Hypoxia Regulates DPP4 Expression, Proteolytic Inactivation, and Shedding from Ovarian Cancer Cells
Abstract
:1. Introduction
2. Results
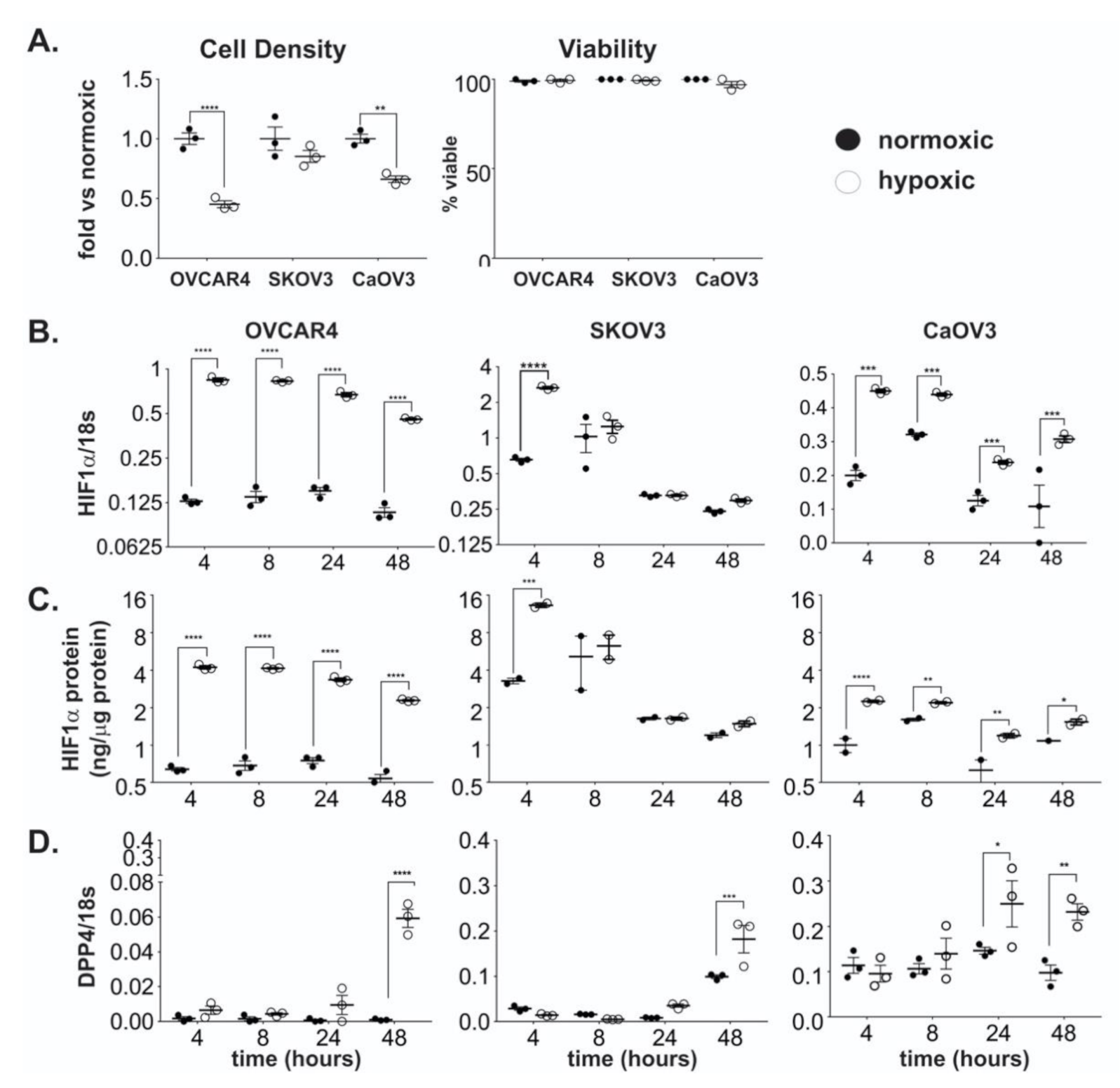
2.1. DPP4 Expression is Upregulated by Hypoxia in Ovarian Cancer Cells
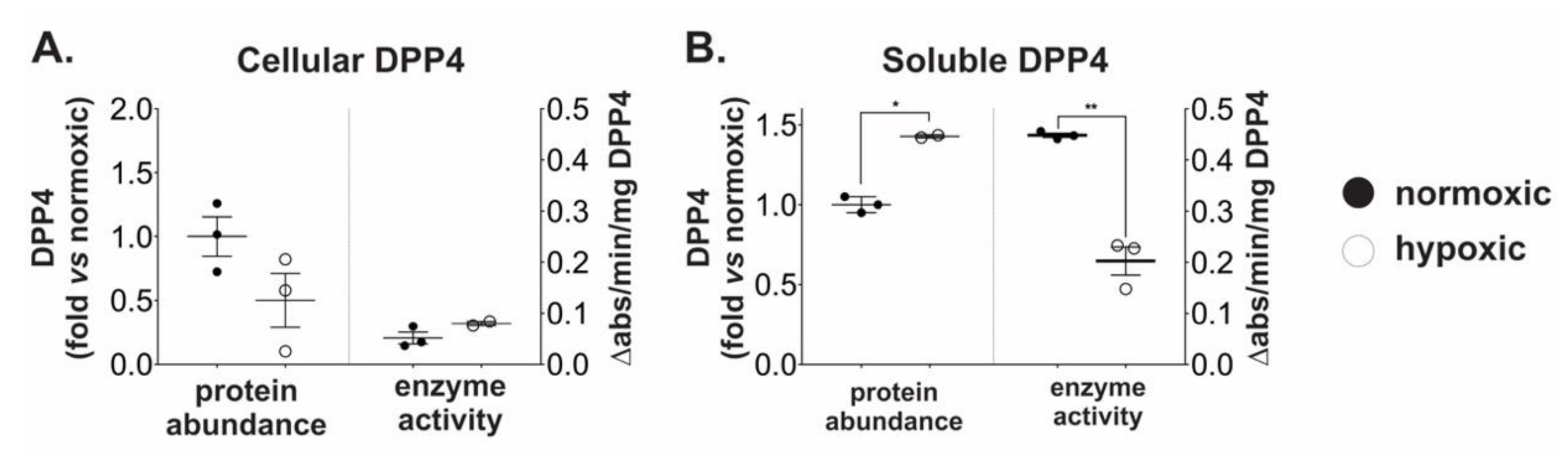
2.2. Chronic Hypoxia Induces DPP4 Shedding from the Surface of Ovarian Cancer Cells
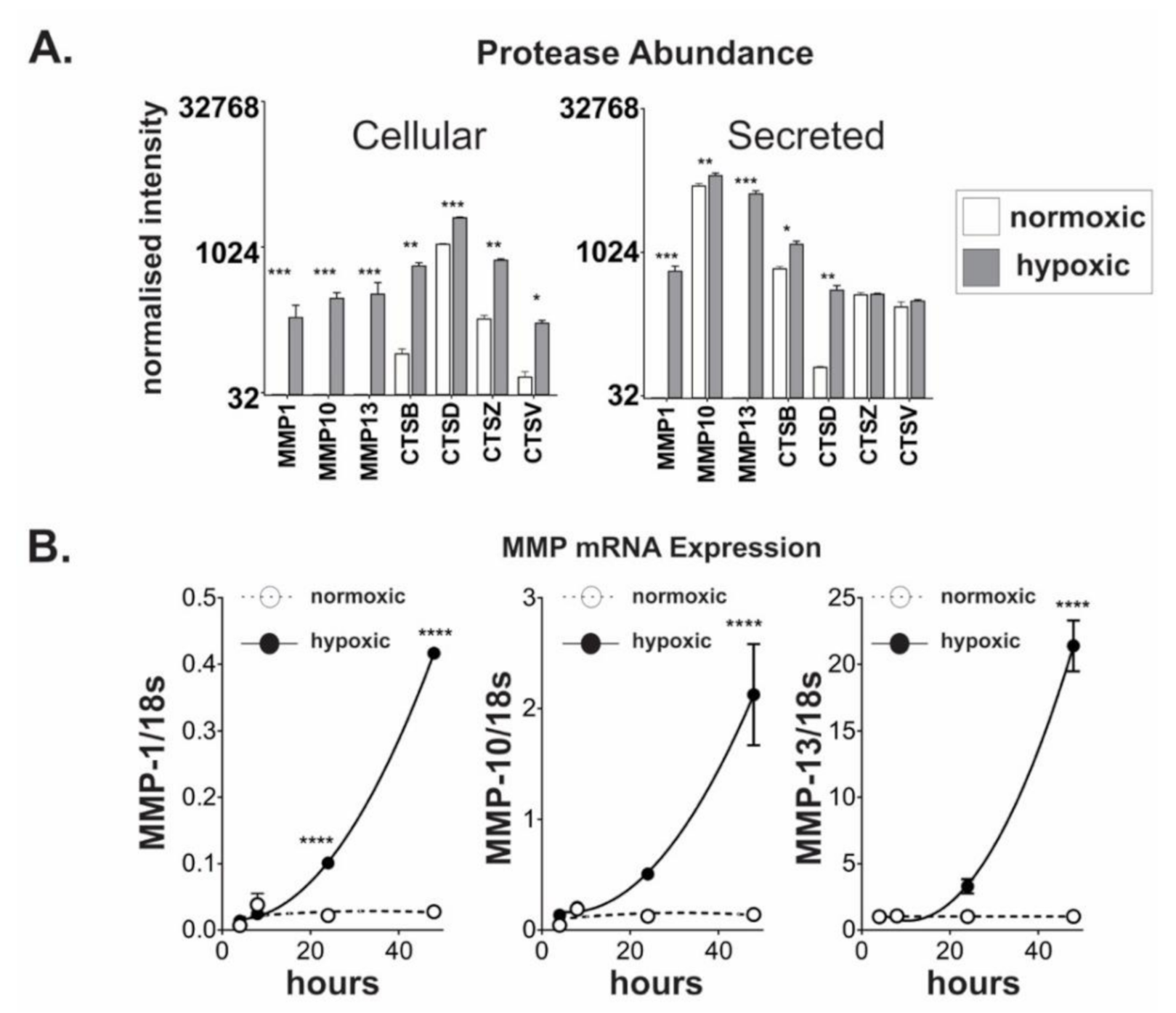
2.3. Chronic Hypoxic Growth Stimulates Matrix Metalloproteinase Expression and Alters DPP4 Release from Cells
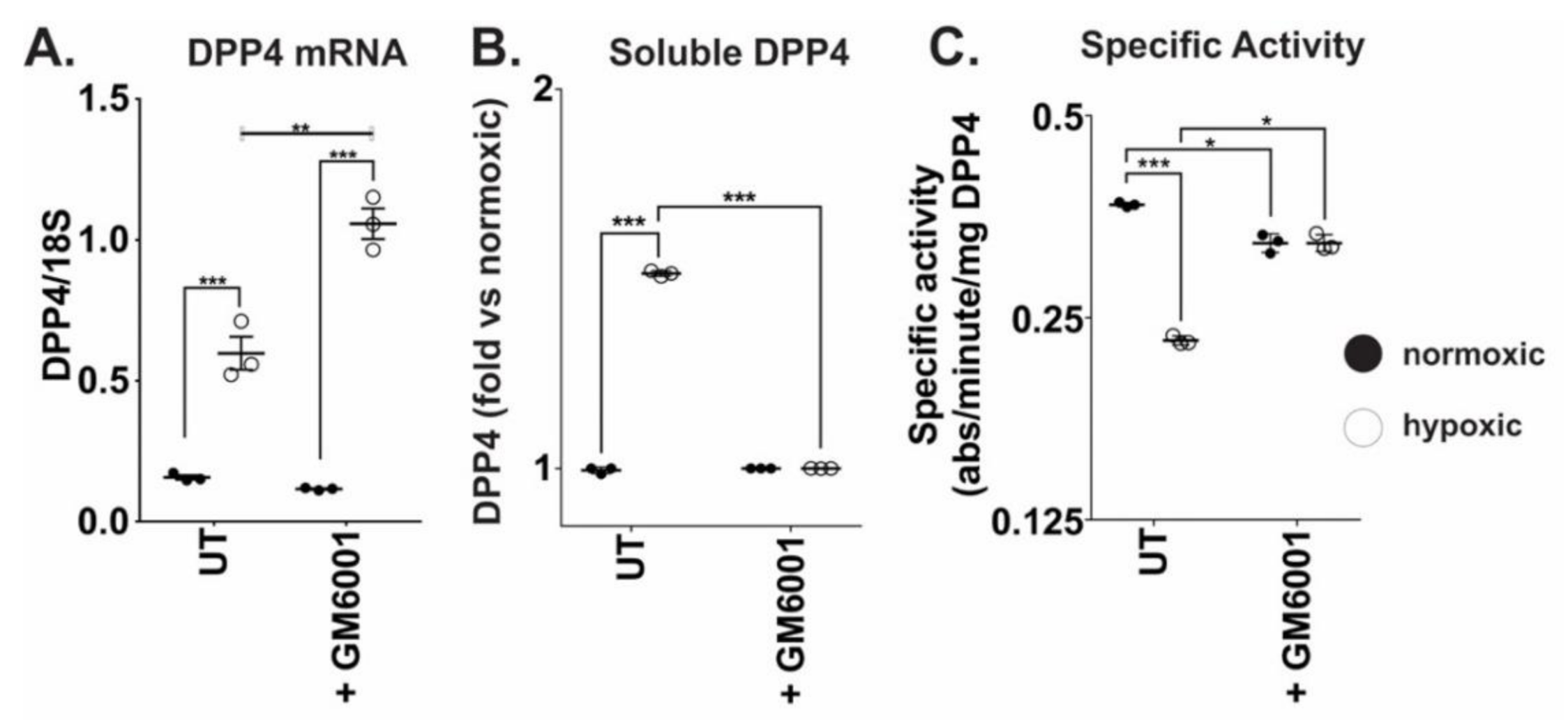
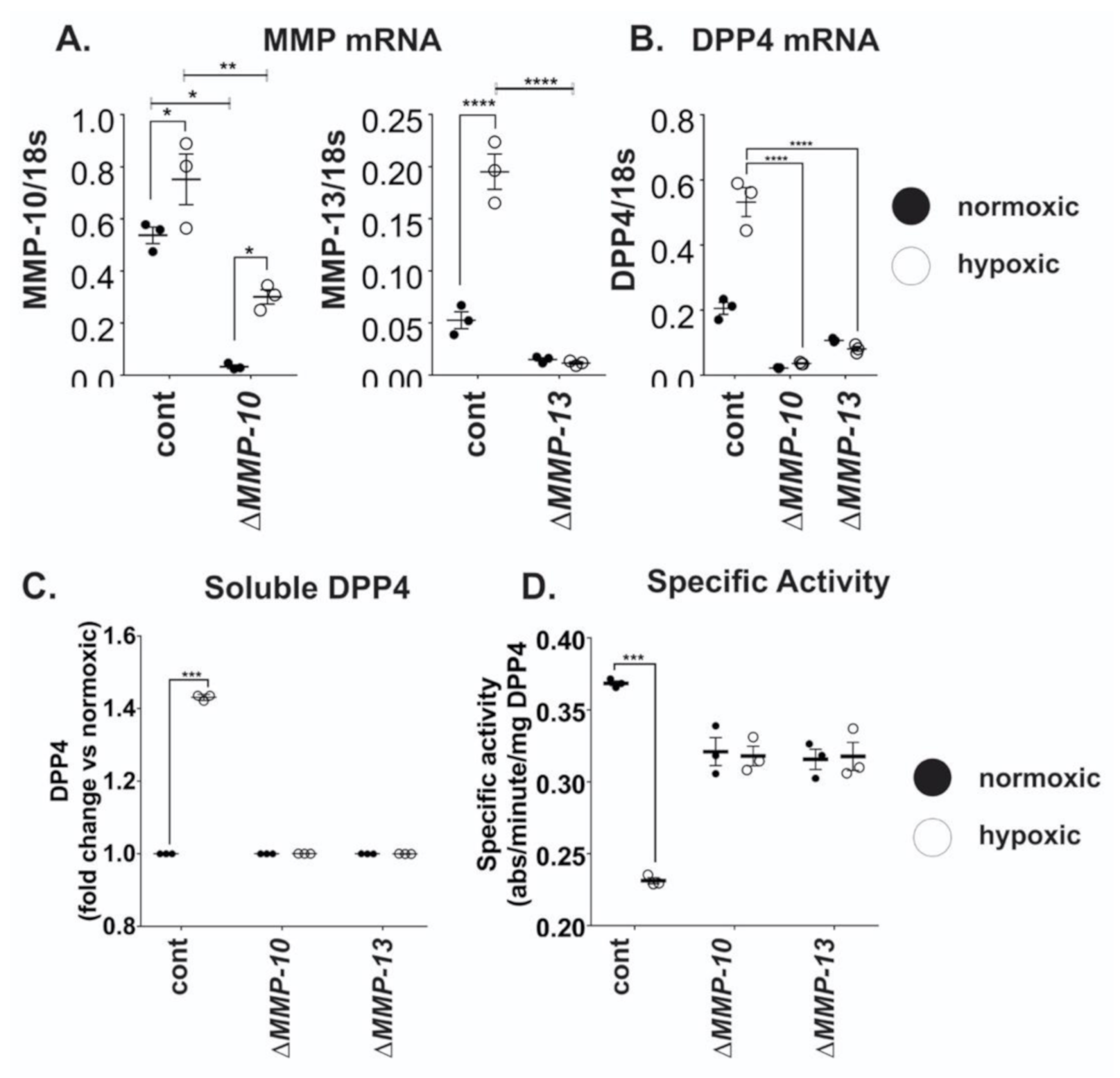
2.4. MMP10 and MMP13 Mediate DPP4 Expression and Release from Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Quantitative Real-Time PCR (qRT-PCR)
4.3. Enzyme Linked Immunosorbent Assay (ELISA)
4.4. DPP4 Enzyme Activity Assays
4.5. Protease Arrays
4.6. Targeted Knockdown of Selected MMPs Using shRNA
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Jayson, G.C.; Kohn, E.C.; Kitchener, H.C.; Ledermann, J.A. Ovarian cancer. Lancet 2014, 384, 1376–1388. [Google Scholar] [CrossRef]
- Gorrell, Mark D. Dipeptidyl peptidase IV and related enzymes in cell biology and liver disorders. Clin. Sci. 2005, 108, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.M.; Yao, T.W.; Chowdhury, S.; Nadvi, N.A.; Osborne, B.; Church, W.B.; McCaughan, G.W.; Gorrell, M.D. The dipeptidyl peptidase IV family in cancer and cell biology. FEBS J. 2010, 277, 1126–1144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Maqsudi, S.; Rainczuk, A.; Duffield, N.; Lawrence, J.; Keane, F.M.; Justa-Schuch, D.; Geiss-Friedlander, R.; Gorrell, M.D.; Stephens, A.N. Identification of novel dipeptidyl peptidase 9 substrates by two-dimensional differential in-gel electrophoresis. FEBS J. 2015, 282, 3737–3757. [Google Scholar] [CrossRef]
- Beckenkamp, A.; Davies, S.; Willig, J.B.; Buffon, A. DPPIV/CD26: A tumor suppressor or a marker of malignancy? Tumour Biol. 2016, 37, 7059–7073. [Google Scholar] [CrossRef]
