Protective Role of St. John’s Wort and Its Components Hyperforin and Hypericin against Diabetes through Inhibition of Inflammatory Signaling: Evidence from In Vitro and In Vivo Studies
Abstract
:1. Introduction
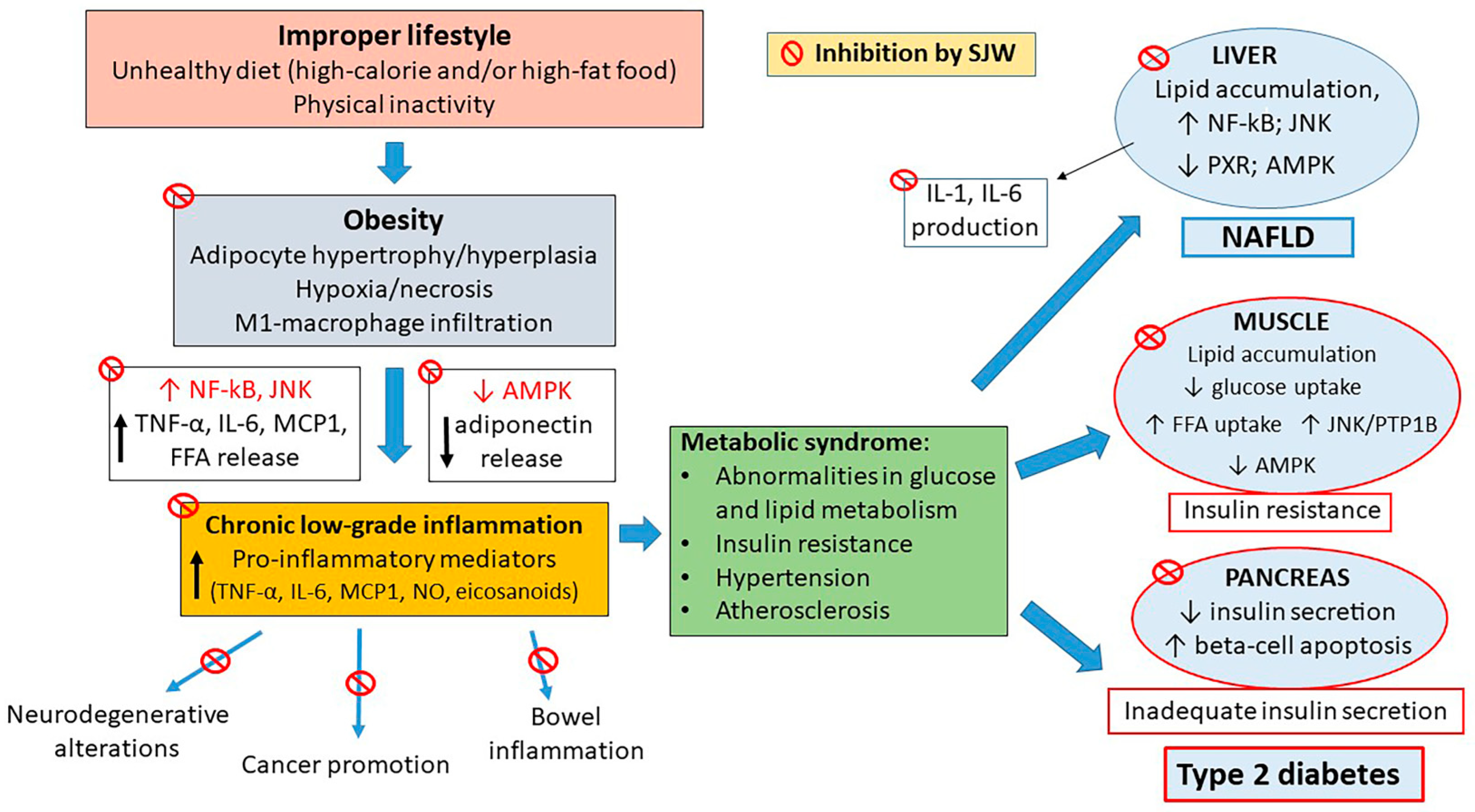
2. Inflammatory Signaling and Diabetes
3. Anti-Inflammatory Drugs against Diabetes
4. SJW and Its Components
5. Mechanisms of the Anti-Inflammatory Activities of SJW and Its Components
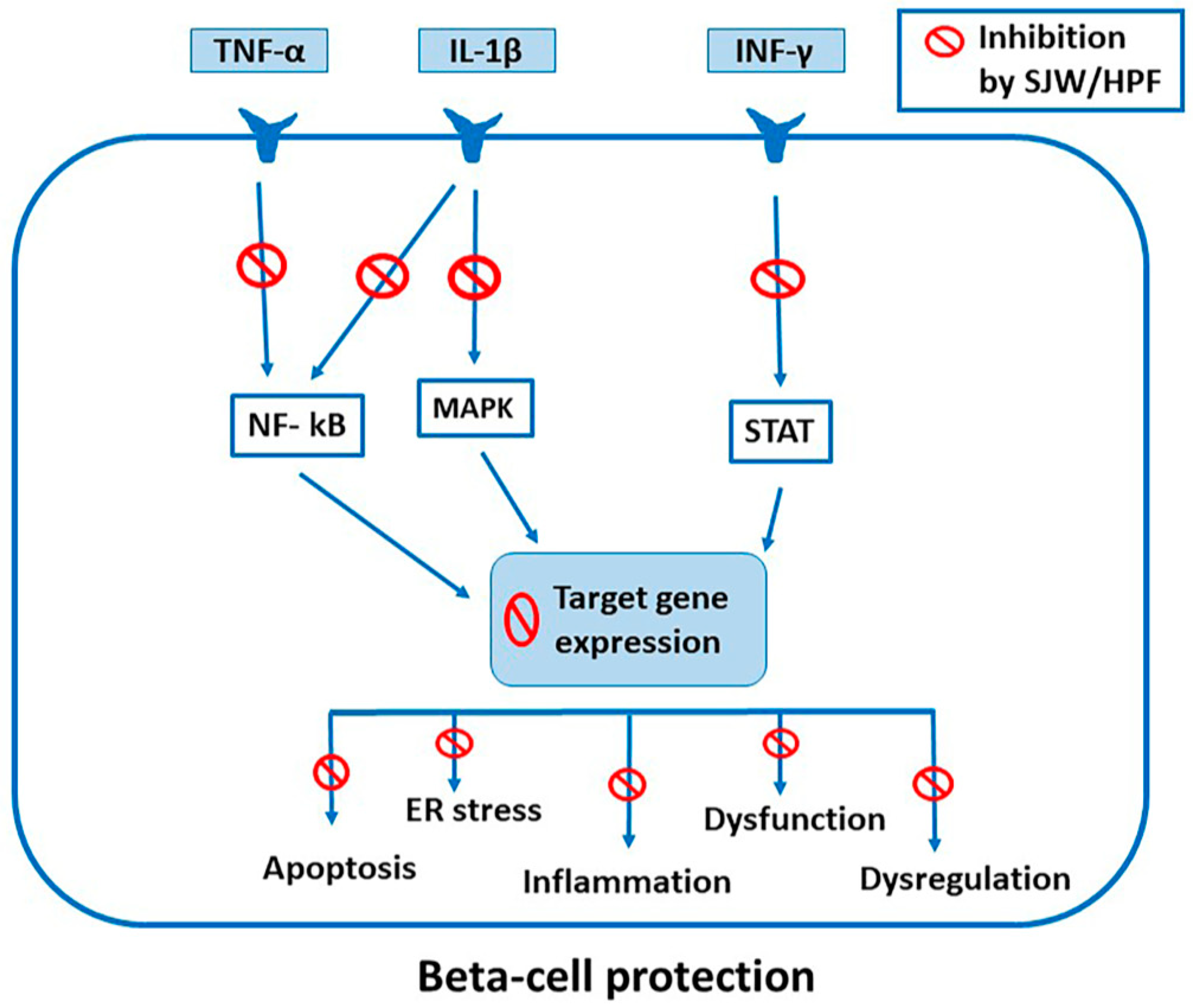
5.1. Inhibition of Cytokine-Induced Activation of STAT, NF-kB and MAPK Signaling
5.2. Inhibition of Phospholipid-Derived Inflammatory Mediators by SJW and HPF
5.3. Free Radical Scavenging and Antioxidant Activity of SJW and HPF
5.4. Activation of AMPK by SJW and Its Components
5.5. Anti-Inflammatory Effects of SJW and HPF Mediated by PXR Activation
5.6. Neuroprotective Effects of SJW and HPF Mediated by Anti-Inflammatory Mechanisms
5.7. Beneficial Effects of SJW and HPF in Various Experimental Models of Acute and Chronic Inflammation
6. Effects of SJW Extract, HPF and HYP in β Cells and Isolated Pancreatic Islets
7. Effects of SJW Extract and HPF in Adipocytes
8. Effects of SJW Extract: In Vivo Studies
9. Effects of SJW on Diabetic Complications
10. Discussion
11. Concluding Remarks and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| T1D | type 1 diabetes mellitus |
| T2D | type 2 diabetes mellitus |
| IFN | interferon |
| IL | interleukin |
| TNF | tumor necrosis factor |
| MCP-1 | monocyte chemoattractant protein-1 |
| M1-macrophages | classically activated M1 macrophages |
| SJW | St. John’s wort |
| HPF | hyperforin |
| HYP | hypericin |
| STAT | signal transducer and activator of transcription |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| JAK | receptor-associated Janus kinases |
| IkB | inhibitor molecules |
| IKK | protein kinase complex |
| iNOS | inducible nitric oxide synthase |
| MAPKs | mitogen-activated protein kinases |
| ERK | extracellular signal-related kinase |
| JNK | c-jun N-terminal kinase |
| ROS | reactive oxygen species |
| RNS | reactive nitrogen species |
| ER | endoplasmic reticulum |
| CHOP | C/EBP homologous protein |
| DP5 | death protein 5 |
| NSAIDs | non-steroidal anti-inflammatory drugs |
| AMPK | adenosine monophosphate-activated protein kinase |
| SOCS3 | suppressor of cytokine signaling 3 |
| DCHA-hyperforin | hyperforin dicyclohexylammonium salt |
| 5-LO | 5-lipoxygenase |
| COX | cyclooxygenase |
| PGE2 | prostaglandin E2 |
| fMLP | N-formyl-methionyl-leucyl-phenylalanine |
| LPS | lipopolysaccharide |
| PMN | polymorphonuclear neutrophils |
| PXR | pregnane X receptor |
| RXR | retinoid X receptor |
| HFD | high-fat-diet |
| AD | Alzheimer’s disease |
| SOD | superoxide dismutase |
| Aβ | amyloid-β-peptide |
| AAP | amyloid precursor protein |
| AST | aspartate aminotransferase |
| ALT | alanine aminotransferase |
| ICAM | intercellular cell adhesion molecule |
| BCL-2 | B-cell lymphoma protein 2 |
| BAX | B-cell lymphoma protein 2 (Bcl-2)-associated X |
| CXCL | C‑X‑C motif chemokine ligand |
| CIITA | immune-transcriptional co-activator |
| Puma | p53-upregulated mediator of apoptosis |
| Bim | B-cell lymphoma 2 interacting mediator of cell death |
| Chop | C/EBP homologous protein |
| PDX-1 | pancreatic duodenal homeobox-1 |
| FFA | free fatty acids |
| IAPP | islet amyloid polypeptide |
| FATP1 | transporter of free fatty acids |
| HYP | hypericin |
| HFHS | high-fat/high-sucrose |
| NAFLD | non-alchoholic liver fatty disease |
| pAMPK | phosphorylated adenosine monophosphate-activated protein kinase |
| PKACs | protein kinase A catalytic |
| CRP | C reactive protein |
| TG | triglycerides |
| LDL | low-density lipoprotein |
| PKA | protein kinase cAMP-dependent |
| TGF-β | transforming growth factor beta |
| TRPC6 | transient receptor potential channel |
| GABA | gamma-aminobutyric acid |
| NMDA | N-methyl-d-aspartate |
| PPAR-γ | peroxisome proliferator-activated receptor-gamma |
| STZ-NA | streptozotocin-nicotinamide |
| AGE | advanced glycation end products |
| TLR | Toll-like receptor |
| CYPs | cytochromes P450s |
| P-gp | P-glycoprotein |
| cAMP | cyclic adenosine monophosphate |
| PTP1B | protein tyrosine phosphatase 1B |
References
- Karczewski, J.; Śledzińska, E.; Baturo, A.; Jończyk, I.; Maleszko, A.; Samborski, P.; Begier-Krasińska, B.; Dobrowolska, A. Obesity and inflammation. Eur. Cytokine Netw. 2018, 29, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Leitner, D.R.; Frühbeck, G.; Yumuk, V.; Schindler, K.; Micic, D.; Woodward, E.; Toplak, H. Obesity and Type 2 Diabetes: Two Diseases with a Need for Combined Treatment Strategies—EASO Can Lead the Way. Obes. Facts 2017, 10, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Lindström, J.; Louheranta, A.; Mannelin, M.; Rastas, M.; Salminen, V.; Eriksson, J.; Uusitupa, M.; Tuomilehto, J.; Finnish Diabetes Prevention Study Group. The Finnish Diabetes Prevention Study (DPS): Lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care 2003, 26, 3230–3236. [Google Scholar]
- Christoffersson, G.; Rodriguez-Calvo, T.; von Herrath, M. Recent advances in understanding Type 1 Diabetes. F1000Research 2016, 5, 110. [Google Scholar] [CrossRef] [Green Version]
- Eguchi, K.; Nagai, R. Islet inflammation in type 2 diabetes and physiology. J. Clin. Invest. 2017, 127, 14–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böni-Schnetzler, M.; Meier, D.T. Islet inflammation in type 2 diabetes. Semin. Immunopathol. 2019, 41, 501–513. [Google Scholar] [CrossRef] [Green Version]
- Carrillo, J.L.M.; Campo, J.O.M.D.; Coronado, O.G.; Gutiérrez, P.T.V.; Cordero, J.F.C.; Juárez, J.V. Adipose Tissue and Inflammation. In Adipose Tissue; Szablewski, L., Ed.; InTech: London, UK, 2018; ISBN 978-1-78923-228-8. [Google Scholar]
- Burhans, M.S.; Hagman, D.K.; Kuzma, J.N.; Schmidt, K.A.; Kratz, M. Contribution of Adipose Tissue Inflammation to the Development of Type 2 Diabetes Mellitus. Compr. Physiol. 2018, 9, 1–58. [Google Scholar]
- Catrysse, L.; van Loo, G. Inflammation and the Metabolic Syndrome: The Tissue-Specific Functions of NF-κB. Trends Cell Biol. 2017, 27, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Cao, H.; Ou, J.; Chen, L.; Zhang, Y.; Szkudelski, T.; Delmas, D.; Daglia, M.; Xiao, J. Dietary polyphenols and type 2 diabetes: Human Study and Clinical Trial. Crit. Rev. Food Sci. Nutr. 2019, 59, 3371–3379. [Google Scholar] [CrossRef]
- Khan, H.; Sureda, A.; Belwal, T.; Çetinkaya, S.; Süntar, İ.; Tejada, S.; Devkota, H.P.; Ullah, H.; Aschner, M. Polyphenols in the treatment of autoimmune diseases. Autoimmun. Rev. 2019, 18, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Song, Z.; Weng, J.; Fantus, I.G. Curcumin and other dietary polyphenols: Potential mechanisms of metabolic actions and therapy for diabetes and obesity. Am. J. Physiol. Endocrinol. Metab. 2018, 314, E201–E205. [Google Scholar] [CrossRef]
- Novelli, M.; Menegazzi, M.; Beffy, P.; Porozov, S.; Gregorelli, A.; Giacopelli, D.; De Tata, V.; Masiello, P. St. John’s wort extract and hyperforin inhibit multiple phosphorylation steps of cytokine signaling and prevent inflammatory and apoptotic gene induction in pancreatic β cells. Int. J. Biochem. Cell Biol. 2016, 81, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Novelli, M.; Beffy, P.; Gregorelli, A.; Porozov, S.; Mascia, F.; Vantaggiato, C.; Masiello, P.; Menegazzi, M. Persistence of STAT-1 inhibition and induction of cytokine resistance in pancreatic β cells treated with St John’s wort and its component hyperforin. J. Pharm. Pharmacol. 2019, 71, 93–103. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.; Hao, F.; Yao, X.; Qiu, Y.; Liu, L.; Wang, S.; Yu, C.; Song, Z.; Bao, Y.; Yi, J.; et al. Hypericin maintians PDX1 expression via the Erk pathway and protects islet β-cells against glucotoxicity and lipotoxicity. Int. J. Biol. Sci. 2019, 15, 1472–1487. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Li, Y.; Bai, M.; Huang, Y.; Yang, H.; Liu, L.; Wang, S.; Yu, C.; Song, Z.; Bao, Y.; et al. Hypericin attenuates nonalcoholic fatty liver disease and abnormal lipid metabolism via the PKA-mediated AMPK signaling pathway in vitro and in vivo. Pharmacol. Res. 2020, 153, 104657. [Google Scholar] [CrossRef]
