Genetic Susceptibility and Protein Expression of Extracellular Matrix Turnover-Related Genes in Oral Submucous Fibrosis
Abstract
:1. Introduction
1.1. Epidemiology and Risk Factor of Oral Submucous Fibrosis (OSMF)
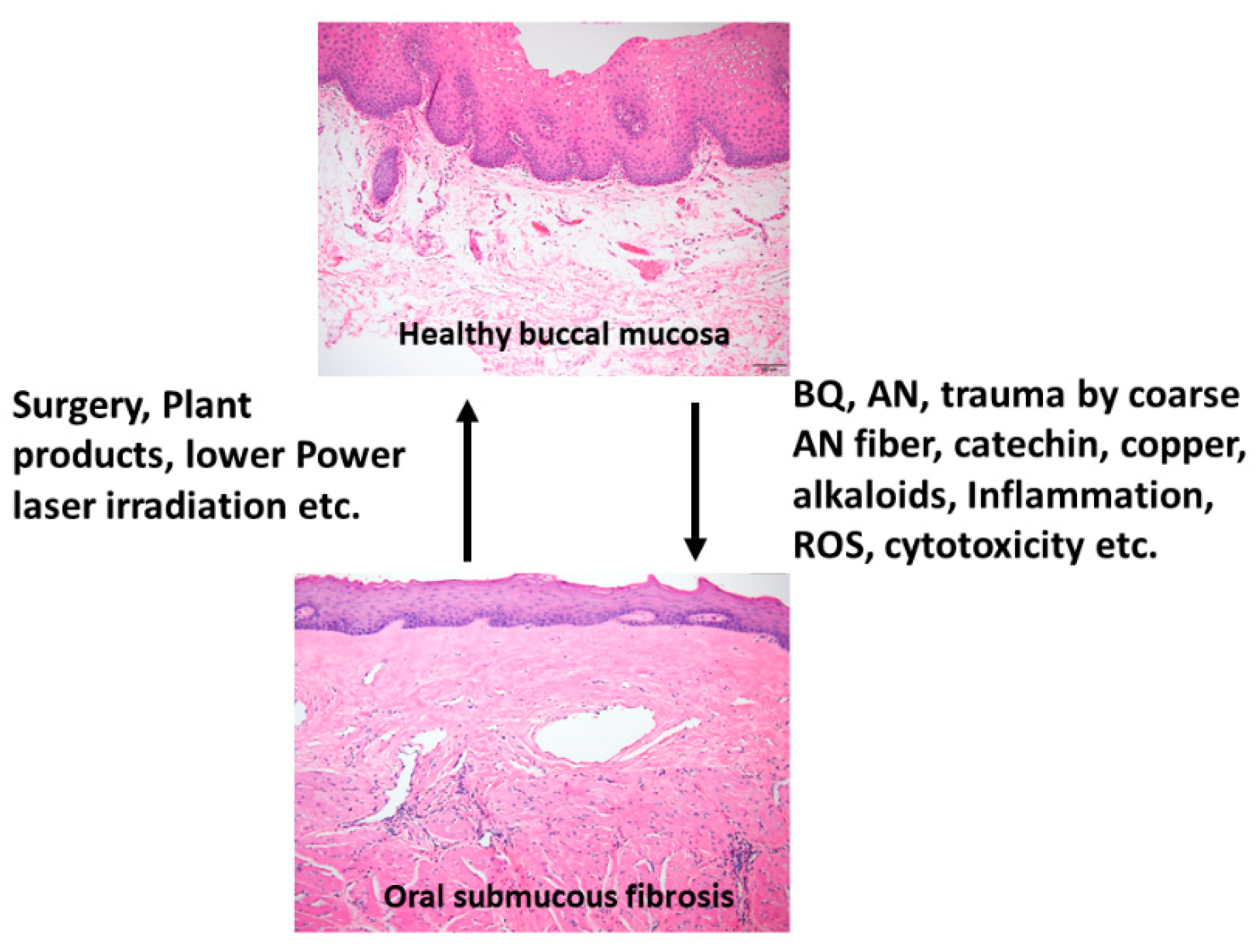
1.2. BQ and OSMF—Etiology, Clinical and Histologic Features
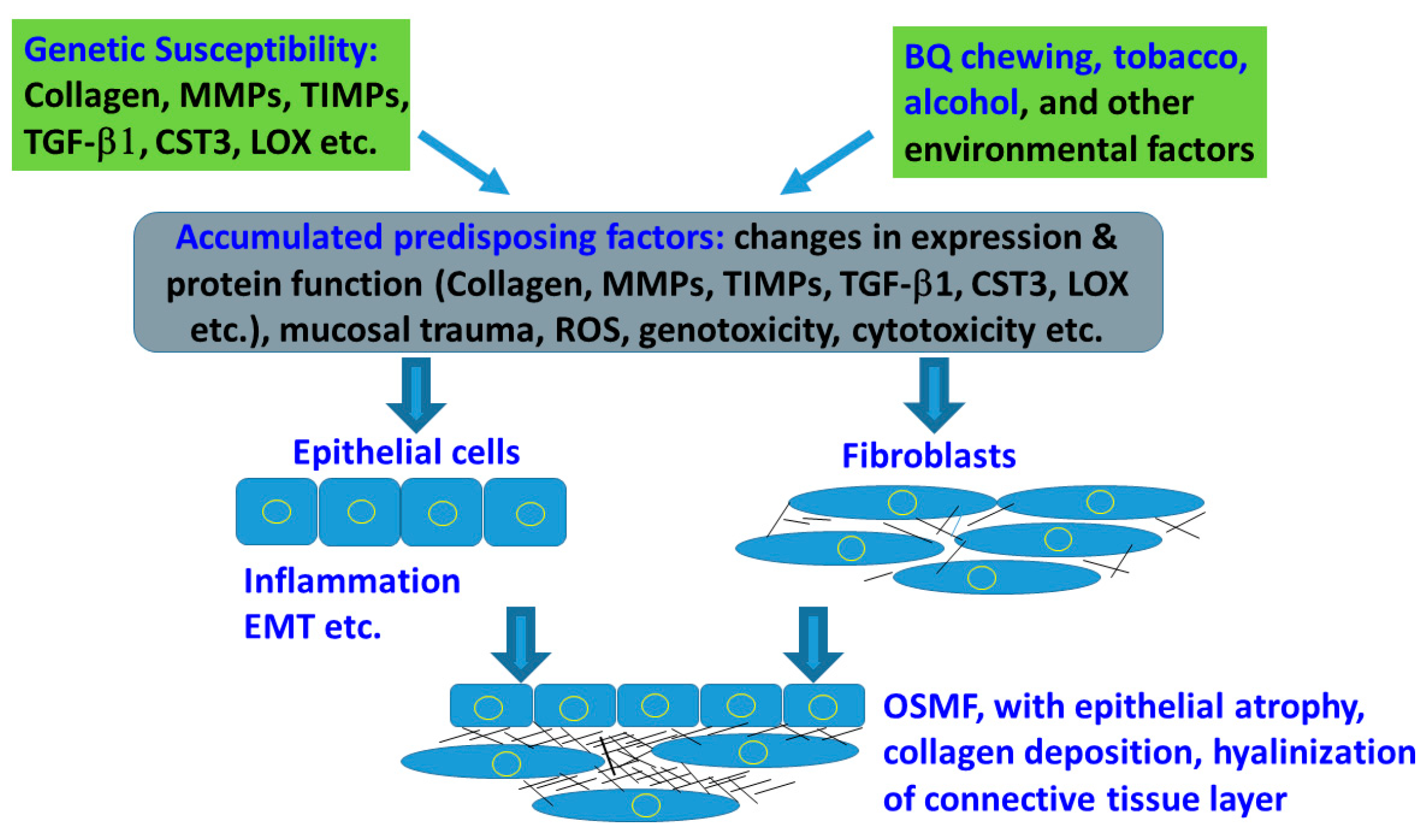
2. Genetic Susceptibility and Expression in Tissue/Organ Fibrosis
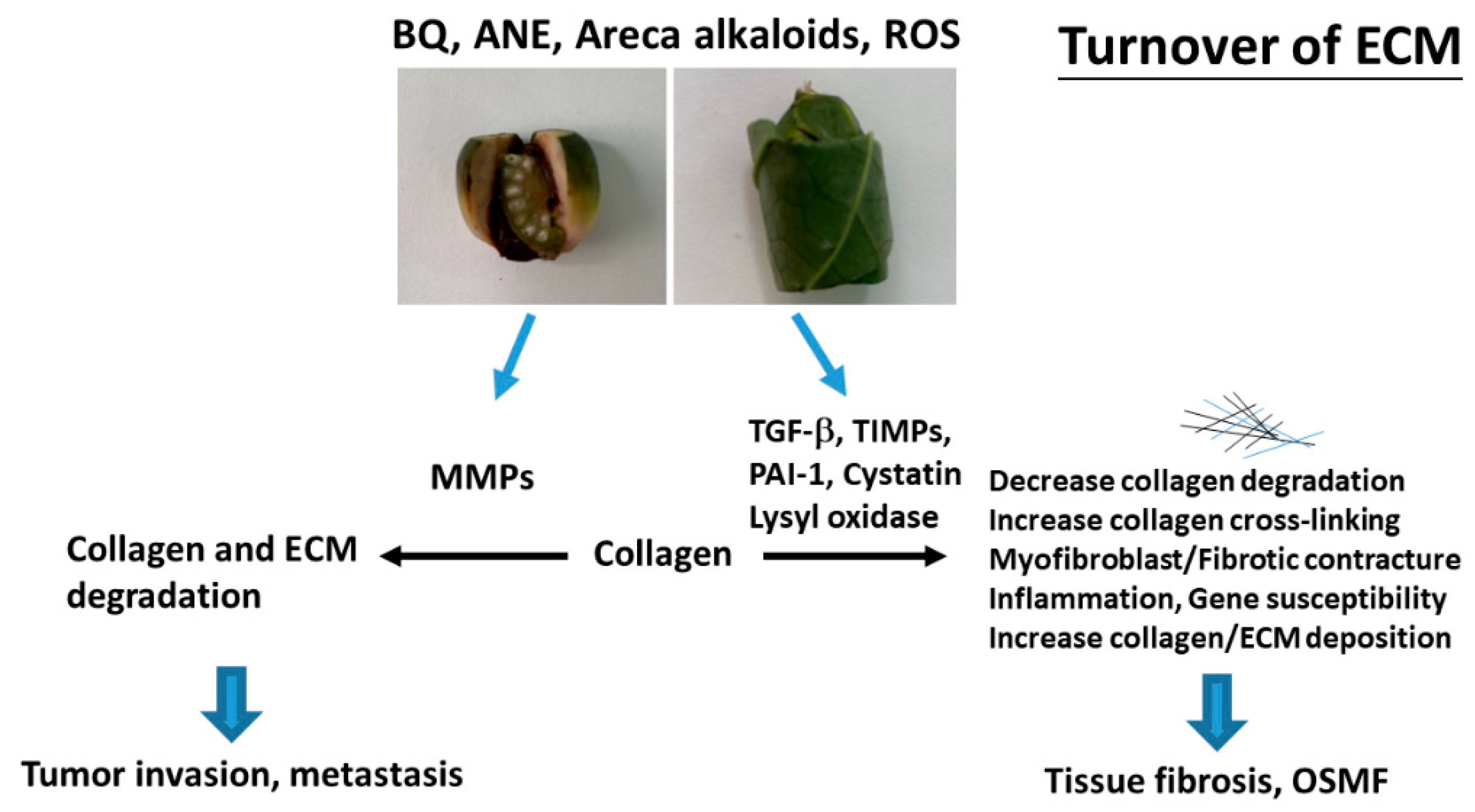
3. BQ and Collagen Turnover
3.1. Collagen-Related Genes
3.2. Role of Collagen 1A1 and Collagen 1A2 (COL1A1 and COL1A2)
4. BQ and MMPs
4.1. Collagenase-1 (COLase, MMP-1)
4.2. MMP-2 and MMP-9 (Gelatinase-A and Gelatinase-B)
4.3. MMP-3 (Stromelysin-1)
5. BQ and TGF-β
6. BQ and Lysyl Oxidase (LOX)
7. BQ and Cystatin C (CST3)
8. BQ and Plasminogen Activator Inhibitor-1 (PAI-1)
9. BQ and TIMPs
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- IARC. Betel-quid and areca-nut chewing and some areca-nut derived nitrosamines. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monogr. Eval. Carcinog. Risks Hum. 2004, 85, 1–334. [Google Scholar]
- Reichart, P.A.; Nguyen, X.H. Betel quid chewing, oral cancer and other oral mucosal diseases in Vietnam: A review. J. Oral Pathol. Med. 2008, 37, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.F.; Wang, Y.H.; Su, N.Y.; Yu, H.C.; Wei, C.Y.; Yu, C.H.; Chang, Y.C. Changes in prevalence of precancerous oral submucous fibrosis from 1996 to 2013 in Taiwan: A nationwide population-based retrospective study. J. Formos. Med. Assoc. 2018, 117, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Chiang, W.F.; Liu, S.Y.; Lin, J.F.; Chiu, S.F.; Gou, S.B.; Chiou, C.T.; Chang, C.H. Malignant development in patients with oral potentially malignant disorders detected through nationwide screening: Outcomes of 5-year follow-up at a single hospital. Head Neck 2020, 42, 67–76. [Google Scholar] [CrossRef]
- Angadi, P.V.; Rekha Krishnapillai, R. Oral submucous fibrosis: A clinicopathologic review of 205 cases in Indians. Oral Maxillofac. Surg. 2011, 15, 15–19. [Google Scholar] [CrossRef]
- Avinash Tejasvi, M.L.; Anulekha, C.K.; Afroze, M.M.; Shenai, K.P.; Chatra, L.; Bhayya, H. A correlation between oral mucosal lesions and various quid-chewing habit patterns: A cross sectional study. J. Cancer Res. Ther. 2019, 15, 620–624. [Google Scholar]
- Jeng, J.H.; Chang, M.C.; Hahn, L.J. Role of areca nut in betel quid-associated chemical carcinogenesis: Current awareness and future perspectives. Oral Oncol. 2001, 37, 477–492. [Google Scholar] [CrossRef]
- Jeng, J.H.; Hahn, L.J.; Lin, B.R.; Hsieh, C.C.; Chan, C.P.; Chang, M.C. Effects of areca nut, inflorescence piper betle extracts and arecoline on cytotoxicity, total and unscheduled DNA synthesis in cultured gingival keratinocytes. J. Oral Pathol. Med. 1999, 28, 64–71. [Google Scholar] [CrossRef]
- Chang, M.C.; Chiang, C.P.; Lin, C.L.; Lee, J.J.; Hahn, L.J.; Jeng, J.H. Cell-mediated immunity and head and neck cancer: With special emphasis on betel quid chewing habit. Oral Oncol. 2005, 41, 757–775. [Google Scholar] [CrossRef]
- Moutasim, K.A.; Jenei, V.; Sapienza, K.; Marsh, D.; Weinreb, P.H.; Violette, S.M.; Lewis, M.P.; Marshall, J.F.; Fortune, F.; Tilakaratne, W.M.; et al. Betel-derived alkaloid up-regulates keratinocyte alphavbeta6 integrin expression and promotes oral submucous fibrosis. J. Pathol. 2011, 223, 366–377. [Google Scholar] [CrossRef]
- Mohiuddin, S.; Fatima, N.; Hosein, S.; Fatima, N. High risk if malignant transformation oral submucous fibrosis in Pakistan females: A potential national disaster. J. Pak. Med. Assoc. 2016, 66, 1362–1366. [Google Scholar] [PubMed]
- Yeh, C.Y.; Lin, C.L.; Chang, M.C.; Chen, H.M.; Kok, S.H.; Chang, S.H.; Kuo, Y.S.; Hahn, L.J.; Chan, C.P.; Lee, J.J.; et al. Differences in oral habit and lymphocyte subpopulation affect malignant transformation of patients with oral precancer. J. Formos. Med. Assoc. 2016, 115, 263–268. [Google Scholar] [CrossRef] [Green Version]
