Distinct Molecular Pattern-Induced Calcium Signatures Lead to Different Downstream Transcriptional Regulations via AtSR1/CAMTA3
Abstract
1. Introduction
2. Results
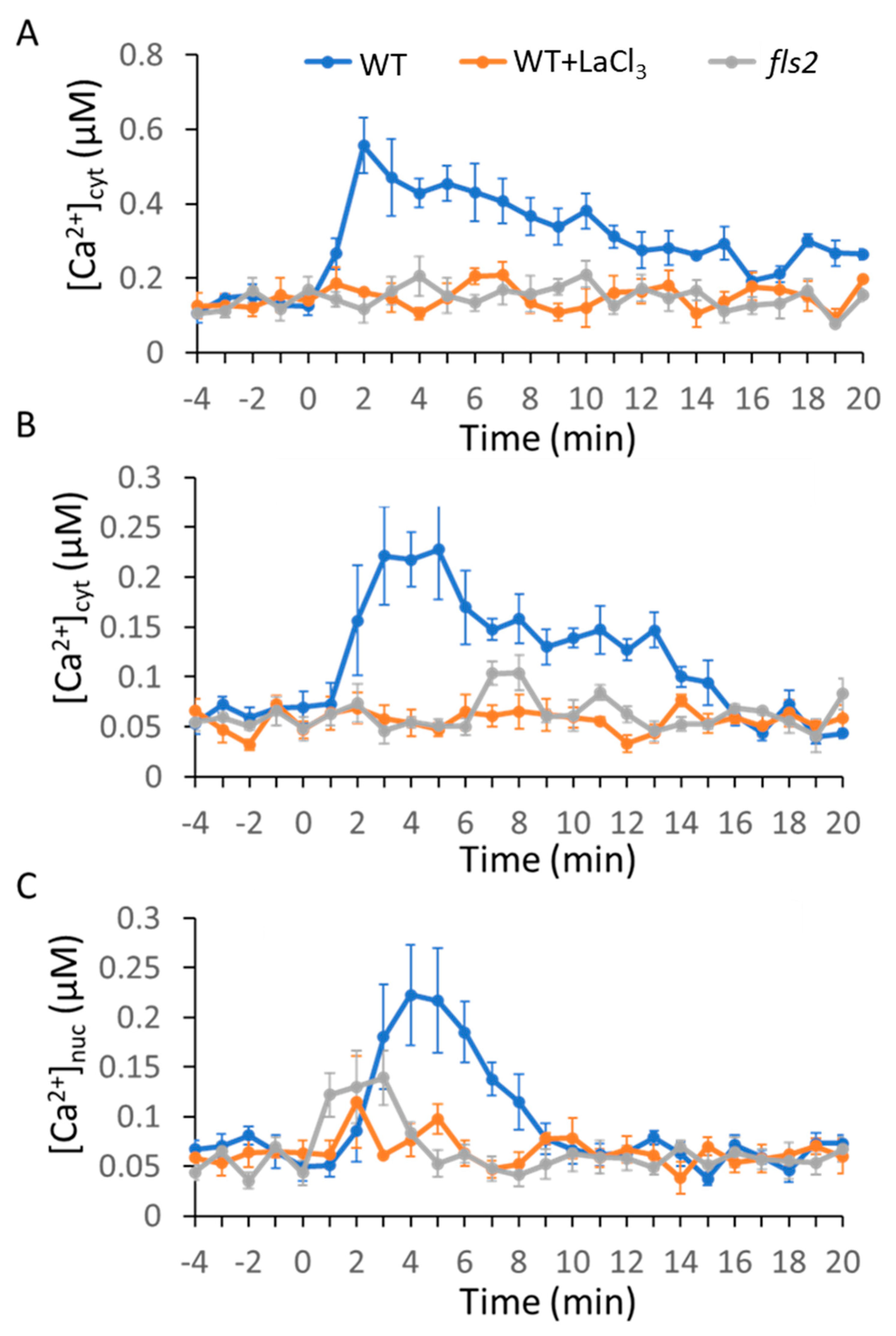
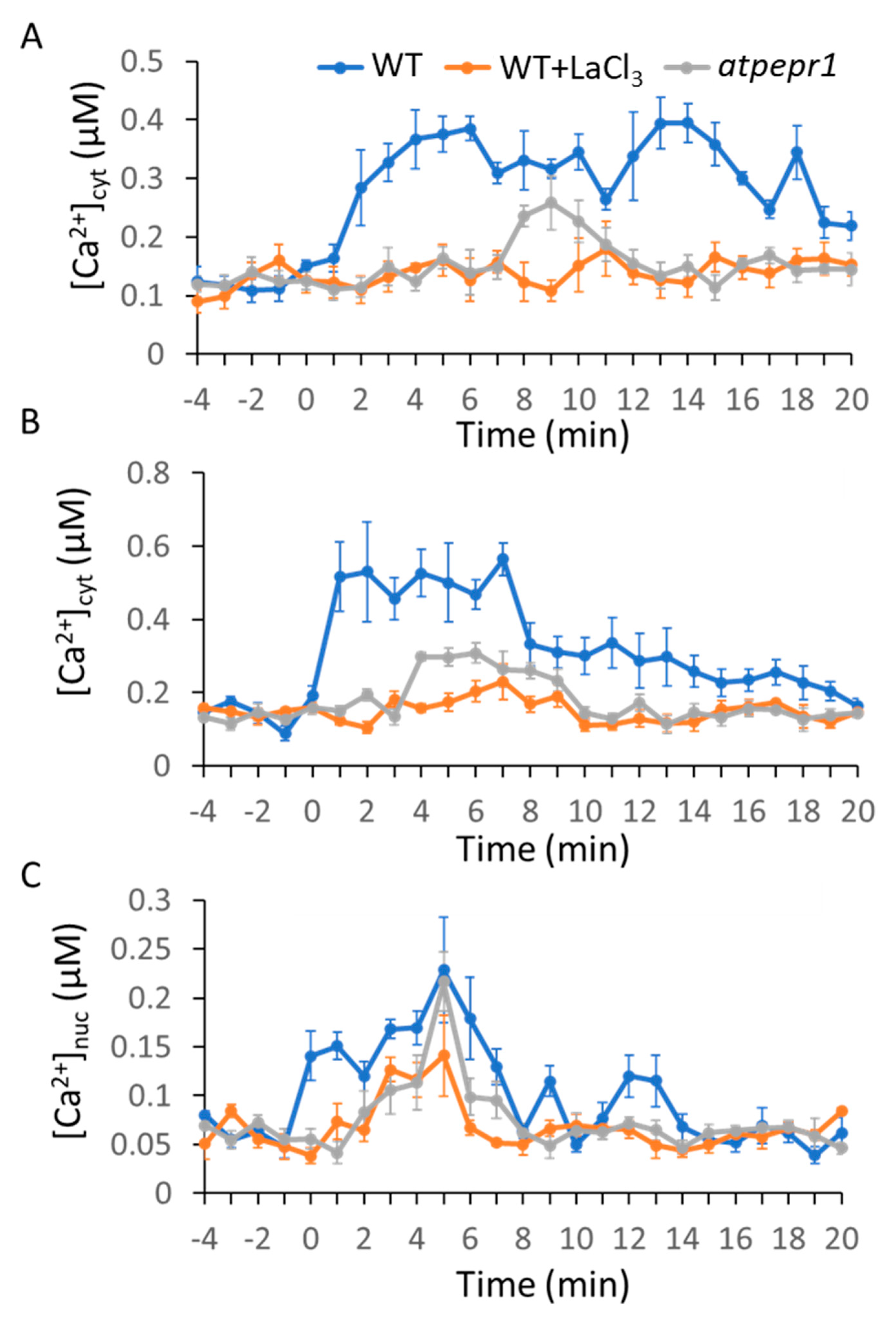
2.1. MAMP/DAMP-Induced Ca2+ Transients in Cytosol
2.2. MAMPs/DAMP-Induced Ca2+ Transients in the Nucleus
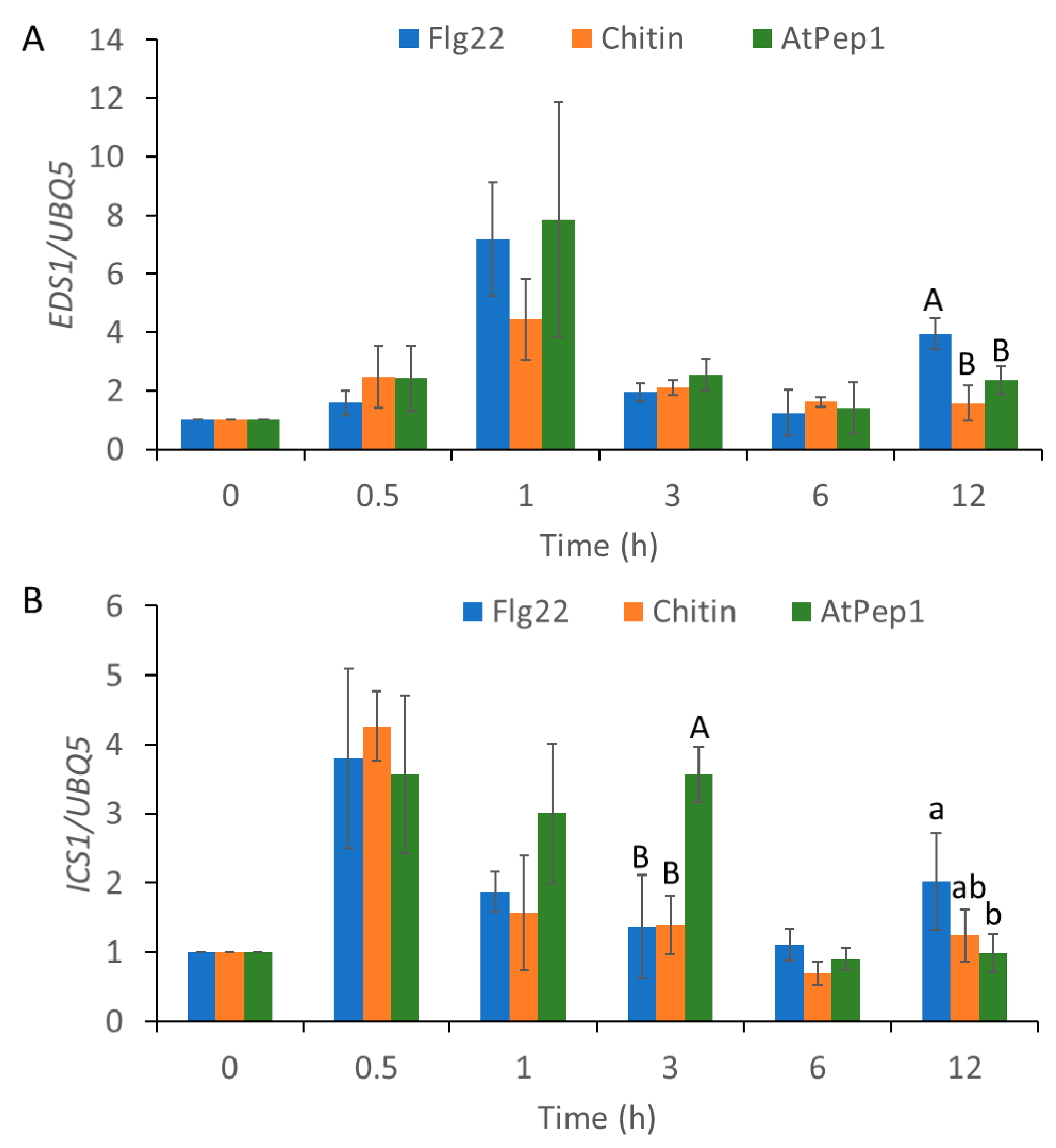
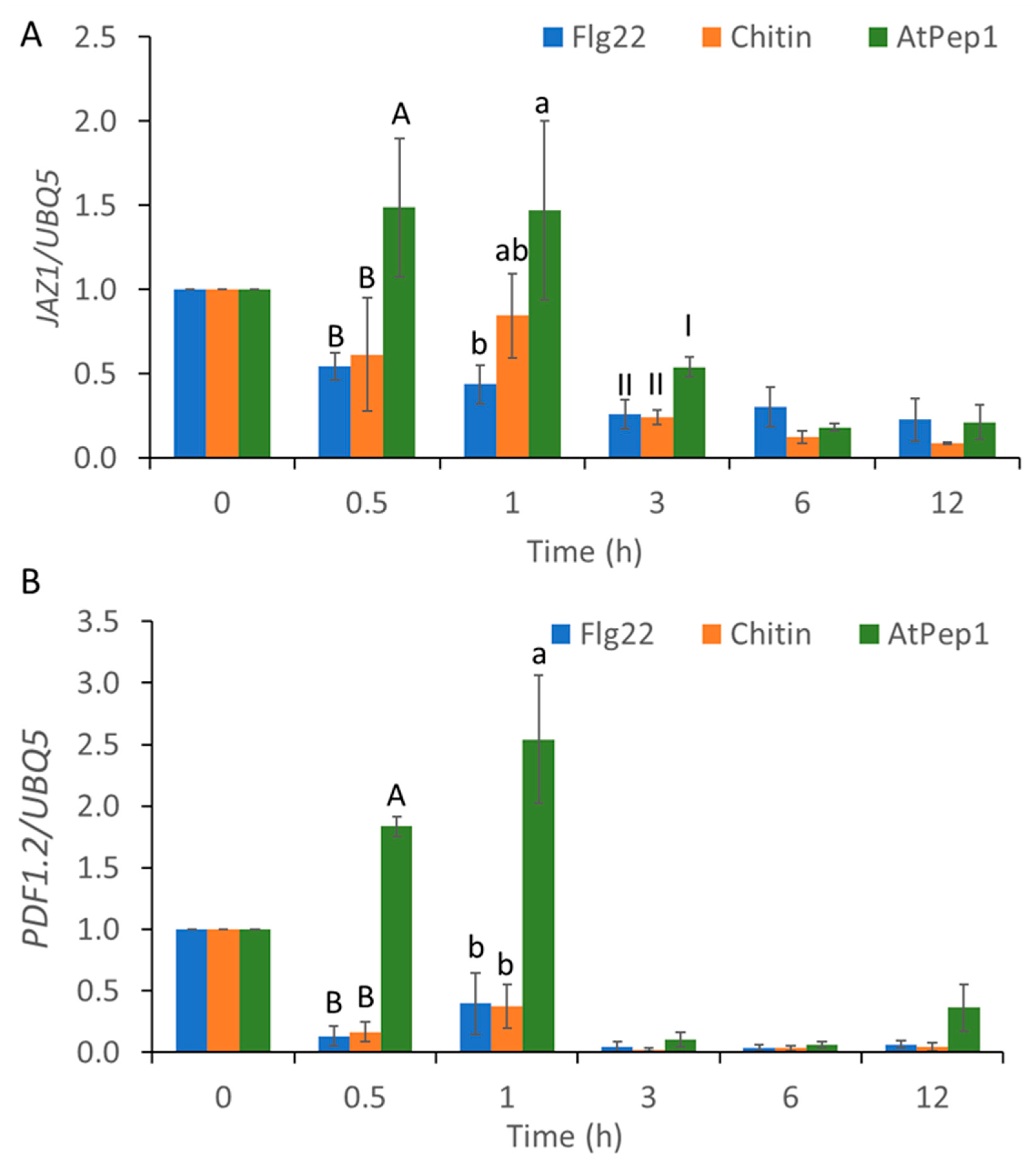
2.3. MAMP/DAMP-Induced Transcriptional Reprogramming of SA-Regulated Genes and JA-Regulated Genes
2.4. AtSR1/CAMTA3 Mediates Pattern-Triggered Transcriptional Reprograming of SA- and JA-Regulated Genes
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Calcium Measurements in Leaf Discs
4.3. Calcium Measurements in Arabidopsis Leaf Protoplasts
4.4. Chemicals, Buffers, and Elicitors
4.5. RNA Preparation and Real-Time PCR Analysis
4.6. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gilroy, S.; Suzuki, N.; Miller, G.; Choi, W.-G.; Toyota, M.; Devireddy, A.R.; Mittler, R. A tidal wave of signals: Calcium and ROS at the forefront of rapid systemic signaling. Trends Plant. Sci. 2014, 19, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Wang, C.; Gao, Q.; Li, L.; Luan, S. Calcium spikes, waves and oscillations in plant development and biotic interactions. Nat. Plants 2020, 6, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, C. Plant pattern-recognition receptors. Trends Immunol. 2014, 35, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Choi, J.; Cao, Y.; Stacey, G. Extracellular ATP acts as a damage-associated molecular pattern (DAMP) signal in plants. Front. Plant. Sci. 2014, 5, 446. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, Y.; Kong, L.; Yu, X.; Feng, B.; Liu, D.; Zhao, B.; Mendes, G.C.; Yuan, P.; Ge, D.; et al. The malectin-like receptor-like kinase LETUM1 modulates NLR protein SUMM2 activation via MEKK2 scaffolding. Nat. Plants 2020, 6, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Chinchilla, D.; Zipfel, C.; Robatzek, S.; Kemmerling, B.; Nürnberger, T.; Jones, J.D.G.; Felix, G.; Boller, T. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nat. Cell Biol. 2007, 448, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Frey, N.F.D.; Mbengue, M.; Kwaaitaal, M.; Nitsch, L.; Altenbach, D.; Häweker, H.; Lozano-Duran, R.; Njo, M.F.; Beeckman, T.; Huettel, B.; et al. Plasma Membrane Calcium ATPases Are Important Components of Receptor-Mediated Signaling in Plant Immune Responses and Development. Plant. Physiol. 