Assessment of NK Cell Activity Based on NK Cell-Specific Receptor Synergy in Peripheral Blood Mononuclear Cells and Whole Blood
Abstract
:1. Introduction
2. Results
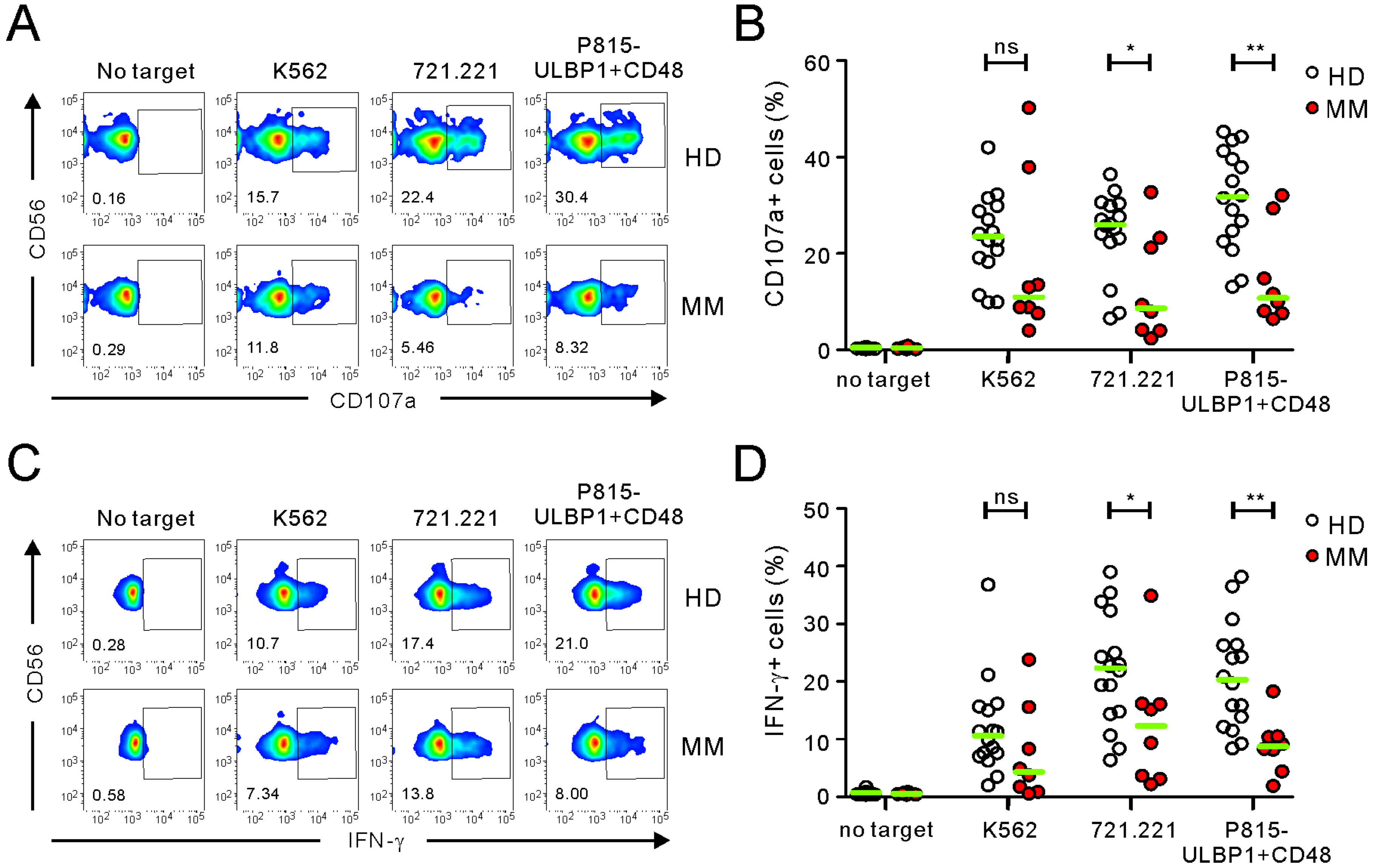
2.1. Comparison of FC-Based NKA Using PBMCs from Healthy Donors and MM Patients
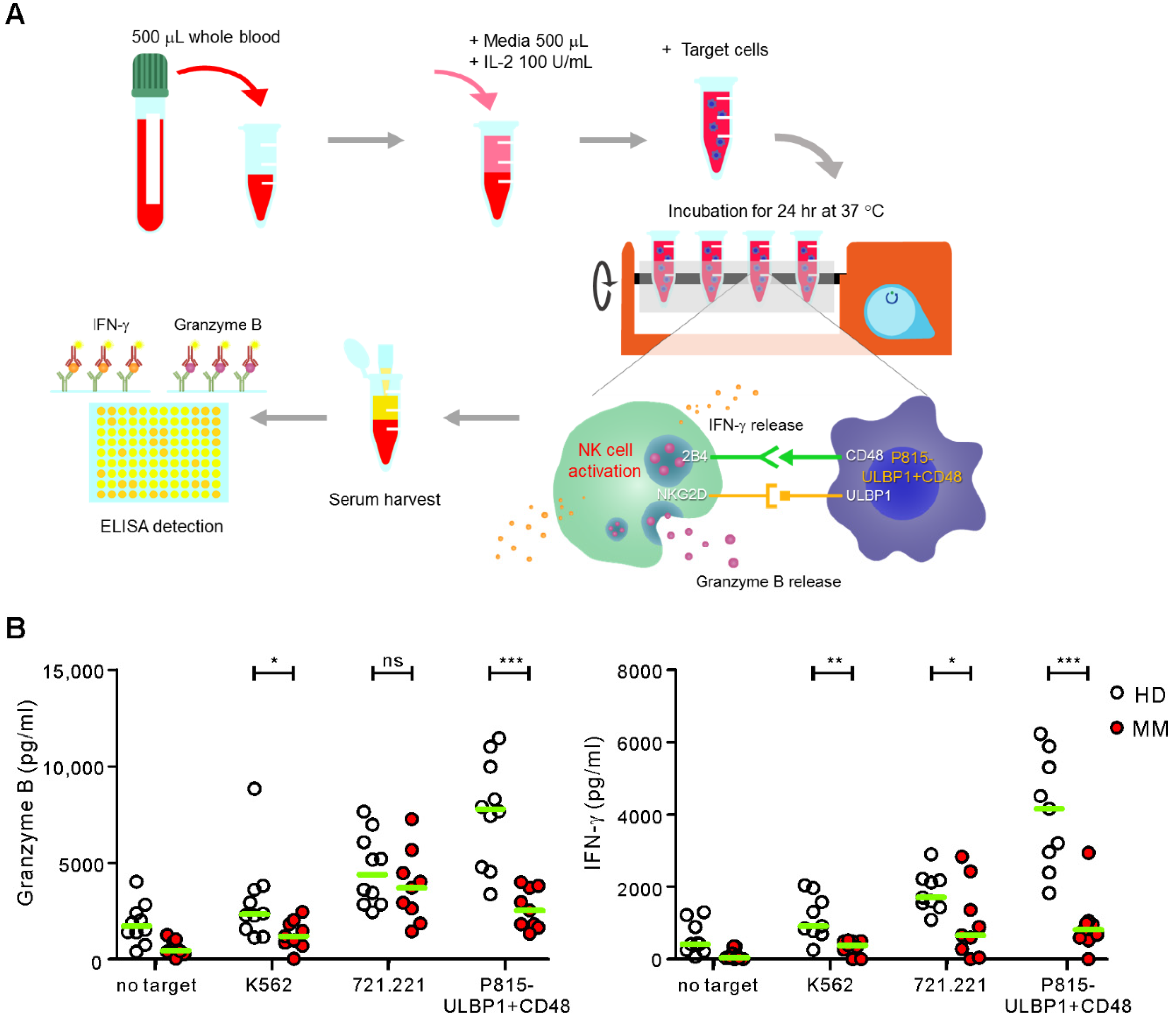
2.2. Comparison of ELISA-Based NKA Using WB Samples from Healthy Donors and MM Patients
2.3. NKG2D Expression Being Associated with NK Cell Degranulation in Healthy Donors and MM Patients
2.4. TGF-β1 Levels Being Related to Impaired NKA in MM
3. Discussion
4. Materials and Methods
4.1. Study Design and Patients
4.2. Target Cell and Culture
4.3. Antibodies
4.4. NK Cell Cytotoxic Degranulation and Intracellular IFN-γ Staining Assay
4.5. Whole Blood (WB) ELISA Assay
4.6. Plasma Cytokine Analysis
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Long, E.O.; Kim, H.S.; Liu, D.; Peterson, M.E.; Rajagopalan, S. Controlling natural killer cell responses: Integration of signals for activation and inhibition. Annu. Rev. Immunol. 2013, 31, 227–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Soto, A.; Gonzalez, S.; Smyth, M.J.; Galluzzi, L. Control of Metastasis by NK Cells. Cancer Cell 2017, 32, 135–154. [Google Scholar] [CrossRef] [PubMed]
- Morvan, M.G.; Lanier, L.L. NK cells and cancer: You can teach innate cells new tricks. Nat. Rev. Cancer 2016, 16, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Lanier, L.L. NK cell recognition. Annu. Rev. Immunol. 2005, 23, 225–274. [Google Scholar] [CrossRef] [PubMed]
- Bryceson, Y.T.; March, M.E.; Ljunggren, H.G.; Long, E.O. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood 2006, 107, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Das, A.; Gross, C.C.; Bryceson, Y.T.; Long, E.O. Synergistic signals for natural cytotoxicity are required to overcome inhibition by c-Cbl ubiquitin ligase. Immunity 2010, 32, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Kwon, H.J.; Choi, G.E.; Ryu, S.; Kwon, S.J.; Kim, S.C.; Booth, C.; Nichols, K.E.; Kim, H.S. Stepwise phosphorylation of p65 promotes NF-kappaB activation and NK cell responses during target cell recognition. Nat. Commun. 2016, 7, 11686. [Google Scholar] [CrossRef]