- Tsai, K.Y.; Su, C.C.; Chiang, C.T.; Tseng, Y.T.; Lian, I.B. Environmental heavy metal as a potential risk factor for the progression of oral potentially malignant disorders in central Taiwan. Cancer Epidemiol. 2017, 47, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Speight, P.M.; Khyrram, S.A.; Kujan, O. Oral potentially malignant disorders: Risk of progression to malignancy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 612–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warnakulasuriya, S.; Johnson, N.W.; van der Waal, I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J. Oral Pathol. Med. 2007, 36, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.H.; Wang, T.H.; Shieh, T.M.; Tseng, Y.H. Oral submucous fibrosis: A review on etiopethogenesis, diagnosis and therapy. Int. J. Mol. Sci. 2019, 20, 2940. [Google Scholar] [CrossRef] [Green Version]
- Tilakaratne, W.M.; Ekanayaka, R.P.; Warnakulasuriya, S. Oral submucous fibrosis: A historical perspective and a review on etiology and pathogenesis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 178–191. [Google Scholar] [CrossRef] [Green Version]
- Nair, U.J.; Obe, G.; Friesen, M.; Goldberg, M.T.; Bartsch, H. Role of lime in the generation of reactive oxygen species from betel quid ingredients. Environ. Health Perspect. 1992, 98, 203–205. [Google Scholar] [CrossRef]
- Ranadive, K.J.; Ranadive, S.N.; Shivapurkar, N.M.; Gothoskar, S.V. Betel quid chewing and oral cancer: Experimental studies on hamsters. Int. J. Cancer 1979, 24, 835–843. [Google Scholar] [CrossRef]
- Jin, Y.T.; Tsai, S.T.; Wong, T.Y.; Chen, F.F.; Chen, R.M. Studies on promoting activity of Taiwan betel quid ingredients in hamster buccal pouch carcinogenesis. Eur. J. Cancer B Oral Oncol. 1996, 32B, 343–346. [Google Scholar] [CrossRef]
- Chang, J.Z.; Yang, W.H.; Deng, Y.T.; Chen, H.M.; Kuo, M.Y. Thrombin-stimulated connective tissue growth factor (CTGF/CCN2) production in human buccal mucosal fibroblasts inhibition by epigallocatechin-3-gallate. Head Neck 2002, 34, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Harvey, W.; Scutt, A.; Meghji, S.; Canniff, J.P. Stimulation of human buccal mucosa fibroblasts in vitro by betel-nut alkaloids. Arch. Oral Biol. 1986, 31, 45–49. [Google Scholar] [CrossRef]
- Chang, M.C.; Lin, L.D.; Wu, H.L.; Ho, Y.S.; Hsien, H.C.; Wang, T.M.; Jeng, P.Y.; Cheng, R.H.; Hahn, L.J.; Jeng, J.H. Areca nut-induced buccal mucosa fibroblasts contraction and its signaling: A potential role in oral submucous fibrosis—A precancer condition. Carcinogenesis 2013, 34, 1096–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trivedy, C.; Meghji, S.; Warnakulasuriya, K.A.; Johnson, N.W.; Harris, M. Copper stimulates human oral fibroblasts in vitro: A role in the pathogenesis of oral submucous fibrosis. J. Oral Pathol. Med. 2001, 30, 465–470. [Google Scholar] [CrossRef]
- Jeng, J.H.; Ho, Y.S.; Chan, C.P.; Wang, Y.J.; Hahn, L.J.; Lei, D.; Hsu, C.C.; Chang, M.C. Areca nut extract up-regulates prostaglandin production, cyclooxygenase-2 mRNA and protein expression of human oral keratinocytes. Carcinogenesis. 2000, 21, 1365–1370. [Google Scholar] [CrossRef]
- Scutt, A.; Meghji, S.; Canniff, J.P.; Harvey, W. Stabilization of collagen by betel nut polyphenols as a mechanism in oral submucous fibrosis. Experientia 1987, 43, 391–393. [Google Scholar] [CrossRef]
- Shieh, D.H.; Chiang, L.C.; Lee, C.H.; Yang, Y.H.; Shieh, T.Y. Effects of arecoline, safrole, and nicotine on collagen phagocytosis by human buccal mucosal fibroblasts as a possible mechanism for oral submucous fibrosis in Taiwan. J. Oral Pathol. Med. 2004, 33, 581–587. [Google Scholar] [CrossRef]
- Basyte-Bacevice, V.; Skieceviciene, J.; Valantiene, I.; Sumskiene, J.; Petrenkiene, V.; Kondrackiene, J.; Petrauskas, D.; Lammert, F.; Kupcinskas, J. TM6SF2 and MBOAT7 gene variants in liver fibrosis and cirrhosis. Int. J. Mol. Sci. 2019, 20, 1277. [Google Scholar] [CrossRef] [Green Version]
- Gui, Z.; Li, W.; Fei, S.; Guo, M.; Chen, H.; Sun, L.; Han, Z.; Tao, J.; Ju, X.; Yang, H. Single nucleotide polymorphisms of ubiquitin-related genes were associated with allograft fibrosis of renal transplant fibrosis. Ann. Transplant. 2019, 24, 553–568. [Google Scholar] [CrossRef]
- Mathai, S.K.; Schwartz, D.A.; Warg, L.A. Genetic susceptibility and pulmonary fibrosis. Curr. Opin. Pulm. Med. 2014, 20, 429–435. [Google Scholar] [CrossRef] [Green Version]
- Angiolilli, C.; Marut, W.; van der Kroef, M.; Chouri, E.; Reedquist, K.A.; Radstake, T.R.D.J. New insight into the genetics and epigenetics of systemic sclerosis. Nat. Rev. Rheumatol. 2018, 14, 657–673. [Google Scholar] [CrossRef]
