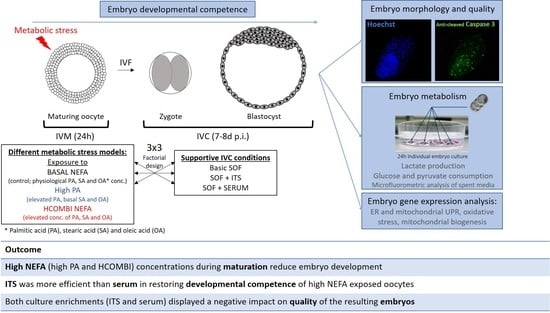
Rescue Potential of Supportive Embryo Culture Conditions on Bovine Embryos Derived from Metabolically Compromised Oocytes
Abstract
:1. Introduction
2. Results
2.1. The Impact of High NEFA Supplementation during IVM
2.1.1. Cumulus Cell Expansion
2.1.2. Embryo Development
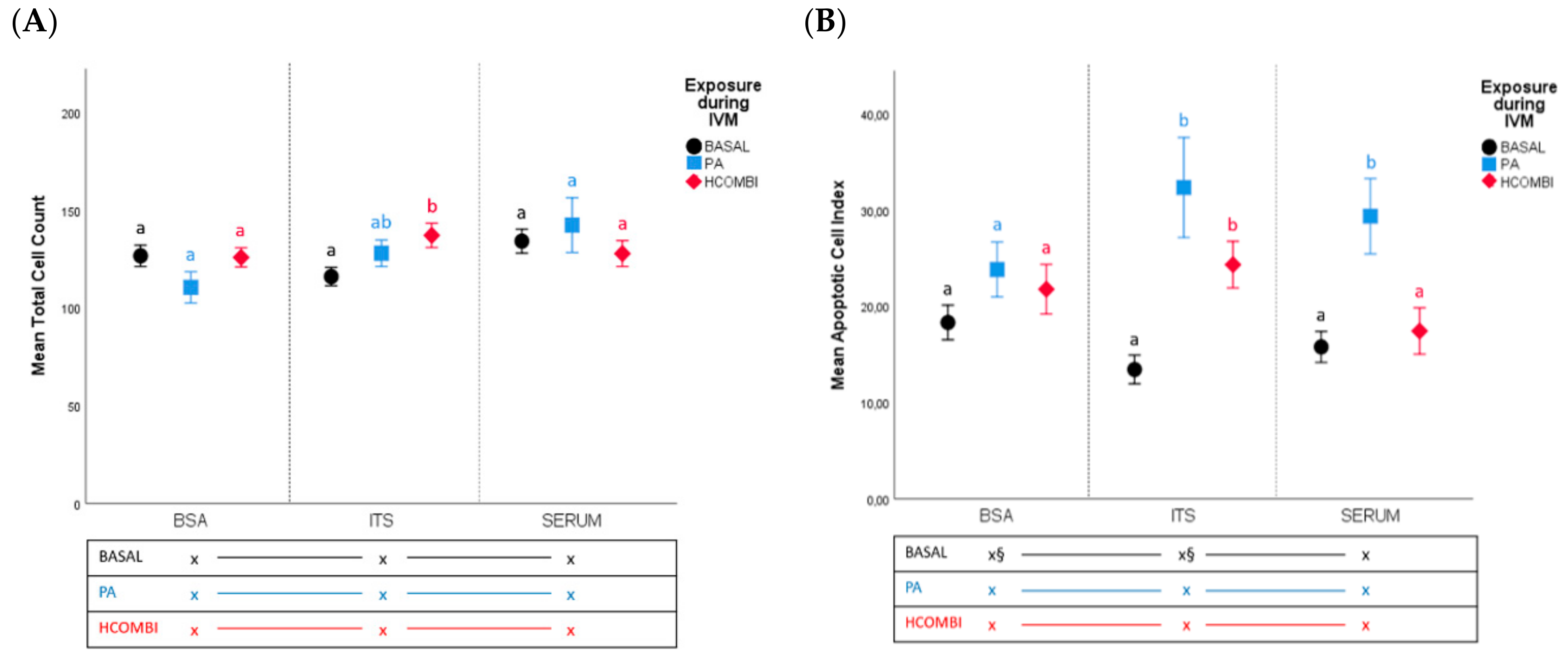
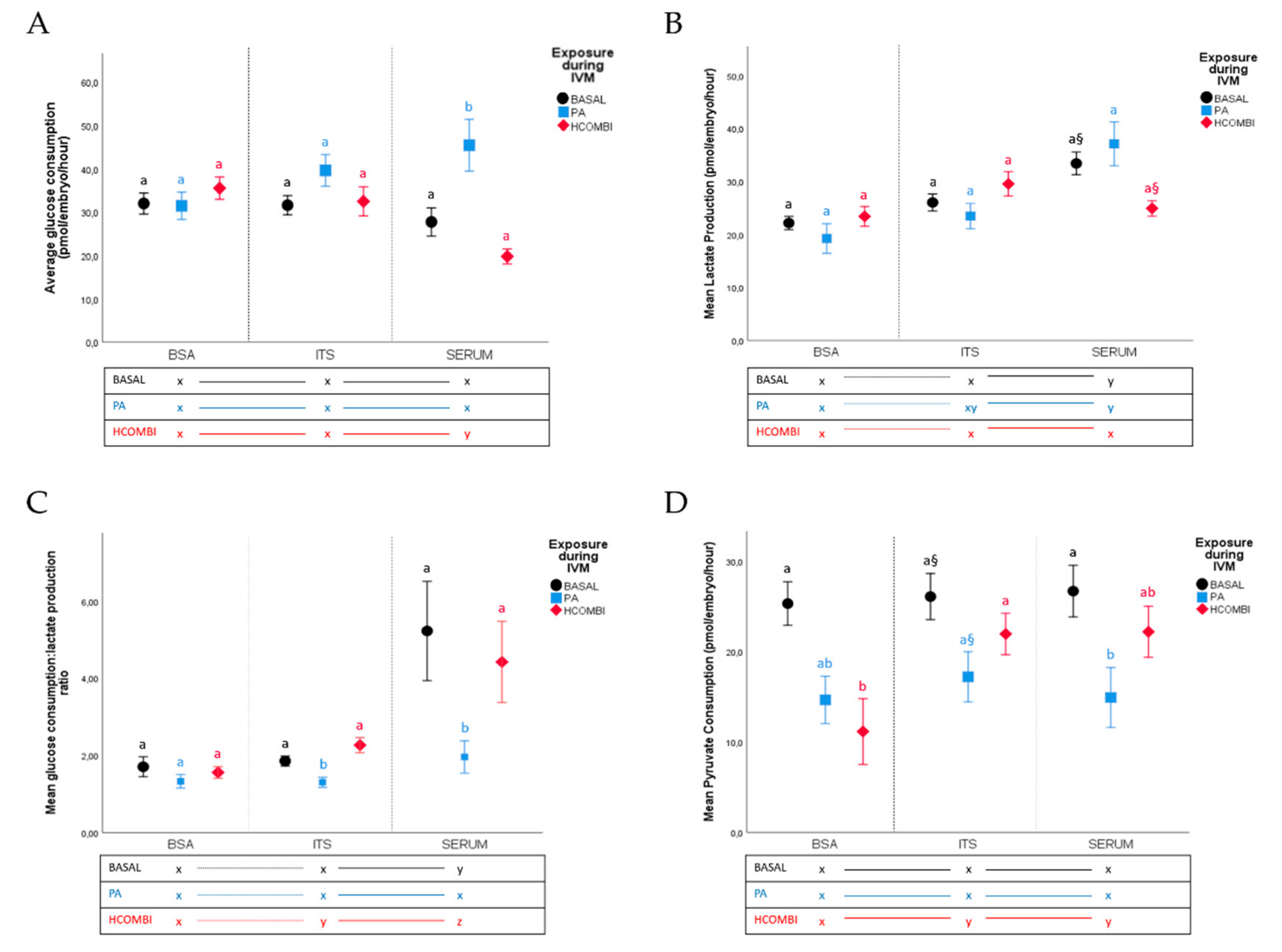
2.2. Embryo Quality
2.2.1. Total Cell Count and Apoptotic Cell Index
2.2.2. Metabolic Activity of Surviving Blastocysts
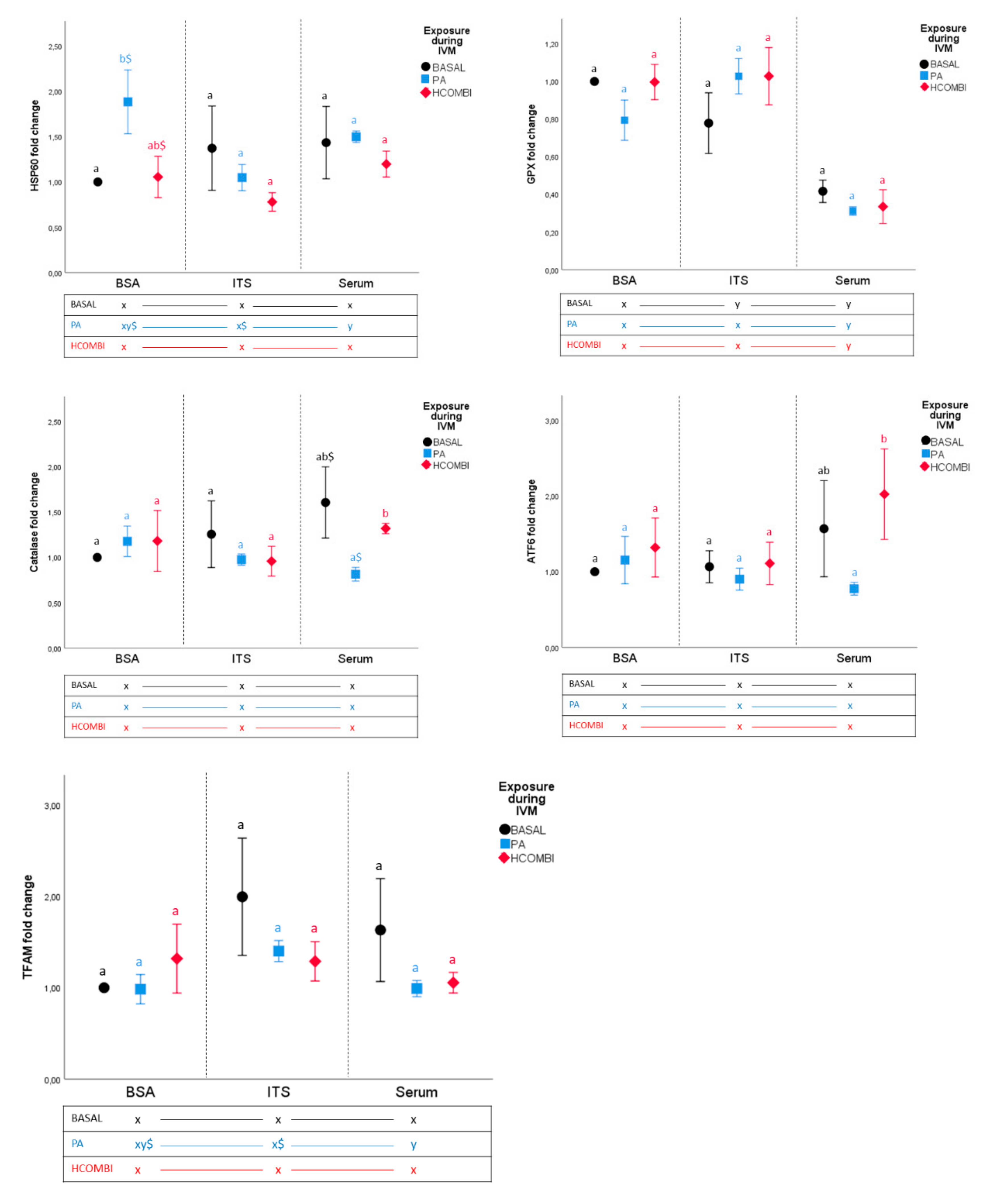
2.2.3. Gene Expression Analysis of Day 8 Blastocysts
3. Discussion
3.1. High NEFA Concentrations during IVM Affect Subsequent Embryo Development but Not Quality (Apoptosis, Cellular Metabolism and Gene Expression Analysis) When Cultured in Non-Supportive Conditions (Basic SOF-BSA Medium)
3.2. Supplementation of IVC Medium with ITS Supports the Development of Lower Quality Oocytes That Otherwise Would Not Have Developed Further
3.3. Supplementation of IVC Medium with Serum Did Not Improve Developmental Competence and Quality of PA-Exposed Oocytes
3.4. Supplementation of Serum to IVC Media of HCOMBI- and BASAL-Exposed Oocytes Alters Cellular Metabolism in Surviving Blastocysts
4. Materials and Methods
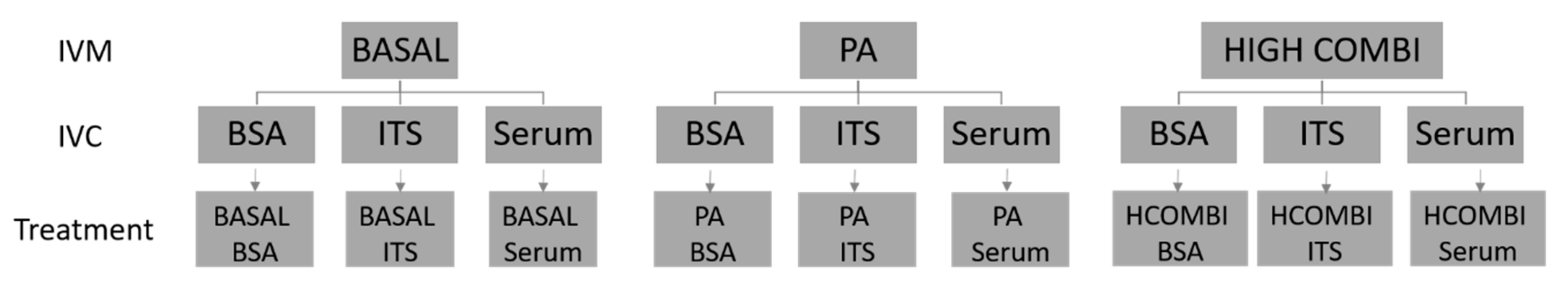
4.1. Experimental Design
4.2. Preparation of NEFA Stocks and NEFA-Supplemented In Vitro Maturation Media
4.3. In Vitro Embryo Production Procedure
4.3.1. Oocyte Collection and In Vitro Maturation (IVM)
4.3.2. In Vitro Embryo Production
4.4. Outcome Parameters
4.4.1. Assessment of Cumulus Cell Expansion
4.4.2. Embryo Developmental Competence
4.4.3. Blastocyst Energy Metabolism: Determination of Pyruvate and Glucose Uptake and Lactate Production
4.4.4. Assessment of Blastocyst Cell Number and Apoptotic Cell Index
4.4.5. Blastocyst RNA Extraction, Reverse Transcription and Quantification of Gene Expression by Quantitative Polymerase Chain Reaction (qPCR)
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Practice Committee of the American Society for Reproductive Medicine. Obesity and reproduction: A committee opinion. Fertil. Steril. 2015, 104, 1116–1126. [Google Scholar] [CrossRef] [Green Version]
- WHO. Low Fertility-the Future of Europe? (Entre Nous); WHO: Geneva, Switzerland, 2006. [Google Scholar]
- Jungheim, E.S.; Louden, E.D.; Chi, M.M.; Frolova, A.I.; Riley, J.K.; Moley, K.H. Preimplantation exposure of mouse embryos to palmitic acid results in fetal growth restriction followed by catch-up growth in the offspring. Biol. Reprod. 2011, 85, 678–683. [Google Scholar] [CrossRef] [Green Version]
