B Cell Activating Factor (BAFF) Is Required for the Development of Intra-Renal Tertiary Lymphoid Organs in Experimental Kidney Transplantation in Rats
Abstract
:1. Introduction
2. Results
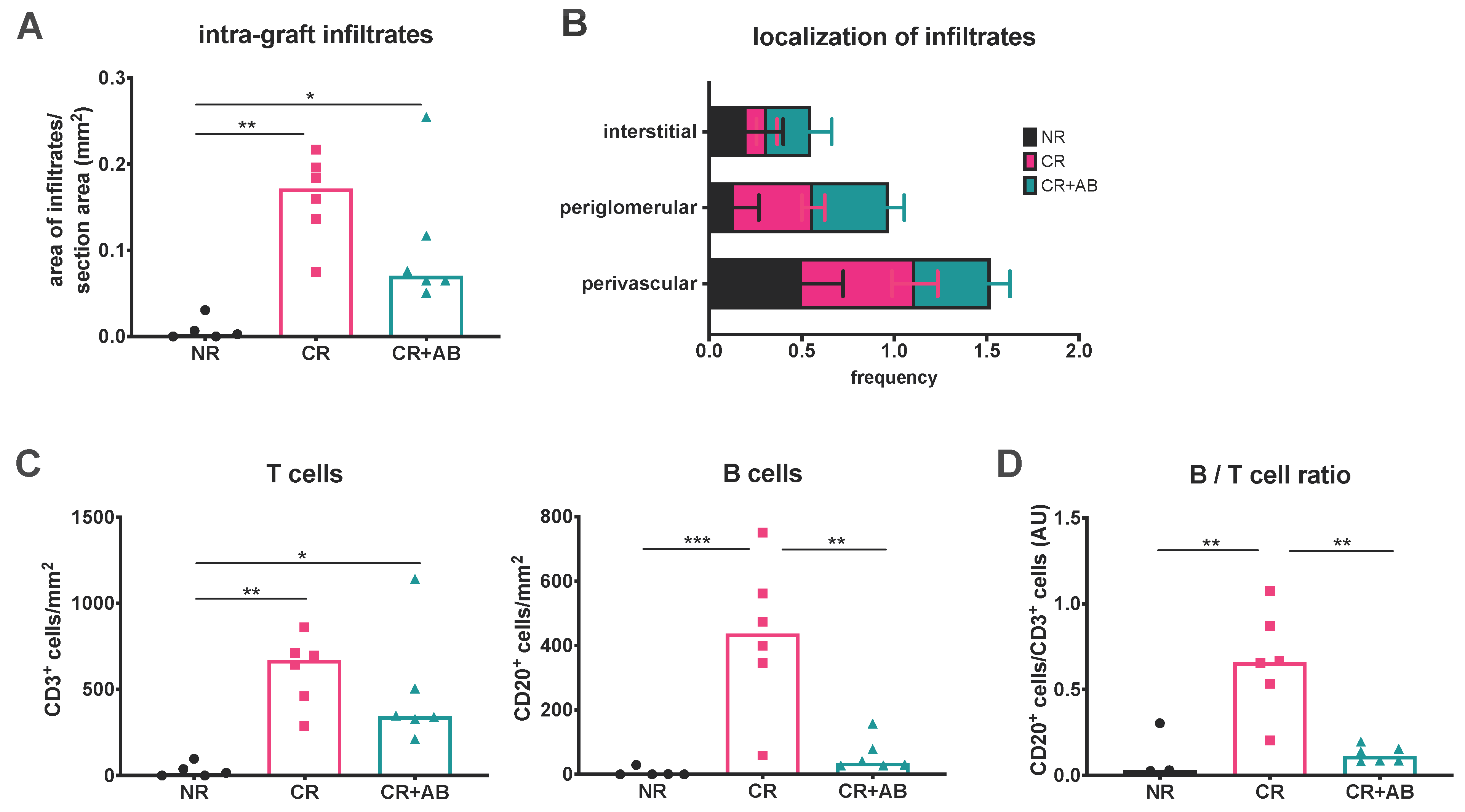
2.1. Anti-BAFF Treatment Alters the T and B Cell Composition of Intra-Renal Infiltrates
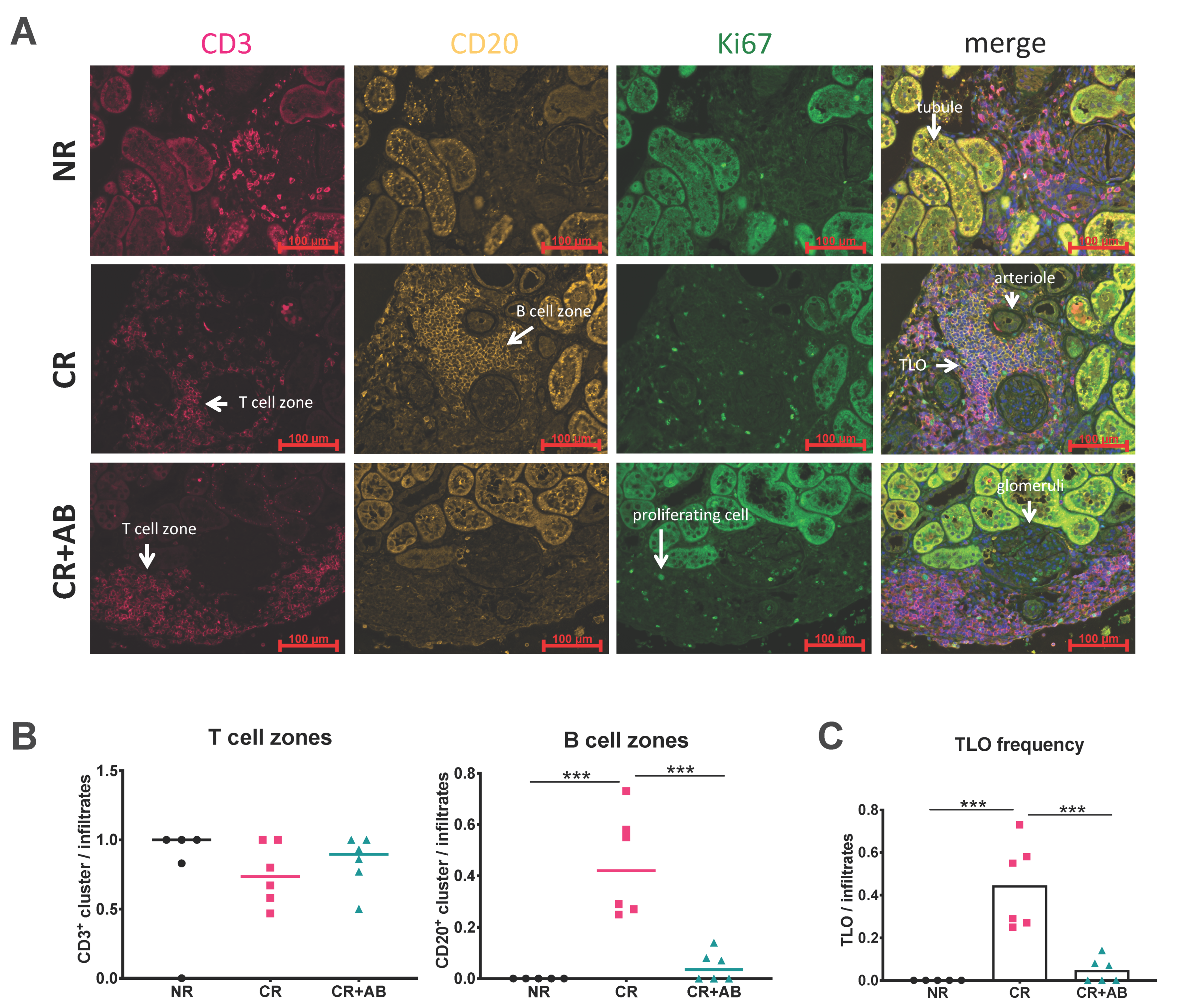
2.2. Anti-BAFF Treatment Interfered with TLO Formation
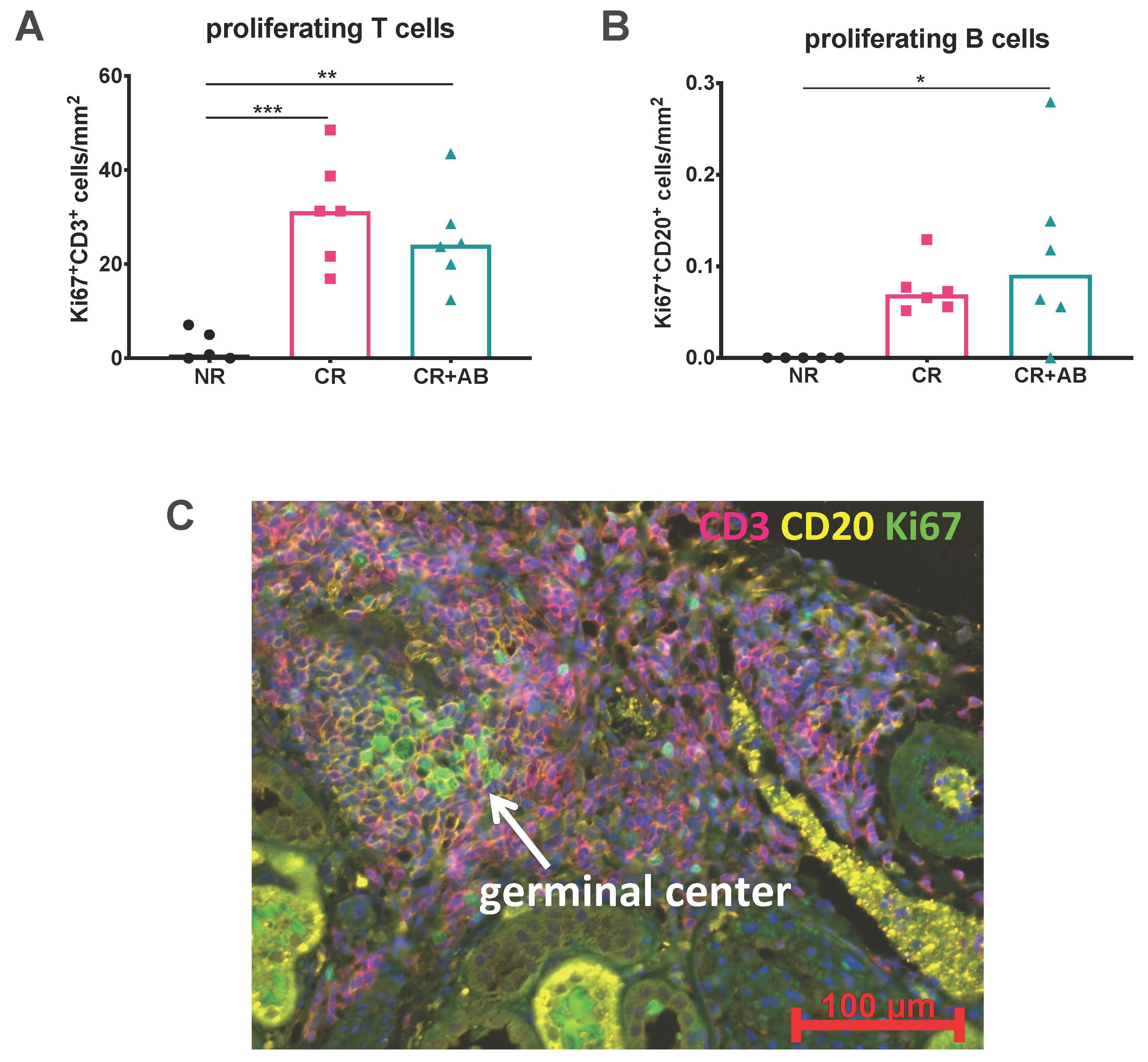
2.3. Proliferation of Intra-Renal T and B Cells Was Not Altered by Anti-BAFF Treatment
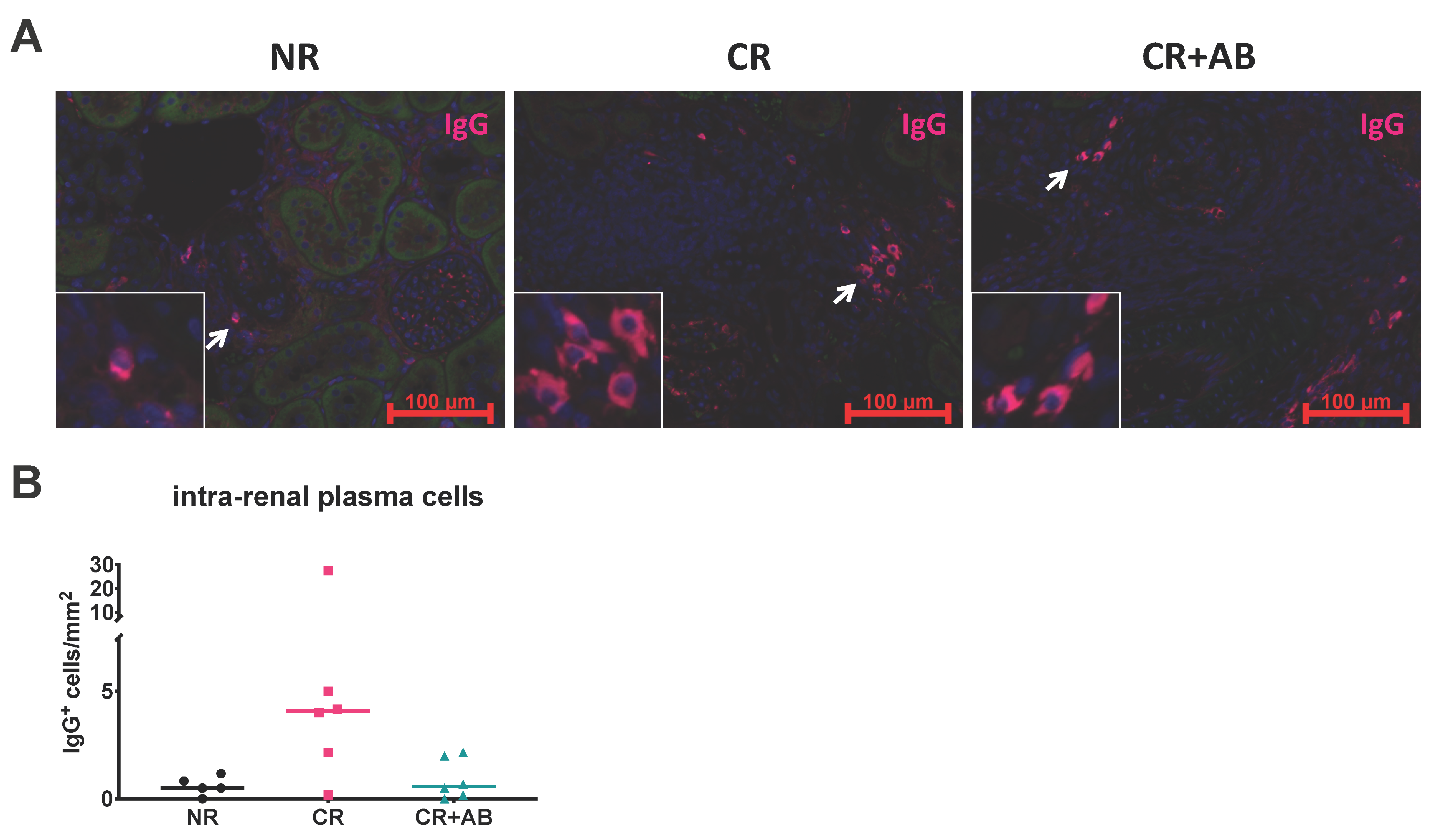
2.4. Effect of Anti-BAFF Treatment on Intra-Renal Plasma Cells
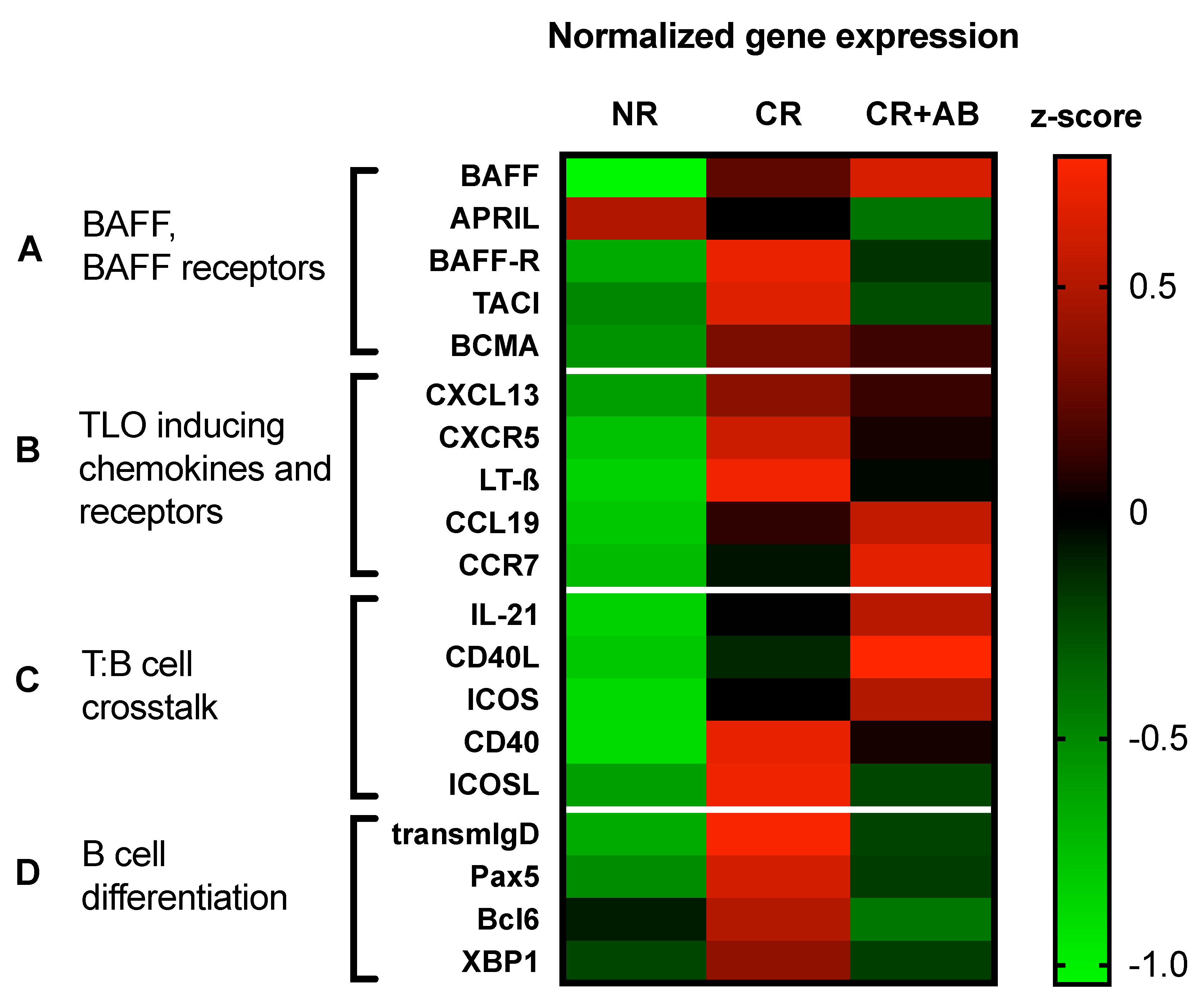
2.5. Anti-BAFF Treatment Regulated Expression of TLO-Promoting Factors and B Cell Differentiation Markers within Allografts
3. Discussion
4. Materials and Methods
4.1. Experimental Kidney Transplantation
4.2. Histology, Immunohistochemistry, and Immunofluorescence
4.3. Real-Time PCR
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AB | antibody |
| ABMR | antibody-mediated rejection |
| APRIL | a proliferation inducing ligand |
| BAFF | B cell activating factor |
| BAFF-R | BAFF receptor |
| Bcl-6 | B cell lymphoma 6 |
| BCMA | B cell maturation antigen |
| BN | Brown Norway rat |
| CD | cluster of differentiation |
| CD40L | CD40 ligand |
| CR | chronic rejection |
| CR + AB | chronic rejection + anti-BAFF antibody |
| CsA | cyclosporine A |
| HPRT | hypoxanthine-guanine-phosphoribosyl-transferase |
| GC | germinal center |
| ICOS | inducible T cell costimulator |
| ICOSL | ICOS ligand |
| IFTA | interstitial fibrosis and tubular atrophy |
| Ig | immunoglobulin |
| IL | interleukin |
| Ktx | kidney transplantation |
| LEW | Lewis rat |
| MHC | major histocompatibility complex |
| NR | no rejection |
| Pax5 | paired box protein 5 |
