Contact-Dependent Growth Inhibition in Bacteria: Do Not Get Too Close!
Abstract
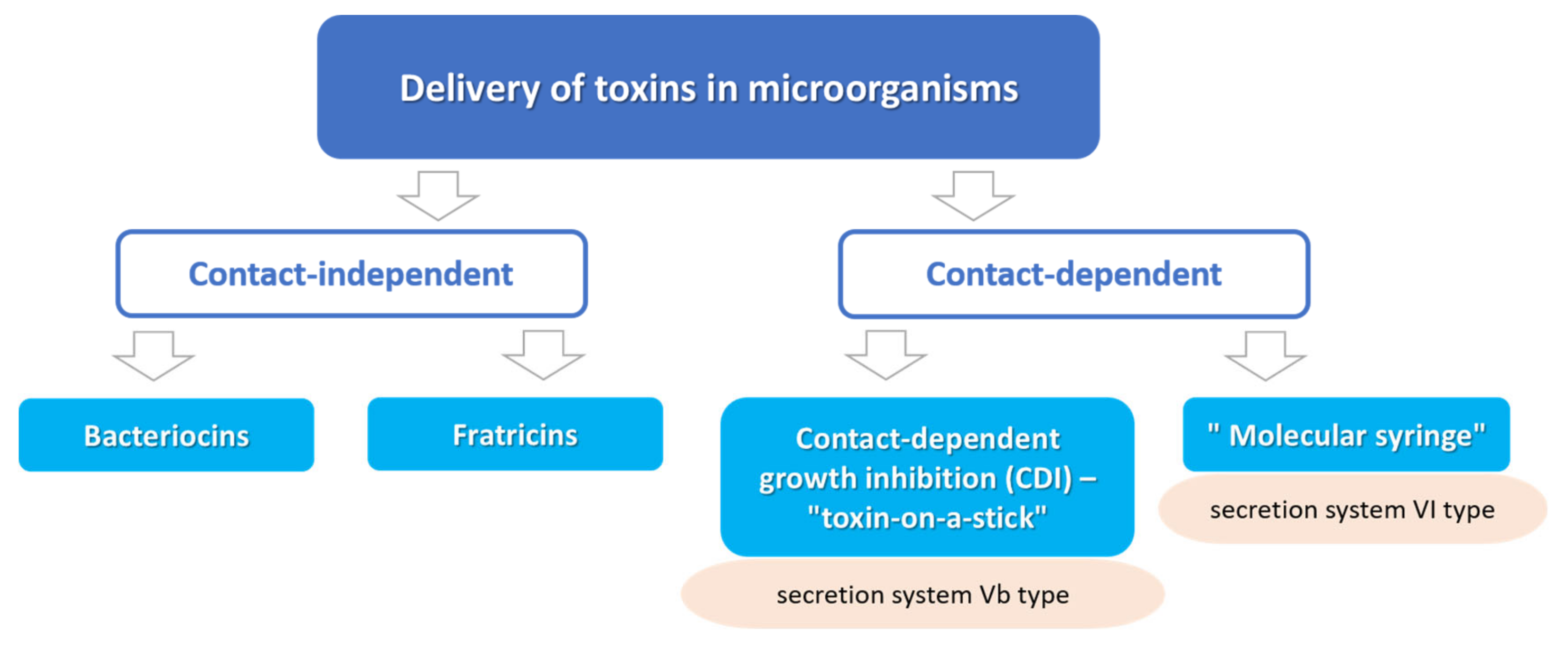
:1. Introduction
2. Contact-Dependent Growth Inhibition in Escherichia coli
3. “Toxin-on-a-Stick”: The Structure of the CdiA Protein
4. CDI Systems in Other Bacterial Species
5. Harpoon with Replaceable Tips: Orphan CDI Modules
6. What Are CDI Systems Designed for?
6.1. Interbacterial Antagonism
6.2. To Kill or to Kiss? Contact-Dependent Signaling
6.3. An Impact of CDI Toxins in Bacterial Populations
7. Modulating the Microbial Communities: The Enemy of My Enemy Is My Friend
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chatterjee, S.; Samal, B.; Singh, P.; Pradhan, B.B.; Verma, R.K. Transition of solitary to biofilm community life style in bacteria: A survival strategy with division of labour. Int. J. Dev. Biol. 2020, 64, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Claessen, D.; Rozen, D.E.; Kuipers, O.P.; Søgaard-Andersen, L.; van Wezel, G.P. Bacterial solutions to multicellularity: A tale of biofilms, filaments and fruiting bodies. Nat. Rev. Microbiol. 2014, 12, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abisado, R.G.; Benomar, S.; Klaus, J.R.; Dandekar, A.A.; Chandler, J.R. Bacterial quorum sensing and microbial community interactions. mBio 2018, 9, e02331-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allocati, N.; Masulli, M.; Di Ilio, C.; De Laurenzi, V. Die for the community: An overview of programmed cell death in bacteria. Cell Death Dis. 2015, 6, e1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popp, P.F.; Mascher, T. Coordinated cell death in isogenic bacterial populations: Sacrificing some for the benefit of many? J. Mol. Biol. 2019, 431, 4656–4669. [Google Scholar] [CrossRef]
- Meade, E.; Slattery, M.A.; Garvey, M. Bacteriocins, potent antimicrobial peptides and the fight against multi drug resistant species: Resistance is futile? Antibiotics 2020, 9, 32. [Google Scholar] [CrossRef] [Green Version]
- Simons, A.; Alhanout, K.; Duval, R.E. Bacteriocins, Antimicrobial peptides from bacterial origin: Overview of their biology and their impact against multidrug-resistant bacteria. Microorganisms 2020, 8, 639. [Google Scholar] [CrossRef]
- Wei, H.; Håvarstein, L.S. Fratricide is essential for efficient gene transfer between pneumococci in biofilms. Appl. Environ. Microbiol. 2012, 78, 5897–5905. [Google Scholar] [CrossRef] [Green Version]
- Claverys, J.-P.; Håvarstein, L.S. Cannibalism and fratricide: Mechanisms and raisons d’être. Nat. Rev. Microbiol. 2007, 5, 219. [Google Scholar] [CrossRef]
- Ikryannikova, L.N.; Kurbatov, L.K.; Soond, S.M.; Zamyatnin, A.A. Harnessing the potential of killers and altruists within the microbial community: A possible alternative to antibiotic therapy? Antibiotics 2019, 8, 230. [Google Scholar] [CrossRef] [Green Version]
- Benz, J.; Meinhart, A. Antibacterial effector/immunity systems: It’s just the tip of the iceberg. Curr. Opin. Microbiol. 2014, 17, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, T.A.; Ahmad, S.; Whitney, J.C. Contact-dependent interbacterial antagonism mediated by protein secretion machines. Trends Microbiol. 2020, 28, 387–400. [Google Scholar] [CrossRef]
- Garcia, E.C. Contact-dependent interbacterial toxins deliver a message. Curr. Opin. Microbiol. 2018, 42, 40–46. [Google Scholar] [CrossRef]