- Leroy, J.L.; Vanholder, T.; Mateusen, B.; Christophe, A.; Opsomer, G.; de Kruif, A.; Genicot, G.; Van Soom, A. Non-esterified fatty acids in follicular fluid of dairy cows and their effect on developmental capacity of bovine oocytes in vitro. Reproduction 2005, 130, 485–495. [Google Scholar] [CrossRef] [Green Version]
- Robker, R.L.; Akison, L.K.; Bennett, B.D.; Thrupp, P.N.; Chura, L.R.; Russell, D.L.; Lane, M.; Norman, R.J. Obese women exhibit differences in ovarian metabolites, hormones, and gene expression compared with moderate-weight women. J. Clin. Endocrinol. Metab. 2009, 94, 1533–1540. [Google Scholar] [CrossRef] [Green Version]
- Valckx, S.D.; De Pauw, I.; De Neubourg, D.; Inion, I.; Berth, M.; Fransen, E.; Bols, P.E.; Leroy, J.L. BMI-related metabolic composition of the follicular fluid of women undergoing assisted reproductive treatment and the consequences for oocyte and embryo quality. Hum. Reprod. 2012, 27, 3531–3539. [Google Scholar] [CrossRef] [Green Version]
- Jungheim, E.S.; Macones, G.A.; Odem, R.R.; Patterson, B.W.; Lanzendorf, S.E.; Ratts, V.S.; Moley, K.H. Associations between free fatty acids, cumulus oocyte complex morphology and ovarian function during in vitro fertilization. Fertil. Steril. 2011, 95, 1970–1974. [Google Scholar] [CrossRef] [Green Version]
- Pasquali, R.; Pelusi, C.; Genghini, S.; Cacciari, M.; Gambineri, A. Obesity and reproductive disorders in women. Hum. Reprod. Update 2003, 9, 359–372. [Google Scholar] [CrossRef] [Green Version]
- Valckx, S.D.; De Bie, J.; Michiels, E.D.; Goovaerts, I.G.; Punjabi, U.; Ramos-Ibeas, P.; Gutierrez-Adan, A.; Bols, P.E.; Leroy, J.L. The effect of human follicular fluid on bovine oocyte developmental competence and embryo quality. Reprod. Biomed. Online 2015, 30, 203–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valckx, S.D.; Arias-Alvarez, M.; De Pauw, I.; Fievez, V.; Vlaeminck, B.; Fransen, E.; Bols, P.E.; Leroy, J.L. Fatty acid composition of the follicular fluid of normal weight, overweight and obese women undergoing assisted reproductive treatment: A descriptive cross-sectional study. Reprod. Biol. Endocrinol. 2014, 12, 13. [Google Scholar] [CrossRef]
- Mirabi, P.; Chaichi, M.J.; Esmaeilzadeh, S.; Ali Jorsaraei, S.G.; Bijani, A.; Ehsani, M.; Hashemi Karooee, S.F. The role of fatty acids on ICSI outcomes: A prospective cohort study. Lipids Health Dis. 2017, 16, 18. [Google Scholar] [CrossRef] [Green Version]