| SLE | systemic lupus erythematosus |
| TACI | transmembrane activator and calcium modulator and cyclophilin ligand interactor |
| TLO | tertiary lymphoid organs |
| XBP-1 | X-box binding protein 1 |
References
- Gondos, A.; Dohler, B.; Brenner, H.; Opelz, G. Kidney graft survival in Europe and the United States: Strikingly different long-term outcomes. Transplantation 2013, 95, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, C.; Loupy, A.; Hill, G.S.; Andrade, J.; Nochy, D.; Antoine, C.; Gautreau, C.; Charron, D.; Glotz, D.; Suberbielle-Boissel, C. Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J. Am. Soc. Nephrol. 2010, 21, 1398–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sellares, J.; Reeve, J.; Loupy, A.; Mengel, M.; Sis, B.; Skene, A.; de Freitas, D.G.; Kreepala, C.; Hidalgo, L.G.; Famulski, K.S.; et al. Molecular diagnosis of antibody-mediated rejection in human kidney transplants. Am. J. Transplant. 2013, 13, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Mannon, R.B.; Matas, A.J.; Grande, J.; Leduc, R.; Connett, J.; Kasiske, B.; Cecka, J.M.; Gaston, R.S.; Cosio, F.; Gourishankar, S.; et al. Inflammation in areas of tubular atrophy in kidney allograft biopsies: A potent predictor of allograft failure. Am. J. Transplant. 2010, 10, 2066–2073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shishido, S.; Asanuma, H.; Nakai, H.; Mori, Y.; Satoh, H.; Kamimaki, I.; Hataya, H.; Ikeda, M.; Honda, M.; Hasegawa, A. The impact of repeated subclinical acute rejection on the progression of chronic allograft nephropathy. J. Am. Soc. Nephrol. 2003, 14, 1046–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, W.D.; Griffin, M.D.; Cornell, L.D.; Cosio, F.G.; Stegall, M.D. Fibrosis with inflammation at one year predicts transplant functional decline. J. Am. Soc. Nephrol. 2010, 21, 1987–1997. [Google Scholar] [CrossRef] [Green Version]
