Association of miR-21-5p, miR-122-5p, and miR-320a-3p with 90-Day Mortality in Cardiogenic Shock
Abstract
1. Introduction
2. Results
2.1. miRNA Association with Baseline Characteristics
2.2. miRNA Characteristics, Mortality, and Prognostic Value at Baseline
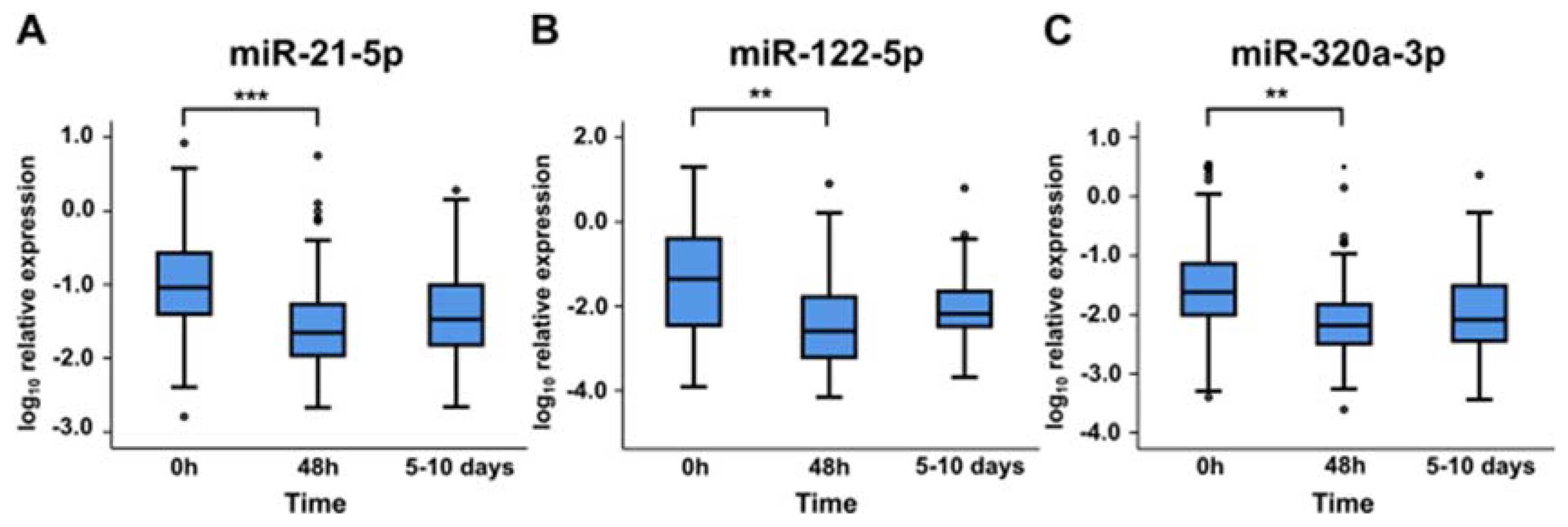
2.3. miRNA Levels at Later Time Points
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reynolds, H.R.; Hochman, J.S. Cardiogenic shock: Current concepts and improving outcomes. Circulation 2008, 117, 686–697. [Google Scholar] [CrossRef] [PubMed]
- Van Diepen, S.; Katz, J.N.; Albert, N.M.; Henry, T.D.; Jacobs, A.K.; Kapur, N.K.; Kilic, A.; Menon, V.; Ohman, E.M.; Sweitzer, N.K.; et al. Contemporary management of cardiogenic shock: A scientific statement from the American Heart Association. Circulation 2017, 136, e232–e268. [Google Scholar] [CrossRef] [PubMed]
- Thiele, H.; Zeymer, U.; Neumann, F.-J.; Ferenc, M.; Olbrich, H.-G.; Hausleiter, J.; Richardt, G.; Hennersdorf, M.; Empen, K.; Fuernau, G.; et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N. Engl. J. Med. 2012, 367, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Thiele, H.; Ohman, E.M.; Desch, S.; Eitel, I.; de Waha, S. Management of cardiogenic shock. Eur. Heart J. 2015, 36, 1223–1230. [Google Scholar] [CrossRef]
- Hochman, J.S.; Buller, C.E.; Sleeper, L.A.; Boland, J.; Dzavik, V.; Sanborn, T.A.; Godfrey, E.; White, H.D.; Lim, J.; LeJemtel, T. Cardiogenic shock complicating acute myocardial infarction—Etiologies, management and outcome: A report from the SHOCK Trial Registry. J. Am. Coll. Cardiol. 2000, 36, 1063–1070. [Google Scholar] [CrossRef]
- Harjola, V.-P.; Lassus, J.; Sionis, A.; Køber, L.; Tarvasmäki, T.; Spinar, J.; Parissis, J.; Banaszewski, M.; Silva-Cardoso, J.; Carubelli, V.; et al. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. Eur. J. Heart Fail. 2015, 17, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Hammond, S.M. An overview of microRNAs. Adv. Drug Deliv. Rev. 2015, 87, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef] [PubMed]
- Creemers, E.E.; Tijsen, A.J.; Pinto, Y.M. Circulating MicroRNAs: Novel biomarkers and extracellular communicators in cardiovascular disease? Circ. Res. 2012, 110, 483–495. [Google Scholar] [CrossRef]
- Wang, G.-K.; Zhu, J.-Q.; Zhang, J.-T.; Li, Q.; Li, Y.; He, J.; Qin, Y.-W.; Jing, Q. Circulating microRNA: A novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur. Heart J. 2010, 31, 659–666. [Google Scholar] [CrossRef]