- O’Gorman, A.; Wallace, M.; Cottell, E.; Gibney, M.J.; McAuliffe, F.M.; Wingfield, M.; Brennan, L. Metabolic profiling of human follicular fluid identifies potential biomarkers of oocyte developmental competence. Reproduction 2013, 146, 389–395. [Google Scholar] [CrossRef] [Green Version]
- Aardema, H.; Vos, P.L.; Lolicato, F.; Roelen, B.A.; Knijn, H.M.; Vaandrager, A.B.; Helms, J.B.; Gadella, B.M. Oleic acid prevents detrimental effects of saturated fatty acids on bovine oocyte developmental competence. Biol. Reprod. 2011, 85, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Marei, W.F.A.; De Bie, J.; Mohey-Elsaeed, O.; Wydooghe, E.; Bols, P.E.J.; Leroy, J. Alpha-linolenic acid protects the developmental capacity of bovine cumulus-oocyte complexes matured under lipotoxic conditions in vitro. Biol. Reprod. 2017, 96, 1181–1196. [Google Scholar] [CrossRef]
- Van Hoeck, V.; Sturmey, R.G.; Bermejo-Alvarez, P.; Rizos, D.; Gutierrez-Adan, A.; Leese, H.J.; Bols, P.E.; Leroy, J.L. Elevated non-esterified fatty acid concentrations during bovine oocyte maturation compromise early embryo physiology. PLoS ONE 2011, 6, e23183. [Google Scholar] [CrossRef]
- Van Hoeck, V.; Leroy, J.L.; Arias Alvarez, M.; Rizos, D.; Gutierrez-Adan, A.; Schnorbusch, K.; Bols, P.E.; Leese, H.J.; Sturmey, R.G. Oocyte developmental failure in response to elevated nonesterified fatty acid concentrations: Mechanistic insights. Reproduction 2013, 145, 33–44. [Google Scholar] [CrossRef] [Green Version]
- Burton, G.J.; Hempstock, J.; Jauniaux, E. Oxygen, early embryonic metabolism and free radical-mediated embryopathies. Reprod. Biomed. Online 2003, 6, 84–96. [Google Scholar] [CrossRef]
- Marei, W.F.A.; Van Raemdonck, G.; Baggerman, G.; Bols, P.E.J.; Leroy, J. Proteomic changes in oocytes after in vitro maturation in lipotoxic conditions are different from those in cumulus cells. Sci. Rep. 2019, 9, 3673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marei, W.F.A.; Van den Bosch, L.; Pintelon, I.; Mohey-Elsaeed, O.; Bols, P.E.J.; Leroy, J. Mitochondria-targeted therapy rescues development and quality of embryos derived from oocytes matured under oxidative stress conditions: A bovine in vitro model. Hum. Reprod. 2019, 34, 1984–1998. [Google Scholar] [CrossRef] [PubMed]
- Desmet, K.L.; Van Hoeck, V.; Gagne, D.; Fournier, E.; Thakur, A.; O’Doherty, A.M.; Walsh, C.P.; Sirard, M.A.; Bols, P.E.; Leroy, J.L. Exposure of bovine oocytes and embryos to elevated non-esterified fatty acid concentrations: Integration of epigenetic and transcriptomic signatures in resultant blastocysts. BMC Genom. 2016, 17, 1004. [Google Scholar] [CrossRef]