- Tse, G.H.; Johnston, C.J.; Kluth, D.; Gray, M.; Gray, D.; Hughes, J.; Marson, L.P. Intrarenal B Cell Cytokines Promote Transplant Fibrosis and Tubular Atrophy. Am. J. Transplant. 2015, 15, 3067–3080. [Google Scholar] [CrossRef]
- Sarwal, M.; Chua, M.S.; Kambham, N.; Hsieh, S.C.; Satterwhite, T.; Masek, M.; Salvatierra, O., Jr. Molecular heterogeneity in acute renal allograft rejection identified by DNA microarray profiling. N. Engl. J. Med. 2003, 349, 125–138. [Google Scholar] [CrossRef] [Green Version]
- Hueso, M.; Navarro, E.; Moreso, F.; O’Valle, F.; Perez-Riba, M.; Del Moral, R.G.; Grinyo, J.M.; Seron, D. Intragraft expression of the IL-10 gene is up-regulated in renal protocol biopsies with early interstitial fibrosis, tubular atrophy, and subclinical rejection. Am. J. Pathol. 2010, 176, 1696–1704. [Google Scholar] [CrossRef] [Green Version]
- Thaunat, O.; Field, A.C.; Dai, J.; Louedec, L.; Patey, N.; Bloch, M.F.; Mandet, C.; Belair, M.F.; Bruneval, P.; Meilhac, O.; et al. Lymphoid neogenesis in chronic rejection: Evidence for a local humoral alloimmune response. Proc. Natl. Acad. Sci. USA 2005, 102, 14723–14728. [Google Scholar] [CrossRef] [Green Version]
- Thaunat, O.; Patey, N.; Caligiuri, G.; Gautreau, C.; Mamani-Matsuda, M.; Mekki, Y.; Dieu-Nosjean, M.C.; Eberl, G.; Ecochard, R.; Michel, J.B.; et al. Chronic rejection triggers the development of an aggressive intragraft immune response through recapitulation of lymphoid organogenesis. J. Immunol. 2010, 185, 717–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thaunat, O.; Graff-Dubois, S.; Brouard, S.; Gautreau, C.; Varthaman, A.; Fabien, N.; Field, A.C.; Louedec, L.; Dai, J.; Joly, E.; et al. Immune responses elicited in tertiary lymphoid tissues display distinctive features. PLoS ONE 2010, 5, e11398. [Google Scholar] [CrossRef]