- Chassaing, B.; Cascales, E. Antibacterial weapons: Targeted destruction in the microbiota. Trends Microbiol. 2018, 26, 329–338. [Google Scholar] [CrossRef] [Green Version]
- Braun, V.; Patzer, S.I. Intercellular communication by related bacterial protein toxins: Colicins, contact-dependent inhibitors, and proteins exported by the type VI secretion system. FEMS Microbiol. Lett. 2013, 345, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Konovalova, A.; Søgaard-Andersen, L. Close encounters: Contact-dependent interactions in bacteria. Mol. Microbiol. 2011, 81, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Garcia, F.; Ruiz-Perez, F.; Cataldi, Á.; Larzábal, M. Type VI secretion system in pathogenic Escherichia coli: Structure, role in virulence, and acquisition. Front. Microbiol. 2019, 10, 1965. [Google Scholar] [CrossRef] [Green Version]
- Cascales, E.; Cambillau, C. Structural biology of type VI secretion systems. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 1102–1111. [Google Scholar] [CrossRef] [Green Version]
- Coulthurst, S.J. The type VI secretion system—A widespread and versatile cell targeting system. Res. Microbiol. 2013, 164, 640–654. [Google Scholar] [CrossRef]
- Cianfanelli, F.R.; Monlezun, L.; Coulthurst, S.J. Aim, Load, Fire: The Type VI Secretion System, a Bacterial Nanoweapon. Trends Microbiol. 2016, 24, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Meuskens, I.; Saragliadis, A.; Leo, J.C.; Linke, D. Type V secretion systems: An overview of passenger domain functions. Front. Microbiol. 2019, 10, 1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, C.S.; Aoki, S.K.; Low, D.A. Bacterial contact-dependent delivery systems. Annu. Rev. Genet. 2010, 44, 71–90. [Google Scholar] [CrossRef] [PubMed]
- Hayes, C.S.; Koskiniemi, S.; Ruhe, Z.C.; Poole, S.J.; Low, D.A. Mechanisms and biological roles of contact-dependent growth inhibition systems. Cold Spring Harb. Perspect. Med. 2014, 4, a010025. [Google Scholar] [CrossRef] [Green Version]
- Bottery, M.J.; Passaris, I.; Dytham, C.; Wood, A.J.; van der Woude, M.W. Spatial organization of expanding bacterial colonies is affected by contact-dependent growth inhibition. Curr. Biol. 2019, 29, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruhe, Z.C.; Low, D.A.; Hayes, C.S. Bacterial contact-dependent growth inhibition. Trends Microbiol. 2013, 21, 230–237. [Google Scholar] [CrossRef] [Green Version]
- Slechta, E.S.; Mulvey, M.A. Contact-dependent inhibition: Bacterial brakes and secret handshakes. Trends Microbiol. 2006, 14, 58–60. [Google Scholar] [CrossRef]
- Danka, E.S.; Garcia, E.C.; Cotter, P.A. Are CDI systems multicolored, facultative, helping greenbeards? Trends Microbiol. 2017, 25, 391–401. [Google Scholar] [CrossRef] [Green Version]
- Jones, A.M.; Garza-Sanchez, F.; So, J.; Hayes, C.S.; Low, D.A. Activation of contact-dependent antibacterial tRNase toxins by translation elongation factors. Proc. Natl. Acad. Sci. USA 2017, 114, E1951–E1957. [Google Scholar] [CrossRef] [Green Version]
- Aoki, S.K.; Diner, E.J.; de Roodenbeke, C.T.; Burgess, B.R.; Poole, S.J.; Braaten, B.A.; Jones, A.M.; Webb, J.S.; Hayes, C.S.; Cotter, P.A.; et al. A Widespread Family of Polymorphic Contact-Dependent Toxin Delivery Systems in Bacteria. Nature 2010, 468, 439–442. [Google Scholar] [CrossRef] [Green Version]