- Marei, W.F.A.; Alvarez, M.A.; Van Hoeck, V.; Gutierrez-Adan, A.; Bols, P.E.J.; Leroy, J. Effect of nutritionally induced hyperlipidaemia on in vitro bovine embryo quality depends on the type of major fatty acid in the diet. Reprod. Fertil. Dev. 2017, 29, 1856–1867. [Google Scholar] [CrossRef]
- Van Hoeck, V.; De Bie, J.; Andries, S.; Merckx, E.; Bols, P.E.J.; Leroy, J.L.M.R. Elevated concentrations of saturated NEFA during bovine in vitro embryo culture compromise pre-implantation embryo development. In Proceedings of the 28th Scientific Meeting AETE, St-Malo, France, 7–8 September 2012. [Google Scholar]
- Rizos, D.; Ward, F.; Duffy, P.; Boland, M.P.; Lonergan, P. Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: Implications for blastocyst yield and blastocyst quality. Mol. Reprod. Dev. 2002, 61, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Lazzari, G.; Colleoni, S.; Lagutina, I.; Crotti, G.; Turini, P.; Tessaro, I.; Brunetti, D.; Duchi, R.; Galli, C. Short-term and long-term effects of embryo culture in the surrogate sheep oviduct versus in vitro culture for different domestic species. Theriogenology 2010, 73, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Sermondade, N.; Delarouziere, V.; Ravel, C.; Berthaut, I.; Verstraete, L.; Mathieu, E.; Antoine, J.M.; Mandelbaum, J. Characterization of a recurrent poor-quality embryo morphology phenotype and zygote transfer as a rescue strategy. Reprod. Biomed. Online 2012, 24, 403–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bavister, B.D. Culture of preimplantation embryos: Facts and artifacts. Hum. Reprod. Update 1995, 1, 91–148. [Google Scholar] [CrossRef]
- George, F.; Daniaux, C.; Genicot, G.; Verhaeghe, B.; Lambert, P.; Donnay, I. Set up of a serum-free culture system for bovine embryos: Embryo development and quality before and after transient transfer. Theriogenology 2008, 69, 612–623. [Google Scholar] [CrossRef]
- Mesalam, A.; Lee, K.L.; Khan, I.; Chowdhury, M.M.R.; Zhang, S.; Song, S.H.; Joo, M.D.; Lee, J.H.; Jin, J.I.; Kong, I.K. A combination of bovine serum albumin with insulin-transferrin-sodium selenite and/or epidermal growth factor as alternatives to fetal bovine serum in culture medium improves bovine embryo quality and trophoblast invasion by induction of matrix metalloproteinases. Reprod. Fertil. Dev. 2019, 31, 333–346. [Google Scholar]
- Van Langendonckt, A.; Donnay, I.; Schuurbiers, N.; Auquier, P.; Carolan, C.; Massip, A.; Dessy, F. Effects of supplementation with fetal calf serum on development of bovine embryos in synthetic oviduct fluid medium. J. Reprod. Fertil. 1997, 109, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Abe, H.; Hoshi, H. Evaluation of bovine embryos produced in high performance serum-free media. J. Reprod. Dev. 2003, 49, 193–202. [Google Scholar] [CrossRef] [Green Version]
