Selective Formation, Reactivity, Redox and Magnetic Properties of MnIII and FeIII Dinuclear Complexes with Shortened Salen-Type Schiff Base Ligands
Abstract
:1. Introduction
2. Results and Discussion
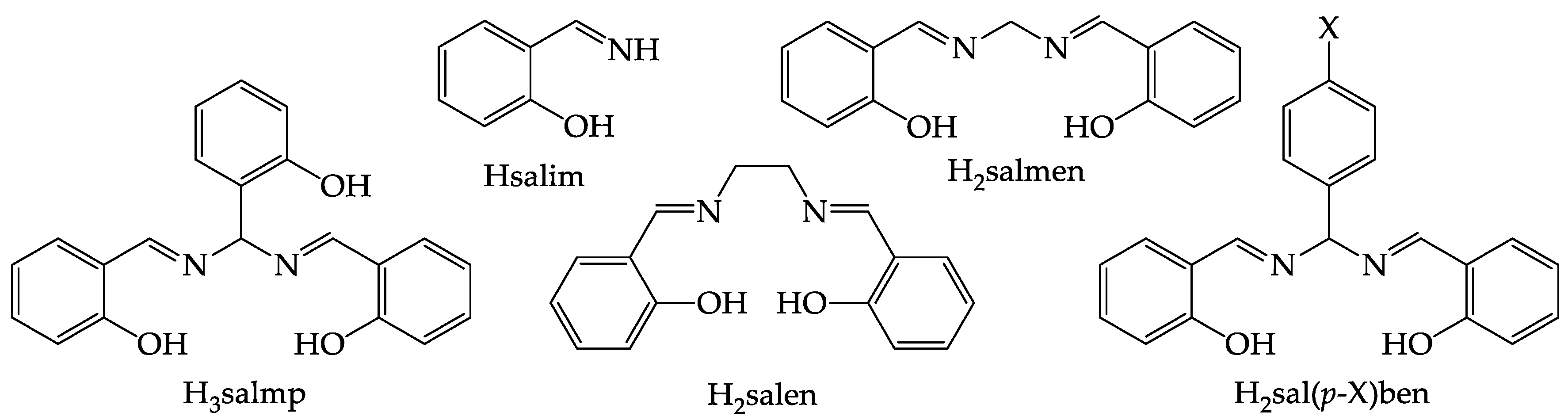
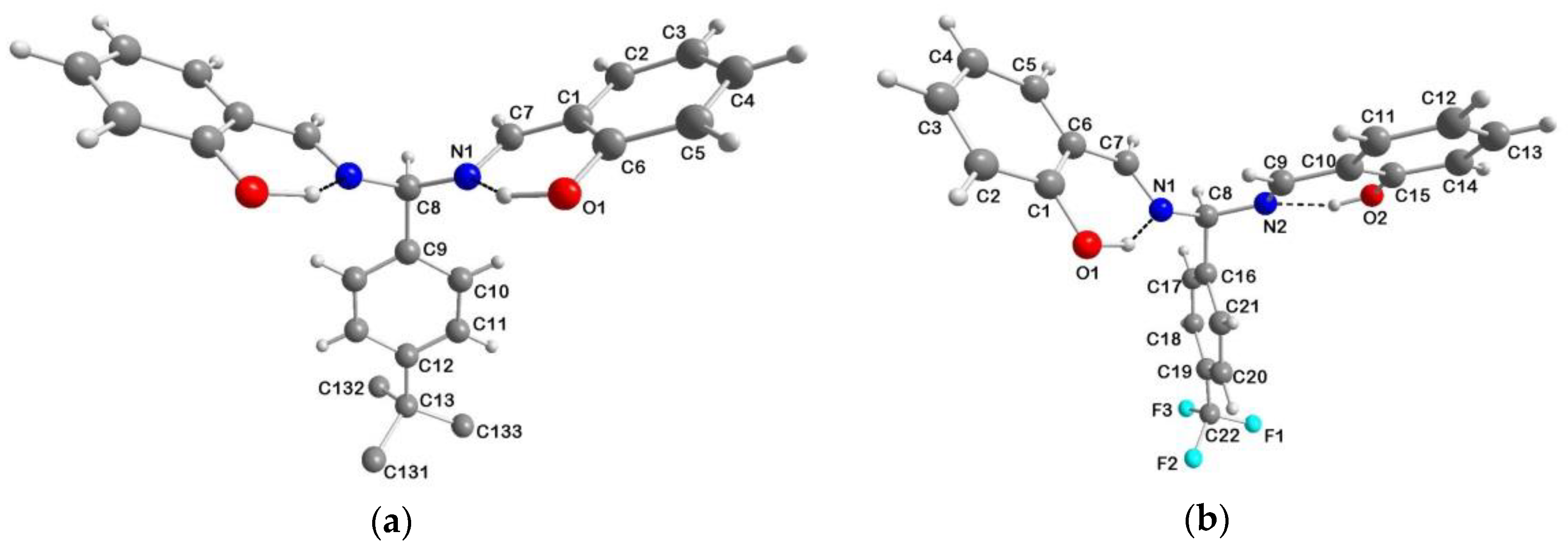
2.1. Synthesis of the Shortened Schiff Bases and Crystal Structures of H2sal(p-X)ben (X = tBu, CF3)
2.2. Synthesis, Isolation and Reactivity of Dinuclear Complexes with H3salmp
M = Mn (1a), Fe (2a); X = AcO, Cl, NO3
[Fe2(μ-salmp)(μ-OMe)(salim)2] (2b) + salH + 6 NaCl + 5 H2O
2.3. Dinuclear Complexes with Tetradentate Ligands and Reactivity in Solution
R = H, Me, Et; M = Mn (3a–c), Fe (4a–c); X = AcO, Cl
X = tBu, Me, H, F, Cl, CF3, NO2; M = Mn (5a–g), Fe (6a–g); X = AcO, NO3
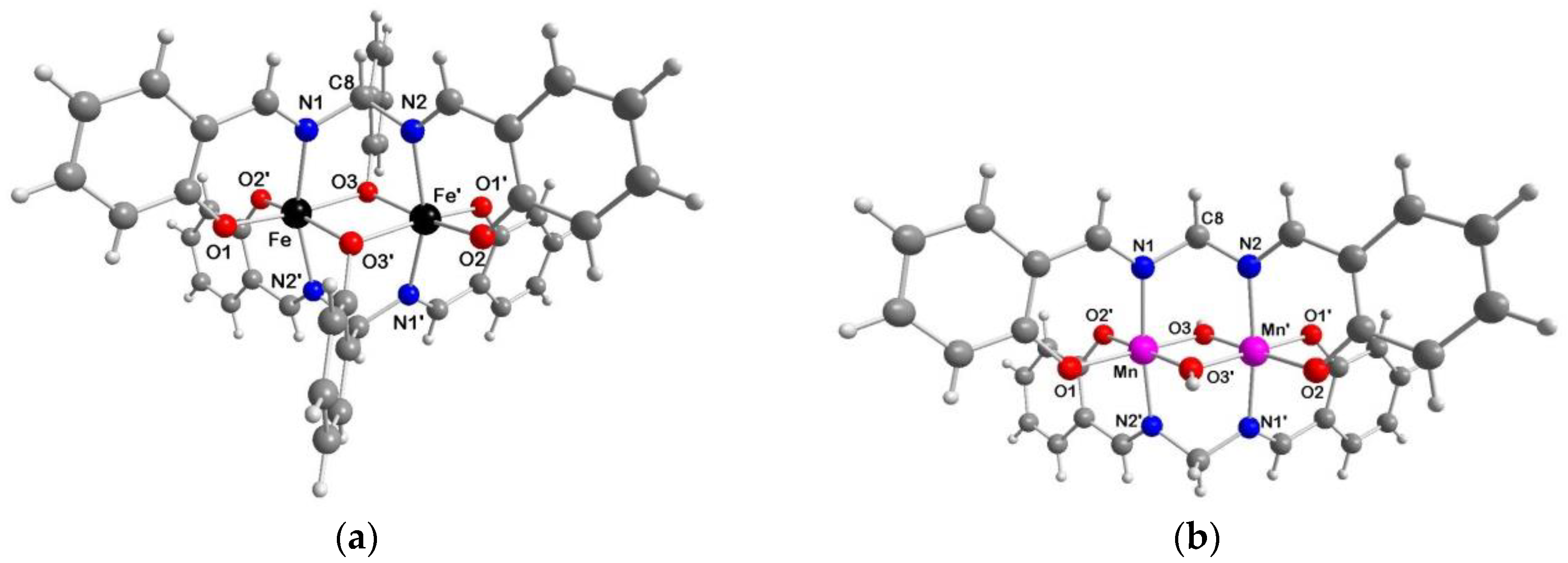
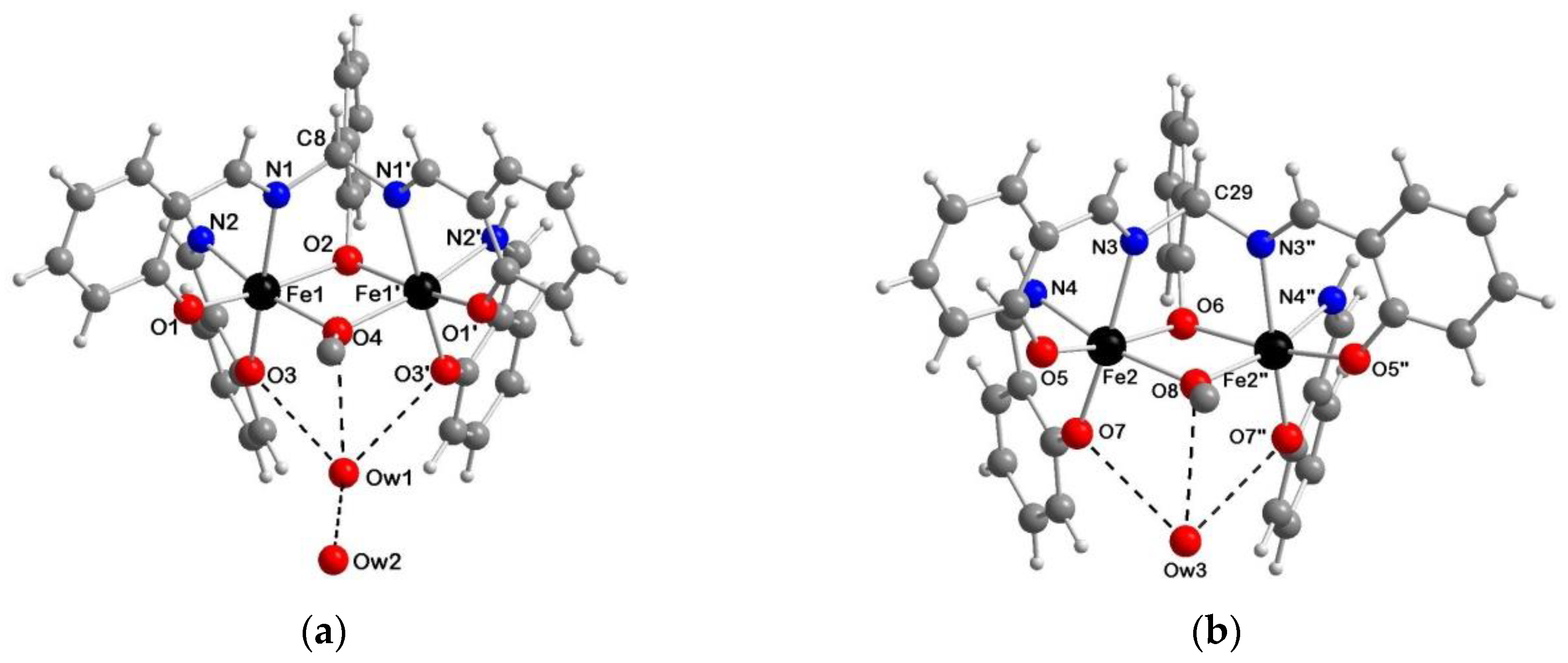
2.4. Molecular Structures of 2a·2AcOEt, 2a·2CH3CN, 2b·1.5H2O and 3c·2DMF
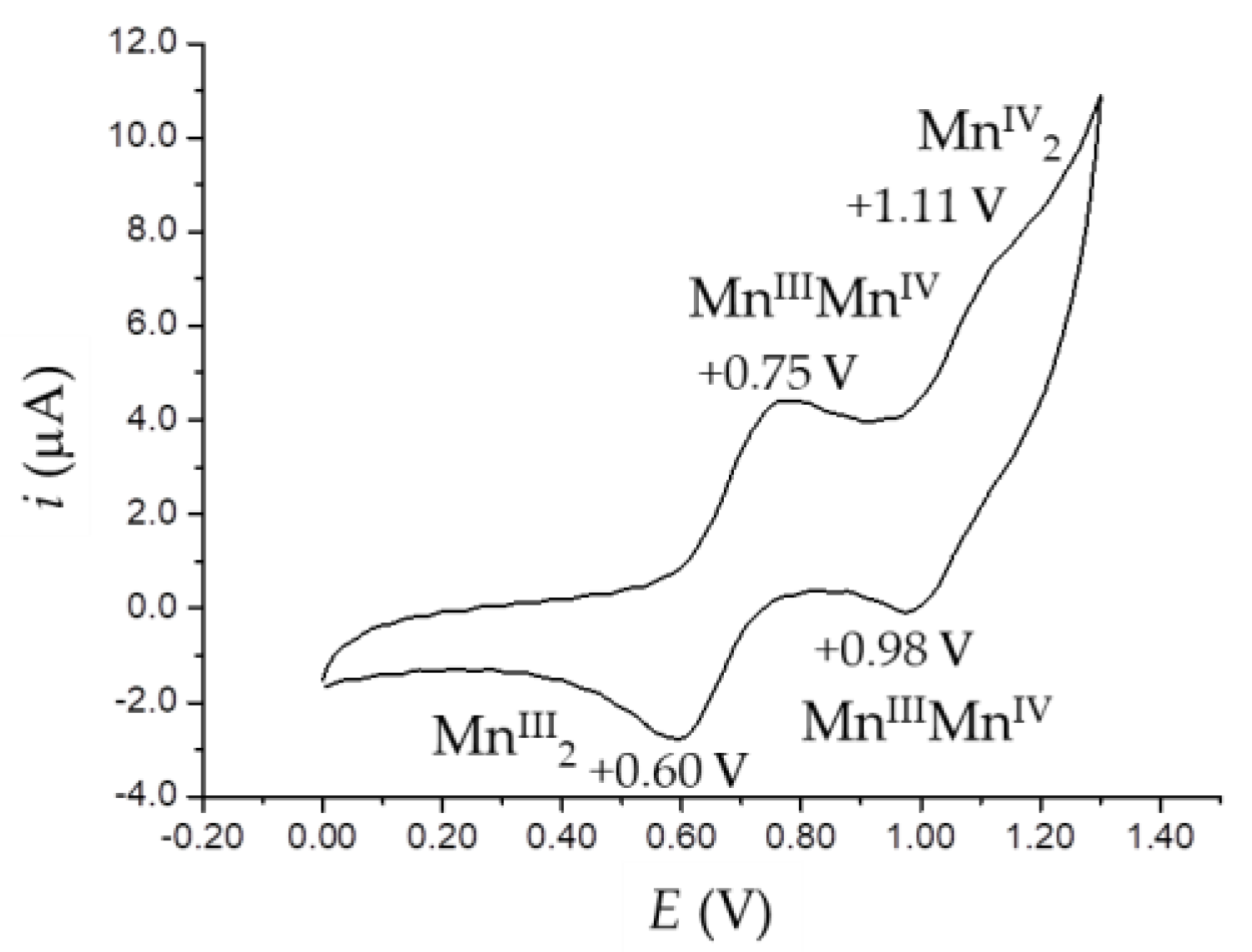
2.5. Cyclic Voltammetry Studies
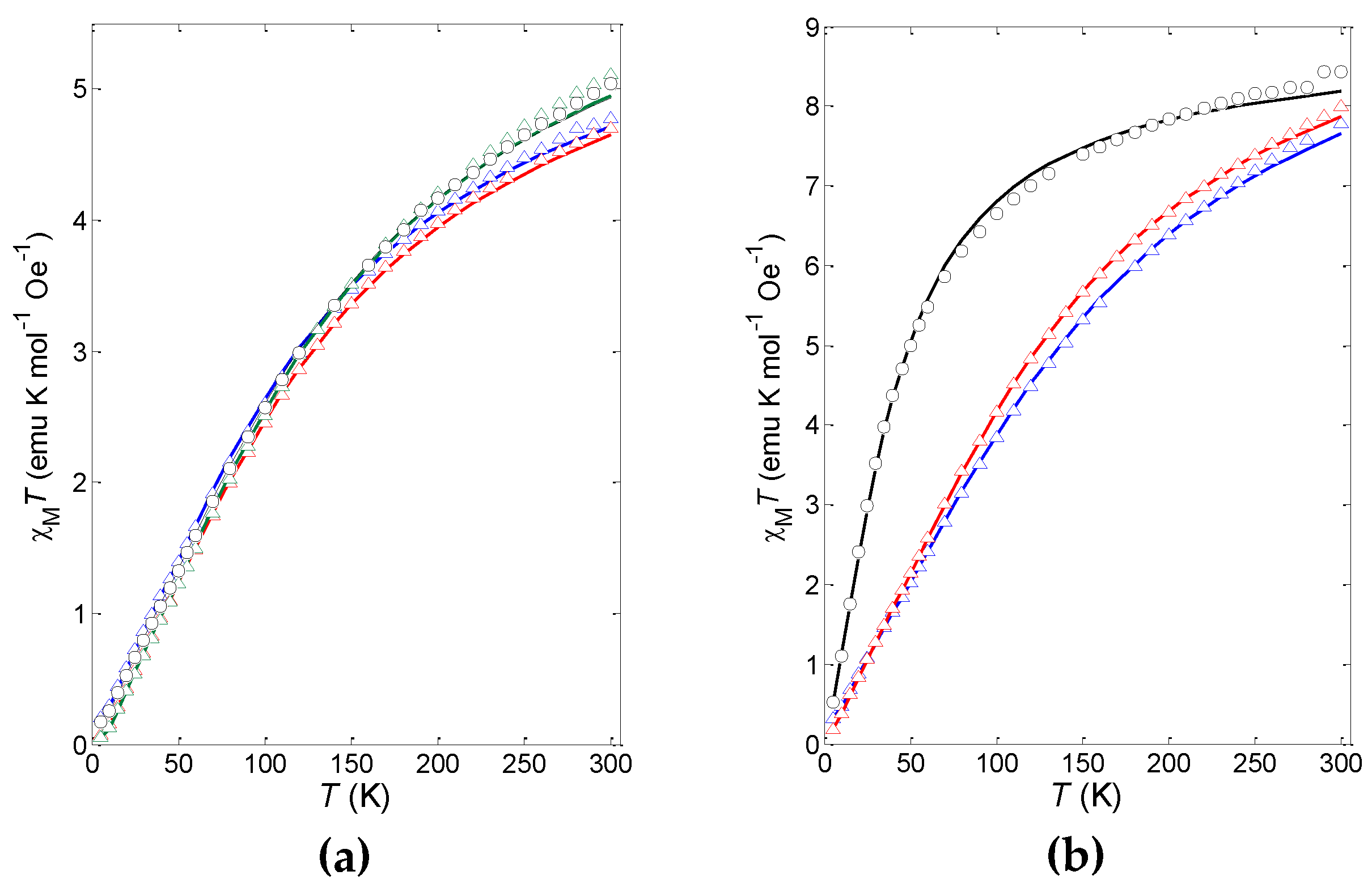
2.6. Magnetic Properties
3. Materials and Methods
3.1. General Procedures
3.2. Synthesis of [Mn2(μ-salmp)2] (1a)
3.3. Synthesis of [Fe2(μ-salmp)2] (2a)
3.4. Synthesis of [Fe2(μ-salmp)(μ-OMe)(salim)2] (2b)
3.5. Synthesis of [Fe2(μ-salmp)(μ-OH)(salim)2] (2c)
3.6. Synthesis of [Mn2(μ-salmen)2(μ-OEt)2] (3a)
3.7. Synthesis of [Fe2(μ-salmen)2(μ-OEt)2] (4a)
3.8. Synthesis of [Mn2(μ-sal(p-tBu)ben)2(μ-OMe)2] (5a)
3.9. Synthesis of [Fe2(μ-sal(p-tBu)ben)2(μ-OMe)2] (6a)
3.10. X-ray Data Collection and Structure Determination
3.11. Cyclic Voltammetry
3.12. Magnetic Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| H3salmp | N,N′-bis(salicylidene)-2-hydroxyphenylmethanediamine |
| H2salmen | N,N′-bis(salicylidene)methanediamine |
| H2sal(p-X)ben | N,N′-bis(salicylidene)-4-X-phenylmethanediamine |
| Hsalim | salicylaldimine |
| salH | salicylaldehyde |
| DMF | dimethylformamide |
| AcOEt | ethyl acetate |
| RT | room temperature |
| MS-ESI | Mass Spectrometry—Electron Spray Ionization |
References
- Tsumaki, T. Nebenvalenzringverbindungen. V. Spektrochemische Untersuchungen über die innerkomplexen Metallsalze von Salicylaldehyd-äthylendiimin und seinen verwandten Verbindungen. Bull. Chem. Soc. Jpn. 1938, 13, 583–591. [Google Scholar] [CrossRef]