2012, 159, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, M.; Yu, L.; Zhou, Z.; Liang, X.; Liu, Z.; Cai, G.; Gao, L.; Zhang, X.; Wang, Y.; et al. The FLS2-Associated Kinase BIK1 Directly Phosphorylates the NADPH Oxidase RbohD to Control Plant Immunity. Cell Host Microbe 2014, 15, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, L.; Macho, A.P.; Han, Z.; Hu, Z.; Zipfel, C.; Zhou, J.-M.; Chai, J. Structural Basis for flg22-Induced Activation of the Arabidopsis FLS2-BAK1 Immune Complex. Science 2013, 342, 624–628. [Google Scholar] [CrossRef]
- Choi, W.-G.; Miller, G.; Wallace, I.; Harper, J.; Mittler, R.; Gilroy, S. Orchestrating rapid long-distance signaling in plants with Ca2+, ROS and electrical signals. Plant. J. 2017, 90, 698–707. [Google Scholar] [CrossRef]
- Marcec, M.J.; Gilroy, S.; Poovaiah, B.; Tanaka, K. Mutual interplay of Ca2+ and ROS signaling in plant immune response. Plant. Sci. 2019, 283, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liang, Y.; Tanaka, K.; Nguyen, C.T.; Jedrzejczak, R.P.; Joachimiak, A.; Stacey, G. The kinase LYK5 is a major chitin receptor in Arabidopsis and forms a chitin-induced complex with related kinase CERK1. eLife 2014, 3, e03766. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Tanaka, K.; Zhang, X.-C.; Son, G.H.; Brechenmacher, L.; Nguyen, T.H.N.; Stacey, G. LYK4, a Lysin Motif Receptor-Like Kinase, Is Important for Chitin Signaling and Plant Innate Immunity in Arabidopsis. Plant. Physiol. 2012, 160, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, C.; Liang, Y.; Stacey, G. Chitin receptor CERK 1 links salt stress and chitin-triggered innate immunity in Arabidopsis. Plant. J. 2017, 89, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, A.; Wong, H.L.; Fujiwara, M.; Okuda, J.; Nishide, K.; Uno, K.; Imai, K.; Umemura, K.; Kawasaki, T.; Kawano, Y.; et al. An OsCEBiP/OsCERK1-OsRacGEF1-OsRac1 Module Is an Essential Early Component of Chitin-Induced Rice Immunity. Cell Host Microbe 2013, 13, 465–476. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Pearce, G.; Ryan, C.A. The cell surface leucine-rich repeat receptor for AtPep1, an endogenous peptide elicitor in Arabidopsis, is functional in transgenic tobacco cells. Proc. Natl. Acad. Sci. USA 2006, 103, 10104–10109. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Huffaker, A.; Bryan, A.C.; Tax, F.E.; Ryan, C.A. PEPR2 Is a Second Receptor for the Pep1 and Pep2 Peptides and Contributes to Defense Responses in Arabidopsis. Plant. Cell 2010, 22, 508–522. [Google Scholar] [CrossRef]
- Qi, Z.; Verma, R.; Gehring, C.; Yamaguchi, Y.; Zhao, Y.; Ryan, C.A.; Berkowitz, G.A. Ca2+ signaling by plant Arabidopsis thaliana Pep peptides depends on AtPepR1, a receptor with guanylyl cyclase activity, and cGMP-activated Ca2+ channels. Proc. Natl. Acad. Sci. USA 2010, 107, 21193–21198. [Google Scholar]
- Ashton, A.R. Guanylyl cyclase activity in plants? Proc. Natl. Acad. Sci. USA 2011, 108, E96. [Google Scholar] [CrossRef]
- Charpentier, M.; Oldroyd, G.E.D. Nuclear Calcium Signaling in Plants. Plant. Physiol. 2013, 163, 496–503. [Google Scholar] [CrossRef]
- Yuan, P.; Yang, T.; Poovaiah, B.W. Calcium Signaling-Mediated Plant Response to Cold Stress. Int. J. Mol. Sci. 2018, 19, 3896. [Google Scholar] [CrossRef]
- Yuan, P.; Du, L.; Poovaiah, B. Ca2+/Calmodulin-Dependent AtSR1/CAMTA3 Plays Critical Roles in Balancing Plant Growth and Immunity. Int. J. Molec. Sci. 2018, 19, 1764. [Google Scholar] [CrossRef]
- Charpentier, M.; Sun, J.; Martins, T.V.; Radhakrishnan, G.V.; Findlay, K.; Soumpourou, E.; Thouin, J.; Véry, A.-A.; Sanders, D.; Morris, R.J.; et al. Nuclear-localized cyclic nucleotide-gated channels mediate symbiotic calcium oscillations. Science 2016, 352, 1102–1105. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Luo, J.; Ning, T.; Cao, W.; Jin, X.; Zhao, H.; Wang, Y.; Han, S. Cytosolic and Nucleosolic Calcium Signaling in Response to Osmotic and Salt Stresses Are Independent of Each Other in Roots of Arabidopsis Seedlings. Front. Plant. Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Poovaiah, B.W.; Du, L.; Wang, H.; Yang, T. Recent Advances in Calcium/Calmodulin-Mediated Signaling with an Emphasis on Plant-Microbe Interactions. Plant. Physiol. 2013, 163, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Edel, K.H.; Marchadier, E.; Brownlee, C.; Kudla, J.; Hetherington, A.M. The Evolution of Calcium-Based Signalling in Plants. Curr. Biol. 2017, 27, R667–R679. [Google Scholar] [CrossRef]
