Manipulation of Axonal Outgrowth via Exogenous Low Forces
Abstract
:1. Introduction
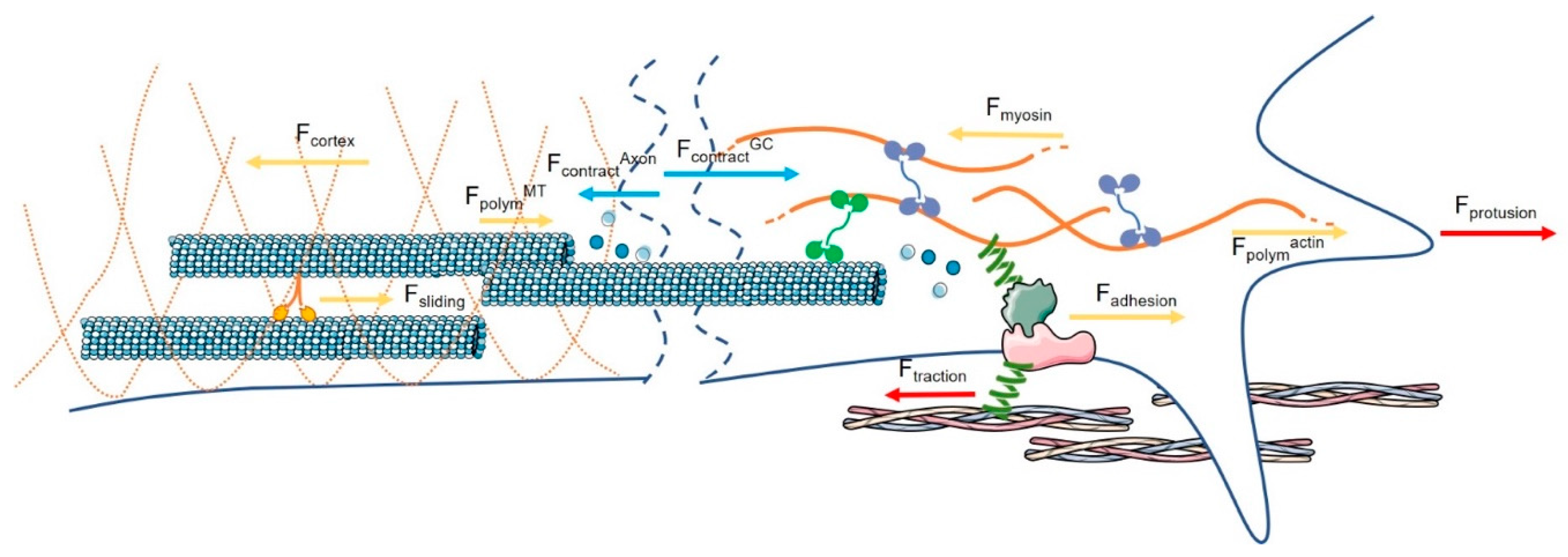
2. Endogenous Force Generation in Axonal Outgrowth
2.1. Axon: The Neuron Machinery That Moves Forward
2.2. The Contractile Force Generated at the GC Is Responsible for the Movement of the GC
2.3. The Contractile Force Generated at the Axon Level Influences the Bulk Translocation
2.4. Variations of Endogenous Force Generation Modulate Axon Growth
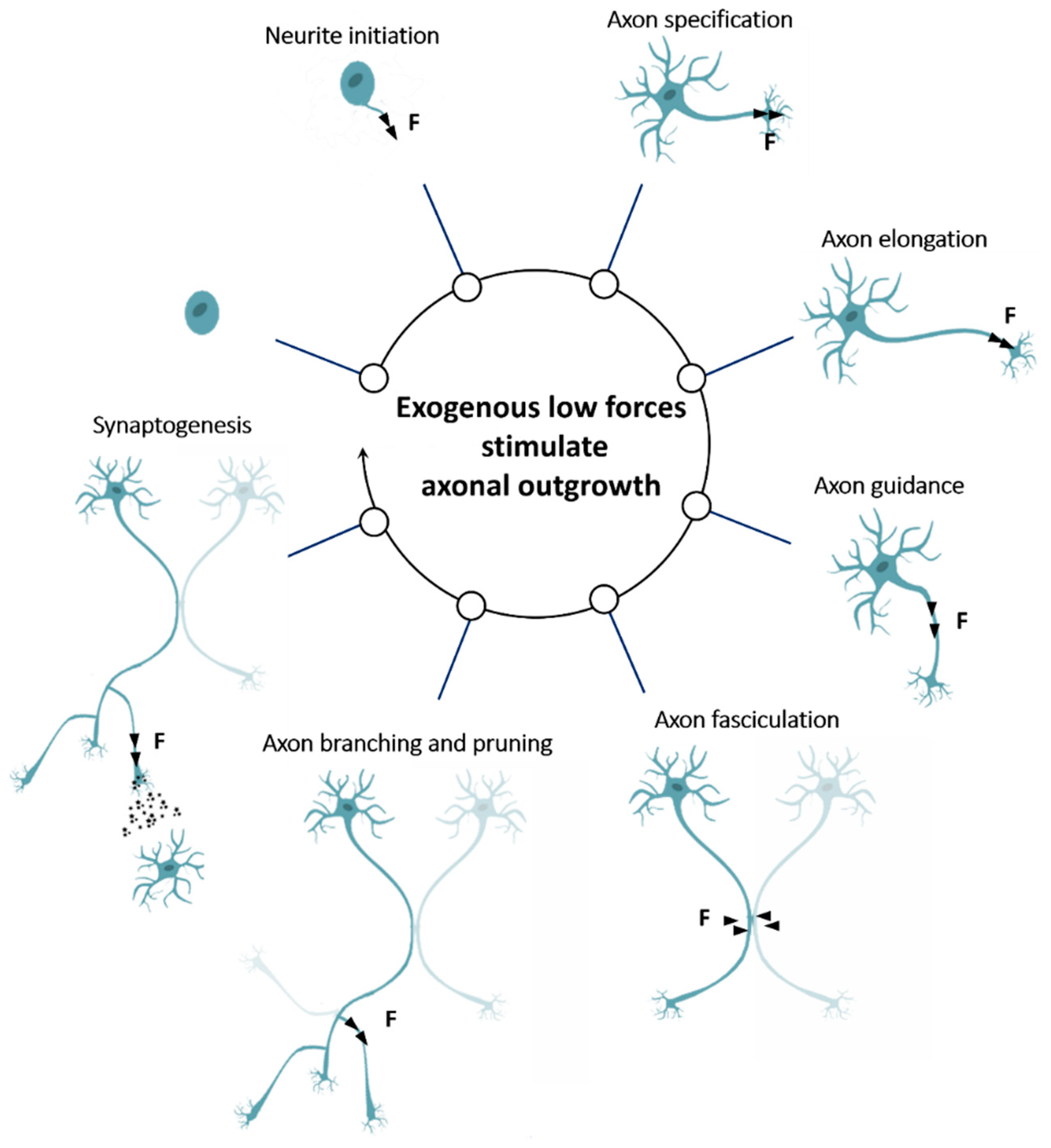
3. Exogenous Low Forces Stimulate Axonal Outgrowth
3.1. Exogenous Force Promotes Neurite Initiation
3.2. Exogenous Force Promotes Axon Specification
3.3. Exogenous Force Promotes Axon Elongation
3.4. Exogenous Force Promotes Axon Guidance
3.5. Exogenous Force Promotes Axon Fasciculation
3.6. Exogenous Force Promotes Axon Branching and Pruning
3.7. Exogenous Force Promotes Synaptogenesis
4. Local and Molecular Effects Triggered by Exogenous Low Forces
4.1. The Birth of “Stretch-Growth”
4.2. Exogenous Forces Affect Cytoskeletal Dynamics
4.3. Involvement of Exogenous Forces in Vesicular Transport
4.4. Exogenous Forces Induce Intracellular Calcium Influx
4.5. Cross-Talk with Other Molecular Pathways
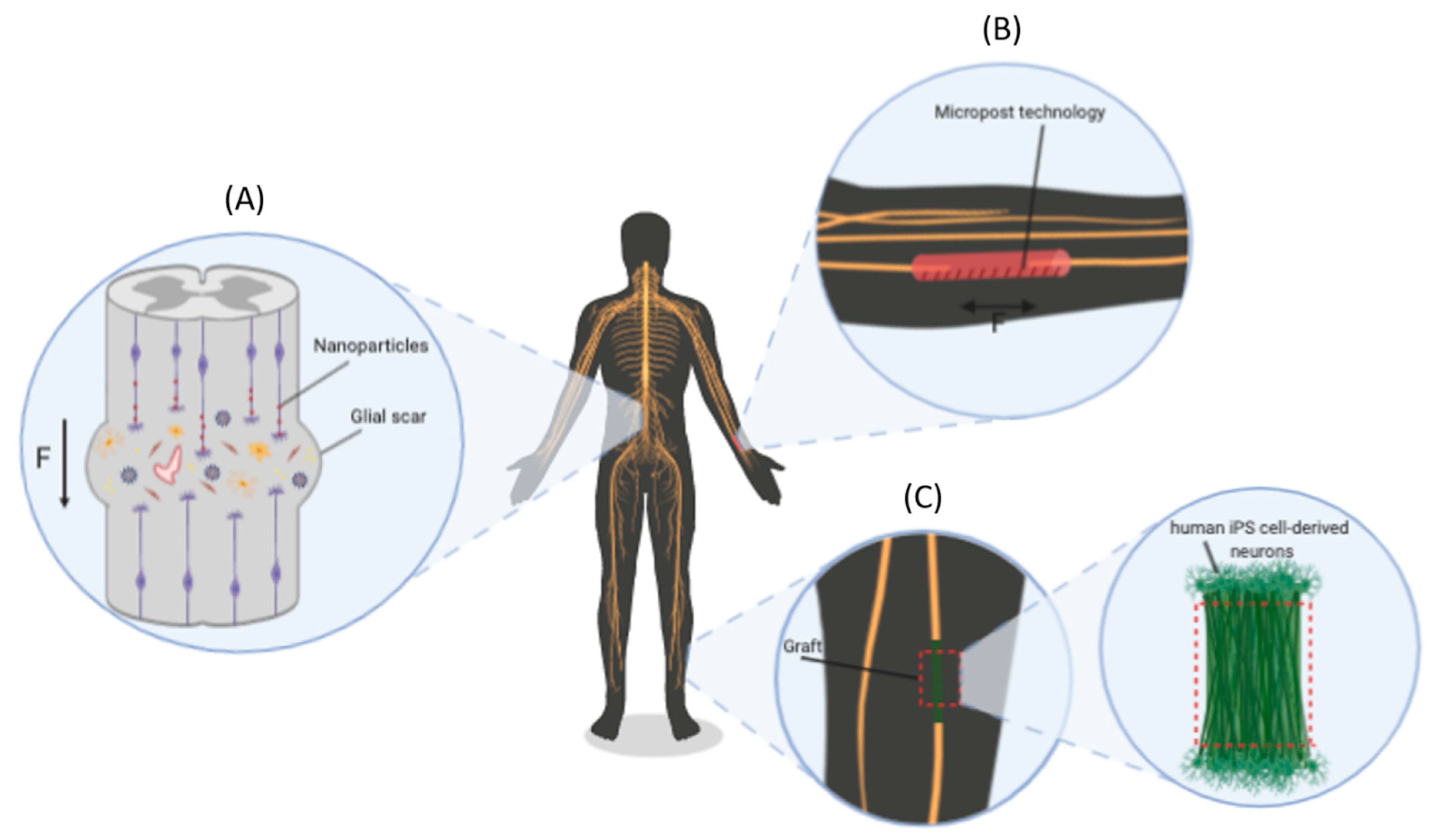
5. Stretch-Growth: Methods and Future Therapeutic Perspectives
5.1. Methods for the Application of Extremely Low Exogenous Forces
5.2. New Therapeutic Perspectives
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM | Atomic force microscopy |
| BDNF | Brain-derived neurotrophic factor |
| BFP | Biomembrane force probe |
| bRBC | Biotinylated red blood cell |
| C | Central |
| CAMs | Cell adhesion molecules |
| CNS | Central nervous system |
| CSPG | Chondroitin sulfate proteoglycans |
| DIV | Days in vitro |
| DRG | Dorsal root ganglia |
| ECM | Extracellular matrix |
| FA | Focal adhesion |
| FAK | Focal adhesion kinase |