- Khodabandehloo, H.; Gorgani-Firuzjaee, S.; Panahi, G.; Meshkani, R. Molecular and cellular mechanisms linking inflammation to insulin resistance and β-cell dysfunction. Transl. Res. 2016, 167, 228–256. [Google Scholar] [CrossRef]
- Berchtold, L.A.; Prause, M.; Størling, J.; Mandrup-Poulsen, T. Cytokines and Pancreatic β-Cell Apoptosis. In Advances in Clinical Chemistry; Elsevier: London, UK, 2016; Volume 75, pp. 99–158. ISBN 978-0-12-804688-3. [Google Scholar]
- Eizirik, D.L.; Colli, M.L.; Ortis, F. The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat. Rev. Endocrinol. 2009, 5, 219–226. [Google Scholar] [CrossRef]
- Prause, M.; Berchtold, L.A.; Urizar, A.I.; Hyldgaard Trauelsen, M.; Billestrup, N.; Mandrup-Poulsen, T.; Størling, J. TRAF2 mediates JNK and STAT3 activation in response to IL-1β and IFNγ and facilitates apoptotic death of insulin-producing β-cells. Mol. Cell. Endocrinol. 2016, 420, 24–36. [Google Scholar] [CrossRef]
- Zhang, Q.; Lenardo, M.J.; Baltimore, D. 30 Years of NF-κB: A Blossoming of Relevance to Human Pathobiology. Cell 2017, 168, 37–57. [Google Scholar] [CrossRef] [Green Version]
- Burke, S.J.; Stadler, K.; Lu, D.; Gleason, E.; Han, A.; Donohoe, D.R.; Rogers, R.C.; Hermann, G.E.; Karlstad, M.D.; Collier, J.J. IL-1β reciprocally regulates chemokine and insulin secretion in pancreatic β-cells via NF-κB. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E715–E726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardozo, A.K.; Heimberg, H.; Heremans, Y.; Leeman, R.; Kutlu, B.; Kruhøffer, M.; Ørntoft, T.; Eizirik, D.L. A comprehensive analysis of cytokine-induced and nuclear factor-kappa B-dependent genes in primary rat pancreatic beta-cells. J. Biol. Chem. 2001, 276, 48879–48886. [Google Scholar] [CrossRef] [Green Version]
- Heimberg, H.; Heremans, Y.; Jobin, C.; Leemans, R.; Cardozo, A.K.; Darville, M.; Eizirik, D.L. Inhibition of cytokine-induced NF-kappaB activation by adenovirus-mediated expression of a NF-kappaB super-repressor prevents beta-cell apoptosis. Diabetes 2001, 50, 2219–2224. [Google Scholar] [CrossRef] [Green Version]
- Pavlovic, D.; Andersen, N.A.; Mandrup-Poulsen, T.; Eizirik, D.L. Activation of extracellular signal-regulated kinase (ERK)1/2 contributes to cytokine-induced apoptosis in purified rat pancreatic beta-cells. Eur. Cytokine Netw. 2000, 11, 267–274. [Google Scholar]
- Saldeen, J.; Lee, J.C.; Welsh, N. Role of p38 mitogen-activated protein kinase (p38 MAPK) in cytokine-induced rat islet cell apoptosis. Biochem. Pharmacol. 2001, 61, 1561–1569. [Google Scholar] [CrossRef]
- Bonny, C.; Oberson, A.; Negri, S.; Sauser, C.; Schorderet, D.F. Cell-permeable peptide inhibitors of JNK: Novel blockers of beta-cell death. Diabetes 2001, 50, 77–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yung, J.H.M.; Giacca, A. Role of c-Jun N-terminal Kinase (JNK) in Obesity and Type 2 Diabetes. Cells 2020, 9, 706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, J.; Lu, S.; Ou, B.; Liu, Q.; Dai, J.; Ji, C.; Zhou, H.; Huang, H.; Ma, Y. The Role of JNk Signaling Pathway in Obesity-Driven Insulin Resistance. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 1399–1406. [Google Scholar] [CrossRef]
- Nguyen, M.T.A.; Favelyukis, S.; Nguyen, A.-K.; Reichart, D.; Scott, P.A.; Jenn, A.; Liu-Bryan, R.; Glass, C.K.; Neels, J.G.; Olefsky, J.M. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J. Biol. Chem. 2007, 282, 35279–35292. [Google Scholar] [CrossRef] [Green Version]
- Han, M.S.; Jung, D.Y.; Morel, C.; Lakhani, S.A.; Kim, J.K.; Flavell, R.A.; Davis, R.J. JNK Expression by Macrophages Promotes Obesity-Induced Insulin Resistance and Inflammation. Science 2013, 339, 218–222. [Google Scholar] [CrossRef] [Green Version]
- MohammadTaghvaei, N.; Taheripak, G.; Taghikhani, M.; Meshkani, R. Palmitate-induced PTP1B expression is mediated by ceramide-JNK and nuclear factor κB (NF-κB) activation. Cell. Signal. 2012, 24, 1964–1970. [Google Scholar] [CrossRef] [PubMed]
- Galic, S.; Fullerton, M.D.; Schertzer, J.D.; Sikkema, S.; Marcinko, K.; Walkley, C.R.; Izon, D.; Honeyman, J.; Chen, Z.-P.; van Denderen, B.J.; et al. Hematopoietic AMPK β1 reduces mouse adipose tissue macrophage inflammation and insulin resistance in obesity. J. Clin. Investig. 2011, 121, 4903–4915. [Google Scholar] [CrossRef] [Green Version]
- Gauthier, M.-S.; O’Brien, E.L.; Bigornia, S.; Mott, M.; Cacicedo, J.M.; Xu, X.J.; Gokce, N.; Apovian, C.; Ruderman, N. Decreased AMP-activated protein kinase activity is associated with increased inflammation in visceral adipose tissue and with whole-body insulin resistance in morbidly obese humans. Biochem. Biophys. Res. Commun. 2011, 404, 382–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Zhang, X.; Wang, H.; Guo, X.; Li, H.; Wang, Y.; Xu, X.; Tan, L.; Mashek, M.; Zhang, C.; et al. AMP-activated protein kinase 1 protects against diet-induced insulin resistance and obesity. Diabetes 2013, 62, 998. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Yang, G.; Kim, Y.; Kim, J.; Ha, J. AMPK activators: Mechanisms of action and physiological activities. Exp. Mol. Med. 2016, 48, e224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenzen, S. Chemistry and biology of reactive species with special reference to the antioxidative defence status in pancreatic β-cells. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1929–1942. [Google Scholar] [CrossRef]
- Roma, L.P.; Jonas, J.-C. Nutrient Metabolism, Subcellular Redox State, and Oxidative Stress in Pancreatic Islets and β-Cells. J. Mol. Biol. 2020, 432, 1461–1493. [Google Scholar] [CrossRef]
- Newsholme, P.; Cruzat, V.F.; Keane, K.N.; Carlessi, R.; de Bittencourt, P.I.H. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem. J. 2016, 473, 4527–4550. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Morgan, M.J.; Choksi, S.; Liu, Z.-G. TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol. Cell 2007, 26, 675–687. [Google Scholar] [CrossRef]
- Issa, N.; Lachance, G.; Bellmann, K.; Laplante, M.; Stadler, K.; Marette, A. Cytokines promote lipolysis in 3T3-L1 adipocytes through induction of NADPH oxidase 3 expression and superoxide production. J. Lipid Res. 2018, 59, 2321–2328. [Google Scholar] [CrossRef] [Green Version]
- Cetkovic-Cvrlje, M.; Eizirik, D.L. TNF-alpha and IFN-gamma potentiate the deleterious effects of IL-1 beta on mouse pancreatic islets mainly via generation of nitric oxide. Cytokine 1994, 6, 399–406. [Google Scholar] [CrossRef]
- Oleson, B.J.; Corbett, J.A. Dual Role of Nitric Oxide in Regulating the Response of β Cells to DNA Damage. Antioxid. Redox Signal. 2018, 29, 1432–1445. [Google Scholar] [CrossRef] [PubMed]
- Meyerovich, K.; Ortis, F.; Allagnat, F.; Cardozo, A.K. Endoplasmic reticulum stress and the unfolded protein response in pancreatic islet inflammation. J. Mol. Endocrinol. 2016, 57, R1–R17. [Google Scholar] [CrossRef] [Green Version]
- Brozzi, F.; Nardelli, T.R.; Lopes, M.; Millard, I.; Barthson, J.; Igoillo-Esteve, M.; Grieco, F.A.; Villate, O.; Oliveira, J.M.; Casimir, M.; et al. Cytokines induce endoplasmic reticulum stress in human, rat and mouse beta cells via different mechanisms. Diabetologia 2015, 58, 2307–2316. [Google Scholar] [CrossRef] [Green Version]
- Gurzov, E.N.; Ortis, F.; Cunha, D.A.; Gosset, G.; Li, M.; Cardozo, A.K.; Eizirik, D.L. Signaling by IL-1beta+IFN-gamma and ER stress converge on DP5/Hrk activation: A novel mechanism for pancreatic beta-cell apoptosis. Cell Death Differ. 2009, 16, 1539–1550. [Google Scholar] [CrossRef]
- Chan, J.Y.; Luzuriaga, J.; Maxwell, E.L.; West, P.K.; Bensellam, M.; Laybutt, D.R. The balance between adaptive and apoptotic unfolded protein responses regulates β-cell death under ER stress conditions through XBP1, CHOP and JNK. Mol. Cell. Endocrinol. 2015, 413, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Meyerovich, K.; Violato, N.M.; Fukaya, M.; Dirix, V.; Pachera, N.; Marselli, L.; Marchetti, P.; Strasser, A.; Eizirik, D.L.; Cardozo, A.K. MCL-1 Is a Key Antiapoptotic Protein in Human and Rodent Pancreatic β-Cells. Diabetes 2017, 66, 2446–2458. [Google Scholar] [CrossRef] [Green Version]
