Blood Profile of Cytokines, Chemokines, Growth Factors, and Redox Biomarkers in Response to Different Protocols of Treadmill Running in Rats
Abstract
:1. Introduction
2. Results
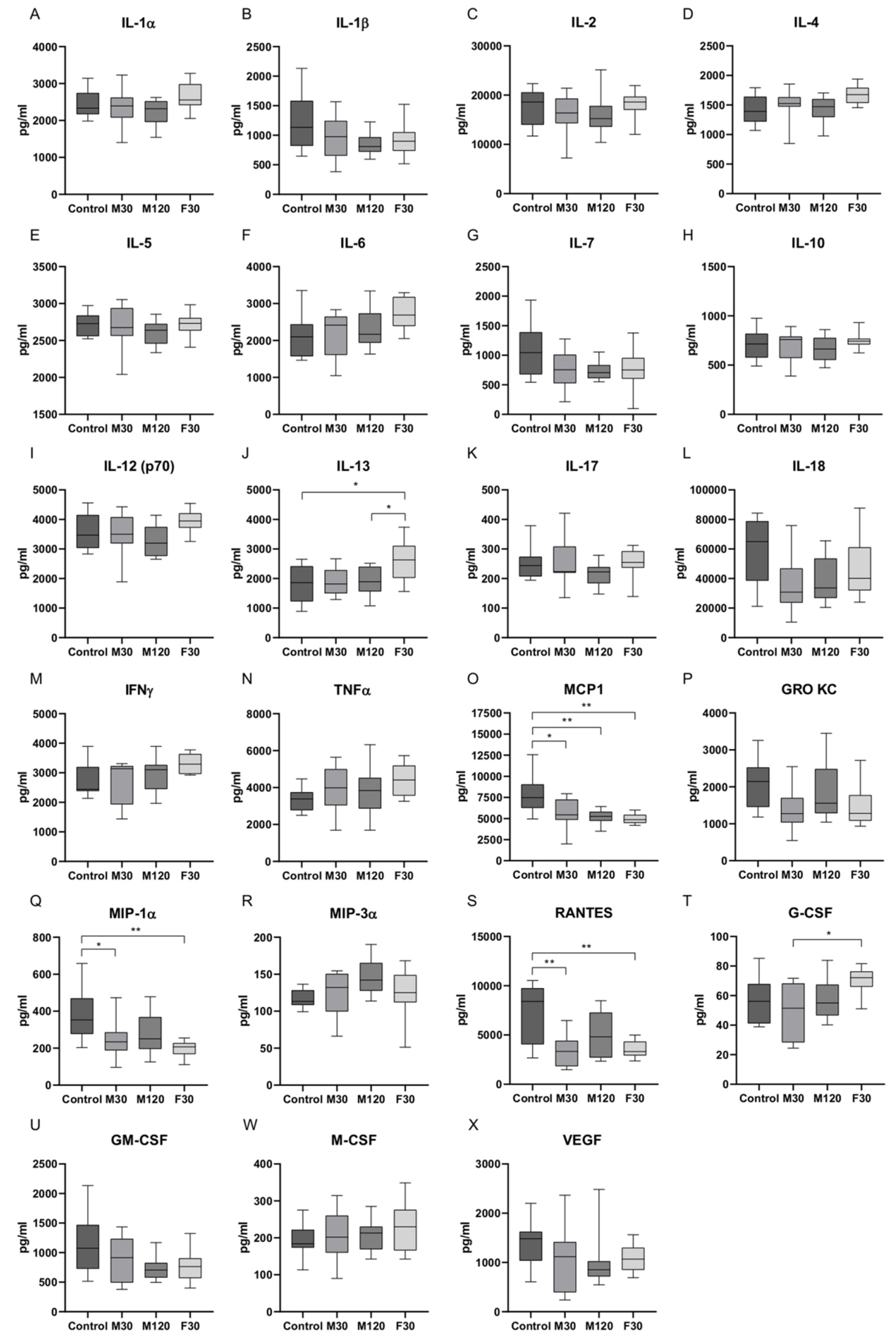
2.1. Serum Concentrations of Cytokines in Response to Treadmill Running
2.2. Enzymatic and Non-Enzymatic Antioxidants, Total Antioxidant/Oxidant Status, Oxidative Damage Products, and Nitrosative Stress in Plasma or Serum of Rats Subjected to Treadmill Running
2.3. Plasma Content and Composition of Free Fatty Acids (FFA) in Rats Subjected to Treadmill Running
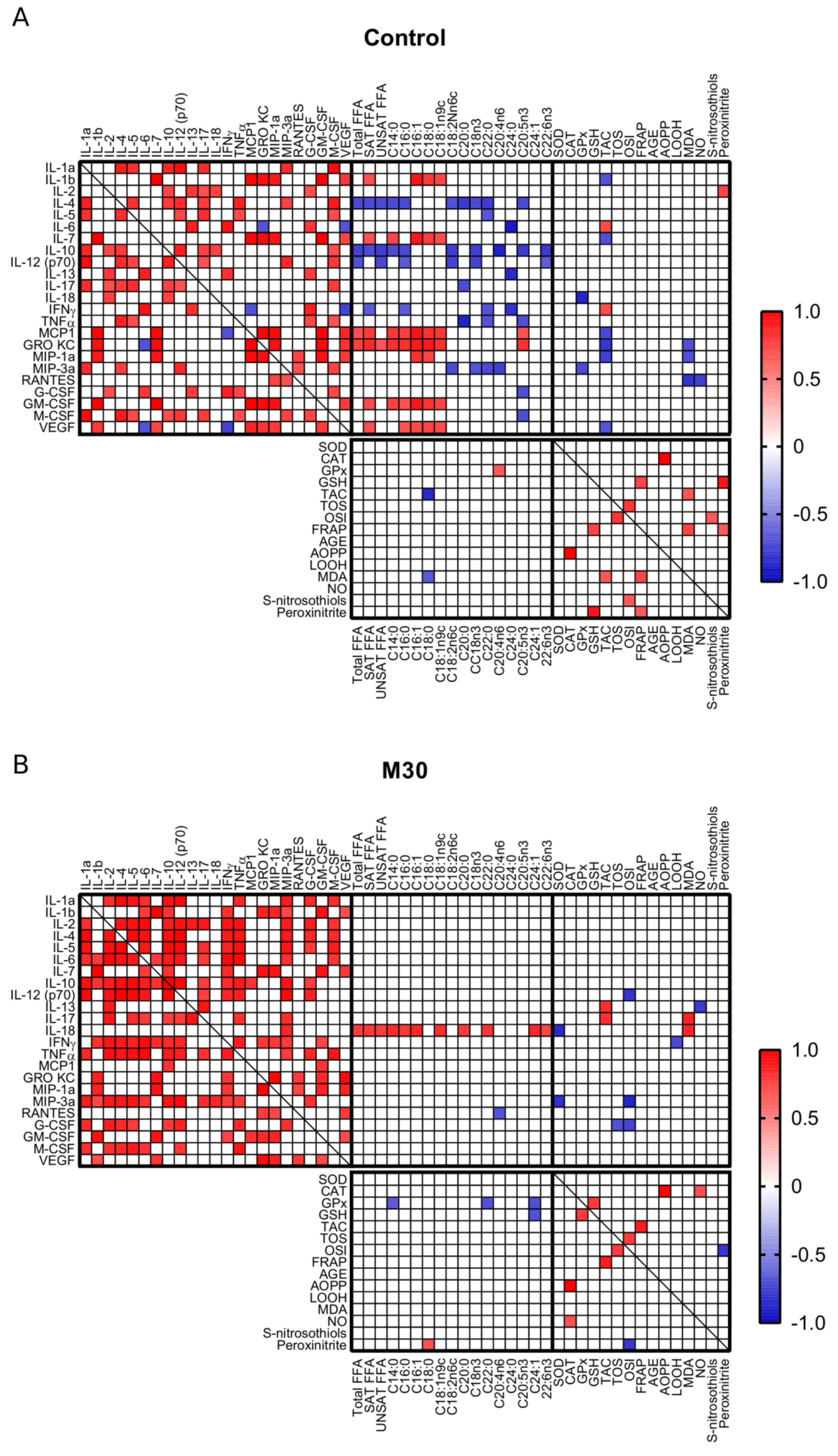
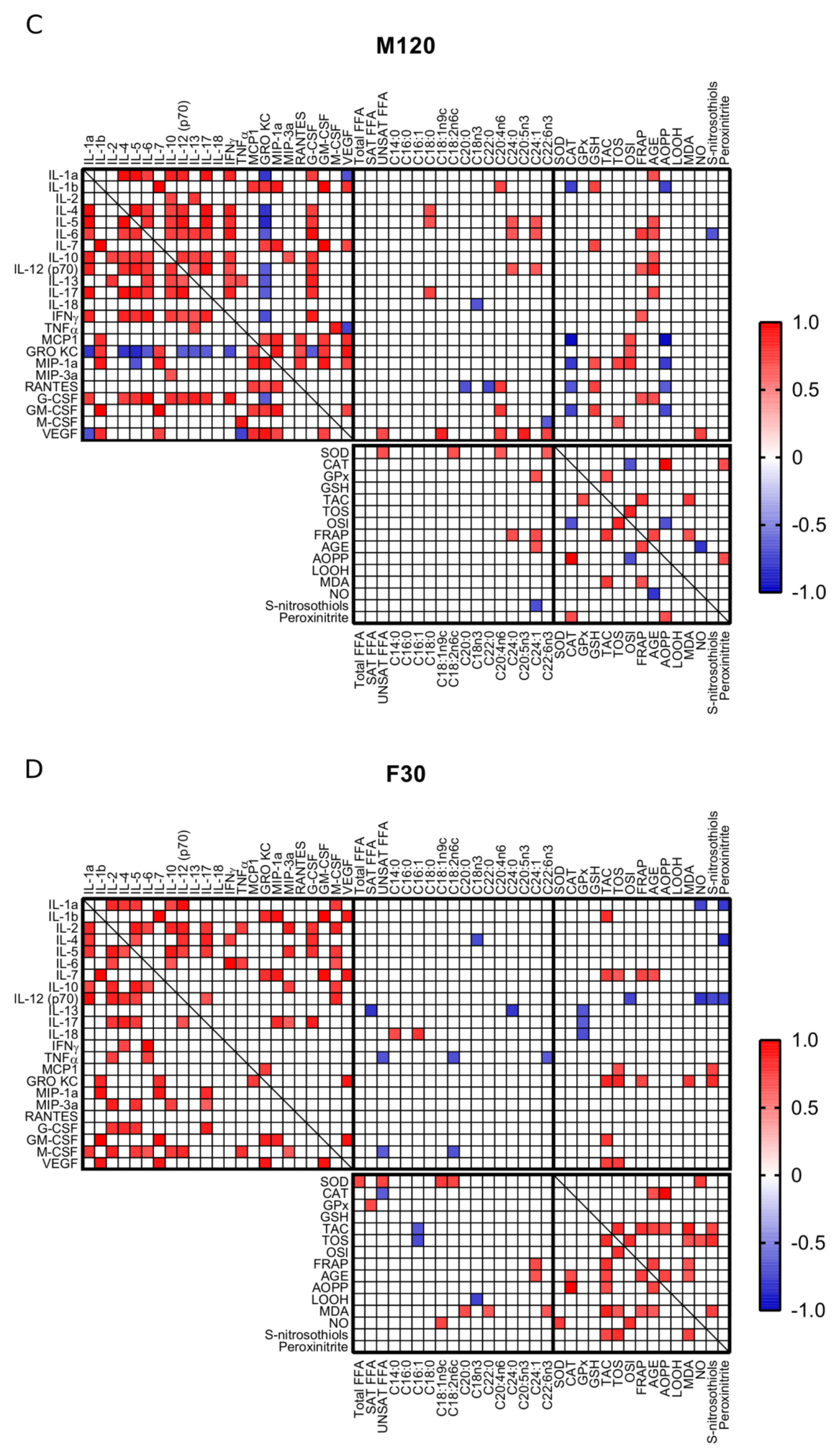
2.4. Correlations
3. Discussion
4. Materials and Methods
4.1. Animal Experiments
- (1)
- Sedentary control.
- (2)
- Rats running on a treadmill set at +10° incline at the speed of 18 m/min for 30 min (M30).
- (3)
- Rats running on a treadmill set at +10° incline at the speed of 18 m/min for 120 min (M120).
- (4)
- Rats running on a treadmill set at +10° incline at the speed of 28 m/min for 30 min (F30).
4.2. Cytokine/Chemokine Detection in Serum by ELISA Multiplex
4.3. Determination of Redox Biomarkers
4.3.1. Antioxidant Barrier
4.3.2. Redox Status
4.3.3. Oxidative Damage Products
4.3.4. Nitrosative Stress
4.4. Plasma Free Fatty Acid Content and Composition
4.5. Data Analysis and Statistics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AGE | advanced glycation end products |
| AMPK | AMP-activated protein kinase |
| AOPP | advanced oxidation protein products |
| CAT | catalase |
| FOXO3a | forkhead box O3a |
| FRAP | ferric reducing ability of plasma |
| G-CSF | granulocyte colony-stimulating factor |
| GM-CSF | granulocyte-macrophage colony stimulating factor |
| GPx | glutathione peroxidase |
| GRO KC/CXCL1 | growth-regulated oncogene/keratinocyte chemoattractant/chemokine (C-X-C motif) ligand 1 |
| GSH | reduced glutathione |
| HO-1 | heme oxygenase-1 |
| IFNγ | interferon γ |
| IL | interleukin |
| IL-1ra | interleukin-1 receptor antagonist |
| JNK | c-Jun N-terminal kinases |
| Km | Michaelis–Menten constant |
| LOOH | lipid hydroperoxides |
| MAA | malondialdehyde acetaldehyde |
| MAPK | mitogen activated protein kinase |
| MCP1/CCL2 | monocyte chemoattractant protein 1/chemokine (C-C motif) ligand 2 |
| M-CSF | macrophage colony-stimulating factor |
| MDA | malondialdehyde |
| MIP-1α/CCL3 | macrophage inflammatory protein-1α/chemokine (C-C motif) ligand 3 |
| MIP-3α/CCL20 | macrophage inflammatory protein-3α/chemokine (C-C motif) ligand 20 |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NEFA | non-esterified fatty acids |
| NF-κB | nuclear factor κB |
| NO | nitric oxide |
| NOS | nitric oxide synthase |
| Nrf2 | nuclear factor-like 2 |
| RAGE | receptor for advanced glycation end products |
| RANTES/CCL5 | regulated on activation, normal T-cell expressed and secreted/chemokine (C-C motif) ligand 5 |
| SOD | superoxide dismutase |
| TNFα | tumor necrosis factor α |
| VEGF | vascular endothelial growth factor |
References
