Impact of Deoxycholic Acid on Oesophageal Adenocarcinoma Invasion: Effect on Matrix Metalloproteinases
Abstract
:1. Introduction
2. Results
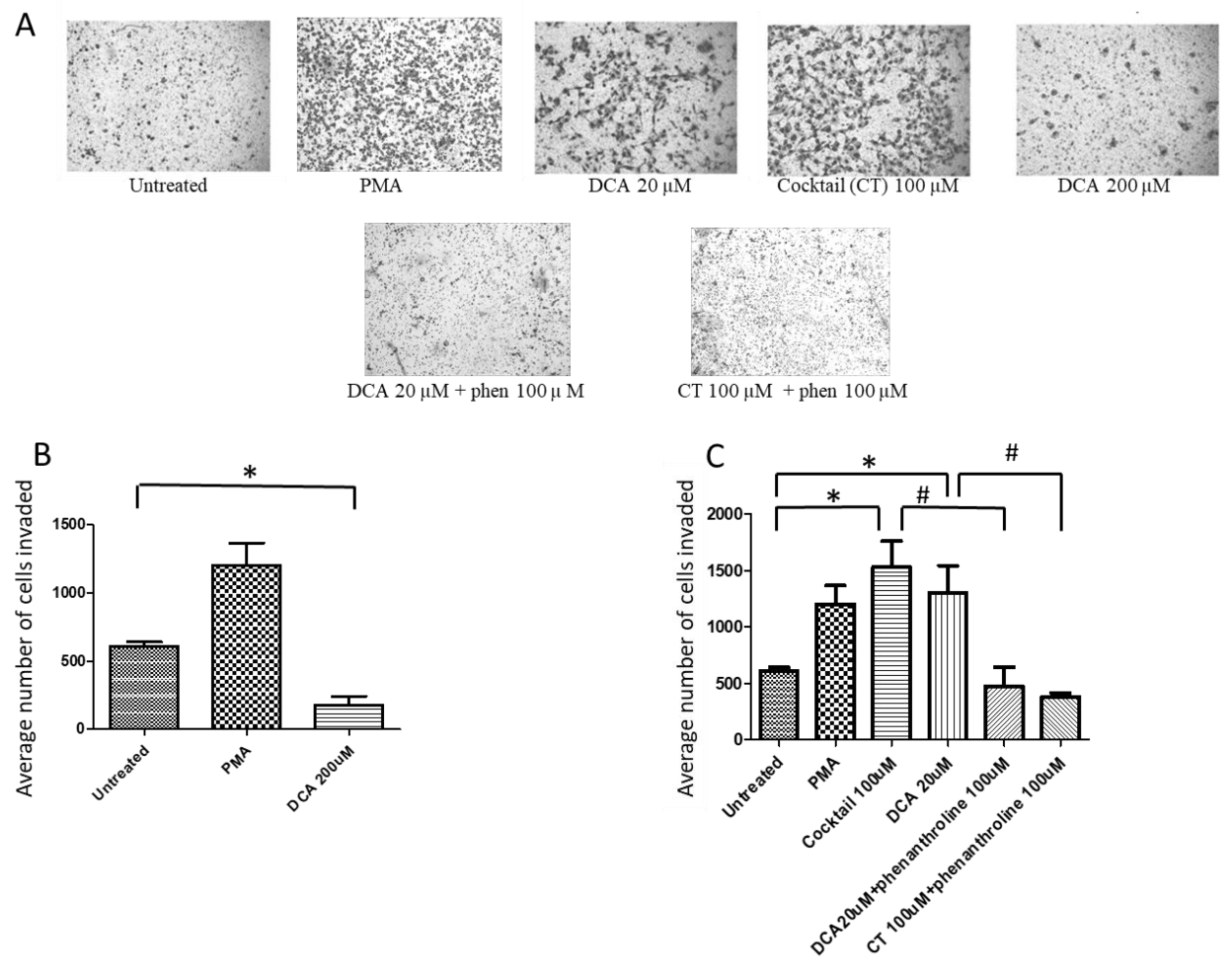
2.1. Bile Acids Promote OAC Cell Invasion
2.2. DCA at High Concentration Reduces Cell Viability
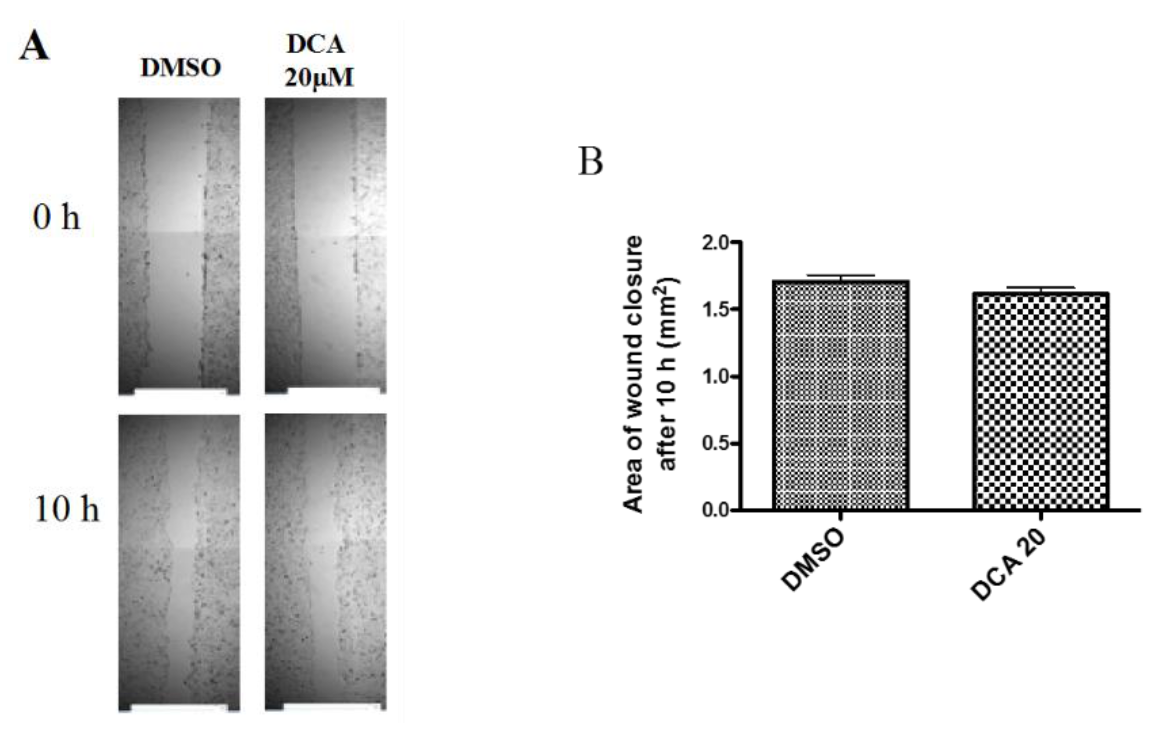
2.3. DCA Does Not Exert Any Effect on 2D Cell Migration
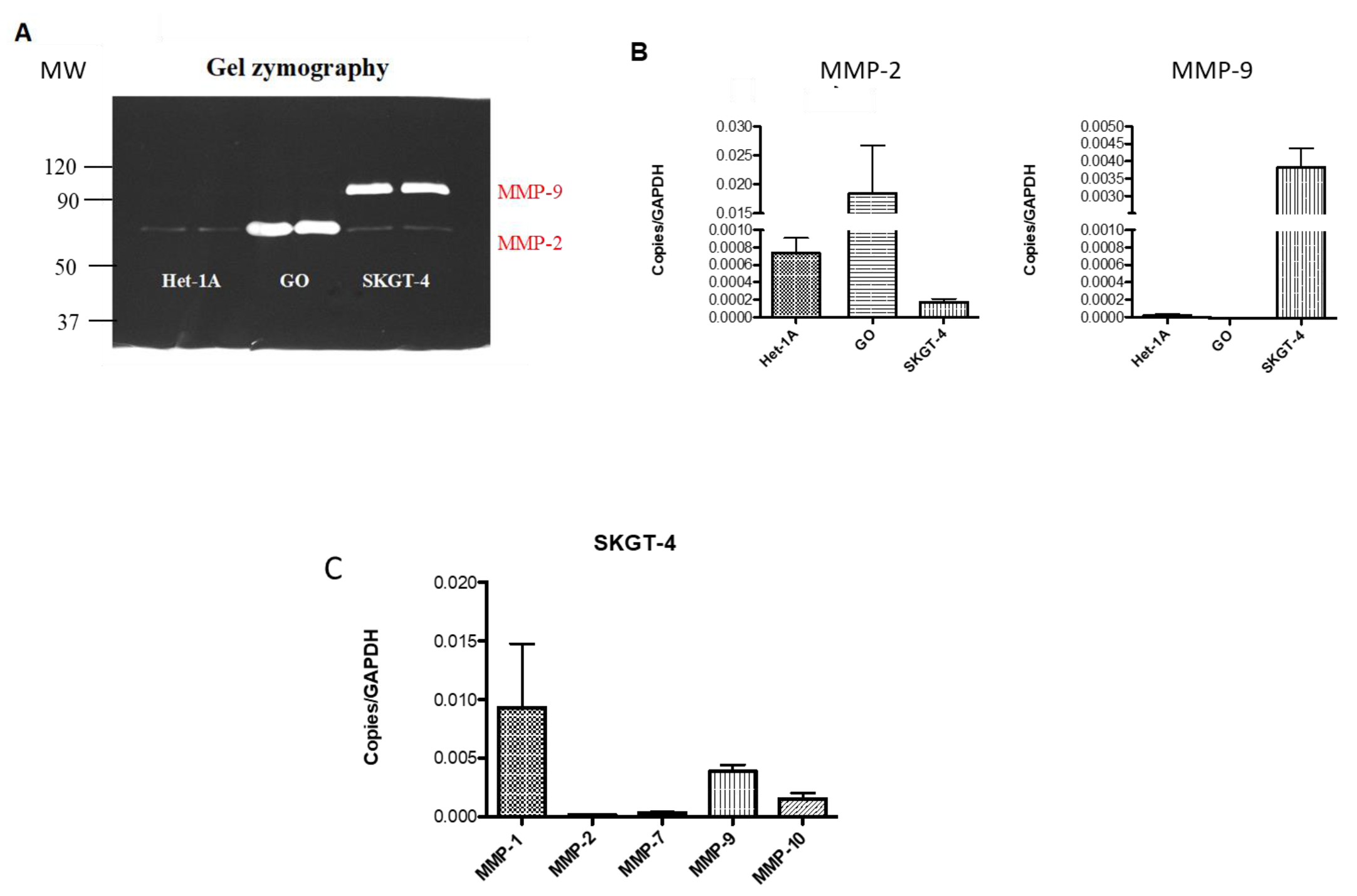
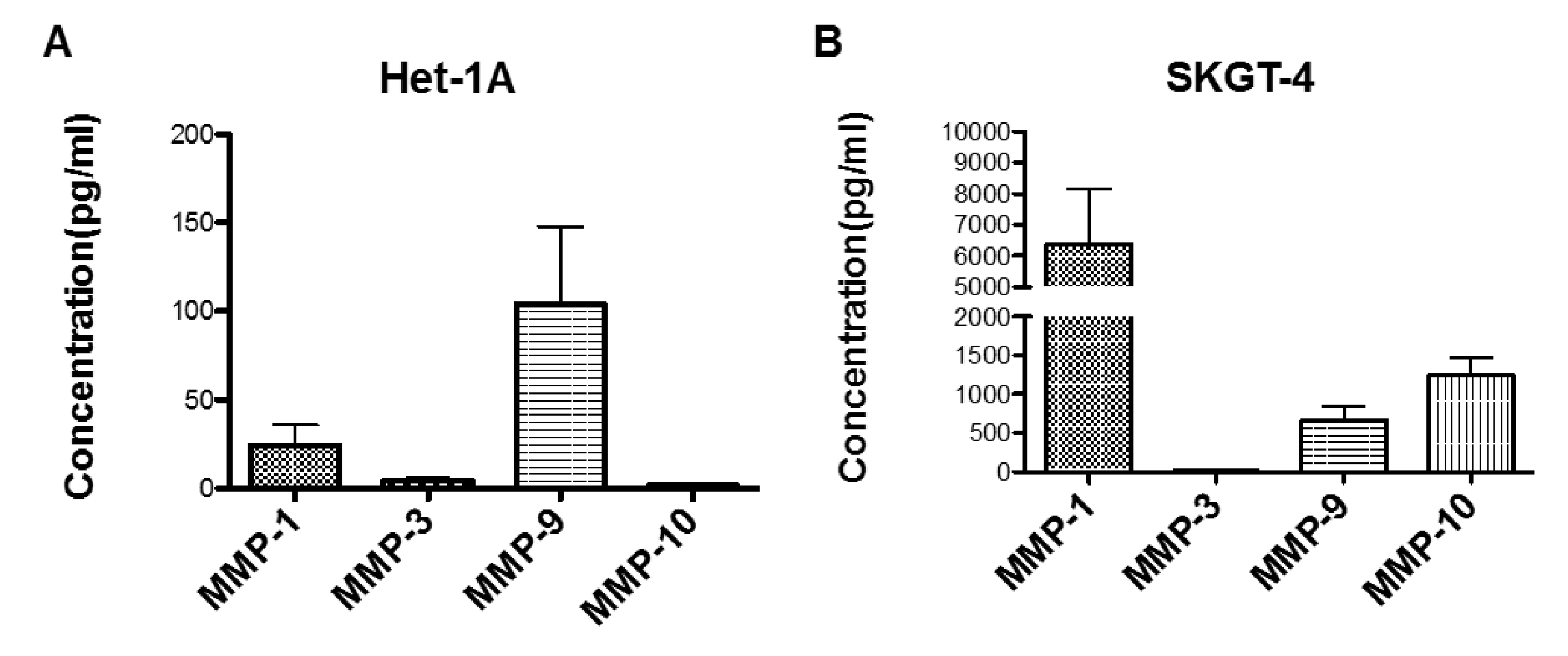
2.4. Profiling MMP Expression and Activity in Oesophageal Epithelial Cell Lines Representing the Normal-BO-OAC Sequence
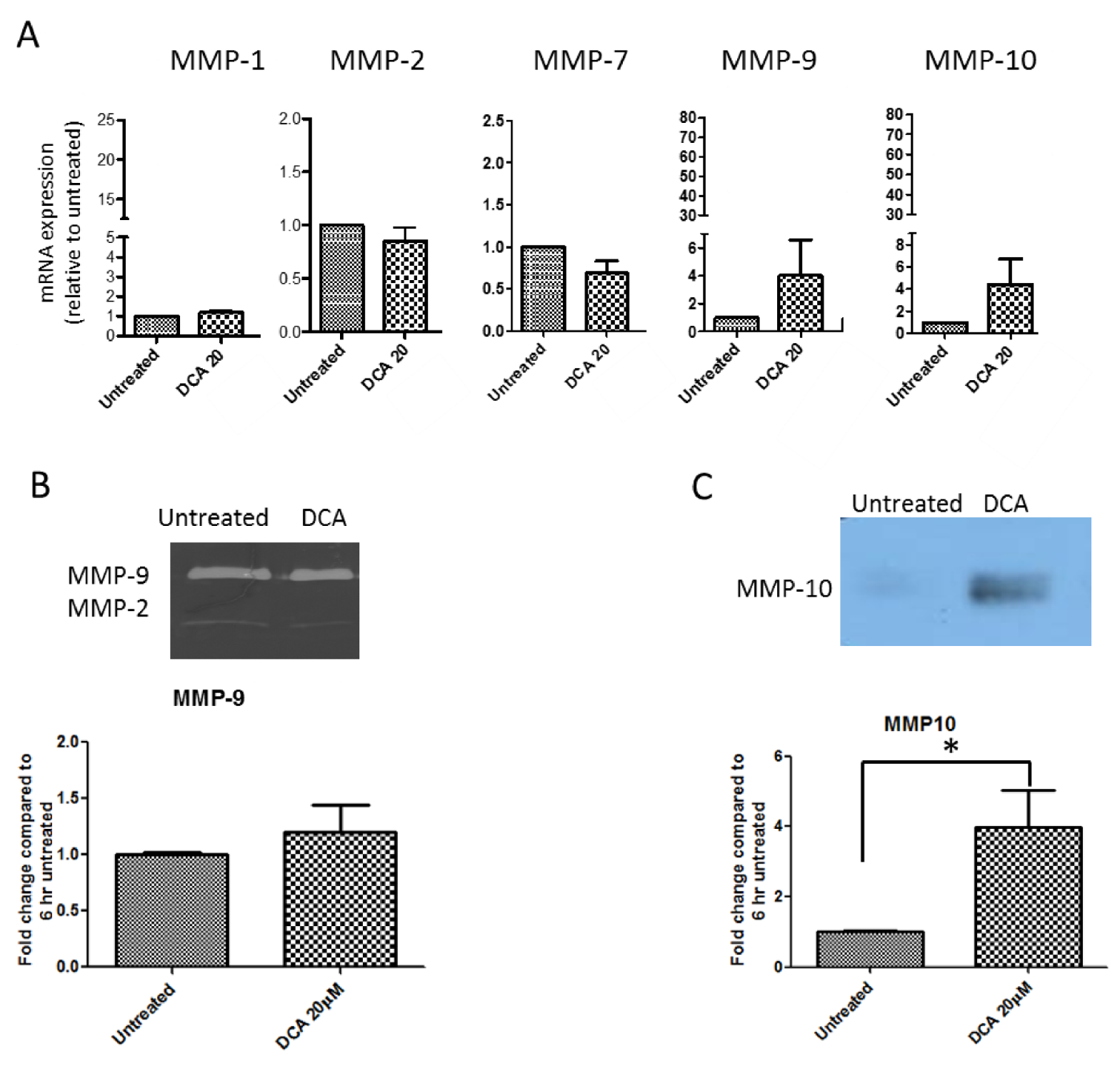
2.5. Measurement of Effects of BAs on MMP mRNA Expression in SKGT-4 Cells
2.6. DCA Up-Regulates MMP-10 Protein Expression in SKGT-4 Cells
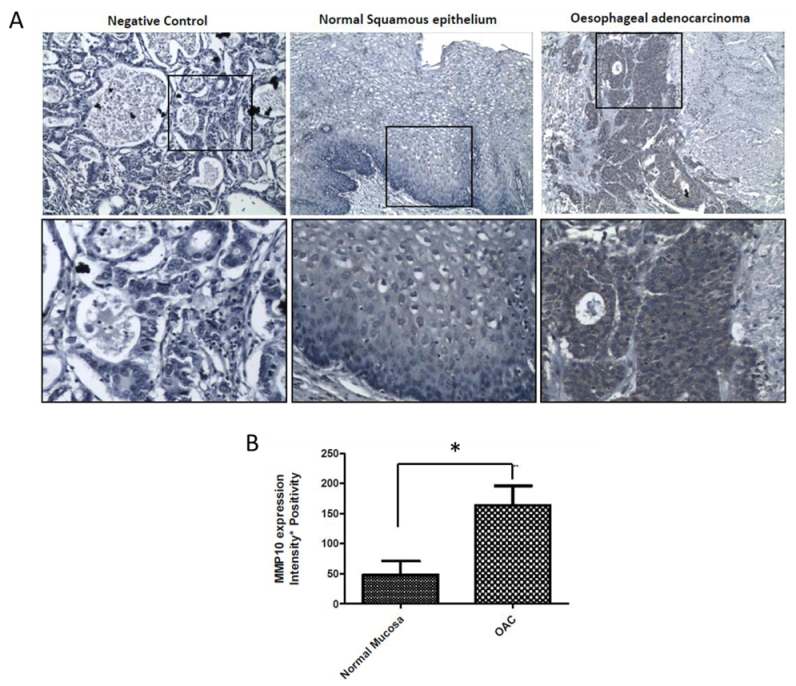
2.7. MMP-10 Is Over-Expressed in OAC Patient Tissue
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Invasion Assay
4.3. Cell Survival
4.4. Assessment of the 2D Migration Using the Scratch Wound Assay
4.5. MMP mRNA Expression Using Quantitative Real-Time PCR
4.6. MMP Activity Detection by Gelatin Zymography
4.7. MMP Secretion Detection by Multiplex ELISA
4.8. MMP Expression Detection by Western Blotting
4.9. Immunohistochemical Staining and Analysis in Human Samples
4.10. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Schlottmann, F.; Patti, M.G.; Shaheen, N.J. From Heartburn to Barrett’s Esophagus, and Beyond. World J. Surg. 2017, 41, 1698–1704. [Google Scholar] [CrossRef]