- Yu, F.; Tian, W.; Luan, S. From Receptor-Like Kinases to Calcium Spikes: What Are the Missing Links? Mol. Plant. 2014, 7, 1501–1504. [Google Scholar] [CrossRef]
- Xie, K.; Chen, J.; Wang, Q.; Yang, Y. Direct Phosphorylation and Activation of a Mitogen-Activated Protein Kinase by a Calcium-Dependent Protein Kinase in Rice. Plant. Cell 2014, 26, 3077–3089. [Google Scholar] [CrossRef]
- Boudsocq, M.; Willmann, M.R.; McCormack, M.P.; Lee, H.; Shan, L.; He, P.; Bush, J.; Cheng, S.-H.; Sheen, J. Differential innate immune signalling via Ca2+ sensor protein kinases. Nat. Cell Biol. 2010, 464, 418–422. [Google Scholar] [CrossRef]
- Dubiella, U.; Seybold, H.; Durian, G.; Komander, E.; Lassig, R.; Witte, C.-P.; Schulze, W.X.; Romeis, T. Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proc. Natl. Acad. Sci. USA 2013, 110, 8744–8749. [Google Scholar] [CrossRef]
- Reddy, A.S.; Ali, G.S.; Celesnik, H.; Day, I.S. Coping with Stresses: Roles of Calcium- and Calcium/Calmodulin-Regulated Gene Expression. Plant. Cell 2011, 23, 2010–2032. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, S.; Ding, P.; Wang, D.; Cheng, Y.T.; He, J.; Gao, M.; Xu, F.; Li, Y.; Zhu, Z.; et al. Control of salicylic acid synthesis and systemic acquired resistance by two members of a plant-specific family of transcription factors. Proc. Natl. Acad. Sci. USA 2010, 107, 18220–18225. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tsuda, K.; Truman, W.; Sato, M.; Nguyen, L.V.; Katagiri, F.; Glazebrook, J. CBP60g and SARD1 play partially redundant critical roles in salicylic acid signaling. Plant. J. 2011, 67, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhang, Y.; Li, Y.; Zhang, Q.; Ding, Y.; Zhang, Y. ChIP-seq reveals broad roles of SARD1 and CBP60g in regulating plant immunity. Nat. Commun. 2015, 6, 10159. [Google Scholar] [CrossRef] [PubMed]
- Truman, W.; Sreekanta, S.; Lu, Y.; Bethke, G.; Tsuda, K.; Katagiri, F.; Glazebrook, J. The CALMODULIN-BINDING PROTEIN60 Family Includes Both Negative and Positive Regulators of Plant Immunity. Plant. Physiol. 2013, 163, 1741–1751. [Google Scholar] [CrossRef] [PubMed]