- Rainczuk, A.; Rao, J.R.; Gathercole, J.L.; Fairweather, N.J.; Chu, S.; Masadah, R.; Jobling, T.W.; Deb-Choudhury, S.; Dyer, J.; Stephens, A.N. Evidence for the antagonistic form of CXC-motif chemokine CXCL10 in serous epithelial ovarian tumours. Int. J. Cancer 2014, 134, 530–541. [Google Scholar] [CrossRef]
- Kajiyama, H.; Shibata, K.; Terauchi, M.; Ino, K.; Nawa, A.; Kikkawa, F. Involvement of DPPIV/CD26 in epithelial morphology and suppressed invasive ability in ovarian carcinoma cells. Ann. N. Y. Acad. Sci. 2006, 1086, 233–240. [Google Scholar] [CrossRef]
- Kajiyama, H.; Kikkawa, F.; Maeda, O.; Suzuki, T.; Ino, K.; Mizutani, S. Increased expression of dipeptidyl peptidase IV in human mesothelial cells by malignant ascites from ovarian carcinoma patients. Oncology 2002, 63, 158–165. [Google Scholar] [CrossRef]
- Kikkawa, F.; Kajiyama, H.; Shibata, K.; Ino, K.; Nomura, S.; Mizutani, S. Dipeptidyl peptidase IV in tumor progression. Biochim. Biophys. Acta-Proteins Proteom. 2005, 1751, 45–51. [Google Scholar] [CrossRef]
- Kim, K.S.; Sengupta, S.; Berk, M.; Kwak, Y.G.; Escobar, P.F.; Belinson, J.; Mok, S.C.; Xu, Y. Hypoxia enhances lysophosphatidic acid responsiveness in ovarian cancer cells and lysophosphatidic acid induces ovarian tumor metastasis in vivo. Cancer Res. 2006, 66, 7983–7990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McEvoy, L.M.; O’Toole, S.A.; Spillane, C.D.; Martin, C.M.; Gallagher, M.F.; Stordal, B.; Blackshields, G.; Sheils, O.; O’Leary, J.J. Identifying novel hypoxia-associated markers of chemoresistance in ovarian cancer. BMC Cancer 2015, 15, 547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braicu, E.I.; Luketina, H.; Richter, R.; Castillo-Tong, D.C.; Lambrechts, S.; Mahner, S.; Concin, N.; Mentze, M.; Zeillinger, R.; Vergote, I.; et al. HIFI α is an independent prognostic factor for overall survival in advanced primary epithelial ovarian cancer–A study of the OVCAD consortium. Onco Targets Ther. 2014, 7, 1563–1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, D.T.; Chun, S.Y.; Burkitt, K.; Abe, M.; Chen, S.; Havre, P.; Mabjeesh, N.J.; Heath, E.I.; Vogelzang, N.J.; Cruz-Correa, M.; et al. Hypoxia-inducible factor-1 target genes as indicators of tumor vessel response to vascular endothelial growth factor inhibition. Cancer Res. 2008, 68, 1872–1880. [Google Scholar] [CrossRef] [Green Version]