- Lambelet, M.; Terra, L.F.; Fukaya, M.; Meyerovich, K.; Labriola, L.; Cardozo, A.K.; Allagnat, F. Dysfunctional autophagy following exposure to pro-inflammatory cytokines contributes to pancreatic β-cell apoptosis. Cell Death Dis. 2018, 9, 96. [Google Scholar] [CrossRef] [PubMed]
- Agius, L.; Ford, B.E.; Chachra, S.S. The Metformin Mechanism on Gluconeogenesis and AMPK Activation: The Metabolite Perspective. Int. J. Mol. Sci. 2020, 21, 3240. [Google Scholar] [CrossRef]
- Hirsch, H.A.; Iliopoulos, D.; Struhl, K. Metformin inhibits the inflammatory response associated with cellular transformation and cancer stem cell growth. Proc. Natl. Acad. Sci. USA 2013, 110, 972–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhury, A.; Duvoor, C.; Reddy Dendi, V.S.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.S.; Montales, M.T.; Kuriakose, K.; et al. Clinical Review of Antidiabetic Drugs: Implications for Type 2 Diabetes Mellitus Management. Front. Endocrinol. 2017, 8, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, S.V.; Inzucchi, S.E.; Echouffo-Tcheugui, J.B.; Tang, F.; Lam, C.S.P.; Sperling, L.S.; Kosiborod, M. Understanding Contemporary Use of Thiazolidinediones. Circ. Heart Fail. 2019, 12, e005855. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.A.; Mattison, D.R.; Azoulay, L.; Krewski, D. Thiazolidinedione drugs in the treatment of type 2 diabetes mellitus: Past, present and future. Crit. Rev. Toxicol. 2018, 48, 52–108. [Google Scholar] [CrossRef]
- Bolen, S.; Feldman, L.; Vassy, J.; Wilson, L.; Yeh, H.-C.; Marinopoulos, S.; Wiley, C.; Selvin, E.; Wilson, R.; Bass, E.B.; et al. Systematic Review: Comparative Effectiveness and Safety of Oral Medications for Type 2 Diabetes Mellitus. Ann. Intern. Med. 2007, 147, 386. [Google Scholar] [CrossRef]
- Donath, M.Y. Multiple benefits of targeting inflammation in the treatment of type 2 diabetes. Diabetologia 2016, 59, 679–682. [Google Scholar] [CrossRef]
- Rehman, K.; Akash, M.S.H. Mechanisms of inflammatory responses and development of insulin resistance: How are they interlinked? J. Biomed. Sci. 2016, 23, 87. [Google Scholar] [CrossRef] [Green Version]
- Bellucci, P.N.; González Bagnes, M.F.; Di Girolamo, G.; González, C.D. Potential Effects of Nonsteroidal Anti-Inflammatory Drugs in the Prevention and Treatment of Type 2 Diabetes Mellitus. J. Pharm. Pract. 2017, 30, 549–556. [Google Scholar] [CrossRef]
- Pollack, R.M.; Donath, M.Y.; LeRoith, D.; Leibowitz, G. Anti-inflammatory Agents in the Treatment of Diabetes and Its Vascular Complications. Diabetes Care 2016, 39 (Suppl. 2), S244–S252. [Google Scholar] [CrossRef] [Green Version]
- Ward, S.G. New drug targets in inflammation: Efforts to expand the anti-inflammatory armoury. Br. J. Pharmacol. 2008, 153 (Suppl. 1), S5–S6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harirforoosh, S.; Asghar, W.; Jamali, F. Adverse effects of nonsteroidal antiinflammatory drugs: An update of gastrointestinal, cardiovascular and renal complications. J. Pharm. Pharm. Sci. Publ. Can. Soc. Pharm. Sci. Soc. Can. Sci. Pharm. 2013, 16, 821–847. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, G.R.; Dandapani, M.; Hardie, D.G. AMPK: Mediating the metabolic effects of salicylate-based drugs? Trends Endocrinol. Metab. TEM 2013, 24, 481–487. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.K.; Ford, R.J.; Desjardins, E.M.; Green, A.E.; Hughes, M.C.; Houde, V.P.; Day, E.A.; Marcinko, K.; Crane, J.D.; Mottillo, E.P.; et al. Salsalate (Salicylate) Uncouples Mitochondria, Improves Glucose Homeostasis, and Reduces Liver Lipids Independent of AMPK-β1. Diabetes 2016, 65, 3352–3361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esser, N.; Paquot, N.; Scheen, A.J. Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease. Expert Opin. Investig. Drugs 2015, 24, 283–307. [Google Scholar] [CrossRef]
- Goldfine, A.B.; Fonseca, V.; Jablonski, K.A.; Pyle, L.; Staten, M.A.; Shoelson, S.E.; TINSAL-T2D (Targeting Inflammation Using Salsalate in Type 2 Diabetes) Study Team. The effects of salsalate on glycemic control in patients with type 2 diabetes: A randomized trial. Ann. Intern. Med. 2010, 152, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Goldfine, A.B.; Fonseca, V.; Jablonski, K.A.; Chen, Y.-D.I.; Tipton, L.; Staten, M.A.; Shoelson, S.E.; Targeting Inflammation Using Salsalate in Type 2 Diabetes Study Team. Salicylate (salsalate) in patients with type 2 diabetes: A randomized trial. Ann. Intern. Med. 2013, 159, 1–12. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, L.A.J. Targeting signal transduction as a strategy to treat inflammatory diseases. Nat. Rev. Drug Discov. 2006, 5, 549–563. [Google Scholar] [CrossRef]
- Everett, B.M.; Donath, M.Y.; Pradhan, A.D.; Thuren, T.; Pais, P.; Nicolau, J.C.; Glynn, R.J.; Libby, P.; Ridker, P.M. Anti-Inflammatory Therapy With Canakinumab for the Prevention and Management of Diabetes. J. Am. Coll. Cardiol. 2018, 71, 2392–2401. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Guo, X.; Zhang, H.; Zhang, X.; Li, Z. The Effect of Diacerein on Type 2 Diabetic Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials with Trial Sequential Analysis. J. Diabetes Res. 2020, 2020, 2593792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belete, T.M. A Recent Achievement in the Discovery and Development of Novel Targets for the Treatment of Type-2 Diabetes Mellitus. J. Exp. Pharmacol. 2020, 12, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Greenbaum, C.; VanBuecken, D.; Lord, S. Disease-Modifying Therapies in Type 1 Diabetes: A Look into the Future of Diabetes Practice. Drugs 2019, 79, 43–61. [Google Scholar] [CrossRef]
- Moran, A.; Bundy, B.; Becker, D.J.; DiMeglio, L.A.; Gitelman, S.E.; Goland, R.; Greenbaum, C.J.; Herold, K.C.; Marks, J.B.; Raskin, P.; et al. Interleukin-1 antagonism in type 1 diabetes of recent onset: Two multicentre, randomised, double-blind, placebo-controlled trials. Lancet Lond. Engl. 2013, 381, 1905–1915. [Google Scholar] [CrossRef] [Green Version]
- Christenhusz, M.J.M.; Byng, J.W. The number of known plants species in the world and its annual increase. Phytotaxa 2016, 261, 201. [Google Scholar] [CrossRef] [Green Version]
- Crockett, S.L.; Robson, N.K.B. Taxonomy and Chemotaxonomy of the Genus Hypericum. Med. Aromat. Plant Sci. Biotechnol. 2011, 5, 1–13. [Google Scholar]
- Monograph. Hypericum perforatum. Altern. Med. Rev. J. Clin. Ther. 2004, 9, 318–325. [Google Scholar]
- Schmidt, M.; Butterweck, V. The mechanisms of action of St. John’s wort: An update. Wien. Med. Wochenschr. 2015, 165, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.; Arnason, J.T.; Roufogalis, B.D. St John’s wort (Hypericum perforatum L.): Botanical, chemical, pharmacological and clinical advances. J. Pharm. Pharmacol. 2019, 71, 1–3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zirak, N.; Shafiee, M.; Soltani, G.; Mirzaei, M.; Sahebkar, A. Hypericum perforatum in the treatment of psychiatric and neurodegenerative disorders: Current evidence and potential mechanisms of action. J. Cell. Physiol. 2019, 234, 8496–8508. [Google Scholar] [CrossRef]
- Oliveira, A.I.; Pinho, C.; Sarmento, B.; Dias, A.C.P. Neuroprotective Activity of Hypericum perforatum and Its Major Components. Front. Plant Sci. 2016, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Apaydin, E.A.; Maher, A.R.; Shanman, R.; Booth, M.S.; Miles, J.N.V.; Sorbero, M.E.; Hempel, S. A systematic review of St. John’s wort for major depressive disorder. Syst. Rev. 2016, 5, 148. [Google Scholar] [CrossRef] [Green Version]
- Napoli, E.; Siracusa, L.; Ruberto, G.; Carrubba, A.; Lazzara, S.; Speciale, A.; Cimino, F.; Saija, A.; Cristani, M. Phytochemical profiles, phototoxic and antioxidant properties of eleven Hypericum species—A comparative study. Phytochemistry 2018, 152, 162–173. [Google Scholar] [CrossRef]
- Bruni, R.; Sacchetti, G. Factors affecting polyphenol biosynthesis in wild and field grown St. John’s Wort (Hypericum perforatum L. Hypericaceae/Guttiferae). Mol. Basel Switz. 2009, 14, 682–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seyis, F.; Yurteri, E.; Özcan, A.; Cirak, C. Altitudinal impacts on chemical content and composition of Hypericum perforatum, a prominent medicinal herb. South Afr. J. Bot. 2020, 135, 391–403. [Google Scholar] [CrossRef]