- Raulet, D.H.; Gasser, S.; Gowen, B.G.; Deng, W.; Jung, H. Regulation of ligands for the NKG2D activating receptor. Annu. Rev. Immunol. 2013, 31, 413–441. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.; Cruz-Munoz, M.E.; Zhong, M.C.; Chen, R.; Latour, S.; Veillette, A. Essential function for SAP family adaptors in the surveillance of hematopoietic cells by natural killer cells. Nat. Immunol. 2009, 10, 973–980. [Google Scholar] [CrossRef]
- Viel, S.; Charrier, E.; Marcais, A.; Rouzaire, P.; Bienvenu, J.; Karlin, L.; Salles, G.; Walzer, T. Monitoring NK cell activity in patients with hematological malignancies. Oncoimmunology 2013, 2, e26011. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Matsuyama, S.; Miyake, S.; Suga, K.; Nakachi, K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: An 11-year follow-up study of a general population. Lancet 2000, 356, 1795–1799. [Google Scholar] [CrossRef]
- Strayer, D.R.; Carter, W.A.; Mayberry, S.D.; Pequignot, E.; Brodsky, I. Low natural cytotoxicity of peripheral blood mononuclear cells in individuals with high familial incidences of cancer. Cancer Res. 1984, 44, 370–374. [Google Scholar] [PubMed]
- Oka, M.; Mitsunaga, H.; Hazama, S.; Yoshino, S.; Suzuki, T. Natural killer activity and serum immunosuppressive acidic protein levels in esophageal and gastric cancers. Surg. Today 1993, 23, 669–674. [Google Scholar] [CrossRef]
- Schantz, S.P.; Shillitoe, E.J.; Brown, B.; Campbell, B. Natural killer cell activity and head and neck cancer: A clinical assessment. J. Natl. Cancer Inst. 1986, 77, 869–875. [Google Scholar]
- Coca, S.; Perez-Piqueras, J.; Martinez, D.; Colmenarejo, A.; Saez, M.A.; Vallejo, C.; Martos, J.A.; Moreno, M. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer 1997, 79, 2320–2328. [Google Scholar] [CrossRef]
- Takeuchi, H.; Maehara, Y.; Tokunaga, E.; Koga, T.; Kakeji, Y.; Sugimachi, K. Prognostic significance of natural killer cell activity in patients with gastric carcinoma: A multivariate analysis. Am. J. Gastroenterol. 2001, 96, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Biron, C.A.; Byron, K.S.; Sullivan, J.L. Severe herpesvirus infections in an adolescent without natural killer cells. N. Engl. J. Med. 1989, 320, 1731–1735. [Google Scholar] [CrossRef]
