Custodiol-N Is Superior to Custodiol® Solution in Experimental Rat Uterus Preservation
Abstract
:1. Introduction
2. Results
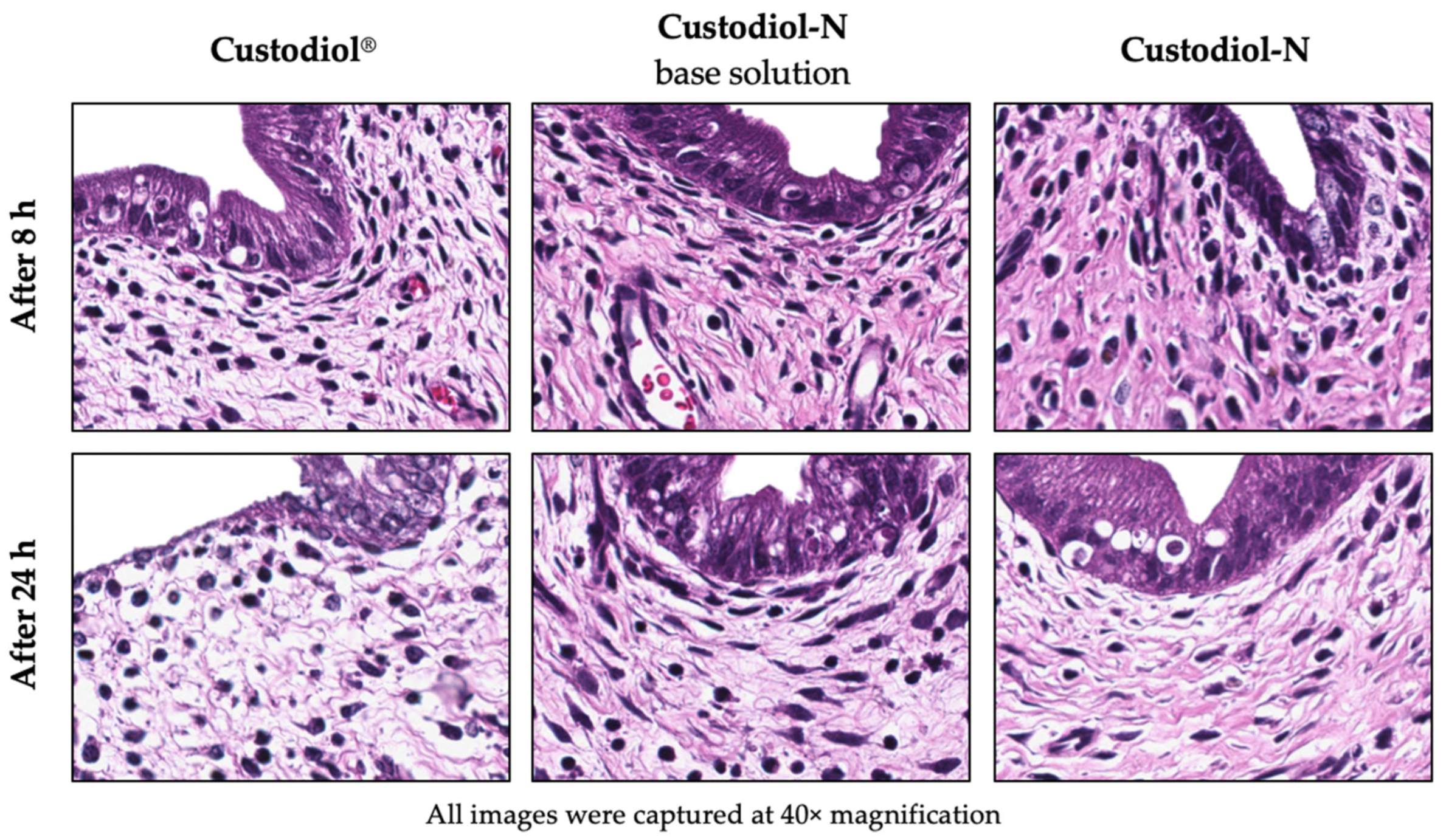
2.1. Histology
2.2. Tissue MPO Expression
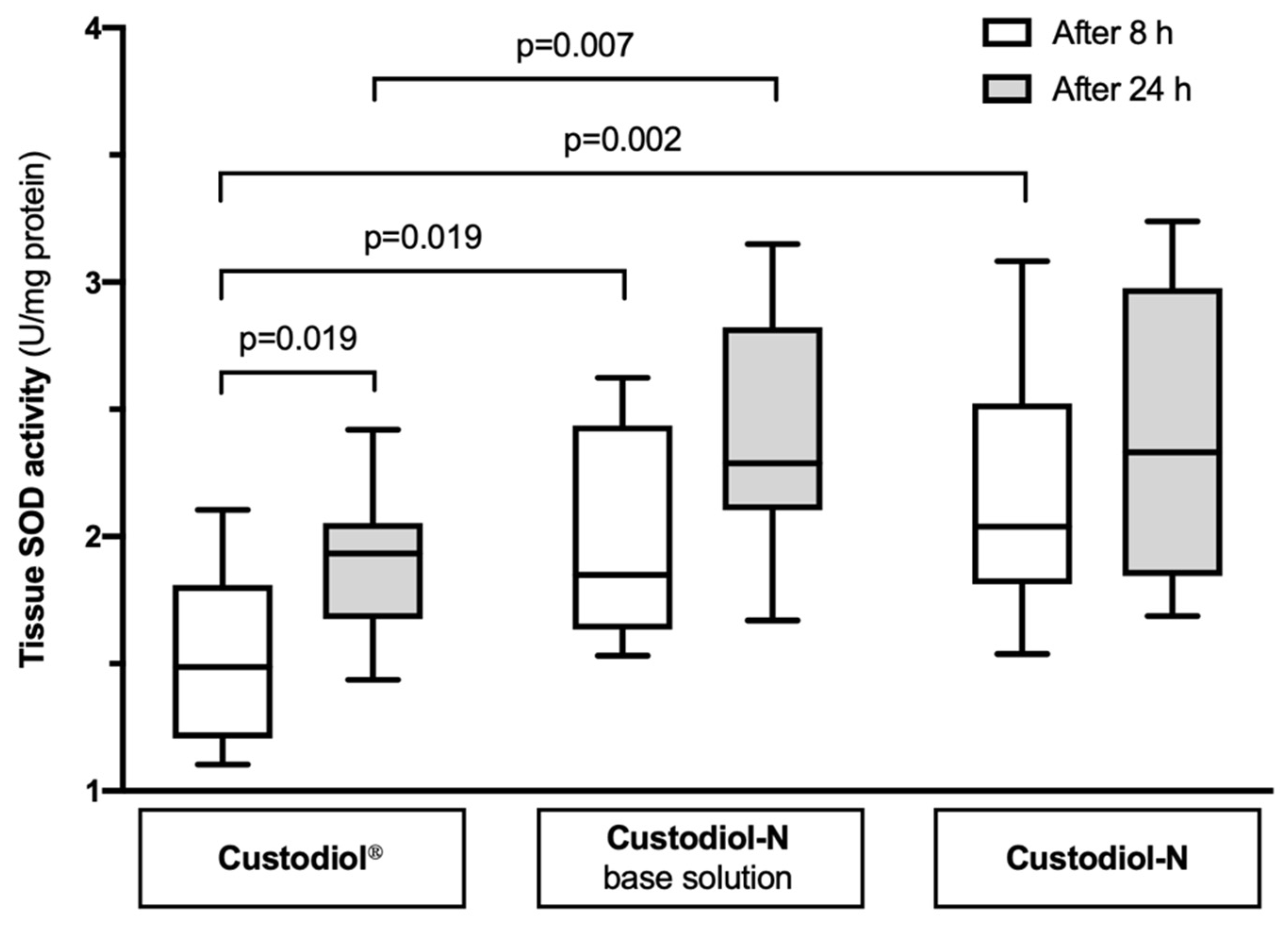
2.3. Tissue SOD Activity
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Uterus Procurement
4.3. Experimental Groups, Static Cold Storage (SCS), and Sampling
4.4. Histology
4.5. Immunohistochemical (IHC) Staining
4.6. Biochemistry
4.7. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AUFI | absolute uterine factor infertility |
| UTx | uterus transplantation |
| H&E | hematoxylin and eosin |
| IHC | immunohistochemistry |
| IRI | ischemia/reperfusion injury |
| MPO | myeloperoxidase |
| ROS | reactive oxygen species |
| SCS | static cold storage |
| SOD | superoxide dismutase |
References
- Groth, K.; Akhi, S.N.; Mölne, J.; Wranning, C.A.; Brännström, M. Effects of immunosuppression by cyclosporine A on allogenic uterine transplant in the rat. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 163, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Zitkute, V.; Kvietkauskas, M.; Leber, B.; Strupas, K.; Stiegler, P.; Schemmer, P. Ischemia and reperfusion injury in uterus transplantation: A comprehensive review. Transplant. Rev. 2020, 34, 100550. [Google Scholar] [CrossRef]
- Brännström, M.; Dahm-Kähler, P.; Greite, R.; Mölne, J.; Diaz-Garcia, C.; Tullius, S.G. Uterus transplantation: A rapidly expanding field. Transplantation 2018, 102, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Brännström, M.; Johannesson, L.; Bokström, H.; Kvarnström, N.; Mölne, J.; Dahm-Kähler, P.; Enskog, A.; Milenkovic, M.; Ekberg, J.; Diaz-Garcia, C.; et al. Livebirth after uterus transplantation. Lancet 2015, 385, 607–616. [Google Scholar] [CrossRef] [Green Version]
- Dion, L.; Tardieu, A.; Piver, P.; Aubard, Y.; Ayoubi, J.; Garbin, O.; Agostini, A.; Collinet, P.; Gauthier, T.; Lavoué, V.; et al. Uterus transplantation: Where do we stand in 2018? J. Gynecol. Obstet. Hum. Reprod. 2019, 48, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Johannesson, L.; Testa, G.; Flyckt, R.; Farrell, R.M.; Quintini, C.; Wall, A.E.; O’Neill, K.E.; Tzakis, A.G.; Richards, E.G.; Gordon, S.M.; et al. Guidelines for standardized nomenclature and reporting in uterus transplantation: An opinion from the United States Uterus Transplant Consortium. Arab. Archaeol. Epigr. 2020. [Google Scholar] [CrossRef]