- Kambe, S.; Takajo, T.; Saito, K.; Hayashi, T.; Sakurai, A.; Midorikawa, H. A Simple synthesis of a new heterocycle from N, N′-Bis [2-hydroxybenzylidene]-2-hydroxy-α, α-tolyldiamine and Carbonyl Compounds. Synthesis 1975, 1975, 802–804. [Google Scholar] [CrossRef]
- Tsumaki, T. Über einige eisenverbindungen mit nebenvalenzringen. Bull. Chem. Soc. Jpn. 1935, 10, 74–81. [Google Scholar] [CrossRef]
- Takajo, T.; Kambe, S.; Ando, W. Synthesis of N,N′-Bis[2-hydroxybenzylidene]arylmethanediamines. Synthesis 1984, 1984, 256–259. [Google Scholar] [CrossRef]
- Choudhary, N.; Hughes, D.L.; Kleinkes, U.; Larkworthy, L.F.; Leigh, G.; Maiwald, M.; Marmion, C.J.; Sanders, J.; Smith, G.W.; Sudbrake, C. New tetradentate Schiff bases, their oxovanadium(IV) complexes, and some complexes of bidentate Schiff bases with vanadium(III). Polyhedron 1997, 16, 1517–1528. [Google Scholar] [CrossRef]
- Novitchi, G.; Shova, S.; Cascaval, A.; Gulea, A. Synthesis, structure and complex formation of N,N′-disalicylidenemethylendiamine (Salmen). Rev. Roum. Chim. 2002, 47, 1027–1035. [Google Scholar]
- Xu, X.; Wang, X.; Liu, H.; You, X. Synthesis, characterization and crystal structure of a binuclear molybdenum(VI) oxo complex of a dianionic tetradentate Schiff-base ligand. J. Chem. Soc. Dalton Trans. 1993, 1377–1379. [Google Scholar] [CrossRef]
- Tsumaki, T.; Antoku, S.; Shito, M. Coordinate valency rings. XXIV. On some inner complex ferric salts of hydrosalicylamide and its derivatives. Bull. Chem. Soc. Jpn. 1960, 33, 1096–1100. [Google Scholar] [CrossRef]
- Batley, G.; Graddon, D. Nickel(II) hydrosalicylamide complexes. Aust. J. Chem. 1967, 20, 1749. [Google Scholar] [CrossRef] [Green Version]
- Ghoreishi, S.M.; Behpour, M.; Mazaheri, S.; Naeimi, H. Uranyl sensor based on a N,N′-bis (salicylidene)-2-hydroxy-phenylmethanediamine and multiwall carbon nanotube electrode. J. Radioanal. Nucl. Chem. 2012, 293, 201–210. [Google Scholar] [CrossRef]
- Pasini, A.; Ferrari, R.P.; Lanfranconi, S.; Pozzi, A.; Laurenti, E.; Moroni, M. Metal complexes of quadridentate Schiff bases which can form a four-membered chelate ring. Inorg. Chim. Acta 1997, 266, 1–3. [Google Scholar] [CrossRef]
- Naeimi, H.; Rabiei, K.; Salimi, F. Rapid, efficient and facile synthesis and characterization of novel Schiff bases and their complexes with transition metal ions. Dyes Pigment. 2007, 75, 294–297. [Google Scholar] [CrossRef]