- Rajendran, R. Vidyarani. Familial occurrence of oral submucous fibrosis. Report of eight families from Northern Kerala, South India. Indian J. Dent. Res. 2004, 15, 139–144. [Google Scholar]
- Hande, A.H.; Chaudhart, M.S.; Gawande, M.N.; Gadbail, A.R.; Zade, P.R.; Bajaj, S.; Patil, S.K.; Tekade, S. Oral submucous fibrosis: An enigmatic morpho-insight. J. Cancer Res. Ther. 2019, 15, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Wollina, U.; Verma, S.B.; Ali, F.M.; Patil, K. Oral submucous fibrosis: An update. Clin. Cosmet. Investig. Dermatol. 2015, 8, 193–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theocharis, A.D.; Manou, D.; Karamanos, N.K. The extracellular matrix as a multitasking player in disease. FEBS J. 2019, 286, 2830–2869. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.H.; Xu, H.X.; Wang, W.Q.; Li, S.; Li, H.; Li, T.J.; Zhang, W.H.; Yu, X.J.; Liu, L. The role of collagen in cancer: From bench to bedside. J. Transl. Med. 2019, 17, 309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asanuma, K.; Shirato, I.; Ishidoh, K.; Kominami, E.; Tomino, Y. Selective modulation of the secretion of proteinases and their inhibitors by growth factors in cultured differentiation podocytes. Kidney Int. 2002, 62, 822–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinemeier, K.M.; Olesen, J.L.; Haddad, F.; Langberg, H.; Kjaer, M.; Baldwin, K.M. Expression of collagen and related growth factors in rat tendon and skeletal muscle in response to specific contraction types. J. Physiol. 2007, 582, 1303–1316. [Google Scholar] [CrossRef]
- Patel, K.; Sebastiani, G. Limitations of non-invasive tests for assessment of liver fibrosis. JHEP Rep. 2020, 2, 100067. [Google Scholar] [CrossRef] [Green Version]
- Chiu, C.J.; Chang, M.L.; Chiang, C.P.; Hahn, L.J.; Hsieh, L.L.; Chen, C.J. Interaction of collagen-related genes and susceptibility to betel quid-induced oral submucous fibrosis. Cancer Epidemiol. Biomark. Prev. 2002, 11, 646–653. [Google Scholar]
- Kamath, V.V. The nature of collagen in oral submucous fibrosis: A systematic review of the literature. Saudi. J. Oral Sci. 2014, 1, 57–64. [Google Scholar] [CrossRef]
- Utsunomiya, H.; Tilakaratne, W.M.; Oshiro, K.; Maruyama, S.; Suzuki, M.; Ida-Yonemochi, H.; Cheng, J.; Saku, T. Extracellular matrix remodeling in oral submucous fibrosis: Its stage-specific modes revealed by immunohistochemistry and in situ hybridization. J. Oral Pathol. Med. 2005, 34, 498–507. [Google Scholar] [CrossRef]
- Reichart, P.A.; van Wyk, C.W.; Becker, J.; Schuppan, D. Distribution of procollagen type III, collagen type VI and tenacin in oral submucous fibrosis (OSF). J. Oral Pathol. Med. 1994, 23, 394–398. [Google Scholar] [CrossRef]
- Kaur, J.; Rao, M.; Chakravarti, N.; Mathur, M.; Shukla, N.K.; Sanwal, B.D.; Ralhan, R. Co-expression of colligin and collagen in oral submucous fibrosis: Plausible role in pathogenesis. Oral Oncol. 2001, 37, 282–287. [Google Scholar] [CrossRef]
- Trivedy, C.; Warnakulasuriya, K.A.; Peters, T.J.; Snekus, R.; Hazarey, V.K.; Johnson, N.W. Raised tissue copper levels in oral submucous fibrosis. J. Oral Pathol. Med. 2000, 29, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Kode, M.A.; Karjodkar, F.R. Estimation of the serum and the salivary trace elements in OSMF patients. J. Clin. Diagnostic Res. 2013, 7, 1215–1218. [Google Scholar] [CrossRef] [PubMed]
- Hosthor, S.S.; Mahesh, P.; Priya, S.A.; Sharada, P.; Jyotsna, M.; Chitra, S. Quantitative analysis of serum levels of trace elements in patients with oral submucous fibrosis and oral squamous cell carcinoma: A randomized cross-sectional study. J. Oral Maxillofac. Pathol. 2014, 18, 46–51. [Google Scholar]
- Sharma, A.; Sahni, P.; Nayak, M.T.; Singhvi, A.; Kumar, R. Identification of the pattern of copper as an etiological factor in oral submucous fibrosis: A cytological study. J. Exp. Ther. Oncol. 2014, 10, 317–323. [Google Scholar] [PubMed]
- Mohammed, F.; Manohar, V.; Jose, M.; Thapasum, A.F.; Mohamed, S.; Shamaz, B.H.; D’Souza, N. Estimation of copper in saliva and areca nut products and its correlation with histological grades of oral submucous fibrosis. J. Oral Pathol. Med. 2015, 44, 208–313. [Google Scholar] [CrossRef]