- Pitzalis, C.; Jones, G.W.; Bombardieri, M.; Jones, S.A. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat. Rev. Immunol. 2014, 14, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Luther, S.A.; Bidgol, A.; Hargreaves, D.C.; Schmidt, A.; Xu, Y.; Paniyadi, J.; Matloubian, M.; Cyster, J.G. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J. Immunol. 2002, 169, 424–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziegler, E.; Gueler, F.; Rong, S.; Mengel, M.; Witzke, O.; Kribben, A.; Haller, H.; Kunzendorf, U.; Krautwald, S. CCL19-IgG prevents allograft rejection by impairment of immune cell trafficking. J. Am. Soc. Nephrol. 2006, 17, 2521–2532. [Google Scholar] [CrossRef]
- Cheng, J.; Torkamani, A.; Grover, R.K.; Jones, T.M.; Ruiz, D.I.; Schork, N.J.; Quigley, M.M.; Hall, F.W.; Salomon, D.R.; Lerner, R.A. Ectopic B-cell clusters that infiltrate transplanted human kidneys are clonal. Proc. Natl. Acad. Sci. USA 2011, 108, 5560–5565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koenig, A.; Thaunat, O. Lymphoid Neogenesis and Tertiary Lymphoid Organs in Transplanted Organs. Front. Immunol. 2016, 7, 646. [Google Scholar] [CrossRef] [Green Version]
- Thaunat, O.; Patey, N.; Gautreau, C.; Lechaton, S.; Fremeaux-Bacchi, V.; Dieu-Nosjean, M.C.; Cassuto-Viguier, E.; Legendre, C.; Delahousse, M.; Lang, P.; et al. B cell survival in intragraft tertiary lymphoid organs after rituximab therapy. Transplantation 2008, 85, 1648–1653. [Google Scholar] [CrossRef]
- Mackay, F.; Figgett, W.A.; Saulep, D.; Lepage, M.; Hibbs, M.L. B-cell stage and context-dependent requirements for survival signals from BAFF and the B-cell receptor. Immunol. Rev. 2010, 237, 205–225. [Google Scholar] [CrossRef]