- Poole, S.J.; Diner, E.J.; Aoki, S.K.; Braaten, B.A.; de Roodenbeke, C.K.; Low, D.A.; Hayes, C.S. Identification of functional toxin/immunity genes linked to contact-dependent growth inhibition (CDI) and rearrangement hotspot (Rhs) systems. PLoS Genet. 2011, 7, e1002217. [Google Scholar] [CrossRef]
- Holberger, L.E.; Garza-Sánchez, F.; Lamoureux, J.; Low, D.A.; Hayes, C.S. A novel family of toxin/antitoxin proteins in Bacillus species. FEBS Lett. 2012, 586, 132–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoki, S.K.; Pamma, R.; Hernday, A.D.; Bickham, J.E.; Braaten, B.A.; Low, D.A. Contact-dependent inhibition of growth in Escherichia coli. Science 2005, 309, 1245–1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoki, S.K.; Poole, S.J.; Hayes, C.S.; Low, D.A. Toxin on a stick: Modular CDI toxin delivery systems play roles in bacterial competition. Virulence 2011, 2, 356–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruhe, Z.C.; Nguyen, J.Y.; Xiong, J.; Koskiniemi, S.; Beck, C.M.; Perkins, B.R.; Low, D.A.; Hayes, C.S. CdiA effectors use modular receptor-binding domains to recognize target bacteria. mBio 2017, 8, e00290-17. [Google Scholar] [CrossRef] [Green Version]
- Ruhe, Z.C.; Subramanian, P.; Song, K.; Nguyen, J.Y.; Stevens, T.A.; Low, D.A.; Jensen, G.J.; Hayes, C.S. Programmed secretion arrest and receptor-triggered toxin export during antibacterial contact-dependent growth inhibition. Cell 2018, 175, 921–933. [Google Scholar] [CrossRef] [Green Version]
- Webb, J.S.; Nikolakakis, K.C.; Willett, J.L.E.; Aoki, S.K.; Hayes, C.S.; Low, D.A. Delivery of CdiA nuclease toxins into target cells during contact-dependent growth inhibition. PLoS ONE 2013, 8, e57609. [Google Scholar] [CrossRef]
- Morse, R.P.; Nikolakakis, K.C.; Willett, J.L.E.; Gerrick, E.; Low, D.A.; Hayes, C.S.; Goulding, C.W. Structural basis of toxicity and immunity in contact-dependent growth inhibition (CDI) systems. Proc. Natl. Acad. Sci. USA 2012, 109, 21480–21485. [Google Scholar] [CrossRef] [Green Version]
- Willett, J.L.E.; Ruhe, Z.C.; Goulding, C.W.; Low, D.A.; Hayes, C.S. Contact-dependent growth inhibition (CDI) and CdiB/CdiA two-partner secretion proteins. J. Mol. Biol. 2015, 427, 3754–3765. [Google Scholar] [CrossRef] [Green Version]
- Guérin, J.; Bigot, S.; Schneider, R.; Buchanan, S.K.; Jacob-Dubuisson, F. Two-partner secretion: Combining efficiency and simplicity in the secretion of large proteins for bacteria-host and bacteria-bacteria interactions. Front. Cell. Infect. Microbiol. 2017, 7, 148. [Google Scholar] [CrossRef]
- Aoki, S.K.; Malinverni, J.C.; Jacoby, K.; Thomas, B.; Pamma, R.; Trinh, B.N.; Remers, S.; Webb, J.; Braaten, B.A.; Silhavy, T.J.; et al. Contact-dependent growth inhibition requires the essential outer membrane protein BamA (YaeT) as the receptor and the inner membrane transport protein AcrB. Mol. Microbiol. 2008, 70, 323–340. [Google Scholar] [CrossRef] [Green Version]