- Abe, H.; Yamashita, S.; Itoh, T.; Satoh, T.; Hoshi, H. Ultrastructure of bovine embryos developed from in vitro-matured and -fertilized oocytes: Comparative morphological evaluation of embryos cultured either in serum-free medium or in serum-supplemented medium. Mol. Reprod. Dev. 1999, 53, 325–335. [Google Scholar] [CrossRef]
- Ferguson, E.M.; Leese, H.J. Triglyceride content of bovine oocytes and early embryos. J. Reprod. Fertil. 1999, 116, 373–378. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Kinoshita, M.; Ohnishi, M.; Fukui, Y. Lipid and fatty acid analysis of fresh and frozen-thawed immature and in vitro matured bovine oocytes. Reproduction 2001, 122, 131–138. [Google Scholar] [CrossRef]
- Roche, M.; Rondeau, P.; Singh, N.R.; Tarnus, E.; Bourdon, E. The antioxidant properties of serum albumin. FEBS Lett. 2008, 582, 1783–1787. [Google Scholar] [CrossRef] [PubMed]
- Augustin, R.; Pocar, P.; Wrenzycki, C.; Niemann, H.; Fischer, B. Mitogenic and anti-apoptotic activity of insulin on bovine embryos produced in vitro. Reproduction 2003, 126, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.; Sato, G. Methods for growth of cultured cells in serum-free medium. Anal. Biochem. 1980, 102, 255–270. [Google Scholar] [CrossRef]
- Goovaerts, I.G.; Leroy, J.L.; Langbeen, A.; Jorssen, E.P.; Bosmans, E.; Bols, P.E. Unravelling the needs of singly in vitro-produced bovine embryos: From cumulus cell co-culture to semi-defined, oil-free culture conditions. Reprod. Fertil. Dev. 2012, 24, 1084–1092. [Google Scholar] [CrossRef]
- Gutteridge, J.M.C. Oxygen free radicals. In Selenium in Medicine and Biology; Springer: Berlin, Germany, 1989; pp. 225–233. [Google Scholar]
- Morbeck, D.E.; Krisher, R.L.; Herrick, J.R.; Baumann, N.A.; Matern, D.; Moyer, T. Composition of commercial media used for human embryo culture. Fertil. Steril. 2014, 102, 759–766.e9. [Google Scholar] [CrossRef]
- De Bie, J.; Marei, W.F.A.; Maillo, V.; Jordaens, L.; Gutierrez-Adan, A.; Bols, P.E.J.; Leroy, J. Differential effects of high and low glucose concentrations during lipolysis-like conditions on bovine in vitro oocyte quality, metabolism and subsequent embryo development. Reprod. Fertil. Dev. 2017, 29, 2284–2300. [Google Scholar] [CrossRef] [Green Version]