- Lavoué, V.; Vigneau, C.; Duros, S.; Boudjema, K.; Levêque, J.; Piver, P.; Aubard, Y.; Gauthier, T. Which donor for uterus transplants: Brain-dead donor or living donor? A systematic review. Transplantation 2017, 101, 267–273. [Google Scholar] [CrossRef]
- Kvarnström, N.; Enskog, A.; Dahm-Kähler, P.; Brännström, M. Live versus deceased donor in uterus transplantation. Fertil. Steril. 2019, 112, 24–27. [Google Scholar] [CrossRef]
- Brännström, M.; Johannesson, L.; Dahm-Kähler, P.; Enskog, A.; Mölne, J.; Kvarnström, N.; Diaz-Garcia, C.; Hanafy, A.; Lundmark, C.; Marcickiewicz, J.; et al. First clinical uterus transplantation trial: A six-month report. Fertil. Steril. 2014, 101, 1228–1236. [Google Scholar] [CrossRef]
- Fageeh, W.; Raffa, H.; Jabbad, H.; Marzouki, A. Transplantation of the human uterus. Int. J. Gynecol. Obstet. 2002, 76, 245–251. [Google Scholar] [CrossRef]
- Brännström, M. Uterus transplantation. Curr. Opin. Organ Transplant. 2015, 20, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Flyckt, R.; Davis, A.; Farrell, R.; Zimberg, S.; Tzakis, A.; Falcone, T. Uterine transplantation: Surgical innovation in the treatment of uterine factor infertility. J. Obstet. Gynaecol. Can. 2018, 40, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Padma, A.M.; Truong, M.; Jar-Allah, T.; Song, M.J.; Oltean, M.; Brännström, M.; Hellström, M. The development of an extended normothermic ex vivo reperfusion model of the sheep uterus to evaluate organ quality after cold ischemia in relation to uterus transplantation. Acta Obstet. Gynecol. Scand. 2019, 98, 1127–1138. [Google Scholar] [CrossRef]
- Tricard, J.; Ponsonnard, S.; Tholance, Y.; Mesturoux, L.; Lachatre, D.; Couquet, C.; Terro, F.; Yardin, C.; Marquet, P.; Piccardo, A.; et al. Uterus tolerance to extended cold ischemic storage after auto-transplantation in ewes. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 214, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Tardieu, A.; Chazelas, P.; Faye, P.-A.; Favreau, F.; Nadal-Desbarats, L.; Sallée, C.; Margueritte, F.; Couquet, C.-Y.; Marquet, P.; Guellec, C.B.-L.; et al. Changes in the metabolic composition of storage solution with prolonged cold ischemia of the uterus. J. Assist. Reprod. Genet. 2019, 36, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Tholance, Y.; Tricard, J.; Chianea, T.; Marquet, P.; Ponsonnard, S.; Sturtz, F.; Piccardo, A.; Gauthier, T. Metabolic alterations of uterine grafts after extended cold ischemic storage: Experimental study in ewes. Mol. Hum. Reprod. 