NAA10 as a New Prognostic Marker for Cancer Progression
Abstract
:1. Introduction
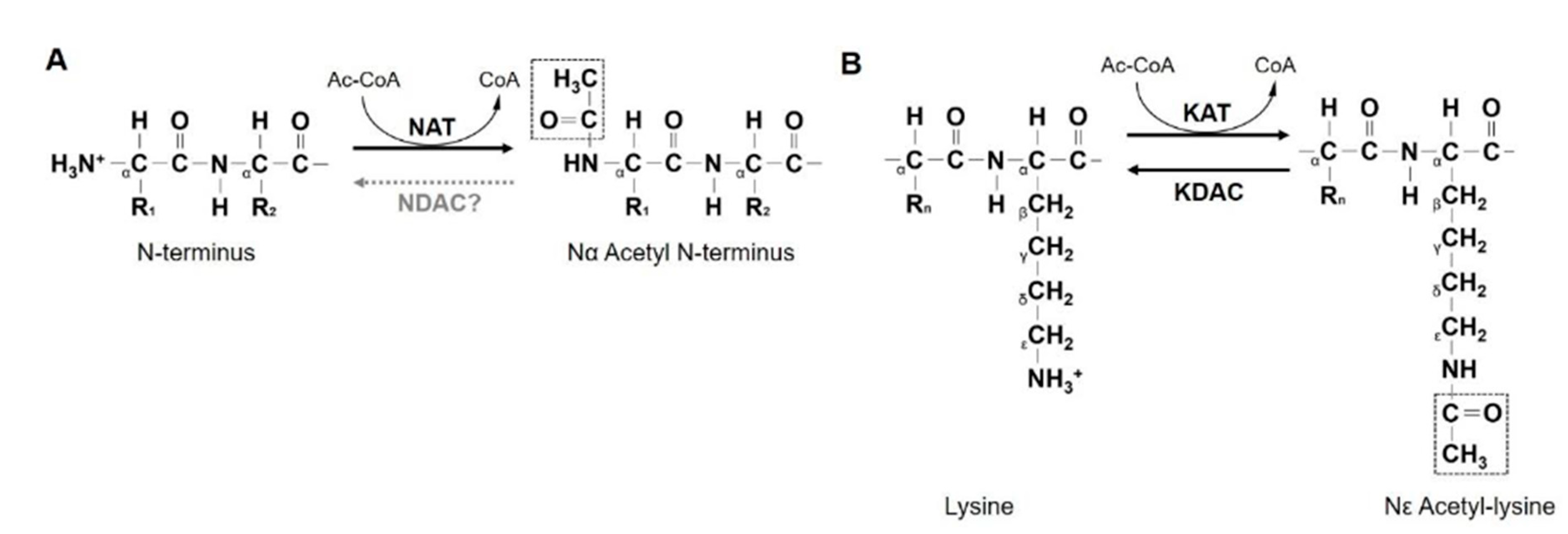
2. NATs
3. NAA10
4. Expression of NAA10 in Cancer
4.1. Breast Cancer (BCa)
4.2. Lung Cancer (LCa)
4.3. Hepatocellular Carcinoma (HCC)
4.4. Colorectal Cancer (CRC)
4.5. Osteosarcoma
4.6. Oral Squamous Cell Carcinoma (OSCC)
4.7. Prostate Cancer (PCa)
5. NAA10 as a Prognostic Marker
5.1. NAA10 and Cancer Survival
5.1.1. BCa
5.1.2. LCa
5.1.3. HCC
5.1.4. CRC
5.1.5. Osteosarcoma
5.1.6. OSCC
5.2. Invasion and Metastasis
5.2.1. BCa
5.2.2. LCa
5.2.3. HCC
5.2.4. Osteosarcoma
5.2.5. OSCC
5.3. Recurrence
6. NAA10 in Tumorigenesis
6.1. NAA10 as an Oncoprotein
6.2. NAA10 as a Tumor Suppressor
7. Other NATs in Cancer
8. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AIF | Apoptosis-inducing factor |
| Apaf-1 | Apoptotic protease-activating factor-1 |
| AR | Androgen receptor |
| ARD1 | Arrest-defective 1 |
| ATD | Acetyltransferase domain |
| AuA | Aurora kinase A |
| BCa | Breast cancer |
| CRC | Colorectal cancer |
| DNMT1 | DNA methyltransferase 1 |
| FIH | Factor Inhibiting HIF |
| HCC | Hepatocellular carcinoma |
| Hsp70 | Heat shock protein 70 |
| HYPK | Huntingtin yeast two-hybrid protein K |
| IDC | Invasive ductal carcinoma |
| IDR | Intrinsically disordered region |
| IKKβ | IκB kinase β |
| KAT | Lysine acetyltransferase |
| KDAC | Lysine deacetylase |
| LCa | Lung cancer |
| MLCK | Myosin light chain kinase |
| MMP-2 | Matrix metalloproteinase-2 |
| MVI | Microvascular invasion |
| NAA10 | N-α-acetyltransferase 10 |
| NAT | N-terminal acetyltransferase |
| OSCC | Oral squamous cell carcinoma |
| PGK1 | Phosphoglycerate kinase 1 |
| PIX | p21-activated kinase-interacting exchange factor |
| PCa | Prostate cancer |
| Runx2 | Runt-related transcription factor 2 |
| STAT5α | Signal transducer and activator of transcription 5α |
References
- Heron, M. Deaths: Leading causes for 2016. Natl. Vital Stat. Rep. 2018, 67, 1–77. [Google Scholar] [PubMed]
- Koncina, E.; Haan, S.; Rauh, S.; Letellier, E. Prognostic and predictive molecular biomarkers for colorectal cancer: Updates and challenges. Cancers 2020, 12, 319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aksnes, H.; Ree, R.; Arnesen, T. Co-translational, post-translational, and non-catalytic roles of N-Terminal acetyltransferases. Mol. Cell 2019, 73, 1097–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.H.; Gong, J.L.; Yu, M.; Yang, H.; Lai, J.H.; Ma, M.X.; Wu, H.; Li, L.; Tan, D.Y. Up-regulation of human arrest-defective 1 protein is correlated with metastatic phenotype and poor prognosis in breast cancer. Asian Pac. J. Cancer Prev. 2011, 12, 1973–1977. [Google Scholar] [PubMed]
- Chien, M.H.; Lee, W.J.; Yang, Y.C.; Tan, P.; Pan, K.F.; Liu, Y.C.; Tsai, H.C.; Hsu, C.H.; Wen, Y.C.; Hsiao, M.; et al. N-alpha-acetyltransferase 10 protein promotes metastasis by stabilizing matrix metalloproteinase-2 protein in human osteosarcomas. Cancer Lett. 2018, 433, 86–98. [Google Scholar] [CrossRef]