- Shaheen, N.J.; Crosby, M.A.; Bozymski, E.M.; Sandler, R.S. Is there publication bias in the reporting of cancer risk in Barrett’s esophagus? Gastroenterology 2000, 119, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Bytzer, P.; Christensen, P.B.; Damkier, P.; Vinding, K.; Seersholm, N. Adenocarcinoma of the esophagus and Barrett’s esophagus: a population-based study. Am. J. Gastroenterol. 1999, 94, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.F. The Function of Bile Sats in Fat Absorption. The Solvent Properties of Dilute Micellar Solutions of Conjugated Biles Salts. Biochem. J. 1963, 89, 57–68. [Google Scholar] [CrossRef] [Green Version]
- Bokkenheuser, V.; Hoshita, T.; Mosbach, E.H. Bacterial 7-dehydroxylation of cholic acid and allocholic acid. J. Lipid Res. 1969, 10, 421–426. [Google Scholar] [PubMed]
- Straub, D.; Elferink, R.P.J.O.; Jansen, P.L.M.; Bergman, J.J.G.H.M.; Parikh, K.; Krishnadath, K.K. Glyco-conjugated bile acids drive the initial metaplastic gland formation from multi-layered glands through crypt-fission in a murine model. PLoS ONE 2019, 14, e0220050. [Google Scholar] [CrossRef] [Green Version]
- McQuaid, K.R.; Laine, L.; Fennerty, M.B.; Souza, R.; Spechler, S.J. Systematic review: the role of bile acids in the pathogenesis of gastro-oesophageal reflux disease and related neoplasia. Aliment. Pharmacol. Ther. 2011, 34, 146–165. [Google Scholar] [CrossRef]
- O’Riordan, J.M.; Abdel-Latif, M.M.; Ravi, N.; McNamara, D.; Byrne, P.J.; McDonald, G.S.A.; Keeling, P.W.N.; Kelleher, D.; Reynolds, J.V. Proinflammatory Cytokine and Nuclear Factor Kappa-B Expression along the Inflammation-Metaplasia-Dysplasia-Adenocarcinoma Sequence in the Esophagus. Am. J. Gastroenterol. 