- Wang, H.; Peng, R.; Wang, J.; Qin, Z.; Xue, L. Circulating microRNAs as potential cancer biomarkers: The advantage and disadvantage. Clin. Epigenetics 2018, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhang, C. MicroRNA-21 in cardiovascular disease. J. Cardiovasc. Transl. Res. 2010, 3, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Kumarswamy, R.; Volkmann, I.; Thum, T. Regulation and function of miRNA-21 in health and disease. RNA Biol. 2011, 8, 706. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Cheng, Y.; Yang, J.; Li, J.; Liu, X.; Wang, X.; Wang, D.; Krall, T.J.; Delphin, E.S.; Zhang, C. MicroRNA expression signature and the role of MicroRNA-21 in the early phase of acute myocardial infarction. J. Biol. Chem. 2009, 284, 29514–29525. [Google Scholar] [CrossRef]
- Chen, C.-H.; Hsu, S.-Y.; Chiu, C.-C.; Leu, S. MicroRNA-21 mediates the protective effect of cardiomyocyte-derived conditioned medium on ameliorating myocardial infarction in rats. Cells 2019, 8, 935. [Google Scholar] [CrossRef]
- Wang, Z.H.; Sun, X.Y.; Li, C.L.; Sun, Y.M.; Li, J.; Wang, L.F.; Li, Z.Q. miRNA-21 expression in the serum of elderly patients with acute myocardial infarction. Med. Sci. Monit. 2017, 23, 5728–5734. [Google Scholar] [CrossRef][Green Version]
- Lagos-Quintana, M.; Rauhut, R.; Yalcin, A.; Meyer, J.; Lendeckel, W.; Tuschl, T. Identification of tissue-specific microRNAs from mouse. Curr. Biol. 2002, 12, 735–739. [Google Scholar] [CrossRef]
- Tsai, W.C.; Hsu, S.D.; Hsu, C.S.; Lai, T.C.; Chen, S.J.; Shen, R.; Huang, Y.; Chen, H.C.; Lee, C.H.; Tsai, T.F.; et al. MicroRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. J. Clin. Investig. 2012, 122, 2884–2897. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, Y.; Zheng, R.; Guo, Y.; Wang, Y.; Guo, H.; Fei, M.; Sun, S. Plasma microRNA-122 as a biomarker for viral-, alcohol-, and chemical-related hepatic diseases. Clin. Chem. 2010, 56, 1830–1838. [Google Scholar] [CrossRef]
- Corsten, M.F.; Dennert, R.; Jochems, S.; Kuznetsova, T.; Devaux, Y.; Hofstra, L.; Wagner, D.R.; Staessen, J.A.; Heymans, S.; Schroen, B. Circulating MicroRNA-208b and MicroRNA-499 reflect myocardial damage in cardiovascular disease. Circ. Cardiovasc. Genet. 2010, 3, 499–506. [Google Scholar] [CrossRef]
- Andersson, P.; Gidlöf, O.; Braun, O.Ö.; Götberg, M.; Van Der Pals, J.; Olde, B.; Erlinge, D. Plasma levels of liver-specific miR-122 is massively increased in a porcine cardiogenic shock model and attenuated by hypothermia. Shock 2012, 37, 234–238. [Google Scholar] [CrossRef]
