The Emerging and Diverse Roles of Bis(monoacylglycero) Phosphate Lipids in Cellular Physiology and Disease
Abstract
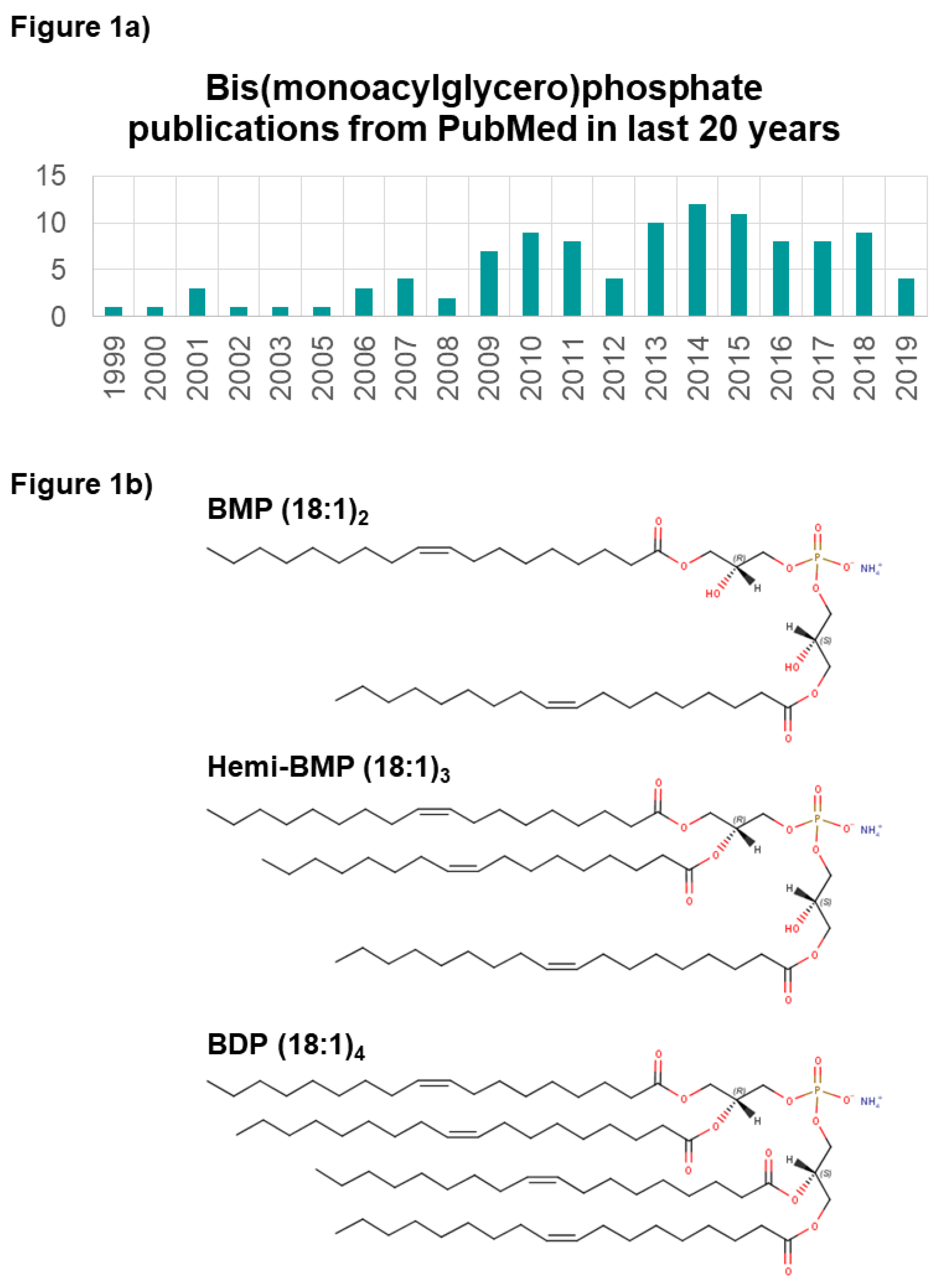
1. Introduction
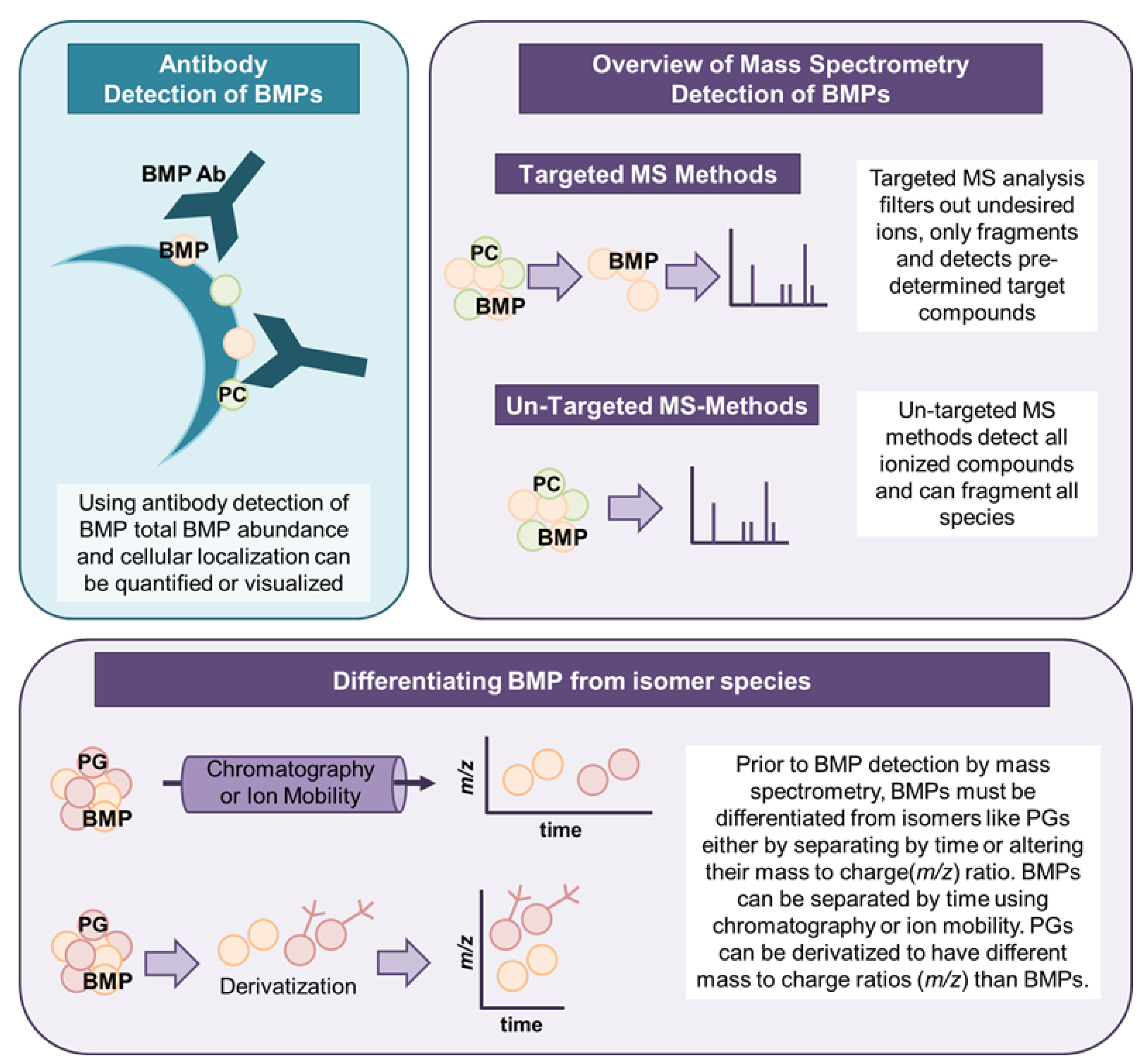
1.1. Mass Spectrometry- and Antibody-Based Measurement of BMPs
1.2. Biosynthesis and Metabolism of BMPs in Diverse Tissues
1.3. BMP Esterified Acyl Chain Composition and Changes
1.4. BMP is a Structural Lipid Important for Lysosomal Protein Membrane Docking and Function
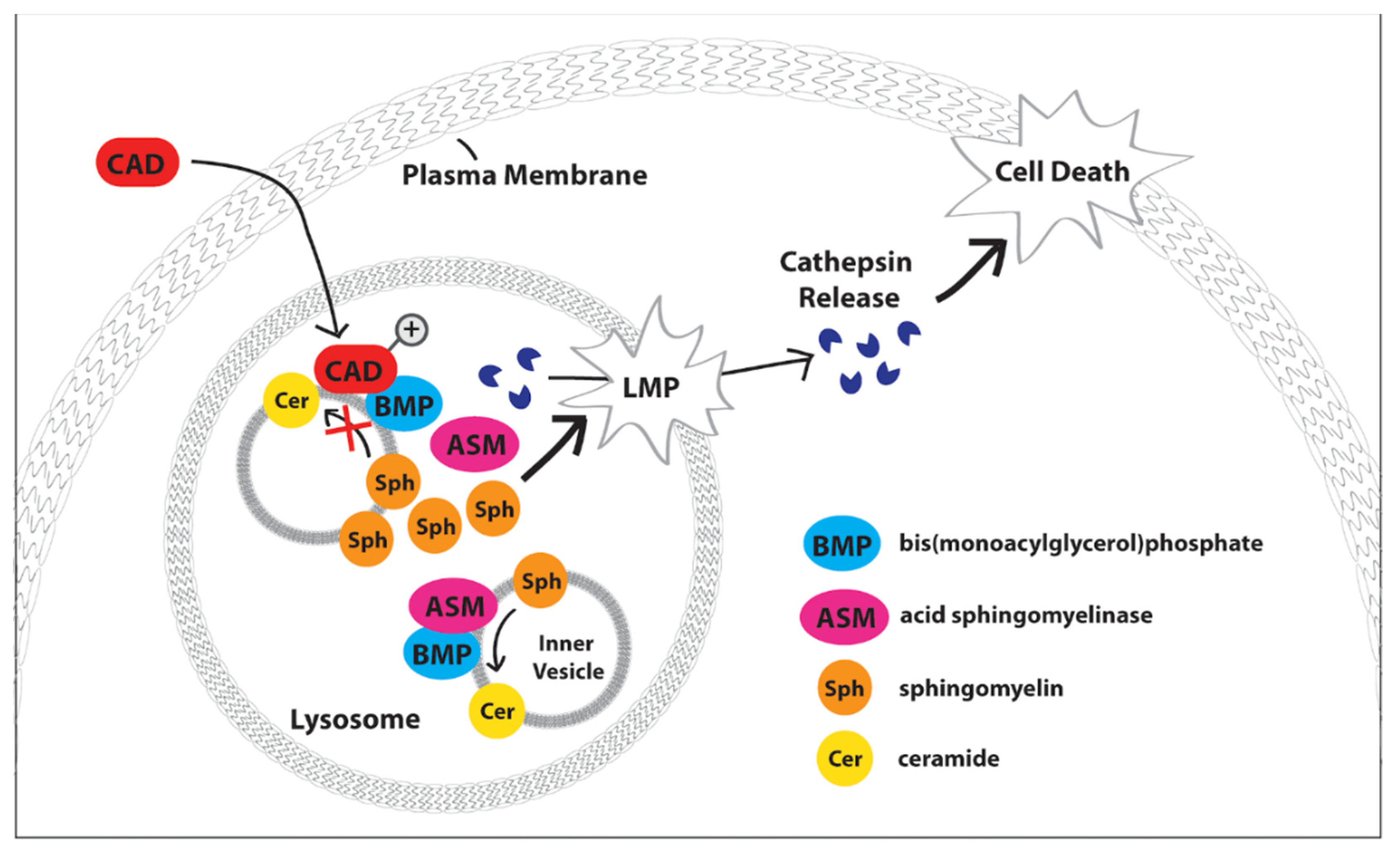
2. Acid Sphingomyelinase
2.1. Niemann-Pick Disease Type C2 Protein (NPC-2)
2.2. Heat Shock Protein 70 (Hsp 70)
2.3. Lysosomal Phospholipase A2 (LPLA2)
2.4. Apoptosis Linked Gene 2 Interacting Protein X (Alix)
3. BMPs and Disease
3.1. Lysosomal Storage Diseases Display Unique BMP Associated Phenotype
3.1.1. GM1 and GM2 Gangliosidosis
3.1.2. Gaucher Disease
3.1.3. INCL, MPS1, MPS2 and Fabry Disease
3.1.4. Niemann-Pick Disease Type C (NPC)
3.2. BMP Modulation in Other Genetic or Acquired Disorders
3.2.1. Stargardt Disease
3.2.2. Antiphospholipid Syndrome (APS)
3.2.3. Phospholipidosis, Cationic Amphiphilic Drugs, and Acquired Lysosomal Storage Diseases
3.2.4. Infection and Inflammation
3.2.5. Age Related Neurological Disease Progression
4. Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meikle, P.J.; Duplock, S.; Blacklock, D.; Whitfield, P.D.; Macintosh, G.; Hopwood, J.J.; Fuller, M. Effect of lysosomal storage on bis(monoacylglycero)phosphate. Biochem. J. 2008, 411, 71–78. [Google Scholar] [CrossRef]
- Gray, D.R.B.G.M. The isolation and characterisation of phosphatidylglycerol and a structural isomer from pig lung. Chem. Phys. Lipids 1967, 1, 254–263. [Google Scholar] [CrossRef]
- Schulze, H.; Sandhoff, K. Lysosomal lipid storage diseases. Cold Spring Harb Perspect. Biol. 2011, 3. [Google Scholar] [CrossRef] [PubMed]
- Record, M.; Amara, S.; Subra, C.; Jiang, G.; Prestwich, G.D.; Ferrato, F.; Carriere, F. Bis (monoacylglycero) phosphate interfacial properties and lipolysis by pancreatic lipase-related protein 2, an enzyme present in THP-1 human monocytes. Biochim. Biophys. Acta 2011, 1811, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Lobasso, S.; Tanzarella, P.; Vergara, D.; Maffia, M.; Cocco, T.; Corcelli, A. Lipid profiling of parkin-mutant human skin fibroblasts. J. Cell Physiol. 2017, 232, 3540–3551. [Google Scholar] [CrossRef] [PubMed]
- Hullin-Matsuda, F.; Luquain-Costaz, C.; Bouvier, J.; Delton-Vandenbroucke, I. Bis(monoacylglycero)phosphate, a peculiar phospholipid to control the fate of cholesterol: Implications in pathology. Prostaglandins Leukot Essent. Fatty Acids 2009, 81, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Gallala, H.D.; Sandhoff, K. Biological function of the cellular lipid BMP-BMP as a key activator for cholesterol sorting and membrane digestion. Neurochem. Res. 2011, 36, 1594–1600. [Google Scholar] [CrossRef] [PubMed]
- Akgoc, Z.; Iosim, S.; Seyfried, T.N. Bis(monoacylglycero)phosphate as a Macrophage Enriched Phospholipid. Lipids 2015, 50, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Akgoc, Z.; Sena-Esteves, M.; Martin, D.R.; Han, X.; d’Azzo, A.; Seyfried, T.N. Bis(monoacylglycero)phosphate: A secondary storage lipid in the gangliosidoses. J. Lipid Res. 2015, 56, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Hostetler, K.Y. Chapter 6 Polyglycerophospholipids: Phosphatidylglycerol, diphosphatidylglycerol and bis (monoacylglycero) phosphate. New Compr. Biochem. 1982, 4, 215–261. [Google Scholar]
