5-Nitro-2,4-Dichloropyrimidine as an Universal Model for Low-Energy Electron Processes Relevant for Radiosensitization
Abstract
:1. Introduction
- 1.
- DEA—dissociaitive electron attachment AB + e− → A + B−,
- 2.
- AEA—asociative electron attachment AB + e− → AB−
- 3.
- DNA sensitization
2. Materials and Methods
2.1. Electron Attachment to Isolated 5-Nitro-2,4-Dichloropyrimidine
2.2. Electron Attachment to Dry and Microhydrated 5-Nitro-2,4-Dichloropyrimidine in Molecular Beam
2.3. Cytotoxicity of 5-Nitro-2,4-Dichloropyrimidine
2.3.1. Cell Lines and Culturing
2.3.2. MTT Assay
3. Results and Discussion
3.1. Parent Anion
3.2. Fragment Anions
3.3. Effect of Microhydration
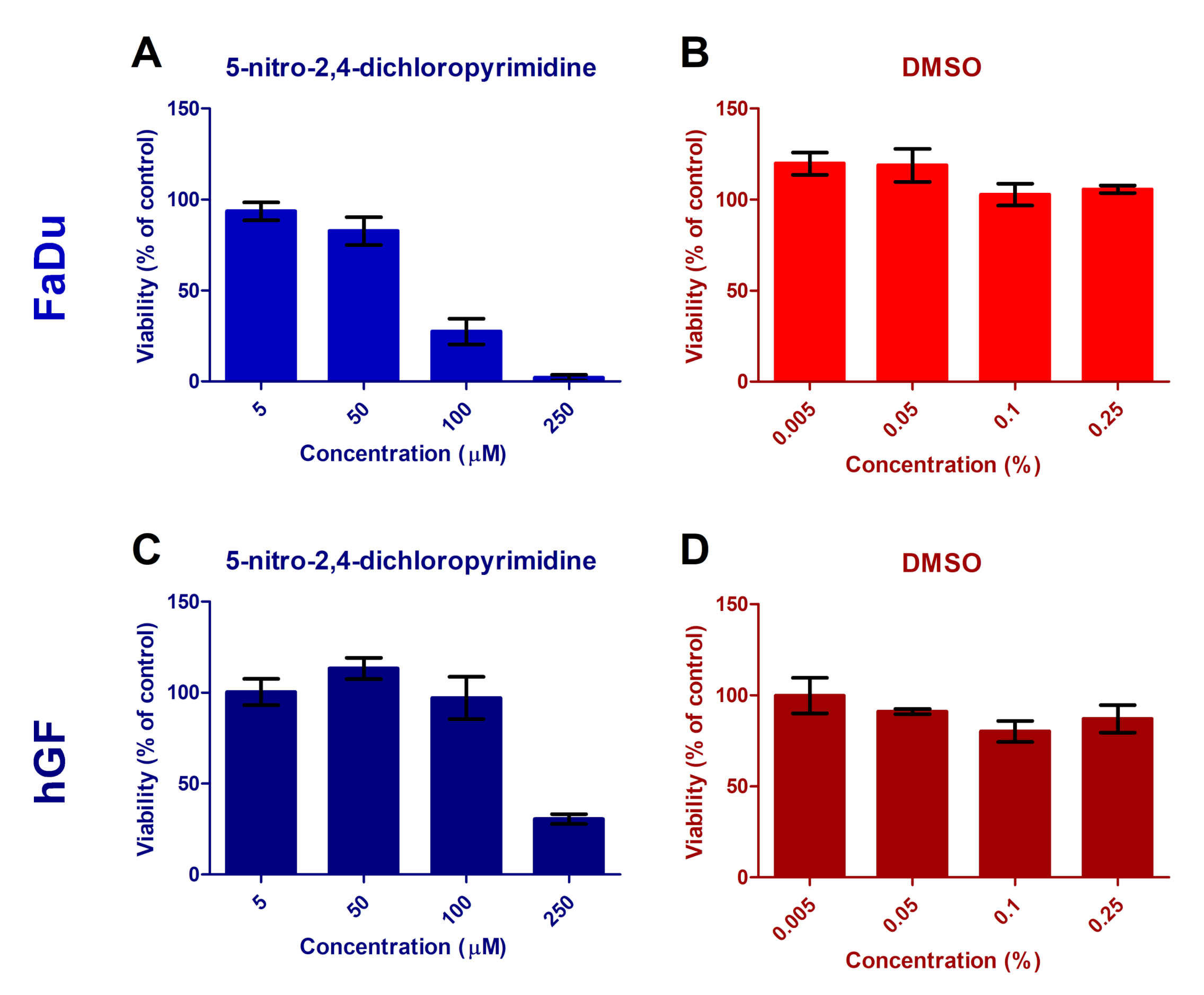
3.4. Cytotoxicity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Seiwert, T.Y.; Salama, J.K.; Vokes, E.E. The concurrent chemoradiation paradigm—General principles. Nat. Clin. Pract. Oncol. 2007, 4, 86. [Google Scholar] [CrossRef]
- Longley, D.; Harkin, P.; Johnston, P. 5-Fluorouracil: Mechanisms of Action and Clinical Strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Peng, J.; Tuttle, P.R.; Ren, Y.; Garcia, C.; Debnath, D.; Rishi, S.; Hanson, C.; Ward, S.; Kumar, A.; et al. Electron-Mediated Aminyl and Iminyl Radicals from C5 Azido-Modified Pyrimidine Nucleosides Augment Radiation Damage to Cancer Cells. Org. Lett. 2018, 20, 7400–7404. [Google Scholar] [CrossRef]
- Makurat, S.; Chomicz-Mańka, L.; Rak, J. Electrophilic 5-Substituted Uracils as Potential Radiosensitizers: A Density Functional Theory Study. ChemPhysChem 2016, 17, 2572–2578. [Google Scholar] [CrossRef]
- Zdrowowicz, M.; Chomicz, L.; Žyndul, M.; Wityk, P.; Rak, J.; Wiegand, T.J.; Hanson, C.G.; Adhikary, A.; Sevilla, M.D. 5-Thiocyanato-2’-deoxyuridine as a possible radiosensitizer: Electron-induced formation of uracil-C5-thiyl radical and its dimerization. Phys. Chem. Chem. Phys. 2015, 17, 16907–16916. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Wei, Y.; Yu, X.; Peng, J.; Leng, X. 3-Bromopyruvic Acid, A Hexokinase II Inhibitor, is an Effective Antitumor Agent on the Hepatoma Cells; in vitro and in vivo Findings. Anti-Cancer Agents Med. Chem. 2014, 771–776. [Google Scholar] [CrossRef]
- Adams, G.E.; Flockhart, I.R.; Smithen, C.E.; Stratford, I.J.; Wardman, P.; Watts, M.E. Electron-Affinic Sensitization: VII. A Correlation between Structures, One-Electron Reduction Potentials, and Efficiencies of Nitroimidazoles as Hypoxic Cell Radiosensitizers. Radiat. Res. 1976, 67, 9–20. [Google Scholar] [CrossRef]