- Ren, X.P.; Wu, J.; Wang, X.; Sartor, M.A.; Qian, J.; Jones, K.; Nicolaou, P.; Pritchard, T.J.; Fan, G.C. MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 20. Circulation 2009, 119, 2357–2366. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.-Q.; Jiang, H.; Lu, Z.-B. MiR-320 regulates cardiomyocyte apoptosis induced by ischemia–reperfusion injury by targeting AKIP1. Cell. Mol. Biol. Lett. 2018, 23, 41. [Google Scholar] [CrossRef] [PubMed]
- Devaux, Y.; Mueller, M.; Haaf, P.; Goretti, E.; Twerenbold, R.; Zangrando, J.; Vausort, M.; Reichlin, T.; Wildi, K.; Moehring, B.; et al. Diagnostic and prognostic value of circulating microRNAs in patients with acute chest pain. J. Intern. Med. 2015, 277, 260–271. [Google Scholar] [CrossRef]
- Cortez-Dias, N.; Costa, M.C.; Carrilho-Ferreira, P.; Silva, D.; Jorge, C.; Calisto, C.; Pessoa, T.; Martins, S.R.; de Sousa, J.C.; da Silva, P.C.; et al. Circulating miR-122-5p/miR-133B ratio is a specific early prognostic biomarker in acute myocardial infarction. Circ. J. 2016, 80, 2183–2191. [Google Scholar] [CrossRef]
- Goldbergova, M.P.; Ipkova, J.; Fedorko, J.; Sevcikova, J.; Parenica, J.; Spinar, J.; Masarik, M.; Vasku, A. MicroRNAs in pathophysiology of acute myocardial infarction and cardiogenic shock. Bratisl. Med. J. 2018, 119, 341–347. [Google Scholar] [CrossRef]
- Gilje, P.; Frydland, M.; Bro-Jeppesen, J.; Dankiewicz, J.; Friberg, H.; Rundgren, M.; Devaux, Y.; Stammet, P.; Al-Mashat, M.; Jögi, J.; et al. The association between plasma miR-122-5p release pattern at admission and all-cause mortality or shock after out-of-hospital cardiac arrest. Biomarkers 2019, 24, 29–35. [Google Scholar] [CrossRef]
- Jäntti, T.; Segersvärd, H.; Tolppanen, H.; Tarvasmäki, T.; Lassus, J.; Devaux, Y.; Vausort, M.; Pulkki, K.; Sionis, A.; Bayes-Genis, A.; et al. Circulating levels of microRNA 423-5p are associated with 90 day mortality in cardiogenic shock. ESC Heart Fail. 2019, 6, 98–102. [Google Scholar] [CrossRef]
- Iborra-Egea, O.; Rueda, F.; Lakkisto, P.; Harjola, V.-P.; García-García, C.; Bayes-Genis, A. Circulating MiRNA Dynamics in ST-Segment Elevation Myocardial Infarction-driven Cardiogenic Shock. Rev. Española Cardiol. 2019, 72, 783–786. [Google Scholar] [CrossRef]