- Liu, N.; Tengstrand, E.A.; Chourb, L.; Hsieh, F.Y. Di-22:6-bis(monoacylglycerol)phosphate: A clinical biomarker of drug-induced phospholipidosis for drug development and safety assessment. Toxicol. Appl. Pharmacol. 2014, 279, 467–476. [Google Scholar] [CrossRef]
- Thompson, K.L.; Haskins, K.; Rosenzweig, B.A.; Stewart, S.; Zhang, J.; Peters, D.; Knapton, A.; Rouse, R.; Mans, D.; Colatsky, T. Comparison of the diagnostic accuracy of di-22:6-bis(monoacylglycerol)phosphate and other urinary phospholipids for drug-induced phospholipidosis or tissue injury in the rat. Int. J. Toxicol. 2012, 31, 14–24. [Google Scholar] [CrossRef]
- Kobayashi, T.; Stang, E.; Fang, K.S.; de Moerloose, P.; Parton, R.G.; Gruenberg, J. A lipid associated with the antiphospholipid syndrome regulates endosome structure and function. Nature 1998, 392, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Bissig, C.; Johnson, S.; Gruenberg, J. Studying lipids involved in the endosomal pathway. Methods Cell Biol. 2012, 108, 19–46. [Google Scholar] [CrossRef]
- Mo, G.C.H.; Yip, C.M. Structural templating of J-aggregates: Visualizing bis(monoacylglycero)phosphate domains in live cells. Biochim. Biophys. Acta Proteins Proteom. 2017, 1865, 1687–1695. [Google Scholar] [CrossRef]
- Kind, T.; Tsugawa, H.; Cajka, T.; Ma, Y.; Lai, Z.; Mehta, S.S.; Wohlgemuth, G.; Barupal, D.K.; Showalter, M.R.; Arita, M.; et al. Identification of small molecules using accurate mass MS/MS search. Mass Spectrom. Rev. 2017. [Google Scholar] [CrossRef]
- Kind, T.; Liu, K.H.; Lee, D.Y.; DeFelice, B.; Meissen, J.K.; Fiehn, O. LipidBlast in silico tandem mass spectrometry database for lipid identification. Nat. Methods 2013, 10, 755–758. [Google Scholar] [CrossRef]
- Cajka, T.; Fiehn, O. LC-MS-Based Lipidomics and Automated Identification of Lipids Using the LipidBlast In-Silico MS/MS Library. Methods Mol. Biol. 2017, 1609, 149–170. [Google Scholar] [CrossRef] [PubMed]
- Kind, T.; Okazaki, Y.; Saito, K.; Fiehn, O. LipidBlast templates as flexible tools for creating new in-silico tandem mass spectral libraries. Anal. Chem. 2014, 86, 11024–11027. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef]
- Hankin, J.A.; Murphy, R.C.; Barkley, R.M.; Gijón, M.A. Ion mobility and tandem mass spectrometry of phosphatidylglycerol and bis (monoacylglycerol) phosphate (BMP). Int. J. Mass Spectrom. 2015, 378, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Züllig, T.; Köfeler, H.C. High resolution mass spectrometry in lipidomics. Mass Spectrom. Rev. 2020. [Google Scholar] [CrossRef]
- Scherer, M.; Schmitz, G.; Liebisch, G. Simultaneous quantification of cardiolipin, bis(monoacylglycero)phosphate and their precursors by hydrophilic interaction LC-MS/MS including correction of isotopic overlap. Anal. Chem. 2010, 82, 8794–8799. [Google Scholar] [CrossRef]