| FXS | Fragile X syndrome |
| GC | Growth cone |
| iPS | Induced pluripotent stem cell |
| MAPs | MT-associated proteins |
| MEMs | Micromechanical systems |
| MNPs | Magnetic nanoparticles |
| MNs | Microneedles |
| MS | Mechanosensitive |
| MT | Microtubule |
| MTW | Magnetic tweezers |
| NGC | Nerve guidance conduit |
| NGF | Nerve growth factor |
| NMII | Non-muscle myosin II |
| NO | Nitric oxide |
| NSs | Nanopatterned scaffolds |
| OT | Optical trap |
| P | Peripheral |
| PAK | P-21 activated kinase |
| PDMS | Polydimethylsiloxane |
| PKC | Protein kinase C |
| PNS | Peripheral nervous system |
| RBI | Restrained bead interaction |
| RF | Retrograde flow |
| RGC | Retinal ganglion cells |
| ROCK | Rho-associated protein kinase |
| RPTP-alpha | Protein-tyrosine phosphatase alpha |
| SACs | Stretch-activated ion channels |
| SCI | Spinal cord injury |
| SEMA3A | Semaphorin-3A |
| sEPSCs | Spontaneous excitatory postsynaptic currents |
| SG | Stretch-growth |
| T | Transition |
| TFM | Traction force microscopy |
References
- Suter, D.M.; Miller, K.E. The emerging role of forces in axonal elongation. Prog. Neurobiol. 2011, 94, 91–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franze, K. The mechanical control of nervous system development. Development 2013, 140, 3069–3077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franze, K.; Janmey, P.A.; Guck, J. Mechanics in Neuronal Development and Repair. Annu. Rev. Biomed. Eng. 2013, 15, 227–251. [Google Scholar] [CrossRef]
- Smith, D.H. Stretch growth of integrated axon tracts: Extremes and exploitations. Prog. Neurobiol. 2009, 89, 231–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, P. Nerve Patterns: The Mechanism of Nerve Growth. Growth: Third Growth Symposium 5. 1941, pp. 163–203. Available online: https://www.worldcat.org/title/nerve-patterns-the-mechanics-of-nerve-growth/oclc/537059321 (accessed on 1 October 2020).