- Spatarescu, I.; Sibiescu, D.; Rosca, I.; Cailean, A.; Cretescu, I.; Secula, M.S. Synthesis and Characterization of a new coordination compounds of Cr(III), Fe(III) and Cu(II) with ligand derived from N,N′-bis (salicylidene)-methinmethyldiamine. Rev. Chim. 2010, 61, 306–310. [Google Scholar]
- Ammar, R.A.A.; Alaghaz, A.-N.M.A. Synthesis, spectroscopic characterization and potentiometric studies of a tetradentate [N2O2] Schiff base, N,N′-bis(2-hydroxybenzylidene)-1,1-diaminoethane and its Co(II), Ni(II), Cu(II) and Zn(II) complexes. Int. J. Electrochem. Sci. 2013, 8, 8686–8699. [Google Scholar]
- Dewan, A.; Bora, U.; Borah, G. A simple and efficient tetradentate Schiff base derived palladium complex for Suzuki-Miyaura reaction in water. Tetrahedron Lett. 2014, 55, 1689–1692. [Google Scholar] [CrossRef]
- Gogoi, A.; Dewan, A.; Borah, G.; Bora, U. A palladium salen complex: An efficient catalyst for the Sonogashira reaction at room temperature. New J. Chem. 2015, 39, 3341–3344. [Google Scholar] [CrossRef]
- Gogoi, A.; Sarmah, G.; Dewan, A.; Bora, U. Unique copper-salen complex: An efficient catalyst for N-arylations of anilines and imidazoles at room temperature. Tetrahedron Lett. 2014, 55, 31–35. [Google Scholar] [CrossRef]
- Downing, R.S.; Urbach, F.L. Circular dichroism of square-planar, tetradentate Schiff base chelates of copper(II). J. Am. Chem. Soc. 1969, 91, 5977–5983. [Google Scholar] [CrossRef]
- Pasini, A.; Demartin, F.; Piovesana, O.; Chiari, B.; Cinti, A.; Crispu, O. Novel copper(II) complexes of “short” salen homologues. Structure and magnetic properties of the tetranuclear complex [Cu2(L2)2]2 [H2L2 = phenyl-N,N′-bis(salicylidene)methanediamine]. J. Chem. Soc. Dalton Trans. 2000, 3467–3472. [Google Scholar] [CrossRef]
- Chiari, B.; Cinti, A.; Crispu, O.; Demartin, F.; Pasini, A.; Piovesana, O. Binuclear Co(II)Co(II), Co(II)Co(III) and Co(III)Co(III) complexes of “short” salen homologues derived from the condensation of salicylaldehyde and methanediamine or phenylmethanediamines. Synthesis, structures and magnetism. J. Chem. Soc. Dalton Trans. 2001, 3611–3616. [Google Scholar] [CrossRef]