- Röhrborn, D.; Eckel, J.; Sell, H. Shedding of dipeptidyl peptidase 4 is mediated by metalloproteases and up-regulated by hypoxia in human adipocytes and smooth muscle cells. FEBS Lett. 2014, 588, 3870–3877. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, H.H.; Velebit, J.; Radic, N.; Francic, V.; Kreft, M.; Zorec, R. Hypoxia Alters the Expression of Dipeptidyl Peptidase 4 and Induces Developmental Remodeling of Human Preadipocytes. J. Diabetes Res. 2016, 2016, 7481470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilan, J.U.; Lu, C.; Galli, S.; Izycka-Swieszewska, E.; Earnest, J.P.; Shabbir, A.; Everhart, L.M.; Wang, S.; Martin, S.; Horton, M.; et al. Hypoxia shifts activity of neuropeptide Y in Ewing sarcoma from growth-inhibitory to growth-promoting effects. Oncotarget 2013, 4, 2487–2501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domcke, S.; Sinha, R.; Levine, D.A.; Sander, C.; Schultz, N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat. Commun. 2013, 4, 2126. [Google Scholar] [CrossRef] [PubMed]
- Beaufort, C.M.; Helmijr, J.C.A.; Piskorz, A.M.; Hoogstraat, M.; Ruigrok-Ritstier, K.; Besselink, N.; Murtaza, M.; van IJcken, W.F.J.; Heine, A.A.J.; Smid, M.; et al. Ovarian cancer cell line panel (OCCP): Clinical importance of in vitro morphological subtypes. PLoS ONE 2014, 9, e103988. [Google Scholar] [CrossRef]
- Shaw, T.J.; Senterman, M.K.; Dawson, K.; Crane, C.A.; Vanderhyden, B.C. Characterization of intraperitoneal, orthotopic, and metastatic xenograft models of human ovarian cancer. Mol. Ther. 2004, 10, 1032–1042. [Google Scholar] [CrossRef]