- Kura, B.; Kalocayova, B.; Devaux, Y.; Bartekova, M. Potential clinical implications of mir-1 and mir-21 in heart disease and cardioprotection. Int. J. Mol. Sci. 2020, 21, 700. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Nie, H.; Zhang, K.; Ma, D.; Yang, G.; Zheng, Z.; Liu, K.; Yu, B.; Zhai, C.; Yang, S. A feedback regulatory loop between HIF-1α and miR-21 in response to hypoxia in cardiomyocytes. FEBS Lett. 2014, 588, 3137–3146. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhu, P.; Yang, J.; Liu, X.; Dong, S.; Wang, X.; Chun, B.; Zhuang, J.; Zhang, C. Ischaemic preconditioning-regulated miR-21 protects heart against ischaemia/reperfusion injury via anti-apoptosis through its target PDCD4. Cardiovasc. Res. 2010, 87, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Dong, T.; Zhou, H.; Wang, L.; Huang, A.; Feng, B.; Quan, Y.; Jin, R.; Zhang, W.; Sun, J.; et al. miR-320a suppresses colorectal cancer progression by targeting Rac1. Carcinogenesis 2013, 35, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zeng, J.; Pan, J.; Geng, X.; Li, L.; Wu, J.; Song, P.; Wang, Y.; Liu, J.; Wang, L. MiR-320a inhibits gastric carcinoma by targeting activity in the FoxM1-P27KIP1 axis. Oncotarget 2016, 7, 29275–29286. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Sun, W.; Yue, S.; Yang, J.; Li, J.; Ma, B.; Wang, J.; Yang, X.; Pu, M.; et al. Loss of exosomal miR-320a from cancer-associated fibroblasts contributes to HCC proliferation and metastasis. Cancer Lett. 2017, 397, 33–42. [Google Scholar] [CrossRef]
- Galeano-Otero, I.; Del Toro, R.; Guisado, A.; Díaz, I.; Mayoral-González, I.; Guerrero-Márquez, F.; Gutiérrez-Carretero, E.; Casquero-Domínguez, S.; Díaz-de la Llera, L.; Barón-Esquivias, G.; et al. Circulating miR-320a as a predictive biomarker for left ventricular remodelling in STEMI patients undergoing primary percutaneous coronary intervention. J. Clin. Med. 2020, 9, 1051. [Google Scholar] [CrossRef]
- Kataja, A.; Tarvasmäki, T.; Lassus, J.; Køber, L.; Sionis, A.; Spinar, J.; Parissis, J.; Carubelli, V.; Cardoso, J.; Banaszewski, M.; et al. Altered mental status predicts mortality in cardiogenic shock—Results from the CardShock study. Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 38–44. [Google Scholar] [CrossRef]
- Zampetaki, A.; Mayr, M. Analytical challenges and technical limitations in assessing circulating MiRNAs. Thromb. Haemost. 2012, 108, 592–598. [Google Scholar]