- Illingsworth, M.L.; Schwartz, L.J.; Jensen, A.J.; Zhu, T.; Knappenberger, E.J.; Sweet, J.E.; Wilkinson, P.S.; Waltermire, B.E.; Rheingold, A.L. Synthesis, structure, and reactivity of bis(N,N′-bis (2-hydroxybenzylidene)-2-hydroxyphenylmethanediaminato)zirconium(IV), a Schiff base complex with 6,4,6-membered chelate rings. Polyhedron 2002, 21, 211–218. [Google Scholar] [CrossRef]
- Li, L.; Zhou, Y.; Yuan, F. Bis(μ-2-{bis-[(2-oxidobenzylidene)amino]methyl}phenolato) bis[(tetrahydrofuran)samarium(III)] tetrahydrofuran disolvate. Acta Crystallogr. Sect. E Struct. Rep. Online 2012, 68, m603–m604. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Cao, Z.-H.; Ge, J.-Y.; Sun, Y.-C.; Ouyang, Z.-W.; Zuo, J.-L.; Wang, Z.-X.; Kurmoo, M. Magnetostructural relationship for μ2-phenoxido bridged ferric dimers. Dalton Trans. 2017, 46, 4317–4324. [Google Scholar] [CrossRef]
- Bill, E.; Krebs, C.; Winter, M.; Gerdan, M.; Trautwein, A.X.; Flörke, U.; Haupt, H.-J.; Chaudhuri, P. A Triangular iron(III) complex potentially relevant to iron(III)-binding sites in Ferreascidin. Chem. Eur. J. 1997, 3, 193–201. [Google Scholar] [CrossRef]
- Chaudhuri, P.; Hess, M.; Weyhermüller, T.; Bill, E.; Haupt, H.-J.; Flörke, U. A novel series of asymmetric trinuclear M(III) complexes (M = Ti, V, Cr, Mn, Fe, Co) with the [M3O2]6+ core by a deoximation reaction exemplified by the V(III) complex. Inorg. Chem. Commun. 1998, 1, 39–42. [Google Scholar] [CrossRef]
- Hendrich, M.P.; Day, E.P.; Wang, C.-P.; Synder, B.S.; Holm, R.H.; Münck, E. Ground-state electronic structures of binuclear iron(II) sites: Experimental protocol and a consistent description of Moessbauer, EPR, and magnetization measurements of the bis(phenolate)-bridged complex [Fe2(salmp)2]2. Inorg. Chem. 1994, 33, 2848–2856. [Google Scholar] [CrossRef]
- Yu, S.B.; Wang, C.P.; Day, E.P.; Holm, R.H. A binuclear manganese system with three isolated oxidation states: Synthesis, structures, and properties of molecules with a Mn2(OR)2 bridge unit containing MnIIIMnIII, MnIIIMnII, and MnIIMnII. Inorg. Chem. 1991, 30, 4067–4074. [Google Scholar] [CrossRef]
- Surerus, K.K.; Münck, E.; Snyder, B.S.; Holm, R.H. A binuclear mixed-valence ferromagnetic iron system with an S = 9/2 ground state and valence trapped and detrapped states. J. Am. Chem. Soc. 1989, 111, 5501–5502. [Google Scholar] [CrossRef]