- Irure-Ventura, J.; San Segundo, D.; Rodrigo, E.; Merino, D.; Belmar-Vega, L.; Ruiz San Millán, J.C.; Valero, R.; Benito, A.; López-Hoyos, M. High Pretransplant BAFF Levels and B-cell Subset Polarized towards a Memory Phenotype as Predictive Biomarkers for Antibody-Mediated Rejection. Int. J. Mol. Sci. 2020, 21, 779. [Google Scholar] [CrossRef] [Green Version]
- Friebus-Kardash, J.; Wilde, B.; Keles, D.; Heinold, A.; Kribben, A.; Witzke, O.; Heinemann, F.M.; Eisenberger, U. Pretransplant serum BAFF levels are associated with pretransplant HLA immunization and renal allograft survival. Transpl. Immunol. 2018, 47, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Kuhne, L.; Jung, B.; Poth, H.; Schuster, A.; Wurm, S.; Ruemmele, P.; Banas, B.; Bergler, T. Renal allograft rejection, lymphocyte infiltration, and de novo donor-specific antibodies in a novel model of non-adherence to immunosuppressive therapy. BMC Immunol. 2017, 18, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steines, L.; Poth, H.; Schuster, A.; Geissler, E.K.; Amann, K.; Banas, B.; Bergler, T. Anti-BAFF Treatment Interferes With Humoral Responses in a Model of Renal Transplantation in Rats. Transplantation 2020, 104, e16–e22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasegawa, J.; Honda, K.; Omoto, K.; Wakai, S.; Shirakawa, H.; Okumi, M.; Ishida, H.; Fuchinoue, S.; Hattori, M.; Tanabe, K. Clinical and Pathological Features of Plasma Cell-Rich Acute Rejection After Kidney Transplantation. Transplantation 2018, 102, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Kwun, J.; Manook, M.; Page, E.; Burghuber, C.; Hong, J.; Knechtle, S.J. Crosstalk Between T and B Cells in the Germinal Center After Transplantation. Transplantation 2017, 101, 704–712. [Google Scholar] [CrossRef] [Green Version]