- Overgaard, J.; Hansen, H.S.; Overgaard, M.; Bastholt, L.; Berthelsen, A.; Specht, L.; Lindeløv, B.; Jørgensen, K. A randomized double-blind phase III study of nimorazole as a hypoxic radiosensitizer of primary radiotherapy in supraglottic larynx and pharynx carcinoma. Results of the Danish Head and Neck Cancer Study (DAHANCA) Protocol 5-85. Radiother. Oncol. 1998, 135–146. [Google Scholar] [CrossRef]
- Sugie, C.; Shibamoto, Y.; Ito, M.; Ogino, H.; Suzuki, H.; Uto, Y.; Nagasawa, H.; Hori, H. Reevaluation of the Radiosensitizing Effects of Sanazole and Nimorazole In Vitro and In Vivo. J. Radiat. Res. 2005, 46, 453–459. [Google Scholar] [CrossRef] [Green Version]
- Epelbaum, R.; Rosenblatt, E.; Nasrallah, S.; Faraggi, D.; Gaitini, D.; Mizrahi, S.; Kuten, A. Phase II study of gemcitabine combined with radiation therapy in patients with localized, unresectable pancreatic cancer. J. Surg. Oncol. 2002, 81, 138–143. [Google Scholar] [CrossRef]
- Cihoric, N.; Tsikkinis, A.; Vlaskou Badra, E.; Glatzer, M.; Novak, U.; Scherz, A.; Shelan, M.; Soldatovic, I.; Yojena, C.K.K.; Aebersold, D.M.; et al. Highly conformal combined radiotherapy with cisplatin and gemcitabine for treatment of loco-regionally advanced cervical cancer—A retrospective study. Radiat. Oncol. 2017, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boudaiffa, B.; Cloutier, P.; Hunting, D.; Huels, M.A.; Sanche, L. Resonant Formation of DNA Strand Breaks by Low-Energy (3 to 20 eV) Electrons. Science 2000, 287, 1658–1660. [Google Scholar]
- Nguyen, J.; Ma, Y.; Luo, T.; Bristow, R.G.; Jaffray, D.A.; Lu, Q.B. Direct Observation of Ultrafast-Electron-Transfer Reactions Unravels High Effectiveness of Reductive DNA Damage. Proc. Natl. Acad. Sci. USA 2011, 108, 11778–11783. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Kumar, A.; Muroya, Y.; Yamashita, S.; Sakurai, T.; Denisov, S.A.; Sevilla, M.D.; Adhikary, A.; Seki, S.; Mostafavi, M. Observation of dissociative quasi-free electron attachment to nucleoside via excited anion radical in solution. Nat. Commun. 2019, 10, 102. [Google Scholar] [CrossRef]
- Schürmann, R.; Vogel, S.; Ebel, K.; Bald, I. The Physico-Chemical Basis of DNA Radiosensitization: Implications for Cancer Radiation Therapy. Chem. A Eur. J. 2018, 24, 10271–10279. [Google Scholar] [CrossRef]
- Fabrikant, I.I.; Eden, S.; Mason, N.J.; Fedor, J. Recent Progress in Dissociative Electron Attachment. Adv. At. Mol. Opt. Phys. 2017, 545–657. [Google Scholar] [CrossRef]