- Lee, S.; Garner, E.I.; Welch, W.R.; Berkowitz, R.S.; Mok, S.C. Over-expression of hypoxia-inducible factor 1 alpha in ovarian clear cell carcinoma. Gynecol. Oncol. 2007, 106, 311–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007, 8, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Filatova, N.A.; Kirpichnikova, K.M.; Vakhromova, E.A.; Gamalei, I.A. [Effect of alpha-lipoic acid on the sensitivity of transformed fibroblasts to lysis by natural killer cells. Comparison with NAC action]. Tsitologiia 2009, 51, 398–402. [Google Scholar] [PubMed]
- Chowdhury, H.H.; Velebit, J.; Mekjavic, I.B.; Eiken, O.; Kreft, M.; Zorec, R. Systemic Hypoxia Increases the Expression of DPP4 in Preadipocytes of Healthy Human Participants. Exp. Clin. Endocrinol. Diabetes 2018, 126, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Lambeir, A.M.; Durinx, C.; Scharpé, S.; De Meester, I. Dipeptidyl-peptidase IV from bench to bedside: An update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit. Rev. Clin. Lab. Sci. 2003, 40, 209–294. [Google Scholar] [CrossRef] [PubMed]
- Bauvois, B.; Djavaheri-Mergny, M.; Rouillard, D.; Dumont, J.; Wietzerbin, J. Regulation of CD26/DPPIV gene expression by interferons and retinoic acid in tumor B cells. Oncogene 2000, 19, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Erickson, R.H.; Lai, R.S.; Kim, Y.S. Role of hepatocyte nuclear factor 1alpha and 1beta in the transcriptional regulation of human dipeptidyl peptidase IV during differentiation of Caco-2 cells. Biochem. Biophys. Res. Commun. 2000, 270, 235. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.K.; Klein, J.B. Analysis of expression and posttranslational modification of proteins during hypoxia. J. Appl. Physiol. 2004, 96, 1178–1186. [Google Scholar] [CrossRef] [Green Version]
- Zhong, J.; Gong, Q.; Goud, A.; Srinivasamaharaj, S.; Rajagopalan, S. Recent Advances in Dipeptidyl-Peptidase-4 Inhibition Therapy: Lessons from the Bench and Clinical Trials. J. Diabetes Res. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Yang, Q.; Lian, X.; Jiang, P.; Cui, J. Hypoxia-Inducible Factor-1alpha (HIF-1alpha) Promotes Hypoxia-Induced Invasion and Metastasis in Ovarian Cancer by Targeting Matrix Metallopeptidase 13 (MMP13). Med. Sci. Monit. 2019, 25, 7202–7208. [Google Scholar] [CrossRef]
