163. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Kirmi, O. Anatomy and imaging of the normal meninges. Semin. Ultrasound CT MR 2009, 30, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Ghannam, J.Y.; Al Kharazi, K.A. Neuroanatomy, Cranial Meninges. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Alves de Lima, K.; Rustenhoven, J.; Kipnis, J. Meningeal immunity and its function in maintenance of the central nervous system in health and disease. Annu. Rev. Immunol. 2020, 38, 597–620. [Google Scholar] [CrossRef]
- Titelbaum, D.S.; Engisch, R.; Schwartz, E.D.; Napoli, S.Q.; Sloane, J.A.; Samaan, S.; Katz, J.D.; Lathi, E.S. Leptomeningeal enhancement on 3D-FLAIR MRI in multiple sclerosis: Systematic observations in clinical practice. J. Neuroimaging 2020. [Google Scholar] [CrossRef] [PubMed]
- Serafini, B.; Rosicarelli, B.; Magliozzi, R.; Stigliano, E.; Aloisi, F. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 2004, 14, 164–174. [Google Scholar] [CrossRef]
- Magliozzi, R.; Howell, O.; Vora, A.; Serafini, B.; Nicholas, R.; Puopolo, M.; Reynolds, R.; Aloisi, F. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 2007, 130, 1089–1104. [Google Scholar] [CrossRef] [PubMed]
- Haider, L.; Zrzavy, T.; Hametner, S.; Höftberger, R.; Bagnato, F.; Grabner, G.; Trattnig, S.; Pfeifenbring, S.; Brück, W.; Lassmann, H. The topograpy of demyelination and neurodegeneration in the multiple sclerosis brain. Brain 2016, 139, 807–815. [Google Scholar] [CrossRef]
- Pikor, N.B.; Prat, A.; Bar-Or, A.; Gommerman, J.L. Meningeal tertiary lymphoid tissues and multiple sclerosis: A gathering place for diverse types of immune cells during CNS autoimmunity. Front. Immunol. 2016, 6, 657. [Google Scholar] [CrossRef]
- Lucchinetti, C.F.; Popescu, B.F.G.; Bunyan, R.F.; Moll, N.M.; Roemer, S.F.; Lassmann, H.; Brück, W.; Parisi, J.E.; Scheithauer, B.W.; Giannini, C.; et al. Inflammatory cortical demyelination in early multiple sclerosis. N. Engl. J. Med. 2011, 365, 2188–2197. [Google Scholar] [CrossRef]
- Aloisi, F.; Pujol-Borrel, R. Lymphoid neogenesis in chronic inflammatory diseases. Nat. Rev. Immunol. 2006, 6, 205–217. [Google Scholar] [CrossRef]
- Pipi, E.; Nayar, S.; Gardner, D.H.; Colafrancesco, S.; Smith, C.; Barone, F. Tertiary lymphoid structures: Autoimmunity goes local. Front. Immunol. 2018, 9, 1952. [Google Scholar] [CrossRef]
- Howell, O.W.; Reeves, C.A.; Nicholas, R.; Carassiti, D.; Radotra, B.; Gentlemen, S.M.; Serafini, B.; Aloisi, F.; Roncaroli, F.; Magliozzi, R.; et al. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain 2011, 134, 2755–2771. [Google Scholar] [CrossRef] [PubMed]
- Bevan, R.J.; Evans, R.; Griffith, L.; Watkins, L.M.; Rees, M.I.; Magliozzi, R.; Allen, I.; McDonnel, G.; Kee, R.; Naughton, M.; et al. Meningeal inflammation and cortical demyelination in acute multiple sclerosis. Ann. Neurol. 2018, 84, 829–842. [Google Scholar] [CrossRef]
- Lehmann-Horn, K.; Wang, S.Z.; Sagan, S.A.; Zamvil, S.S.; von Budingen, H.C. B cell repertoire expansion occurs in meningeal ectopic lymphoid tissue. JCI Insight 2016, 1, e87234. [Google Scholar] [CrossRef]
- Reali, C.; Magliozzi, R.; Roncaroli, F.; Nicholas, R.; Howell, O.W.; Reynolds, R. B cell rich meningeal inflammation associates with increased spinal cord pathology in multiple sclerosis. Brain Pathol. 2020, 30, 779–793. [Google Scholar] [CrossRef] [PubMed]
- Kidd, D.; Barkhof, F.; McConnell, R.; Algra, P.R.; Allen, I.V.; Revesz, T. Cortical lesions in multiple sclerosis. Brain 1999, 122, 17–26. [Google Scholar] [CrossRef]
- Peterson, J.W.; Bo, L.; Mork, S.; Chang, A.; Trapp, B.D. Transected neurites, apoptotic neurons and reduced inflammation in cortical MS lesions. Ann. Neurol. 2001, 50, 389–400. [Google Scholar] [CrossRef]
- Bø, L.; Vedeler, C.A.; Nyland, H.I.; Trapp, B.D.; Mørk, S.J. Subpial demyelination in the cerebral cortex of multiple sclerosis patients. J. Neuropathol. Exp. Neurol. 2003, 62, 723–732. [Google Scholar] [CrossRef]