- Ruhe, Z.C.; Wallace, A.B.; Low, D.A.; Hayes, C.S. Receptor polymorphism restricts contact-dependent growth inhibition to members of the same species. mBio 2013, 4, e00480-13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck, C.M.; Willett, J.L.E.; Cunningham, D.A.; Kim, J.J.; Low, D.A.; Hayes, C.S. CdiA effectors from uropathogenic Escherichia coli use heterotrimeric osmoporins as receptors to recognize target bacteria. PLoS Pathog. 2016, 12, e1005925. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.K.; Webb, J.S.; Braaten, B.A.; Low, D.A. Contact-dependent growth inhibition causes reversible metabolic downregulation in Escherichia coli. J. Bacteriol. 2009, 191, 1777–1786. [Google Scholar] [CrossRef] [Green Version]
- Diner, E.J.; Beck, C.M.; Webb, J.S.; Low, D.A.; Hayes, C.S. Identification of a target cell permissive factor required for contact-dependent growth inhibition (CDI). Genes Dev. 2012, 26, 515–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; de Souza, R.F.; Anantharaman, V.; Iyer, L.M.; Aravind, L. Polymorphic toxin systems: Comprehensive characterization of trafficking modes, processing, mechanisms of action, immunity and ecology using comparative genomics. Biol. Direct. 2012, 7, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virtanen, P.; Wäneskog, M.; Koskiniemi, S. Class II contact-dependent growth inhibition (CDI) systems allow for broad-range cross-species toxin delivery within the Enterobacteriaceae family. Mol. Microbiol. 2019, 111, 1109–1125. [Google Scholar] [CrossRef] [Green Version]
- Ruhe, Z.C.; Nguyen, J.Y.; Chen, A.J.; Leung, N.Y.; Hayes, C.S.; Low, D.A. CDI systems are stably maintained by a cell-contact mediated surveillance mechanism. PLoS Genet. 2016, 12, e1006145. [Google Scholar] [CrossRef] [PubMed]
- Willett, J.L.E.; Gucinski, G.C.; Fatherree, J.P.; Low, D.A.; Hayes, C.S. Contact-dependent growth inhibition toxins exploit multiple independent cell-entry pathways. PNAS 2015, 112, 11341–11346. [Google Scholar] [CrossRef] [Green Version]
- Kaundal, S.; Uttam, M.; Thakur, K.G. Dual role of a biosynthetic enzyme, CysK, in contact dependent growth inhibition in bacteria. PLoS ONE 2016, 11, e0159844. [Google Scholar] [CrossRef]
- Michalska, K.; Gucinski, G.C.; Garza-Sanchez, F.; Johnson, P.M.; Stols, L.M.; Eschenfeldt, W.H.; Babnigg, G.; Low, D.A.; Goulding, C.W.; Joachimiak, A.; et al. Structure of a novel antibacterial toxin that exploits elongation factor Tu to cleave specific transfer RNAs. Nucl. Acids Res. 2017, 45, 10306–10320. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Fang, Q.; Tu, Q.; Liu, C.; Yin, J.; Yin, Y.; Xia, L.; Bian, X.; Zhang, Y. Identification of a contact-dependent growth inhibition system in the probiotic Escherichia coli Nissle 1917. Fems Microbiol. Lett. 2018, 365, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, M.S.; Garcia, E.C.; Cotter, P.A. The Burkholderia bcpAIOB genes define unique classes of two-partner secretion and contact dependent dependent growth inhibition systems. PLoS Genet. 2012, 8, e1002877. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.S.; Garcia, E.C.; Cotter, P.A. Kind discrimination and competitive exclusion mediated by contact-dependent growth inhibition systems shape biofilm community structure. PLoS Pathog. 2014, 10, e1004076. [Google Scholar] [CrossRef] [PubMed]