- Jiang, B.; Ren, T.; Dong, B.; Qu, L.; Jin, G.; Li, J.; Qu, H.; Meng, L.; Liu, C.; Wu, J.; et al. Peptide mimic isolated by autoantibody reveals human arrest defective 1 overexpression is associated with poor prognosis for colon cancer patients. Am. J. Pathol. 2010, 177, 1095–1103. [Google Scholar] [CrossRef]
- Shim, J.H.; Chung, Y.H.; Kim, J.A.; Lee, D.; Kim, K.M.; Lim, Y.S.; Lee, H.C.; Lee, Y.S.; Yu, E.; Lee, Y.J. Clinical implications of arrest-defective protein 1 expression in hepatocellular carcinoma: A novel predictor of microvascular invasion. Dig. Dis. 2012, 30, 603–608. [Google Scholar] [CrossRef]
- Lee, C.F.; Ou, D.S.; Lee, S.B.; Chang, L.H.; Lin, R.K.; Li, Y.S.; Upadhyay, A.K.; Cheng, X.; Wang, Y.C.; Hsu, H.S.; et al. hNaa10p contributes to tumorigenesis by facilitating DNMT1-mediated tumor suppressor gene silencing. J. Clin. Investig. 2010, 120, 2920–2930. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wang, Z.; Guo, J.; Li, Y.; Bavarva, J.H.; Qian, C.; Brahimi-Horn, M.C.; Tan, D.; Liu, W. Inactivation of androgen-induced regulator ARD1 inhibits androgen receptor acetylation and prostate tumorigenesis. Proc. Natl. Acad. Sci. USA 2012, 109, 3053–3058. [Google Scholar] [CrossRef] [Green Version]
- Minguez, P.; Letunic, I.; Parca, L.; Bork, P. PTMcode: A database of known and predicted functional associations between post-translational modifications in proteins. Nucleic Acids Res. 2013, 41, D306–D311. [Google Scholar] [CrossRef] [Green Version]
- Minguez, P.; Parca, L.; Diella, F.; Mende, D.R.; Kumar, R.; Helmer-Citterich, M.; Gavin, A.C.; van Noort, V.; Bork, P. Deciphering a global network of functionally associated post-translational modifications. Mol. Syst. Biol. 2012, 8, 599–612. [Google Scholar] [CrossRef]
- Ree, R.; Varland, S.; Arnesen, T. Spotlight on protein N-terminal acetylation. Exp. Mol. Med. 2018, 50, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnaudo, N.; Fernandez, I.S.; McLaughlin, S.H.; Peak-Chew, S.Y.; Rhodes, D.; Martino, F. The N-terminal acetylation of Sir3 stabilizes its binding to the nucleosome core particle. Nat. Struct. Mol. Biol. 2013, 20, 1119–1121. [Google Scholar] [CrossRef] [PubMed]
- Aksnes, H.; Hole, K.; Arnesen, T. Molecular, cellular, and physiological significance of N-terminal acetylation. Int. Rev. Cell Mol. Biol. 2015, 316, 267–305. [Google Scholar]
- Raychaudhuri, S.; Sinha, M.; Mukhopadhyay, D.; Bhattacharyya, N.P. HYPK, a Huntingtin interacting protein, reduces aggregates and apoptosis induced by N-terminal Huntingtin with 40 glutamines in Neuro2a cells and exhibits chaperone-like activity. Hum. Mol. Genet. 2008, 17, 240–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, C.S.; Shemorry, A.; Varshavsky, A. N-terminal acetylation of cellular proteins creates specific degradation signals. Science 2010, 327, 973–977. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, P.; Ha, E.; Vo, T.T.L.; Seo, J.H. Diverse roles of arrest defective 1 in cancer development. Arch. Pharm. Res. 2019, 42, 1040–1051. [Google Scholar] [CrossRef]