2019, 25, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Tardieu, A.; Dion, L.; Lavoué, V.; Chazelas, P.; Marquet, P.; Piver, P.; Sallée, C.; Aubard, Y.; Le Guellec, C.; Favreau, F.; et al. The key role of warm and cold ischemia in uterus transplantation: A review. J. Clin. Med. 2019, 8, 760. [Google Scholar] [CrossRef] [Green Version]
- Wranning, C.A.; Mölne, J.; El-Akouri, R.; Kurlberg, G.; Brännström, M. Short-term ischaemic storage of human uterine myometrium—Basic studies towards uterine transplantation. Hum. Reprod. 2005, 20, 2736–2744. [Google Scholar] [CrossRef] [Green Version]
- Obara, H.; Kisu, I.; Kato, Y.; Yamada, Y.; Matsubara, K.; Emoto, K.; Adachi, M.; Matoba, Y.; Umene, K.; Nogami, Y.; et al. Surgical technique for allogeneic uterus transplantation in macaques. Sci. Rep. 2016, 6, 35989. [Google Scholar] [CrossRef] [Green Version]
- Brännström, M.; Bokström, H.; Dahm-Kähler, P.; Diaz-Garcia, C.; Ekberg, J.; Enskog, A.; Hagberg, H.; Johannesson, L.; Kvarnström, N.; Mölne, J.; et al. One uterus bridging three generations: First live birth after mother-to-daughter uterus transplantation. Fertil. Steril. 2016, 106, 261–266. [Google Scholar] [CrossRef] [Green Version]
- Castellón, L.A.R.; Amador, M.I.G.; González, R.E.D.; Eduardo, M.S.J.; Díaz-García, C.; Kvarnström, N.; Bränström, M. The history behind successful uterine transplantation in humans. JBRA Assist. Reprod. 2017, 21, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Xue, T.; Yang, H.; Zhao, G.-Y.; Zhang, G.; Lu, Z.-H.; Huang, Y.-H.; Ma, X.-D.; Liu, H.-X.; Liang, S.-R.; et al. Modified uterine Allotransplantation and immunosuppression procedure in the sheep model. PLoS ONE 2013, 8, e81300. [Google Scholar] [CrossRef] [PubMed]
- Ejzenberg, D.; Andraus, W.; Mendes, L.R.B.C.; Ducatti, L.; Song, A.; Tanigawa, R.; Rocha-Santos, V.; Arantes, R.M.; Soares, J.M.; Serafini, P.C.; et al. Livebirth after uterus transplantation from a deceased donor in a recipient with uterine infertility. Lancet 2018, 392, 2697–2704. [Google Scholar] [CrossRef]
- Johannesson, L.; Enskog, A.; Molne, J.; Diaz-Garcia, C.; Hanafy, A.; Dahm-Kahler, P.; Tekin, A.; Tryphonopoulos, P.; Morales, P.; Rivas, K.; et al. Preclinical report on allogeneic uterus transplantation in non-human primates. Hum. Reprod. 2012, 28, 189–198. [Google Scholar] [CrossRef] [Green Version]