- Nikolakakis, K.; Amber, S.; Wilbur, J.S.; Diner, E.J.; Aoki, S.K.; Poole, S.J.; Tuanyok, A.; Keim, P.S.; Peacock, S.; Hayes, C.S.; et al. The toxin/immunity network of Burkholderia pseudomallei contact-dependent growth inhibition (CDI) systems. Mol. Microbiol. 2012, 84, 516–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, E.C.; Anderson, M.S.; Hagar, J.A.; Cotter, P.A. Burkholderia BcpA mediates biofilm formation independently of interbacterial contact-dependent growth inhibition. Mol. Microbiol. 2013, 89, 1213–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, E.C.; Perault, A.I.; Marlatt, S.A.; Cotter, P.A. Interbacterial signaling via Burkholderia contact-dependent growth inhibition system proteins. Proc. Nat. Acad. Sci. USA 2016, 113, 8296–8301. [Google Scholar] [CrossRef] [Green Version]
- Koskiniemi, S.; Garza-Sánchez, F.; Edman, N.; Chaudhuri, S.; Poole, S.J.; Manoil, C.; Hayes, C.S.; Low, D.A. Genetic analysis of the CDI pathway from Burkholderia pseudomallei 1026b. PLoS ONE 2015, 10, e0120265. [Google Scholar] [CrossRef]
- Perault, A.I.; Cotter, P.A. Three distinct contact-dependent growth inhibition systems mediate interbacterial competition by the cystic fibrosis pathogen Burkholderia dolosa. J. Bacteriol. 2018, 200, e00428-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers-Morales, T.; Oates, E.; Byrd, M.S.; Garcia, E.C. Burkholderia cepacia complex contact-dependent growth inhibition systems mediate interbacterial competition. J. Bacteriol. 2019, 201, e00012-19. [Google Scholar] [CrossRef] [Green Version]
- Tan, K.; Johnson, P.M.; Stols, L.; Boubion, B.; Eschenfeldt, W.; Babnigg, G.; Hayes, C.S.; Joachimiak, A.; Goulding, C.W. The structure of a contact-dependent growth inhibition (CDI) immunity protein from Neisseria meningitidis MC58. Acta Cryst. F 2015, F71, 702–709. [Google Scholar] [CrossRef] [Green Version]
- Mercy, C.; Ize, B.; Salcedo, S.P.; de Bentzmann, S.; Bigot, S. Functional characterization of Pseudomonas contact dependent growth inhibition (CDI) systems. PLoS ONE 2016, 11, e0147435. [Google Scholar] [CrossRef]
- Melvin, J.A.; Gaston, J.R.; Phillips, S.N.; Springer, M.J.; Marshall, C.W.; Shanks, R.M.Q.; Bomberger, J.M. Pseudomonas aeruginosa contact-dependent growth inhibition plays dual role in host-pathogen interactions. MSphere 2017, 2, e00336-17. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.P.; Hauser, A.R. Diversity of contact-dependent growth inhibition systems of Pseudomonas aeruginosa. J. Bacteriol. 2019, 201, e00776-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, J.P.; Ozer, E.A.; Minasov, G.; Shuvalova, L.; Kiryukhina, O.; Satchell, K.J.F.; Hauser, A.R. A comparative genomics approach identifies contact-dependent growth inhibition as a virulence determinant. Proc. Nat. Acad. Sci. USA 2020, 117, 6811–6821. [Google Scholar] [CrossRef] [PubMed]
- Harding, C.M.; Pulido, M.R.; Di Venanzio, G.; Kinsella, R.L.; Webb, A.I.; Scott, N.E.; Pachón, J.; Feldman, M.F. Pathogenic Acinetobacter species have a functional type I secretion system and contact-dependent inhibition systems. J. Biol. Chem. 2017, 292, 9075–9087. [Google Scholar] [CrossRef] [Green Version]
- De Gregorio, E.; Esposito, E.P.; Zarrilli, R.; Di Nocera, P.P. Contact dependent growth inhibition proteins in Acinetobacter baylyi ADP1. Curr. Microbiol. 2018, 75, 1434–1440. [Google Scholar] [CrossRef] [Green Version]
- De Gregorio, E.; Zarrilli, R.; Di Nocera, P.P. Contact-dependent growth inhibition systems in Acinetobacter. Sci. Rep. 2019, 9, 154. [Google Scholar] [CrossRef] [Green Version]