- Silva, B.A.; Ferreres, F.; Malva, J.O.; Dias, A.C.P. Phytochemical and antioxidant characterization of Hypericum perforatum alcoholic extracts. Food Chem. 2005, 90, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Scotti, F.; Löbel, K.; Booker, A.; Heinrich, M. St. John’s Wort (Hypericum perforatum) Products—How Variable Is the Primary Material? Front. Plant Sci. 2018, 9, 1973. [Google Scholar] [CrossRef] [Green Version]
- Rusalepp, L.; Raal, A.; Püssa, T.; Mäeorg, U. Comparison of chemical composition of Hypericum perforatum and H. maculatum in Estonia. Biochem. Syst. Ecol. 2017, 73, 41–46. [Google Scholar] [CrossRef]
- Cossuta, D.; Vatai, T.; Báthori, M.; Hohmann, J.; Keve, T.; Simándi, B. Extraction of hyperforin and hypericin from st. John’s wort (Hypericum perforatum L.) with different solvents: Extraction of hyperforin and hypericin. J. Food Process Eng. 2012, 35, 222–235. [Google Scholar] [CrossRef]
- Gaid, M.; Biedermann, E.; Füller, J.; Haas, P.; Behrends, S.; Krull, R.; Scholl, S.; Wittstock, U.; Müller-Goymann, C.; Beerhues, L. Biotechnological production of hyperforin for pharmaceutical formulation. Eur. J. Pharm. Biopharm. 2018, 126, 10–26. [Google Scholar] [CrossRef]
- Jucá, M.M.; Cysne Filho, F.M.S.; de Almeida, J.C.; da Silva Mesquita, D.; de Moraes Barriga, J.R.; Dias, K.C.F.; Barbosa, T.M.; Vasconcelos, L.C.; Leal, L.K.A.M.; Ribeiro, J.E.; et al. Flavonoids: Biological activities and therapeutic potential. Nat. Prod. Res. 2020, 34, 692–705. [Google Scholar]
- Almeida, A.F.; Borge, G.I.A.; Piskula, M.; Tudose, A.; Tudoreanu, L.; Valentová, K.; Williamson, G.; Santos, C.N. Bioavailability of Quercetin in Humans with a Focus on Interindividual Variation: Variability in quercetin bioavailability. Compr. Rev. Food Sci. Food Saf. 2018, 17, 714–731. [Google Scholar] [CrossRef] [Green Version]
- Lü, P. Inhibitory effects of hyperoside on lung cancer by inducing apoptosis and suppressing inflammatory response via caspase-3 and NF-κB signaling pathway. Biomed. Pharmacother. Biomed. Pharmacother. 2016, 82, 216–225. [Google Scholar] [CrossRef]
- Wu, W.; Xie, Z.; Zhang, Q.; Ma, Y.; Bi, X.; Yang, X.; Li, B.; Chen, J. Hyperoside Ameliorates Diabetic Retinopathy via Anti-Oxidation, Inhibiting Cell Damage and Apoptosis Induced by High Glucose. Front. Pharmacol. 2020, 11, 797. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.; Shimizu, R.; Kanemaru, M.; Suzuki, Y.; Moriwaki, M.; Mizukami, H. Enzymatically modified isoquercitrin, alpha-oligoglucosyl quercetin 3-O-glucoside, is absorbed more easily than other quercetin glycosides or aglycone after oral administration in rats. Biol. Pharm. Bull. 2009, 32, 2034–2040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crozier, A.; Del Rio, D.; Clifford, M.N. Bioavailability of dietary flavonoids and phenolic compounds. Mol. Aspects Med. 2010, 31, 446–467. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, S.; Wu, Y.; Shen, X.; Xu, S.; Guo, Z.; Zhang, R.; Xing, D. The Physiologic Activity and Mechanism of Quercetin-Like Natural Plant Flavonoids. Curr. Pharm. Biotechnol. 2020, 21, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Shen, S.C.; Lee, W.R.; Hou, W.C.; Yang, L.L.; Lee, T.J. Inhibition of nitric oxide synthase inhibitors and lipopolysaccharide induced inducible NOS and cyclooxygenase-2 gene expressions by rutin, quercetin, and quercetin pentaacetate in RAW 264.7 macrophages. J. Cell. Biochem. 2001, 82, 537–548. [Google Scholar] [CrossRef]
- Singer, A.; Wonnemann, M.; Müller, W.E. Hyperforin, a major antidepressant constituent of St. John’s Wort, inhibits serotonin uptake by elevating free intracellular Na+1. J. Pharmacol. Exp. Ther. 1999, 290, 1363–1368. [Google Scholar]
- Albert, D.; Zündorf, I.; Dingermann, T.; Müller, W.E.; Steinhilber, D.; Werz, O. Hyperforin is a dual inhibitor of cyclooxygenase-1 and 5-lipoxygenase. Biochem. Pharmacol. 2002, 64, 1767–1775. [Google Scholar] [CrossRef]
- Feisst, C.; Pergola, C.; Rakonjac, M.; Rossi, A.; Koeberle, A.; Dodt, G.; Hoffmann, M.; Hoernig, C.; Fischer, L.; Steinhilber, D.; et al. Hyperforin is a novel type of 5-lipoxygenase inhibitor with high efficacy in vivo. Cell. Mol. Life Sci. CMLS 2009, 66, 2759–2771. [Google Scholar] [CrossRef]
- Jensen, A.G.; Hansen, S.H.; Nielsen, E.O. Adhyperforin as a contributor to the effect of Hypericum perforatum L. in biochemical models of antidepressant activity. Life Sci. 2001, 68, 1593–1605. [Google Scholar] [CrossRef]
- Biber, A.; Fischer, H.; Römer, A.; Chatterjee, S.S. Oral bioavailability of hyperforin from hypericum extracts in rats and human volunteers. Pharmacopsychiatry 1998, 31 (Suppl. 1), 36–43. [Google Scholar] [CrossRef]
- Schulz, H.-U.; Schürer, M.; Bässler, D.; Weiser, D. Investigation of the bioavailability of hypericin, pseudohypericin, hyperforin and the flavonoids quercetin and isorhamnetin following single and multiple oral dosing of a hypericum extract containing tablet. Arzneimittelforschung 2005, 55, 15–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chrubasik-Hausmann, S.; Vlachojannis, J.; McLachlan, A.J. Understanding drug interactions with St John’s wort (Hypericum perforatum L.): Impact of hyperforin content. J. Pharm. Pharmacol. 2019, 71, 129–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orth, H.C.; Rentel, C.; Schmidt, P.C. Isolation, purity analysis and stability of hyperforin as a standard material from Hypericum perforatum L. J. Pharm. Pharmacol. 1999, 51, 193–200. [Google Scholar] [CrossRef]
- Gobbi, M.; Moia, M.; Funicello, M.; Riva, A.; Morazzoni, P.; Mennini, T. In vitro effects of the dicyclohexylammonium salt of hyperforin on interleukin-6 release in different experimental models. Planta Med. 2004, 70, 680–682. [Google Scholar] [CrossRef]
- Füller, J.; Kellner, T.; Gaid, M.; Beerhues, L.; Müller-Goymann, C.C. Stabilization of hyperforin dicyclohexylammonium salt with dissolved albumin and albumin nanoparticles for studying hyperforin effects on 2D cultivation of keratinocytes in vitro. Eur. J. Pharm. Biopharm. 2018, 126, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Gartner, M.; Müller, T.; Simon, J.C.; Giannis, A.; Sleeman, J.P. Aristoforin, a novel stable derivative of hyperforin, is a potent anticancer agent. Chembiochem Eur. J. Chem. Biol. 2005, 6, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Kitanov, G.M. Hypericin and pseudohypericin in some Hypericum species. Biochem. Syst. Ecol. 2001, 29, 171–178. [Google Scholar] [CrossRef]
- Jendželovská, Z.; Jendželovský, R.; Kuchárová, B.; Fedoročko, P. Hypericin in the Light and in the Dark: Two Sides of the Same Coin. Front. Plant Sci. 2016, 7, 560. [Google Scholar] [CrossRef] [Green Version]
- Menegazzi, M.; Novelli, M.; Beffy, P.; D’Aleo, V.; Tedeschi, E.; Lupi, R.; Zoratti, E.; Marchetti, P.; Suzuki, H.; Masiello, P. Protective effects of St. John’s wort extract and its component hyperforin against cytokine-induced cytotoxicity in a pancreatic beta-cell line. Int. J. Biochem. Cell Biol. 2008, 40, 1509–1521. [Google Scholar] [CrossRef]
- Novelli, M.; Beffy, P.; Menegazzi, M.; De Tata, V.; Martino, L.; Sgarbossa, A.; Porozov, S.; Pippa, A.; Masini, M.; Marchetti, P.; et al. St. John’s wort extract and hyperforin protect rat and human pancreatic islets against cytokine toxicity. Acta Diabetol. 2014, 51, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Tedeschi, E.; Menegazzi, M.; Margotto, D.; Suzuki, H.; Förstermann, U.; Kleinert, H. Anti-inflammatory actions of St. John’s wort: Inhibition of human inducible nitric-oxide synthase expression by down-regulating signal transducer and activator of transcription-1alpha (STAT-1alpha) activation. J. Pharmacol. Exp. Ther. 2003, 307, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Hammer, K.D.P.; Yum, M.-Y.; Dixon, P.M.; Birt, D.F. Identification of JAK-STAT pathways as important for the anti-inflammatory activity of a Hypericum perforatum fraction and bioactive constituents in RAW 264.7 mouse macrophages. Phytochemistry 2010, 71, 716–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammer, K.D.P.; Hillwig, M.L.; Solco, A.K.S.; Dixon, P.M.; Delate, K.; Murphy, P.A.; Wurtele, E.S.; Birt, D.F. Inhibition of prostaglandin E(2) production by anti-inflammatory hypericum perforatum extracts and constituents in RAW264.7 Mouse Macrophage Cells. J. Agric. Food Chem. 2007, 55, 7323–7331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koeberle, A.; Rossi, A.; Bauer, J.; Dehm, F.; Verotta, L.; Northoff, H.; Sautebin, L.; Werz, O. Hyperforin, an Anti-Inflammatory Constituent from St. John’s Wort, Inhibits Microsomal Prostaglandin E(2) Synthase-1 and Suppresses Prostaglandin E(2) Formation in vivo. Front. Pharmacol. 2011, 2, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordzieke, D.E.; Medraño-Fernandez, I. The Plasma Membrane: A Platform for Intra- and Intercellular Redox Signaling. Antioxid. Basel Switz. 2018, 7, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Hunt, E.J.; Lester, C.E.; Lester, E.A.; Tackett, R.L. Effect of St. John’s wort on free radical production. Life Sci. 2001, 69, 181–190. [Google Scholar] [CrossRef]