- Krautler, N.J.; Suan, D.; Butt, D.; Bourne, K.; Hermes, J.R.; Chan, T.D.; Sundling, C.; Kaplan, W.; Schofield, P.; Jackson, J.; et al. Differentiation of germinal center B cells into plasma cells is initiated by high-affinity antigen and completed by Tfh cells. J. Exp. Med. 2017, 214, 1259–1267. [Google Scholar] [CrossRef] [Green Version]
- Baddoura, F.K.; Nasr, I.W.; Wrobel, B.; Li, Q.; Ruddle, N.H.; Lakkis, F.G. Lymphoid neogenesis in murine cardiac allografts undergoing chronic rejection. Am. J. Transplant. 2005, 5, 510–516. [Google Scholar] [CrossRef]
- Parsons, R.F.; Vivek, K.; Redfield, R.R.; 3rd Migone, T.S.; Cancro, M.P.; Naji, A.; Noorchashm, H. B-lymphocyte homeostasis and BLyS-directed immunotherapy in transplantation. Transplant. Rev. 2010, 24, 207–221. [Google Scholar] [CrossRef] [Green Version]
- Ngo, V.N.; Korner, H.; Gunn, M.D.; Schmidt, K.N.; Riminton, D.S.; Cooper, M.D.; Browning, J.L.; Sedgwick, J.D.; Cyster, J.G. Lymphotoxin alpha/beta and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J. Exp. Med. 1999, 189, 403–412. [Google Scholar] [CrossRef] [Green Version]
- Grabner, R.; Lotzer, K.; Dopping, S.; Hildner, M.; Radke, D.; Beer, M.; Spanbroek, R.; Lippert, B.; Reardon, C.A.; Getz, G.S.; et al. Lymphotoxin beta receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE-/- mice. J. Exp. Med. 2009, 206, 233–248. [Google Scholar] [CrossRef] [Green Version]
- Vinuesa, C.G.; Linterman, M.A.; Yu, D.; MacLennan, I.C. Follicular Helper T Cells. Annu. Rev. Immunol. 2016, 34, 335–368. [Google Scholar] [CrossRef] [PubMed]
- Reimold, A.M.; Iwakoshi, N.N.; Manis, J.; Vallabhajosyula, P.; Szomolanyi-Tsuda, E.; Gravallese, E.M.; Friend, D.; Grusby, M.J.; Alt, F.; Glimcher, L.H. Plasma cell differentiation requires the transcription factor XBP-1. Nature 2001, 412, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Wehner, J.R.; Fox-Talbot, K.; Halushka, M.K.; Ellis, C.; Zachary, A.A.; Baldwin, W.M., 3rd. B cells and plasma cells in