- Roussin, M.; Rabarioelina, S.; Cluzeau, L.; Cayron, J.; Lesterlin, C.; Salcedo, S.P.; Bigot, S. Identification of a contact-dependent growth inhibition (CDI) system that reduces biofilm formation and host cell adhesion of Acinetobacter baumannii DSM30011 strain. Front. Microbiol. 2019, 10, 2450. [Google Scholar] [CrossRef]
- Whitney, J.C.; Peterson, S.B.; Kim, J.; Pazos, M.; Verster, A.J.; Radey, M.C.; Kulasekara, H.D.; Ching, M.Q.; Bullen, N.P.; Bryant, D.; et al. A broadly distributed toxin family mediates contact-dependent antagonism between gram-positive bacteria. eLife 2017, 6, e26938. [Google Scholar] [CrossRef]
- Beck, C.M.; Morse, R.P.; Cunningham, D.A.; Iniguez, A.; Low, D.A.; Goulding, C.W.; Hayes, C.S. CdiA from Enterobacter cloacae delivers a toxic ribosomal RNase into target bacteria. Structure 2014, 22, 707–718. [Google Scholar] [CrossRef] [Green Version]
- Ogier, J.-C.; Duvic, B.; Lanois, A.; Givaudan, A.; Gaudriault, S. A new member of the growing family of contact-dependent growth inhibition systems in Xenorhabdus doucetiae. PLoS ONE 2016, 11, e0167443. [Google Scholar] [CrossRef] [PubMed]
- Paschen, S.A.; Waizenegger, T.; Stan, T.; Preuss, M.; Cyrklaff, M.; Hell, K.; Rapaport, D.; Neupert, W. Evolutionary conservation of biogenesis of β-barrel membrane proteins. Nature 2003, 426, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Voulhoux, R.; Bos, M.P.; Geurtsen, J.; Mols, M.; Tommassen, J. Role of a highly conserved bacterial protein in outer membrane protein assembly. Science 2003, 299, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Malinverni, J.; Ruiz, N.; Kim, S.; Silhavy, T.J.; Kahne, D. Identification of a multicomponent complex required for outer membrane biogenesis in Escherichia coli. Cell 2005, 121, 235–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojas, C.M.; Ham, J.H.; Deng, W.L.; Doyle, J.J.; Collmer, A. HecA, a member of a class of adhesins produced by diverse pathogenic bacteria, contributes to the attachment, aggregation, epidermal cell killing, and virulence phenotypes of Erwinia chrysanthemi EC16 on Nicotiana clevelandii seedlings. PNAS 2002, 99, 13142–13147. [Google Scholar] [CrossRef] [Green Version]
- Guilhabert, M.R.; Kirkpatrick, B.C. Identification of Xylella fastidiosa antivirulence genes: Hemagglutinin adhesins contribute to X. fastidiosa biofilm maturation and colonization and attenuate virulence. Mol. Plant Microbe Interact. 2005, 18, 856–868. [Google Scholar] [CrossRef] [Green Version]
- Killiny, N.; Almeida, R.P.P. Xylella fastidiosa afimbrial adhesins mediate cell transmission to plants by leafhopper vectors. Appl. Environ. Microbiol. 2009, 75, 521–528. [Google Scholar] [CrossRef] [Green Version]
- Killiny, N.; Almeida, R.P.P. Factors affecting the initial adhesion and retention of the plant pathogen Xylella fastidiosa in the foregut of an insect vector. Appl. Environ. Microbiol. 2014, 80, 420–426. [Google Scholar] [CrossRef] [Green Version]
- Neil, R.B.; Apicella, M.A. Role of HrpA in biofilm formation of Neisseria meningitidis and regulation of the hrpBAS transcripts. Infect. Immun. 2009, 77, 2285–2293. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, C.; Turner, D.; Boesl, M.; Abele, M.; Frosch, M.; Kurzai, O. A functional two-partner secretion system contributes to adhesion of Neisseria meningitidis to epithelial cells. J. Bacteriol. 2007, 189, 7968–7976. [Google Scholar] [CrossRef] [Green Version]