- Lee, K.E.; Heo, J.E.; Kim, J.M.; Hwang, C.S. N-Terminal acetylation-targeted N-End rule proteolytic system: The Ac/N-End rule pathway. Mol. Cells 2016, 39, 169–178. [Google Scholar]
- Lee, D.; Jang, M.K.; Seo, J.H.; Ryu, S.H.; Kim, J.A.; Chung, Y.H. ARD1/NAA10 in hepatocellular carcinoma: Pathways and clinical implications. Exp. Mol. Med. 2018, 50, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Whiteway, M.; Szostak, J.W. The ARD1 gene of yeast functions in the switch between the mitotic cell cycle and alternative developmental pathways. Cell 1985, 43, 483–492. [Google Scholar] [CrossRef]
- Arnesen, T.; Van Damme, P.; Polevoda, B.; Helsens, K.; Evjenth, R.; Colaert, N.; Varhaug, J.E.; Vandekerckhove, J.; Lillehaug, J.R.; Sherman, F.; et al. Proteomics analyses reveal the evolutionary conservation and divergence of N-terminal acetyltransferases from yeast and humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8157–8162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DePaolo, J.S.; Wang, Z.; Guo, J.; Zhang, G.; Qian, C.; Zhang, H.; Zabaleta, J.; Liu, W. Acetylation of androgen receptor by ARD1 promotes dissociation from HSP90 complex and prostate tumorigenesis. Oncotarget 2016, 7, 71417–71428. [Google Scholar] [CrossRef] [Green Version]
- Yoon, H.; Kim, H.L.; Chun, Y.S.; Shin, D.H.; Lee, K.H.; Shin, C.S.; Lee, D.Y.; Kim, H.H.; Lee, Z.H.; Ryoo, H.M.; et al. NAA10 controls osteoblast differentiation and bone formation as a feedback regulator of Runx2. Nat. Commun. 2014, 5, 5176. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.H.; Park, J.H.; Lee, E.J.; Vo, T.T.; Choi, H.; Kim, J.Y.; Jang, J.K.; Wee, H.J.; Lee, H.S.; Jang, S.H.; et al. ARD1-mediated Hsp70 acetylation balances stress-induced protein refolding and degradation. Nat. Commun. 2016, 7, 12882. [Google Scholar] [CrossRef] [Green Version]
- Qian, X.; Li, X.; Lu, Z. Protein kinase activity of the glycolytic enzyme PGK1 regulates autophagy to promote tumorigenesis. Autophagy 2017, 13, 1246–1247. [Google Scholar] [CrossRef] [Green Version]
- Arnesen, T.; Anderson, D.; Baldersheim, C.; Lanotte, M.; Varhaug, J.E.; Lillehaug, J.R. Identification and characterization of the human ARD1-NATH protein acetyltransferase complex. Biochem. J. 2005, 386, 433–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.H.; Seo, J.H.; Wee, H.J.; Vo, T.T.; Lee, E.J.; Choi, H.; Cha, J.H.; Ahn, B.J.; Shin, M.W.; Bae, S.J.; et al. Nuclear translocation of hARD1 contributes to proper cell cycle progression. PLoS ONE 2014, 9, e105185. [Google Scholar] [CrossRef] [PubMed]