- Galon, Y.; Nave, R.; Boyce, J.M.; Nachmias, D.; Knight, M.R.; Fromm, H. Calmodulin-binding transcription activator (CAMTA) 3 mediates biotic defense responses inArabidopsis. FEBS Lett. 2008, 582, 943–948. [Google Scholar] [CrossRef]
- Du, L.; Ali, G.S.; Simons, K.A.; Hou, J.; Yang, T.; Reddy, A.S.N.; Poovaiah, B.W. Ca2+/calmodulin regulates salicylic-acid-mediated plant immunity. Nature 2009, 457, 1154–1158. [Google Scholar] [CrossRef]
- Nie, H.; Zhao, C.; Wu, G.; Wu, Y.; Chen, Y.; Tang, D. SR1, a Calmodulin-Binding Transcription Factor, Modulates Plant Defense and Ethylene-Induced Senescence by Directly Regulating NDR1 and EIN3. Plant. Physiol. 2012, 158, 1847–1859. [Google Scholar] [CrossRef]
- Kim, Y.; Park, S.; Gilmour, S.J.; Thomashow, M.F. Roles of CAMTA transcription factors and salicylic acid in configuring the low-temperature transcriptome and freezing tolerance of Arabidopsis. Plant. J. 2013, 75, 364–376. [Google Scholar] [CrossRef]
- Yuan, P.; Tanaka, K.; Du, L.; Poovaiah, B. Calcium Signaling in Plant Autoimmunity: A Guard Model for AtSR1/CAMTA3-Mediated Immune Response. Mol. Plant. 2018, 11, 637–639. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, Y.; Tang, S.; Pan, J.; Yu, Y.; Han, J.; Li, Y.; Du, X.; Nan, Z.; Sun, Q. AtCNGC2 is involved in jasmonic acid-induced calcium mobilization. J. Exp. Bot. 2015, 67, 809–819. [Google Scholar] [CrossRef]
- Liu, J.; Lenzoni, G.; Knight, M.R. Design Principle for Decoding Calcium Signals to Generate Specific Gene Expression Via Transcription. Plant. Physiol. 2019, 182, 1743–1761. [Google Scholar] [CrossRef]
- Lenzoni, G.; Liu, J.; Knight, M.R. Predicting plant immunity gene expression by identifying the decoding mechanism of calcium signatures. New Phytol. 2017, 217, 1598–1609. [Google Scholar] [CrossRef]
- Maintz, J.; Cavdar, M.; Tamborski, J.; Kwaaitaal, M.; Huisman, R.; Meesters, C.; Kombrink, E.; Panstruga, R. Comparative Analysis of MAMP-induced Calcium Influx in Arabidopsis Seedlings and Protoplasts. Plant. Cell Physiol. 2014, 55, 1813–1825. [Google Scholar] [CrossRef] [PubMed]
- Moeder, W.; Urquhart, W.; Ung, H.; Yoshioka, K. The Role of Cyclic Nucleotide-Gated Ion Channels in Plant Immunity. Mol. Plant. 2011, 4, 442–452. [Google Scholar] [CrossRef]
- Yuan, P.; Jauregui, E.; Du, L.; Tanaka, K.; Poovaiah, B.W. Calcium signatures and signaling events orchestrate plant–microbe interactions. Curr. Opin. Plant. Biol. 2017, 38, 173–183. [Google Scholar] [CrossRef]