- Vosse, C.; Wienken, C.; Cadenas, C.; Hayen, H. Separation and identification of phospholipids by hydrophilic interaction liquid chromatography coupled to tandem high resolution mass spectrometry with focus on isomeric phosphatidylglycerol and bis(monoacylglycero)phosphate. J. Chromatogr. A 2018, 1565, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Baronas, E.T.; Lee, J.W.; Alden, C.; Hsieh, F.Y. Biomarkers to monitor drug-induced phospholipidosis. Toxicol. Appl. Pharmacol. 2007, 218, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Lecommandeur, E.; Baker, D.; Cox, T.M.; Nicholls, A.W.; Griffin, J.L. Alterations in endo-lysosomal function induce similar hepatic lipid profiles in rodent models of drug-induced phospholipidosis and Sandhoff disease. J. Lipid Res. 2017, 58, 1306–1314. [Google Scholar] [CrossRef]
- Lee, J.C.; Yang, J.S.; Moon, M.H. Simultaneous Relative Quantification of Various Polyglycerophospholipids with Isotope-Labeled Methylation by Nanoflow Ultrahigh Performance Liquid Chromatography-Tandem Mass Spectrometry. Anal. Chem. 2019, 91, 6716–6723. [Google Scholar] [CrossRef]
- Wang, X.; Schmitt, M.V.; Xu, L.; Jiao, Y.; Guo, L.; Lienau, P.; Reichel, A.; Liu, X. Quantitative molecular tissue atlas of Bis(monoacylglycero)phosphate and phosphatidylglycerol membrane lipids in rodent organs generated by methylation assisted high resolution mass spectrometry. Anal. Chim. Acta 2019, 1084, 60–70. [Google Scholar] [CrossRef]
- Wang, M.; Palavicini, J.P.; Cseresznye, A.; Han, X. Strategy for Quantitative Analysis of Isomeric Bis(monoacylglycero)phosphate and Phosphatidylglycerol Species by Shotgun Lipidomics after One-Step Methylation. Anal. Chem. 2017, 89, 8490–8495. [Google Scholar] [CrossRef]
- Kobayashi, T.; Beuchat, M.H.; Chevallier, J.; Makino, A.; Mayran, N.; Escola, J.M.; Lebrand, C.; Cosson, P.; Kobayashi, T.; Gruenberg, J. Separation and characterization of late endosomal membrane domains. J. Biol. Chem. 2002, 277, 32157–32164. [Google Scholar] [CrossRef]
- Hullin-Matsuda, F.; Kawasaki, K.; Delton-Vandenbroucke, I.; Xu, Y.; Nishijima, M.; Lagarde, M.; Schlame, M.; Kobayashi, T. De novo biosynthesis of the late endosome lipid, bis(monoacylglycero)phosphate. J. Lipid Res. 2007, 48, 1997–2008. [Google Scholar] [CrossRef] [PubMed]
- Goursot, A.; Mineva, T.; Bissig, C.; Gruenberg, J.; Salahub, D.R. Structure, dynamics, and energetics of lysobisphosphatidic acid (LBPA) isomers. J. Phys. Chem. B 2010, 114, 15712–15720. [Google Scholar] [CrossRef]
- Pribasnig, M.A.; Mrak, I.; Grabner, G.F.; Taschler, U.; Knittelfelder, O.; Scherz, B.; Eichmann, T.O.; Heier, C.; Grumet, L.; Kowaliuk, J.; et al. alpha/beta Hydrolase Domain-containing 6 (ABHD6) Degrades the Late Endosomal/Lysosomal Lipid Bis(monoacylglycero)phosphate. J. Biol. Chem. 2015, 290, 29869–29881. [Google Scholar] [CrossRef]
- Marrs, W.R.; Horne, E.A.; Ortega-Gutierrez, S.; Cisneros, J.A.; Xu, C.; Lin, Y.H.; Muccioli, G.G.; Lopez-Rodriguez, M.L.; Stella, N. Dual inhibition of alpha/beta-hydrolase domain 6 and fatty acid amide hydrolase increases endocannabinoid levels in neurons. J. Biol. Chem. 2011, 286, 28723–28728. [Google Scholar] [CrossRef]
- Thomas, G.; Betters, J.L.; Lord, C.C.; Brown, A.L.; Marshall, S.; Ferguson, D.; Sawyer, J.; Davis, M.A.; Melchior, J.T.; Blume, L.C.; et al. The serine hydrolase ABHD6 Is a critical regulator of the metabolic syndrome. Cell Rep. 2013, 5, 508–520. [Google Scholar] [CrossRef]