- Bald, I.; Čurík, R.; Kopyra, J.; Tarana, M. Dissociative Electron Attachment to Biomolecules. In Nanoscale Insights into Ion-Beam Cancer Therapy; Solov’yov, A.V., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 159–207. [Google Scholar] [CrossRef]
- Gorfinkiel, J.D.; Ptasinska, S. Electron Scattering from Molecules and Molecular Aggregates of Biological Relevance. J. Phys. B At. Mol. Opt. Phys. 2017, 50, 182001. [Google Scholar] [CrossRef] [Green Version]
- Chomicz, L.; Zdrowowicz, M.; Kasprzykowski, F.; Rak, J.; Buonaugurio, A.; Wang, Y.; Bowen, K.H. How to Find Out Whether a 5-Substituted Uracil Could Be a Potential DNA Radiosensitizer. J. Phys. Chem. Lett. 2013, 4, 2853–2857. [Google Scholar] [CrossRef]
- von Sonntag, C. Free-Radical-Induced DNA Damage and Its Repair; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Alizadeh, E.; Orlando, T.M.; Sanche, L. Biomolecular Damage Induced by Ionizing Radiation: The Direct and Indirect Effects of Low-Energy Electrons on DNA. Annu. Rev. Phys. Chem. 2015, 66, 379–398. [Google Scholar] [CrossRef]
- Choudhari, S.K.; Chaudhary, M.; Bagde, S.; Gadbail, A.R.; Joshi, V. Nitric oxide and cancer: A review. World J. Surg. Oncol. 2013, 11, 118. [Google Scholar] [CrossRef] [Green Version]
- Huerta, S.; Chilka, S.; Bonavida, B. Nitric oxide donors: Novel cancer therapeutics (Review). Int. J. Oncol. 2008, 33, 909–927. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Fu, J.; Zhang, Y. Nitric Oxide Donor-Based Cancer Therapy: Advances and Prospects. J. Med. Chem. 2017, 60, 7617–7635. [Google Scholar] [CrossRef]
- Kopyra, J.; Koenig-Lehmann, C.; Bald, I.; Illenberger, E. A Single Slow Electron Triggers the Loss of Both Chlorine Atoms from the Anticancer Drug Cisplatin: Implications for Chemoradiation Therapy. Angew. Chem. Int. Ed. 2009, 48, 7904–7907. [Google Scholar] [CrossRef] [PubMed]
- Rak, J.; Chomicz, L.; Wiczk, J.; Westphal, K.; Zdrowowicz, M.; Wityk, P.; Żyndul, M.; Makurat, S.; Golon, L. Mechanisms of Damage to DNA Labeled with Electrophilic Nucleobases Induced by Ionizing or UV Radiation. J. Phys. Chem. B 2015, 119, 8227–8238. [Google Scholar] [CrossRef]
- Wang, C.R.; Lu, Q.B. Real-Time Observation of a Molecular Reaction Mechanism of Aqueous 5-Halo-2’-deoxyuridines under UV/Ionizing Radiation. Angew. Chem. Int. Ed. 2007, 46, 6316–6320. [Google Scholar] [CrossRef]