- Aksnes, H.; Van Damme, P.; Goris, M.; Starheim, K.K.; Marie, M.; Stove, S.I.; Hoel, C.; Kalvik, T.V.; Hole, K.; Glomnes, N.; et al. An organellar nalpha-acetyltransferase, naa60, acetylates cytosolic N termini of transmembrane proteins and maintains Golgi integrity. Cell Rep. 2015, 10, 1362–1374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, P.E.; Dyson, H.J. Intrinsically disordered proteins in cellular signalling and regulation. Nat. Rev. Mol. Cell Biol. 2015, 16, 18–29. [Google Scholar] [CrossRef]
- Seo, J.H.; Cha, J.H.; Park, J.H.; Jeong, C.H.; Park, Z.Y.; Lee, H.S.; Oh, S.H.; Kang, J.H.; Suh, S.W.; Kim, K.H.; et al. Arrest defective 1 autoacetylation is a critical step in its ability to stimulate cancer cell proliferation. Cancer Res. 2010, 70, 4422–4432. [Google Scholar] [CrossRef] [Green Version]
- Qian, X.; Li, X.; Cai, Q.; Zhang, C.; Yu, Q.; Jiang, Y.; Lee, J.H.; Hawke, D.; Wang, Y.; Xia, Y.; et al. Phosphoglycerate kinase 1 phosphorylates beclin1 to induce autophagy. Mol. Cell 2017, 65, 917–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, H.P.; Lee, D.F.; Xia, W.; Lai, C.C.; Li, L.Y.; Hung, M.C. Phosphorylation of ARD1 by IKKbeta contributes to its destabilization and degradation. Biochem. Biophys. Res. Commun. 2009, 389, 156–161. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; Chun, Y.S.; Huh, J.; Park, J.W. FIH permits NAA10 to catalyze the oxygen-dependent lysyl-acetylation of HIF-1α. Redox Biol. 2018, 19, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.T.L.; Park, J.H.; Lee, E.J.; Nguyen, Y.T.K.; Han, B.W.; Nguyen, H.T.T.; Mun, K.C.; Ha, E.; Kwon, T.K.; Kim, K.W.; et al. Characterization of lysine acetyltransferase activity of recombinant human ARD1/NAA10. Molecules 2020, 25, 588. [Google Scholar] [CrossRef] [Green Version]
- Liszczak, G.; Arnesen, T.; Marmorstein, R. Structure of a ternary Naa50p (NAT5/SAN) N-terminal acetyltransferase complex reveals the molecular basis for substrate-specific acetylation. J. Biol. Chem. 2011, 286, 37002–37010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottlieb, L.; Marmorstein, R. Structure of human NatA and its regulation by the huntingtin interacting protein HYPK. Structure 2018, 26, 925–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, H.P.; Lee, D.F.; Chen, C.T.; Liu, M.; Chou, C.K.; Lee, H.J.; Du, Y.; Xie, X.; Wei, Y.; Xia, W.; et al. ARD1 stabilization of TSC2 suppresses tumorigenesis through the mTOR signaling pathway. Sci. Signal. 2010, 3, ra9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, K.T.; Tan, C.T.; Johansson, G.; Lee, J.M.; Yang, P.W.; Lu, H.Y.; Chen, C.K.; Su, J.L.; Chen, P.B.; Wu, Y.L.; et al. N-alpha-acetyltransferase 10 protein suppresses cancer cell metastasis by binding PIX proteins and inhibiting Cdc42/Rac1 activity. Cancer Cell 2011, 19, 218–231. [Google Scholar] [CrossRef] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [Green Version]