- Bottemanne, P.; Paquot, A.; Ameraoui, H.; Alhouayek, M.; Muccioli, G.G. The alpha/beta-hydrolase domain 6 inhibitor WWL70 decreases endotoxin-induced lung inflammation in mice, potential contribution of 2-arachidonoylglycerol, and lysoglycerophospholipids. FASEB J. 2019. [Google Scholar] [CrossRef]
- Grabner, G.F.; Fawzy, N.; Pribasnig, M.; Trieb, M.; Taschler, U.; Holzer, M.; Schweiger, M.; Wolinski, H.; Kolb, D.; Horvath, A.; et al. Metabolic disease and ABHD6 alter the circulating bis(monoacylglycerol)phosphate profile in mice and humans. J. Lipid Res. 2019. [Google Scholar] [CrossRef]
- Ito, M.; Tchoua, U.; Okamoto, M.; Tojo, H. Purification and properties of a phospholipase A2/lipase preferring phosphatidic acid, bis(monoacylglycerol) phosphate, and monoacylglycerol from rat testis. J. Biol. Chem. 2002, 277, 43674–43681. [Google Scholar] [CrossRef] [PubMed]
- Blankman, J.L.; Long, J.Z.; Trauger, S.A.; Siuzdak, G.; Cravatt, B.F. ABHD12 controls brain lysophosphatidylserine pathways that are deregulated in a murine model of the neurodegenerative disease PHARC. Proc. Natl. Acad. Sci. USA 2013, 110, 1500–1505. [Google Scholar] [CrossRef] [PubMed]
- Brotherus, J.; Renkonen, O.; Fischer, W.; Herrmann, J. Novel stereoconfiguration in lyso-bis-phosphatidic acid of cultured BHK-cells. Chem. Phys. Lipids 1974, 13, 178–182. [Google Scholar] [CrossRef]
- Mason, R.J.; Stossel, T.P.; Vaughan, M. Lipids of alveolar macrophages, polymorphonuclear leukocytes, and their phagocytic vesicles. J. Clin. Invest. 1972, 51, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Cochran, F.R.; Connor, J.R.; Roddick, V.L.; Waite, B.M. Lyso(bis)phosphatidic acid: A novel source of arachidonic acid for oxidative metabolism by rabbit alveolar macrophages. Biochem. Biophys. Res. Commun. 1985, 130, 800–806. [Google Scholar] [CrossRef]
- Wherrett, J.R.; Huterer, S. Bis-(monoacylglyceryl)-phosphate of rat and human liver: Fatty acid composition and NMR spectroscopy. Lipids 1973, 8, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, J.; Zemski Berry, K.A.; Hullin-Matsuda, F.; Makino, A.; Michaud, S.; Geloen, A.; Murphy, R.C.; Kobayashi, T.; Lagarde, M.; Delton-Vandenbroucke, I. Selective decrease of bis(monoacylglycero)phosphate content in macrophages by high supplementation with docosahexaenoic acid. J. Lipid Res. 2009, 50, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Hein, L.K.; Duplock, S.; Fuller, M. Selective reduction of bis(monoacylglycero)phosphate ameliorates the storage burden in a THP-1 macrophage model of Gaucher disease. J. Lipid Res. 2013, 54, 1691–1697. [Google Scholar] [CrossRef]
- Petersen, N.H.; Olsen, O.D.; Groth-Pedersen, L.; Ellegaard, A.M.; Bilgin, M.; Redmer, S.; Ostenfeld, M.S.; Ulanet, D.; Dovmark, T.H.; Lonborg, A.; et al. Transformation-associated changes in sphingolipid metabolism sensitize cells to lysosomal cell death induced by inhibitors of acid sphingomyelinase. Cancer Cell 2013, 24, 379–393. [Google Scholar] [CrossRef]