- Clancy, N.T.; Saso, S.; Stoyanov, D.; Sauvage, V.; Corless, D.J.; Boyd, M.; Noakes, D.E.; Thum, M.-Y.; Ghaem-Maghami, S.; Smith, J.R.; et al. Multispectral imaging of organ viability during uterine transplantation surgery in rabbits and sheep. J. Biomed. Opt. 2016, 21, 106006. [Google Scholar] [CrossRef] [PubMed]
- Kniepeiss, D.; Houben, P.; Stiegler, P.; Berghold, A.; Riedl, R.; Kahn, J.; Schemmer, P. A prospective, randomized, single-blind, multicentre, phase III study on organ preservation with Custodiol-N solution compared with Custodiol(R) solution in organ transplantation (kidney, liver and pancreas). Trials 2020, 21, 62. [Google Scholar] [CrossRef]
- Kahn, J.; Schemmer, P. Comprehensive review on Custodiol-N (HTK-N) and its molecular side of action for organ preservation. Curr. Pharm. Biotechnol. 2018, 18, 1237–1248. [Google Scholar] [CrossRef]
- Pless, G.; Sauer, I.M.; Rauen, U. Improvement of the cold storage of isolated human hepatocytes. Cell Transplant. 2012, 21, 23–37. [Google Scholar] [CrossRef]
- Rauen, U.; Petrat, F.; Li, T.; De Groot, H. Hypothermia injury/cold-induced apoptosis--evidence of an increase in chelatable iron causing oxidative injury in spite of low O2-/H2O2 formation. FASEB J. 2000, 14, 1953–1964. [Google Scholar]
- Liu, Q.; Bruns, H.; Schultze, D.; Xue, Y.; Zorn, M.; Flechtenmacher, C.; Straub, B.K.; Rauen, U.; Schemmer, P. HTK-N, a modified HTK solution, decreases preservation injury in a model of microsteatotic rat liver transplantation. Langenbeck’s Arch. Surg. 2012, 397, 1323–1331. [Google Scholar] [CrossRef]
- Veres, G.; Radovits, T.; Merkely, B.; Karck, M.; Szabo, G. Custodiol-N, the novel cardioplegic solution reduces ischemia/reperfusion injury after cardiopulmonary bypass. J. Cardiothorac. Surg. 2015, 10, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loganathan, S.; Radovits, T.; Hirschberg, K.; Korkmaz, S.; Koch, A.; Karck, M.; Szabó, G. Effects of Custodiol-N, a novel organ preservation solution, on ischemia/reperfusion injury. J. Thorac. Cardiovasc. Surg. 2010, 139, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Pizanis, N.; Gillner, S.; Kamler, M.; De Groot, H.; Jakob, H.; Rauen, U. Cold-induced injury to lung epithelial cells can be inhibited by iron chelators—Implications for lung preservation. Eur. J. Cardio-Thorac. Surg. 2011, 40, 948–955. [Google Scholar] [CrossRef] [Green Version]