- Kutzelnigg, A.; Lucchinetti, C.F.; Stadelmann, C.; Brück, W.; Rauschka, H.; Bergmann, M.; Schmidbauer, M.; Parisi, J.E.; Lassmann, H. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 2005, 128, 2705–2712. [Google Scholar] [CrossRef]
- Haider, L.; Simeonidou, C.; Steinberger, G.; Hametner, S.; Grigoriadis, N.; Deretzi, G.; Kovacs, G.G.; Kutzelnigg, A.; Lassmann, H.; Frischer, J.M. Multiple sclerosis deep grey matter: The relation between demyelination, neurodegeneration, inflammation and iron. J. Neurol. Neurosurg. Psychiatry 2014, 85, 1386–1395. [Google Scholar] [CrossRef]
- Bø, L.; Vedeler, C.A.; Nyland, H.; Trapp, B.D.; Mork, S.J. Intracortical multiple sclerosis lesions are not associated with increased lymphocyte infiltration. Mult. Scler. 2003, 9, 323–331. [Google Scholar] [CrossRef]
- Brink, B.P.; Veerhuis, R.; Breij, E.C.; van der Valk, P.; Dijkstra, D.; Bö, L. The pathology of multiple sclerosis is location-dependent: No significant complement activation is detected in purely cortical lesions. J. Neuropathol. Exp. Neurol. 2005, 6, 147–155. [Google Scholar] [CrossRef]
- Van Horssen, J.; Brink, B.P.; de Vries, H.E. The blood-brain barrier in cortical multiple sclerosis lesions. J. Neuropathol. Exp. Neurol. 2007, 66, 321–328. [Google Scholar] [CrossRef]
- Ransohoff, R.M.; Kivisakk, P.; Kidd, G. Three or more routes for leukocyte migration into the central nervous system. Nat. Rev. Immunol. 2003, 3, 569–581. [Google Scholar] [CrossRef]
- Mainero, C.; Louapre, C.; Govindarajan, S.T.; Giannì, C.; Nielsen, A.S.; Cohe-Adad, J.; Sloane, J.; Kinkel, R.P. A gradient in cortical pathology in multiple sclerosis by in vivo quantitative 7 T imaging. Brain 2015, 138, 932–945. [Google Scholar] [CrossRef] [PubMed]
- Samson, R.S.; Cardoso, M.J.; Muhlert, N.; Sethi, V.; Wheeler-Kingshott, C.A.M.; Ron, M.; Ourselin, S.; Miller, D.H.; Chard, D.T. Investigation of outer cortical magnetisation transfer ratio abnormalities in multiple sclerosis clinical subgroups. Mult. Scler. 2014, 20, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Pardini, M.; Yaldizli, Ö.; Sethi, V.; Muhlert, N.; Wheeler-Kingshott, C.A.M.; Samson, R.S.; Miller, D.H.; Chard, D.T. Magnetization transfer ratio measures in normal-appearing white matter show periventricular gradient abnormalities in multiple sclerosis. Brain 2015, 138, 1239–1246. [Google Scholar] [CrossRef]
- Brown, J.W.L.; Chowdhury, A.; Kanber, B.; Prados Carrasco, F.; Eshaghi, A.; Sudre, C.H.; Pardini, M.; Samson, R.S.; van de Pavert, S.H.; Wheeler-Kingshott, C.G. Magnetisation transfer ratio abnormalities in primary and secondary progressive multiple sclerosis. Mult. Scler. 2020, 26, 679–687. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carrol, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Fredman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet. Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Deisenhammer, F.; Zetterberg, H.; Fitzner, B.; Zettl, U.K. The cerebrospinal fluid in multiple sclerosis. Front. Immunol. 2019, 12, 726. [Google Scholar] [CrossRef]
- Magliozzi, R.; Howell, O.W.; Nicholas, R.; Cruciani, C.; Castellaro, M.; Romualdi, C.; Rossi, S.; Pitteri, M.; Benedetti, M.D.; Gajofatto, A.; et al. Inflammatory intrathecal profiles and cortical damage in multiple sclerosis. Ann. Neurol. 2018, 83, 739–755. [Google Scholar] [CrossRef]
- Magliozzi, R.; Scalfari, A.; Pisani, A.I.; Ziccardi, S.; Marastoni, D.; Pizzini, F.B.; Bajrami, A.; Tamanti, A.; Guandalini, M.; Bonomi, S.; et al. The CSF profile linked to cortical damage predicts multiple sclerosis activity. Ann. Neurol. 2020. [Google Scholar] [CrossRef]
- Gardner, C.; Magliozzi, R.; Durrenberger, P.F.; Howell, O.W.; Rundle, J.; Reynolds, R. Cortical grey matter demyelination can be induced by elevated pro-inflammatory cytokines in the subarachnoid space of MOG-immunized rats. Brain 2013, 136, 3596–3608. [Google Scholar] [CrossRef] [PubMed]