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.I.; Bourgeois, J.M.; Nederveen, J.P.; Leite, M.R.; Hettinga, B.P.; Bujak, A.L.; May, L.; Lin, E.; Crozier, M.; Rusiecki, D.R.; et al. Lifelong aerobic exercise protects against inflammaging and cancer. PLoS ONE 2019, 14, e0210863. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Ishihara, K.; Tekus, E.; Varga, C.; Posa, A.; Balogh, L.; Boldogh, I.; Koltai, E. Exercise, oxidants, and antioxidants change the shape of the bell-shaped hormesis curve. Redox Biol. 2017, 12, 285–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiraly, M.A.; Campbell, J.; Park, E.; Bates, H.E.; Yue, J.T.Y.; Rao, V.; Matthews, S.G.; Bikopoulos, G.; Rozakis-Adcock, M.; Giacca, A.; et al. Exercise maintains euglycemia in association with decreased activation of c-Jun NH2-terminal kinase and serine phosphorylation of IRS-1 in the liver of ZDF rats. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E671–E682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, K.; Tominaga, T.; Ruhee, R.T.; Ma, S. Characterization and Modulation of Systemic Inflammatory Response to Exhaustive Exercise in Relation to Oxidative Stress. Antioxidants 2020, 9, 401. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, K.; Nakaji, S.; Yamada, M.; Totsuka, M.; Sato, K.; Sugawara, K. Systemic inflammatory response to exhaustive exercise. Cytokine kinetics. Exerc. Immunol. Rev. 2002, 8, 6–48. [Google Scholar] [PubMed]
- Varamenti, E.; Tod, D.; Pullinger, S.A. Redox Homeostasis and Inflammation Responses to Training in Adolescent Athletes: A Systematic Review and Meta-analysis. Sports Med. Open 2020, 6, 1–17. [Google Scholar] [CrossRef]
- Yavari, A.; Javadi, M.; Mirmiran, P.; Bahadoran, Z. Exercise-Induced Oxidative Stress and Dietary antioxidants. Asian J. Sports Med. 2015, 6, e24898. [Google Scholar] [CrossRef] [Green Version]
- Bulua, A.C.; Simon, A.; Maddipati, R.; Pelletier, M.; Park, H.; Kim, K.-Y.; Sack, M.N.; Kastner, D.L.; Siegel, R.M. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J. Exp. Med. 2011, 208, 519–533. [Google Scholar] [CrossRef]
- Zalewska, A.; Szarmach, I.; Żendzian-Piotrowska, M.; Maciejczyk, M. The Effect of N-Acetylcysteine on Respiratory Enzymes, ADP/ATP Ratio, Glutathione Metabolism, and Nitrosative Stress in the Salivary Gland Mitochondria of Insulin Resistant Rats. Nutrients 2020, 12, 458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, S.; Tominaga, T.; Kanda, K.; Sugama, K.; Omae, C.; Hashimoto, S.; Aoyama, K.; Yoshikai, Y.; Suzuki, K. Effects of an 8-Week Protein Supplementation Regimen with Hyperimmunized Cow Milk on Exercise-Induced Organ Damage and Inflammation in Male Runners: A Randomized, Placebo Controlled, Cross-Over Study. Biomedicines 2020, 8, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zińczuk, J.; Maciejczyk, M.; Zaręba, K.; Pryczynicz, A.; Dymicka-Piekarska, V.; Kamińska, J.; Koper-Lenkiewicz, O.; Matowicka-Karna, J.; Kędra, B.; Zalewska, A.; et al. Pro-Oxidant Enzymes, Redox Balance and Oxidative Damage to Proteins, Lipids and DNA in Colorectal Cancer Tissue. Is Oxidative Stress Dependent on Tumour Budding and Inflammatory Infiltration? Cancers 2020, 12, 1636. [Google Scholar] [CrossRef]
- Klimiuk, A.; Zalewska, A.; Sawicki, R.; Knapp, M.; Maciejczyk, M. Salivary Oxidative Stress Increases with the Progression of Chronic Heart Failure. J. Clin. Med. 2020, 9, 769. [Google Scholar] [CrossRef] [Green Version]