- Wang, T.H.; Hsia, S.M.; Shieh, T.M. Lysyl oxidase and the tumor microenvironment. Int. J. Mol. Sci. 2016, 18, 62. [Google Scholar] [CrossRef] [Green Version]
- Trivedy, C.; Baldwin, D.; Warnakulasuriya, S.; Johnson, N.W.; Peters, T. Copper content in Areca catechu (betel nut) products and oral submucous fibrosis. Lancet 1997, 349, 1447. [Google Scholar] [CrossRef]
- Ovet, H.; Oztay, F. The copper chelator tetrathiomolybdate regressed bleomycin-induced pulmonary fibrosis in mice, by reducing lysyl oxidase expressions. Biol. Trace Elem. Res. 2014, 162, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.Y.; Chen, H.M.; Hahn, L.J.; Hsieh, C.C.; Chiang, C.P. Collagen biosynthesis in human oral submucous fibrosis fibroblast cultures. J. Dent. Res. 1995, 74, 1783–1788. [Google Scholar] [CrossRef]
- Jeng, J.H.; Lan, W.H.; Hahn, L.J.; Hsieh, C.C.; Kuo, M.Y. Inhibition of the migration, attachment, spreading, growth and collagen synthesis of human gingival fibroblasts by arecoline, a major areca alkaloid, in vitro. J. Oral Pathol. Med. 1996, 25, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Krishnakumar, K.; Ramadoss, R.; Krishnan, R.; Sukhija, H. In vitro quantification of collagen and Snail1 gene expression in experimentally induced fibrosis by arecoline and commercial smokeless tobacco products. Asian Pac. J. Cancer Prev. 2020, 21, 1143–1148. [Google Scholar] [CrossRef] [Green Version]
- Arpino, V.; Brock, M.; Gill, S.E. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol. 2015, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Roeb, E. Matrix metalloproteinases and liver fibrosis (translational aspects). Matrix Biol. 2018, 463–473. [Google Scholar] [CrossRef]
- Chaudhary, A.K.; Pandya, S.; Mehrotra, R.; Singh, M.; Singh, M. Role of functional polymorphism of matrix metalloproteinase-2 (-1306 C/T and -168 G/T) and MMP-9 (-1562 C/T) promoter in oral submucous fibrosis and head and neck squamous cell carcinoma in an Indian population. Biomarkers 2011, 16, 577–586. [Google Scholar] [CrossRef]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [Green Version]
- Brinckerhoff, C.E.; Rutter, J.L.; Benbow, U. Interstitial collagenases as markers of tumor progression. Clin. Cancer Res. 2000, 6, 4823–4830. [Google Scholar]
- Kumar, V.; Abbas, A.K.; Fausto, N. Robbins and Cortan Pathologic Basis of Disease, 7th ed.; Saunders: Philadelphia, PA, USA, 2004; pp. 269–342. [Google Scholar]
- Shieh, T.Y.; Yang, J.F. Collagenase activity in oral submucous fibrosis. Proc. Natl. Sci. Counc. Repub. China Part B Life Sci. 1992, 16, 106–110. [Google Scholar]
- Mishra, G.; Ranganathan, K. Matrix metalloproteinase-1 expression in oral submucous fibrosis: An immunohistochemical study. Indian J. Dent. Res. 2010, 21, 320–325. [Google Scholar] [CrossRef]
- Rajendran, R.; Rajeesh, M.P.K.; Shaikh, S.; Pillai, S.M.R. Expression of matrix metalloproteinases (MMP1, MMP-2 and MMP-9) and their inhibitors (TIMP-1 and TIMP-2) in oral submucous fibrosis. Indian J. Dent. Res. 2006, 17, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Liu, S.Y.; Lin, M.H.; Chiang, W.F.; Chen, T.C.; Huang, W.T.; Chou, D.S.; Chiu, C.T.; Liu, Y.C. Upregulation of matrix metalloproteinase-1 (MMP-1) expression in oral carcinomas of betel quid (BQ) users: Roles of BQ ingredients in the acceleration of tumour cell motility through MMP-1. Arch. Oral Biol. 2008, 53, 810–818. [Google Scholar] [CrossRef]
- Lin, S.C.; Chung, M.Y.; Huang, J.W.; Shieh, T.M.; Liu, C.J.; Chang, K.W. Correlation between functional genotypes in the matrix metalloproteinases-1 promoter and risk of oral squamous cell carcinomas. J. Oral Pathol. Med. 2004, 33, 323–326. [Google Scholar] [CrossRef]
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef] [Green Version]
- Nelson, A.R.; Fingleton, B.; Rothenberg, M.L.; Matrisian, L.M. Matrix metalloproteinases: Biologic activity and clinical implications. J. Clin. Oncol. 2000, 18, 1135–1149. [Google Scholar] [CrossRef] [PubMed]