- Gidlöf, O.; Andersson, P.; Van Der Pals, J.; Götberg, M.; Erlinge, D. Cardiospecific microRNA plasma levels correlate with troponin and cardiac function in patients with ST elevation myocardial infarction, are selectively dependent on renal elimination, and can be detected in urine samples. Cardiology 2011, 118, 217–226. [Google Scholar] [CrossRef]
- Neal, C.S.; Michael, M.Z.; Pimlott, L.K.; Yong, T.Y.; Li, J.Y.Z.; Gleadle, J.M. Circulating microRNA expression is reduced in chronic kidney disease. Nephrol. Dial. Transpl. 2011, 26, 3794–3802. [Google Scholar] [CrossRef]
- Szelenberger, R.; Kacprzak, M.; Saluk-Bijak, J.; Zielinska, M.; Bijak, M. Plasma MicroRNA as a novel diagnostic. Clin. Chim. Acta 2019, 499, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Hinkel, R.; Ramanujam, D.; Kaczmarek, V.; Howe, A.; Klett, K.; Beck, C.; Dueck, A.; Thum, T.; Laugwitz, K.L.; Maegdefessel, L.; et al. AntimiR-21 prevents myocardial dysfunction in a pig model of ischemia/reperfusion injury. J. Am. Coll. Cardiol. 2020, 75, 1788–1800. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Tarvasmäki, T.; Haapio, M.; Mebazaa, A.; Sionis, A.; Silva-Cardoso, J.; Tolppanen, H.; Lindholm, M.G.; Pulkki, K.; Parissis, J.; Harjola, V.-P.; et al. Acute kidney injury in cardiogenic shock: Definitions, incidence, haemodynamic alterations, and mortality. Eur. J. Heart Fail. 2018, 20, 572–581. [Google Scholar] [CrossRef]
- Devaux, Y.; Vausort, M.; Goretti, E.; Nazarov, P.V.; Azuaje, F.; Gilson, G.; Corsten, M.F.; Schroen, B.; Lair, M.L.; Heymans, S.; et al. Use of circulating microRNAs to diagnose acute myocardial infarction. Clin. Chem. 2012, 58, 559–567. [Google Scholar] [CrossRef] [PubMed]
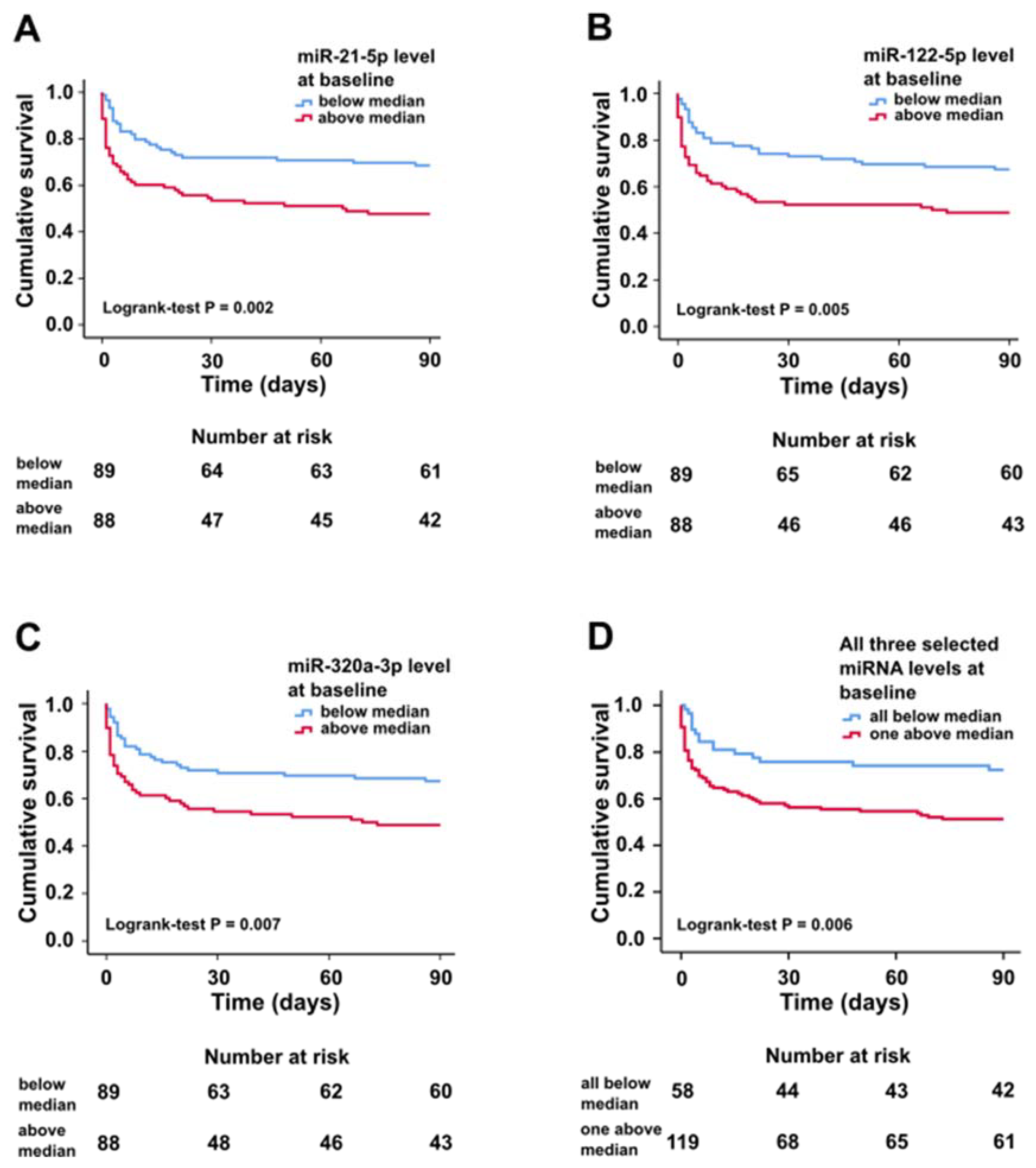
| Variable | All (n = 179) | All Selected miRNA Below Median (n = 59) | One or More of Selected miRNA above Median (n = 120) | p-Value |
|---|---|---|---|---|
| Age, years | 66 (12) | 64 (13) | 67 (12) | 0.096 |
| Women, n (%) | 47 (26) | 11 (19) | 36 (30) | 0.105 |
| BMI, kg/m2 | 26.9 (4.2) | 26.2 (4.3) | 27.3 (4.1) | 0.101 |
| Prior MI, n (%) | 45 (25) | 14 (24) | 31 (26) | 0.757 |
| Prior CABG, n (%) | 11 (6) | 5 (9) | 6 (5) | 0.363 |
| Clinical Characteristics | ||||
| ACS etiology, n (%) | 143 (80) | 45 (76) | 98 (82) | 0.395 |
| Altered mental state at presentation, n (%) | 118 (67) | 34 (58) | 84 (71) | 0.719 |
| Oliguria, n (%) | 94 (53) | 29 (49) | 65 (56) | 0.424 |
| LVEF, % | 33 (14) | 33 (14) | 33 (14) | 0.734 |
| Biochemical Findings | ||||
| eGFR, mL/min/1.73 m2 | 63 (30) | 72 (29) | 58 (29) | 0.003 |