- Lu, Q.B.; Kalantari, S.; Wang, C.R. Electron Transfer Reaction Mechanism of Cisplatin with DNA at the Molecular Level. Mol. Pharm. 2007, 4, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, H.S.; Smith, K.C.; Tomlin, P.A. Effect of Halogenated Pyrimidines on Radiosensitivity of E. coli. Radiat. Res. 1962, 16, 98–113. [Google Scholar] [CrossRef]
- Meißner, R.; Kočišek, J.; Feketeová, L.; Fedor, J.; Fárník, M.; Limão-Vieira, P.; Illenberger, E.; Denifl, S. Low-energy electrons transform the nimorazole molecule into a radiosensitiser. Nat. Commun. 2019, 10, 2388. [Google Scholar] [CrossRef]
- Poštulka, J.; Slavíček, P.; Fedor, J.; Fárník, M.; Kočišek, J. Energy Transfer in Microhydrated Uracil, 5-Fluorouracil, and 5-Bromouracil. J. Phys. Chem. B 2017, 121, 8965–8974. [Google Scholar] [CrossRef]
- Verkhovtsev, A.; Surdutovich, E.; Solov’yov, A.V. Multiscale approach predictions for biological outcomes in ion-beam cancer therapy. Sci. Rep. 2016, 27654. [Google Scholar] [CrossRef] [Green Version]
- Rezaee, M.; Hunting, D.J.; Sanche, L. New Insights into the Mechanism Underlying the Synergistic Action of Ionizing Radiation With Platinum Chemotherapeutic Drugs: The Role of Low-Energy Electrons. Int. J. Radiat. Oncol. Biol. Phys. 2013, 847–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reimitz, D.; Davídková, M.; Mestek, O.; Pinkas, J.; Kočišek, J. Radiomodifying effects of RAPTA C and CDDP on DNA strand break induction. Radiat. Phys. Chem. 2017, 229–234. [Google Scholar] [CrossRef]
- Dong, Y.; Zhou, L.; Tian, Q.; Zheng, Y.; Sanche, L. Chemoradiation Cancer Therapy: Molecular Mechanisms of Cisplatin Radiosensitization. J. Phys. Chem. C 2017, 121, 17505–17513. [Google Scholar] [CrossRef]
- Wagner, C.; Wagenknecht, H.A. Reductive Electron Transfer in Phenothiazine-Modified DNA Is Dependent on the Base Sequence. Chem. A Eur. J. 2005, 11, 1871–1876. [Google Scholar] [CrossRef]
- Xiao, F.; Luo, X.; Fu, X.; Zheng, Y. Cleavage Enhancement of Specific Chemical Bonds in DNA by Cisplatin Radiosensitization. J. Phys. Chem. B 2013, 117, 4893–4900. [Google Scholar] [CrossRef]
- Park, Y.; Polska, K.; Rak, J.; Wagner, J.R.; Sanche, L. Fundamental Mechanisms of DNA Radiosensitization: Damage Induced by Low-Energy Electrons in Brominated Oligonucleotide Trimers. J. Phys. Chem. B 2012, 116, 9676–9682. [Google Scholar] [CrossRef]
- Rezaee, M.; Sanche, L.; Hunting, D.J. Cisplatin Enhances the Formation of DNA Single- and Double-Strand Breaks by Hydrated Electrons and Hydroxyl Radicals. Radiat. Res. 2013, 179, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Rackwitz, J.; Ranković, M.L.; Milosavljević, A.R.; Bald, I. A novel setup for the determination of absolute cross sections for low-energy electron induced strand breaks in oligonucleotides—The effect of the radiosensitizer 5-fluorouracil*. Eur. Phys. J. D 2017, 32. [Google Scholar] [CrossRef]