- Ren, T.; Jiang, B.; Jin, G.; Li, J.; Dong, B.; Zhang, J.; Meng, L.; Wu, J.; Shou, C. Generation of novel monoclonal antibodies and their application for detecting ARD1 expression in colorectal cancer. Cancer Lett. 2008, 264, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, Q.; Niu, J.; Li, B.; Jiang, D.; Wan, Z.; Yang, Q.; Jiang, F.; Wei, P.; Bai, S. microRNA-342-5p and miR-608 inhibit colon cancer tumorigenesis by targeting NAA10. Oncotarget 2016, 7, 2709–2720. [Google Scholar] [CrossRef] [Green Version]
- Dorfel, M.J.; Lyon, G.J. The biological functions of Naa10—From amino-terminal acetylation to human disease. Gene 2015, 567, 103–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.N.; Kweon, H.Y.; Oh, G.T. N-α-acetyltransferase 10 (NAA10) in development: The role of NAA10. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef]
- Markopoulos, A.K. Current aspects on oral squamous cell carcinoma. Open Dent. J. 2012, 6, 126–130. [Google Scholar] [CrossRef]
- Zeng, Y.; Zheng, J.; Zhao, J.; Jia, P.R.; Yang, Y.; Yang, G.J.; Ma, J.F.; Gu, Y.Q.; Xu, J. High expression of Naa10p associates with lymph node metastasis and predicts favorable prognosis of oral squamous cell carcinoma. Tumour. Biol. 2016, 37, 6719–6728. [Google Scholar] [CrossRef] [PubMed]
- Merriel, S.W.D.; Funston, G.; Hamilton, W. Prostate cancer in primary care. Adv. Ther. 2018, 35, 1285–1294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lonergan, P.E.; Tindall, D.J. Androgen receptor signaling in prostate cancer development and progression. J. Carcinog. 2011, 10, 20. [Google Scholar]
- Zhou, Y.; Bolton, E.C.; Jones, J.O. Androgens and androgen receptor signaling in prostate tumorigenesis. J. Mol. Endocrinol. 2015, 54, R15–R29. [Google Scholar] [CrossRef] [Green Version]
- Lavery, D.N.; Bevan, C.L. Androgen receptor signalling in prostate cancer: The functional consequences of acetylation. J. Biomed. Biotechnol. 2011, 2011, 862125. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Min, L.; Han, Y.; Meng, L.; Liu, C.; Xie, Y.; Dong, B.; Wang, L.; Jiang, B.; Xu, H.; et al. Inhibition of STAT5a by Naa10p contributes to decreased breast cancer metastasis. Carcinogenesis 2014, 35, 2244–2253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozada, E.M.; Andrysik, Z.; Yin, M.; Redilla, N.; Rice, K.; Stambrook, P.J. Acetylation and deacetylation of Cdc25A constitutes a novel mechanism for modulating Cdc25A functions with implications for cancer. Oncotarget 2016, 7, 20425–20439. [Google Scholar] [CrossRef]
- Park, Y.H.; Seo, J.H.; Park, J.H.; Lee, H.S.; Kim, K.W. Hsp70 acetylation prevents caspase-dependent/independent apoptosis and autophagic cell death in cancer cells. Int. J. Oncol. 2017, 51, 573–578. [Google Scholar] [CrossRef] [Green Version]
- Vo, T.T.L.; Park, J.H.; Seo, J.H.; Lee, E.J.; Choi, H.; Bae, S.J.; Le, H.; An, S.; Lee, H.S.; Wee, H.J.; et al. ARD1-mediated aurora kinase A acetylation promotes cell proliferation and migration. Oncotarget 2017, 8, 57216–57230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, D.H.; Chun, Y.S.; Lee, K.H.; Shin, H.W.; Park, J.W. Arrest defective-1 controls tumor cell behavior by acetylating myosin light chain kinase. PLoS ONE 2009, 4, e7451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, J.H.; Park, J.W.; Chun, Y.S. Human arrest defective 1 acetylates and activates beta-catenin, promoting lung cancer cell proliferation. Cancer Res. 2006, 66, 10677–10682. [Google Scholar] [CrossRef] [Green Version]