2005, 100, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, G.J.S.; Mikhail, J.; Alhamdani, A.; Brown, T.H.; Caplin, S.; Manson, J.M.; Bowden, R.; Toffazal, N.; Griffiths, A.P.; Parry, J.M.; et al. Immunohistochemical study of nuclear factor-kappaB activity and interleukin-8 abundance in oesophageal adenocarcinoma; a useful strategy for monitoring these biomarkers. J. Clin. Pathol. 2007, 60, 1232–1237. [Google Scholar] [CrossRef] [Green Version]
- Huo, X.; Zhang, X.; Yu, C.; Zhang, Q.; Cheng, E.; Wang, D.H.; Pham, T.H.; Spechler, S.J.; Souza, R.F. In oesophageal squamous cells exposed to acidic bile salt medium, omeprazole inhibits IL-8 expression through effects on nuclear factor-κB and activator protein-1. Gut 2014, 63, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Juergens, S.; Zhang, X.; Rezaei, D.; Yu, C.; Strauch, E.D.; Wang, J.Y.; Cheng, E.; Meyer, F.; Wang, D.H.; et al. Deoxycholic acid causes DNA damage while inducing apoptotic resistance through NF-κB activation in benign Barrett’s epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G278–G286. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Williams, V.A.; Gellersen, O.; Jones, C.; Watson, T.J.; Peters, J.H. The Pathogenesis of Barrett’s Esophagus: Secondary Bile Acids Upregulate Intestinal Differentiation Factor CDX2 Expression in Esophageal Cells. J. Gastrointest. Surg. 2007, 11, 827–834. [Google Scholar] [CrossRef]
- Mutoh, H.; Sakamoto, H.; Hayakawa, H.; Arao, Y.; Satoh, K.; Nokubi, M.; Sugano, K. The intestine-specific homeobox gene Cdx2 induces expression of the basic helix–loop–helix transcription factor Math1. Differentiation 2006, 74, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Chinyama, C.N.; Marshall, R.E.; Owen, W.J.; Mason, R.C.; Kothari, D.; Wilkinson, M.L.; Sanderson, J.D. Expression of MUC1 and MUC2 mucin gene products in Barrett’s metaplasia, dysplasia and adenocarcinoma: An immunopathological study with clinical correlation. Histopathology 1999, 35, 517–524. [Google Scholar] [CrossRef]
- Wu, J.; Gong, J.; Geng, J.; Song, Y. Deoxycholic acid induces the overexpression of intestinal mucin, MUC2, via NF-kB signaling pathway in human esophageal adenocarcinoma cells. BMC Cancer 2008, 8, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishijima, K.; Miwa, K.; Miyashita, T.; Kinami, S.; Ninomiya, I.; Fushida, S.; Fujimura, T.; Hattori, T. Impact of the Biliary Diversion Procedure on Carcinogenesis in Barrett’s Esophagus Surgically Induced by Duodenoesophageal Reflux in Rats. Ann. Surg. 2004, 240, 57–67. [Google Scholar] [CrossRef]
- Sun, D.; Wang, X.; Gai, Z.; Song, X.; Jia, X.; Tian, H. Bile acids but not acidic acids induce Barrett’s esophagus. Int. J. Clin. Exp. Pathol. 2015, 8, 1384–1392. [Google Scholar]
- John, A.; Tuszynski, G. The role of matrix metalloproteinases in tumor angiogenesis and tumor metastasis. Pathol. Oncol. Res. 2001, 7, 14–23. [Google Scholar] [CrossRef]
- Sanita, P.; Angelucci, A. Roles of Metalloproteases in Metastatic Niche. Curr. Mol. Med. 2011, 11, 609–622. [Google Scholar]
- Stetler-Stevenson, W.G. The Role of Matrix Metalloproteinases in Tumor Invasion, Metastasis, and Angiogenesis. Surg. Oncol. Clin. North Am. 2001, 10, 383–392. [Google Scholar] [CrossRef]