- Kaspar, F.; Jelinek, H.F.; Perkins, S.; Al-Aubaidy, H.A.; DeJong, B.; Butkowski, E. Acute-Phase Inflammatory Response to Single-Bout HIIT and Endurance Training: A Comparative Study. Mediat. Inflamm. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Duzova, H.; Karakoc, Y.; Emre, M.H.; Dogan, Z.Y.; Kilinc, E. Effects of Acute Moderate and Strenuous Exercise Bouts on IL-17 Production and Inflammatory Response in Trained Rats. J. Sports Sci. Med. 2009, 8, 219–224. [Google Scholar]
- Bloomer, R.J.; Falvo, M.J.; Fry, A.C.; Schilling, B.K.; Smith, W.A.; Moore, C.A. Oxidative Stress Response in Trained Men following Repeated Squats or Sprints. Med. Sci. Sports Exerc. 2006, 38, 1436–1442. [Google Scholar] [CrossRef] [Green Version]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, K. Chronic Inflammation as an Immunological Abnormality and Effectiveness of Exercise. Biomolecules 2019, 9, 223. [Google Scholar] [CrossRef] [Green Version]
- He, F.; Li, J.; Liu, Z.; Chuang, C.-C.; Yang, W.; Zuo, L. Redox Mechanism of Reactive Oxygen Species in Exercise. Front. Physiol. 2016, 7, 486. [Google Scholar] [CrossRef] [Green Version]
- Mika, A.; Macaluso, F.; Barone, R.; Di Felice, V.; Sledzinski, T. Effect of Exercise on Fatty Acid Metabolism and Adipokine Secretion in Adipose Tissue. Front. Physiol. 2019, 10, 26. [Google Scholar] [CrossRef] [Green Version]
- Mikłosz, A.; Łukaszuk, B.; Baranowski, M.; Chabowski, A.; Górski, J. Assessment of the Main Compounds of the Lipolytic System in Treadmill Running Rats: Different Response Patterns between the Right and Left Ventricle. Int. J. Mol. Sci. 2019, 20, 2556. [Google Scholar] [CrossRef] [Green Version]
- Simon, M.C.; Bilan, S.; Nowotny, B.; Dickhaus, T.; Burkart, V.; Schloot, N.C. Fatty acids modulate cytokine and chemokine secretion of stimulated human whole blood cultures in diabetes. Clin. Exp. Immunol. 2013, 172, 383–393. [Google Scholar] [CrossRef]
- Nassar, A.L.D.S.; Marot, L.P.; Ovidio, P.P.; De Castro, G.S.F.; Jordão, A.A. Oxidative stress and fatty acid profile in Wistar rats subjected to acute food restriction and refeeding with high-fat diets. Acta Cir. Bras. 2014, 29, 178–185. [Google Scholar] [CrossRef] [Green Version]
- Little, H.C.; Tan, S.Y.; Cali, F.M.; Rodríguez, S.; Lei, X.; Wolfe, A.; Hug, C.; Wong, G.W. Multiplex Quantification Identifies Novel Exercise-regulated Myokines/Cytokines in Plasma and in Glycolytic and Oxidative Skeletal Muscle. Mol. Cell. Proteom. 2018, 17, 1546–1563. [Google Scholar] [CrossRef] [Green Version]
- Ostrowski, K.; Rohde, T.; Asp, S.; Schjerling, P.; Pedersen, B.K. Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J. Physiol. 1999, 515, 287–291. [Google Scholar] [CrossRef]
- JanssenDuijghuijsen, L.M.; Keijer, J.; Mensink, M.; Lenaerts, K.; Ridder, L.; Nierkens, S.; Kartaram, S.W.; Verschuren, M.C.M.; Pieters, R.H.H.; Bas, R.; et al. Adaptation of exercise-induced stress in well-trained healthy young men. Exp. Physiol. 2017, 102, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Terink, R.; Bongers, C.C.W.G.; Witkamp, R.F.; Mensink, M.; Eijsvogels, T.M.; Klein Gunnewiek, J.M.T.; Hopman, M.T.E. Changes in cytokine levels after prolonged and repeated moderate intensity exercise in middle-aged men and women. Transl. Sports Med. 2018, 1, 110–119. [Google Scholar] [CrossRef] [Green Version]
- Vasconcelos, E.D.S.; Salla, R.F. Role of interleukin-6 and interleukin-15 in exercise. MOJ Immunol. 2018, 6, 11–13. [Google Scholar] [CrossRef]