- Neri, L.; Lasa, M.; Elosegui-Artola, A.; D’Avola, D.; Carte, B.; Gazquez, C.; Alve, S.; Roca-Cusachs, P.; Inarrairaegui, M.; Herrero, J.; et al. NatB-mediated protein N-alpha-terminal acetylation is a potential therapeutic target in hepatocellular carcinoma. Oncotarget 2017, 8, 40967–40981. [Google Scholar] [CrossRef] [Green Version]
- Varland, S.; Myklebust, L.M.; Goksoyr, S.O.; Glomnes, N.; Torsvik, J.; Varhaug, J.E.; Arnesen, T. Identification of an alternatively spliced nuclear isoform of human N-terminal acetyltransferase Naa30. Gene 2018, 644, 27–37. [Google Scholar] [CrossRef]
- Starheim, K.K.; Gromyko, D.; Evjenth, R.; Ryningen, A.; Varhaug, J.E.; Lillehaug, J.R.; Arnesen, T. Knockdown of human N α-terminal acetyltransferase complex C leads to p53-dependent apoptosis and aberrant human Arl8b localization. Mol. Cell. Biol. 2009, 29, 3569–3581. [Google Scholar] [CrossRef] [Green Version]
- Van Damme, P.; Kalvik, T.V.; Starheim, K.K.; Jonckheere, V.; Myklebust, L.M.; Menschaert, G.; Varhaug, J.E.; Gevaert, K.; Arnesen, T. A role for human N-alpha acetyltransferase 30 (Naa30) in maintaining mitochondrial integrity. Mol. Cell. Proteom. 2016, 15, 3361–3372. [Google Scholar] [CrossRef] [Green Version]
- Mughal, A.A.; Grieg, Z.; Skjellegrind, H.; Fayzullin, A.; Lamkhannat, M.; Joel, M.; Ahmed, M.S.; Murrell, W.; Vik-Mo, E.O.; Langmoen, I.A.; et al. Knockdown of NAT12/NAA30 reduces tumorigenic features of glioblastoma-initiating cells. Mol. Cancer 2015, 14, 160. [Google Scholar] [CrossRef] [Green Version]
- Pavlou, D.; Kirmizis, A. Depletion of histone N-terminal-acetyltransferase Naa40 induces p53-independent apoptosis in colorectal cancer cells via the mitochondrial pathway. Apoptosis 2016, 21, 298–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, J.; Chen, A.; Deng, Y.; Liu, M.; Wang, Y.; Wang, Y.; Nie, M.; Wang, C.; Ding, H.; Yao, B.; et al. NatD promotes lung cancer progression by preventing histone H4 serine phosphorylation to activate Slug expression. Nat. Commun. 2017, 8, 928. [Google Scholar] [CrossRef]
- Drazic, A.; Aksnes, H.; Marie, M.; Boczkowska, M.; Varland, S.; Timmerman, E.; Foyn, H.; Glomnes, N.; Rebowski, G.; Impens, F.; et al. NAA80 is actin’s N-terminal acetyltransferase and regulates cytoskeleton assembly and cell motility. Proc. Natl. Acad. Sci. USA 2018, 115, 4399–4404. [Google Scholar] [CrossRef] [Green Version]
- Vo, T.T.L.; Jeong, C.H.; Lee, S.; Kim, K.W.; Ha, E.; Seo, J.H. Versatility of ARD1/NAA10-mediated protein lysine acetylation. Exp. Mol. Med. 2018, 50, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Prognosis | Cancer Type | Clinical Outcome | Reference |