- Bibby, B.A.S.; Miranda, C.S.; Reynolds, J.V.; Cawthorne, C.J.; Maher, S.G. Silencing microRNA-330-5p increases MMP1 expression and promotes an invasive phenotype in oesophageal adenocarcinoma. BMC Cancer 2019, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.; Lazariotou, M.; Kircher, S.; Stuermer, L.; Reiber, C.; Höfelmayr, A.; Gattenlöhner, S.; Otto, C.; Germer, C.T.; Von Rahden, B.H.A. MMP-1 is a (pre-)invasive factor in Barrett-associated esophageal adenocarcinomas and is associated with positive lymph node status. J. Transl. Med. 2010, 8, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, N.; Zhang, R.; Wu, J.; Pu, Y.; Yin, X.; Cheng, Y.; Wu, J.; Feng, C.; Luo, Y.; Zhang, J. Interleukin-17A promotes esophageal adenocarcinoma cell invasiveness through ROS-dependent, NF-κB-mediated MMP-2/9 activation. Oncol. Rep. 2017, 37, 1779–1785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garalla, H.M.; Lertkowit, N.; Tiszlavicz, L.; Reisz, Z.; Holmberg, C.; Beynon, R.; Simpson, D.; Varga, A.; Kumar, J.D.; Dodd, S.; et al. Matrix metalloproteinase (MMP)-7 in Barrett’s esophagus and esophageal adenocarcinoma: Expression, metabolism, and functional significance. Physiol. Rep. 2018, 6, e13683. [Google Scholar] [CrossRef]
- Allott, E.H.; Lysaght, J.; Cathcart, M.C.; Donohoe, C.L.; Cummins, R.; McGarrigle, S.A.; Kay, E.; Reynolds, J.V.; Pidgeon, G.P. MMP9 expression in oesophageal adenocarcinoma is upregulated with visceral obesity and is associated with poor tumour differentiation. Mol. Carcinog. 2013, 52, 144–154. [Google Scholar] [CrossRef]