- Rauen, U.; Kerkweg, U.; De Groot, H. Iron-dependent vs. iron-independent cold-induced injury to cultured rat hepatocytes: A comparative study in physiological media and organ preservation solutions. Cryobiology 2007, 54, 77–86. [Google Scholar] [CrossRef]
- Radovits, T.; Lin, L.-N.; Zotkina, J.; Koch, A.; Rauen, U.; Köhler, G.; Karck, M.; Szabo, G. Endothelial dysfunction after long-term cold storage in HTK organ preservation solutions: Effects of iron Chelators and N-α-acetyl-l-histidine. J. Heart Lung Transplant. 2008, 27, 208–216. [Google Scholar] [CrossRef]
- Stegemann, J.; Hirner, A.; Rauen, U.; Minor, T. Use of a new modified HTK solution for machine preservation of marginal liver grafts. J. Surg. Res. 2010, 160, 155–162. [Google Scholar] [CrossRef]
- Gharagozloo, F.; Melendez, F.J.; Hein, R.A.; Shemin, R.J.; Disesa, V.J.; Cohn, L.H. The effect of superoxide dismutase and catalase on the extended preservation of the ex vivo heart for transplantation. J. Thorac. Cardiovasc. Surg. 1988, 95, 1008–1013. [Google Scholar] [CrossRef]
- Wu, M.-Y.; Yiang, G.-T.; Liao, W.-T.; Tsai, A.P.-Y.; Cheng, Y.-L.; Cheng, P.-W.; Li, C.-Y. Current mechanistic concepts in ischemia and reperfusion injury. Cell. Physiol. Biochem. 2018, 46, 1650–1667. [Google Scholar] [CrossRef] [PubMed]
- Latchana, N. Preservation solutions used during abdominal transplantation: Current status and outcomes. World J. Transplant. 2015, 5, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Hostert, L.; Pratt, J.R.; Billar, K.J.; Potts, D.J.; Lodge, J.P.A.; Ahmad, L.H.N. A pathophysiologic study of the kidney tubule to optimize organ preservation solutions. Kidney Int. 2004, 66, 77–90. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, T.; Sumimoto, R.; Shimada, H.; Kamada, N.; Nakagawara, G. Effect of sugars in the preservation solution on liver storage in rats. Cryobiology 1991, 28, 428–435. [Google Scholar] [CrossRef]
- El-Akouri, R.R.; Wranning, C.A.; Mölne, J.; Kurlberg, G.; Brännström, M. Pregnancy in transplanted mouse uterus after long-term cold ischaemic preservation. Hum. Reprod. 2003, 18, 2024–2030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ersoy, G.S.; Eken, M.; Cevik, O.; Çilingir, Ö.T.; Tal, R. Mycophenolate mofetil attenuates uterine ischaemia/reperfusion injury in a rat model. Reprod. Biomed. Online 2017, 34, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Garcia, C.; Akhi, S.N.; Martínez-Varea, A.; Brännström, M. The effect of warm ischemia at uterus transplantation in a rat model. Acta Obstet. Gynecol. Scand. 2012, 92, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [Green Version]