- Ruthel, G.; Hollenbeck, P.J. Growth Cones Are Not Required for Initial Establishment of Polarity or Differential Axon Branch Growth in Cultured Hippocampal Neurons. J. Neurosci. 2000, 20, 2266–2274. [Google Scholar] [CrossRef]
- O’Toole, M.; Lamoureux, P.; Miller, K.E. Measurement of Subcellular Force Generation in Neurons. Biophys. J. 2015, 108, 1027–1037. [Google Scholar] [CrossRef] [Green Version]
- Craig, E.M.; Van Goor, D.; Forscher, P.; Mogilner, A. Membrane Tension, Myosin Force, and Actin Turnover Maintain Actin Treadmill in the Nerve Growth Cone. Biophys. J. 2012, 102, 1503–1513. [Google Scholar] [CrossRef] [Green Version]
- Miller, K.E.; Suter, D.M. An Integrated Cytoskeletal Model of Neurite Outgrowth. Front. Cell. Neurosci. 2018, 12. [Google Scholar] [CrossRef]
- Lowery, L.A.; Vactor, D. Van The trip of the tip: Understanding the growth cone machinery. Nat. Rev. Mol. Cell Biol. 2009, 10, 332–343. [Google Scholar] [CrossRef]
- Betz, T.; Koch, D.; Lu, Y.-B.; Franze, K.; Kas, J.A. Growth cones as soft and weak force generators. Proc. Natl. Acad. Sci. USA 2011, 108, 13420–13425. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Guo, S.S.; Fässler, R. Integrin-mediated mechanotransduction. J. Cell Biol. 2016, 215, 445–456. [Google Scholar] [CrossRef]
- Mitchison, T.; Kirschner, M. Cytoskeletal dynamics and nerve growth. Neuron 1988, 1, 761–772. [Google Scholar] [CrossRef]
- Kerstein, P.C.; Nichol, R.H.; Gomez, T.M. Mechanochemical regulation of growth cone motility. Front. Cell. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [Green Version]
- Mogilner, A.; Oster, G. Force Generation by Actin Polymerization II: The Elastic Ratchet and Tethered Filaments. Biophys. J. 2003, 84, 1591–1605. [Google Scholar] [CrossRef] [Green Version]
- Polackwich, R.J.; Koch, D.; McAllister, R.; Geller, H.M.; Urbach, J.S. Traction force and tension fluctuations in growing axons. Front. Cell. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.C.; Suter, D.M. Quantitative analysis of microtubule dynamics during adhesion-mediated growth cone guidance. Dev. Neurobiol. 2008, 68, 1363–1377. [Google Scholar] [CrossRef] [Green Version]
- Schaefer, A.W.; Schoonderwoert, V.T.G.; Ji, L.; Mederios, N.; Danuser, G.; Forscher, P. Coordination of actin filament and microtubule dynamics during neurite outgrowth. Dev. Cell 2008, 15, 146–162. [Google Scholar] [CrossRef] [Green Version]
- Mutalik, S.P.; Joseph, J.; Pullarkat, P.A.; Ghose, A. Cytoskeletal Mechanisms of Axonal Contractility. Biophys. J. 2018, 115, 713–724. [Google Scholar] [CrossRef] [Green Version]
- Oelz, D.B.; del Castillo, U.; Gelfand, V.I.; Mogilner, A. Microtubule Dynamics, Kinesin-1 Sliding, and Dynein Action Drive Growth of Cell Processes. Biophys. J. 2018, 115, 1614–1624. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, F.J.; Hughey, J.; Wittmann, T.; Hyman, A.; Greaser, M.; Baas, P.W. Motor proteins regulate force interactions between microtubules and microfilaments in the axon. Nat. Cell Biol. 2000, 2, 276–280. [Google Scholar] [CrossRef]
- Roossien, D.H.; Lamoureux, P.; Miller, K.E. Cytoplasmic dynein pushes the cytoskeletal meshwork forward during axonal elongation. J. Cell Sci. 2014, 127, 3593–3602. [Google Scholar] [CrossRef] [Green Version]
- Jakobs, M.; Franze, K.; Zemel, A. Force generation by molecular-motor-powered microtubule bundles; implications for neuronal polarization and growth. Front. Cell. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.; Fox, P.; Lakonishok, M.; Davidson, M.W.; Gelfand, V.I. Initial Neurite Outgrowth in Drosophila Neurons Is Driven by Kinesin-Powered Microtubule Sliding. Curr. Biol. 2013, 23, 1018–1023. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Medina, B.; Buendía Padilla, M.; Gutiérrez-Esparza, A.J.; Oaxaca Camacho, A.R. Differential effect of multiple kinesin motors on run length, force and microtubule binding rate. Biophys. Chem. 2018, 242, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Athamneh, A.I.M.; Cartagena-Rivera, A.X.; Raman, A.; Suter, D.M. Substrate Deformation Predicts Neuronal Growth Cone Advance. Biophys. J. 2015, 109, 1358–1371. [Google Scholar] [CrossRef] [Green Version]
- Chan, C.E.; Odde, D.J. Traction Dynamics of Filopodia on Compliant Substrates. Science 2008, 322, 1687–1691. [Google Scholar] [CrossRef]
- Elosegui-Artola, A.; Oria, R.; Chen, Y.; Kosmalska, A.; Pérez-González, C.; Castro, N.; Zhu, C.; Trepat, X.; Roca-Cusachs, P. Mechanical regulation of a molecular clutch defines force transmission and transduction in response to matrix rigidity. Nat. Cell Biol. 2016, 18, 540–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeMali, K.A.; Sun, X.; Bui, G.A. Force Transmission at Cell–Cell and Cell–Matrix Adhesions. Biochemistry 2014, 53, 7706–7717. [Google Scholar] [CrossRef] [PubMed]
- del Rio, A.; Perez-Jimenez, R.; Liu, R.; Roca-Cusachs, P.; Fernandez, J.M.; Sheetz, M.P. Stretching Single Talin Rod Molecules Activates Vinculin Binding. Science 2009, 323, 638–641. [Google Scholar] [CrossRef]
- Grashoff, C.; Hoffman, B.D.; Brenner, M.D.; Zhou, R.; Parsons, M.; Yang, M.T.; McLean, M.A.; Sligar, S.G.; Chen, C.S.; Ha, T.; et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 2010, 466, 263–266. [Google Scholar] [CrossRef] [Green Version]
- Sawada, Y.; Tamada, M.; Dubin-Thaler, B.J.; Cherniavskaya, O.; Sakai, R.; Tanaka, S.; Sheetz, M.P. Force Sensing by Mechanical Extension of the Src Family Kinase Substrate p130Cas. Cell 2006, 127, 1015–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrlicher, A.J.; Nakamura, F.; Hartwig, J.H.; Weitz, D.A.; Stossel, T.P. Mechanical strain in actin networks regulates FilGAP and integrin binding to filamin A. Nature 2011, 478, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Razinia, Z.; Mäkelä, T.; Ylänne, J.; Calderwood, D.A. Filamins in Mechanosensing and Signaling. Annu. Rev. Biophys. 2012, 41, 227–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, J.P.; Robles, E.; Ducharme-Smith, A.; Gomez, T.M. Focal adhesion kinase modulates Cdc42 activity downstream of positive and negative axon guidance cues. J. Cell Sci. 2012, 125, 2918–2929. [Google Scholar] [CrossRef] [Green Version]
- Kostic, A.; Sap, J.; Sheetz, M.P. RPTPalpha is required for rigidity-dependent inhibition of extension and differentiation of hippocampal neurons. J. Cell Sci. 2007, 120, 3895–3904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Toole, M.; Latham, R.; Baqri, R.M.; Miller, K.E. Modeling mitochondrial dynamics during in vivo axonal elongation. J. Theor. Biol. 2008, 255, 369–377. [Google Scholar] [CrossRef]
- Chaudhary, A.R.; Berger, F.; Berger, C.L.; Hendricks, A.G. Tau directs intracellular trafficking by regulating the forces exerted by kinesin and dynein teams. Traffic 2018, 19, 111–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bashaw, G.J.; Klein, R. Signaling from Axon Guidance Receptors. Cold Spring Harb. Perspect. Biol. 2010, 2, a001941. [Google Scholar] [CrossRef] [Green Version]