- Cleto, L.S.; Oleto, A.F.; Sousa, L.P.; Barreto, T.O.; Cruz, J.S.; Penaforte, C.L.; Magalhães, J.C.; Sousa-Franco, J.; Pinto, K.M.C.; Campi-Azevedo, A.C.; et al. Plasma cytokine response, lipid peroxidation and NF-κB activation in skeletal muscle following maximumprogressive swimming. Braz. J. Med Biol. Res. 2011, 44, 546–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, K. Characterization of Exercise-Induced Cytokine Release, the Impacts on the Body, the Mechanisms and Modulations. Int. J. Sports Exerc. Med. 2019, 5. [Google Scholar] [CrossRef] [Green Version]
- Steensberg, A.; Febbraio, M.A.; Osada, T.; Schjerling, P.; Van Hall, G.; Saltin, B.; Pedersen, B.K. Interleukin-6 production in contracting human skeletal muscle is influenced by pre-exercise muscle glycogen content. J. Physiol. 2001, 537, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, N.H.; Stanya, K.J.; Hyde, A.L.; Chalom, M.M.; Alexander, R.K.; Liou, Y.-H.; Starost, K.A.; Gangl, M.R.; Jacobi, D.; Liu, S.; et al. Interleukin-13 drives metabolic conditioning of muscle to endurance exercise. Science 2020, 368. [Google Scholar] [CrossRef]
- Lin, S.; Hou, J.; Xiang, F.; Zhang, X.; Che, L.; Lin, Y.; Xu, S.; Wu, D.; Fang, Z. Reproductive stage associated changes in plasma fatty acid profile and proinflammatory cytokine expression in rat mammary glands. Anim. Nutr. 2016, 2, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Ruhee, R.T.; Ma, S.; Suzuki, K. Protective Effects of Sulforaphane on Exercise-Induced Organ Damage via Inducing Antioxidant Defense Responses. Antioxidants 2020, 9, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinberg, J.G.; Ba, A.; Brégeon, F.; Delliaux, S.; Jammes, Y. Cytokine and Oxidative Responses to Maximal Cycling Exercise in Sedentary Subjects. Med. Sci. Sports Exerc. 2007, 39, 964–968. [Google Scholar] [CrossRef]
- Xiao, N.-N. Effects of Resveratrol Supplementation on Oxidative Damage and Lipid Peroxidation Induced by Strenuous Exercise in Rats. Biomol. Ther. 2015, 23, 374–378. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.H.; Lee, Y.R.; Park, H.G.; Lee, W.L. The effects of either resveratrol or exercise on macrophage infiltration and switching from M1 to M2 in high fat diet mice. J. Exerc. Nutr. Biochem. 2015, 19, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Huang, D.; Ransohoff, R.M.; Zhou, L. Acute skeletal muscle injury: CCL2 expression by both monocytes and injured muscle is required for repair. FASEB J. 2011, 25, 3344–3355. [Google Scholar] [CrossRef] [Green Version]
- Sell, H.; Dietze-Schroeder, D.; Kaiser, U.; Eckel, J. Monocyte Chemotactic Protein-1 Is a Potential Player in the Negative Cross-Talk between Adipose Tissue and Skeletal Muscle. Endocrinology 2006, 147, 2458–2467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catoire, M.; Mensink, M.; Kalkhoven, E.; Schrauwen, P.; Kersten, S. Identification of human exercise-induced myokines using secretome analysis. Physiol. Genom. 2014, 46, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.M.; Suzuki, K.; Hordern, M.; Wilson, G.; Nosaka, K.; Coombes, J.S. Plasma cytokine changes in relation to exercise intensity and muscle damage. Eur. J. Appl. Physiol. 