| hsTnT, ng/L | 2190 (388–5418) | 1473 (407–5419) | 2427 (386–5417) | 0.645 |
| NT-proBNP, ng/L | 2710 (585–9434) | 2475 (942–7487) | 2759 (563–9716) | 0.888 |
| ALT, U/L | 44 (20–92) | 21 (11–42) | 66 (29–129) | <0.001 |
| Blood lactate, mmol/L | 2.7 (1.7–5.7) | 2.1 (1.4–3.1) | 3.4 (2.1–6.7) | <0.001 |
| CRP, mg/L | 16 (4–54) | 17 (5–48) | 15 (4–60) | 0.925 |
| Variable | All (n = 179) | miRNA Below Median (n = 59) | miRNA above Median (n = 120) | p-Value |
|---|---|---|---|---|
| miR-21-5p | ||||
| eGFR, mL/min/1.73 m2 | 63 (30) | 70 (31) | 55 (26) | 0.001 |
| Blood lactate, mmol/L | 2.7 (1.7–5.7) | 2.2 (1.3–3.2) | 3.7 (2.3–6.7) | 0.001 |
| ALT, U/L | 44 (20–92) | 31 (17–66) | 71 (28–129) | <0.001 |
| In hospital mortality, n (%) | 67 (37) | 27 (30) | 40 (45) | 0.038 |
| Acute kidney injury, * n (%) | 67 (44) | 29 (35) | 38 (55) | 0.015 |
| 90-day mortality, n (%) | 74 (42) | 28 (31) | 46 (52) | 0.005 |
| miR-122-5p | ||||
| Altered mental state, n (%) | 118 (67) | 50 (56) | 68 (77) | 0.003 |
| eGFR, mL/min/1.73 m2 | 63 (30) | 67 (29) | 58 (30) | 0.041 |
| Blood lactate, mmol/L | 2.7 (1.7–5.7) | 2.1 (1.4–3.1) | 5.0 (2.4–8.2) | <0.001 |
| ALT, U/L | 44 (20–92) | 21 (12–42) | 88 (49–175) | <0.001 |
| Total bilirubin, μμmol/L | 9.6 (5.7–15.4) | 8.6 (5.7–12.7) | 10.5 (6.1–20.3) | 0.047 |
| In hospital mortality; n (%) | 67 (37) | 26 (29) | 41 (46) | 0.018 |
| 90-day mortality; n (%) | 74 (42) | 29 (33) | 45 (51) | 0.012 |
| miR-320a-3p | ||||
| Cardiac index, # L/min/m2 | 2.2 (0.9) | 2.5 (1.0) | 1.8 (0.6) | 0.021 |
| eGFR, mL/min/1.73 m2 | 63 (30) | 68 (30) | 58 (28) | 0.024 |
| Blood lactate, mmol/L | 2.7 (1.7–5.7) | 2.4 (1.5–4.3) | 3.5 (2.1–6.5) | 0.015 |
| ALT, U/L | 44 (20–92) | 31 (17–69) | 58 (29–129) | <0.001 |
| 90-day mortality; n (%) | 74 (42) | 29 (33) | 45 (51) | 0.012 |
| miR-21-5p | miR-122-5p | miR-320a-3p | ALT | Creatinine | Lactate | hsTnT | NT-proBNP | |
|---|---|---|---|---|---|---|---|---|
| miR-21-5p | 1.00 | 0.66 *** | 0.90 *** | 0.39 *** | 0.17 * | 0.28 *** | 0.12 | −0.02 |
| miR-122-5p | 1.00 | 0.67 *** | 0.72 *** | 0.16 * | 0.50 *** | 0.00 | −0.08 | |
| miR-320a-3p | 1.00 | 0.39 *** | 0.18 * | 0.25 ** | 0.11 | −0.08 |
| Variable | Hazard Ratio (95% CI) | p-Value |
|---|---|---|
| miR-21-5p | ||
| miR-21-5p level above median | 2.10 (1.26–3.49) | 0.004 |
| Age | 1.02 (0.99–1.04) | 0.183 |
| Altered mental state | 1.92 (1.01–3.65) | 0.048 |
| Previous MI or CABG | 1.74 (1.05–2.87) | 0.031 |
| ACS etiology | 1.52 (0.76–3.04) | 0.240 |