- Toriyama, M.; Kozawa, S.; Sakumura, Y.; Inagaki, N. Conversion of a Signal into Forces for Axon Outgrowth through Pak1-Mediated Shootin1 Phosphorylation. Curr. Biol. 2013, 23, 529–534. [Google Scholar] [CrossRef] [Green Version]
- Turney, S.G.; Ahmed, M.; Chandrasekar, I.; Wysolmerski, R.B.; Goeckeler, Z.M.; Rioux, R.M.; Whitesides, G.M.; Bridgman, P.C. Nerve growth factor stimulates axon outgrowth through negative regulation of growth cone actomyosin restraint of microtubule advance. Mol. Biol. Cell 2016, 27, 500–517. [Google Scholar] [CrossRef]
- Myers, J.P.; Gomez, T.M. Focal Adhesion Kinase Promotes Integrin Adhesion Dynamics Necessary for Chemotropic Turning of Nerve Growth Cones. J. Neurosci. 2011, 31, 13585–13595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, S. Rac1 and RhoA Promote Neurite Outgrowth through Formation and Stabilization of Growth Cone Point Contacts. J. Neurosci. 2006, 26, 1418–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, A.C.; Holt, C.E. Local translation and directional steering in axons. EMBO J. 2007, 26, 3729–3736. [Google Scholar] [CrossRef]
- van Horck, F.P.; Weinl, C.; Holt, C.E. Retinal axon guidance: Novel mechanisms for steering. Curr. Opin. Neurobiol. 2004, 14, 61–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaub, B.M.; Kasuba, K.C.; Mace, E.; Strittmatter, T.; Laskowski, P.R.; Geissler, S.A.; Hierlemann, A.; Fussenegger, M.; Roska, B.; Müller, D.J. Neurons differentiate magnitude and location of mechanical stimuli. Proc. Natl. Acad. Sci. USA 2020, 117, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Gomez, T.M. Filopodial Calcium Transients Promote Substrate-Dependent Growth Cone Turning. Science 2001, 291, 1983–1987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robles, E.; Huttenlocher, A.; Gomez, T.M. Filopodial Calcium Transients Regulate Growth Cone Motility and Guidance through Local Activation of Calpain. Neuron 2003, 38, 597–609. [Google Scholar] [CrossRef] [Green Version]
- Kerstein, P.C.; Jacques-Fricke, B.T.; Rengifo, J.; Mogen, B.J.; Williams, J.C.; Gottlieb, P.A.; Sachs, F.; Gomez, T.M. Mechanosensitive TRPC1 Channels Promote Calpain Proteolysis of Talin to Regulate Spinal Axon Outgrowth. J. Neurosci. 2013, 33, 273–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glading, A.; Lauffenburger, D.A.; Wells, A. Cutting to the chase: Calpain proteases in cell motility. Trends Cell Biol. 2002, 12, 46–54. [Google Scholar] [CrossRef]
- Yip, A.K.; Iwasaki, K.; Ursekar, C.; Machiyama, H.; Saxena, M.; Chen, H.; Harada, I.; Chiam, K.-H.; Sawada, Y. Cellular Response to Substrate Rigidity Is Governed by Either Stress or Strain. Biophys. J. 2013, 104, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Kilinc, D. The Emerging Role of Mechanics in Synapse Formation and Plasticity. Front. Cell. Neurosci. 2018, 12. [Google Scholar] [CrossRef]
- Gahl, T.J.; Kunze, A. Force-mediating magnetic nanoparticles to engineer neuronal cell function. Front. Neurosci. 2018, 12. [Google Scholar] [CrossRef] [PubMed]
- Athamneh, A.I.M.; Suter, D.M. Quantifying mechanical force in axonal growth and guidance. Front. Cell. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajagopalan, J.; Tofangchi, A.A.; Saif, M.T. Drosophila Neurons Actively Regulate Axonal Tension In Vivo. Biophys. J. 2010, 99, 3208–3215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dotti, C.; Sullivan, C.; Banker, G. The establishment of polarity by hippocampal neurons in culture. J. Neurosci. 1988, 8, 1454–1468. [Google Scholar] [CrossRef] [Green Version]
- Bray, D. Axonal growth in response to experimentally applied mechanical tension. Dev. Biol. 1984, 102, 379–389. [Google Scholar] [CrossRef]
- Lamoureux, P.; Altun-Gultekin, Z.F.; Lin, C.; Wagner, J.A.; Heidemann, S.R. Rac is required for growth cone function but not neurite assembly. J. Cell Sci. 1997, 110, 635–641. [Google Scholar]
- Chada, S.; Lamoureux, P.; Buxbaum, R.E.; Heidemann, S.R. Cytomechanics of neurite outgrowth from chick brain neurons. J. Cell Sci. 1997, 110, 1179–1186. [Google Scholar]
- Lamoureux, P.; Buxbaum, R.E.; Heidemann, S.R. Direct evidence that growth cones pull. Nature 1989, 340, 159–162. [Google Scholar] [CrossRef]
- Zheng, J.; Lamoureux, P.; Santiago, V.; Dennerll, T.; Buxbaum, R.; Heidemann, S. Tensile regulation of axonal elongation and initiation. J. Neurosci. 1991, 11, 1117–1125. [Google Scholar] [CrossRef]
- Fass, J.N.; Odde, D.J. Tensile Force-Dependent Neurite Elicitation via Anti-β1 Integrin Antibody-Coated Magnetic Beads. Biophys. J. 2003, 85, 623–636. [Google Scholar] [CrossRef] [Green Version]
- Magdesian, M.H.; Lopez-Ayon, G.M.; Mori, M.; Boudreau, D.; Goulet-Hanssens, A.; Sanz, R.; Miyahara, Y.; Barrett, C.J.; Fournier, A.E.; De Koninck, Y.; et al. Rapid Mechanically Controlled Rewiring of Neuronal Circuits. J. Neurosci. 2016, 36, 979–987. [Google Scholar] [CrossRef] [Green Version]
- Fukata, Y.; Kimura, T.; Kaibuchi, K. Axon specification in hippocampal neurons. Neurosci. Res. 2002, 43, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Lamoureux, P.; Ruthel, G.; Buxbaum, R.E.; Heidemann, S.R. Mechanical tension can specify axonal fate in hippocampal neurons. J. Cell Biol. 2002, 159, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Kunze, A.; Tseng, P.; Godzich, C.; Murray, C.; Caputo, A.; Schweizer, F.E.; Di Carlo, D. Engineering Cortical Neuron Polarity with Nanomagnets on a Chip. ACS Nano 2015, 9, 3664–3676. [Google Scholar] [CrossRef]
- González-Billault, C.; Engelke, M.; Jiménez-Mateos, E.M.; Wandosell, F.; Cáceres, A.; Avila, J. Participation of structural microtubule-associated proteins (MAPs) in the development of neuronal polarity. J. Neurosci. Res. 2002, 67, 713–719. [Google Scholar] [CrossRef]
- Schelski, M.; Bradke, F. Neuronal polarization: From spatiotemporal signaling to cytoskeletal dynamics. Mol. Cell. Neurosci. 2017, 84, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Dennerll, T.J.; Lamoureux, P.; Buxbaum, R.E.; Heidemann, S.R. The cytomechanics of axonal elongation and retraction. J. Cell Biol. 1989, 109, 3073–3083. [Google Scholar] [CrossRef] [Green Version]
- Heidemann, S.R.; Lamoureux, P.; Buxbaum, R.E. Cytomechanics of axonal development. Cell Biochem. Biophys. 1997, 27, 135–155. [Google Scholar] [CrossRef]
- Lamoureux, P.; Heidemann, S.R.; Martzke, N.R.; Miller, K.E. Growth and elongation within and along the axon. Dev. Neurobiol. 2010, 70, 135–149. [Google Scholar] [CrossRef]