coronaries of chronically rejected cardiac transplants. Transplantation 2010, 89, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Einecke, G.; Reeve, J.; Mengel, M.; Sis, B.; Bunnag, S.; Mueller, T.F.; Halloran, P.F. Expression of B cell and immunoglobulin transcripts is a feature of inflammation in late allografts. Am. J. Transplant. 2008, 8, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
- Desvaux, D.; Le Gouvello, S.; Pastural, M.; Abtahi, M.; Suberbielle, C.; Boeri, N.; Rémy, P.; Salomon, L.; Lang, P.; Baron, C. Acute renal allograft rejections with major interstitial oedema and plasma cell-rich infiltrates: High gamma-interferon expression and poor clinical outcome. Nephrol. Dial. Transplant. 2004, 19, 933–939. [Google Scholar] [CrossRef] [Green Version]
- Hamada, A.M.; Yamamoto, I.; Kawabe, M.; Katsumata, H.; Yamakawa, T.; Katsuma, A.; Nakada, Y.; Kobayashi, A.; Koike, Y.; Miki, J.; et al. Clinicopathological features and outcomes of kidney allografts in plasma cell-rich acute rejection: A case series. Nephrology 2018, 23, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Banham, G.D.; Flint, S.M.; Torpey, N.; Lyons, P.A.; Shanahan, D.N.; Gibson, A.; Watson CJ, E.; O’Sullivan, A.M.; Chadwick, J.A.; Foster, K.E.; et al. Belimumab in kidney transplantation: An experimental medicine, randomised, placebo-controlled phase 2 trial. Lancet 2018, 391, 2619–2630. [Google Scholar] [CrossRef] [Green Version]
- Bergler, T.; Hoffmann, U.; Bergler, E.; Jung, B.; Banas, M.C.; Reinhold, S.W.; Kramer, B.K.; Banas, B. Toll-like receptor 4 in experimental kidney transplantation: Early mediator of endogenous danger signals. Nephron Exp. Nephrol. 2012, 121, e59–e70. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, U.; Bergler, T.; Jung, B.; Steege, A.; Pace, C.; Rummele, P.; Reinhold, S.; Kruger, B.; Kramer, B.K.; Banas, B. Comprehensive morphometric analysis of mononuclear cell infiltration during experimental renal allograft rejection. Transpl. Immunol. 2013, 28, 24–31. [Google Scholar] [CrossRef]