- Kirkegaard, T.; Roth, A.G.; Petersen, N.H.; Mahalka, A.K.; Olsen, O.D.; Moilanen, I.; Zylicz, A.; Knudsen, J.; Sandhoff, K.; Arenz, C.; et al. Hsp70 stabilizes lysosomes and reverts Niemann-Pick disease-associated lysosomal pathology. Nature 2010, 463, 549–553. [Google Scholar] [CrossRef]
- Kolter, T.; Sandhoff, K. Lysosomal degradation of membrane lipids. FEBS Lett. 2010, 584, 1700–1712. [Google Scholar] [CrossRef]
- Chevallier, J.; Chamoun, Z.; Jiang, G.; Prestwich, G.; Sakai, N.; Matile, S.; Parton, R.G.; Gruenberg, J. Lysobisphosphatidic acid controls endosomal cholesterol levels. J. Biol. Chem. 2008, 283, 27871–27880. [Google Scholar] [CrossRef]
- Bissig, C.; Lenoir, M.; Velluz, M.C.; Kufareva, I.; Abagyan, R.; Overduin, M.; Gruenberg, J. Viral infection controlled by a calcium-dependent lipid-binding module in ALIX. Dev. Cell 2013, 25, 364–373. [Google Scholar] [CrossRef]
- Savic, R.; Schuchman, E.H. Use of acid sphingomyelinase for cancer therapy. Adv. Cancer Res. 2013, 117, 91–115. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.; Ziobro, R.; Becker, K.A.; Kolesnick, R.; Gulbins, E. Acid sphingomyelinase. Handb. Exp. Pharmacol. 2013, 77–88. [Google Scholar] [CrossRef]
- Jenkins, R.W.; Canals, D.; Hannun, Y.A. Roles and regulation of secretory and lysosomal acid sphingomyelinase. Cell Signal. 2009, 21, 836–846. [Google Scholar] [CrossRef]
- Oninla, V.O.; Breiden, B.; Babalola, J.O.; Sandhoff, K. Acid sphingomyelinase activity is regulated by membrane lipids and facilitates cholesterol transfer by NPC2. J. Lipid Res. 2014, 55, 2606–2619. [Google Scholar] [CrossRef] [PubMed]
- Enkavi, G.; Mikkolainen, H.; Gungor, B.; Ikonen, E.; Vattulainen, I. Concerted regulation of npc2 binding to endosomal/lysosomal membranes by bis(monoacylglycero)phosphate and sphingomyelin. PLoS Comput. Biol. 2017, 13, e1005831. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.C.; Appelqvist, H.; Nilsson, C.; Kagedal, K.; Roberg, K.; Ollinger, K. Regulation of apoptosis-associated lysosomal membrane permeabilization. Apoptosis 2010, 15, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Schaloske, R.H.; Dennis, E.A. The phospholipase A2 superfamily and its group numbering system. Biochim. Biophys. Acta 2006, 1761, 1246–1259. [Google Scholar] [CrossRef] [PubMed]
- Shayman, J.A.; Kelly, R.; Kollmeyer, J.; He, Y.; Abe, A. Group XV phospholipase A(2), a lysosomal phospholipase A(2). Prog. Lipid Res. 2011, 50, 1–13. [Google Scholar] [CrossRef]
- Hiraoka, M.; Abe, A.; Lu, Y.; Yang, K.; Han, X.; Gross, R.W.; Shayman, J.A. Lysosomal phospholipase A2 and phospholipidosis. Mol. Cell. Biol. 2006, 26, 6139–6148. [Google Scholar] [CrossRef]
- Abe, A.; Shayman, J.A. The role of negatively charged lipids in lysosomal phospholipase A2 function. J. Lipid Res. 2009, 50, 2027–2035. [Google Scholar] [CrossRef]
- Anderson, D.M.G.; Ablonczy, Z.; Koutalos, Y.; Hanneken, A.M.; Spraggins, J.M.; Calcutt, M.W.; Crouch, R.K.; Caprioli, R.M.; Schey, K.L. Bis(monoacylglycero)phosphate lipids in the retinal pigment epithelium implicate lysosomal/endosomal dysfunction in a model of Stargardt disease and human retinas. Sci. Rep. 2017, 7, 17352. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.M.; Lambertsen, K.L.; Clausen, B.H.; Meyer, M.; Bhandari, D.R.; Larsen, S.T.; Poulsen, S.S.; Spengler, B.; Janfelt, C.; Hansen, H.S. Mass spectrometry imaging of biomarker lipids for phagocytosis and signalling during focal cerebral ischaemia. Sci. Rep. 2016, 6, 39571. [Google Scholar] [CrossRef]