- Nargis, T.; Kumar, K.; Ghosh, A.R.; Sharma, A.; Rudra, D.; Sen, D.; Chakrabarti, S.; Mukhopadhyay, S.; Ganguly, D.; Chakrabarti, P. KLK5 induces shedding of DPP4 from circulatory Th17 cells in type 2 diabetes. Mol. Metab. 2017, 6, 1529–1539. [Google Scholar] [CrossRef] [PubMed]
- Fasolato, S.; Trevellin, E.; Ruvoletto, M.; Granzotto, M.; Zanus, G.; Boscaro, E.; Babetto, E.; Terrin, L.; Battocchio, M.A.; Ciscato, F.; et al. SerpinB3 induces dipeptidyl-peptidase IV/CD26 expression and its metabolic effects in hepatocellular carcinoma. Life Sci. 2018, 200, 134. [Google Scholar] [CrossRef] [PubMed]
- Fortelny, N.; Cox, J.H.; Kappelhoff, R.; Starr, A.E.; Lange, P.F.; Pavlidis, P.; Overall, C.M. Network Analyses Reveal Pervasive Functional Regulation Between Proteases in the Human Protease Web (Analysis of the Interconnected Human Protease Web). PLoS Biol. 2014, 12, e1001869. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.-C.; Cheung, A.H.-K.; Wong, S.K.-M.; Wan, T.M.-H.; Ng, L.; Chow, A.K.-M.; Cheng, N.S.-M.; Pak, R.C.-H.; Li, H.-S.; Man, J.H.-W.; et al. Prognostic significance of CD26 in patients with colorectal cancer. PLoS ONE 2014, 9, e98582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larrinaga, G.; Perez, I.; Sanz, B.; Beitia, M.; Errarte, P.; Fernandez, A.; Blanco, L.; Etxezarraga, M.C.; Gil, J.; Lopez, J.I. Dipeptidyl-Peptidase IV Activity Is Correlated with Colorectal Cancer Prognosis. PLoS ONE 2015, 10, e0119436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matić, I.Z.; Ðorđić, M.; Grozdanić, N.; Damjanović, A.; Kolundžija, B.; Erić-Nikolić, A.; Džodić, R.; Šašić, M.; Nikolić, S.; Dobrosavljević, D.; et al. Serum activity of DPPIV and its expression on lymphocytes in patients with melanoma and in people with vitiligo. BMC Immunol. 2012, 13, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordero, O.J.; Salgado, F.J.; Nogueira, M. On the origin of serum CD26 and its altered concentration in cancer patients. Cancer Immunol. Immunother. 2009, 58, 1723. [Google Scholar] [CrossRef]