- Steketee, M.B.; Moysidis, S.N.; Jin, X.-L.; Weinstein, J.E.; Pita-Thomas, W.; Raju, H.B.; Iqbal, S.; Goldberg, J.L. Nanoparticle-mediated signaling endosome localization regulates growth cone motility and neurite growth. Proc. Natl. Acad. Sci. USA 2011, 108, 19042–19047. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.H.; Wolf, J.A.; Meaney, D.F. A New Strategy to Produce Sustained Growth of Central Nervous System Axons: Continuous Mechanical Tension. Tissue Eng. 2001, 7, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Heidemann, S.R.; Bray, D. Tension-driven axon assembly: A possible mechanism. Front. Cell. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfister, B.J. Extreme Stretch Growth of Integrated Axons. J. Neurosci. 2004, 24, 7978–7983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loverde, J.R.; Pfister, B.J. Developmental axon stretch stimulates neuron growth while maintaining normal electrical activity, intracellular calcium flux, and somatic morphology. Front. Cell. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steketee, M.B.; Oboudiyat, C.; Daneman, R.; Trakhtenberg, E.; Lamoureux, P.; Weinstein, J.E.; Heidemann, S.; Barres, B.A.; Goldberg, J.L. Regulation of Intrinsic Axon Growth Ability at Retinal Ganglion Cell Growth Cones. Investig. Opthalmol. Vis. Sci. 2014, 55, 4369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vincentiis, S.; Falconieri, A.; Mainardi, M.; Cappello, V.; Scribano, V.; Bizzarri, R.; Storti, B.; Dente, L.; Costa, M.; Raffa, V. Extremely Low Forces Induce Extreme Axon Growth. J. Neurosci. 2020, 40, 4997–5007. [Google Scholar] [CrossRef]
- Raffa, V.; Falcone, F.; De Vincentiis, S.; Falconieri, A.; Calatayud, M.P.; Goya, G.F.; Cuschieri, A. Piconewton Mechanical Forces Promote Neurite Growth. Biophys. J. 2018, 115, 2026–2033. [Google Scholar] [CrossRef] [Green Version]
- Abraham, J.-A.; Linnartz, C.; Dreissen, G.; Springer, R.; Blaschke, S.; Rueger, M.A.; Fink, G.R.; Hoffmann, B.; Merkel, R. Directing Neuronal Outgrowth and Network Formation of Rat Cortical Neurons by Cyclic Substrate Stretch. Langmuir 2019, 35, 7423–7431. [Google Scholar] [CrossRef]
- Katiyar, K.S.; Struzyna, L.A.; Das, S.; Cullen, D.K. Stretch growth of motor axons in custom mechanobioreactors to generate long-projecting axonal constructs. J. Tissue Eng. Regen. Med. 2019, 13, 2040–2054. [Google Scholar] [CrossRef]
- Tay, A.; Kunze, A.; Murray, C.; Di Carlo, D. Induction of Calcium Influx in Cortical Neural Networks by Nanomagnetic Forces. ACS Nano 2016, 10, 2331–2341. [Google Scholar] [CrossRef]
- Tay, A.; Di Carlo, D. Magnetic Nanoparticle-Based Mechanical Stimulation for Restoration of Mechano-Sensitive Ion Channel Equilibrium in Neural Networks. Nano Lett. 2017, 17, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, B.; Xu, H.; Du, S.; Liu, T.; Ren, J.; Zhang, J.; Zhang, H.; Liu, Y.; Lu, L. Growth and elongation of axons through mechanical tension mediated by fluorescent-magnetic bifunctional Fe3O4·Rhodamine 6G@PDA superparticles. J. Nanobiotechnol. 2020, 18, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suter, D.M.; Forscher, P. An emerging link between cytoskeletal dynamics and cell adhesion molecules in growth cone guidance. Curr. Opin. Neurobiol. 1998, 8, 106–116. [Google Scholar] [CrossRef]
- Wu, T.; Nieminen, T.A.; Mohanty, S.; Miotke, J.; Meyer, R.L.; Rubinsztein-Dunlop, H.; Berns, M.W. A photon-driven micromotor can direct nerve fibre growth. Nat. Photonics 2012, 6, 62–67. [Google Scholar] [CrossRef]
- Riggio, C.; Calatayud, M.P.; Giannaccini, M.; Sanz, B.; Torres, T.E.; Fernández-Pacheco, R.; Ripoli, A.; Ibarra, M.R.; Dente, L.; Cuschieri, A.; et al. The orientation of the neuronal growth process can be directed via magnetic nanoparticles under an applied magnetic field. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1549–1558. [Google Scholar] [CrossRef]
- Davis, O.; Merrison-Hort, R.; Soffe, S.R.; Borisyuk, R. Studying the role of axon fasciculation during development in a computational model of the Xenopus tadpole spinal cord. Sci. Rep. 2017, 7, 13551. [Google Scholar] [CrossRef]
- Šmít, D.; Fouquet, C.; Pincet, F.; Zapotocky, M.; Trembleau, A. Axon tension regulates fasciculation/defasciculation through the control of axon shaft zippering. eLife 2017, 6. [Google Scholar] [CrossRef]
- Luo, L.; O’Leary, D.D.M. Axon retraction and degeneration in development and disease. Annu. Rev. Neurosci. 2005, 28, 127–156. [Google Scholar] [CrossRef] [Green Version]
- Low, L.K.; Cheng, H.-J. Axon pruning: An essential step underlying the developmental plasticity of neuronal connections. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1531–1544. [Google Scholar] [CrossRef] [Green Version]
- Bray, D. Mechanical tension produced by nerve cells in tissue culture. J. Cell Sci. 1979, 37, 391–410. [Google Scholar] [PubMed]
- Dent, E.W.; Kalil, K. Axon Branching Requires Interactions between Dynamic Microtubules and Actin Filaments. J. Neurosci. 2001, 21, 9757–9769. [Google Scholar] [CrossRef]
- Kapitein, L.C.; Hoogenraad, C.C. Building the Neuronal Microtubule Cytoskeleton. Neuron 2015, 87, 492–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalil, K.; Dent, E.W. Branch management: Mechanisms of axon branching in the developing vertebrate CNS. Nat. Rev. Neurosci. 2014, 15, 7–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anava, S.; Greenbaum, A.; Jacob, E.B.; Hanein, Y.; Ayali, A. The Regulative Role of Neurite Mechanical Tension in Network Development. Biophys. J. 2009, 96, 1661–1670. [Google Scholar] [CrossRef] [Green Version]
- Franze, K.; Gerdelmann, J.; Weick, M.; Betz, T.; Pawlizak, S.; Lakadamyali, M.; Bayer, J.; Rillich, K.; Gögler, M.; Lu, Y.-B.; et al. Neurite Branch Retraction Is Caused by a Threshold-Dependent Mechanical Impact. Biophys. J. 2009, 97, 1883–1890. [Google Scholar] [CrossRef] [Green Version]
- Tessier-Lavigne, M.; Goodman, C.S. The Molecular Biology of Axon Guidance. Science 1996, 274, 1123–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, W.W.; Li, T.C.; Rubakhin, S.S.; Chiba, A.; Sweedler, J.V.; Saif, T.A. Mechanical Tension Modulates Local and Global Vesicle Dynamics in Neurons. Cell. Mol. Bioeng. 2012, 5, 155–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siechen, S.; Yang, S.; Chiba, A.; Saif, T. Mechanical tension contributes to clustering of neurotransmitter vesicles at presynaptic terminals. Proc. Natl. Acad. Sci. USA 2009, 106, 12611–12616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, B.-M.; Grinnell, A.D. Kinetics, Ca 2+ Dependence, and Biophysical Properties of Integrin-Mediated Mechanical Modulation of Transmitter Release from Frog Motor Nerve Terminals. J. Neurosci. 1997, 17, 904–916. [Google Scholar] [CrossRef] [Green Version]
- Fan, A.; Stebbings, K.A.; Llano, D.A.; Saif, T. Stretch induced hyperexcitability of mice callosal pathway. Front. Cell. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mutalik, S.P.; Ghose, A. Axonal cytomechanics in neuronal development. J. Biosci. 2020, 45, 64. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.M.; Grinnell, A.D. Integrins and modulation of transmitter release from motor nerve terminals by stretch. Science 1995. [Google Scholar] [CrossRef]
- Fatt, P.; Katz, B. Spontaneous subthreshold activity at motor nerve endings. J. Physiol. 1952, 117, 109–128. [Google Scholar] [CrossRef]