- Wittemer, C.; Ohl, J.; Bailly, M.; Bettahar-Lebugle, K.; Nisand, I. Does body mass index of infertile women have an impact on IVF procedure and outcome? J. Assist. Reprod. Genet. 2000, 17, 547–552. [Google Scholar] [CrossRef]
- Paczkowski, M.; Schoolcraft, W.B.; Krisher, R.L. Fatty acid metabolism during maturation affects glucose uptake and is essential to oocyte competence. Reproduction 2014, 148, 429–439. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Kim, E.; Hyun, S.H. Toxicity evaluation of ethanol treatment during in vitro maturation of porcine oocytes and subsequent embryonic development following parthenogenetic activation and in vitro fertilization. Int. J. Mol. Med. 2014, 34, 1372–1380. [Google Scholar] [CrossRef]
- Bowles, C.; Lishman, A. Attempts to improve the yield of bovine blastocysts by incorporating insulin, selenium and transferrin in the in vitro system. S. Afr. J. Anim. Sci. 1998, 28, 30–37. [Google Scholar] [CrossRef]
- Shamsuddin, M.; Larsson, B.; Gustafsson, H.; Rodriguez-Martinez, H. A serum-free, cell-free culture system for development of bovine one-cell embryos up to blastocyst stage with improved viability. Theriogenology 1994, 41, 1033–1043. [Google Scholar] [CrossRef]
- Krisher, R.L.; Prather, R.S. A role for the Warburg effect in preimplantation embryo development: Metabolic modification to support rapid cell proliferation. Mol. Reprod. Dev. 2012, 79, 311–320. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.L.; Wang, Y.B.; Jiao, G.Z.; Kong, D.L.; Li, Q.; Li, H.; Zheng, L.L.; Tan, J.H. Effects of glucose metabolism during in vitro maturation on cytoplasmic maturation of mouse oocytes. Sci. Rep. 2016, 6, 20764. [Google Scholar] [CrossRef] [Green Version]
- Gardner, D.K.; Harvey, A.J. Blastocyst metabolism. Reprod. Fertil. Dev. 2015, 27, 638–654. [Google Scholar] [CrossRef]
- Khera, A.; Dong, L.F.; Holland, O.; Vanderlelie, J.; Pasdar, E.A.; Neuzil, J.; Perkins, A.V. Selenium supplementation induces mitochondrial biogenesis in trophoblasts. Placenta 2015, 36, 863–869. [Google Scholar] [CrossRef]
- Jeong, Y.W.; Hossein, M.S.; Bhandari, D.P.; Kim, Y.W.; Kim, J.H.; Park, S.W.; Lee, E.; Park, S.M.; Jeong, Y.I.; Lee, J.Y.; et al. Effects of insulin-transferrin-selenium in defined and porcine follicular fluid supplemented IVM media on porcine IVF and SCNT embryo production. Anim. Reprod. Sci. 2008, 106, 13–24. [Google Scholar] [CrossRef]