- Hardie, D.G. AMPK: A target for drugs and natural products with effects on both diabetes and cancer. Diabetes 2013, 62, 2164–2172. [Google Scholar] [CrossRef] [Green Version]
- Wiechmann, K.; Müller, H.; Fischer, D.; Jauch, J.; Werz, O. The acylphloroglucinols hyperforin and myrtucommulone A cause mitochondrial dysfunctions in leukemic cells by direct interference with mitochondria. Apoptosis Int. J. Program. Cell Death 2015, 20, 1508–1517. [Google Scholar] [CrossRef]
- You, M.-K.; Kim, H.-J.; Kook, J.H.; Kim, H.-A. St. John’s Wort Regulates Proliferation and Apoptosis in MCF-7 Human Breast Cancer Cells by Inhibiting AMPK/mTOR and Activating the Mitochondrial Pathway. Int. J. Mol. Sci. 2018, 19, 966. [Google Scholar] [CrossRef] [Green Version]
- Moore, L.B.; Goodwin, B.; Jones, S.A.; Wisely, G.B.; Serabjit-Singh, C.J.; Willson, T.M.; Collins, J.L.; Kliewer, S.A. St. John’s wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc. Natl. Acad. Sci. USA 2000, 97, 7500–7502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hennessy, M.; Kelleher, D.; Spiers, J.P.; Barry, M.; Kavanagh, P.; Back, D.; Mulcahy, F.; Feely, J. St Johns wort increases expression of P-glycoprotein: Implications for drug interactions. Br. J. Clin. Pharmacol. 2002, 53, 75–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Xie, W. Targeting xenobiotic receptors PXR and CAR for metabolic diseases. Trends Pharmacol. Sci. 2012, 33, 552–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brill, M.J.E.; Diepstraten, J.; van Rongen, A.; van Kralingen, S.; van den Anker, J.N.; Knibbe, C.A.J. Impact of obesity on drug metabolism and elimination in adults and children. Clin. Pharmacokinet. 2012, 51, 277–304. [Google Scholar] [CrossRef]
- Cheng, J.; Shah, Y.M.; Gonzalez, F.J. Pregnane X receptor as a target for treatment of inflammatory bowel disorders. Trends Pharmacol. Sci. 2012, 33, 323–330. [Google Scholar] [CrossRef] [Green Version]
- Rosette, C.; Agan, F.J.; Rosette, N.; Moro, L.; Mazzetti, A.; Hassan, C.; Gerloni, M. Rifamycin SV exhibits strong anti-inflammatory in vitro activity through pregnane X receptor stimulation and NFκB inhibition. Drug Metab. Pharmacokinet. 2019, 34, 172–180. [Google Scholar] [CrossRef]
- Zhou, C.; Tabb, M.M.; Nelson, E.L.; Grün, F.; Verma, S.; Sadatrafiei, A.; Lin, M.; Mallick, S.; Forman, B.M.; Thummel, K.E.; et al. Mutual repression between steroid and xenobiotic receptor and NF-kappaB signaling pathways links xenobiotic metabolism and inflammation. J. Clin. Investig. 2006, 116, 2280–2289. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.; Ke, S.; Liu, D.; Sheng, T.; Thomas, P.E.; Rabson, A.B.; Gallo, M.A.; Xie, W.; Tian, Y. Role of NF-kappaB in regulation of PXR-mediated gene expression: A mechanism for the suppression of cytochrome P-450 3A4 by proinflammatory agents. J. Biol. Chem. 2006, 281, 17882–17889. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.-Y.; Yan, Y.-J.; Li, Y.-H.; Lv, L. Reversing effects of ginsenosides on LPS-induced hepatic CYP3A11/3A4 dysfunction through the pregnane X receptor. J. Ethnopharmacol. 2019, 229, 246–255. [Google Scholar] [CrossRef]
- Lv, C.; Huang, L. Xenobiotic receptors in mediating the effect of sepsis on drug metabolism. Acta Pharm. Sin. B 2020, 10, 33–41. [Google Scholar] [CrossRef]
- Jamwal, R.; de la Monte, S.M.; Ogasawara, K.; Adusumalli, S.; Barlock, B.B.; Akhlaghi, F. Nonalcoholic Fatty Liver Disease and Diabetes Are Associated with Decreased CYP3A4 Protein Expression and Activity in Human Liver. Mol. Pharm. 2018, 15, 2621–2632. [Google Scholar] [CrossRef] [PubMed]
- Gravel, S.; Chiasson, J.-L.; Turgeon, J.; Grangeon, A.; Michaud, V. Modulation of CYP450 Activities in Patients With Type 2 Diabetes. Clin. Pharmacol. Ther. 2019, 106, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Van Dyken, P.; Lacoste, B. Impact of Metabolic Syndrome on Neuroinflammation and the Blood-Brain Barrier. Front. Neurosci. 2018, 12, 930. [Google Scholar] [CrossRef] [PubMed]
- Borshchev, Y.Y.; Uspensky, Y.P.; Galagudza, M.M. Pathogenetic pathways of cognitive dysfunction and dementia in metabolic syndrome. Life Sci. 2019, 237, 116932. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Kumar, M.; Zhu, Y. Protective Effect of Hyperforin on β Amyloid Protein Induced Apoptosis in PC12 Cells and Colchicine Induced Alzheimer’s Disease: An Anti-oxidant and Anti-inflammatory Therapy. J. Oleo Sci. 2018, 67, 1443–1453. [Google Scholar] [CrossRef] [Green Version]
- Dinamarca, M.C.; Cerpa, W.; Garrido, J.; Hancke, J.L.; Inestrosa, N.C. Hyperforin prevents beta-amyloid neurotoxicity and spatial memory impairments by disaggregation of Alzheimer’s amyloid-beta-deposits. Mol. Psychiatry 2006, 11, 1032–1048. [Google Scholar] [CrossRef]
- Dinamarca, M.C.; Arrázola, M.; Toledo, E.; Cerpa, W.F.; Hancke, J.; Inestrosa, N.C. Release of acetylcholinesterase (AChE) from beta-amyloid plaques assemblies improves the spatial memory impairments in APP-transgenic mice. Chem. Biol. Interact. 2008, 175, 142–149. [Google Scholar] [CrossRef]
- Cerpa, W.; Hancke, J.L.; Morazzoni, P.; Bombardelli, E.; Riva, A.; Marin, P.P.; Inestrosa, N.C. The hyperforin derivative IDN5706 occludes spatial memory impairments and neuropathological changes in a double transgenic Alzheimer’s mouse model. Curr. Alzheimer Res. 2010, 7, 126–133. [Google Scholar] [CrossRef]
- Qin, W.; Ho, L.; Pompl, P.N.; Peng, Y.; Zhao, Z.; Xiang, Z.; Robakis, N.K.; Shioi, J.; Suh, J.; Pasinetti, G.M. Cyclooxygenase (COX)-2 and COX-1 potentiate beta-amyloid peptide generation through mechanisms that involve gamma-secretase activity. J. Biol. Chem. 2003, 278, 50970–50977. [Google Scholar] [CrossRef] [Green Version]
- Dash, P.K.; Moore, A.N. Enhanced processing of APP induced by IL-1 beta can be reduced by indomethacin and nordihydroguaiaretic acid. Biochem. Biophys. Res. Commun. 1995, 208, 542–548. [Google Scholar] [CrossRef]
- Brenn, A.; Grube, M.; Jedlitschky, G.; Fischer, A.; Strohmeier, B.; Eiden, M.; Keller, M.; Groschup, M.H.; Vogelgesang, S. St. John’s Wort reduces beta-amyloid accumulation in a double transgenic Alzheimer’s disease mouse model-role of P-glycoprotein. Brain Pathol. Zurich Switz. 2014, 24, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Cirrito, J.R.; Deane, R.; Fagan, A.M.; Spinner, M.L.; Parsadanian, M.; Finn, M.B.; Jiang, H.; Prior, J.L.; Sagare, A.; Bales, K.R.; et al. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J. Clin. Investig. 2005, 115, 3285–3290. [Google Scholar] [CrossRef] [Green Version]
- Chai, A.B.; Leung, G.K.F.; Callaghan, R.; Gelissen, I.C. P-glycoprotein: A role in the export of amyloid-β in Alzheimer’s disease? FEBS J. 2020, 287, 612–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarz, D.; Kisselev, P.; Roots, I. St. John’s wort extracts and some of their constituents potently inhibit ultimate carcinogen formation from benzo[a]pyrene-7,8-dihydrodiol by human CYP1A1. Cancer Res. 2003, 63, 8062–8068. [Google Scholar] [PubMed]