- James, R.E.; Schalks, R.; Browne, E.; Eleftheriadou, I.; Munoz, C.P.; Mazarakis, N.D.; Reynolds, R. Persistent elevation of intrathecal pro-inflammatory cytokines leads to multiple sclerosis-like cortical demyelination and neurodegeneration. Acta. Neuropathol. Commun. 2020, 8, 66. [Google Scholar] [CrossRef]
- Huang, J.; Khademi, M.; Fugger, L.; Lindhe, Ö.; Novakova, L.; Axelsson, M.; Malmeström, C.; Constantinescu, C.; Lycke, J.; Piehl, F.; et al. Inflammation-related plasma and CSF biomarkers for multiple sclerosis. Proc. Natl. Acad. Sci. USA 2020, 117, 12952–12960. [Google Scholar] [CrossRef]
- Javed, K.; Reddy, V.; Lui, F. Neuroanatomy, Choroid Plexus. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Kaiser, K.; Bryja, V. Choroid plexus: The orchestrator of long-range signalling within the CNS. Int. J. Mol. Sci. 2020, 21, 4760. [Google Scholar] [CrossRef]
- Baruch, K.; Schwartz, M. CNS-specific T cells shape brain function via the choroid plexus. Brain Behav. Immun. 2013, 34, 11–16. [Google Scholar] [CrossRef]
- Ghersi-Egea, J.F.; Strazielle, N.; Catala, M.; Silva-Vargas, V.; Doetsch, F.; Engelhardt, B. Molecular anatomy and functions of the choroidal blood-cerebrospinal fluid barrier in health and disease. Acta. Neuropathol. 2018, 135, 337–361. [Google Scholar] [CrossRef]
- Hatrock, D.; Caporicci-Dinucci, N.; Stratton, J.A. Ependymal cells and multiple sclerosis: Proposing a relationship. Neural. Regen. Res. 2020, 15, 263–264. [Google Scholar]
- Engelhardt, B.; Wolburg-Buchholz, K.; Wolburg, H. Involvement of the choroid plexus in central nervous system inflammation. Microsc. Res. Tech. 2001, 52, 112–129. [Google Scholar] [CrossRef]
- Strominger, I.; Elyahu, Y.; Berner, O.; Reckhow, J.; Mittal, K.; Nemirovsky, A.; Monsonego, A. The choroid plexus functions as a niche for T-Cell stimulation within the Central Nervous System. Front. Immunol. 2018, 9, 1066. [Google Scholar] [CrossRef]
- Rodríguez-Lorenzo, S.; Konings, J.; van der Pol, S.; Kamermans, A.; Amor, S.; van Horssen, J.; Witte, M.E.; Kooij, G.; de Vries, H.E. Inflammation of the choroid plexus in progressive multiple sclerosis: Accumulation of granulocytes and T cells. Acta. Neuropathol. Commun. 2020, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Vercellino, M.; Votta, B.; Condello, C.; Piacentino, C.; Romagnolo, A.; Merola, A.; Capello, E.; Mancardi, G.L.; Mutani, R.; Giordana, M.T.; et al. Involvement of the choroid plexus in multiple sclerosis autoimmune inflammation: A neuropathological study. J. Neuroimmunol. 2008, 199, 133–141. [Google Scholar] [CrossRef]
- Giunti, D.; Borsellino, G.; Benelli, R.; Marchese, M.; Capello, E.; Valle, M.T.; Pedemonte, E.; Noonan, D.; Albini, A.; Bernardi, G.; et al. Phenotypic and functional analysis of T cells homing into the CSF of subjects with inflammatory diseases of the CNS. J. Leukoc. Biol. 2003, 73, 584–590. [Google Scholar] [CrossRef]
- Kivisäkk, P.; Mahad, D.J.; Callahan, M.K.; Trebst, C.; Tucky, B.; Wei, T.; Wu, L.; Baekkevold, E.S.; Lassmann, H.; Staugaitis, S.M.; et al. Human cerebrospinal fluid central memory CD4+ T cells: Evidence for trafficking through choroid plexus and meninges via P-selectin. Proc. Natl. Acad. Sci. USA 2003, 100, 8389–8394. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monaco, S.; Nicholas, R.; Reynolds, R.; Magliozzi, R. Intrathecal Inflammation in Progressive Multiple Sclerosis. Int. J. Mol. Sci. 2020, 21, 8217. https://doi.org/10.3390/ijms21218217
Monaco S, Nicholas R, Reynolds R, Magliozzi R. Intrathecal Inflammation in Progressive Multiple Sclerosis. International Journal of Molecular Sciences. 2020; 21(21):8217. https://doi.org/10.3390/ijms21218217
Chicago/Turabian StyleMonaco, Salvatore, Richard Nicholas, Richard Reynolds, and Roberta Magliozzi. 2020. "Intrathecal Inflammation in Progressive Multiple Sclerosis" International Journal of Molecular Sciences 21, no. 21: 8217. https://doi.org/10.3390/ijms21218217
APA StyleMonaco, S., Nicholas, R., Reynolds, R., & Magliozzi, R. (2020). Intrathecal Inflammation in Progressive Multiple Sclerosis. International Journal of Molecular Sciences, 21(21), 8217. https://doi.org/10.3390/ijms21218217