- Grinnell, A.D.; Chen, B.-M.; Kashani, A.; Lin, J.; Suzuki, K.; Kidokoro, Y. The role of integrins in the modulation of neurotransmitter release from motor nerve terminals by stretch and hypertonicity. J. Neurocytol. 2003, 32, 489–503. [Google Scholar] [CrossRef]
- Dobrunz, L.E. Release probability is regulated by the size of the readily releasable vesicle pool at excitatory synapses in hippocampus. Int. J. Dev. Neurosci. 2002, 20, 225–236. [Google Scholar] [CrossRef]
- Brown, A. Slow axonal transport: Stop and go traffic in the axon. Nat. Rev. Mol. Cell Biol. 2000, 1, 153–156. [Google Scholar] [CrossRef]
- Roy, S.; Coffee, P.; Smith, G.; Liem, R.K.H.; Brady, S.T.; Black, M.M. Neurofilaments Are Transported Rapidly But Intermittently in Axons: Implications for Slow Axonal Transport. J. Neurosci. 2000, 20, 6849–6861. [Google Scholar] [CrossRef] [Green Version]
- Feldman, E.L.; Axelrod, D.; Schwartz, M.; Heacock, A.M.; Agranoff, B.W. Studies on the localization of newly added membrane in growing neurites. J. Neurobiol. 1981, 12, 591–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson, L.J.; Tzima, E.; Reader, J.S. Mechanical Forces and Their Effect on the Ribosome and Protein Translation Machinery. Cells 2020, 9, 650. [Google Scholar] [CrossRef] [Green Version]
- Pita-Thomas, W.; Steketee, M.B.; Moysidis, S.N.; Thakor, K.; Hampton, B.; Goldberg, J.L. Promoting filopodial elongation in neurons by membrane-bound magnetic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 559–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Rooij, R.; Kuhl, E.; Miller, K.E. Modeling the Axon as an Active Partner with the Growth Cone in Axonal Elongation. Biophys. J. 2018, 115, 1783–1795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, T.J.; Jackson, D.R.; Smith, R.D.; Shannon, S.F.; Cremo, C.R.; Baker, J.E. Actin Sliding Velocities are Influenced by the Driving Forces of Actin-Myosin Binding. Cell. Mol. Bioeng. 2013, 6, 26–37. [Google Scholar] [CrossRef] [Green Version]
- Glogauer, M.; Arora, P.; Yao, G.; Sokholov, I.; Ferrier, J.; McCulloch, C.A.G. Calcium ions and tyrosine phosphorylation interact coordinately with actin to regulate cytoprotective responses to stretching. J. Cell Sci. 1997, 110, 11–21. [Google Scholar]
- Deshpande, V.S.; McMeeking, R.M.; Evans, A.G. A bio-chemo-mechanical model for cell contractility. Proc. Natl. Acad. Sci. USA 2006, 103, 14015–14020. [Google Scholar] [CrossRef] [Green Version]
- Shingyoji, C.; Nakano, I.; Inoue, Y.; Higuchi, H. Dynein arms are strain-dependent direction-switching force generators. Cytoskeleton 2015, 72, 388–401. [Google Scholar] [CrossRef] [PubMed]
- Hamant, O.; Inoue, D.; Bouchez, D.; Dumais, J.; Mjolsness, E. Are microtubules tension sensors? Nat. Commun. 2019, 10, 2360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sleigh, J.N.; Rossor, A.M.; Fellows, A.D.; Tosolini, A.P.; Schiavo, G. Axonal transport and neurological disease. Nat. Rev. Neurol. 2019, 15, 691–703. [Google Scholar] [CrossRef]
- Ahmed, W.W.; Saif, T.A. Active transport of vesicles in neurons is modulated by mechanical tension. Sci. Rep. 2015, 4, 4481. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, W.W.; Williams, B.J.; Silver, A.M.; Saif, T.A. Measuring nonequilibrium vesicle dynamics in neurons under tension. Lab Chip 2013, 13, 570. [Google Scholar] [CrossRef]
- Loverde, J.R.; Ozoka, V.C.; Aquino, R.; Lin, L.; Pfister, B.J. Live Imaging of Axon Stretch Growth in Embryonic and Adult Neurons. J. Neurotrauma 2011, 28, 2389–2403. [Google Scholar] [CrossRef]
- Kunze, A.; Murray, C.T.; Godzich, C.; Lin, J.; Owsley, K.; Tay, A.; Di Carlo, D. Modulating motility of intracellular vesicles in cortical neurons with nanomagnetic forces on-chip. Lab Chip 2017, 17, 842–854. [Google Scholar] [CrossRef]
- Chowdary, P.D.; Xie, C.; Osakada, Y.; Che, D.L.; Cui, B. Magnetic Manipulation of Axonal Transport in Live Neurons. Biophys. J. 2013, 104, 652a. [Google Scholar] [CrossRef] [Green Version]
- Chowdary, P.C.; McGuire, A.; Lee, Y.; Che, D.; Hanson, L.; Osakada, Y.; Ooi, C.; Xie, C.; Wang, S.X.; Cui, B. Magnetic manipulation of axonal endosome transport in live neurons. bioRxiv 2019, 733253. [Google Scholar] [CrossRef]
- Morris, C.E. Voltage-gated channel mechanosensitivity: Fact or friction? Front. Physiol. 2011. [Google Scholar] [CrossRef] [Green Version]
- Tyler, W.J. The mechanobiology of brain function. Nat. Rev. Neurosci. 2012, 13, 867–878. [Google Scholar] [CrossRef]
- Rosenberg, S.S.; Spitzer, N.C. Calcium Signaling in Neuronal Development. Cold Spring Harb. Perspect. Biol. 2011, 3, a004259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valenzuela, J.I.; Perez, F. Diversifying the secretory routes in neurons. Front. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [Green Version]
- Kilinc, D.; Blasiak, A.; O’Mahony, J.J.; Lee, G.U. Low Piconewton Towing of CNS Axons against Diffusing and Surface-Bound Repellents Requires the Inhibition of Motor Protein-Associated Pathways. Sci. Rep. 2015, 4, 7128. [Google Scholar] [CrossRef]
- Suter, D.M.; Errante, L.D.; Belotserkovsky, V.; Forscher, P. The Ig Superfamily Cell Adhesion Molecule, apCAM, Mediates Growth Cone Steering by Substrate–Cytoskeletal Coupling. J. Cell Biol. 1998, 141, 227–240. [Google Scholar] [CrossRef] [Green Version]
- Cojoc, D.; Difato, F.; Ferrari, E.; Shahapure, R.B.; Laishram, J.; Righi, M.; Di Fabrizio, E.M.; Torre, V. Properties of the Force Exerted by Filopodia and Lamellipodia and the Involvement of Cytoskeletal Components. PLoS ONE 2007, 2, e1072. [Google Scholar] [CrossRef]
- Chighizola, M.; Dini, T.; Lenardi, C.; Milani, P.; Podestà, A.; Schulte, C. Mechanotransduction in neuronal cell development and functioning. Biophys. Rev. 2019, 11, 701–720. [Google Scholar] [CrossRef] [PubMed]
- Elting, M.W.; Spudich, J.A. Future Challenges in Single-Molecule Fluorescence and Laser Trap Approaches to Studies of Molecular Motors. Dev. Cell 2012, 23, 1084–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bard, L.; Boscher, C.; Lambert, M.; Mege, R.-M.; Choquet, D.; Thoumine, O. A Molecular Clutch between the Actin Flow and N-Cadherin Adhesions Drives Growth Cone Migration. J. Neurosci. 2008, 28, 5879–5890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gosse, C.; Croquette, V. Magnetic Tweezers: Micromanipulation and Force Measurement at the Molecular Level. Biophys. J. 2002, 82, 3314–3329. [Google Scholar] [CrossRef] [Green Version]
- Grevesse, T.; Dabiri, B.E.; Parker, K.K.; Gabriele, S. Opposite rheological properties of neuronal microcompartments predict axonal vulnerability in brain injury. Sci. Rep. 2015, 5, 9475. [Google Scholar] [CrossRef] [Green Version]
- Janssen, X.J.A.; Lipfert, J.; Jager, T.; Daudey, R.; Beekman, J.; Dekker, N.H. Electromagnetic Torque Tweezers: A Versatile Approach for Measurement of Single-Molecule Twist and Torque. Nano Lett. 2012, 12, 3634–3639. [Google Scholar] [CrossRef] [PubMed]