- Lemmen, J.; Tozakidis, I.E.P.; Galla, H.-J. Pregnane X receptor upregulates ABC-transporter Abcg2 and Abcb1 at the blood-brain barrier. Brain Res. 2013, 1491, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Vogelgesang, S.; Cascorbi, I.; Schroeder, E.; Pahnke, J.; Kroemer, H.K.; Siegmund, W.; Kunert-Keil, C.; Walker, L.C.; Warzok, R.W. Deposition of Alzheimer’s beta-amyloid is inversely correlated with P-glycoprotein expression in the brains of elderly non-demented humans. Pharmacogenetics 2002, 12, 535–541. [Google Scholar] [CrossRef]
- Chiu, C.; Miller, M.C.; Monahan, R.; Osgood, D.P.; Stopa, E.G.; Silverberg, G.D. P-glycoprotein expression and amyloid accumulation in human aging and Alzheimer’s disease: Preliminary observations. Neurobiol. Aging 2015, 36, 2475–2482. [Google Scholar] [CrossRef]
- Park, R.; Kook, S.-Y.; Park, J.-C.; Mook-Jung, I. Aβ1-42 reduces P-glycoprotein in the blood-brain barrier through RAGE-NF-κB signaling. Cell Death Dis. 2014, 5, e1299. [Google Scholar] [CrossRef]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 575–590. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [Green Version]
- Alasmari, F.; Ashby, C.R.; Hall, F.S.; Sari, Y.; Tiwari, A.K. Modulation of the ATP-Binding Cassette B1 Transporter by Neuro-Inflammatory Cytokines: Role in the Pathogenesis of Alzheimer’s Disease. Front. Pharmacol. 2018, 9, 658. [Google Scholar] [CrossRef]
- Iqbal, M.; Ho, H.L.; Petropoulos, S.; Moisiadis, V.G.; Gibb, W.; Matthews, S.G. Pro-inflammatory cytokine regulation of P-glycoprotein in the developing blood-brain barrier. PLoS ONE 2012, 7, e43022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dell’Aica, I.; Niero, R.; Piazza, F.; Cabrelle, A.; Sartor, L.; Colalto, C.; Brunetta, E.; Lorusso, G.; Benelli, R.; Albini, A.; et al. Hyperforin blocks neutrophil activation of matrix metalloproteinase-9, motility and recruitment, and restrains inflammation-triggered angiogenesis and lung fibrosis. J. Pharmacol. Exp. Ther. 2007, 321, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, M.S.N.; Cardoso, R.D.R.; Fattori, V.; Arakawa, N.S.; Tomaz, J.C.; Lopes, N.P.; Casagrande, R.; Verri, W.A. Hypericum perforatum Reduces Paracetamol-Induced Hepatotoxicity and Lethality in Mice by Modulating Inflammation and Oxidative Stress. Phytother. Res. PTR 2015, 29, 1097–1101. [Google Scholar] [CrossRef] [PubMed]
- Paterniti, I.; Briguglio, E.; Mazzon, E.; Galuppo, M.; Oteri, G.; Cordasco, G.; Cuzzocrea, S. Effects of Hypericum Perforatum, in a rodent model of periodontitis. BMC Complement. Altern. Med. 2010, 10, 73. [Google Scholar] [CrossRef] [Green Version]
- Zdunić, G.; Godevac, D.; Milenković, M.; Vucićević, D.; Savikin, K.; Menković, N.; Petrović, S. Evaluation of Hypericum perforatum oil extracts for an antiinflammatory and gastroprotective activity in rats. Phytother. Res. PTR 2009, 23, 1559–1564. [Google Scholar] [CrossRef]
- Dost, T.; Ozkayran, H.; Gokalp, F.; Yenisey, C.; Birincioglu, M. The effect of Hypericum perforatum (St. John’s Wort) on experimental colitis in rat. Dig. Dis. Sci. 2009, 54, 1214–1221. [Google Scholar] [CrossRef]
- Hu, Z.-P.; Yang, X.-X.; Chan, S.Y.; Xu, A.-L.; Duan, W.; Zhu, Y.-Z.; Sheu, F.-S.; Boelsterli, U.A.; Chan, E.; Zhang, Q.; et al. St. John’s wort attenuates irinotecan-induced diarrhea via down-regulation of intestinal pro-inflammatory cytokines and inhibition of intestinal epithelial apoptosis. Toxicol. Appl. Pharmacol. 2006, 216, 225–237. [Google Scholar] [CrossRef]
- Menegazzi, M.; Di Paola, R.; Mazzon, E.; Muià, C.; Genovese, T.; Crisafulli, C.; Suzuki, H.; Cuzzocrea, S. Hypericum perforatum attenuates the development of carrageenan-induced lung injury in mice. Free Radic. Biol. Med. 2006, 40, 740–753. [Google Scholar] [CrossRef]
- Di Paola, R.; Mazzon, E.; Muià, C.; Crisafulli, C.; Genovese, T.; Di Bella, P.; Esposito, E.; Menegazzi, M.; Meli, R.; Suzuki, H.; et al. Protective effect of Hypericum perforatum in zymosan-induced multiple organ dysfunction syndrome: Relationship to its inhibitory effect on nitric oxide production and its peroxynitrite scavenging activity. Nitric Oxide Biol. Chem. 2007, 16, 118–130. [Google Scholar] [CrossRef]
- Genovese, T.; Mazzon, E.; Di Paola, R.; Muià, C.; Crisafulli, C.; Menegazzi, M.; Malleo, G.; Suzuki, H.; Cuzzocrea, S. Hypericum perforatum attenuates the development of cerulein-induced acute pancreatitis in mice. Shock Augusta Ga 2006, 25, 161–167. [Google Scholar] [CrossRef]
- Genovese, T.; Mazzon, E.; Menegazzi, M.; Di Paola, R.; Muià, C.; Crisafulli, C.; Bramanti, P.; Suzuki, H.; Cuzzocrea, S. Neuroprotection and enhanced recovery with hypericum perforatum extract after experimental spinal cord injury in mice. Shock Augusta Ga 2006, 25, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, Ü.S.; Nazıroğlu, M.; Şenol, N.; Ghazizadeh, V. Hypericum perforatum Attenuates Spinal Cord Injury-Induced Oxidative Stress and Apoptosis in the Dorsal Root Ganglion of Rats: Involvement of TRPM2 and TRPV1 Channels. Mol. Neurobiol. 2016, 53, 3540–3551. [Google Scholar] [CrossRef] [PubMed]
- De Paola, R.; Muià, C.; Mazzon, E.; Genovese, T.; Crisafulli, C.; Menegazzi, M.; Caputi, A.P.; Suzuki, H.; Cuzzocrea, S. Effects of Hypericum perforatum extract in a rat model of ischemia and reperfusion injury. Shock Augusta Ga 2005, 24, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Cakir, M.; Duzova, H.; Baysal, I.; Gül, C.C.; Kuşcu, G.; Kutluk, F.; Çakin, H.; Şeker, Ş.; İlbeği, E.; Uslu, S.; et al. The effect of hypericum perforatum on kidney ischemia/reperfusion damage. Ren. Fail. 2017, 39, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Uslusoy, F.; Nazıroğlu, M.; Övey, İ.S.; Sönmez, T.T. Hypericum perforatum L. supplementation protects sciatic nerve injury-induced apoptotic, inflammatory and oxidative damage to muscle, blood and brain in rats. J. Pharm. Pharmacol. 2019, 71, 83–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eizirik, D.L.; Moore, F.; Flamez, D.; Ortis, F. Use of a systems biology approach to understand pancreatic beta-cell death in Type 1 diabetes. Biochem. Soc. Trans. 2008, 36, 321–327. [Google Scholar] [CrossRef]
- Kutlu, B.; Cardozo, A.K.; Darville, M.I.; Kruhøffer, M.; Magnusson, N.; Ørntoft, T.; Eizirik, D.L. Discovery of gene networks regulating cytokine-induced dysfunction and apoptosis in insulin-producing INS-1 cells. Diabetes 2003, 52, 2701–2719. [Google Scholar] [CrossRef] [Green Version]
- Barthson, J.; Germano, C.M.; Moore, F.; Maida, A.; Drucker, D.J.; Marchetti, P.; Gysemans, C.; Mathieu, C.; Nuñez, G.; Jurisicova, A.; et al. Cytokines tumor necrosis factor-α and interferon-γ induce pancreatic β-cell apoptosis through STAT1-mediated Bim protein activation. J. Biol. Chem. 2011, 286, 39632–39643. [Google Scholar] [CrossRef] [Green Version]
- Kraus, B.; Wolff, H.; Elstner, E.F.; Heilmann, J. Hyperforin is a modulator of inducible nitric oxide synthase and phagocytosis in microglia and macrophages. Naunyn. Schmiedebergs Arch. Pharmacol. 2010, 381, 541–553. [Google Scholar] [CrossRef]
- Hosogai, N.; Fukuhara, A.; Oshima, K.; Miyata, Y.; Tanaka, S.; Segawa, K.; Furukawa, S.; Tochino, Y.; Komuro, R.; Matsuda, M.; et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 2007, 56, 901–911. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Gao, Z.; He, Q.; Zhou, D.; Guo, Z.; Ye, J. Role of hypoxia in obesity-induced disorders of glucose and lipid metabolism in adipose tissue. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E333–E342. [Google Scholar] [CrossRef] [Green Version]
- Fuster, J.J.; Ouchi, N.; Gokce, N.; Walsh, K. Obesity-Induced Changes in Adipose Tissue Microenvironment and Their Impact on Cardiovascular Disease. Circ. Res. 2016, 118, 1786–1807. [Google Scholar] [CrossRef] [Green Version]
- Di Prospero, N.A.; Artis, E.; Andrade-Gordon, P.; Johnson, D.L.; Vaccaro, N.; Xi, L.; Rothenberg, P. CCR2 antagonism in patients with type 2 diabetes mellitus: A randomized, placebo-controlled study. Diabetes Obes. Metab. 2014, 16, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Menne, J.; Eulberg, D.; Beyer, D.; Baumann, M.; Saudek, F.; Valkusz, Z.; Więcek, A.; Haller, H.; Emapticap Study Group. C-C motif-ligand 2 inhibition with emapticap pegol (NOX-E36) in type 2 diabetic patients with albuminuria. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2017, 32, 307–315. [Google Scholar]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Appari, M.; Channon, K.M.; McNeill, E. Metabolic Regulation of Adipose Tissue Macrophage Function in Obesity and Diabetes. Antioxid. Redox Signal. 2018, 29, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Olefsky, J.M.; Glass, C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010, 72, 219–246. [Google Scholar] [CrossRef] [PubMed]