- Samus, N.M.; Strelkov, E.A.; Tsapkov, V.I.; Gulya, A.P. Lanthanide Complexes with N,N′-Bis(2- hydroxybenzylidene)-2-hydroxyphenylmethanediamine. Russ. J. Coord. Chem. 2004, 30, 275–279. [Google Scholar] [CrossRef]
- Li, L.; Yuan, F. Unexpected C=N bond cleavage in H3salmp: Synthesis and structures of [LnNa(salmp)(sal)]2·6EtOH (Ln = Sm, Er; H3salmp = 2-bis(salicylidieneamino)methylphenol; sal = salicylaldehydato). J. Struct. Chem. 2017, 58, 1472–1476. [Google Scholar] [CrossRef]
- Chiari, B.; Cinti, A.; Crispu, O.; Demartin, F.; Pasini, A.; Piovesana, O. New pentanuclear mixed valence Co(II)–Co(III) complexes of “short” salen homologues. J. Chem. Soc. Dalton Trans. 2002, 4672–4677. [Google Scholar] [CrossRef]
- Spatarescu, I.; Sibiescu, D.; Rosca, I. The characterization and the synthesis of a new coordination compound for removal of Cr(III) from wastewaters. Environ. Eng. Manag. J. 2010, 9, 443–447. [Google Scholar] [CrossRef]
- Pollock, C.J.; Lancaster, K.M.; Finkelstein, K.D.; Debeer, S. Study of iron dimers reveals angular dependence of valence-to-core X-ray emission spectra. Inorg. Chem. 2014, 53, 10378–10385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snyder, B.S.; Patterson, G.S.; Abrahamson, A.J.; Holm, R.H. Binuclear iron system ferromagnetic in three oxidation states: Synthesis, structures, and electronic aspects of molecules with a Fe2(OR)2 bridge unit containing Fe(III,III), Fe(III,II), and Fe(II,II). J. Am. Chem. Soc. 1989, 111, 5214–5223. [Google Scholar] [CrossRef]
- Nabulsi, N.A.R.; Fronczek, F.R.; Gandour, R.D. Two polymorphs of 2-bis[(2-hydroxyphenylmethylene) amino]methylphenol. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1988, 44, 1086–1089. [Google Scholar] [CrossRef] [Green Version]
- Stuart, B. Infrared Spectroscopy: Fundamentals and Applications; Analytical Techniques in the Sciences; J. Wiley: Chichester, UK; Hoboken, NJ, USA, 2004; ISBN 978-0-470-85427-3. [Google Scholar]
- Rigamonti, L.; Forni, A.; Pievo, R.; Reedijk, J.; Pasini, A. Copper(II) compounds with NNO tridentate Schiff base ligands: Effect of subtle variations in ligands on complex formation, structures and magnetic properties. Inorg. Chim. Acta 2012, 387, 373–382. [Google Scholar] [CrossRef]