- Price, S.J.; Greaves, D.R.; Watkins, H. Identification of novel, functional genetic variants in the human matrix metalloproteinase-2 gene: Role of Sp1 in allele-specific transcriptional regulation. J. Biol. Chem. 2001, 276, 7549–7558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.C.; Lo, S.S.; Liu, C.J.; Chung, M.Y.; Huang, J.W.; Chang, K.W. Functional genotype in matrix metalloproteinases-2 promoter is a risk factor for oral carcinogenesis. J. Oral Pathol. Med. 2004, 33, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Witty, J.P.; Foster, S.A.; Stricklin, G.P.; Matrisian, L.M.; Stern, P.H. Parathyroid hormone-induced resorption in fetal rat limb bones is associated with production of the metalloproteinases collagenase and gelatinase B. J. Bone Miner. Res. 1996, 11, 72–78. [Google Scholar] [CrossRef]
- Venugopal, A.; Uma Maheswari, T.N. Expression of matrix metalloproteinase-9 in oral potentially malignant disorders: A systemic review. J. Oral Maxillofac. Pathol. 2016, 20, 474–479. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.C.; Yang, S.F.; Tai, K.W.; Chou, M.Y.; Hsieh, Y.S. Increased tissue inhibitor of metalloproteinase-1 expression and inhibition of gelatinase A activity in buccal mucosal fibroblasts by arecoline as possible mechanisms for oral submucous fibrosis. Oral Oncol. 2002, 38, 195–200. [Google Scholar] [CrossRef]
- Chang, M.C.; Chan, C.P.; Wang, W.T.; Chang, B.E.; Lee, J.J.; Tseng, S.K.; Yeung, S.Y.; Hahn, L.J.; Jeng, J.H. Toxicity of areca nut ingredients: Activation of CHK1/CHK2, induction of cell cycle arrest, and regulation of MMP-9 and TIMPs production in SAS epithelial cells. Head Neck 2013, 35, 1295–1302. [Google Scholar] [CrossRef]
- Chang, M.C.; Pan, Y.H.; Wu, H.L.; Lu, Y.J.; Liao, W.C.; Yeh, C.Y.; Lee, J.J.; Jeng, J.H. Stimulation of MMP-9 of oral epithelial cells by areca nut extract is related to TGF-β1/Smad2-dependent and –independent pathways and prevented by betel leaf extract, hydroxychavicol and melatonin. Aging US 2019, 11, 11624–11639. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.; Baber, J.; Fujii, T.; Coito, A.J. Matrix metalloproteinase in liver injury, repair and fibrosis. Matrix Biol. 2015, 147–156. [Google Scholar] [CrossRef]
- Tu, H.F.; Wu, C.H.; Kao, S.Y.; Liu, C.J.; Liu, T.Y.; Lui, M.T. Functional -1562 C-to-T polymorphism in matrix metalloproteinase-9 (MMP-9) promoter is associated with the risk for oral squamous cell carcinoma in younger male areca users. J. Oral Pathol. Med. 2007, 36, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Ye, S. Polymorphism in matrix metalloproteinase gene promoters: Implication in regulation of gene expression and susceptibility of various diseases. Matrix Biol. 2000, 19, 623–639. [Google Scholar] [CrossRef]
- Tu, H.F.; Liu, C.J.; Chang, C.S.; Lui, M.T.; Kao, S.Y.; Chang, C.P.; Liu, T.Y. The functional (-1171 5A-6A) polymorphisms of matrix metalloproteinase 3 gene as a risk factor for oral submucous fibrosis among male areca users. J. Oral Pathol. Med. 2006, 35, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.K.; Singh, M.; Bharti, A.C.; Singh, M.; Shukla, S.; Singh, A.K.; Mehrotra, R. Synergistic effect of stromelysin-1 (matrix metalloproteinase-3) promoter (-1171 5A -> 6A) polymorphism in oral submucous fibrosis and head and neck lesions. BMC Cancer 2010, 10, 369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zade, P.R.; Gosavi, S.R.; Hazarey, V.K.; Ganvir, S.M. Matrix metalloproteinases-3 gene-promoter polymorphism as a risk factor in oral submucous fibrosis in an Indian population: A pilot study. J. Investig. Clin. Dent. 2017, 8, e12228. [Google Scholar] [CrossRef]
- Chen, Q.; Lee, C.E.; Denard, B.; Ye, J. Sustained induction of collagen synthesis by TGF-β requires regulated intramembrane proteolysis of CREB3L1. PLoS ONE 2014, 9, e108528. [Google Scholar] [CrossRef] [PubMed]
- Kale, A.D.; Mane, D.R.; Shukla, D. Expression of transforming growth factor β and its correlation with lipodystrophy in oral submucous fibrosis: An immunohistochemical study. Med. Oral Patol. Oral Cir. Bucal. 2013, 18, e12–e18. [Google Scholar] [CrossRef] [PubMed]
- Thangjam, G.S.; Agarwal, P.; Balapure, A.K.; Rao, S.G.; Kondaiah, P. Regulation of extracellular matrix genes by arecoline in primary gingival fibroblasts requires epithelial factors. J. Periodont. Res. 2009, 44, 736–743. [Google Scholar] [CrossRef]