- Rackwitz, J.; Kopyra, J.; Dąbkowska, I.; Ebel, K.; Ranković, M.L.; Milosavljević, A.R.; Bald, I. Sensitizing DNA Towards Low-Energy Electrons with 2-Fluoroadenine. Angew. Chem. Int. Ed. 2016, 55, 10248–10252. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 Revision E.01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Pshenichnyuk, S.A.; Vorob’ev, A.S.; Modelli, A. Resonance electron attachment and long-lived negative ions of phthalimide and pyromellitic diimide. J. Chem. Phys. 2011, 135, 184301. [Google Scholar] [CrossRef]
- Asfandiarov, N.L.; Pshenichnyuk, S.A.; Rakhmeyev, R.G.; Tuktarov, R.F.; Zaitsev, N.L.; Vorob’ev, A.S.; Kočišek, J.; Fedor, J.; Modelli, A. 4-Bromobiphenyl: Long-lived molecular anion formation and competition between electron detachment and dissociation. J. Chem. Phys. 2019, 150, 114304. [Google Scholar] [CrossRef]
- Kočišek, J.; Pysanenko, A.; Fárník, M.; Fedor, J. Microhydration Prevents Fragmentation of Uracil and Thymine by Low-Energy Electrons. J. Phys. Chem. Lett. 2016, 7, 3401–3405. [Google Scholar] [CrossRef] [PubMed]
- Asfandiarov, N.L.; Pshenichnyuk, S.A.; Vorob’ev, A.S.; Nafikova, E.P.; Elkin, Y.N.; Pelageev, D.N.; Koltsova, E.A.; Modelli, A. Electron attachment to some naphthoquinone derivatives: Long-lived molecular anion formation. Rapid Commun. Mass Spectrom. 2014, 28, 1580–1590. [Google Scholar] [CrossRef]
- Asfandiarov, N.L.; Pshenichnyuk, S.A.; Vorob’ev, A.S.; Nafikova, E.P.; Modelli, A. Electron affinity evaluation for nitrobenzene derivatives using negative ion lifetime data. Rapid Commun. Mass Spectrom. 2015, 29, 910–912. [Google Scholar] [CrossRef]
- Schürmann, R.; Tsering, T.; Tanzer, K.; Denifl, S.; Kumar, S.V.K.; Bald, I. Resonant Formation of Strand Breaks in Sensitized Oligonucleotides Induced by Low-Energy Electrons (0.5–9 eV). Angew. Chem. Int. Ed. 2017, 56, 10952–10955. [Google Scholar] [CrossRef]
- Chen, E.C.; Chen, E.S. Electron affinities from gas chromatography electron capture detector and negative ion mass spectrometry responses and complementary methods. J. Chromatogr. A 2018, 1–17. [Google Scholar] [CrossRef]
- Kočišek, J.; Grygoryeva, K.; Lengyel, J.; Fárník, M.; Fedor, J. Effect of Cluster Environment on the Electron Attachment to 2-Nitrophenol. Eur. Phys. J. D 2016, 70, 98. [Google Scholar] [CrossRef]