- Meikle, P.J.; Hopwood, J.J.; Clague, A.E.; Carey, W.F. Prevalence of lysosomal storage disorders. JAMA 1999, 281, 249–254. [Google Scholar] [CrossRef]
- Vanier, M.T. Biochemical studies in Niemann-Pick disease. I. Major sphingolipids of liver and spleen. Biochim. Biophys. Acta 1983, 750, 178–184. [Google Scholar] [CrossRef]
- Tan, M.A.; Fuller, M.; Zabidi-Hussin, Z.A.; Hopwood, J.J.; Meikle, P.J. Biochemical profiling to predict disease severity in metachromatic leukodystrophy. Mol. Genet. Metab. 2010, 99, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Garcia, A.; Pham, M.M.; Crowson, C.S.; Amin, S.; Moder, K.G.; Pruthi, R.K.; Warrington, K.J.; Matteson, E.L. The Epidemiology of Antiphospholipid Syndrome. A Population-Based Study. Arthritis Rheumatol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Tanna, P.; Strauss, R.W.; Fujinami, K.; Michaelides, M. Stargardt disease: Clinical features, molecular genetics, animal models and therapeutic options. Br. J. Ophthalmol. 2017, 101, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Platt, F.M.; d’Azzo, A.; Davidson, B.L.; Neufeld, E.F.; Tifft, C.J. Lysosomal storage diseases. Nat. Rev. Dis. Primers. 2018, 4, 27. [Google Scholar] [CrossRef]
- Saville, J.T.; Lehmann, R.J.; Derrick-Roberts, A.L.K.; Fuller, M. Selective normalisation of regional brain bis(monoacylglycero)phosphate in the mucopolysaccharidosis 1 (Hurler) mouse. Exp. Neurol. 2016, 277, 68–75. [Google Scholar] [CrossRef]
- Sandhoff, K.; Harzer, K. Gangliosides and gangliosidoses: Principles of molecular and metabolic pathogenesis. J. Neurosci. 2013, 33, 10195–10208. [Google Scholar] [CrossRef]
- Wilkening, G.; Linke, T.; Sandhoff, K. Lysosomal degradation on vesicular membrane surfaces. Enhanced glucosylceramide degradation by lysosomal anionic lipids and activators. J. Biol. Chem. 1998, 273, 30271–30278. [Google Scholar] [CrossRef] [PubMed]
- Remmel, N.; Locatelli-Hoops, S.; Breiden, B.; Schwarzmann, G.; Sandhoff, K. Saposin B mobilizes lipids from cholesterol-poor and bis(monoacylglycero)phosphate-rich membranes at acidic pH. Unglycosylated patient variant saposin B lacks lipid-extraction capacity. FEBS J. 2007, 274, 3405–3420. [Google Scholar] [CrossRef] [PubMed]
- Matzner, U.; Breiden, B.; Schwarzmann, G.; Yaghootfam, A.; Fluharty, A.L.; Hasilik, A.; Sandhoff, K.; Gieselmann, V. Saposin B-dependent reconstitution of arylsulfatase A activity in vitro and in cell culture models of metachromatic leukodystrophy. J. Biol. Chem. 2009, 284, 9372–9381. [Google Scholar] [CrossRef] [PubMed]
- Kakela, R.; Somerharju, P.; Tyynela, J. Analysis of phospholipid molecular species in brains from patients with infantile and juvenile neuronal-ceroid lipofuscinosis using liquid chromatography-electrospray ionization mass spectrometry. J. Neurochem. 2003, 84, 1051–1065. [Google Scholar] [CrossRef]
- Papandreou, A.; Gissen, P. Diagnostic workup and management of patients with suspected Niemann-Pick type C disease. Ther. Adv. Neurol. Disord. 2016, 9, 216–229. [Google Scholar] [CrossRef]