- Barreira da Silva, R.; Laird, M.E.; Yatim, N.; Fiette, L.; Ingersoll, M.A.; Albert, M.L. Dipeptidylpeptidase 4 inhibition enhances lymphocyte trafficking, improving both naturally occurring tumor immunity and immunotherapy. Nat. Immunol. 2015, 16, 850–858. [Google Scholar] [CrossRef]
- Rainczuk, A.; Rao, J.; Gathercole, J.; Stephens, A.N. The emerging role of CXC chemokines in epithelial ovarian cancer. Reproduction 2012, 144, 303–317. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.-X.; Pedersen, E.; Shiozawa, Y.; Havens, A.; Jung, Y.; Wang, J.; Pienta, K.; Taichman, R. CD26/dipeptidyl peptidase IV regulates prostate cancer metastasis by degrading SDF-1/CXCL12. Off. J. Metastasis Res. Soc. 2008, 25, 765–776. [Google Scholar] [CrossRef]
- Russo, J.W.; Gao, C.; Bhasin, S.S.; Voznesensky, O.S.; Calagua, C.; Arai, S.; Nelson, P.S.; Montgomery, B.; Mostaghel, E.A.; Corey, E.; et al. Downregulation of Dipeptidyl Peptidase 4 Accelerates Progression to Castration-Resistant Prostate Cancer. Cancer Res. 2018, 78, 6354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busek, P.; Stremenova, J.; Sromova, L.; Hilser, M.; Balaziova, E.; Kosek, D.; Trylcova, J.; Strnad, H.; Krepela, E.; Sedo, A. Dipeptidyl peptidase-IV inhibits glioma cell growth independent of its enzymatic activity. Int. J. Biochem. Cell Biol. 2012, 44, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Busek, P.; Stremenova, J.; Krepela, E.; Sedo, A. Modulation of substance P signaling by dipeptidyl peptidase-IV enzymatic activity in human glioma cell lines. Physiol. Res. 2008, 57, 443–449. [Google Scholar] [PubMed]
- Tseng, C.-H. Sitagliptin May Reduce Breast Cancer Risk in Women With Type 2 Diabetes. Clin. Breast Cancer 2017, 17, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Hollande, C.; Boussier, J.; Ziai, J.; Nozawa, T.; Bondet, V.; Phung, W.; Lu, B. Inhibition of the dipeptidyl peptidase DPP4 (CD26) reveals IL-33-dependent eosinophil-mediated control of tumor growth. Nat. Immunol. 