- Clemons, N.J.; Shannon, N.B.; Abeyratne, L.R.; Walker, C.E.; Saadi, A.; O’Donovan, M.L.; Lao-Sirieix, P.P.; Fitzgerald, R.C. Nitric oxide-mediated invasion in Barrett’s high-grade dysplasia and adenocarcinoma. Carcinogenesis 2010, 31, 1669–1675. [Google Scholar] [CrossRef] [Green Version]
- Halvorsen, B.; Staff, A.C.; Ligaarden, S.; Prydz, K.; Kolset, S.O. Lithocholic acid and sulphated lithocholic acid differ in the ability to promote matrix metalloproteinase secretion in the human colon cancer cell line CaCo-2. Biochem. J. 2000, 349, 189–193. [Google Scholar] [CrossRef]
- Nehra, D.; Howell, P.; Williams, C.P.; Pye, J.K.; Beynon, J. Toxic bile acids in gastro-oesophageal reflux disease: Influence of gastric acidity. Gut 1999, 44, 598–602. [Google Scholar] [CrossRef]
- Darragh, J.; Hunter, M.; Pohler, E.; Nelson, L.; Dillon, J.F.; Nenutil, R.; Vojtesek, B.; Ross, P.E.; Kernohan, N.; Hupp, T.R. The calcium-binding domain of the stress protein SEP53 is required for survival in response to deoxycholic acid-mediated injury. FEBS J. 2006, 273, 1930–1947. [Google Scholar] [CrossRef]
- Radziwon-Balicka, A.; Santos-Martinez, M.J.; Corbalan, J.J.; O’Sullivan, S.; Treumann, A.; Gilmer, J.F.; Radomski, M.W.; Medina, C. Mechanisms of platelet-stimulated colon cancer invasion: Role of clusterin and thrombospondin 1 in regulation of the P38MAPK-MMP-9 pathway. Carcinogenesis 2014, 35, 324–332. [Google Scholar] [CrossRef]
- Pai, R.; Tarnawski, A.S.; Tran, T. Deoxycholic acid activates beta-catenin signaling pathway and increases colon cell cancer growth and invasiveness. Mol. Biol. Cell 2004, 15, 2156–2163. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Friedl, P. Interstitial cell migration: Integrin-dependent and alternative adhesion mechanisms. Cell Tissue Res. 2010, 339, 83–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, K.; Lindert, M.T.; Krause, M.; Alexander, S.; Riet, J.T.; Willis, A.L.; Hoffman, R.M.; Figdor, C.G.; Weiss, S.J.; Friedl, P. Physical limits of cell migration: Control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J. Cell Biol. 2013, 201, 1069–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef] [Green Version]
- Winer, A.; Adams, S.; Mignatti, P. Matrix Metalloproteinase Inhibitors in Cancer Therapy: Turning Past Failures into Future Successes. Mol. Cancer Ther. 2018, 17, 1147–1155. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.-D.; Song, K.-S.; Li, G.; Choi, H.; Park, H.-D.; Lim, K.; Hwang, B.-D.; Yoon, W.-H. Activity and expression of urokinase-type plasminogen activator and matrix metalloproteinases in human colorectal cancer. BMC Cancer 2006, 6, 211. [Google Scholar] [CrossRef] [Green Version]
- Duggan, S.; Behan, F.M.; Kirca, M.; Smith, S.M.; Reynolds, J.V.; Long, A.; Kelleher, D. An integrative genomic approach in oesophageal cells identifies TRB3 as a bile acid responsive gene, downregulated in Barrett’s oesophagus, which regulates NF-κB activation and cytokine levels. Carcinogenesis 2010, 31, 936–945. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Roth, M.J.; Dawsey, S.M.; Yan, W.; Rodriguez-Canales, J.; Erickson, H.S.; Shuang-Mei, Z.; Goldstein, A.M.; Taylor, P.R.; Richardson, A.M.; et al. Increased matrix metalloproteinase activation in esophageal squamous cell carcinoma. J. Transl. Med. 2010, 8, 91. [Google Scholar] [CrossRef] [Green Version]