- Gottig, N.; Garavaglia, B.S.; Garofalo, C.G.; Orellano, E.G.; Ottado, J. A filamentous hemagglutinin-like protein of Xanthomonas axonopodis pv. citri, the phytopathogen responsible for citrus canker, is involved in bacterial virulence. PLoS ONE 2009, 4, e4358. [Google Scholar] [CrossRef]
- Ruhe, Z.C.; Townsley, L.; Wallace, A.B.; King, A.; Van der Woude, M.W.; Low, D.A.; Yildiz, F.H.; Hayes, C.S. CdiA promotes receptor-independent intercellular adhesion. Mol Microbiol. 2015, 98, 175–192. [Google Scholar] [CrossRef]
- Wall, D. Kin recognition in bacteria. Annu. Rev. Microbiol. 2016, 70, 143–160. [Google Scholar] [CrossRef] [Green Version]
- Blanchard, A.E.; Celik, V.; Lu, T. Extinction, coexistence, and localized patterns of a bacterial population with contact-dependent inhibition. BMC Syst. Biol. 2014, 8, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, A.; Baltekin, Ö.; Wäneskog, M.; Elkhalifa, D.; Hammarlöf, D.L.; Elf, J.; Koskiniemi, S. Contact-dependent growth inhibition induces high levels of antibiotic-tolerant persister cells in clonal bacterial populations. EMBO J. 2018, e98026. [Google Scholar] [CrossRef]
- Sana, T.G.; Lugo, K.A.; Monack, D.M. T6SS: The bacterial “fight club” in the host gut. PLoS Pathog. 2017, 13, e1006325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coyne, M.J.; Comstock, L.E. Type VI secretion systems and the gut microbiota. Microbiol. Spectr. 2019, 7, 1–11. [Google Scholar] [CrossRef]
- Russell, A.B.; Wexler, A.G.; Harding, B.N.; Whitney, J.C.; Bohn, A.J.; Goo, Y.A.; Tran, B.Q.; Barry, N.A.; Zheng, H.; Peterson, S.B.; et al. A type VI secretion-related pathway in Bacteroidetes mediates interbacterial antagonism. Cell Host Microbe 2014, 16, 227–236. [Google Scholar] [CrossRef] [Green Version]
- Sassone-Corsi, M.; Nuccio, S.P.; Liu, H.; Hernandez, D.; Vu, C.T.; Takahashi, A.A.; Edwards, R.A.; Raffatellu, M. Microcins mediate competition among Enterobacteriaceae in the inflamed gut. Nature 2016, 540, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Barbour, A.; Wescombe, P.; Smith, L. Evolution of lantibiotic salivaricins: New weapons to fight infectious diseases. Trends Microbiol. 2020, 28, 578–593. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Khampang, P.; Erbe, C.; Kumar, S.; Taylor, S.R.; Kerschner, J.E. Nontypeable Haemophilus influenzae inhibits autolysis and fratricide of Streptococcus pneumoniae in vitro. Microbes Infect. 2014, 16, 203–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Microorganism | CDI-Related Findings | Reference | |
|---|---|---|---|
| Escherichia | E. coli strain EC93 | The first description of the CDI phenomenon. | [32] |
| E. coli | CDI toxins/receptors complexes. | [40,41,42] | |
| E. coli | Mechanisms of CDI regulation. | [28,35,36,38,43,47,48] | |
| E. coli, B. pseudomallei | CDI toxin/immunity protein complexes. | [30,37] | |
| E. coli strain 536 (UPEC536) | CysK enzyme stabilizes the complex of CdiA-CT with the immunity protein CdiI. | [44,49] | |
| E. coli strain NC101 | CDI toxin/immunity protein/elongation factor Tu complex. | [50] | |
| E. coli strain Nissle 1917 | Identification of functioning cdiA-CT/cdiI modules. | [51] | |