- Gust, A.A.; Biswas, R.; Lenz, H.D.; Rauhut, T.; Ranf, S.; Kemmerling, B.; Götz, F.; Glawischnig, E.; Lee, J.; Felix, G.; et al. Bacteria-derived Peptidoglycans Constitute Pathogen-associated Molecular Patterns Triggering Innate Immunity inArabidopsis. J. Biol. Chem. 2007, 282, 32338–32348. [Google Scholar] [CrossRef]
- Choi, W.-G.; Swanson, S.J.; Gilroy, S. High-resolution imaging of Ca2+, redox status, ROS and pH using GFP biosensors. Plant. J. 2012, 70, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Kwaaitaal, M.; Huisman, R.; Maintz, J.; Reinstädler, A.; Panstruga, R. Ionotropic glutamate receptor (iGluR)-like channels mediate MAMP-induced calcium influx in Arabidopsis thaliana. Biochem. J. 2011, 440, 355–373. [Google Scholar] [CrossRef]
- Tanaka, K.; Choi, J.; Stacey, G. Aequorin Luminescence-Based Functional Calcium Assay for Heterotrimeric G-Proteins in Arabidopsis. In G Protein-Coupled Receptor Signaling in Plants: Methods and Protocols; Running, M.P., Ed.; Humana Press: Totowa, NJ, USA, 2013; pp. 45–54. [Google Scholar] [CrossRef]
- Jha, S.K.; Sharma, M.; Pandey, G.K. Role of Cyclic Nucleotide Gated Channels in Stress Management in Plants. Curr. Genom. 2016, 17, 315–329. [Google Scholar] [CrossRef]
- Saand, M.A.; Xu, Y.-P.; Munyampundu, J.-P.; Li, W.; Zhang, X.-R.; Cai, X.-Z. Phylogeny and evolution of plant cyclic nucleotide-gated ion channel (CNGC) gene family and functional analyses of tomatoCNGCs. DNA Res. 2015, 22, 471–483. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Munemasa, S.; Nishimura, N.; Ren, H.-M.; Robert, N.; Han, M.; Puzõrjova, I.; Kollist, H.; Lee, S.; Mori, I.; et al. Identification of Cyclic GMP-Activated Nonselective Ca2+-Permeable Cation Channels and Associated CNGC5 and CNGC6 Genes in Arabidopsis Guard Cells. Plant. Physiol. 2013, 163, 578–590. [Google Scholar] [CrossRef] [PubMed]
- DeFalco, T.A.; Moeder, W.; Yoshioka, K. Opening the Gates: Insights into Cyclic Nucleotide-Gated Channel-Mediated Signaling. Trends Plant. Sci. 2016, 21, 903–906. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, M. Calcium Signals in the Plant Nucleus: Origin and Function. J. Experim. Bot. 2018, 69, 4165–4173. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.-L.; Shi, Z.; Bao, Y.; Yan, J.; Yang, Z.; Yu, H.; Li, Y.; Gou, M.; Wang, S.; Zou, B.; et al. Calcium Pumps and Interacting BON1 Protein Modulate Calcium Signature, Stomatal Closure, and Plant Immunity. Plant. Physiol. 2017, 175, 424–437. [Google Scholar] [CrossRef] [PubMed]