- Kilinc, D.; Lee, G.U. Advances in magnetic tweezers for single molecule and cell biophysics. Integr. Biol. 2014, 6, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Goya, G.F.; Calatayud, M.P.; Sanz, B.; Giannaccini, M.; Raffa, V.; Torres, T.E.; Ibarra, M.R. Magnetic nanoparticles for magnetically guided therapies against neural diseases. MRS Bull. 2014, 39, 965–969. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.-Y.; Zhang, Y.-Y.; Xie, J.; Li, C.-X.; Chen, W.-Y.; Liu, B.-L.; Wu, X.; Li, S.-N.; Huo, B.; Jiang, L.-H.; et al. Stiff substrates enhance cultured neuronal network activity. Sci. Rep. 2015, 4, 6215. [Google Scholar] [CrossRef] [Green Version]
- Baranes, K.; Hibsh, D.; Cohen, S.; Yamin, T.; Efroni, S.; Sharoni, A.; Shefi, O. Comparing Transcriptome Profiles of Neurons Interfacing Adjacent Cells and Nanopatterned Substrates Reveals Fundamental Neuronal Interactions. Nano Lett. 2019, 19, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Grzywa, E.L.; Lee, A.C.; Lee, G.U.; Suter, D.M. High-resolution analysis of neuronal growth cone morphology by comparative atomic force and optical microscopy. J. Neurobiol. 2006, 66, 1529–1543. [Google Scholar] [CrossRef] [PubMed]
- Gourier, C.; Jegou, A.; Husson, J.; Pincet, F. A Nanospring Named Erythrocyte. The Biomembrane Force Probe. Cell. Mol. Bioeng. 2008, 1, 263–275. [Google Scholar] [CrossRef]
- Fouquet, C.; Trembleau, A. Preparation and Manipulation of Olfactory Epithelium Explant Cultures for Measurement of the Mechanical Tension of Individual Axons Using the Biomembrane Force Probe. Bio-Protocol 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Hällström, W.; Lexholm, M.; Suyatin, D.B.; Hammarin, G.; Hessman, D.; Samuelson, L.; Montelius, L.; Kanje, M.; Prinz, C.N. Fifteen-Piconewton Force Detection from Neural Growth Cones Using Nanowire Arrays. Nano Lett. 2010, 10, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Schierbaum, N.; Rheinlaender, J.; Schäffer, T.E. Combined atomic force microscopy (AFM) and traction force microscopy (TFM) reveals a correlation between viscoelastic material properties and contractile prestress of living cells. Soft Matter 2019, 15, 1721–1729. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Kramer, C.M.; Chen, C.S.; Reich, D.H. Probing cellular traction forces with magnetic nanowires and microfabricated force sensor arrays. Nanotechnology 2012, 23, 075101. [Google Scholar] [CrossRef] [Green Version]
- Bradbury, E.J.; Burnside, E.R. Moving beyond the glial scar for spinal cord repair. Nat. Commun. 2019, 10, 3879. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, F.; Cheng, F.; Ying, L.; Wang, C.; Shi, K.; Wang, J.; Xia, K.; Gong, Z.; Huang, X.; et al. Strategies and prospects of effective neural circuits reconstruction after spinal cord injury. Cell Death Dis. 2020, 11, 439. [Google Scholar] [CrossRef]
- Hellal, F.; Hurtado, A.; Ruschel, J.; Flynn, K.C.; Laskowski, C.J.; Umlauf, M.; Kapitein, L.C.; Strikis, D.; Lemmon, V.; Bixby, J.; et al. Microtubule Stabilization Reduces Scarring and Causes Axon Regeneration After Spinal Cord Injury. Science 2011, 331, 928–931. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Li, D.; Farrelly, O.; Miles, L.; Li, F.; Kim, S.E.; Lo, T.Y.; Wang, F.; Li, T.; Thompson-Peer, K.L.; et al. The Mechanosensitive Ion Channel Piezo Inhibits Axon Regeneration. Neuron 2019, 102, 373–389. [Google Scholar] [CrossRef] [Green Version]
- Gaudin, R.; Knipfer, C.; Henningsen, A.; Smeets, R.; Heiland, M.; Hadlock, T. Approaches to peripheral nerve repair: Generations of biomaterial conduits yielding to replacing autologous nerve grafts in craniomaxillofacial surgery. BioMed Res. Int. 2016, 2016, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Vigani, B.; Rossi, S.; Sandri, G.; Bonferoni, M.; Ferrari, F. Design and criteria of electrospun fibrous scaffolds for the treatment of spinal cord injury. Neural Regen. Res. 2017, 12, 1786. [Google Scholar] [CrossRef]
- Ezra, M.; Bushman, J.; Shreiber, D.; Schachner, M.; Kohn, J. Porous and Nonporous Nerve Conduits: The Effects of a Hydrogel Luminal Filler with and Without a Neurite-Promoting Moiety. Tissue Eng. Part A 2016, 22, 818–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Ruiter, G.C.W.; Malessy, M.J.A.; Yaszemski, M.J.; Windebank, A.J.; Spinner, R.J. Designing ideal conduits for peripheral nerve repair. Neurosurg. Focus 2009, 26, E5. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Lim, S.H.; Mao, H.-Q.; Chew, S.Y. Current applications and future perspectives of artificial nerve conduits. Exp. Neurol. 2010, 223, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Liu, T.; Chew, S.Y. The application of nanofibrous scaffolds in neural tissue engineering. Adv. Drug Deliv. Rev. 2009, 61, 1055–1064. [Google Scholar] [CrossRef]
- Hong, Y.; Huber, A.; Takanari, K.; Amoroso, N.J.; Hashizume, R.; Badylak, S.F.; Wagner, W.R. Mechanical properties and in vivo behavior of a biodegradable synthetic polymer microfiber–extracellular matrix hydrogel biohybrid scaffold. Biomaterials 2011, 32, 3387–3394. [Google Scholar] [CrossRef] [Green Version]
- Pellico, J.; Ellis, C.M.; Davis, J.J. Nanoparticle-Based Paramagnetic Contrast Agents for Magnetic Resonance Imaging. Contrast Media Mol. Imaging 2019, 2019, 1845637. [Google Scholar] [CrossRef]
- Hetzel, D.; Strauss, W.; Bernard, K.; Li, Z.; Urboniene, A.; Allen, L.F. A Phase III, randomized, open-label trial of ferumoxytol compared with iron sucrose for the treatment of iron deficiency anemia in patients with a history of unsatisfactory oral iron therapy. Am. J. Hematol. 2014, 89, 646–650. [Google Scholar] [CrossRef] [Green Version]
- Schwenk, M.H. Ferumoxytol: A New Intravenous Iron Preparation for the Treatment of Iron Deficiency Anemia in Patients with Chronic Kidney Disease. Pharmacotherapy 2010, 30, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhou, Z.; Mao, H.; Yang, L. Magnetic nanoparticles for precision oncology: Theranostic magnetic iron oxide nanoparticles for image-guided and targeted cancer therapy. Nanomedicine 2017, 12, 73–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subramanian, M.; Miaskowski, A.; Jenkins, S.I.; Lim, J.; Dobson, J. Remote manipulation of magnetic nanoparticles using magnetic field gradient to promote cancer cell death. Appl. Phys. A 2019, 125, 226. [Google Scholar] [CrossRef] [Green Version]
- Cortajarena, A.L.; Ortega, D.; Ocampo, S.M.; Gonzalez-García, A.; Couleaud, P.; Miranda, R.; Belda-Iniesta, C.; Ayuso-Sacido, A. Engineering Iron Oxide Nanoparticles for Clinical Settings. Nanobiomedicine 2014, 1, 2. [Google Scholar] [CrossRef] [Green Version]
- Kevadiya, B.D.; Ottemann, B.M.; Thomas, M.B.; Mukadam, I.; Nigam, S.; McMillan, J.E.; Gorantla, S.; Bronich, T.K.; Edagwa, B.; Gendelman, H.E. Neurotheranostics as personalized medicines. Adv. Drug Deliv. Rev. 2019, 148, 252–289. [Google Scholar] [CrossRef] [PubMed]