- Desmet, K.L.J.; Marei, W.F.A.; Richard, C.; Sprangers, K.; Beemster, G.T.S.; Meysman, P.; Laukens, K.; Declerck, K.; Vanden Berghe, W.; Bols, P.E.J.; et al. Oocyte maturation under lipotoxic conditions induces carryover transcriptomic and functional alterations during post-hatching development of good-quality blastocysts: Novel insights from a bovine embryo-transfer model. Hum. Reprod. 2020, 35, 293–307. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Das, N.; Mandala, A.; Mukhopadhyay, S.; Roy, S.S. Fenofibrate Reverses Palmitate Induced Impairment in Glucose Uptake in Skeletal Muscle Cells by Preventing Cytosolic Ceramide Accumulation. Cell. Physiol. Biochem. 2015, 37, 1315–1328. [Google Scholar] [CrossRef]
- Ferguson, E.M.; Leese, H.J. A potential role for triglyceride as an energy source during bovine oocyte maturation and early embryo development. Mol. Reprod. Dev. 2006, 73, 1195–1201. [Google Scholar] [CrossRef]
- Sturmey, R.G.; Leese, H.J. Role of glucose and fatty acid metabolism in porcine early embryo development. Reprod. Fertil. Dev. 2008, 20, 149. [Google Scholar] [CrossRef]
- Lim, J.H.; Gerhart-Hines, Z.; Dominy, J.E.; Lee, Y.; Kim, S.; Tabata, M.; Xiang, Y.K.; Puigserver, P. Oleic acid stimulates complete oxidation of fatty acids through protein kinase A-dependent activation of SIRT1-PGC1alpha complex. J. Biol. Chem. 2013, 288, 7117–7126. [Google Scholar] [CrossRef] [Green Version]
- Wrenzycki, C.; Herrmann, D.; Carnwath, J.W.; Niemann, H. Alterations in the relative abundance of gene transcripts in preimplantation bovine embryos cultured in medium supplemented with either serum or PVA. Mol. Reprod. Dev. 1999, 53, 8–18. [Google Scholar] [CrossRef]
- Steenbergen, R.; Oti, M.; Ter Horst, R.; Tat, W.; Neufeldt, C.; Belovodskiy, A.; Chua, T.T.; Cho, W.J.; Joyce, M.; Dutilh, B.E.; et al. Establishing normal metabolism and differentiation in hepatocellular carcinoma cells by culturing in adult human serum. Sci. Rep. 2018, 8, 11685. [Google Scholar] [CrossRef]
- Leroy, J.L.M.R.; De Bie, J.; Jordaens, L.; Desmet, K.; Smits, A.; Marei, W.F.A.; Bols, P.E.J.; Hoeck, V.V. Negative energy balance and metabolic stress in relation to oocyte and embryo quality: An update on possible pathways reducing fertility in dairy cows. Anim. Reprod. 2017, 14, 497–506. [Google Scholar] [CrossRef]
- Harvey, A.J. The role of oxygen in ruminant preimplantation embryo development and metabolism. Anim. Reprod. Sci. 2007, 98, 113–128. [Google Scholar] [CrossRef]
- Marei, W.F.; Ghafari, F.; Fouladi-Nashta, A.A. Role of hyaluronic acid in maturation and further early embryo development of bovine oocytes. Theriogenology 2012, 78, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Stringfellow, D.A.; Givens, M.D.; Society, I.E.T. Manual of the International Embryo Transfer Society: A Procedural Guide and General Information for the Use of Embryo Transfer Technology Emphasizing Sanitary Procedures; International Embryo Transfer Society: Savory, IL, USA, 2010. [Google Scholar]