2019, 20, 257. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.J.; Lee, Q.; Wong, A.C.; Spann, B.C.; Vincent, J.N.; Wong, J.J.; Schlitzer, A.; Gorrell, M.D.; Weninger, W.; Roediger, B. Differential chemokine receptor expression and usage by pre-cDC1 and pre-cDC2. Immunol. Cell Biol. 2018, 96, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Augoff, K.; Hryniewicz-Jankowska, A.; Tabola, R.; Czapla, L.; Szelachowski, P.; Wierzbicki, J.; Grabowski, K.; Sikorski, A.F. Upregulated expression and activation of membrane-associated proteases in esophageal squamous cell carcinoma. Oncol. Rep. 2014, 31, 2820–2826. [Google Scholar] [CrossRef]
- Beckenkamp, A.; Willig, J.B.; Santana, D.B.; Nascimento, J.; Paccez, J.D.; Zerbini, L.F.; Bruno, A.N.; Pilger, D.A.; Wink, M.R.; Buffon, A. Differential Expression and Enzymatic Activity of DPPIV/CD26 Affects Migration Ability of Cervical Carcinoma Cells. PLoS ONE 2015, 10, e0134305. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Wang, T.Y.; Liu, C.L.; Chien, M.N.; Chen, M.J.; Hsu, Y.C.; Leung, C.H.; Cheng, S.P. Dipeptidyl Peptidase IV as a Prognostic Marker and Therapeutic Target in Papillary Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2017, 102, 2930–2940. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.H.; Iyer, D.N.; Lam, C.S.; Ng, L.; Wong, S.K.M.; Lee, H.S.; Wan, T.; Man, J.; Chow, A.K.M.; Poon, R.T.; et al. Emergence of CD26+ Cancer Stem Cells with Metastatic Properties in Colorectal Carcinogenesis. Int. J. Mol. Sci. 2017, 18, 1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raspadori, D.; Pacelli, P.; Sicuranza, A.; Abruzzese, E.; Iurlo, A.; Cattaneo, D.; Gozzini, A.; Galimberti, S.; Barate, C.; Pregno, P.; et al. Flow Cytometry Assessment of CD26(+) Leukemic Stem Cells in Peripheral Blood: A Simple and Rapid New Diagnostic Tool for Chronic Myeloid Leukemia. Cytom. B Clin. Cytom. 