- Giannaccini, M.; Calatayud, M.P.; Poggetti, A.; Corbianco, S.; Novelli, M.; Paoli, M.; Battistini, P.; Castagna, M.; Dente, L.; Parchi, P.; et al. Magnetic Nanoparticles for Efficient Delivery of Growth Factors: Stimulation of Peripheral Nerve Regeneration. Adv. Healthc. Mater. 2017, 6, 1601429. [Google Scholar] [CrossRef]
- Marcus, M.; Smith, A.; Maswadeh, A.; Shemesh, Z.; Zak, I.; Motiei, M.; Schori, H.; Margel, S.; Sharoni, A.; Shefi, O. Magnetic Targeting of Growth Factors Using Iron Oxide Nanoparticles. Nanomaterials 2018, 8, 707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannaccini, M.; Usai, A.; Chiellini, F.; Guadagni, V.; Andreazzoli, M.; Ori, M.; Pasqualetti, M.; Dente, L.; Raffa, V. Neurotrophin-conjugated nanoparticles prevent retina damage induced by oxidative stress. Cell. Mol. Life Sci. 2018, 75, 1255–1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sniadecki, N.J.; Anguelouch, A.; Yang, M.T.; Lamb, C.M.; Liu, Z.; Kirschner, S.B.; Liu, Y.; Reich, D.H.; Chen, C.S. Magnetic microposts as an approach to apply forces to living cells. Proc. Natl. Acad. Sci. USA 2007, 104, 14553–14558. [Google Scholar] [CrossRef] [Green Version]
- Rajagopalan, J.; Saif, M.T.A. MEMS sensors and microsystems for cell mechanobiology. J. Micromech. Microeng. 2011, 21, 054002. [Google Scholar] [CrossRef] [Green Version]
- Unal, M.; Alapan, Y.; Jia, H.; Varga, A.G.; Angelino, K.; Aslan, M.; Sayin, I.; Han, C.; Jiang, Y.; Zhang, Z.; et al. Micro and Nano-Scale Technologies for Cell Mechanics. Nanobiomedicine 2014, 1, 5. [Google Scholar] [CrossRef]
- Chen, H.I.; Jgamadze, D.; Lim, J.; Mensah-Brown, K.; Wolf, J.A.; Mills, J.A.; Smith, D.H. Functional Cortical Axon Tracts Generated from Human Stem Cell-Derived Neurons. Tissue Eng. Part A 2019, 25, 736–745. [Google Scholar] [CrossRef] [PubMed]

| Methods | Quantification | Biological Model | Effect | Ref. |
|---|---|---|---|---|
| MN | N/A | Chick DRG (E7–E12) | Axon branching | [92] |
| 40–1000 µm h−1 | Chick DRG (E10–E12) | Neurite initiation/axon elongation/axon branching | [57] | |
| 102–103 pN | Chick DRG (E12) | GC-mediated axonal elongation | [60] | |
| 102–103 pN | PC12 cells + Chick DRG (E10–E12) | Axon elongation/axon pruning | [69] | |
| 100–103 pN | Chick DRG (E10–E12) | Neurite initiation/neurite elongation | [61] | |
| 101–103 pN | Chick sensory neurons (E7–E8) | Neurite initiation/axon elongation | [59] | |
| 102–104 pN | PC12 cells | Neurite initiation/axon elongation | [58] | |
| ~102 pN | Rat hippocampal neurons (E18–E19) | Neurite to axon specification | [65] | |
| 103 pN | Rat hippocampal neurons (E18) | GC motility + neurite extension | [135] | |
| 10−4–103 pN | Rat RGC (E13–P8) | Axon elongation | [77] | |
| 102–105 pN | Aplysia bag cell neurons | GC traction force + cytoskeletal dynamics | [26] | |
| RBI | 190–310 µm h−1 | Aplysia bag cell neurons | GC motility + cytoskeletal dynamics | [131] |
| N/A | Aplysia bag cell neurons | Regulation of MT behavior during neuronal growth | [18] | |
| 2.5–100 µm h−1 | Chick DRG (E12) | Axon elongation | [71] | |
| AFM | ~103 pN | Fly embryo motoneurons | Synaptic vesicle accumulation | [100] |
| 102–105 pN | Aplysia bag cell neurons | GC traction force + cytoskeletal dynamics | [26] | |
| ~102 pN | Rat sensory neurons (E17–E18) | Neurite initiation/axon elongation/network formation | [63] | |
| BFP | 101–103 pN | Mouse olfactory sensory neurons (E13.5) | Axon fasciculation/defasciculation | [89] |
| OT | ~100 pN | Rat hippocampal neurons (E18) | GC motility + neurite extension | [135] |
| 10−1 pN | Carassius auratus retinal ganglion cells | Axon orientation | [86] | |
| MTW | 100–103 pN | Chick sensory neuron (E7–E8) | Neurite initiation/axon elongation | [62] |
| 100–102 pN | Mouse cortical neurons (E14) | Mechanochemical axon elongation | [130] | |
| 5–400 pN µm−2 | Rat cortical neurons (E18) | Study of rheological properties | [137] | |
| MNP | 101–102 pN | Rat RGC (E20–P8) | Axon elongation | [72] |
| ~100–101 pN | Neuron-like PC12 cells | Axon orientation | [87] | |
| 100–103 pN | Rat cortical neurons (E18) | Axon specification/axon orientation | [66] | |
| ~100–101 pN | Rat RGC (P0-P4) | Directional filopodia elongation/actin cytoskeleton polymerization | [112] | |
| 102–103 pN | Rat cortical neurons (E18) | Axon elongation/intracellular Ca2+ influx induction | [82] | |
| 102–103 pN | Rat cortical neurons (E18) | Intracellular Ca2+ influx induction | [83] | |
| ~100–102 pN | Rat cortical neurons (E18) | Vesicle speed alteration | [123] | |
| pN | PC12 cells + SH-SY5Y | Axon elongation | [79] | |
| ~100–101 pN | Rat DRG neurons (E18) | Axonal transport alteration | [125] | |
| pN | PC12 cells + rat DRG (P1–3) | Axon elongation | [84] | |
| 100–101 pN | Mouse hippocampal neurons (P0–P1) | Axon elongation/branching/axon excitability | [78] | |
| TFM | 10–2000pN µm−2 | NG108-15 cells | Axon branching/axon pruning | [97] |
| 103 pN | Adult mouse DRG + adult mouse SCG | GC pathfinding | [146] | |
| MEM | 84 µm h−1 | Rat cortical neurons + differentiated human neurons from NT2 cell line | Axon elongation | [73] |
| 0.2–2% | Rat DRG (E15) | Axonal elongation | [75] | |
| 42–250 µm h−1 | Rat DRG (E15) | Mitochondrial transport alteration | [122] | |
| 0–20% | Drosophila embryo motor neurons + Aplysia neurons | Synaptic vesicle accumulation | [99,120,121] | |
| 2.5–4.2% | One-month mouse brain slices | Axon excitability | [102] | |
| 5–52% | Rat DRG (E16) | Axonal elongation | [76] | |
| 7–28% | Rat cortical neurons (E18–E19) | Axon elongation/axon branching/axon orientation | [80] | |
| 4.2–83.3 µm h−1 | Rat DRG (E16) + rat motor neurons (E16) | Axon elongation/axon fasciculation | [81] | |
| NS | N/A | Adult locust frontal ganglions | Axon branching/axon pruning | [96] |
| N/A | Mouse hippocampal neurons P1 + PC12 cells | Neuronal network activity | [141] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Vincentiis, S.; Falconieri, A.; Scribano, V.; Ghignoli, S.; Raffa, V. Manipulation of Axonal Outgrowth via Exogenous Low Forces. Int. J. Mol. Sci. 2020, 21, 8009. https://doi.org/10.3390/ijms21218009
De Vincentiis S, Falconieri A, Scribano V, Ghignoli S, Raffa V. Manipulation of Axonal Outgrowth via Exogenous Low Forces. International Journal of Molecular Sciences. 2020; 21(21):8009. https://doi.org/10.3390/ijms21218009
Chicago/Turabian StyleDe Vincentiis, Sara, Alessandro Falconieri, Vincenzo Scribano, Samuele Ghignoli, and Vittoria Raffa. 2020. "Manipulation of Axonal Outgrowth via Exogenous Low Forces" International Journal of Molecular Sciences 21, no. 21: 8009. https://doi.org/10.3390/ijms21218009
APA StyleDe Vincentiis, S., Falconieri, A., Scribano, V., Ghignoli, S., & Raffa, V. (2020). Manipulation of Axonal Outgrowth via Exogenous Low Forces. International Journal of Molecular Sciences, 21(21), 8009. https://doi.org/10.3390/ijms21218009