2005, 95, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Trøseid, M.; Lappegård, K.T.; Claudi, T.; Damås, J.K.; Mørkrid, L.; Brendberg, R.; Mollnes, T.E. Exercise reduces plasma levels of the chemokines MCP-1 and IL-8 in subjects with the metabolic syndrome. Eur. Hear. J. 2004, 25, 349–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leggate, M.; Carter, W.G.; Evans, M.J.C.; Vennard, R.A.; Sribala-Sundaram, S.; Nimmo, M.A. Determination of inflammatory and prominent proteomic changes in plasma and adipose tissue after high-intensity intermittent training in overweight and obese males. J. Appl. Physiol. 2012, 112, 1353–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baturcam, E.; Abubaker, J.; Tiss, A.; Abu-Farha, M.; Khadir, A.; Al-Ghimlas, F.; Al-Khairi, I.; Cherian, P.; Elkum, N.; Hammad, M.; et al. Physical Exercise Reduces the Expression of RANTES and Its CCR5 Receptor in the Adipose Tissue of Obese Humans. Mediat. Inflamm. 2014, 2014. [Google Scholar] [CrossRef]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta Mol. Cell Res. 2014, 1843, 2563–2582. [Google Scholar] [CrossRef] [Green Version]
- Azizbeigi, K.; Stannard, S.R.; Atashak, S.; Haghighi, M.M. Antioxidant enzymes and oxidative stress adaptation to exercise training: Comparison of endurance, resistance, and concurrent training in untrained males. J. Exerc. Sci. Fit. 2014, 12, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Nocella, C.; Cammisotto, V.; Pigozzi, F.; Borrione, P.; Fossati, C.; D’Amico, A.; Cangemi, R.; Peruzzi, M.; Gobbi, G.; Ettorre, E.; et al. Impairment between Oxidant and Antioxidant Systems: Short- and Long-term Implications for Athletes’ Health. Nutrients 2019, 11, 1353. [Google Scholar] [CrossRef] [Green Version]
- Lushchak, V.I. Classification of oxidative stress based on its intensity. EXCLI J. 2014, 13, 922–937. [Google Scholar]
- Świderska, M.; Maciejczyk, M.; Zalewska, A.; Pogorzelska, J.; Flisiak, R.; Chabowski, A. Oxidative stress biomarkers in the serum and plasma of patients with non-alcoholic fatty liver disease (NAFLD). Can plasma AGE be a marker of NAFLD? Oxidative stress biomarkers in NAFLD patients. Free. Radic. Res. 2019, 53, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Pietarinen-Runtti, P.; Lakari, E.; Raivio, K.O.; Kinnula, V.L. Expression of antioxidant enzymes in human inflammatory cells. Am. J. Physiol. Cell Physiol. 2000, 278, C118–C125. [Google Scholar] [CrossRef]
- Prigol, M.; Luchese, C.; Nogueira, C.W. Antioxidant effect of diphenyl diselenide on oxidative stress caused by acute physical exercise in skeletal muscle and lungs of mice. Cell Biochem. Funct. 2009, 27, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, C.G.; Qi, Z.; Cui, D.; Ding, S. Acute Exercise Induced Mitochondrial H2O2 Production in Mouse Skeletal Muscle: Association with p66Shcand FOXO3a Signaling and Antioxidant Enzymes. Oxidative Med. Cell. Longev. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Gottfredsen, R.H.; Larsen, U.G.; Enghild, J.J.; Petersen, S.V. Hydrogen peroxide induce modifications of human extracellular superoxide dismutase that results in enzyme inhibition. Redox Biol. 2013, 1, 24–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tryfidou, D.V.; McClean, C.; Nikolaidis, M.G.; Davison, G.W. DNA Damage Following Acute Aerobic Exercise: A Systematic Review and Meta-analysis. Sports Med. 2020, 50, 103–127. [Google Scholar] [CrossRef] [Green Version]