- Guerif, F.; McKeegan, P.; Leese, H.J.; Sturmey, R.G. A simple approach for COnsumption and RElease (CORE) analysis of metabolic activity in single mammalian embryos. PLoS ONE 2013, 8, e67834. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

| Maturation | Culture | Oocytes Used | Cleaved Embryos | Two-Cell Stage | Four-Cell Plus | Fragmented Embryos | Day 7 Blastocysts | Day 8 Blastocysts |
|---|---|---|---|---|---|---|---|---|
| BASAL | BSA | 515 | 395 a (76.69%) | 48 a§ (9.32%) | 255 a (49.51%) | 63 (12.23%) | 105 a (20.38%) | 125 a§ (24.27%) |
| PA | BSA | 270 | 176 b (65.18%) | 42 b (15.55%) | 81 b (30.00%) | 35 (12.96%) | 30 b§ (11.11%) | 37 b (13.70%) |
| HCOMBI | BSA | 527 | 377 ab (71.53%) | 63 ab§ (11.95%) | 221 c (41.93%) | 65 (12.33%) | 87 ab§ (16.50%) | 96 ab§ (18.21%) |
| BASAL | ITS | 510 | 406 §$ (79.6%) | 63 (12.35%) | 250 x (49.01%) | 56§ (10.98%) | 124 (24.31%) | 139 (27.25%) |
| PA | ITS | 278 | 200 $ (71.94%) | 43 (15.46%) | 110 y (39.56%) | 30 (10.79%) | 52 (18.7%) | 68 (24.46%) |
| HCOMBI | ITS | 525 | 387 § (73.71%) | 46 (8.76%) | 235 xy (44.76%) | 84 § (16.00%) | 101 (19.23%) | 120 (22.85%) |
| BASAL | Serum | 488 | 348 jk§ (71.31%) | 73 j (14.95%) | 182 j (37.29%) | 55 (11.27%) | 119 j (24.39%) | 136 j (27.86%) |
| PA | Serum | 247 | 156 j§ (63.15%) | 54 k (21.86%) | 53 k (21.45%) | 30 (12.14%) | 30 k (12.14%) | 45 k (18.21%) |
| HCOMBI | Serum | 377 | 275 k (72.94%) | 55 jk (14.58%) | 149 j (39.52%) | 41 (10.87%) | 88 j (23.34%) | 91 jk (24.13%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smits, A.; Leroy, J.L.M.R.; Bols, P.E.J.; De Bie, J.; Marei, W.F.A. Rescue Potential of Supportive Embryo Culture Conditions on Bovine Embryos Derived from Metabolically Compromised Oocytes. Int. J. Mol. Sci. 2020, 21, 8206. https://doi.org/10.3390/ijms21218206
Smits A, Leroy JLMR, Bols PEJ, De Bie J, Marei WFA. Rescue Potential of Supportive Embryo Culture Conditions on Bovine Embryos Derived from Metabolically Compromised Oocytes. International Journal of Molecular Sciences. 2020; 21(21):8206. https://doi.org/10.3390/ijms21218206
Chicago/Turabian StyleSmits, Anouk, Jo L. M. R. Leroy, Peter E. J. Bols, Jessie De Bie, and Waleed F. A. Marei. 2020. "Rescue Potential of Supportive Embryo Culture Conditions on Bovine Embryos Derived from Metabolically Compromised Oocytes" International Journal of Molecular Sciences 21, no. 21: 8206. https://doi.org/10.3390/ijms21218206