- Wang, C.R.; Nguyen, J.; Lu, Q.B. Bond Breaks of Nucleotides by Dissociative Electron Transfer of Nonequilibrium Prehydrated Electrons: A New Molecular Mechansim for Reductive DNA Damage. J. Am. Chem. Soc. 2009, 131, 11320–11322. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.B. Effects and applications of ultrashort-lived prehydrated electrons in radiation biology and radiotherapy of cancer. Mutat. Res. Rev. Mutat. Res. 2010, 190–199. [Google Scholar] [CrossRef]
- Vrána, O.; Brabec, V. The Effect of Combined Treatment with Platinum Complexes and Ionizing Radiation on DNA in Vitro. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1986, 50, 995–1007. [Google Scholar] [CrossRef]
- Berzinsh, U.; Gustafsson, M.; Hanstorp, D.; Klinkmüller, A.; Ljungblad, U.; Mårtensson-Pendrill, A.M. Isotope shift in the electron affinity of chlorine. Phys. Rev. A 1995, 51, 231–238. [Google Scholar] [CrossRef] [Green Version]
- Ervin, K.M.; Ho, J.; Lineberger, W.C. Ultraviolet photoelectron spectrum of nitrite anion. J. Phys. Chem. 1988, 92, 5405–5412. [Google Scholar] [CrossRef]
- Ma, J.; Wang, F.; Denisov, S.A.; Adhikary, A.; Mostafavi, M. Reactivity of Prehydrated Electrons Toward Nucleobases and Nucleotides in Aqueous Solution. Sci. Adv. 2017, 3, e1701669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spisz, P.; Zdrowowicz, M.; Kozak, W.; Chomicz-Mańka, L.; Falkiewicz, K.; Makurat, S.; Sikorski, A.; Wyrzykowski, D.; Rak, J.; Arthur-Baidoo, E.; et al. Uracil-5-yl O-Sulfamate: An Illusive Radiosensitizer. Pitfalls in Modeling the Radiosensitizing Derivatives of Nucleobases. J. Phys. Chem. B 2020, 124, 5600–5613. [Google Scholar] [CrossRef] [PubMed]
- Neustetter, M.; Aysina, J.; da Silva, F.F.; Denifl, S. The Effect of Solvation on Electron Attachment to Pure and Hydrated Pyrimidine Clusters. Angew. Chem. Int. Ed. 2015, 54, 9124–9126. [Google Scholar] [CrossRef] [Green Version]
- Kočišek, J.; Sedmidubská, B.; Indrajith, S.; Fárník, M.; Fedor, J. Electron Attachment to Microhydrated Deoxycytidine Monophosphate. J. Phys. Chem. B 2018, 122, 5212–5217. [Google Scholar] [CrossRef]
- Kumar, A.; Walker, J.A.; Bartels, D.M.; Sevilla, M.D. A Simple ab Initio Model for the Hydrated Electron That Matches Experiment. J. Phys. Chem. A 2015, 119, 9148–9159. [Google Scholar] [CrossRef] [Green Version]
- Egger, A.E.; Hartinger, C.G.; Hamidane, H.B.; Tsybin, Y.O.; Keppler, B.K.; Dyson, P.J. High Resolution Mass Spectrometry for Studying the Interactions of Cisplatin with Oligonucleotides. Inorg. Chem. 2008, 47, 10626–10633. [Google Scholar] [CrossRef]