- DeFalco, T.A.; Marshall, C.B.; Munro, K.; Kang, H.-G.; Moeder, W.; Ikura, M.; Snedden, W.A.; Yoshioka, K. Multiple Calmodulin-binding Sites Positively and Negatively Regulate Arabidopsis CYCLIC NUCLEOTIDE-GATED CHANNEL12. Plant. Cell 2016. [Google Scholar] [CrossRef][Green Version]
- Zipfel, C.; Oldroyd, G.E.D. Plant signalling in symbiosis and immunity. Nat. Cell Biol. 2017, 543, 328–336. [Google Scholar] [CrossRef]
- Gong, B.-Q.; Xue, J.; Zhang, N.; Xu, L.; Yao, X.; Yang, Q.-J.; Yu, Y.; Wang, H.-B.; Zhang, D.; Li, J.-F. Rice Chitin Receptor OsCEBiP Is Not a Transmembrane Protein but Targets the Plasma Membrane via a GPI Anchor. Molec. Plant. 2017, 10, 767–770. [Google Scholar] [CrossRef]
- Nagano, M.; Ishikawa, T.; Fujiwara, M.; Fukao, Y.; Kawano, Y.; Kawai-Yamada, M.; Shimamoto, K. Plasma Membrane Microdomains Are Essential for Rac1-RbohB/H-Mediated Immunity in Rice. Plant. Cell 2016, 28, 1966–1983. [Google Scholar] [CrossRef]
- Lachaud, C.; Da Silva, D.; Cotelle, V.; Thuleau, P.; Xiong, T.C.; Jauneau, A.; Brière, C.; Graziana, A.; Bellec, Y.; Faure, J.-D.; et al. Nuclear calcium controls the apoptotic-like cell death induced by d-erythro-sphinganine in tobacco cells. Cell Calcium 2010, 47, 92–100. [Google Scholar] [CrossRef]
- Liu, J.; Whalley, H.J.; Knight, M.R. Combining modelling and experimental approaches to explain how calcium signatures are decoded by calmodulin-binding transcription activators ( CAMTA s) to produce specific gene expression responses. New Phytol. 2015, 208, 174–187. [Google Scholar] [CrossRef]
- Denoux, C.; Galletti, R.; Mammarella, N.; Gopalan, S.; Werck, D.; De Lorenzo, G.; Ferrari, S.; Ausubel, F.M.; Dewdney, J. Activation of Defense Response Pathways by OGs and Flg22 Elicitors in Arabidopsis Seedlings. Mol. Plant. 2008, 1, 423–445. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Seo, M.; Takebayashi, Y.; Kamiya, Y.; Riemann, M.; Nick, P. Jasmonates are induced by the PAMP flg22 but not the cell death-inducing elicitor Harpin in Vitis rupestris. Protoplasma 2016, 254, 271–283. [Google Scholar] [CrossRef]