- Orange, J.S. Human natural killer cell deficiencies and susceptibility to infection. Microbes Infect. 2002, 4, 1545–1558. [Google Scholar] [CrossRef]
- Jurisic, V.; Srdic, T.; Konjevic, G.; Markovic, O.; Colovic, M. Clinical stage-depending decrease of NK cell activity in multiple myeloma patients. Med. Oncol. 2007, 24, 312–317. [Google Scholar] [CrossRef]
- Jun, E.; Song, A.Y.; Choi, J.W.; Lee, H.H.; Kim, M.Y.; Ko, D.H.; Kang, H.J.; Kim, S.W.; Bryceson, Y.; Kim, S.C.; et al. Progressive Impairment of NK Cell Cytotoxic Degranulation Is Associated With TGF-beta1 Deregulation and Disease Progression in Pancreatic Cancer. Front. Immunol. 2019, 10, 1354. [Google Scholar] [CrossRef]
- Claus, M.; Greil, J.; Watzl, C. Comprehensive analysis of NK cell function in whole blood samples. J. Immunol. Methods 2009, 341, 154–164. [Google Scholar] [CrossRef]
- Haque, K.; Truman, C.; Dittmer, I.; Laundy, G.; Denning-Kendall, P.; Hows, J.; Feest, T.; Bradley, B. Modified cytotoxic T lymphocyte precursor frequency assay by measuring released europium in a time resolved fluorometer. Arch. Immunol. Ther. Exp. (Warsz.) 1997, 45, 37–42. [Google Scholar]
- Bryceson, Y.T.; Fauriat, C.; Nunes, J.M.; Wood, S.M.; Bjorkstrom, N.K.; Long, E.O.; Ljunggren, H.G. Functional analysis of human NK cells by flow cytometry. Methods Mol. Biol. 2010, 612, 335–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kane, K.L.; Ashton, F.A.; Schmitz, J.L.; Folds, J.D. Determination of natural killer cell function by flow cytometry. Clin. Diagn. Lab. Immunol. 1996, 3, 295–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alter, G.; Malenfant, J.M.; Altfeld, M. CD107a as a functional marker for the identification of natural killer cell activity. J. Immunol. Methods 2004, 294, 15–22. [Google Scholar] [CrossRef]
- Dons’koi, B.V.; Chernyshov, V.P.; Osypchuk, D.V. Measurement of NK activity in whole blood by the CD69 up-regulation after co-incubation with K562, comparison with NK cytotoxicity assays and CD107a degranulation assay. J. Immunol. Methods 2011, 372, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Tremblay-McLean, A.; Coenraads, S.; Kiani, Z.; Dupuy, F.P.; Bernard, N.F. Expression of ligands for activating natural killer cell receptors on cell lines commonly used to assess natural killer cell function. BMC Immunol. 2019, 20, 8. [Google Scholar] [CrossRef] [Green Version]