- Sørensen, B.S.; Busk, M.; Olthof, N.; Speel, E.J.; Horsman, M.R.; Alsner, J.; Overgaard, J. Radiosensitivity and effect of hypoxia in HPV positive head and neck cancer cells. Radiother. Oncol. 2013, 500–505. [Google Scholar] [CrossRef]
- Wen, J.; Tong, Y.; Zu, Y. Low Concentration DMSO Stimulates Cell Growth and In vitro Transformation of Human Multiple Myeloma Cells. JAMMR 2014, 5, 65–74. [Google Scholar] [CrossRef]
- Singh, M.; McKenzie, K.; Xiaoling, M. Effect of dimethyl sulfoxide on in vitro proliferation of skin fibroblast cells. J. Biotech Res. 2017, 8, 78–82. [Google Scholar]
- Galvao, J.; Davis, B.; Tilley, M.; Normando, E.; Duchen, M.R.; Cordeiro, M.F. Unexpected low-dose toxicity of the universal solvent DMSO. Faseb J. 2014, 28, 1317–1330. [Google Scholar] [CrossRef]
- Vondráček, J.; Souček, K.; Sheard, M.A.; Chramostová, K.; Andrysík, Z.; Hofmanová, J.; Kozubík, A. Dimethyl sulfoxide potentiates death receptor-mediated apoptosis in the human myeloid leukemia U937 cell line through enhancement of mitochondrial membrane depolarization. Leuk. Res. 2006, 81–89. [Google Scholar] [CrossRef]
- Verheijen, M.; Lienhard, M.; Schrooders, Y.; Clayton, O.; Nudischer, R.; Boerno, S.; Timmermann, B.; Selevsek, N.; Schlapbach, R.; Gmuender, H.; et al. DMSO induces drastic changes in human cellular processes and epigenetic landscape in vitro. Sci. Rep. 2019, 9, 4641. [Google Scholar] [CrossRef] [Green Version]
- Roots, R.; Okada, S. Protection of DNA Molecules of Cultured Mammalian Cells from Radiation-induced Single-strand Scissions by Various Alcohols and SH Compounds. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1972. [Google Scholar] [CrossRef]
- Kashino, G.; Liu, Y.; Suzuki, M.; Masunaga, S.I.; Kinashi, Y.; Ono, K.; Tano, K.; Watanabe, M. An Alternative Mechanism for Radioprotection by Dimethyl Sulfoxide; Possible Facilitation of DNA Double-strand Break Repair. J. Radiat. Res. 2010, 51, 733–740. [Google Scholar] [CrossRef] [Green Version]
- Peng, R.; Zhang, W.; Zuo, Z.; Shan, Y.; Liu, X.; Tang, Y.; Yu, Z.; Wang, L.; Cong, Y. Dimethyl sulfoxide, a potent oral radioprotective agent, confers radioprotection of hematopoietic stem and progenitor cells independent of apoptosis. Free Radic. Biol. Med. 2020, 1–11. [Google Scholar] [CrossRef]
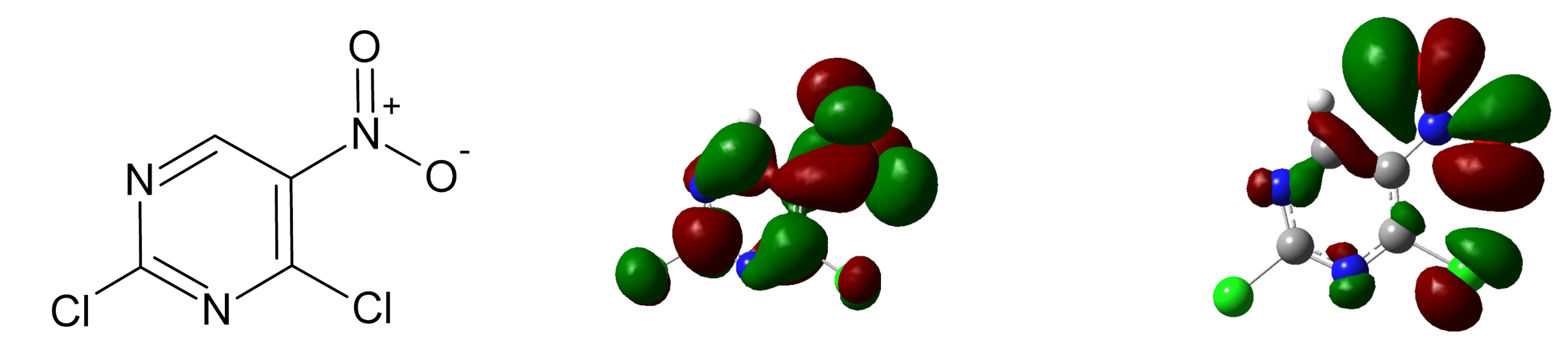
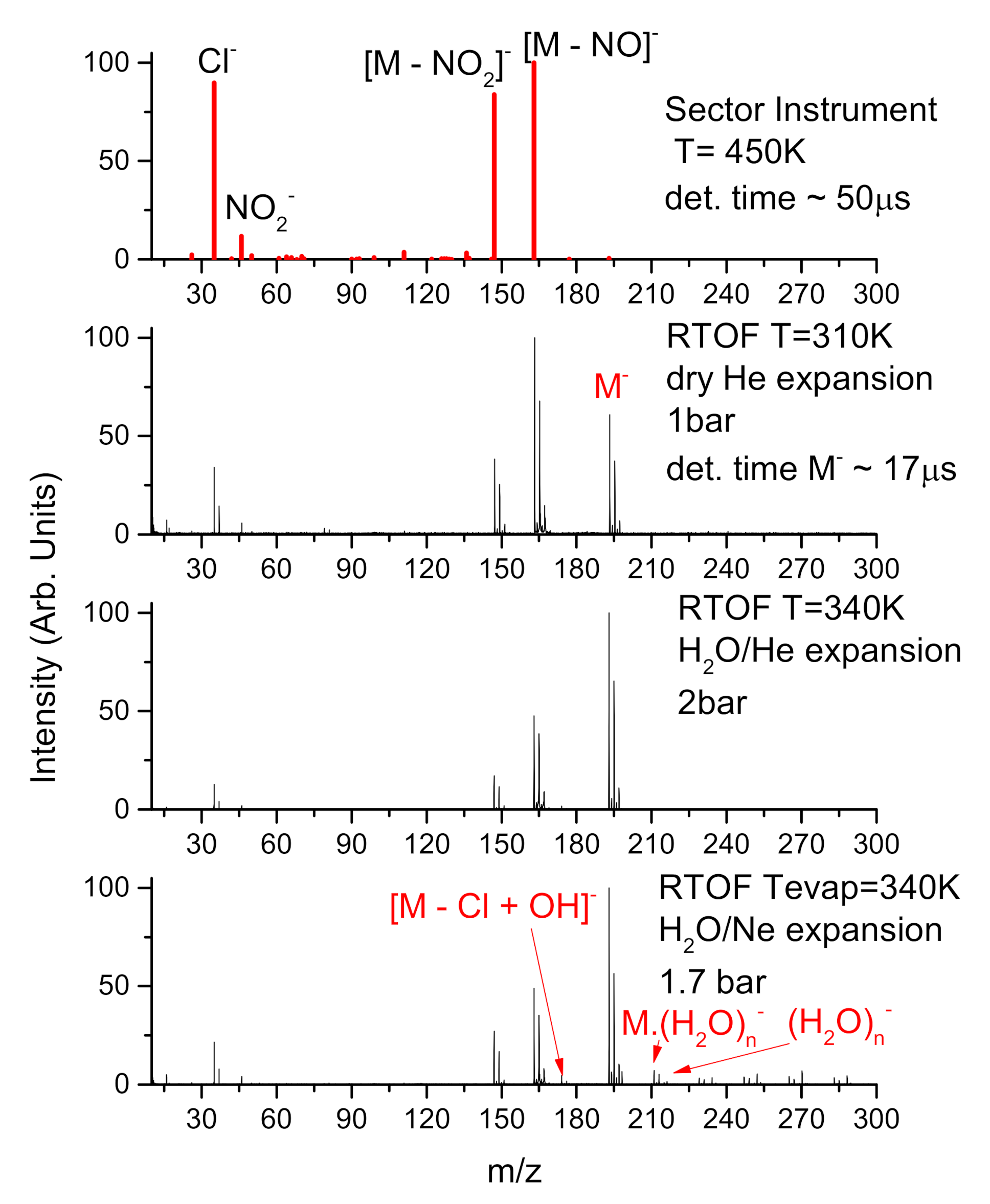