| Group | Abbreviation | n= |
|---|---|---|
| Ktx CsA 10 mg/kg/d d56 (no rejection) | NR | 5 |
| Ktx CsA 5 mg/kg/2nd d d56 (chronic rejection) | CR | 6 |
| Ktx CsA 5 mg/kg/2nd d d56 + anti-BAFF antibody (chronic rejection + anti-BAFF antibody) | CR + AB | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steines, L.; Poth, H.; Herrmann, M.; Schuster, A.; Banas, B.; Bergler, T. B Cell Activating Factor (BAFF) Is Required for the Development of Intra-Renal Tertiary Lymphoid Organs in Experimental Kidney Transplantation in Rats. Int. J. Mol. Sci. 2020, 21, 8045. https://doi.org/10.3390/ijms21218045
Steines L, Poth H, Herrmann M, Schuster A, Banas B, Bergler T. B Cell Activating Factor (BAFF) Is Required for the Development of Intra-Renal Tertiary Lymphoid Organs in Experimental Kidney Transplantation in Rats. International Journal of Molecular Sciences. 2020; 21(21):8045. https://doi.org/10.3390/ijms21218045
Chicago/Turabian StyleSteines, Louisa, Helen Poth, Marlene Herrmann, Antonia Schuster, Bernhard Banas, and Tobias Bergler. 2020. "B Cell Activating Factor (BAFF) Is Required for the Development of Intra-Renal Tertiary Lymphoid Organs in Experimental Kidney Transplantation in Rats" International Journal of Molecular Sciences 21, no. 21: 8045. https://doi.org/10.3390/ijms21218045
APA StyleSteines, L., Poth, H., Herrmann, M., Schuster, A., Banas, B., & Bergler, T. (2020). B Cell Activating Factor (BAFF) Is Required for the Development of Intra-Renal Tertiary Lymphoid Organs in Experimental Kidney Transplantation in Rats. International Journal of Molecular Sciences, 21(21), 8045. https://doi.org/10.3390/ijms21218045