- Russo, L.; Lumeng, C.N. Properties and functions of adipose tissue macrophages in obesity. Immunology 2018, 155, 407–417. [Google Scholar] [CrossRef]
- Jiao, P.; Ma, J.; Feng, B.; Zhang, H.; Diehl, J.A.; Chin, Y.E.; Yan, W.; Xu, H. FFA-induced adipocyte inflammation and insulin resistance: Involvement of ER stress and IKKβ pathways. Obes. Silver Spring Md 2011, 19, 483–491. [Google Scholar] [CrossRef]
- Huang, S.; Rutkowsky, J.M.; Snodgrass, R.G.; Ono-Moore, K.D.; Schneider, D.A.; Newman, J.W.; Adams, S.H.; Hwang, D.H. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. J. Lipid Res. 2012, 53, 2002–2013. [Google Scholar] [CrossRef] [Green Version]
- Kadowaki, T.; Yamauchi, T.; Kubota, N.; Hara, K.; Ueki, K.; Tobe, K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Investig. 2006, 116, 1784–1792. [Google Scholar] [CrossRef] [Green Version]
- Zoico, E.; Garbin, U.; Olioso, D.; Mazzali, G.; Fratta Pasini, A.M.; Di Francesco, V.; Sepe, A.; Cominacini, L.; Zamboni, M. The effects of adiponectin on interleukin-6 and MCP-1 secretion in lipopolysaccharide-treated 3T3-L1 adipocytes: Role of the NF-kappaB pathway. Int. J. Mol. Med. 2009, 24, 847–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maedler, K.; Sergeev, P.; Ris, F.; Oberholzer, J.; Joller-Jemelka, H.I.; Spinas, G.A.; Kaiser, N.; Halban, P.A.; Donath, M.Y. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J. Clin. Investig. 2002, 110, 851–860. [Google Scholar] [CrossRef]
- Hatano, T.; Sameshima, Y.; Kawabata, M.; Yamada, S.; Shinozuka, K.; Nakabayashi, T.; Mizuno, H. St. John’s wort promotes adipocyte differentiation and modulates NF-κB activation in 3T3-L1 cells. Biol. Pharm. Bull. 2014, 37, 1132–1138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Husain, G.M.; Singh, P.N.; Kumar, V. Beneficial effects of a standardized Hypericum perforatum extract in rats with experimentally induced hyperglycemia. Drug Discov. Ther. 2009, 3, 215–220. [Google Scholar] [PubMed]
- Can, Ö.D.; Öztürk, Y.; Öztürk, N.; Sagratini, G.; Ricciutelli, M.; Vittori, S.; Maggi, F. Effects of treatment with St. John’s Wort on blood glucose levels and pain perceptions of streptozotocin-diabetic rats. Fitoterapia 2011, 82, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Husain, G.M.; Chatterjee, S.S.; Singh, P.N.; Kumar, V. Beneficial effect of Hypericum perforatum on depression and anxiety in a type 2 diabetic rat model. Acta Pol. Pharm. 2011, 68, 913–918. [Google Scholar] [PubMed]
- Arokiyaraj, S.; Balamurugan, R.; Augustian, P. Antihyperglycemic effect of Hypericum perforatum ethyl acetate extract on streptozotocin-induced diabetic rats. Asian Pac. J. Trop. Biomed. 2011, 1, 386–390. [Google Scholar]
- Tian, J.; Tao, R.; Zhang, X.; Liu, Q.; He, Y.-B.; Su, Y.; Ji, T.; Ye, F. Effect of Hypericum perforatum L. extract on insulin resistance and lipid metabolic disorder in high-fat-diet induced obese mice. Phytother. Res. PTR 2015, 29, 86–92. [Google Scholar] [CrossRef] [Green Version]
- Abd El Motteleb, D.M.; Abd El Aleem, D.I. Renoprotective effect of Hypericum perforatum against diabetic nephropathy in rats: Insights in the underlying mechanisms. Clin. Exp. Pharmacol. Physiol. 2017, 44, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Madiraju, A.K.; Alves, T.; Zhao, X.; Cline, G.W.; Zhang, D.; Bhanot, S.; Samuel, V.T.; Kibbey, R.G.; Shulman, G.I. Argininosuccinate synthetase regulates hepatic AMPK linking protein catabolism and ureagenesis to hepatic lipid metabolism. Proc. Natl. Acad. Sci. USA 2016, 113, E3423–E3430. [Google Scholar] [CrossRef] [Green Version]
- Stage, T.B.; Damkier, P.; Christensen, M.M.H.; Nielsen, L.B.-K.; Højlund, K.; Brøsen, K. Impaired Glucose Tolerance in Healthy Men Treated with St. John’s Wort. Basic Clin. Pharmacol. Toxicol. 2016, 118, 219–224. [Google Scholar] [CrossRef] [Green Version]
- Rysä, J.; Buler, M.; Savolainen, M.J.; Ruskoaho, H.; Hakkola, J.; Hukkanen, J. Pregnane X receptor agonists impair postprandial glucose tolerance. Clin. Pharmacol. Ther. 2013, 93, 556–563. [Google Scholar] [CrossRef]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed. Pharmacother. 2019, 112, 108615. [Google Scholar] [CrossRef]
- Altıparmak, M.; Eskitaşçıoğlu, T. Comparison of Systemic and Topical Hypericum Perforatum on Diabetic Surgical Wounds. J. Investig. Surg. 2018, 31, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Farsak, M.; Özdağli, G.; Özmüş, D.; Çömelekoğlu, Ü.; Yalın, S.; Bozdoğan Arpacı, R.; Gen, R.; Kanık, A.; Ümit Talas, D. Effects of Hypericum perforatum on an Experimentally Induced Diabetic Wound in a Rat Model. Wounds Compend. Clin. Res. Pract. 2017, 29, E10–E17. [Google Scholar]
- Iabichella, M.L. The use of an extract of Hypericum perforatum and Azadirachta indica in advanced diabetic foot: An unexpected outcome. BMJ Case Rep. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Yadollah-Damavandi, S.; Chavoshi-Nejad, M.; Jangholi, E.; Nekouyian, N.; Hosseini, S.; Seifaee, A.; Rafiee, S.; Karimi, H.; Ashkani-Esfahani, S.; Parsa, Y.; et al. Topical Hypericum perforatum Improves Tissue Regeneration in Full-Thickness Excisional Wounds in Diabetic Rat Model. Evid. Based Complement. Altern. Med. ECAM 2015, 2015, 245328. [Google Scholar] [CrossRef] [Green Version]
- Wölfle, U.; Seelinger, G.; Schempp, C. Topical Application of St. Johnʼs Wort (Hypericum perforatum). Planta Med. 2013, 80, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Khadem Nezhad, S.; Taghavi Zenouz, A.; Aghazadeh, M.; Samadi Kafil, H. Strong antimicrobial activity of Hypericum perforatum L. against oral isolates of Lactobacillus spp. Cell. Mol. Biol. 2017, 63, 58. [Google Scholar] [CrossRef]
- Chen, H.; Muhammad, I.; Zhang, Y.; Ren, Y.; Zhang, R.; Huang, X.; Diao, L.; Liu, H.; Li, X.; Sun, X.; et al. Antiviral Activity Against Infectious Bronchitis Virus and Bioactive Components of Hypericum perforatum L. Front. Pharmacol. 2019, 10, 1272. [Google Scholar] [CrossRef] [PubMed]
- Saddiqe, Z.; Naeem, I.; Maimoona, A. A review of the antibacterial activity of Hypericum perforatum L. J. Ethnopharmacol. 2010, 131, 511–521. [Google Scholar] [CrossRef]
- Güneş, S.; Tıhmınlıoğlu, F. Hypericum perforatum incorporated chitosan films as potential bioactive wound dressing material. Int. J. Biol. Macromol. 2017, 102, 933–943. [Google Scholar] [CrossRef]
- Gunes, S.; Tamburaci, S.; Tihminlioglu, F. A novel bilayer zein/MMT nanocomposite incorporated with H. perforatum oil for wound healing. J. Mater. Sci. Mater. Med. 2019, 31, 7. [Google Scholar] [CrossRef]
- Galeotti, N. Hypericum perforatum (St John’s wort) beyond depression: A therapeutic perspective for pain conditions. J. Ethnopharmacol. 2017, 200, 136–146. [Google Scholar] [CrossRef]
- Galeotti, N.; Maidecchi, A.; Mattoli, L.; Burico, M.; Ghelardini, C. St. John’s Wort seed and feverfew flower extracts relieve painful diabetic neuropathy in a rat model of diabetes. Fitoterapia 2014, 92, 23–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sindrup, S.H.; Madsen, C.; Bach, F.W.; Gram, L.F.; Jensen, T.S. St. John’s wort has no effect on pain in polyneuropathy. Pain 2001, 91, 361–365. [Google Scholar] [CrossRef]
- Hayes, H.L.; Moss, L.G.; Schisler, J.C.; Haldeman, J.M.; Zhang, Z.; Rosenberg, P.B.; Newgard, C.B.; Hohmeier, H.E. Pdx-1 Activates Islet- and -Cell Proliferation via a Mechanism Regulated by Transient Receptor Potential Cation Channels 3 and 6 and Extracellular Signal-Regulated Kinases 1 and 2. Mol. Cell. Biol. 2013, 33, 4017–4029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef] [Green Version]