- Garcia, D.; Erkan, D. Diagnosis and Management of the Antiphospholipid Syndrome. N. Engl. J. Med. 2018, 378, 2010–2021. [Google Scholar] [CrossRef]
- Galve-de Rochemonteix, B.; Kobayashi, T.; Rosnoblet, C.; Lindsay, M.; Parton, R.G.; Reber, G.; de Maistre, E.; Wahl, D.; Kruithof, E.K.; Gruenberg, J.; et al. Interaction of anti-phospholipid antibodies with late endosomes of human endothelial cells. Arter. Thromb Vasc. Biol. 2000, 20, 563–574. [Google Scholar] [CrossRef]
- Anderson, N.; Borlak, J. Drug-induced phospholipidosis. FEBS Lett. 2006, 580, 5533–5540. [Google Scholar] [CrossRef]
- Funk, R.S.; Krise, J.P. Cationic amphiphilic drugs cause a marked expansion of apparent lysosomal volume: Implications for an intracellular distribution-based drug interaction. Mol. Pharm. 2012, 9, 1384–1395. [Google Scholar] [CrossRef]
- Rampanelli, E.; Ochodnicky, P.; Vissers, J.P.; Butter, L.M.; Claessen, N.; Calcagni, A.; Kors, L.; Gethings, L.A.; Bakker, S.J.; de Borst, M.H.; et al. Excessive dietary lipid intake provokes an acquired form of lysosomal lipid storage disease in the kidney. J. Pathol. 2018, 246, 470–484. [Google Scholar] [CrossRef]
- Grabner, G.F.; Fawzy, N.; Schreiber, R.; Pusch, L.M.; Bulfon, D.; Koefeler, H.; Eichmann, T.O.; Lass, A.; Schweiger, M.; Marsche, G.; et al. Metabolic regulation of the lysosomal cofactor bis(monoacylglycero)phosphate in mice. J. Lipid Res. 2020. [Google Scholar] [CrossRef]
- Salata, C.; Baritussio, A.; Munegato, D.; Calistri, A.; Ha, H.R.; Bigler, L.; Fabris, F.; Parolin, C.; Palu, G.; Mirazimi, A. Amiodarone and metabolite MDEA inhibit Ebola virus infection by interfering with the viral entry process. Pathog. Dis. 2015, 73. [Google Scholar] [CrossRef]
- Carriere, F.; Longhi, S.; Record, M. The endosomal lipid bis(monoacylglycero) phosphate as a potential key player in the mechanism of action of chloroquine against SARS-COV-2 and other enveloped viruses hijacking the endocytic pathway. Biochimie 2020. [Google Scholar] [CrossRef]
- Roth, S.L.; Whittaker, G.R. Promotion of vesicular stomatitis virus fusion by the endosome-specific phospholipid bis(monoacylglycero)phosphate (BMP). FEBS Lett. 2011, 585, 865–869. [Google Scholar] [CrossRef]
- Nixon, R.A. The role of autophagy in neurodegenerative disease. Nat. Med. 2013, 19, 983–997. [Google Scholar] [CrossRef]
- Yamashima, T. Can ‘calpain-cathepsin hypothesis’ explain Alzheimer neuronal death? Ageing Res. Rev. 2016, 32, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.M.; Lasiecka, Z.M.; Xu, Y.; Neufeld, J.; Shahriar, S.; Simoes, S.; Chan, R.B.; Oliveira, T.G.; Small, S.A.; Di Paolo, G. Neuronal lysosomal dysfunction releases exosomes harboring APP C-terminal fragments and unique lipid signatures. Nat. Commun. 2018, 9, 291. [Google Scholar] [CrossRef]
- Skotland, T.; Hessvik, N.P.; Sandvig, K.; Llorente, A. Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J. Lipid Res. 2019, 60, 9–18. [Google Scholar] [CrossRef]
- Alcalay, R.N.; Hsieh, F.; Tengstrand, E.; Padmanabhan, S.; Baptista, M.; Kehoe, C.; Narayan, S.; Boehme, A.K.; Merchant, K. Higher Urine bis(Monoacylglycerol)Phosphate Levels in LRRK2 G2019S Mutation Carriers: Implications for Therapeutic Development. Mov. Disord. 2020, 35, 134–141. [Google Scholar] [CrossRef]