| Components (mmol/L) | Custodiol® | Custodiol-N Base Solution | Custodiol-N |
|---|---|---|---|
| Sodium | 16 | 16 | 16 |
| Potassium | 10 | 10 | 10 |
| Magnesium | 4 | 8 | 8 |
| Calcium | 0.015 | 0.02 | 0.02 |
| Chloride | 50 | 30 | 30 |
| L-Histidine | 198 | 124 | 124 |
| N-α-acetyl-L-Histidine | – | 57 | 57 |
| Aspartate | 1 | 5 | 5 |
| Tryptophan | 2 | 2 | 2 |
| α-Ketoglutarate | 2 | 2 | 2 |
| Arginine | – | 3 | 3 |
| Alanine | – | 5 | 5 |
| Sucrose | – | 33 | 33 |
| Mannitol | 30 | – | – |
| Glycine | – | 10 | 10 |
| Deferoxamine | – | – | 0.025 |
| LK-615 | – | – | 0.0075 |
| Histological Findings | Score | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Edema | <5% | <5–15% | 15–30% | >30% |
| Necrosis | Absent | <15% | 15–30% | >30% |
| Smooth muscle contraction | Absent | Present | ||
| Impaired basement membrane integrity | Absent | Present | ||
| Endometrial cells loss | Absent | Present | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zitkute, V.; Kvietkauskas, M.; Maskoliunaite, V.; Leber, B.; Ramasauskaite, D.; Strupas, K.; Stiegler, P.; Schemmer, P. Custodiol-N Is Superior to Custodiol® Solution in Experimental Rat Uterus Preservation. Int. J. Mol. Sci. 2020, 21, 8015. https://doi.org/10.3390/ijms21218015
Zitkute V, Kvietkauskas M, Maskoliunaite V, Leber B, Ramasauskaite D, Strupas K, Stiegler P, Schemmer P. Custodiol-N Is Superior to Custodiol® Solution in Experimental Rat Uterus Preservation. International Journal of Molecular Sciences. 2020; 21(21):8015. https://doi.org/10.3390/ijms21218015
Chicago/Turabian StyleZitkute, Viktorija, Mindaugas Kvietkauskas, Vygante Maskoliunaite, Bettina Leber, Diana Ramasauskaite, Kestutis Strupas, Philipp Stiegler, and Peter Schemmer. 2020. "Custodiol-N Is Superior to Custodiol® Solution in Experimental Rat Uterus Preservation" International Journal of Molecular Sciences 21, no. 21: 8015. https://doi.org/10.3390/ijms21218015
APA StyleZitkute, V., Kvietkauskas, M., Maskoliunaite, V., Leber, B., Ramasauskaite, D., Strupas, K., Stiegler, P., & Schemmer, P. (2020). Custodiol-N Is Superior to Custodiol® Solution in Experimental Rat Uterus Preservation. International Journal of Molecular Sciences, 21(21), 8015. https://doi.org/10.3390/ijms21218015