- Rigamonti, L.; Forni, A. Effect of crystal packing and coordinated solvent molecules on metal-ligand bond distances in linear trinuclear nickel compounds with bridging acetato and Schiff base ligands. Inorg. Chim. Acta 2018, 473, 216–222. [Google Scholar] [CrossRef]
- Miyasaka, H.; Saitoh, A.; Abe, S. Magnetic assemblies based on Mn(III) salen analogues. Coord. Chem. Rev. 2007, 251, 2622–2664. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; Wiley-VCH: Weinheim, Germany, 1993. [Google Scholar]
- Adebayo, O.A.; Abboud, K.A.; Christou, G. Mn3 single-molecule magnets and Mn6/Mn9 clusters from the use of methyl 2-pyridyl ketone oxime in manganese phosphinate and phosphonate chemistry. Inorg. Chem. 2017, 56, 11352–11364. [Google Scholar] [CrossRef]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A powerful new program for the analysis of anisotropic monomeric and exchange-coupled polynuclear d- and f-block complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef]
- Rigamonti, L.; Forni, A.; Sironi, M.; Ponti, A.; Ferretti, A.M.; Baschieri, C.; Pasini, A. Experimental and theoretical investigations on magneto-structural correlation in trinuclear copper(II) hydroxido propellers. Polyhedron 2018, 145, 22–34. [Google Scholar] [CrossRef]
- Bhargavi, G.; Rajasekharan, M.; Costes, J.-P.; Tuchagues, J.-P. Synthesis, crystal structure and magnetic properties of dimeric MnIII Schiff base complexes including pseudohalide ligands: Ferromagnetic interactions through phenoxo bridges and single molecule magnetism. Polyhedron 2009, 28, 1253–1260. [Google Scholar] [CrossRef]
- Barros, W.P.; Inglis, R.; Nichol, G.S.; Rajeshkumar, T.; Rajaraman, G.; Piligkos, S.; Stumpf, H.O.; Brechin, E.K. From antiferromagnetic to ferromagnetic exchange in a family of oxime-based MnIII dimers: A magneto-structural study. Dalton Trans. 2013, 42, 16510–16517. [Google Scholar] [CrossRef] [Green Version]
- Comar, P.; Rajeshkumar, T.; Nichol, G.S.; Pitak, M.B.; Coles, S.J.; Rajaraman, G.; Brechin, E.K. Switching the orientation of Jahn–Teller axes in oxime-based MnIII dimers and its effect upon magnetic exchange: A combined experimental and theoretical study. Dalton Trans. 2015, 44, 19805–19811. [Google Scholar] [CrossRef] [Green Version]
- SMART, SAINT and SADABS; Bruker AXS Inc.: Madison, WI, USA, 1997.