| Protein | Disease Implicated in | Disease Prevalence (Births) | BMP Levels, Side Chain Composition and Tissue Localization |
|---|---|---|---|
| ASM (Acid sphingomyelinase) | NPA and NPB (Niemann Pick A and B) [3] and Cancer [49] | 1–248,000 [64] | Significantly elevated in spleen and liver [65], as well as plasma [1] in patients with NPA and NPB. Di-18:1 BMP and di-18:2 BMP were the predominant side chains in plasma [1]. |
| Acid-beta-glucosidase | Gaucher disease [45] | 1–57,000 [64] | Di-18:1 BMP is significantly elevated in plasma samples from patients with Gaucher disease [1]. |
| NPC-1 and NPC-2 | NPC (Niemann Pick C) [3] | 1–211,000 [64] | Significantly elevated in spleen and liver [65], as well as plasma [1] in patients with NPC. BMP di-18:1 was the most prevalent side chain in plasma [1]. |
| GM1-B-Galactosidase | GM1 gangliosidosis [3] | 1–384,000 [64] | Significantly elevated in human brain samples from postmortem patients with GM1 gangliosidosis [9]. BMP di-22:6, di-18:0 and di-18:1 were the most abundant species [9]. |
| Arylsulfatase A | Metachromatic Leukodystrophy [3] | 1–92,000 [64] | No significant elevation in plasma [1] but significant elevation in urine observed, however, PG and BMP were undifferentiated in the study’s Mass Spectrometry analysis, so BMP confirmation is unclear [66] |
| Alpha-galactosidase A | Fabry disease [3] | 1–117,000 [64] | Significantly elevated in cultured skin fibroblasts from patients with Fabry disease, with di-22:6 BMP then di-18:1 BMPs as the most abundant species [1]. |
| B2GP1 (Beta-2-glycoprotein-1) | APS (Antiphospholipid syndrome) [6] | 1–2000 [67] | NA |
| IGF2/MPR (insulin like growth factor/mannose-6-phosphate receptor) | APS (Antiphospholipid syndrome) [6] | 1–2000 [67] | NA |
| ABCA4 (ATP-binding cassette, sub family A, member 4) | Stargardt [61] | 1–9000 [62] | Di-22:6 BMP and BMPs with C20:4 side chains were significantly elevated in human retina tissues from patients with Stargardt disease [63]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Showalter, M.R.; Berg, A.L.; Nagourney, A.; Heil, H.; Carraway, K.L., III; Fiehn, O. The Emerging and Diverse Roles of Bis(monoacylglycero) Phosphate Lipids in Cellular Physiology and Disease. Int. J. Mol. Sci. 2020, 21, 8067. https://doi.org/10.3390/ijms21218067
Showalter MR, Berg AL, Nagourney A, Heil H, Carraway KL III, Fiehn O. The Emerging and Diverse Roles of Bis(monoacylglycero) Phosphate Lipids in Cellular Physiology and Disease. International Journal of Molecular Sciences. 2020; 21(21):8067. https://doi.org/10.3390/ijms21218067
Chicago/Turabian StyleShowalter, Megan R., Anastasia L. Berg, Alexander Nagourney, Hailey Heil, Kermit L. Carraway, III, and Oliver Fiehn. 2020. "The Emerging and Diverse Roles of Bis(monoacylglycero) Phosphate Lipids in Cellular Physiology and Disease" International Journal of Molecular Sciences 21, no. 21: 8067. https://doi.org/10.3390/ijms21218067
APA StyleShowalter, M. R., Berg, A. L., Nagourney, A., Heil, H., Carraway, K. L., III, & Fiehn, O. (2020). The Emerging and Diverse Roles of Bis(monoacylglycero) Phosphate Lipids in Cellular Physiology and Disease. International Journal of Molecular Sciences, 21(21), 8067. https://doi.org/10.3390/ijms21218067