- Stanke-Labesque, F.; Gautier-Veyret, E.; Chhun, S.; Guilhaumou, R. Inflammation is a major regulator of drug metabolizing enzymes and transporters: Consequences for the personalization of drug treatment. Pharmacol. Ther. 2020, 215, 107627. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.-C.; Lin, C.-J. The regulation of drug-metabolizing enzymes and membrane transporters by inflammation: Evidences in inflammatory diseases and age-related disorders. J. Food Drug Anal. 2019, 27, 48–59. [Google Scholar] [CrossRef] [PubMed]
- El-Ghiaty, M.A.; Shoieb, S.M.; El-Kadi, A.O.S. Cytochrome P450-mediated drug interactions in COVID-19 patients: Current findings and possible mechanisms. Med. Hypotheses 2020, 144, 110033. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, P.; Cheng, Y.; Wang, P.; Ma, X.; Liu, M.; Wang, X.; Xu, F. Diet-induced obese alters the expression and function of hepatic drug-metabolizing enzymes and transporters in rats. Biochem. Pharmacol. 2019, 164, 368–376. [Google Scholar] [CrossRef]
- Woolsey, S.J.; Mansell, S.E.; Kim, R.B.; Tirona, R.G.; Beaton, M.D. CYP3A Activity and Expression in Nonalcoholic Fatty Liver Disease. Drug Metab. Dispos. Biol. Fate Chem. 2015, 43, 1484–1490. [Google Scholar] [CrossRef] [Green Version]
- Ghose, R.; Omoluabi, O.; Gandhi, A.; Shah, P.; Strohacker, K.; Carpenter, K.C.; McFarlin, B.; Guo, T. Role of high-fat diet in regulation of gene expression of drug metabolizing enzymes and transporters. Life Sci. 2011, 89, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Masiello, P.; Novelli, M.; Beffy, P.; Menegazzi, M. Can Hypericum perforatum (SJW) prevent cytokine storm in COVID-19 patients? Phytother. Res. PTR 2020, 34, 1471–1473. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.; Appendino, G.; Efferth, T.; Fürst, R.; Izzo, A.A.; Kayser, O.; Pezzuto, J.M.; Viljoen, A. Best practice in research—Overcoming common challenges in phytopharmacological research. J. Ethnopharmacol. 2020, 246, 112230. [Google Scholar] [CrossRef]
- Barnes, J.; Anderson, L.A.; Phillipson, J.D. St John’s wort (Hypericum perforatum L.): A review of its chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 2001, 53, 583–600. [Google Scholar] [CrossRef] [Green Version]
- Ng, Q.X.; Venkatanarayanan, N.; Ho, C.Y.X. Clinical use of Hypericum perforatum (St John’s wort) in depression: A meta-analysis. J. Affect. Disord. 2017, 210, 211–221. [Google Scholar] [CrossRef]
- Satia, J.A.; Littman, A.; Slatore, C.G.; Galanko, J.A.; White, E. Associations of herbal and specialty supplements with lung and colorectal cancer risk in the VITamins and Lifestyle study. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1419–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kandel, B.A.; Ekins, S.; Leuner, K.; Thasler, W.E.; Harteneck, C.; Zanger, U.M. No activation of human pregnane X receptor by hyperforin-related phloroglucinols. J. Pharmacol. Exp. Ther. 2014, 348, 393–400. [Google Scholar] [CrossRef] [Green Version]
- Leuner, K.; Kazanski, V.; Müller, M.; Essin, K.; Henke, B.; Gollasch, M.; Harteneck, C.; Müller, W.E. Hyperforin--a key constituent of St. John’s wort specifically activates TRPC6 channels. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2007, 21, 4101–4111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Napoli E 2018 [82] | Bruni R 2009 [83] | Seyis F 2020 [84] | ||
|---|---|---|---|---|
| mg/g Dry Weight | mg/g Dry Weight | mg/g Dry Matter | High Yielding Plant Part | |
| Naphtodianthrones | ||||
| Pseudohypericin | 5.14 | 0.1–12 | 0.05–6.75 | Dark glands in leaf and petal margin; stamens |
| Hypericin | 3.69 | 0.1–7 | 0.01–2.77 | |
| Acylphloroglucinols | ||||
| Hyperforin | 41.0 | 0.3–150 | 2.15–28.1 | Flowering tops; sepals; translucent glands in leaves |
| Adhyperforin | 4.68 | |||
| Flavonoids | ||||
| Catechins | 0.02 | 1.41–8.7 | Floral dehiscent leaves: sepals, stamens, petals. Likely accumulation in vacuoles | |
| Quercetin-3-O-galactoside | 4.34 | |||
| Quercetin-3-O-glucoside | 1.87 | |||
| Quercetin-3-O-rhamnoside | 2.13 | |||
| Quercetin | 0.30 | 0.05–6.04 | ||
| Isoquercitrin | 0.15–6.99 | |||
| Hyperoside | 1–25 | 1.70–22.3 | ||
| Rutin | 0–35 | 0 | ||
| Phenylpropanes | ||||
| Chlorogenic acid | 0.42–10.55 | Flowers and leaves | ||
| Neochlorogenic acid | 0.37–4.25 | |||
| Biflavones | ||||
| Biapigenin | 4.56 | 0.3–10.2 | Trace-2.65 | Floral dehiscent leaves: sepals, stamens, petals. |
| Amentoflavone | 0.18 | 0–1.8 |
| Dosages | Models | Effects | Hypothesized Mechanisms | Refs. |
|---|---|---|---|---|
| SJW standard extract 100, 200 and 300 mg/kg b.w. daily oral administration for 14 days | STZ-NA diabetic rats | Dose-dependent reduction of fasting blood glucose levels | Antioxidant and free radical scavenging properties; stimulation by of muscarinic M3 receptor in β cells and increased insulin release; activation by HPF of TRPC6 cation channels and increased glucose-stimulated insulin secretion. | Husain GM 2009 [188] |
| SJW standard extract 125 or 250 mg/kg b.w. daily i.p. administration for one week | STZ diabetic rats | Dose-dependent decrease in hyperglycemia; restoration of metabolic parameters and improvement of decreased body weights | Can ÖD 2011 [189] | |
| SJW oral suspension in 0.3% carboxy-methyl cellulose 100 and 200 mg/kg b.w. daily for 15 days | High-fat-diet-fed rats Fructose-fed rats | Decrease in plasma glucose and insulin levels; improvement of lipid abnormalities; prevention of weight increase | Reduction of appetite and food intake mediated by serotonin increase. | Husain GM 2011 [190] |
| SJW ethyl acetate extract 50, 100 and 200 mg/kg b.w. daily i.p. administration for 15 days | STZ diabetic rats | Decrease in blood glucose, serum triglycerides and total cholesterol; increase in plasma insulin and muscle and liver glycogen content | Increase of insulin secretion by the remaining β cells; enhanced muscle and liver glycogen content; decline in glucose-6-phosphatase activity and gluconeogenesis. | Arokiyaraj S 2011 [191] |
| SJW extract containing mainly hypericin analogues 50 and 200 mg/kg b.w. daily by gastric gavage for three weeks | High-fat-diet-fed C57BL/6J mice | Improvement of hyperinsulinemia, hyperglycemia, insulin tolerance and dyslipidemia | Increase in insulin sensitivity and fatty acid oxidation through PTP1B inhibition. | Tian J 2015 [192] |
| SJW standard extract 50, 100 and 200 mg/kg b.w. daily by gastric gavage for eight weeks | STZ-NA diabetic rats | Decrease in hyperglycemia and increase in insulinemia; protection against nephropathy | Same mechanisms as in [191] and [192]. | Abd El Motteleb 2017 [193] |
| Hypericin 0.5–2 mg/kg b.w. daily i.p. administration for either 90 or 30 days | High-fat/high-sucrose-fed mice | Prevention in weight gain; decrease in fasting hyperglycemia; improvement of glucose and insulin intolerance. | Reduction of gluco- and lipo-toxicity; improvement in β-cell function; maintenance of β-cell mass; prevention of insulin resistance | Liang C 2019 [16] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novelli, M.; Masiello, P.; Beffy, P.; Menegazzi, M. Protective Role of St. John’s Wort and Its Components Hyperforin and Hypericin against Diabetes through Inhibition of Inflammatory Signaling: Evidence from In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2020, 21, 8108. https://doi.org/10.3390/ijms21218108
Novelli M, Masiello P, Beffy P, Menegazzi M. Protective Role of St. John’s Wort and Its Components Hyperforin and Hypericin against Diabetes through Inhibition of Inflammatory Signaling: Evidence from In Vitro and In Vivo Studies. International Journal of Molecular Sciences. 2020; 21(21):8108. https://doi.org/10.3390/ijms21218108
Chicago/Turabian StyleNovelli, Michela, Pellegrino Masiello, Pascale Beffy, and Marta Menegazzi. 2020. "Protective Role of St. John’s Wort and Its Components Hyperforin and Hypericin against Diabetes through Inhibition of Inflammatory Signaling: Evidence from In Vitro and In Vivo Studies" International Journal of Molecular Sciences 21, no. 21: 8108. https://doi.org/10.3390/ijms21218108
APA StyleNovelli, M., Masiello, P., Beffy, P., & Menegazzi, M. (2020). Protective Role of St. John’s Wort and Its Components Hyperforin and Hypericin against Diabetes through Inhibition of Inflammatory Signaling: Evidence from In Vitro and In Vivo Studies. International Journal of Molecular Sciences, 21(21), 8108. https://doi.org/10.3390/ijms21218108