- Brandt, C.S.; Baratin, M.; Yi, E.C.; Kennedy, J.; Gao, Z.; Fox, B.; Haldeman, B.; Ostrander, C.D.; Kaifu, T.; Chabannon, C.; et al. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J. Exp. Med. 2009, 206, 1495–1503. [Google Scholar] [CrossRef] [Green Version]
- Parolini, S.; Bottino, C.; Falco, M.; Augugliaro, R.; Giliani, S.; Franceschini, R.; Ochs, H.D.; Wolf, H.; Bonnefoy, J.Y.; Biassoni, R.; et al. X-linked lymphoproliferative disease. 2B4 molecules displaying inhibitory rather than activating function are responsible for the inability of natural killer cells to kill Epstein-Barr virus-infected cells. J. Exp. Med. 2000, 192, 337–346. [Google Scholar] [CrossRef]
- Horowitz, A.; Strauss-Albee, D.M.; Leipold, M.; Kubo, J.; Nemat-Gorgani, N.; Dogan, O.C.; Dekker, C.L.; Mackey, S.; Maecker, H.; Swan, G.E.; et al. Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Sci. Transl. Med. 2013, 5, 208ra145. [Google Scholar] [CrossRef] [Green Version]
- Lisovsky, I.; Isitman, G.; Bruneau, J.; Bernard, N.F. Functional analysis of NK cell subsets activated by 721.221 and K562 HLA-null cells. J. Leukoc. Biol. 2015, 97, 761–767. [Google Scholar] [CrossRef] [Green Version]
- Bryceson, Y.T.; Ljunggren, H.G.; Long, E.O. Minimal requirement for induction of natural cytotoxicity and intersection of activation signals by inhibitory receptors. Blood 2009, 114, 2657–2666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Audenaerde, J.R.M.; Roeyen, G.; Darcy, P.K.; Kershaw, M.H.; Peeters, M.; Smits, E.L.J. Natural killer cells and their therapeutic role in pancreatic cancer: A systematic review. Pharmacol. Ther. 2018, 189, 31–44. [Google Scholar] [CrossRef]
- Fauriat, C.; Mallet, F.; Olive, D.; Costello, R.T. Impaired activating receptor expression pattern in natural killer cells from patients with multiple myeloma. Leukemia 2006, 20, 732–733. [Google Scholar] [CrossRef] [PubMed]