| Burkholderia | B. thailandensis | The “Burkholderia” type of CDI locus is firstly defined. Expression of bcpAIOB genes is required for autoaggregation and adhesion on abiotic surfaces. | [52] |
| B. pseudomallei | Ten subtypes of CDI systems within the B. pseudomallei species were identified. | [54] | |
| B. thailandensis | CdiA toxins probably participate not only in interbacterial competition but also in cooperation and recognition of “self” bacteria from “non-self”. | [53,55,56] | |
| B. thailandensis | The mechanism of CDI toxin delivery can differ even between closely related species. | [57] | |
| B. dolosa | Identification of three functioning CDI systems. | [58] | |
| B. multivorans, B. thailandensis | Non-pathogenic B. thailandensis uses CDI to control the growth of pathogenic B. multivorans during co-cultivation. | [59] | |
| Pseudomonas | P. aeruginosa strain PAO1 | Identification of multiple cdi loci in the Pseudomonas genomes. CDI systems are involved in the processes of adhesion and biofilm formation. | [61] |
| P. aeruginosa | CDI system is vital for virulence of multidrug-resistant P. aeruginosa in acute/chronic infection. | [62] | |
| P. aeruginosa | Identification of CDI genes in P. aeruginosa genomes. | [63] | |
| P. aeruginosa | CDI systems have a toxic effect on mammalian cell culture and increase the virulence of P. aeruginosa strains in the mouse bacteremia model. | [64] | |
| Acinetobacter | A. baumannii, A. nosocomialis | Identification of functioning CDI systems. | [65] |
| Acinetobacter spp. | Identification of >40 variants of cdi loci within the genus Acinetobacter | [67] | |
| A. baylyi strain ADP1 | Both variants of CDI systems discovered play no roles in biofilm formation or adhesion to epithelial cells. | [66] | |
| A. baumannii strain DSM30011 | Identification of two types of CDI toxins. The functioning of CDI systems represses biofilm formation and adhesion to the host cells. | [68] | |
| Neisseria meningitidis | The crystal structure of the CdiI immunity protein. | [60] | |
| Enterobacter cloacae | CdiA-CTECL/immunity protein complex. | [70] | |
| Xenorhabdus doucetiae | Identification of the cdiBCAI locus in the genomes of Xenorhabdus and Photorhabdus luminescens. | [71] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikryannikova, L.N.; Kurbatov, L.K.; Gorokhovets, N.V.; Zamyatnin, A.A., Jr. Contact-Dependent Growth Inhibition in Bacteria: Do Not Get Too Close! Int. J. Mol. Sci. 2020, 21, 7990. https://doi.org/10.3390/ijms21217990
Ikryannikova LN, Kurbatov LK, Gorokhovets NV, Zamyatnin AA Jr. Contact-Dependent Growth Inhibition in Bacteria: Do Not Get Too Close! International Journal of Molecular Sciences. 2020; 21(21):7990. https://doi.org/10.3390/ijms21217990
Chicago/Turabian StyleIkryannikova, Larisa N., Leonid K. Kurbatov, Neonila V. Gorokhovets, and Andrey A. Zamyatnin, Jr. 2020. "Contact-Dependent Growth Inhibition in Bacteria: Do Not Get Too Close!" International Journal of Molecular Sciences 21, no. 21: 7990. https://doi.org/10.3390/ijms21217990
APA StyleIkryannikova, L. N., Kurbatov, L. K., Gorokhovets, N. V., & Zamyatnin, A. A., Jr. (2020). Contact-Dependent Growth Inhibition in Bacteria: Do Not Get Too Close! International Journal of Molecular Sciences, 21(21), 7990. https://doi.org/10.3390/ijms21217990