- Kamath, V.V.; Krishnamurthy, S.; Satelur, K.P.; Rajkumar, K. Transforming growth factor-β1 and TGF-β2 act synergistically in the fibrotic pathway in oral submucous fibrosis: An immunohistochemical observation. Indian J. Med. Paediatr. Oncol. 2015, 36, 111–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Ling, T.; Wu, H. Expression of transforming growth factor beta 1 in keratinocytes of oral submucous fibrosis tissue. Zhonghua Kou Qiang Yi Xue Za Zhi 1997, 32, 239–241. [Google Scholar]
- Das, R.K.; Pal, M.; Barui, A.; Paul, R.R.; Chakraborty, C.; Ray, A.K.; Sengupta, S.; Chatterjee, I. Assessment of malignant potential of oral submucous fibrosis through evaluation of p63, E-cadherin and CD105 expression. J. Clin. Pathol. 2010, 63, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Anura, A.; Das, R.K.; Pal, M.; Paul, R.R.; Ray, A.K.; Chatterjee, J. Correlated analysis of semi-quantitative immunohistochemical features of E-cadherin, VEGF and CD105 in assessing malignant potentiality of oral submucous fibrosis. Pathol. Res. Pract. 2014, 210, 1054–1063. [Google Scholar] [CrossRef]
- Gadbail, A.R.; Chaudhary, M.; Sarode, S.C.; Gondivkar, S.; Tekade, S.A.; Zade, P.; Hande, A.; Sarode, G.S.; Patil, S. Ki67, CD105, and α-SMA expression supports the transformation relevant dysplastic features in the atrophic epithelium of oral submucous fibrosis. PLoS ONE 2018, 13, e0200171. [Google Scholar] [CrossRef]
- Gadbail, A.R.; Chaudhary, M.S.; Sarode, S.C.; Gondivkar, S.M.; Belekar, L.; Mankar-Gadbail, M.P.; Dande, R.; Tekade, S.A.; Yuwanati, M.B.; Patil, S. Ki67, CD105 and α-smooth muscle actin expression in disease progression model of oral submucous fibrosis. J. Investig. Clin. Dent. 2019, 10, e12443. [Google Scholar] [CrossRef]
- Pitiyage, G.N.; Lim, K.P.; Gemenitzidis, E.; The, M.T.; Waseem, A.; Prime, S.S.; Tilakaratne, W.M.; Fortune, F.; Parkinson, E.K. Increased secretion of tissue inhibitors of metalloproteinases 1 and 2 (TIMPs-1 and -2) in fibroblasts are early indicators of oral submucous fibrosis and ageing. J. Oral Pathol. Med. 2012, 41, 454–462. [Google Scholar] [CrossRef]
- Khan, I.; Agarwal, P.; Thangjam, G.S.; Radhesh, R.; Rao, S.G.; Kondaiah, P. Role of TGF-β and BMP7 in the pathogenesis of oral submucous fibrosis. Growth Factors 2011, 29, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Kumar, N.; Pant, I.; Narra, S.; Kondaiah, P. Activation of TGF-β pathway by areca nut constituents: A possible cause of oral submucous fibrosis. PLoS ONE 2012, 7, e51806. [Google Scholar] [CrossRef] [Green Version]
- Kondaiah, P.; Pant, I.; Khan, I. Molecular pathways regulated by areca nut in the etiopathogenesis of oral submucous fibrosis. Periodontology 2000 2019, 80, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Maria, S.; Kamath, V.V.; Satelur, K.; Rajkumar, K. Evaluation of transforming growth factor beta1 gene in oral submucous fibrosis induced in Spraque-Dawley rats by injections of areca nut and pan masala (commercial areca nut product) extracts. J. Cancer Res. Ther. 2016, 12, 379–385. [Google Scholar]
- Zagabathina, S.; Ramadoss, R.; Ah, H.P.; Krishnan, R. Comparative evaluation of SMAD-2 expression in oral submucous fibrosis and reactive oral lesions. Asian Pac. J. Cancer Prev. 2020, 21, 399–403. [Google Scholar] [CrossRef]
- Gupta, S.; Ghosh, S.; Gupta, S.; Sakhuja, P. Effect of curcumin on the expression of p53, transforming growth factor-β and inducible nitric oxide synthase in oral submucous fibrosis: A pilot study. J. Investig. Clin. Dent. 2017, 8. [Google Scholar] [CrossRef]
- Hu, X.; Xiong, H.F.; Wang, W.M.; Huang, L.; Mao, T.; Yang, L.D.; Wang, C.; Huang, D.N.; Wu, J.; Xia, K.; et al. Study on the expression and function of smad family member 7 in oral submucous fibrosis and oral squamous cell carcinoma. Arch. Oral Biol 2020, 112, 104687. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.H.; Chu, P.M.; Hsieh, P.L.; Yang, H.W.; Chueh, P.J.; Huang, Y.F.; Liao, Y.W.; Yu, C.C. Galbridin inhibits the activation of myofibroblasts in human fibrotic buccal mucosal fibroblasts through TGF-β/smad signaling. Environ. Toxicol. 2018, 33, 248–255. [Google Scholar] [CrossRef]
- Samarakoon, R.; Overstreet, J.M.; Higgins, P.J. TGF-β signaling in tissue fibrosis: Redox controls, target genes and therapeutics opportunities. Cell. Signal. 2013, 25, 264–268. [Google Scholar] [CrossRef] [Green Version]