- Jinushi, M.; Vanneman, M.; Munshi, N.C.; Tai, Y.T.; Prabhala, R.H.; Ritz, J.; Neuberg, D.; Anderson, K.C.; Carrasco, D.R.; Dranoff, G. MHC class I chain-related protein A antibodies and shedding are associated with the progression of multiple myeloma. Proc. Natl. Acad. Sci. USA 2008, 105, 1285–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellone, G.; Aste-Amezaga, M.; Trinchieri, G.; Rodeck, U. Regulation of NK cell functions by TGF-beta 1. J. Immunol. 1995, 155, 1066–1073. [Google Scholar] [PubMed]
- Viel, S.; Marcais, A.; Guimaraes, F.S.; Loftus, R.; Rabilloud, J.; Grau, M.; Degouve, S.; Djebali, S.; Sanlaville, A.; Charrier, E.; et al. TGF-beta inhibits the activation and functions of NK cells by repressing the mTOR pathway. Sci. Signal 2016, 9, ra19. [Google Scholar] [CrossRef]
- Bromelow, K.V.; Galea-Lauri, J.; O’Brien, M.E.; Souberbielle, B.E. A highly sensitive whole blood natural killer cell assay. J. Immunol. Methods 1998, 217, 177–184. [Google Scholar] [CrossRef]
- Mhatre, S.; Madkaikar, M.; Ghosh, K.; Desai, M.; Pujari, V.; Gupta, M. Rapid flow cytometry based cytotoxicity assay for evaluation of NK cell function. Indian J. Exp. Biol. 2014, 52, 983–988. [Google Scholar]
- Boehm, U.; Klamp, T.; Groot, M.; Howard, J.C. Cellular responses to interferon-gamma. Annu. Rev. Immunol. 1997, 15, 749–795. [Google Scholar] [CrossRef]
- Chowdhury, D.; Lieberman, J. Death by a thousand cuts: Granzyme pathways of programmed cell death. Annu. Rev. Immunol. 2008, 26, 389–420. [Google Scholar] [CrossRef] [Green Version]
- Fauriat, C.; Long, E.O.; Ljunggren, H.G.; Bryceson, Y.T. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood 2010, 115, 2167–2176. [Google Scholar] [CrossRef] [Green Version]
- Fehniger, T.A.; Shah, M.H.; Turner, M.J.; VanDeusen, J.B.; Whitman, S.P.; Cooper, M.A.; Suzuki, K.; Wechser, M.; Goodsaid, F.; Caligiuri, M.A. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: Implications for the innate immune response. J. Immunol. 1999, 162, 4511–4520. [Google Scholar] [PubMed]
- Lee, S.B.; Cha, J.; Kim, I.K.; Yoon, J.C.; Lee, H.J.; Park, S.W.; Cho, S.; Youn, D.Y.; Lee, H.; Lee, C.H.; et al. A high-throughput assay of NK cell activity in whole blood and its clinical application. Biochem. Biophys. Res. Commun. 2014, 445, 584–590. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H.S.; Lee, J.M.; Park, K.H.; Choi, A.R.; Yoon, J.H.; Ryu, H.; Oh, E.J. Natural Killer Cell Function Tests by Flowcytometry-Based Cytotoxicity and IFN-gamma Production for the Diagnosis of Adult Hemophagocytic Lymphohistiocytosis. Int. J. Mol. Sci. 2019, 20, 5413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frassanito, M.A.; Silvestris, F.; Cafforio, P.; Silvestris, N.; Dammacco, F. IgG M-components in active myeloma patients induce a down-regulation of natural killer cell activity. Int. J. Clin. Lab. Res. 1997, 27, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.M.; Devarakonda, S.; Bumma, N.; Chaudhry, M.; Benson, D.M., Jr. Potential of NK cells in multiple Myeloma therapy. Expert Rev. Hematol. 2019, 12, 425–435. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, G.E.; Lee, B.-J.; Kwon, S.W.; Lee, S.-H.; Kim, H.S.; Jang, Y.J. Natural killer cells regulate eosinophilic inflammation in chronic rhinosinusitis. Sci. Rep. 2016, 6, 27615. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.M.; Yi, E.; Cho, H.; Choi, W.S.; Ko, D.-H.; Yoon, D.H.; Hwang, S.-H.; Kim, H.S. Assessment of NK Cell Activity Based on NK Cell-Specific Receptor Synergy in Peripheral Blood Mononuclear Cells and Whole Blood. Int. J. Mol. Sci. 2020, 21, 8112. https://doi.org/10.3390/ijms21218112
Kim JM, Yi E, Cho H, Choi WS, Ko D-H, Yoon DH, Hwang S-H, Kim HS. Assessment of NK Cell Activity Based on NK Cell-Specific Receptor Synergy in Peripheral Blood Mononuclear Cells and Whole Blood. International Journal of Molecular Sciences. 2020; 21(21):8112. https://doi.org/10.3390/ijms21218112
Chicago/Turabian StyleKim, Jung Min, Eunbi Yi, Hyungwoo Cho, Woo Seon Choi, Dae-Hyun Ko, Dok Hyun Yoon, Sang-Hyun Hwang, and Hun Sik Kim. 2020. "Assessment of NK Cell Activity Based on NK Cell-Specific Receptor Synergy in Peripheral Blood Mononuclear Cells and Whole Blood" International Journal of Molecular Sciences 21, no. 21: 8112. https://doi.org/10.3390/ijms21218112
APA StyleKim, J. M., Yi, E., Cho, H., Choi, W. S., Ko, D.-H., Yoon, D. H., Hwang, S.-H., & Kim, H. S. (2020). Assessment of NK Cell Activity Based on NK Cell-Specific Receptor Synergy in Peripheral Blood Mononuclear Cells and Whole Blood. International Journal of Molecular Sciences, 21(21), 8112. https://doi.org/10.3390/ijms21218112