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Crystallogr. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, C71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic corrections and Pascal’s constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]
- Leopardi, G. Canti; Starita, S., Ed.; Napoli, Italy, 1835. [Google Scholar]
| Formula | Compound Code |
|---|---|
| [M2(μ-salmp)2] | 1a (M = Mn), 2a (M = Fe) |
| [Fe2(μ-salmp)(μ-OR)(salim)2] | 2b–c (R = Me, H) |
| [Mn2(μ-salmen)2(μ-OR)2] | 3a–c (R = Et, Me, H) |
| [Fe2(μ-salmen)2(μ-OR)2] | 4a–c (R = Et, Me, H) |
| [Mn2(μ-sal(p-X)ben)2(μ-OMe)2] | 5a–g (X = tBu, Me, H, F, Cl, CF3, NO2) |
| [Fe2(μ-sal(p-X)ben)2(μ-OMe)2] | 6a–g (X = tBu, Me, H, F, Cl, CF3, NO2) 1 |
| Parameter | 2a·2AcOEt 3 | 2a·2CH3CN 3 | 2a·2DMF 3 | 1a·2CH3CN 4 | 3c·2DMF 4 |
|---|---|---|---|---|---|
| M–O1 | 1.897(2) | 1.896(2) | 1.894(3) | 1.857(3) | 1.9025(7) |
| M–O2’ | 1.917(2) | 1.905(2) | 1.921(3) | 1.952(3) | 1.9123(6) |
| M–N1 | 2.142(2) | 2.164(2) | 2.156(3) | 2.088(3) | 2.0018(7) |
| M–N2’ | 2.149(2) | 2.144(2) | 2.138(3) | 2.196(3) | 2.0027(7) |
| M–O3 | 2.033(2) | 2.028(2) | 2.023(2) | 2.239(3) | 1.8006(6) |
| M–O3’ | 2.059(2) | 2.057(2) | 2.064(2) | 1.900(3) | 1.7973(6) |
| M···M’ | 3.071(1) | 3.091(2) | 3.063(1) | 3.111(1) | 2.6357(4) |
| M–O3–M’ | 97.24(9) | 98.32(7) | 97.06(9) | 97.1(1) | 94.20(2) |
| O3–M–O3’ | 82.76(9) | 81.68(7) | 82.94(9) | 82.9(1) | 85.80(2) |
| O1–M–O2’ | 94.17(12) | 94.24(8) | 93.6(1) | 93.3(1) | 88.79(3) |
| O2’–M–O3 | 91.78(10) | 92.07(8) | 91.6(1) | 93.0(1) | 91.89(3) |
| O1–M–O3’ | 92.68(10) | 93.69(7) | 93.4(1) | 91.0(1) | 93.54(3) |
| O2’–M–N2’ | 87.04(10) | 86.97(7) | 88.3(1) | 86.5(1) | 90.82(3) |
| O2’–M–N1 | 107.03(10) | 107.62(8) | 101.3(1) | 113.2(1) | 95.02(3) |
| O1–M–N2’ | 102.91(9) | 102.64(7) | 108.4(1) | 95.4(2) | 91.82(3) |
| O1–M–N1 | 88.42(10) | 87.86(7) | 87.0(1) | 90.9(1) | 91.78(3) |
| O3–M–N2’ | 85.07(9) | 86.42(7) | 86.9(1) | 79.9(1) | 88.50(3) |
| O3–M–N1 | 82.47(9) | 81.89(7) | 82.5(1) | 80.1(1) | 87.83(3) |
| O3’–M–N2’ | 82.37(9) | 81.75(7) | 81.8(1) | 85.2(1) | 86.87(3) |
| O3’–M–N1 | 82.44(9) | 82.44(7) | 81.8(1) | 86.4(1) | 87.15(3) |
| O2’–M–O3’ | 168.44(9) | 167.40(6) | 170.5(1) | 166.0(1) | 176.76(3) |
| O1–M–O3 | 170.27(9) | 169.21(6) | 167.8(1) | 173.6(1) | 179.25(3) |
| N1–M–N2’ | 161.42(9) | 161.53(7) | 161.4(1) | 159.1(1) | 173.20(3) |
| Molecule A | Value | Molecule B | Value |
|---|---|---|---|
| Fe1–O1 | 1.917(3) | Fe2–O5 | 1.908(4) |
| Fe1–O2 | 2.069(3) | Fe2–O6 | 2.065(3) |
| Fe1–O3 | 1.940(3) | Fe2–O7 | 1.935(4) |
| Fe1–O4 | 1.985(2) | Fe2–O8 | 1.990(3) |
| Fe1–N1 | 2.175(3) | Fe2–N3 | 2.163(4) |
| Fe1–N2 | 2.086(4) | Fe2–N4 | 2.081(4) |
| Fe1···Fe1’ 1 | 3.149(1) | Fe2···Fe2” 2 | 3.151(1) |
| Fe1–O2–Fe1’ 1 | 99.1(2) | Fe2–O6–Fe2” 2 | 99.4(2) |
| Fe1–O4–Fe1’ 1 | 105.0(2) | Fe2–O8–Fe2” 2 | 104.7(2) |
| O1–Fe1–N2 | 95.05(15) | O5–Fe2–N4 | 93.42(18) |
| N2–Fe1–O2 | 89.20(14) | N4–Fe2–O6 | 87.43(17) |