- Rajendran, R.; Harish, R.K.; Anil, S.; Vidyadharan, R.; Banerjee, M. Transforming growth factor-β-1 polymorphisms are infrequent but exist at selected loci in oral submucous fibrosis. Indian J. Dent. Res. 2010, 21, 413–419. [Google Scholar]
- Hsu, H.J.; Yang, Y.H.; Shieh, T.Y.; Chen, C.H.; Kao, Y.H.; Yang, C.F.; Ko, Y.C. Role of cytokine gene (interferon-γ, transforming growth factor-β1, tumor necrosis factor-α, interleukin-6, and interleukin-10) polymorphisms in the risk of oral precancerous lesions in Taiwanese. Kaohsiung J. Med. Sci. 2014, 30, 551–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.J.; Long, J.H.; Wang, X.Y.; Sun, Y. Association of the plasminogen activator inhibitor-1 (PAI-1) gene -675 4G/5G and -844 A/G promoter polymorphism with risk of keloid in Chinese ham population. Med. Sci. Monit. 2014, 20, 2069–2073. [Google Scholar] [PubMed] [Green Version]
- Guo, D.C.; Regalado, E.S.; Gong, L.; Duan, X.; Santos-Cortez, R.L.; Arnaud, P.; Ren, Z.; Cai, B.; Hostetler, E.M.; Moran, R.; et al. LOX mutations predispose to thoracic aortic aneurysms and dissections. Circ. Res. 2016, 118, 928–934. [Google Scholar] [CrossRef] [Green Version]
- Shieh, T.M.; Lin, S.C.; Liu, C.J.; Chang, S.S.; Ku, T.H.; Chang, K.W. Association of expression aberrances and genetic polymorphisms of lysyl oxidase with areca-associated oral tumorigenesis. Clin. Cancer Res. 2007, 13, 4378–4385. [Google Scholar] [CrossRef] [Green Version]
- Chitty, J.L.; Setargew, Y.F.I.; Cox, T.R. Targeting the lysyl oxidases in tumour desmoplasia. Biochem. Soc. Trans. 2019, 47, 1661–1678. [Google Scholar] [CrossRef]
- Chaurasia, A.; Singh, N.; Sahu, D.; Mishra, A. Comparative evaluation of role of lysyl oxidase gene (LOXG473A) expression in pathogenesis and malignant transformation of oral submucous fibrosis. J. Clin. Exp. Dent. 2019, 11, e858–e864. [Google Scholar] [CrossRef]
- Ma, R.H.; Tsai, C.C.; Shieh, T.Y. Increased lysyl oxidase activity in fibroblasts cultured from oral submucous fibrosis associated with betel nut chewing in Taiwan. J. Oral Pathol. Med. 1995, 24, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Tilakaratne, W.M.; Klinikowski, M.F.; Saku, T.; Peters, T.J.; Warnakulasuriya, S. Oral submucous fibrosis: Review on aetiology and pathogenesis. Oral Oncol. 2006, 42, 561–568. [Google Scholar] [CrossRef]
- Shieh, T.M.; Tu, H.F.; Ku, T.H.; Chang, S.S.; Chang, K.W.; Liu, C.J. Association between lysyl oxidase polymorphisms and oral submucous fibrosis in older male areca chewers. J. Oral Pathol. Med. 2009, 38, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Thorawat, A.; Nandimath, K.; Hiremath, S.; Naikmasur, V.G. Molecular screening of lysyl oxidase G473A polymorphisms in oral submucous fibrosis. J. Oral Maxillofac. Pathol. 2014, 18, 207–210. [Google Scholar] [CrossRef] [Green Version]
- Eley, B.M.; Cox, S.W. Advanced in periodontal diagnosis 6. Proteolytic and hydrolytic enzymes of inflammatory cell origin. Br. Dent. J. 1998, 184, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.A.; Kim, D.H.; Oh, K.H.; Han, S.Y.; Han, K.H. The role of cathepsin B in peritoneal fibrosis due to peritoneal dialysis. Int. J. Nephrol. 2019, 2019, 4150656. [Google Scholar] [CrossRef] [PubMed]
- Lalmanach, G.; Saidi, A.; Marchand-Adam, S.; Lecaille, F.; Kasabova, M. Cysteine cathepsin and cystatins: From ancillary tasks to prominent status in lung diseases. Biol. Chem. 2015, 396, 111–130. [Google Scholar] [CrossRef] [PubMed]
- Breznik, B.; Mitrovic, A.; Lah, T.T.; Kos, J. Cystatins in cancer progression: More than just cathepsin inhibitors. Biochimie 2019, 166, 233–250. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.H.; Yang, S.F.; Chang, Y.C. The upregulation of cystatin C in oral submucous fibrosis. Oral Oncol. 2007, 43, 680–685. [Google Scholar]
- Vigneswaran, N.; Wu, J.; Zacharias, W.G. Upregulation of cystatin M during the progression of oropharyngeal squamous cell carcinoma from primary tumor to metastasis. Oral Oncol. 2003, 39, 559–568. [Google Scholar] [CrossRef]
- Carnielli, C.M.; Macedo, C.C.S.; De Rossi, T.; Granato, D.C.; Rivera, C.; Domingues, R.R.; Pauletti, B.A.; Yokoo, S.; Heberle, H.; Busso-Lopes, A.F.; et al. Combining discovery and targeted proteomics reveals a prognostic signature in oral cancer. Nat. Commun. 2018, 9, 3598. [Google Scholar] [CrossRef]