- Aslam, S.N.; Erbs, G.; Morrissey, K.L.; Newman, M.-A.; Chinchilla, D.; Boller, T.; Molinaro, A.; Jackson, R.W.; Cooper, R.M. Microbe-associated molecular pattern (MAMP) signatures, synergy, size and charge: Influences on perception or mobility and host defence responses. Mol. Plant. Pathol. 2009, 10, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Mélida, H.; Sopeña-Torres, S.; Bacete, L.; Garrido-Arandia, M.; Jordá, L.; López, G.; Muñoz-Barrios, A.; Pacios, L.F.; Molina, A. Non-branched β-1,3-glucan oligosaccharides trigger immune responses in Arabidopsis. Plant. J. 2017, 93, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; An, C.; Park, S.; Gilmour, S.J.; Wang, L.; Renna, L.; Brandizzi, F.; Grumet, R.; Thomashow, M. CAMTA-Mediated Regulation of Salicylic Acid Immunity Pathway Genes in Arabidopsis Exposed to Low Temperature and Pathogen Infection. Plant. Cell 2017. [Google Scholar] [CrossRef]
- Knight, H.; Trewavas, A.J.; Knight, M.R. Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant. Cell 1996, 8, 489–503. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, P.; Jewell, J.B.; Behera, S.; Tanaka, K.; Poovaiah, B.W. Distinct Molecular Pattern-Induced Calcium Signatures Lead to Different Downstream Transcriptional Regulations via AtSR1/CAMTA3. Int. J. Mol. Sci. 2020, 21, 8163. https://doi.org/10.3390/ijms21218163
Yuan P, Jewell JB, Behera S, Tanaka K, Poovaiah BW. Distinct Molecular Pattern-Induced Calcium Signatures Lead to Different Downstream Transcriptional Regulations via AtSR1/CAMTA3. International Journal of Molecular Sciences. 2020; 21(21):8163. https://doi.org/10.3390/ijms21218163
Chicago/Turabian StyleYuan, Peiguo, Jeremy B. Jewell, Smrutisanjita Behera, Kiwamu Tanaka, and B. W. Poovaiah. 2020. "Distinct Molecular Pattern-Induced Calcium Signatures Lead to Different Downstream Transcriptional Regulations via AtSR1/CAMTA3" International Journal of Molecular Sciences 21, no. 21: 8163. https://doi.org/10.3390/ijms21218163
APA StyleYuan, P., Jewell, J. B., Behera, S., Tanaka, K., & Poovaiah, B. W. (2020). Distinct Molecular Pattern-Induced Calcium Signatures Lead to Different Downstream Transcriptional Regulations via AtSR1/CAMTA3. International Journal of Molecular Sciences, 21(21), 8163. https://doi.org/10.3390/ijms21218163