2019, 294–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shishido, A.; Mori, S.; Yokoyama, Y.; Hamada, Y.; Minami, K.; Qian, Y.; Wang, J.; Hirose, H.; Wu, X.; Kawaguchi, N.; et al. Mesothelial cells facilitate cancer stemlike properties in spheroids of ovarian cancer cells. Oncol. Rep. 2018, 40, 2105–2114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarraj, M.A.; Chua, H.K.; Umbers, A.; Loveland, K.L.; Findlay, J.K.; Stenvers, K.L. Differential expression of TGFBR3 (betaglycan) in mouse ovary and testis during gonadogenesis. Growth Factors 2007, 25, 334–345. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
| Gene | Accession Number | Primer Sequences 5′–3′ | |
|---|---|---|---|
| Forward | Reverse | ||
| 18S | NR_003286.4 | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG |
| HIF-1α | NM_001530 | CAGCTATTTGCGTGTGAGGA | CCTCATGGTCACATGGATGA |
| DPP4 | NM_001935 | ATGCCAGGAGGAAGGAATCT | TATAGAGGGGCAGACCAGGA |
| MMP1 | NM_002421 | GGACAACTCTCCTTTTGATGGA | CAAAGCCCCGATATCAGTAGAA |
| MMP10 | NM_002425 | CACAGTTTGGCTCATGCCTA | TGCCATTCACATCATCTTGC |
| MMP13 | NM_002427 | GACCCTGGAGCACTCATGTT | TCCTCGGAGACTGGTAATGG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moffitt, L.R.; Bilandzic, M.; Wilson, A.L.; Chen, Y.; Gorrell, M.D.; Oehler, M.K.; Plebanski, M.; Stephens, A.N. Hypoxia Regulates DPP4 Expression, Proteolytic Inactivation, and Shedding from Ovarian Cancer Cells. Int. J. Mol. Sci. 2020, 21, 8110. https://doi.org/10.3390/ijms21218110
Moffitt LR, Bilandzic M, Wilson AL, Chen Y, Gorrell MD, Oehler MK, Plebanski M, Stephens AN. Hypoxia Regulates DPP4 Expression, Proteolytic Inactivation, and Shedding from Ovarian Cancer Cells. International Journal of Molecular Sciences. 2020; 21(21):8110. https://doi.org/10.3390/ijms21218110
Chicago/Turabian StyleMoffitt, Laura R., Maree Bilandzic, Amy L. Wilson, Yiqian Chen, Mark D. Gorrell, Martin K. Oehler, Magdalena Plebanski, and Andrew N. Stephens. 2020. "Hypoxia Regulates DPP4 Expression, Proteolytic Inactivation, and Shedding from Ovarian Cancer Cells" International Journal of Molecular Sciences 21, no. 21: 8110. https://doi.org/10.3390/ijms21218110