- Greenhill, C. Esophageal cancer: Matrix metalloproteinase 10 is associated with survival in patients with esophageal squamous cell carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 63. [Google Scholar]
- Sargent, J.M.; Taylor, C.G. Appraisal of the MTT assay as a rapid test of chemosensitivity in acute myeloid leukaemia. Br. J. Cancer 1989, 60, 206–210. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.; Wang, J.; Pigott, M.T.; Docherty, N.; Boyle, N.; Lis, S.K.; Gilmer, J.F.; Medina, C. Inhibition of matrix metalloproteinase-9 by a barbiturate-nitrate hybrid ameliorates dextran sulphate sodium-induced colitis: Effect on inflammation-related genes. Br. J. Pharmacol. 2017, 174, 512–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quilty, F.; Byrne, A.-M.; Aird, J.; El Mashad, S.; Parra-Blanco, A.; Long, A.; Gilmer, J.F.; Medina, C. Impact of Deoxycholic Acid on Oesophageal Adenocarcinoma Invasion: Effect on Matrix Metalloproteinases. Int. J. Mol. Sci. 2020, 21, 8042. https://doi.org/10.3390/ijms21218042
Quilty F, Byrne A-M, Aird J, El Mashad S, Parra-Blanco A, Long A, Gilmer JF, Medina C. Impact of Deoxycholic Acid on Oesophageal Adenocarcinoma Invasion: Effect on Matrix Metalloproteinases. International Journal of Molecular Sciences. 2020; 21(21):8042. https://doi.org/10.3390/ijms21218042
Chicago/Turabian StyleQuilty, Fran, Anne-Marie Byrne, John Aird, Sheeren El Mashad, Adolfo Parra-Blanco, Aideen Long, John F Gilmer, and Carlos Medina. 2020. "Impact of Deoxycholic Acid on Oesophageal Adenocarcinoma Invasion: Effect on Matrix Metalloproteinases" International Journal of Molecular Sciences 21, no. 21: 8042. https://doi.org/10.3390/ijms21218042