|---|---|---|---|
| Overall Survival | Breast cancer | Low | Wang et al., 2011 [4] |
| High | Kuo et al., 2010 [37] | ||
| High | Zeng et al., 2014 [51] | ||
| Colon cancer | Low | Jiang et al., 2010 [6] | |
| HCC | Low | Lee et al., 2018 [19] | |
| Lung cancer | Low | Lee et al., 2010 [8] | |
| High | Hua et al., 2011 [38] | ||
| OSCC | High | Zeng et al., 2016 [46] | |
| Osteosarcoma | Low | Chien et al., 2018 [5] | |
| Invasiveness | HCC | MVI | Shim et al., 2012 [7] |
| Metastasis | Breast cancer | High | Wang et al., 2011 [4] |
| Low | Kuo et al., 2010 [37] | ||
| Low | Zeng et al., 2011 [51] | ||
| Lung cancer | Low | Hua et al., 2011 [38] | |
| OSCC | Low | Zeng et al., 2016 [46] | |
| Osteosarcoma | High | Chien et al., 2018 [5] | |
| Recurrence | Breast cancer | High | Wang et al., 2011 [4] |
| HCC | High | Lee et al., 2018 [19] | |
| OSCC | Low | Zeng et al., 2016 [46] |
| Role | Target Protein | Function | Effect of NAA10 | Activity | Reference |
|---|---|---|---|---|---|
| Oncoprotein | β-catenin | Proliferation | Acetylation | Activated | Lim et al., 2016 [6] |
| Cdc25A | Proliferation | Acetylation | Activated | Lozada et al., 2016 [52] | |
| AR | Proliferation | Acetylation | Activated | DePaolo et al., 2016 [22] | |
| PGK1 | Autophagy | Acetylation at K388 | Activated | Qian et al., 2017 [25] | |
| Hsp70 | Apoptosis | Acetylation at K77 | Activated | Park et al., 2017 [53] | |
| DNMT1 | Migration | Interaction | Activated | Lee et al., 2010 [8] | |
| AuA | Migration | Acetylation at K75/K125 | Activated | Vo et al., 2017 [54] | |
| MMP-2 | Migration | Stabilization | Activated | Chien et al., 2018 [5] | |
| Tumor suppressor | TSC2 | Autophagy | Acetylation | Activated | Kuo et al., 2010 [37] |
| MLCK | Migration | Acetylation at K608 | Inactivated | Shin et al., 2009 [55] | |
| PIX | Migration | Interaction | Inactivated | Hua et al., 2011 [38] | |
| STAT5α | Migration | Interaction | Inactivated | Zeng et al., 2014 [51] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.M.; Ha, E.; Kim, J.; Cho, C.; Shin, S.-J.; Seo, J.H. NAA10 as a New Prognostic Marker for Cancer Progression. Int. J. Mol. Sci. 2020, 21, 8010. https://doi.org/10.3390/ijms21218010
Kim SM, Ha E, Kim J, Cho C, Shin S-J, Seo JH. NAA10 as a New Prognostic Marker for Cancer Progression. International Journal of Molecular Sciences. 2020; 21(21):8010. https://doi.org/10.3390/ijms21218010
Chicago/Turabian StyleKim, Sun Myung, Eunyoung Ha, Jinyoung Kim, Chiheum Cho, So-Jin Shin, and Ji Hae Seo. 2020. "NAA10 as a New Prognostic Marker for Cancer Progression" International Journal of Molecular Sciences 21, no. 21: 8010. https://doi.org/10.3390/ijms21218010
APA StyleKim, S. M., Ha, E., Kim, J., Cho, C., Shin, S.-J., & Seo, J. H. (2020). NAA10 as a New Prognostic Marker for Cancer Progression. International Journal of Molecular Sciences, 21(21), 8010. https://doi.org/10.3390/ijms21218010