- Powers, S.K.; Jackson, M.J. Exercise-Induced Oxidative Stress: Cellular Mechanisms and Impact on Muscle Force Production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choromańska, B.; Myśliwiec, P.; Łuba, M.; Wojskowicz, P.; Myśliwiec, H.; Choromańska, K.; Żendzian-Piotrowska, M.; Dadan, J.; Zalewska, A.; Maciejczyk, M. Impact of Weight Loss on the Total Antioxidant/Oxidant Potential in Patients with Morbid Obesity—A Longitudinal Study. Antioxidants 2020, 9, 376. [Google Scholar] [CrossRef]
- Catalá, A. Five Decades with Polyunsaturated Fatty Acids: Chemical Synthesis, Enzymatic Formation, Lipid Peroxidation and Its Biological Effects. J. Lipids 2013, 2013, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Nam, T.-G. Lipid Peroxidation and Its Toxicological Implications. Toxicol. Res. 2011, 27, 1–6. [Google Scholar] [CrossRef]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, H.; Khan, M.F. Differential oxidative modification of proteins in MRL+/+ and MRL/lpr mice: Increased formation of lipid peroxidation-derived aldehyde-protein adducts may contribute to accelerated onset of autoimmune response. Free Radic. Res. 2012, 46, 1472–1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachur, J.A.; Garcia, S.B.; Vannucchi, H.; Jordao, A.A.; Chiarello, P.G.; Zucoloto, S. Anti-oxidative systems in rat skeletal muscle after acute physical exercise. Appl. Physiol. Nutr. Metab. 2007, 32, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Ghiasi, R.; Mohammadi, M.; Helan, J.A.; Jozani, S.R.J.; Mohammadi, S.; Ghiasi, A.; Naderi, R. Influence of Two Various Durations of Resistance Exercise on Oxidative Stress in the Male Rat’s Hearts. J. Cardiovasc. Thorac. Res. 2015, 7, 149–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souissi, W.; Bouzid, M.A.; Farjallah, M.A.; Mahmoud, L.B.; Boudaya, M.; Engel, F.A.; Sahnoun, Z. Effect of different running exercise modalities on post-exercise oxidative stress markers in trained athletes. Int. J. Environ. Res. Public Health 2020, 17, 3729. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Żebrowska, E.; Chabowski, A. Insulin Resistance and Oxidative Stress in the Brain: What’s New? Int. J. Mol. Sci. 2019, 20, 874. [Google Scholar] [CrossRef] [Green Version]
- Choromańska, B.; Myśliwiec, P.; Łuba, M.; Wojskowicz, P.; Myśliwiec, H.; Choromańska, K.; Dadan, J.; Zalewska, A.; Maciejczyk, M. The Impact of Hypertension and Metabolic Syndrome on Nitrosative Stress and Glutathione Metabolism in Patients with Morbid Obesity. Oxidative Med. Cell. Longev. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Ott, C.; Jacobs, K.; Haucke, E.; Santos, A.N.; Grune, T.; Simm, A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014, 2, 411–429. [Google Scholar] [CrossRef] [Green Version]
- Sponder, M.; Campean, I.-A.; Emich, M.; Fritzer-Szekeres, M.; Litschauer, B.; Graf, S.; Dalos, D.; Strametz-Juranek, J. Long-term physical activity leads to a significant increase in serum sRAGE levels: A sign of decreased AGE-mediated inflammation due to physical activity? Heart Vessel. 2018, 33, 893–900. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.M.; Han, K.A.; Ahn, H.J.; Hwang, S.Y.; Hong, H.C.; Choi, H.Y.; Yang, S.J.; Yoo, H.J.; Baik, S.H.; Choi, D.S.; et al. Effects of Exercise on sRAGE Levels and Cardiometabolic Risk Factors in Patients with Type 2 Diabetes: A Randomized Controlled Trial. J. Clin. Endocrinol. Metab. 2012, 97, 3751–3758. [Google Scholar] [CrossRef] [Green Version]