| LVEF | 0.97 (0.95–0.99) | 0.002 |
| Lactate | 1.08 (1.03–1.14) | 0.001 |
| eGFR | 0.99 (0.98–1.00) | 0.049 |
| ALT | 1.00 (1.00–1.00) | 0.301 |
| miR-122-5p | ||
| miR-122-5p level above median | 1.33 (0.76–2.34) | 0.321 |
| Age | 1.02 (0.99–1.04) | 0.250 |
| Altered mental state | 1.71 (0.88–3.29) | 0.111 |
| Previous MI or CABG | 1.77 (1.06–2.94) | 0.028 |
| ACS etiology | 1.59 (0.78–3.23) | 0.198 |
| LVEF | 0.98 (0.96–1.00) | 0.014 |
| Lactate | 1.09 (1.03–1.14) | 0.001 |
| eGFR | 0.99 (0.98–1.00) | 0.038 |
| ALT | 1.00 (1.00–1.00) | 0.481 |
| miR-320a-3p | ||
| miR-320a-3p level above median | 2.01 (1.21–3.31) | 0.007 |
| Age | 1.02 (0.99–1.05) | 0.140 |
| Altered mental state | 1.72 (0.91–3.27) | 0.098 |
| Previous MI or CABG | 1.79 (1.08–2.97) | 0.024 |
| ACS etiology | 1.48 (0.74–2.98) | 0.271 |
| LVEF | 0.97 (0.95–0.99) | 0.006 |
| Lactate | 1.09 (1.04–1.14) | 0.001 |
| eGFR | 0.99 (0.98–1.00) | 0.051 |
| ALT | 1.00 (1.00–1.00) | 0.339 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hänninen, M.; Jäntti, T.; Tolppanen, H.; Segersvärd, H.; Tarvasmäki, T.; Lassus, J.; Vausort, M.; Devaux, Y.; Sionis, A.; Tikkanen, I.; et al. Association of miR-21-5p, miR-122-5p, and miR-320a-3p with 90-Day Mortality in Cardiogenic Shock. Int. J. Mol. Sci. 2020, 21, 7925. https://doi.org/10.3390/ijms21217925
Hänninen M, Jäntti T, Tolppanen H, Segersvärd H, Tarvasmäki T, Lassus J, Vausort M, Devaux Y, Sionis A, Tikkanen I, et al. Association of miR-21-5p, miR-122-5p, and miR-320a-3p with 90-Day Mortality in Cardiogenic Shock. International Journal of Molecular Sciences. 2020; 21(21):7925. https://doi.org/10.3390/ijms21217925
Chicago/Turabian StyleHänninen, Mikko, Toni Jäntti, Heli Tolppanen, Heli Segersvärd, Tuukka Tarvasmäki, Johan Lassus, Mélanie Vausort, Yvan Devaux, Alessandro Sionis, Ilkka Tikkanen, and et al. 2020. "Association of miR-21-5p, miR-122-5p, and miR-320a-3p with 90-Day Mortality in Cardiogenic Shock" International Journal of Molecular Sciences 21, no. 21: 7925. https://doi.org/10.3390/ijms21217925
APA StyleHänninen, M., Jäntti, T., Tolppanen, H., Segersvärd, H., Tarvasmäki, T., Lassus, J., Vausort, M., Devaux, Y., Sionis, A., Tikkanen, I., Harjola, V.-P., Lakkisto, P., & for the CardShock Study Group. (2020). Association of miR-21-5p, miR-122-5p, and miR-320a-3p with 90-Day Mortality in Cardiogenic Shock. International Journal of Molecular Sciences, 21(21), 7925. https://doi.org/10.3390/ijms21217925