| O2–Fe1–O4 | 77.17(12) | O6–Fe2–O8 | 77.20(15) |
| O4–Fe1–O1 | 98.27(15) | O8–Fe2–O5 | 101.20(18) |
| O1–Fe1–N1 | 86.93(14) | O5–Fe2–N3 | 86.42(16) |
| O1–Fe1–O3 | 98.41(13) | O5–Fe2–O7 | 97.23(15) |
| N2–Fe1–N1 | 88.33(15) | N4–Fe2–N3 | 85.93(19) |
| N2–Fe1–O3 | 86.05(14) | N4–Fe2–O7 | 86.84(18) |
| O2–Fe1–N1 | 83.15(15) | O6–Fe2–N3 | 83.68(19) |
| O2–Fe1–O3 | 91.89(15) | O6–Fe2–O7 | 92.79(19) |
| O4–Fe1–N1 | 89.41(15) | O8–Fe2–N3 | 89.42(18) |
| O4–Fe1–O3 | 94.91(15) | O8–Fe2–O7 | 96.72(18) |
| O1–Fe1–O2 | 169.10(15) | O5–Fe2–O6 | 169.98(19) |
| N2–Fe1–O4 | 166.35(14) | N4–Fe2–O8 | 164.35(17) |
| N1–Fe1–O3 | 172.56(14) | N3–Fe2–O7 | 172.08(18) |
| Compound | Redox Couple | Epa | Epc | ΔE | E½ |
|---|---|---|---|---|---|
| 3b | MnIV/MnIII | +0.68 | +0.63 | 0.05 | +0.66 |
| MnIII/MnII | −0.00 | −0.30 | 0.30 | −0.15 | |
| – 1 | −0.90 | – | – | ||
| 4b | FeIII/FeII | – 1 | −0.77 | – | – |
| 5a | MnIV/MnIII | +0.75 | +0.60 | 0.15 | +0.68 |
| +1.11 | +0.98 | 0.13 | +1.05 | ||
| MnIII/MnII | – 1 | −0.41 | – | – | |
| – 1 | −0.97 | – | – | ||
| 6a | FeIII/FeII | – 1 | −0.70 | – | – |
| – 1 | −0.84 | – | – |
| Compound | J (cm−1) | g1 | f2 |
|---|---|---|---|
| 3b | –13.64(6) | 2.05 | 2.8% |
| 4b | –2.88(2) | 2.02 | 1.0% |
| 5a | –12.95(4) | 1.98 | 1.2% |
| 5e | –12.25(5) | 1.97 | 3.4% |
| 5f | –13.61(8) | 2.07 | 0.8% |
| 6a | –9.517(8) | 2.20 | 1.6% |
| 6e | –10.71(3) | 2.21 | 2.9% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rigamonti, L.; Zardi, P.; Carlino, S.; Demartin, F.; Castellano, C.; Pigani, L.; Ponti, A.; Ferretti, A.M.; Pasini, A. Selective Formation, Reactivity, Redox and Magnetic Properties of MnIII and FeIII Dinuclear Complexes with Shortened Salen-Type Schiff Base Ligands. Int. J. Mol. Sci. 2020, 21, 7882. https://doi.org/10.3390/ijms21217882
Rigamonti L, Zardi P, Carlino S, Demartin F, Castellano C, Pigani L, Ponti A, Ferretti AM, Pasini A. Selective Formation, Reactivity, Redox and Magnetic Properties of MnIII and FeIII Dinuclear Complexes with Shortened Salen-Type Schiff Base Ligands. International Journal of Molecular Sciences. 2020; 21(21):7882. https://doi.org/10.3390/ijms21217882
Chicago/Turabian StyleRigamonti, Luca, Paolo Zardi, Stefano Carlino, Francesco Demartin, Carlo Castellano, Laura Pigani, Alessandro Ponti, Anna Maria Ferretti, and Alessandro Pasini. 2020. "Selective Formation, Reactivity, Redox and Magnetic Properties of MnIII and FeIII Dinuclear Complexes with Shortened Salen-Type Schiff Base Ligands" International Journal of Molecular Sciences 21, no. 21: 7882. https://doi.org/10.3390/ijms21217882
APA StyleRigamonti, L., Zardi, P., Carlino, S., Demartin, F., Castellano, C., Pigani, L., Ponti, A., Ferretti, A. M., & Pasini, A. (2020). Selective Formation, Reactivity, Redox and Magnetic Properties of MnIII and FeIII Dinuclear Complexes with Shortened Salen-Type Schiff Base Ligands. International Journal of Molecular Sciences, 21(21), 7882. https://doi.org/10.3390/ijms21217882