- Calvert, J.W.; Condit, M.E.; Aragón, J.P.; Nicholson, C.K.; Moody, B.F.; Hood, R.L.; Sindler, A.L.; Gundewar, S.; Seals, D.R.; Barouch, L.A.; et al. Exercise Protects Against Myocardial Ischemia–Reperfusion Injury via Stimulation of β 3 -Adrenergic Receptors and Increased Nitric Oxide Signaling: Role of Nitrite and Nitrosothiols. Circ. Res. 2011, 108, 1448–1458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, Y.H.; Betik, A.C.; McConell, G.K. Role of nitric oxide in skeletal muscle glucose uptake during exercise. Exp. Physiol. 2014, 99, 1569–1573. [Google Scholar] [CrossRef] [PubMed]
- Dyakova, E.Y.; Kapilevich, L.V.; Shylko, V.G.; Popov, S.V.; Anfinogenova, Y. Physical exercise associated with NO production: Signaling pathways and significance in health and disease. Front. Cell Dev. Biol. 2015, 3, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erusalimsky, J.D.; Moncada, S. Nitric Oxide and Mitochondrial Signaling: From physiology to pathophysiology. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2524–2531. [Google Scholar] [CrossRef]
- Steensberg, A.; Keller, C.; Hillig, T.; Frøsig, C.; Wojtaszewski, J.F.P.; Pedersen, B.K.; Pilegaard, H.; Sander, M. Nitric oxide production is a proximal signaling event controlling exercise-induced mRNA expression in human skeletal muscle. FASEB J. 2007, 21, 2683–2694. [Google Scholar] [CrossRef]
- Tsukiyama, Y.; Ito, T.; Nagaoka, K.; Eguchi, E.; Ogino, K. Effects of exercise training on nitric oxide, blood pressure and antioxidant enzymes. J. Clin. Biochem. Nutr. 2017, 60, 180–186. [Google Scholar] [CrossRef] [Green Version]
- Salanova, M.; Schiffl, G.; Gutsmann, M.; Felsenberg, D.; Furlan, S.; Volpe, P.; Clarke, A.; Blottner, D. Nitrosative stress in human skeletal muscle attenuated by exercise countermeasure after chronic disuse. Redox Biol. 2013, 1, 514–526. [Google Scholar] [CrossRef]
- Shepherd, R.E.; Gollnick, P.D. Oxygen uptake of rats at different work intensities. Pflügers Arch. Eur. J. Physiol. 1976, 362, 219–222. [Google Scholar] [CrossRef]
- Choromańska, B.; Myśliwiec, P.; Łuba, M.; Wojskowicz, P.; Dadan, J.; Myśliwiec, H.; Choromańska, K.; Zalewska, A.; Maciejczyk, M. A Longitudinal Study of the Antioxidant Barrier and Oxidative Stress in Morbidly Obese Patients after Bariatric Surgery. Does the Metabolic Syndrome Affect the Redox Homeostasis of Obese People? J. Clin. Med. 2020, 9, 976. [Google Scholar] [CrossRef] [Green Version]
- Maciejczyk, M.; Taranta-Janusz, K.; Wasilewska, A.; Kossakowska, A.; Zalewska, A. A Case-Control Study of Salivary Redox Homeostasis in Hypertensive Children. Can Salivary Uric Acid be a Marker of Hypertension? J. Clin. Med. 2020, 9, 837. [Google Scholar] [CrossRef] [Green Version]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [CrossRef]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Toczewska, J.; Maciejczyk, M.; Konopka, T.; Zalewska, A. Total Oxidant and Antioxidant Capacity of Gingival Crevicular Fluid and Saliva in Patients with Periodontitis: Review and Clinical Study. Antioxidants 2020, 9, 450. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Kalousová, M.; Skrha, J.; Zima, T. Advanced glycation end-products and advanced oxidation protein products in patients with diabetes mellitus. Physiol. Res. 2002, 51, 597–604. [Google Scholar]