| m/z | Assigned Ion | Peak Energy (eV) | Relative Intensity (Max = 100) | Thermodynamic Threshold (eV) |
|---|---|---|---|---|
| 193 | M | 0 | 1.1 | EAa = 2.122 |
| 177 | [M – O] | 0 | <0.1 | |
| 2.7 | 0.1 | 1.336 | ||
| 163 | [M – NO] | 0 | 100 | −2.902 |
| 147 | [M – NO] | 0 | 75 | −0.044 |
| 2 | 4.9 | |||
| 146 | [M – HNO] | 0 | <0.1 | |
| 2.5 | <0.1 | 1.222 | ||
| 4.1 | <0.1 | |||
| 128 | [M – Cl – NO] | 0 | 0.2 | 0.965 |
| 127 | [M – HCl– NO] | 0 | <0.1 | |
| 2.3 | 0.1 | |||
| 3.9 | <0.1 | |||
| 126 | [M – Cl – O] | 0 | <0.1 | |
| 2.7 | 0.1 | |||
| 8.5 | <0.1 | |||
| 122 | [M – ClH] | 0 | <0.1 | |
| 2.6 | 0.1 | 2.425 | ||
| 111 | [M – HCl – NO] | 2.8 | 1.4 | |
| 99 | CNCl | 2.8 | 0.3 | |
| 93 | [M – 2Cl – NO] | 2.9 | 0.1 | |
| 92 | ClNCHNO | 3.2 | 0.1 | |
| 90 | ClCHNO | 2.8 sh. | ||
| 4 | <0.1 | |||
| 6–9 broad | ||||
| 71 | ClH | 0 | <0.1 | |
| 2.8 | 0.1 | 2.758 | ||
| 3.9 sh. | ||||
| 70 | Cl | 2.9 sh. | 2.471 | |
| 3.9 | 0.3 | |||
| 6–9 broad | ||||
| 68 | CNO | 0 | <0.1 | |
| 2.7 | <0.1 | |||
| 66 | CNO | 2.9 | ||
| 3.9 sh. | ||||
| 64 | C3N | 3.9 | 0.2 | |
| or ClNH | 5.5 sh. | |||
| 6–9 broad | ||||
| 61 | ClCN | 0 | <0.1 | |
| 3 | 0.1 | |||
| 3.9 | 0.1 | |||
| 6.7 | <0.1 | |||
| 50 | CN | 4 | 0.3 | |
| 7–10 broad | ||||
| 46 | NO | 2.8 | 2.3 | |
| 3.9 sh. | ||||
| 6.2 sh. | ||||
| 42 | OCN | 0 | <0.1 | |
| 3.7 | 0.1 | |||
| 35 | Cl | 0.15 | 5.4 | |
| 2.7 | 22 | |||
| 3.8 sh. | ||||
| 5–9 broad | ||||
| 26 | CN | 0 | <0.1 | |
| 2.8 | 0.2 | |||
| 3.9 | 0.2 | |||
| 6.2 sh. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luxford, T.F.M.; Pshenichnyuk, S.A.; Asfandiarov, N.L.; Perečko, T.; Falk, M.; Kočišek, J. 5-Nitro-2,4-Dichloropyrimidine as an Universal Model for Low-Energy Electron Processes Relevant for Radiosensitization. Int. J. Mol. Sci. 2020, 21, 8173. https://doi.org/10.3390/ijms21218173
Luxford TFM, Pshenichnyuk SA, Asfandiarov NL, Perečko T, Falk M, Kočišek J. 5-Nitro-2,4-Dichloropyrimidine as an Universal Model for Low-Energy Electron Processes Relevant for Radiosensitization. International Journal of Molecular Sciences. 2020; 21(21):8173. https://doi.org/10.3390/ijms21218173
Chicago/Turabian StyleLuxford, Thomas F. M., Stanislav A. Pshenichnyuk, Nail L. Asfandiarov, Tomáš Perečko, Martin Falk, and Jaroslav Kočišek. 2020. "5-Nitro-2,4-Dichloropyrimidine as an Universal Model for Low-Energy Electron Processes Relevant for Radiosensitization" International Journal of Molecular Sciences 21, no. 21: 8173. https://doi.org/10.3390/ijms21218173