- Flevaris, P.; Vaughan, D. The role of plasminogen activator inhibitor type-1 in fibrosis. Semin. Thromb. Hemost. 2017, 43, 169–177. [Google Scholar] [CrossRef]
- Samarakoon, R.; Higgins, S.P.; Higgins, C.E.; Higgins, P.J. The TGF-β1/p53/PAI-1 signaling axis in vascular senescence: Role of caveolin-1. Biomolecules 2019, 9, 341. [Google Scholar] [CrossRef] [Green Version]
- Samarakoon, R.; Higgins, P.J. Integration of non-SMAD and SMAD signaling in TGF-beta1-induced plasminogen activator inhibitor type-1 gene expression in vascular smooth muscle cells. Thromb. Haemost. 2008, 100, 976–983. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.F.; Hsieh, Y.S.; Tsai, C.H.; Chou, M.Y.; Chang, Y.C. The upregulation of type I plasminogen activator inhibitor in oral submucous fibrosis. Oral Oncol. 2003, 39, 367–372. [Google Scholar] [CrossRef]
- Yang, S.F.; Hsieh, Y.S.; Tsai, C.H.; Chen, Y.J.; Chang, Y.C. Increased plasminogen activator inhibitor/tissue type plasminogen activator ratio in oral submucous fibrosis. Oral Dis. 2007, 13, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.H.; Lee, S.S.; Chang, Y.C. Hypoxic regulation of plasminogen activator inhibitor-1 expression in human buccal mucosa fibroblasts stimulated with arecoline. J. Oral Pathol. Med. 2015, 44, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.F.; Yu, G.T.; Wang, W.M.; Liu, B.; Sun, Z.J. Prognostic and predictive values of SPP1, PAI and caveolin-1 in patients with oral squamous cell carcinoma. Int. J. Clin. Exp. Pathol. 2014, 7, 6032–6039. [Google Scholar] [PubMed]
- Li, X.X.; Li, N.; Ban, C.J.; Zhu, M.; Xiao, B.; Dai, H.P. Idiopathic pulmonary fibrosis in relation to gene polymorphisms of transforming growth factor-β1 and plasminogen activator inhibitor 1. Chin. Med. J. 2011, 124, 1923–1927. [Google Scholar] [PubMed]
- El Edel, R.H.; Essa, E.S.; Essa, A.S.; Hegazy, S.A.; El Rowedy, D.I. Serum PAI-1 and PAI-1 4G/5G polymorphism in hepatitis C virus-induced cirrhosis and hepatitis C virus-induced hepatocellular carcinoma patients. Viral Immunol. 2016, 29, 510–515. [Google Scholar] [CrossRef]
- Vairaktaris, E.; Serefoglou, Z.; Avgoustidis, D.; Yapijakis, C.; Critselis, E.; Vylliotis, A.; Spyridonidou, S.; Derka, S.; Vassiliou, S.; Nkenke, E.; et al. Gene polymorphisms related to angiogenesis, inflammation and thrombosis that influence risk for oral cancer. Oral Oncol. 2009, 45, 247–253. [Google Scholar] [CrossRef]
- Vylliotis, A.; Yapijakis, C.; Nkenke, E.; Nisyrios, T.; Avgoustidis, D.; Adamopoulou, M.; Ragos, V.; Vassiliou, S.; Koronellos, N.; Vairakraris, E. Effect of thrombosis-related gene polymorphisms upon oral cancer: A regression analysis. Anticancer Res. 2013, 33, 4033–4039. [Google Scholar]
- Shrestha, A.; Carnelio, S. Evaluation of matrix metalloproteinases-2 (MMP-2) and tissue inhibitors of metalloproteinases-2 (TIMP-2) in oral submucous fibrosis and their correlation with disease severity. Kathmandu Univ. Med. J. 2013, 11, 274–281. [Google Scholar] [CrossRef]
- Shieh, D.H.; Chiang, L.C.; Shieh, T.Y. Augmented mRNA expression of tissue inhibitor of metalloproteinase-1 in buccal mucosal fibroblasts by arecoline and safrole as a possible pathogenesis for oral submucous fibrosis. Oral Oncol. 2003, 39, 728–735. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, R.-H.; Wang, Y.-P.; Chang, J.Y.-F.; Pan, Y.-H.; Chang, M.-C.; Jeng, J.-H. Genetic Susceptibility and Protein Expression of Extracellular Matrix Turnover-Related Genes in Oral Submucous Fibrosis. Int. J. Mol. Sci. 2020, 21, 8104. https://doi.org/10.3390/ijms21218104
Cheng R-H, Wang Y-P, Chang JY-F, Pan Y-H, Chang M-C, Jeng J-H. Genetic Susceptibility and Protein Expression of Extracellular Matrix Turnover-Related Genes in Oral Submucous Fibrosis. International Journal of Molecular Sciences. 2020; 21(21):8104. https://doi.org/10.3390/ijms21218104
Chicago/Turabian StyleCheng, Ru-Hsiu, Yi-Ping Wang, Julia Yu-Fong Chang, Yu-Hwa Pan, Mei-Chi Chang, and Jiiang-Huei Jeng. 2020. "Genetic Susceptibility and Protein Expression of Extracellular Matrix Turnover-Related Genes in Oral Submucous Fibrosis" International Journal of Molecular Sciences 21, no. 21: 8104. https://doi.org/10.3390/ijms21218104