- Zalewska, A.; Maciejczyk, M.; Szulimowska, J.; Imierska, M.; Błachnio-Zabielska, A. High-Fat Diet Affects Ceramide Content, Disturbs Mitochondrial Redox Balance, and Induces Apoptosis in the Submandibular Glands of Mice. Biomolecules 2019, 9, 877. [Google Scholar] [CrossRef] [Green Version]
- Nourooz-Zadeh, J.; Tajaddini-Sarmadi, J.; Wolff, S.P.; Birlouez-Aragon, I. Measurement of Hydroperoxides in Edible Oils Using the Ferrous Oxidation in Xylenol Orange Assay. J. Agric. Food Chem. 1995, 43, 17–21. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef]
- Grisham, M.B.; Johnson, G.G.; Lancaster, J.R. Quantitation of nitrate and nitrite in extracellular fluids. Methods Enzymol. 1996, 268, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Wink, D.A.; Kim, S.; Coffin, D.; Cook, J.C.; Vodovotz, Y.; Chistodoulou, D.; Jourd’Heuil, D.; Grisham, M.B. Detection of S-nitrosothiols by fluorometric and colorimetric methods. Methods Enzymol. 1999, 301, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.S.; Ischiropoulos, H.; Zhu, L.; Van Der Woerd, M.; Smith, C.; Chen, J.; Harrison, J.; Martin, J.C.; Tsai, M. Kinetics of superoxide dismutase- and iron-catalyzed nitration of phenolics by peroxynitrite. Arch. Biochem. Biophys. 1992, 298, 438–445. [Google Scholar] [CrossRef]

| FFA | Control | M30 | M120 | F30 |
|---|---|---|---|---|
| SAT | 69.41 (55.90–85.09) | 97.08 (86.00–119.84) * | 242.07 (159.10–279.02) *,$ | 139.10 (128.14–148.59) *,# |
| UNSAT | 73.55 (58.64–98.49) | 126.82 (108.18–154.22) * | 183.41 (160.34–211.27) *,$ | 170.25 (148.93–178.21) *,# |
| Total | 140.18 (116.72–183.53) | 224.45 (194.18–274.06) * | 401.42 (373.91–458.89) *,$ | 306.85 (289.08–324.31) *,# |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Supruniuk, E.; Maciejczyk, M.; Zalewska, A.; Górski, J.; Chabowski, A. Blood Profile of Cytokines, Chemokines, Growth Factors, and Redox Biomarkers in Response to Different Protocols of Treadmill Running in Rats. Int. J. Mol. Sci. 2020, 21, 8071. https://doi.org/10.3390/ijms21218071
Supruniuk E, Maciejczyk M, Zalewska A, Górski J, Chabowski A. Blood Profile of Cytokines, Chemokines, Growth Factors, and Redox Biomarkers in Response to Different Protocols of Treadmill Running in Rats. International Journal of Molecular Sciences. 2020; 21(21):8071. https://doi.org/10.3390/ijms21218071
Chicago/Turabian StyleSupruniuk, Elżbieta, Mateusz Maciejczyk, Anna Zalewska, Jan Górski, and Adrian Chabowski. 2020. "Blood Profile of Cytokines, Chemokines, Growth Factors, and Redox Biomarkers in Response to Different Protocols of Treadmill Running in Rats" International Journal of Molecular Sciences 21, no. 21: 8071. https://doi.org/10.3390/ijms21218071
APA StyleSupruniuk, E., Maciejczyk, M., Zalewska, A., Górski, J., & Chabowski, A. (2020). Blood Profile of Cytokines, Chemokines, Growth Factors, and Redox Biomarkers in Response to Different Protocols of Treadmill Running in Rats. International Journal of Molecular Sciences, 21(21), 8071. https://doi.org/10.3390/ijms21218071







